Abstract
Objectives:
The objectives of this study were to define appropriate criteria for assessing the presence of lymphedema, and to report the prevalence and risk factors for development of upper limb lymphedema after level I–III axillary dissection for melanoma.
Summary Background Data:
The lack of a consistent and reliable objective definition for lymphedema remains a significant barrier to appreciating its prevalence after axillary dissection for melanoma (or breast carcinoma).
Methods:
Lymphedema was assessed in 107 patients (82 male, 25 female) who had previously undergone complete level I–III axillary dissection. Of the 107 patients, 17 had also received postoperative axillary radiotherapy. Arm volume was measured using a water displacement technique. Change in volume of the arm on the side of the dissection was referenced to the volume of the other (control) arm. Volume measurements were corrected for the effect of handedness using corrections derived from a control group. Classification and regression tree (CART) analysis was used to determine a threshold fractional arm volume increase above which volume changes were considered to indicate lymphedema.
Results:
Based on the CART analysis results, lymphedema was defined as an increase in arm volume greater than 16% of the volume of the control arm. Using this definition, lymphedema prevalence for patients in the present study was 10% after complete level I–III axillary dissection for melanoma and 53% after additional axillary radiotherapy. Radiotherapy and wound complications were independent risk factors for the development of lymphedema.
Conclusions:
A suggested objective definition for arm lymphedema after axillary dissection is an arm volume increase of greater than 16% of the volume of the control arm.
Based on a new, objective definition, the prevalence of upper limb lymphedema was assessed in 107 patients after complete level I–III axillary lymph node dissection for melanoma. Lymphedema was present in 10% of patients who had surgery only and in 53% of patients who had adjuvant axillary radiotherapy after their surgery.
In 1921, Halsted1 first described upper limb lymphedema as a complication of radical mastectomy, referring to the phenomenon as “elephantiasis chirurgica.” Since that time, many studies have reported a wide range of both subjective and objective prevalence rates for arm lymphedema after removal of lymph nodes from the axilla for treatment of breast carcinoma. These reported prevalence rates range from 2% to 56%2 with various surgical procedures and follow-up periods. Two recent reviews of the breast surgery literature have presented overall upper limb lymphedema prevalence rates of 20%3 and 26%2 after axillary surgery.
Axillary lymph node dissection for the treatment of melanoma is usually more extensive than for breast carcinoma. A complete level I–III dissection is performed routinely for melanoma in most institutions, whereas it is common practice for only level I or level I and II axillary nodes to be removed for breast carcinoma.4,5 There have been few studies attempting to quantify lymphedema prevalence after the more radical level I–III node dissections performed for melanoma.
Urist et al6 reported an arm lymphedema prevalence of only 1% after radical axillary dissection for melanoma. The assessment of lymphedema in their study was based on the difference between arm circumference measurements taken from the arm on the side of the dissection and the other arm. Other studies have relied on clinical judgment alone to assess lymphedema, reported prevalence rates ranging from 0% to 12%.7–9
It is extremely difficult to make meaningful comparisons between the existing studies of lymphedema prevalence in patients with either melanoma or breast carcinoma because, in addition to variations in patient populations and treatment factors, the criteria used to define lymphedema in these studies are highly variable. Moreover, these definitions are either arbitrary or subjective, and their clinical significance is questionable. Because the choice of definition for lymphedema is the final determinant of the prevalence values, application of a consistent definition is essential if relationships between patient and treatment factors and the development of lymphedema are to be fully appreciated.
The main objectives of the present study were to define appropriate criteria for diagnosing the presence of upper limb lymphedema based on upper limb volume measurements and to report the prevalence of the condition in a representative group of melanoma patients who had undergone either a complete level I–III axillary dissection only or a level I–III axillary dissection and postoperative axillary radiotherapy. This study also aimed to present an overview of the patients’ subjective assessments of postoperative morbidity and to explore how this related to the objective assessment of lymphedema.
PATIENTS AND METHODS
Patients
The prevalence of upper limb lymphedema was assessed in 107 patients who had previously undergone a complete lymph node dissection of levels I, II, and III of the axilla for melanoma. Patients were enrolled in the study and had their arms measured on the same day that they presented for a routine follow-up visit at the Sydney Melanoma Unit (SMU), Royal Prince Alfred Hospital, between 1999 and 2002. Arm measurements were made during clinic time (9 am–5 pm), and patients were not given instructions with regard to activity before measurement. No patients wore a compression garment on the day of arm measurement. Patients in whom at least 4 months had elapsed after their surgery were considered eligible for inclusion in the study. Of these, 82 were male and 25 were female. The median age at the time of measurement was 56.9 years (range, 27–86 years). Of the 107 patients in the study, 90 had undergone axillary dissection only and 17 had received axillary radiotherapy subsequent to axillary dissection.
