Abstract
Objective:
The objective of this study was to investigate the effects of 2 levels of intraoperative fluid administration on perioperative physiology and outcome after laparoscopic cholecystectomy.
Summary Background Data:
Intraoperative fluid administration is variable as a result of limited knowledge of physiological and clinical effects of different fluid substitution regimens.
Methods:
In a double-blind study, 48 ASA I–II patients undergoing laparoscopic cholecystectomy were randomized to 15 mL/kg (group 1) or 40 mL/kg (group 2) intraoperative administration of lactated Ringer’s solution (LR). All other aspects of perioperative management as well as preoperative fluid status were standardized. Primary outcome parameters were assessed repeatedly for the first 24 postoperative hours and included pulmonary function (spirometry), exercise capacity (submaximal treadmill test), cardiovascular hormonal responses, balance function, pain, nausea and vomiting, recovery, and hospital stay.
Results:
Intraoperative administration of 40 mL/kg compared with 15 mL/kg LR led to significant improvements in postoperative pulmonary function and exercise capacity and a reduced stress response (aldosterone, antidiuretic hormone, and angiotensin II). Nausea, general well-being, thirst, dizziness, drowsiness, fatigue, and balance function were also significantly improved, as well as significantly more patients fulfilled discharge criteria and were discharged on the day of surgery with the high-volume fluid substitution.
Conclusions:
Intraoperative administration of 40 mL/kg compared with 15 mL/kg LR improves postoperative organ functions and recovery and shortens hospital stay after laparoscopic cholecystectomy.
In a double-blind clinical trial of 48 patients undergoing laparoscopic cholecystectomy, administration of 40 mL/kg compared with 15 mL/kg lactated Ringer’s solution intraoperatively led to significant improvements in perioperative organ functions, recovery, and hospital stay.
Limited scientific evidence on optimal intraoperative fluid management has resulted in large variations of administered fluid regimens in daily practice. Currently, there is a tendency toward liberal perioperative fluid administration,1 and in laparoscopic cholecystectomy, administration of 1 to 4 L of crystalloid has been reported.1 The stress response to surgery profoundly alters fluid homeostasis leading to fluid conservation. Primary mediators are aldosterone, antidiuretic hormone, and the renin–angiotensin system. However, the effect of different fluid regimens on these hormonal responses to surgery is largely unknown.1 Although perioperative administration of high volumes may be deleterious in connection with major surgical procedures,1,2 studies in minor (ambulatory) surgery suggest that fluid substitution aiming to correct preoperative dehydration may improve some parameters of recovery (drowsiness and dizziness).3
We therefore investigated the effects of intraoperative fluid administration with 2 different volumes of Ringer’s lactate (LR) (40 mL/kg and 15 mL/kg) for laparoscopic cholecystectomy on pulmonary function, exercise capacity, stress responses (aldosterone, antidiuretic hormone [ADH], angiotensin II, atrial natriuretic peptide [ANP], and renin), and balance function as the primary outcome assessments. Furthermore, pain, nausea, vomiting, hospital stay, and recovery were assessed.
METHODS
In a randomized, double-blind trial, we studied 48 consecutive patients scheduled for elective laparoscopic cholecystectomy from October 23, 2001, to August 20, 2002. Exclusion criteria were: weight >100 kg; age >70 or <18 years; pregnancy or lactation; ongoing infection (elevated C-reactive protein); inability to perform the preoperative test program; conversion of the operation from laparoscopic to open procedure; history of cardiovascular, pulmonary, or endocrine disease; and regular intake of any medication except anticonceptive pills, postmenopausal estrogen supplementation, or selective serotonin reuptake inhibitors (SSRIs). Furthermore, patients operated in the afternoon were excluded. During the study period, 159 patients underwent elective laparoscopic cholecystectomy. Seventy-nine patients did not meet the inclusion criteria. Of the remaining 80 patients, 28 refused to participate in the study, 1 had surgery postponed to the afternoon, and 3 were excluded as a result of unavailability of the investigators, leaving 48 patients for randomization. All randomized patients completed the study. The Regional Ethics Committee approved the study, and subjects gave written, informed consent before inclusion.
