Abstract
Objective:
Determine the histologic response-rate (complete versus partial tumor extinction) after single radiofrequency ablation (RFA) of small hepatocellular carcinoma (HCC) arising in cirrhosis. Investigate possible predictors of response and assess efficacy and safety of RFA as a bridge to liver transplantation (OLT).
Background:
RFA has become the elective treatment of local control of HCC, although histologic data supporting radiologic assessment of response are rare and prospective studies are lacking. Prognostic impact of repeated RFA for HCC persistence is also undetermined.
Methods:
Percentage of RFA-induced necrosis and tumor persistence-rate at various intervals from treatment was studied in 60 HCC (median: 3 cm; Milan-Criteria IN: 80%) isolated in 50 consecutive cirrhotic patients undergoing OLT. Single-session RFA was the only treatment planned before OLT. Histologic response determined on explanted livers was related to 28 variables and to pre-OLT CT scan.
Results:
Mean interval RFA→OLT was 9.5 months. Post-RFA complete response rate was 55%, rising to 63% for HCC ≤3 cm. Tumor size was the only prognostic factor significantly related to response (P = 0.007). Tumor satellites and/or new HCC foci (56 nodules) were unaffected by RFA and significantly correlated with HCC >3 cm (P = 0.05). Post-RFA tumor persistence probability increased with time (12 months: 59%; 18 months: 70%). Radiologic response rate was 70%, not significantly different from histology. Major post-RFA morbidity was 8%. No mortality, Child deterioration, patient withdrawal because of tumor progression was observed. Post-OLT 3-year patient/graft survival was 83%.
Conclusions:
RFA is a safe and effective treatment of small HCC in cirrhotics awaiting OLT, although tumor size (>3 cm) and time from treatment (>1 year) predict a high risk of tumor persistence in the targeted nodule. RFA should not be considered an independent therapy for HCC.
Radiofrequency ablation is a safe and effective treatment of small hepatocellular carcinoma in cirrhotics awaiting liver transplantation. Tumors beyond 3 cm and time from treatment longer than 1 year predict high rate of tumor persistence in the targeted nodule. Ablative procedures should not be considered an independent therapy for HCC.
The application of radiofrequency ablation (RFA) to the treatment of human liver tumors was pioneered in the early 1990s both in Europe and the USA.1–3 Since then, technology of alternating electric current and electromagnetic energy delivery within living tissues, as well as different clinical applications and approaches (percutaneous versus laparoscopic or laparotomic4–7) have been tested, with an estimated advantage of RFA over liver resection in terms of posttreatment morbidity and mortality, hospital stay, and costs.7–9 A possible gain in both life expectancy and cost per year of life saved has been envisaged for adjuvant percutaneous alcohol injection (PEI) and presumably for RFA in patients awaiting liver transplantation10 and in considerable number of liver units, RFA has become the elective treatment of local control of HCC, even in patients eligible for liver resection or transplantation.11–14
Despite its easy handling and use in different specialties, very little information exists on the efficacy of RFA in terms of pathologic confirmation of complete tumor extinction in every treated nodule.15,16
Moreover, the real impact of RFA on HCC prognosis and long-term survival of patients is difficult to assess, due to the widespread practice of retreating tumor nodules in which persistence of neoplastic tissue is suspected, whether at contrast enhanced ultrasound or CT/NMR imaging.17–21
In fact, the ultimate oncologic question on RFA is focused on the possible relationships between tumor characteristics (such as size, growth pattern, or location within the liver) and predictability of response obtained after each procedure.
It has to be emphasized that the differences in assessing tumor response (histology versus imaging) continues to hamper an unbiased comparison between liver resection and RFA as the most effective treatment of HCC, when liver transplantation is not recommended.11,15,16,22 However, the model of liver transplantation offers a quite unique opportunity to validate RFA efficacy in treating HCC: in fact, the entire cirrhotic liver and the previously “ablated” tumor can be studied with a detailed pathology analysis focused on both treatment-induced necrosis and possible tumor characteristics related to response.
On such assumptions, we conducted a prospective study in which RFA was given as the only treatment of HCC in cirrhotic patients awaiting orthotopic liver transplantation (OLT). The unforeseeable time period from RFA to tumor removal at the time of transplant offered also an opportunity for estimating the probability of residual HCC in the site of treatment at various time intervals from RFA procedures.