All patients had undergone full axillary dissection (levels I–III) as previously described elsewhere.10 This involved complete clearance of the axillary contents to the apex of the axilla (defined by the subclavius tendon), exposing the axillary vein, and preserving the thoracodorsal and long thoracic nerves when appropriate. The intercostobrachial nerve was routinely sacrificed. Pectoralis minor muscle was routinely detached superiorly from the coracoid process and divided inferiorly near its attachment to the chest wall. It was then removed as part of the axillary node dissection specimen, in turn allowing en-bloc excision of the level III nodes medial to the superior part of the pectoralis minor. Before 1987, the sternocostal head of pectoralis major was routinely detached from the humerus and reattached at the completion of the procedure (3 patients had their surgery before 1987). All but 9 patients had their surgery performed by SMU surgeons. The non-SMU surgeons had all been trained at SMU and performed the surgery according to the standard SMU protocol outlined here.
Measurements for the present study were carried out a median 36.4 months (range, 4–492 months; mean ± standard deviation [SD], 58 ± 66 months) from the date of the axillary node dissection. Thirty patients had undergone a sentinel node biopsy (SNB) procedure involving removal of 1 or more axillary nodes before completion of axillary dissection. This occurred (mean ± SD) 0.8 ± 0.4 months before the completion of axillary dissection. Three patients had undergone an ipsilateral neck dissection after their axillary surgery. Four patients had an incomplete axillary dissection procedure performed by surgeons outside SMU before having a completion level I–III dissection performed by surgeons at SMU. In these 4 cases, the date of surgery was taken as the date of the second dissection procedure, which occurred at a mean interval of 18 months after the first procedure.
One patient had received adjuvant chemotherapy and 6 had received adjuvant immunotherapy by the time they were assessed for this study. In those patients who received axillary radiotherapy, the course of radiotherapy was completed (mean ± SD) 2 ± 1 month after the axillary dissection (excluding 1 patient who had delayed radiotherapy, completed 41 months after surgery). Ten patients received a total of 3300–5400 Gy in 5–6 fractions, and 7 received 46–48 Gy in 20–23 fractions.
To provide some index of disease burden, so that its possible relevance might be assessed, the proportion of lymph nodes that were positive for melanoma was calculated from the total number of nodes recovered from the axilla for each patient. In the 30 patients who had an axillary SNB before full axillary dissection, the number of lymph nodes recovered during SNB was added to the number recovered during the completion axillary dissection to derive an overall proportion of positive nodes. All lymph node specimens recovered during both SNB and axillary dissection were examined by SMU pathologists.
Objective Measurements
The volume of each arm was measured using a water displacement technique. Standardization was achieved by measuring volume up to the midpoint between the lateral epicondyle of the humerus and the acromion of the shoulder (MP) and also to a point 15 cm above the lateral epicondyle of the humerus (C-15). These points were marked with the elbow at a right angle and the shoulder in the anatomic position (Fig. 1). The arm was immersed in a tank of warm water to each marked level and the displaced volume measured, as per the method of Kettle et al,11 but based on the technique first described by Archimedes (290–211 BC).
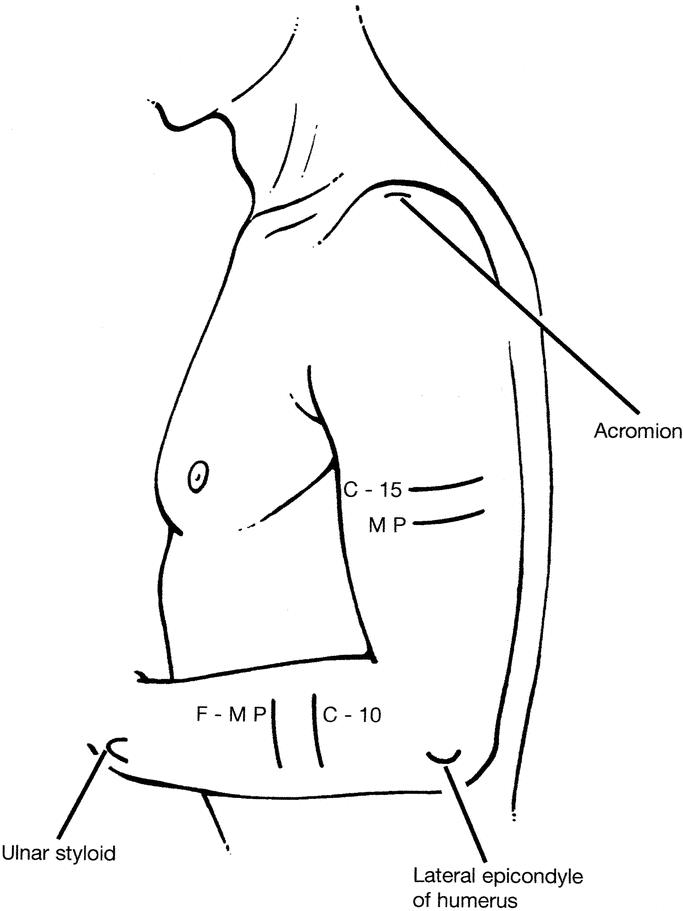
FIGURE 1. Reference points for arm volume and circumference measurements were marked with the elbow at a right angle and the shoulder in the anatomic position. MP, midpoint between the lateral epicondyle of the humerus and the acromion of the shoulder; C-15, 15 cm above the lateral epicondyle of the humerus; F-MP, midpoint between the tip of the ulnar styloid process and the lateral epicondyle of the humerus; C-10, 10 cm below the lateral epicondyle of the humerus.