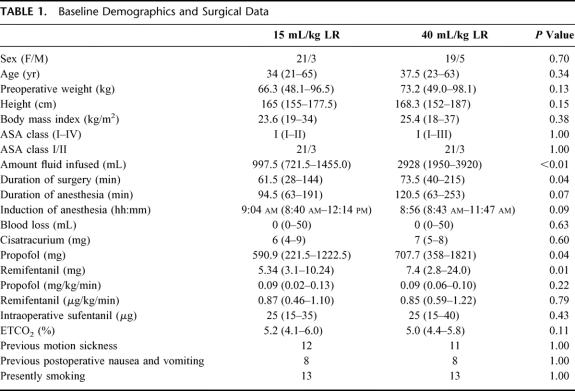
Patient demographics are shown in Table 1. Three patients in each group were classified as ASA II as a result of a body mass index >30 kg/m2.
TABLE 1. Baseline Demographics and Surgical Data
Laparoscopic cholecystectomy was performed in a semiambulatory setting (patients were encouraged but not forced to be discharged on the day of surgery) in a public university hospital with unselected patient referral. The day of the operation was defined as day 0. Preoperative fluid status was standardized in all patients by ensuring that all fasted from midnight before the operation (all surgery performed between 8 and 12 am) and drank 175 mL of water at 6 am on the morning of the operation. On arrival in the operating room, patients were randomized by the sealed envelope method (serially numbered, sealed, and opaque envelopes based on an externally generated computer-generated list of random numbers) to intraoperative infusion of 15 mL/kg (group 1) or 40 mL/kg (group 2) LR (composition: Na+ 130 mmol/l, K+ 4 mmol/l, chloride 109 mmol/l, lactate 28 mmol/l, calcium 1.4 mmol/l). The randomization code was kept separate and not known to any of the investigators until the study was completed. Double blinding was achieved by hiding the fluid infusion bags in opaque sacks ensuring blinding of the surgeons, the patients, and the investigators obtaining the data (B.K., K.G.N., K.H.). The fluid administration was controlled and administered by an anesthesiologist (D.S.C., C.L.) not involved in patient assessments. The investigators obtaining patient data (B.K., K.G.N., K.H.) were not present during the fluid infusion. The allocated amount of LR was infused at a constant rate over 1.5 hours starting immediately before induction of anesthesia. After termination of the infusion, the fluid bags were discarded and the peripheral venous line closed. All patients received a similar general anesthesia with remifentanil, propofol, and muscle relaxants for tracheal intubation. Immediately before induction of anesthesia, 8 mg dexamethasone was given to all patients. Ventilation (O2/air: 1:2) was adjusted to keep end-tidal CO2 4.5–5.5%. At the end of surgery, 30 mg ketorolac, 4 mg ondansetron, and 0.3–0.5 μg/kg sufentanil were administered intravenously. Laparoscopic technique and multimodal pain management was applied as described previously.4
In the recovery room, personnel were unaware of the fluid regimen, and pain treatment was standardized with sufentanil (first choice) and morphine. On-demand antiemetic treatment was standardized and consisted of 4 mg intravenous ondansetron once (first choice) and thereafter 0.625 mg intravenous droperidol (second choice). Patients were allowed (but not forced) to drink fluids after surgery without restrictions. Fluid was ingested from standard cups and the amount was registered 0–4 hours after surgery. Discharge from the hospital was planned to 4 hours after surgery on achieving a score of ≥9 according to the previously validated Postanesthesia Discharge Scoring System (PADS).5 A ward nurse not involved in the study determined the PADS score and discharged the patient.
Study assessments took place preoperatively, 0, 1, 2, 4, and 24 hours after surgery. If discharged, patients returned to the hospital for the 24-hour assessment.
Pulmonary Function
Pulmonary function (forced expiratory volume in the first second [FEV1], forced vital capacity [FVC], and peak expiratory flow [PEF]) was measured preoperatively, 1, 2, 4, and 24 hours after surgery as described previously.6
Weight
The patients were weighed with standardized hospital clothing preoperatively 4 and 24 hours after surgery.
Exercise Capacity
A submaximal treadmill exercise test was performed on a Quinton Club Track 612 (Bothell, WA) treadmill as previously described and validated6 preoperatively, 4 and 24 hours after surgery.