MATERIALS AND METHODS
Patients and HCC Characteristics
From March 1998 to February 2003, a consecutive series of 50 cirrhotic patients with concurrent HCC underwent RFA while awaiting liver transplantation at the National Cancer Institute of Milan.
According to the Child-Pugh scoring system,23 the series included 11 class A (22%), 30 class B (60%), and 9 class C patients (18%). Cirrhosis was related to HBV infection in 24 cases (48%), HCV in 22 cases (44%), HBV plus HCV in 3 (6%), and cryptogenic in 1 case (2%).
Pretransplant tumor staging included contrast enhanced ultrasound, total body double/triple phase helical CT scan, bone scan, brain and cholangio-NMR, together with upper and lower G.I. tract endoscopy. Confirmation biopsy of HCC was not requested by pretransplant protocol, although it was obtained in 6 cases (12%) of doubtful imaging diagnosis associated with normal α-fetoprotein (AFP) serum-level.24
RFA Protocol and Technique
All patients with HCC in the setting of cirrhosis evaluated for OLT were considered eligible for RFA, unless severe liver failure (Child-Pugh >11, MELD score >24) discouraged any treatment attempt, while waiting transplantation.23,25
The specific requirement of the study protocol was that RFA had to be applied only once and for all in each targeted HCC, although multiple needle insertions in the tumor were allowed as a part of each treatment session.
Even though RFA was elected to be the unique pretransplant treatment in all patients, each procedure was technically performed on the basis of patient (and tumor) characteristics, as a result of a multidisciplinary discussion among surgeons and interventional radiologists. Percutaneous approach for RFA was applied in 29 patients (58%), while the remaining 21 underwent RFA in the operating room, under general anesthesia as a part of pretransplant laparoscopic video-assisted staging and treatment of HCC, using intraoperative ultrasound. Among them, there were 3 cases in which transplant candidacy was decided after liver resection of HCC associated with RFA of a second unresectable tumor nodule. Depending on device availability, operator preference and tumor location, RFA was performed in 42 cases (84%) using a “hook-tip” needle housing 4 or 7 retractable curve electrodes (RITA Medical System, Mountain View, CA), while in 8 cases (16% of HCC nodules) ablation was achieved using a “cool-tip” needle housing an exposed 2- to 3-cm electrode and an internal water cooling system (Radionics TM, Burlington, MA). In all instances, RFA was performed with real-time US guidance using a 3.5-MHz convex-probe (HDI 5000, ATL Ultrasound, Bathell, WA; or Aloka 2000, Aloka, Tokyo, Japan) and a guide device incorporated into the US probe in case of percutaneous puncture or during intraoperative approach.
In case of laparoscopic RFA, the US probe was applied directly onto the liver surface, through a 12-mm trocar placed in the right upper quadrant of the abdomen, while the electrode placement into the tumor was directed by hand.4,5 Grounding was achieved by 2 dispersive pads of at least 400 cm2 attached to the patient's thighs, and during the procedures local tissue temperature was continuously monitored, as well as tissue impedance.
During RFA application, the appearance and progression of hyperechogenicity in the targeted tumor was used to guide the duration of therapy. In fact, RFA was applied until the tumor appeared completely hyperechoic for an application time of energy lasting an average of 12 minutes. In case of repositioning of the RFA electrode, that was directed to the area of tumor where hyperechogenicity was not evident after the first needle placement.
Color-Doppler flow imaging was also performed to assess loss of hypervascularity in the tumor after treatment, and in some recent cases, this was achieved through real-time contrast-enhanced US (Sonovue, Bracco, Milan, Italy).
The study was performed with the approval of the institutional scientific and ethic committees, and a written informed consent was obtained from every patient prior to treatment.
Post-RFA Imaging, Liver Transplantation, and Follow-up
The consistency of response to RFA was evaluated by contrast-enhanced CT on the day following treatment. In case of confirmation of short-term effect on the targeted areas, further CT was planned 3 and 6 months after treatment, namely, at the conventional interval accepted as highly predictive for correct determination of tumor necrosis.26,27 Though always performed at each follow-up visit, conventional ultrasound was not used to evaluate post-RFA response during pretransplant follow-up. Liver transplantation was performed according to standardized procedures, and follow-up was run in a dedicated outpatient clinic.