Arm circumference measurements were made at MP and C-15. In addition, forearm circumference measurements were made at the midpoint between the tip of the ulnar styloid process and the lateral epicondyle of the humerus (F-MP) and at a point 10 cm below the lateral epicondyle of the humerus (C-10). Circumference measurements made at all 4 of these points on the arm were summed (Sum). These points were marked with the arm positioned as previously described.
To account for the natural asymmetry of the arms as a result of right or lefthandedness, arm volume and circumference measurements were made in a control group of 21 healthy volunteers: 11 females, 10 males, 18 righthanded, 3 lefthanded, median age 38 years (range, 23–68 years). The mean difference in volume (or circumference) measurements between the dominant arm and the other arm in this control group was calculated for each measurement point, and this was subtracted from the volume (or circumference) measurement of each patient's dominant arm made at the corresponding measurement point.
Defining Lymphedema
After correction for the effect of right of lefthandedness, change in arm volume was expressed as a fraction of the volume of the arm on the nondissected side. Lymphedema was then defined as a fractional volume increase of the arm on the dissected side in excess of a critical discriminator value determined objectively by classification and regression tree (CART) analysis.12
CART is a nonparametric, recursive-partitioning algorithm that yields a tree-structured rule for prediction. This method allows comparison of multiple “predictor” variables to derive an optimal discriminator value in 1 or more of these variables. The relationship of patients’ particular “predictor” values to the optimal discriminator then allows their assignment to a categorical outcome. In our application, the “predictor” variables were the fractional arm volume increases for each patient and whether they had actually noticed an increase in the size of their arm. An optimal discriminator in the form of a threshold fractional arm volume increase was determined, and patients with a fractional arm volume increase greater than this value were then considered to have lymphedema.
To achieve this end, CART analysis begins with the complete patient group and proceeds to split the group into descendent subsets. The aim is to select optimal discriminator values for splits yielding descending subsets “purer” with respect to the original classification problem. Thus, each subset should contain as many as possible patients belonging to 1 particular class (patients who had a fractional arm volume increase in the higher end of the spectrum and who had noticed an increase in the size of their arm) and as few as possible belonging to remaining classes (patients who had a fractional arm volume increase in the lower end of the spectrum and who had not noticed an increase in the size of their arm). The optimal discriminator values were derived by testing all possible discriminators in the analysis process and were not predetermined by the investigators. The sensitivity, specificity, false-positive and false-negative rates, and the overall misclassification rate for the final classifications were calculated.
It should be noted, however, that because there is no acceptable “gold standard” definition for the presence of lymphedema in the literature to date, it is not possible to compare the definition derived from CART analysis in the present study with any generally accepted ideal.
To determine if approximation of arm volume by arm circumference measurement could provide a convenient alternative to direct volume measurement, lymphedema prevalence values were also calculated from the circumference measurements for comparison. Differences in circumference between arms (measured at MP, C-15, and Sum) were also expressed as fractions of the corresponding arm circumference (or Sum thereof) on the nondissected side and the same analysis applied to derive appropriate threshold fractional circumference increase values for each measurement point (or Sum thereof). Lymphedema prevalence values were then calculated based on these threshold values. In addition, actual volume and circumference measurements were correlated for each arm separately as follows: circumference versus volume (both measured at MP) and Sum of the 4 circumferences versus volume measured at MP.
Subjective Measurements
Patients completed a brief questionnaire that asked for their assessment of problems experienced after surgery. Patients were asked if they perceived a functional deficit impacting on their ability to perform normal daily activities, if they had noticed an increase in the size of their arm, and if they had experienced any postoperative wound complications (specifically, infection, hematoma, and/or seroma formation).
Statistical Analysis
Patients were considered in 2 groups: those who had undergone axillary surgery only (n = 90) and those who had received postoperative radiotherapy to the axilla in addition to axillary surgery (n = 17). Arm volume and circumference measurements were analyzed with repeated measures analysis of variance, treating “arm” as the within-subject factor and “group” as the between-subject factor. When significant interaction was detected, stratified analysis was carried out, which included testing the difference between arms using a paired t test for the 2 groups separately as well as testing whether the “difference between the 2 arms” was significantly different between the 2 groups using an unpaired t test. The significance of differences in proportions in patient groups was assessed using a chi-squared analysis. Associations between volume and circumference measurements were made using the Pearson correlation coefficient. Differences between independent correlations were tested using Fisher's z transformation. Differences between dependent correlations were tested using a formula described by Glass and Stanley.13 Factors associated with the presence of lymphedema and subjective functional deficit were identified using logistic regression analyses.