Recovery Parameters
Self-reported registrations of pain, nausea, appetite, general well-being, thirst, headache, dizziness, and drowsiness were evaluated using a 100-mm visual analog scale (VAS) (0 = no symptoms, 100 = worst symptoms possible) before surgery, 1, 2, 4, and 24 hours after surgery, and in the evening of days 0, 1, 2, and 3. Number of vomiting episodes were registered at the same time points (1, 2, 3, 4, or >4 episodes). Fatigue was evaluated on a 10-point fatigue scale (1 = no fatigue and 10 = worst fatigue imaginable) before surgery and 4 and 24 hours after surgery and after discharge in the evening of days 0, 1, 2, and 3.7
Balance Assessments
Balance function was assessed with a Basic Balance Master system (NeuroCom International Inc., Clackamas, US), including 15 tests (3 static and 12 dynamic) as previously described8 preoperatively, 4 and 24 hours after surgery. As a result of the multiple balance assessments obtained by this method, we determined a priori that a minimum of 5 of the 15 tests (including at least 1 static and 1 dynamic balance assessment) should be significantly altered to conclude that overall balance function was significantly altered.
Hormonal Responses
Venous blood was drawn from a cubital vein immediately before induction of anesthesia, at the end of surgery (immediately after closing the incision), 1 and 2 hours postoperatively. A total of 28 mL was drawn at each sampling into tubes containing appropriate additives (EDTA and aprotinin). The concentrations of peptide hormones, antidiuretic hormone (ADH), angiotensin II, and atrial natriuretic peptide (ANP) in plasma was measured after extraction9 as previously described.10 The antibody trapping method of Poulsen and Jørgensen11 was used to determine renin activity in plasma. A commercial kit (Coat-A-Count Aldosterone; Diagnostic Products Corp., Los Angeles, CA) was used to measure levels of aldosterone concentrations in plasma.
Statistics
Data were analyzed on an intention-to-treat basis using nonparametric statistical methods. Data are presented as median (range). P <0.05 was considered significant. Continuous data were compared with Mann-Whitney’s test. Categorical data were compared using Fisher exact test. Outcome assessments, including multiple measurements (balance function and visual analog scales), were analyzed with summary measures to avoid multiple comparisons. VAS were analyzed by comparing the area under the curve (AUC) (VAS analyses specifically separated into early [0–4 hours] and late [evening days 0–3] postoperative period). Furthermore, hormone concentrations at the individual time points compared with baseline values were compared with Wilcoxon”s test for paired observations.
Calculation of sample size was based on the hypothesis that administration of 15 mL/kg LR may lead to an improvement in pulmonary function. A previous study found a reduction in pulmonary function (FVC) after laparoscopic cholecystectomy from 3.8 to 2.3 (40%) (standard deviation, 0.8).12 We considered a reduction in the decrease in postoperative pulmonary function (FVC) by 50% (from a 40% to a 20% reduction) clinically relevant. With a power to detect a minimal relevant difference (MIREDIF) between the 2 groups of 80% and a level of significance of 0.05, 21 patients were needed in each group. To counter for potential patient exclusion after randomization, we decided to include 24 patients in each group. The CONSORT guidelines13 were followed for the report of this trial.