Assessment of RFA Therapeutic Efficacy in the Removed Liver
Two pathologists examined each surgical specimen under senior staff supervision (S.A.), unaware of pretransplantation imaging and RFA modality. Timing of histologic evaluation was not within investigators’ control, since it was only dependent on liver graft availability and transplantation date.
Besides cirrhosis and fibrosis score determination, the liver graft was also investigated for the presence of vital satellites (ie, small tumors close to the main HCC or within the same segment) and for other HCC foci in other segments.
To do that, the removed liver was fixed in 10% formalin and then cut into slices of about 0.8 to 1 cm through sagittal planes, similar to the CT scan. Each suspected neoplastic nodule, as well as all tumors targeted by RFA, was identified for conventional optical microscopy after hematoxylin-eosin staining. Sampling procedures were intended to prepare slices of 1 cm2 as for a systematic mapping of the neoplastic nodule at the level of its maximum diameter. An average of 15 slides per tumor nodule was prepared, with the overall percentage of post-RFA necrosis calculated in each HCC summing the percentage of vital tissue determined in each cm2. Necrosis was defined as the complete absence of cells or the presence of necrotic or amorphous material. Noteworthy, cellular staining faded with time toward a “thermal fixation” of the tissue treated with RFA,28 and consequently small islands of affected “ghost cells” within region of coagulative necrosis were not considered as vital tissue (Fig. 1A, B).
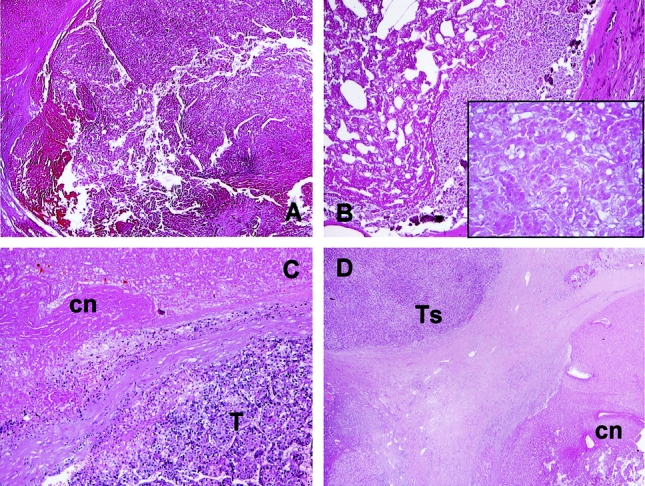
FIGURE 1. Patterns of response after radiofrequency ablation of hepatocellular carcinoma (histologic determination on explanted livers). Complete response (A, B). A, Intense eosinophilic staining of coagulative necrosis with no residual tumor cells in an HCC nodule 4 months after RFA (H&E, × 20). B, Coagulative necrosis surrounded by fibrous pseudocapsula 1 year after RFA (H&E, × 40); at higher magnification (inset, × 100) preservation of the tissue architecture with islands of fading “ghost” hepatocytes are observed. Partial response (C, D). C, Residual vital tumor (T) is detected inside coagulative necrosis (cn) induced by radiofrequency ablation performed 13 months previously, with a fibrous band dividing the 2 areas (H&E, × 40). D, Coagulative necrosis surrounded by tumor capsule, with a small HCC satellite (Ts), just outside the rim of the RFA-extinguished tumor (H&E, × 100).
On the contrary, the diagnosis of “vital” neoplasia was based on the evidence of normal hepatocytes and HCC cells exhibiting (at H&E) a homogeneous eosinophilic staining within cytoplasm and dense basophilic staining of their nuclei.16 Such occurrences were defined as tumor persistence and considered as partial response to RFA (Fig. 1C, D).
Tumor extension was also monitored through AFP serum level determination the day of RFA and every month after treatment. In doing that, biochemical response to RFA was also graded and classified as 50% greater or smaller with respect to the baseline level.