RESULTS
Effect of Handedness
The volume of the dominant arm was greater than the other arm by 2.5% (approximately 50 mL) in the control group.
Lymphedema Definition
The threshold fractional arm volumes determined by separate CART analyses for each measurement point were 15.7% and 18.0% for MP and C-15, respectively. The sensitivity, specificity, false-positive and false-negative, and overall misclassification rates were MP: 0.35, 0.98, 0.06, 0.35, and 0.30, respectively; and C-15: 0.23, 1.0, 0, 0.38, and 0.34, respectively. The classification tree for MP is shown in Figure 2.
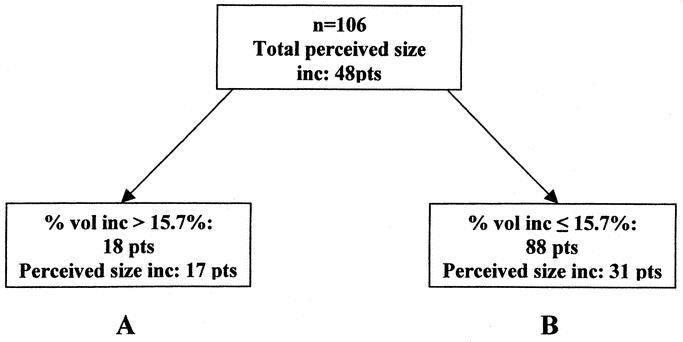
FIGURE 2. Classification tree for arm volume measured at MP. An arm volume increase of >15.7% the volume of the control arm was determined by CART analysis to provide the optimal point of discrimination, allowing patients to be partitioned into 2 groups (A and B) on the basis of arm volume measurements. Group A contains as many as possible patients who experienced larger arm volume increases and who actually noticed this themselves, and group B contains as many as possible patients who experienced smaller arm volume increases (if at all) and who did not notice any volume change themselves. Of 106 patients (MP volume measurements were unavailable from 1 patient), 48 perceived an increase in arm volume. When these patients were partitioned, group A contained 18 patients with arm volume increases above 15.7%, 17 of these patients perceiving a volume increase, whereas group B contained 88 patients with volume increases less than 15.7%, 57 of these patients not perceiving any volume change.
The same analysis of the circumference measurements yielded threshold fractional circumference values of 3.2%, 3.3%, and 3.8% for MP, C-15, and Sum, respectively. The sensitivity, specificity, false-positive and false-negative, and overall misclassification rates were MP: 0.45, 0.88, 0.25, 0.34, and 0.32, respectively; C-15: 0.46, 0.88, 0.24, 0.33, and 0.31, respectively; and Sum: 0.55, 0.95, 0.10, 0.28, and 0.23, respectively.
Lymphedema Prevalence and Severity
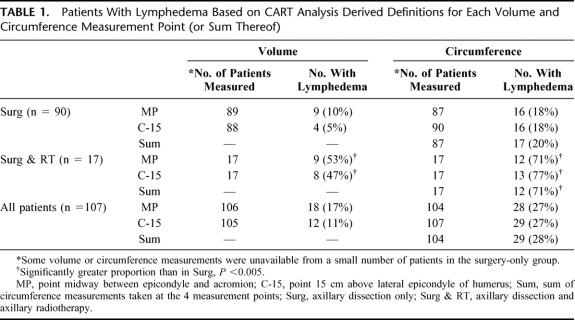
Lymphedema prevalence values, based on the definitions derived from CART analysis of data from each measurement point (or sum thereof), are summarized in Table 1. Based on the lymphedema definition and volume measurements for MP, the prevalence of lymphedema was 10% in patients who had axillary surgery only and 53% in patients who had additional radiotherapy.
TABLE 1. Patients With Lymphedema Based on CART Analysis Derived Definitions for Each Volume and Circumference Measurement Point (or Sum Thereof)
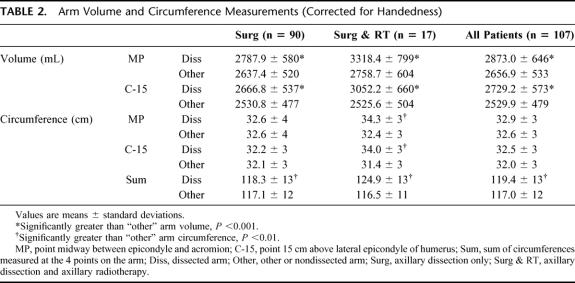
Arm volume and circumference measurements, corrected for the effect of handedness, are summarized in Table 2. In all patients, the volume of the arm on the side of the dissection was significantly greater than that of the other arm when measured at either MP or C-15. The circumference of the arm on the side of the dissection was significantly greater than that of the other arm only in patients who had both surgery and radiotherapy when measured at either MP or C-15. The Sum of the 4 arm circumference measurements was significantly greater for the arm on the side of the dissection in all patients.