RESULTS
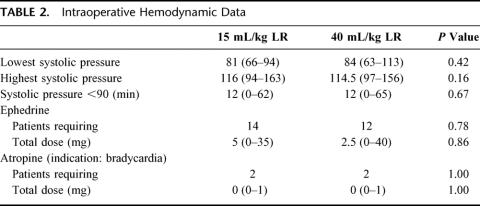
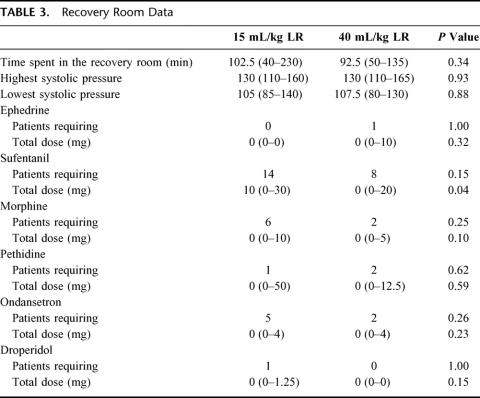
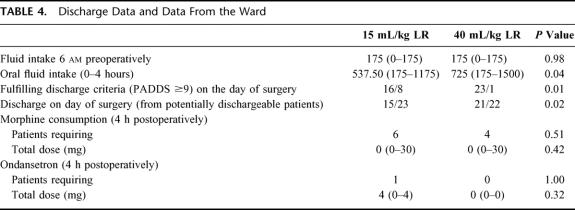
Duration of surgery was significantly longer in group 2 (high volume), which is why significantly larger amounts of propofol and remifentanil were administered intraoperatively in group 2 compared with group 1 (Table 1). In group 1, median 997.5 mL LR was administered intraoperatively compared with 2928 mL in group 2 (Table 1). Consequently, there was a significantly larger increase in weight in group 2 compared with group 1 (2.2 versus 0.8 kg 4 hours postoperatively, and 1.4 versus 0.75 kg 24 hours postoperatively, P <0.01). Intraoperative hemodynamic data did not differ between the groups (Table 2). In the recovery room, significantly smaller amounts of sufentanil was administered in group 2 (Table 3), as well as fewer patients (although insignificant) required morphine, ondansetron, and sufentanil (Table 3). Postoperative oral fluid intake was significantly larger in group 2 compared with group 1 (Table 4). Significantly more patients fulfilled the discharge criteria on the day of surgery in group 2 compared with group 1 (23 of 24 in group 2 versus 16 of 24 in group 1, P <0.02). In 3 patients (1 in group 1 and 2 in group 2), overnight hospital stay was mandatory because these patients were living alone (all 3 patients fulfilled the discharge criteria at the day of surgery). Thus, 22 patients in group 2 and 23 patients in group 1 were eligible for discharge on the day of surgery, and of these patients, significantly more (21 of 22) in group 2 were actually discharged on the day of surgery compared with group 1 in which 15 of 23 patients were discharged on the day of surgery (P <0.03) (Table 4).
TABLE 2. Intraoperative Hemodynamic Data
TABLE 3. Recovery Room Data
TABLE 4. Discharge Data and Data From the Ward
Pulmonary Function
Pulmonary function did not differ between the groups preoperatively. FVC was significantly improved 2 hours postoperatively in group 2 compared with group 1 and FEV1 was significantly improved 2 and 4 hours postoperatively in group 2 compared with group 1 (Fig. 1). There was no difference in peak flow (Fig. 1) at any time point between the groups.
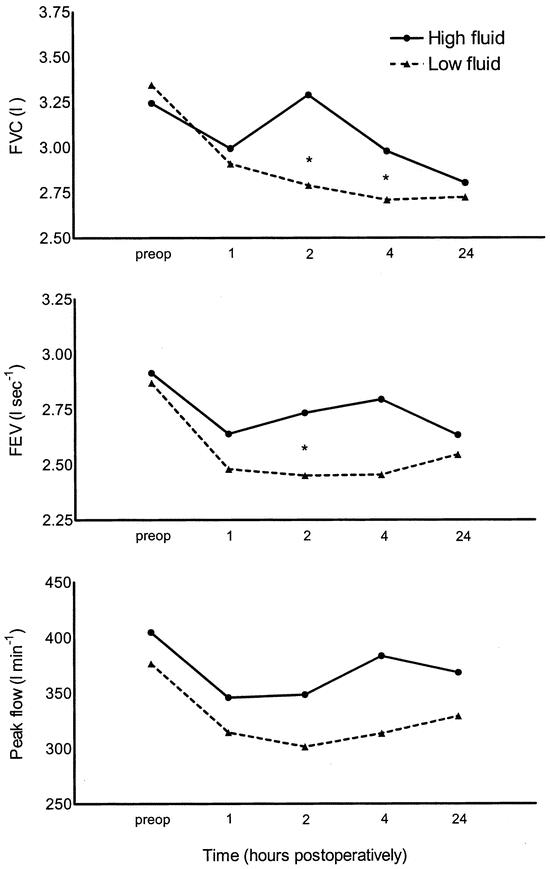
FIGURE 1. Effect of 40 mL/kg (high fluid) versus 15 mL/kg (low fluid) lactated Ringer’s solution on pulmonary function after laparoscopic cholecystectomy. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second. *P <0.05 between groups.