Statistical Methods
A total of 28 variables were collected from patients, tumor, and treatment characteristics (Tables 1–3) and correlated to RFA efficacy, expressed as complete necrosis of the treated tumors at histology. Since some patients were treated for multiple lesions, to account for possible correlation within the same subject, the above analysis was carried out by means of a generalized linear model with logit link within a “generalized estimating equations” approach.29
TABLE 1. Radiofrequency Thermal Ablation (RFA) Treatment of HCC in 50 Cirrhotic Patients Awaiting OLT: Patients’ Demographics, Transplant and Tumor Characteristics
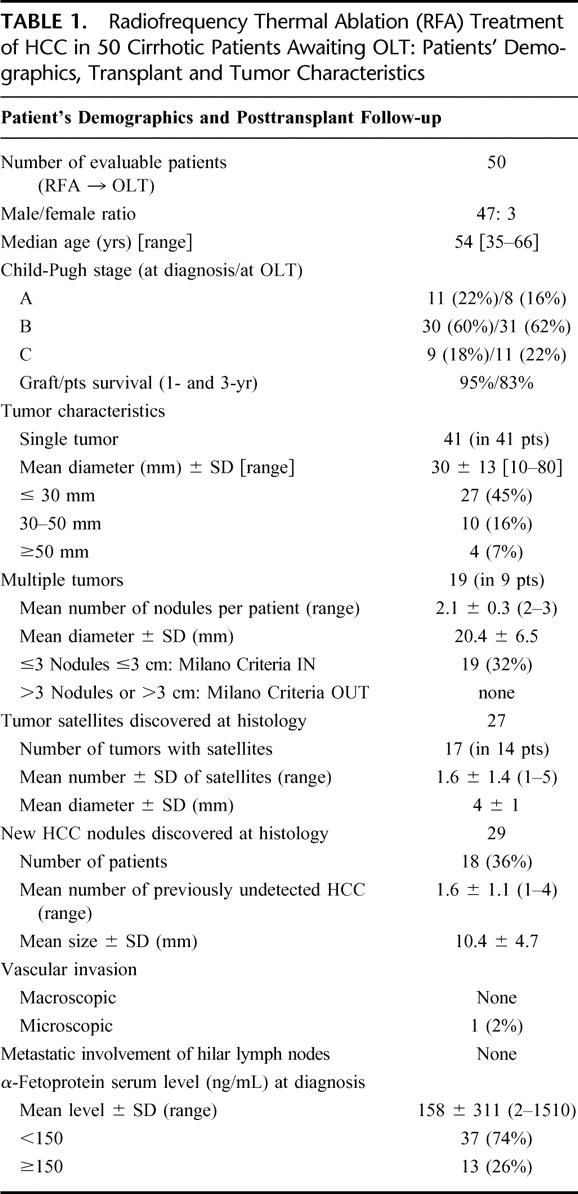
TABLE 2. Radiofrequency Thermal Ablation (RFA) Treatment of HCC in 50 Cirrhotic Patients Awaiting OLT: Treatment Characteristics, Morbidity and Mortality
TABLE 3. Postradiofrequency Thermal Ablation (RFA) Response Determined at Histology (60 HCC in 50 Explanted Livers)

To estimate the probability of residual HCC during the waiting time for liver transplantation, the event time was computed as the interval from the date of RFA to the date of either clinical (at CT scan during follow-up) or histologic (at liver transplantation) confirmation. The analysis was carried out by means of a Weibull regression model for survival data, in which detection-event times in patients with residual tumor at liver transplantation were considered as “left-censored” data. This was done to account for the fact that the true time of recurrence could be in fact any time between the RFA and liver transplantation. The Weibull model was chosen as the best fitting from a number of parametric models. From the Weibull shape and scale parameter estimates, cumulative probability curve of post-RFA residual HCC was computed and plotted (Fig. 2). Analyses were carried out using SAS30 and S-plus31 statistical packages.
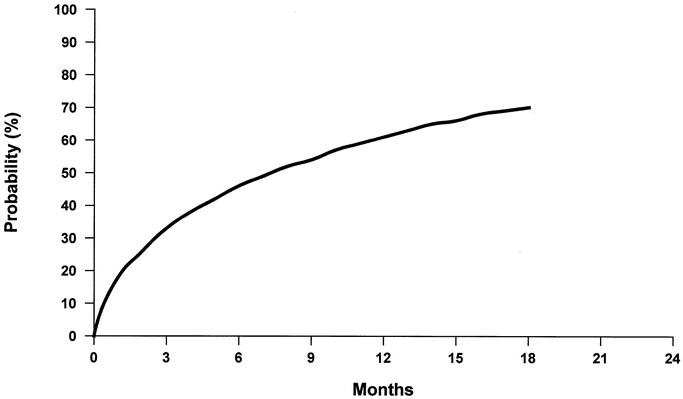
FIGURE 2. Cumulative probability of hepatocellular carcinoma persistence after radiofrequency ablation. The unforeseeable time period from RFA to tumor removal (at the time of liver transplantation) offered the opportunity for estimating the probability of residual HCC in the targeted nodule. The cumulative probability curve shows an increased risk of post-RFA incomplete necrosis and HCC persistence that increases with time (see text).