TABLE 2. Arm Volume and Circumference Measurements (Corrected for Handedness)
Lymphedema severity, reflected by the magnitude of the volume difference between arms, was significantly greater in patients who received surgery and radiotherapy, compared with those who had surgery only (mean ± SD: MP: 559.7 mL ± 433 mL versus 150.5 ± 200, C-15: 526.6 ± 427 versus 136.1 ± 199, both P < 0.001). The magnitude of the difference between arms for all circumference measurements (MP, C-15, and Sum) was significantly greater in patients receiving surgery and radiotherapy compared with those who received surgery only (mean ± SD: MP: 1.9 cm ± 2 cm versus 0.0 ± 1, C-15: 2.6 ± 2 versus 0.1 ± 1, Sum: 8.5 ± 7 versus 1.2 ± 4, all P < 0.001).
Single volume and circumference measurements made at MP for each arm were significantly correlated (arm on dissected side: r = 0.80; other arm: r = 0.79, both P < 0.001). The Sum of the 4 circumference measurements and volume at MP for each arm were also significantly correlated (arm on dissected side: r = 0.91; other arm: r = 0.90, both P < 0.001). However, when these correlations were compared, correlations involving the summed circumference measurements were significantly better than those involving only a single circumference measurement in both the arms on the dissected side and the other arms (both P < 0.05).
Risk Factors
The following factors were analyzed for their possible association with the presence of lymphedema (based on volumes measured at MP): gender, age, time from surgery, occurrence of wound complications after surgery, whether the dissection was on the side of the dominant arm, radiotherapy, and the proportion of positive lymph nodes removed from the axilla. Radiotherapy (odds ratio [OR], 14.3; 95% confidence interval [CI], 2.8–75.0; P < 0.01) and wound complications (OR, 9.1; 95% CI, 2.2–36.8; P < 0.01) were found to be independently associated with the presence of lymphedema. Age was of borderline significance (OR, 1.1; 95% CI, 1.00–1.12; P = 0.057).
There was no difference in the proportions of patients with defined lymphedema between those who underwent a SNB before their axillary dissection and those who did not.
Subjective Data
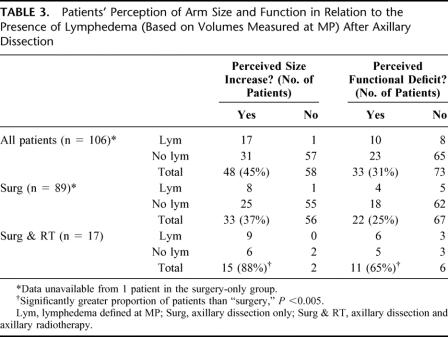
Patients’ perceptions of the size and function of the arm on the side of the axillary dissection and the relationship of this to the presence of lymphedema (based on volumes measured at MP) are summarized in Table 3. Forty-five percent of all patients perceived an increase in the size of the arm on the side of the dissection, and 31% of all patients considered they had some functional deficit in this arm. These proportions were significantly higher among patients who had surgery and radiotherapy compared with surgery only. There was no significant difference in the proportions of males and females who perceived either a size increase or a functional deficit. Perceived functional deficit did not correlate well with actual lymphedema measured at MP, r = 0.24. However, when the genders were considered separately, the correlation was slightly better in males than females (r = 0.26 versus 0.16), although this difference was not significant. There was no significant difference in the proportion of patients who perceived functional deficit after having had surgery on the side of their dominant arm compared with those who had surgery on the side of their nondominant arm.
TABLE 3. Patients’ Perception of Arm Size and Function in Relation to the Presence of Lymphedema (Based on Volumes Measured at MP) After Axillary Dissection
The following factors were analyzed for their possible association with the perceived loss of arm function after surgery: lymphedema (based on volumes measured at MP), gender, age, time from surgery, occurrence of wound complications after surgery, whether the dissection was on the side of the dominant arm, radiotherapy, and the proportion of positive lymph nodes removed from the axilla. Only radiotherapy (OR, 9.9; 95% CI, 1.8–53.1; P < 0.01) was independently associated with perceived functional deficit.
DISCUSSION
Lymphedema is a serious and unpleasant complication of axillary or groin lymph node dissection and represents an added physical and psychologic burden for a patient already attempting to cope with a diagnosis of cancer. Lymphedema is disfiguring, may interfere with limb function, cause a chronic feeling of heaviness and discomfort, and may result in psychologic morbidity and psychosocial maladjustment.14 Moreover, chronic lymphedema may predispose to recurrent infection,3 and even the development of lymphangiosarcoma,15,16 a rare, but fatal disease (Fig. 3).