Exercise Capacity
Exercise capacity did not differ between the groups preoperatively. Four hours postoperatively, exercise capacity was significantly higher in group 2 with 61.5 W (range, 0–129 W) compared with 25.5 W (range, 0–116 W) in group 1 (P = 0.03) (Fig. 2). Twenty-four hours after surgery, exercise capacity did not differ between groups.
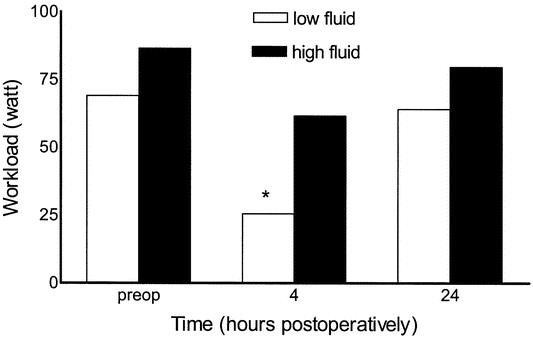
FIGURE 2. Effect of 40 mL/kg (high fluid) versus 15 mL/kg (low fluid) lactated Ringer”s solution on exercise capacity after laparoscopic cholecystectomy. *P <0.05 between groups.
Balance Function
Preoperative balance function did not differ between the groups. Balance function was significantly better 4 hours postoperatively in group 2 compared with group 1 (6 in 15 tests were significantly better—1 in 3 static tests and 5 in 12 dynamic tests) (data not shown). There was no significant difference in any balance function assessments between the groups 24 hours postoperatively.
Recovery Parameters
Thirst, nausea, dizziness, and drowsiness were significantly reduced and general well-being was significantly better in group 2 compared with group 1 in both the early (0–4 hours after surgery) and the late (from the evening on the day of surgery to the evening on the third postoperative day) postoperative period (nausea and general well-being data: early and late nausea reduced from 11 to 0 and 76 to 0, respectively, in group 2 [median AUC values, P <0.05 between groups]), early and late general well-being improved from 163 to 80 and 3518 to 1962, respectively, in group 2 [median AUC values, P <0.05 between groups]). Fatigue was significantly reduced in the late but not early postoperative period in group 2. No differences in either early or late pain, vomiting, appetite, or headache were seen.
Hormonal Responses
There were no significant differences between the plasma concentrations of any of the measured hormones preoperatively. The significant aldosterone, ADH, and angiotensin II response to surgery in group 1 was suppressed in group 2 (Fig. 3A), whereas the ANP response was insignificantly higher in group 2 (Fig. 3B).

FIGURE 3. A and B: Effect of 40 mL/kg (high fluid) versus 15 mL/kg (low fluid) lactated Ringer”s solution on hormonal responses after laparoscopic cholecystectomy. *Significant elevation (P <0.05) compared with baseline values. #Significant decrease (P <0.05) compared with baseline values. ¤Significant difference (P <0.05) between groups. ADH, antidiuretic hormone; ANP, atrial natriuretic peptide.