RESULTS
Patients and Posttransplant Tumor Staging
A summary of the general characteristics of the 50 patients treated with a single session of RFA while on the waiting list for liver transplantation is reported in Table 1.
The large majority of subjects were men (94%) in their fifties (53±7), and candidacy for transplantation was decided in 60% of them as Child-Pugh class B patients. Overall, 42 out of 50 (84%) became Child-Pugh class B-C by the time of transplantation.
The most common cause of cirrhosis was viral hepatitis, with a comparable number of HBV and HCV etiologies (48% versus 44%); moreover, an association with alcohol consumption was noted in 15 cases (30%). All patients were candidated to OLT according to the Milan Criteria for small HCC in cirrhosis (single nodule ≤5 cm or multiple ≤3 nodules, each ≤3 cm)32 and a total of 60 hypervascular nodules of HCC underwent RFA as the unique antitumor treatment, while the patients were on the waiting list. Characteristics of the HCC nodules targeted by pretransplant RFA are summarized in Table 2.
Briefly, the average size of treated tumors was 27.5 mm, being 77% of them (46 nodules) ≤30 mm in diameter. The average number of neoplastic tumor nodules per patient was 1.2 (range 1–3), since in 41 patients the HCC occurred as a single lesion and in 9 patients as multiple disease (2 or 3 tumors, mean number: 2.1). Fourteen patients (28%) presented with microscopic tumoral deposits closely related and often coupled with the capsule of the main HCC, the median number of such tumor satellites being 2 (range 1–5).
In about one third of the series (18 pts: 36%) new HCC foci were discovered in the explanted cirrhotic livers: such understaging of tumor extension was referred to a median of 1 additional nodule of HCC (range 1–4) with a diameter of 10 mm (range 3–14).
There was a close relationship between the size of HCC and the discovery of either neoplastic satellites (close to the largest nodule) or new HCCs in other segments. In fact, about half (57%) of the newly discovered vital satellites and/or new HCC foci (32 out of 56 being “very small” tumors) were associated with the 14 nodules larger than 3 cm, while the remaining 43% (24 out of 56) were distributed among the 46 HCCs ≤3 cm (P < 0.001).
In a single case, microscopic tumor emboli were detected at histology (microvascular invasion), while none of patients suffered macroscopic vascular invasion or metastatic involvement of the hilar lymph nodes.
Mean AFP serum level at the time of pretransplant RFA was 158 ng/mL (range: 2–1510) with 10 patients (20%) showing AFP ≥300 ng/mL, and only 3 cases ≥1000 ng/mL.
Safety and Therapeutic Assessment of RFA Efficacy at Histology
The average hospital stay for pretransplant RFA was 2.5 days (range: 0–10). In the 60 HCCs treated with RFA, a low rate of periprocedural complications was observed, since only 8% of patients had major post-treatment problems requiring hospital admission and medical therapy (Table 2). Minor complications (mainly fever and pain in the right upper abdominal quadrant) were registered in 10 patients (20%), who did not change their regular outpatient schedule.33,34
There was no mortality directly related to RFA, although in 1 patient (2%) an ongoing hepatic deterioration led to transplantation ahead of time: 50 days after RFA.
The rate of morbidity was not significantly different between percutaneous and laparoscopic approach to RFA,4–7 needle type, or number of needle applications during each procedure. Although there was a slight increase in Child-Pugh score at the time of transplantation compared with pre-RFA values (Table 1), that was not significantly different (7.4 ± 1.8 versus 7.5 ± 2.0, respectively).
The median interval from RFA to OLT was 9.5 months (range 2–47), and none of the patients was excluded from transplant operation because of accelerated tumor progression or occurrence of distant metastases while on the waiting list following RFA treatment.