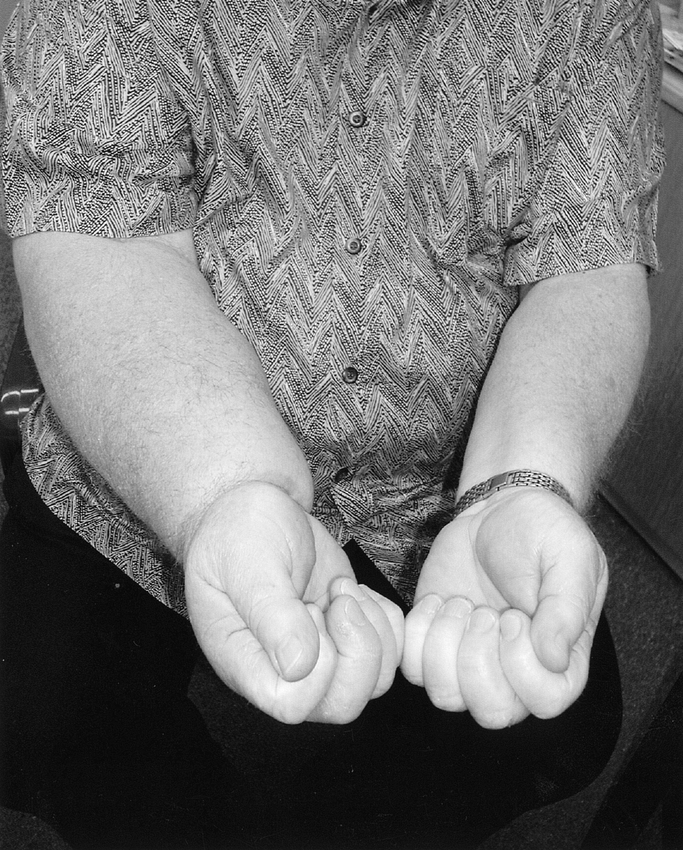
FIGURE 3. Chronic lymphedema of the right upper limb in a 53-year-old man 6 years after level I–III axillary dissection and postoperative radiotherapy for metastatic melanoma.
In the present study, lymphedema was objectively defined as an increase in the volume of the arm on the side of the dissection greater than 16% of the volume of the other arm when measuring arm volume to the midpoint between the lateral epicondyle of the humerus and the acromion of the shoulder. This definition was based on the results of CART analysis of measured arm volume changes together with patients’ perceptions of any arm volume changes after complete level I–III axillary dissection for melanoma.
Based on this definition, the prevalence of arm lymphedema was 10% in 90 patients who underwent complete level I–III axillary dissection only and 53% in an additional 17 patients who underwent axillary radiotherapy after their axillary dissections. The overall prevalence in the entire patient group was 17% (n = 107).
Previous attempts to objectively measure lymphedema prevalence have relied on definitions such as an absolute volume difference of greater than 200 mL between the arm on the dissected side and the other arm6,17,18 and an increase in the absolute circumference of the dissected arm above that of the other arm.6 Variations in the definitions applied in these previous studies greatly limit the comparisons that may be made between them. In addition, such definitions are purely arbitrary and rely on differences in absolute volumes or circumferences, which do not account for the size of the patient. It is difficult to interpret the significance of lymphedema prevalence values derived from such definitions, because the defined condition may not necessarily be of clinical significance in all patients. In a large male, for example, a volume difference between arms of 250 mL may go unnoticed, whereas in a small female, the same difference may contribute to significant functional restriction as a result of a heavy swollen limb.
The definition for lymphedema applied in the present study relies on the change in volume of the arm on the side of the dissection being expressed as a fraction of the other arm volume, thereby accounting for the size of the patient. Moreover, using the 2 different “predictor” variables (actual volume changes and patients’ perception of any volume changes) to determine thresholds for arm volume increases allowed determination of a more clinically meaningful categorical outcome. Patients with defined lymphedema in the present study were more likely to have actually noticed the swelling themselves, and therefore, had the condition impact on their lives in some form.
Lymphedema was defined as a fractional arm volume increase in excess of 15.7% for volumes measured at MP and 18.0% for volumes measured at C-15. The slight difference in threshold values determined by separate CART analyses of data from each measurement point resulted in lower lymphedema prevalence values calculated from C-15 volume measurements compared with MP measurements (Table 1). This small difference in threshold values is most likely a result of magnification of a small degree of measurement error and anatomic variation between the arms of most patients in each CART analysis procedure. Despite the effect on prevalence values, however, there appears to be no benefit in using 1 of these measurement points over the other, and we recommend the use of MP for simplicity and consistency.
Approximation of arm volume by measuring arm circumference does not provide a comparable method for determining lymphedema prevalence. Lymphedema prevalence values derived from circumference measurements are overestimated. Measuring circumference in more than 1 location (instead of relying on a single value) appears to provide a better approximation of arm volume; however, analysis of summed circumference values does not yield comparable lymphedema prevalence values.