DISCUSSION
In summary, we found that intraoperative administration of 40 mL/kg compared with 15 mL/kg LR led to significant improvements in pulmonary function, exercise capacity, balance function, subjective recovery measures (nausea, general well-being, thirst, dizziness, drowsiness, and fatigue) together with a significantly reduced surgical stress response assessed by changes in hormones influencing fluid homeostasis, and a shortened hospital stay in patients undergoing laparoscopic cholecystectomy. Perioperative pulmonary function was improved by up to 50% by fluid administration in this study, a clinically important improvement, which is comparable in size to the improvement in postoperative pulmonary function seen with laparoscopic surgery compared with open surgery per se.14 Furthermore, the amount of sufentanil administered in the recovery room was significantly decreased in the high-volume group and medication for nausea (ondansetron) was also decreased, although not significantly in the high-volume group. These results were obtained despite that the group allocated to 40 mL/kg LR had longer duration of surgery and larger amounts of administered anesthetics. We deliberately chose our fluid regimens to reflect daily clinical practice for fluid administration in “minor” surgical procedures, in which fluid is often administered according to local guidelines and traditions and not in response to specifically obtained physiological parameters. Our results may therefore have broad applicability for daily anesthetic practice in these procedures. However, as a result of concerns of adverse effects of administration of the high volume,1 we only included healthy (ASA I/II) patients, and only 50% of our patient population were eligible for inclusion. Thus, our results may not be applicable to patients with cardiovascular or other systemic diseases or patients undergoing major surgery. Our hypothesis was that intraoperative fluid administration leading to perioperative fluid excess (ie, in excess of normohydration) may adversely affect perioperative organ functions and delay recovery, and that restriction of intraoperative fluid administration may improve these parameters. In this study, high-dose intraoperative fluid administration did not alter postoperative complications as previously suggested.1 Thus, our findings reject our hypothesis. We therefore conclude that high-dose fluid administration could in fact potentially decrease complications (primarily nausea and pain) and improve the perioperative course after laparoscopic cholecystectomy; however, additional studies are needed to further establish the optimal amount of fluid to be administered intraoperatively in laparoscopic cholecystectomy as well as other procedures.
Elective laparoscopic cholecystectomy may be considered a minor- to moderate-sized surgical procedure, with insignificant blood and fluid losses, and the perioperative fluid requirements to maintain normohydration may, therefore, approximate the preoperative fasting deficit. Presuming a fasting deficit of 1 L, this would be covered by the 15 mL/kg LR administered in group 1 together with an oral intake of 175 mL fluid on the morning of surgery and patients being scheduled for morning procedures. The demonstrated benefits of administering 40 mL/kg LR are difficult to explain, but may include preoperative dehydration or surgically induced internal shifts of fluid resulting in increased fluid requirements.
The risk of a type I error, thus accepting a “false-positive” result, is minimized by the observation of several concomitant significant positive findings of high fluid administration on vasoactive hormonal reduction, perioperative physiology, and clinical outcome (hospital stay).
Our study included measurements of various hormones known to influence fluid and electrolyte balance (renin, aldosterone, angiotensin II, ADH, and ANP). The well-known stress response in these hormones may be mediated by an obligatory stress-induced neurogenic reflex15 or from a physiological feedback mechanism leading to a high response when functional deficits in volume or electrolytes are apparent or a decrease when fluid or electrolyte excess occurs. The relative role of these mechanisms after surgical stress has not been clarified,15 but our results of a reduced hormonal stress response in plasma concentrations of ADH, aldosterone, and angiotensin II together with a higher ANP concentration with 40 mL/kg LR suggest that a feedback mechanism is in action. However, whether the altered hormonal responses represent a physiological response to a functional deficit or a response to fluid excess with 40 mL/kg LR cannot be answered, although the beneficial effects of the high-volume regimen on organ functions and recovery suggest that it serves to correct a functional deficit. In this context, it is interesting that other hormonal responses to surgery, ie, cortisol, cannot be modified by glucocorticoid administration,15 suggesting that this response may entirely be released by a neural reflex mechanism rather than be a physiological feedback mechanism.
The significantly earlier hospital discharge seen in group 2 may have major clinical implications, because the treatment (fluid administration) is cheap and the potential cost reduction substantial.
Previously, numerous studies have compared different types of fluids such as colloids, crystalloids, or hypertonic solutions for perioperative fluid replacement.16 However, the amounts of fluid administered have varied according to the type of volume replacement, and conclusions on the appropriate amount of fluid to administer obviously cannot be made from these studies. Several randomized studies in high-risk patients have demonstrated that goal-directed fluid therapy may improve outcome.17,18 However, these studies have primarily focused on administration of fluid according to an algorithm involving invasive monitoring (esophageal Doppler), and the results may not be applicable to general daily practice in minor to moderately sized surgical procedures in low-risk patients. The decrease in the inflammatory response to surgery seen with minimally invasive surgery14 may result in small perioperative fluid shifts. However, in major abdominal surgery, with a substantially larger stress response and altered capillary permeability, the effects of high intraoperative fluid volumes may differ from the positive effects on organ functions we found in the present setting. Thus, the length of postoperative ileus has been found to correlate with the amounts of fluid administered postoperatively (low fluid administration resulting in shortening of postoperative ileus).2 Future studies should therefore address the effects of different intraoperative fluid administration regimens on organ functions and outcome in major surgical procedures and in high-risk patients with comorbidity. The present study did not support the original hypothesis that lower fluid volumes would improve outcomes in laparoscopic cholecystectomy. It is possible that a larger fluid administration may actually enhance clinical results.
In summary, intraoperative administration of 40 mL/kg compared with 15 mL/kg LR for laparoscopic cholecystectomy improves recovery of perioperative organ functions and reduces hospital stay.
ACKNOWLEDGMENTS
The authors thank the anesthesia nurses and anesthesiologists at the department of Anesthesiology 532, Hvidovre University Hospital, Denmark, for helpful assistance.
Footnotes
The study was supported by grants from the University of Copenhagen and the Danish Research Council (no. 22-01-0160).
Reprints: Kathrine Holte, MD, Department of Surgical Gastroenterology, Hvidovre University Hospital, DK-2650 Hvidovre, Denmark. E-mail: kathrine.holte@dadlnet.dk.
REFERENCES
- 1.Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89:622–632. [DOI] [PubMed] [Google Scholar]
- 2.Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002;359:1812–1818. [DOI] [PubMed] [Google Scholar]
- 3.Holte K, Kehlet H. Compensatory fluid administration for preoperative dehydration—does it improve outcome? Acta Anaesthesiol Scand. 2002;46:1089–1093. [DOI] [PubMed] [Google Scholar]
- 4.Bisgaard T, Klarskov B, Rosenberg J, et al. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain. 2001;90:261–269. [DOI] [PubMed] [Google Scholar]
- 5.Marshall SI, Chung F. Discharge criteria and complications after ambulatory surgery. Anesth Analg. 1999;88:508–517. [DOI] [PubMed] [Google Scholar]
- 6.Bisgaard T, Klarskov B, Kehlet H, et al. Recovery after uncomplicated laparoscopic cholecystectomy. Surgery. 2002;132:817–825. [DOI] [PubMed] [Google Scholar]
- 7.Christensen T, Bendix T, Kehlet H. Fatigue and cardiorespiratory function following abdominal surgery. Br J Surg. 1982;69:417–419. [DOI] [PubMed] [Google Scholar]
- 8.Persson F, Kristensen BB, Lund C, et al. Postural stability after inguinal herniorrhaphy under local infiltration anaesthesia. Eur J Surg. 2001;167:449–452. [DOI] [PubMed] [Google Scholar]
- 9.Emmeluth C, Bie P. Effects, release and disposal of endothelin-1 in conscious dogs. Acta Physiol Scand. 1992;146:197–204. [DOI] [PubMed] [Google Scholar]
- 10.Bie P, Sandgaard NC. Determinants of the natriuresis after acute, slow sodium loading in conscious dogs. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1–R10. [DOI] [PubMed] [Google Scholar]
- 11.Poulsen K, Jorgensen J. An easy radioimmunological microassay of renin activity, concentration and substrate in human and animal plasma and tissues based on angiotensin I trapping by antibody. J Clin Endocrinol Metab. 1974;39:816–825. [DOI] [PubMed] [Google Scholar]
- 12.Mimica Z, Biocic M, Bacic A, et al. Laparoscopic and laparotomic cholecystectomy: a randomized trial comparing postoperative respiratory function. Respiration. 2000;67:153–158. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191–1194. [PubMed] [Google Scholar]
- 14.Kehlet H. Surgical stress response: does endoscopic surgery confer an advantage? World J Surg. 1999;23:801–807. [DOI] [PubMed] [Google Scholar]
- 15.Kehlet H. Modification of responses to surgery by neural blockade: clinical implications. In: Cousins MJ, Bridenbaugh PO, eds. Neural Blockade in Clinical Anesthesia and Management of Pain. Philadelphia: Lippincott-Raven; 1998:129–175. [Google Scholar]
- 16.Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ. 1998;316:961–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan TJ, Soppitt A, Maroof M, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97:820–826. [DOI] [PubMed] [Google Scholar]
- 18.Sinclair S, James S, Singer M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. BMJ. 1997;315:909–912. [DOI] [PMC free article] [PubMed] [Google Scholar]