Postoperative axillary radiotherapy is clearly a major risk factor for the development of lymphedema in patients with melanoma and also appears to be associated with more severe lymphedema. These findings are in agreement with many studies of patients with breast cancer.19–22 Two recent studies in patients with melanoma reported high incidences of arm lymphedema, based on clinical judgment, after axillary dissection and radiotherapy. Ballo et al23 reported a 5-year actuarial lymphedema rate of 41%, and Stevens et al24 observed lymphedema in 58% of patients who survived for 2 years without recurrence.
The occurrence of postoperative wound complications (infection, hematoma, and/or seroma formation) reported by the patient was also identified as an independent risk factor for the development of lymphedema. Indeed, postoperative wound complications, especially infection, have often been put forward as etiologic factors for postoperative lymphedema.1,20 Age was of borderline significance as an independent risk factor for the development of lymphedema in the present study; however, increasing age has previously been suggested to be related to a higher incidence of postoperative wound complications after axillary dissection for melanoma.6 Gender, time from surgery, and whether the dissection was performed on the side of the dominant arm had no bearing on the occurrence of lymphedema. Disease burden, as indicated by the proportion of lymph nodes positive for melanoma recovered from the axilla, also had no bearing on the occurrence of lymphedema. Treatment factors, and complications thereof, thus appear to be the prime determinants of the development of lymphedema in patients with melanoma.
Although it is not possible to make definitive conclusions based on the results of the present study, it is likely that differences in general management principles for melanoma versus breast carcinoma affect the postoperative lymphedema rate. Studies in patients with breast carcinoma, in which lymphedema has been defined as an increase in arm volume of greater than 200 mL on the side of the surgery, have reported prevalence values of 25.5%,19 10%,20 and 7.6%.18 When this same definition is applied to the patients with melanoma in the present study, the prevalence values are 32% for surgery only, 77% for surgery and axillary radiotherapy, and 39% overall. Despite the limitations of this definition and differences in patient populations, it is of interest to note that prevalence values derived from our patient group are greater than those reported in patients with breast carcinoma using the same method. Removal of only the level I and II axillary nodes (below the lateral border and behind the pectoralis minor, respectively) is frequently performed in the treatment of breast carcinoma,5 whereas all 3 levels of axillary nodes (including the highest axillary nodes medial to the superior attachment of pectoralis minor) are usually removed for melanoma.4 Furthermore, irradiation of the axilla after axillary surgery is unusual in the treatment of breast cancer.
In the present study, 31% of patients felt that their ability to perform daily activities had been impaired after the axillary dissection. However, the perception of functional deficit did not correlate well with actual lymphedema. This suggests that postoperative factors other than just a swollen and heavy limb may contribute to the perception of functional deficit.
Radiotherapy was identified as an independent risk factor for perceived functional deficit. Although radiotherapy clearly increases the risk of a swollen and heavy arm after axillary dissection, it is also generally accepted that radiotherapy increases the severity of a number of postoperative complications such as pain, numbness, and scarring,5 which may represent some of the factors that also contribute to the perception of functional deficit.
Wrone et al25 have suggested that the SNB procedure may present a small risk for the development of lymphedema. In their review of 235 patients after SNB, 1.7% were judged to have developed mild lymphedema on clinical observation. The SNB procedures, however, were not confined to the axilla, and almost half of the cases of lymphedema occurred in the lower limb after biopsy of nodes in the inguinal region. Some patients (28%) in our study underwent an axillary SNB before axillary dissection; however, this did not effect on the development of lymphedema.
In conclusion, the findings of the present study suggest that an appropriate definition for arm lymphedema after axillary dissection is an arm volume increase of greater than 16% of the volume of the control arm when measuring arm volume to the midpoint between the lateral epicondyle of the humerus and the acromion of the shoulder. Based on this definition, the lymphedema prevalence was 10% after complete level I–III axillary dissection for melanoma and 53% after additional postoperative axillary radiotherapy. Postoperative axillary radiotherapy and surgical wound complications are independent risk factors for the development of lymphedema. Subjective functional deficit after level I–III axillary dissection does not correlate well with actual lymphedema defined in the present study.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the assistance of Cheryl Daley and Leanne Watson with data collection.
Footnotes
Reprints: John F. Thompson, MD., Sydney Melanoma Unit, Sydney Cancer Centre, Gloucester House, Royal Prince Alfred Hospital, Camperdown, NSW, 2050, Australia. E-mail: thompson@smu.org.au.
REFERENCES
- 1.Halsted WS. The swelling of the arm after operations for cancer of the breast—elephantiasis chirurgica—its cause and prevention. Bulletin of the Johns Hopkins Hospital. 1921;32:309–313. [Google Scholar]
- 2.Erickson VS, Pearson ML, Ganz PA, et al. Arm edema in breast cancer patients. J Natl Cancer Inst. 2001;93:96–111. [DOI] [PubMed] [Google Scholar]
- 3.Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83:2776–2781. [DOI] [PubMed] [Google Scholar]
- 4.Mansfield PF, Ames FC, Balch CM. Axillary lymph node dissection. In: Balch CM, Houghton AN, Sober AJ, et al., eds. Cutaneous Melanoma, 3rd ed. St. Louis: Quality Medical Publishing; 1998:259–268. [Google Scholar]
- 5.Winer EP, Morrow M, Osbourne CK, et al. Cancer of the breast. In: Devita VT, Hellman S, Rosenberg S, eds. Cancer: Principles and Practice of Oncology, 5th ed. Philadelphia: Lippincott-Raven; 1997:1541–1602. [Google Scholar]
- 6.Urist MM, Maddox WA, Kennedy JE, et al. Patient risk factors and surgical morbidity after regional lymphadenectomy in 204 melanoma patients. Cancer. 1983;51:2152–2156. [DOI] [PubMed] [Google Scholar]
- 7.Karakousis CP, Henna MA, Emrich LJ, et al. Axillary node dissection in malignant melanoma: results and complications. Surgery. 1990;108:10–17. [PubMed] [Google Scholar]
- 8.Holms EC, Moseley HS, Morton DL, et al. A rational approach to the surgical management of melanoma. Ann Surg. 1977;186:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris MN, Gumport SL, Maiwandi H. Axillary node dissection for melanoma. Surg Gynecol Obstet. 1972;135:936–940. [PubMed] [Google Scholar]
- 10.Ollila DW, McCarthy WH, Felger EA. Therapeutic axillary lymph node dissection for metastatic melanoma. In: Thompson JF, Kroon BBR, Morton DL, eds. Textbook of Melanoma. London: Martin-Dunitz; 2003. In press.
- 11.Kettle JH, Rundle FF, Oddie TH. Measurement of upper limb volumes: a clinical method. Aust N Z J Surg. 1958;27:263–270. [PubMed] [Google Scholar]
- 12.Breiman L, Friedman JH, Olshen RA, et al. Classification and Regression Trees. Belmont: Wadsworth International Group; 1984. [Google Scholar]
- 13.Glass GV, Stanley JC. Statistical Methods in Education and Psychology. Englewood Cliffs, NJ: Prentice Hall; 1970. [Google Scholar]
- 14.Tobin MB, Lacey HJ, Meyer L, et al. The psychological morbidity of breast cancer related arm swelling. Cancer. 1993;72:3248–3252. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson P, Holmberg E, Samuelsson A, et al. Soft tissue sarcoma after treatment for breast cancer—a Swedish population based study. Eur J Cancer. 1998;34:2068–2075. [DOI] [PubMed] [Google Scholar]
- 16.Bisceglia M, Attino V, D'Addetta C, et al. Early stage Stewart-Treves syndrome: report of two cases and review of the literature. Pathologica. 1996;88:483–490. [PubMed] [Google Scholar]
- 17.Ivens D, Hoe AL, Podd TJ, et al. Assessment of morbidity from complete axillary dissection. Br J Cancer. 1992;66:136–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoe AL, Iven D, Royle GT, et al. Incidence of arm swelling following axillary clearance for breast cancer. Br J Surg. 1992;79:261–262. [DOI] [PubMed] [Google Scholar]
- 19.Kissin MW, Querci della Rovere G, Easton D, et al. Risk of lymphedema following the treatment of breast cancer. Br J Surg. 1986;73:580–584. [DOI] [PubMed] [Google Scholar]
- 20.Segerström K, Bjerle P, Graffman S, et al. Factors that influence the incidence of brachial oedema after treatment for breast cancer. Scand J Plast Reconstr Surg Hand Surg. 1992;26:223–227. [DOI] [PubMed] [Google Scholar]
- 21.Ryttov N, Holm NV, Qvist N, et al. Influence of adjuvant irradiation on the development of late arm lymphedema and impaired shoulder mobility after mastectomy for carcinoma of the breast. Acta Oncol. 1988;27:667–670. [DOI] [PubMed] [Google Scholar]
- 22.Borup Christensen S, Lundgren E. Sequelae of axillary dissection vs. axillary sampling with or without irradiation for breast cancer. A randomised trial. Acta Chir Scand. 1989;155:515–519. [PubMed] [Google Scholar]
- 23.Ballo MT, Strom EA, Zagars GK, et al. Adjuvant irradiation for axillary metastases from malignant melanoma. Int J Radiat Oncol Biol Phys. 2002;52:964–972. [DOI] [PubMed] [Google Scholar]
- 24.Stevens G, Thompson JF, Firth I, et al. Locally advanced melanoma. Results of postoperative hypofractionated radiation therapy. Cancer. 2000;88:88–94. [DOI] [PubMed] [Google Scholar]
- 25.Wrone DA, Tanabe KK, Cosimi AB, et al. Lymphedema after sentinel lymph node biopsy for cutaneous melanoma: a report of five cases. Arch Dermatol. 2000;136:511–514. [DOI] [PubMed] [Google Scholar]