The efficacy of RFA was studied in the explanted liver as the capability to induce complete versus partial necrosis in the targeted tumor nodules. Histologically proven response to RFA was then related to a large series of 28 variables deducible from patient tumor and treatment characteristics (with the 10 most relevant ones reported in Table 3).
Overall, 33 out of 60 “ablated” HCCs (55%) resulted completely necrotic at histology, while in the remaining 27 (45%) some residual tumor tissue was confirmed. When vital tumoral cells were detected, an average of 65% of the total tumor volume (range 10% to 95%) was occupied by neoplastic (persistent) tissue.
At statistical analysis, tumor size came out as the unique prognostic factor significantly related to complete response to RFA (P = 0.007), while other tumor characteristics (ie, capsule, satellites, multifocality) or AFP variations before and after RFA, or grading of the associated liver cirrhosis (ie, Child-Pugh score or fibrosis score) did not influence the response to treatment.
When response to RFA was correlated with time, the cumulative probability curve expressing HCC persistence in the treated tumor nodule was calculated (Fig. 2). In HCC nodules treated with a single RFA application, the risk of incomplete necrosis and tumor persistence increased with time and in fact, the cumulative probability of HCC persistence in the treated nodules 12 and 18 months after RFA reached 59% and 70%, respectively.
Imaging Assessment of RFA Effect
In all cases, satisfactory early response signs and correct targeting of RFA were confirmed by 2 radiologists on staff at 24 to 36 hours CT (rim of hyperattenuation surrounding the region of coagulated tumor), with the exception of the 3 pts treated during laparotomy, who were not studied early after surgery. Pretransplant assessment of RFA efficacy, namely a helical CT performed at least 6 months after treatment, was available in only 44 out of 60 treated HCCs, being the other 16 nodules related to patients transplanted at earlier time intervals.
During pretransplant period, CT scan was able to detect partial response to radiofrequency in 30% of the treated HCCs (13 out of 44), while complete tumor extinction was inferred by the absence of any hypervascularization and/or presence of tumor size reduction in the remaining 31 nodules (complete response rate: 70%).
As reported in Table 3, although there was a trend toward an overestimation of RFA efficacy at CT scan, the comparison between the pretransplant imaging and posttransplant histologic response failed to reach statistical significance.
The mean percentage of necrosis induced by RFA in treated nodules was 93.6 ± 12.9% at CT scan and 87.3 ± 22.2% at histology, giving a specificity of helical CT (complete versus partial response) of 73%, and a sensitivity in predicting the percentage of vital or persistent tumor of 64%.
Clinical Outcome
Previous treatment with RFA did not harm transplantation procedures, with the exception of 3 patients who had a significantly longer operation time due to previous liver resection associated with ablation of the residual tumor nodule. Six out of 50 cases (12%) received a right-liver graft (either from in vivo splitting of cadaveric donor or through living related donation). There was no significant difference in posttransplant outcome of such group of recipient when compared with candidates receiving a whole liver graft.
Posttransplant immunosuppression was based on cyclosporine (CsA, 22 pts: 44%) or tacrolimus (Tac, 28 pts: 56%) monotherapy associated with rapid withdrawal of steroids (within 2 months from OLT). In 8 patients (16%) with deteriorated posttransplant renal function, mycophenolate mofetil was added during follow-up at the times of significant reduction (>50%) of CsA or Tac dosage.
After a median posttransplant follow up of 22 months, the 1- and 3-year actuarial patient survival was 95% and 83%, and graft survival was exactly alike, since no patient underwent retransplantation.
During posttransplant follow-up, 4 deaths were registered: 2 for sepsis and brain hemorrhage (at 2 and 18 months) and 2 for HCC recurrence (at 8 and 31 months). Both tumor recurrences involved the liver graft and were also associated with pulmonary or peritoneal metastases. Interestingly, tumor recurrence was registered only in patients with HCC >5 cm who had partial response after RFA, while none of the patients with complete necrosis after successful RFA suffered tumor recurrence after liver transplantation.
DISCUSSION
Liver transplantation has become a widely accepted therapy for small HCC arising in cirrhosis, namely for patients carrying single tumor ≤5 cm or multiple ≤3 nodules ≤3 cm in size.32,35 In recent years, with refinements of posttransplant management and immunosuppression,36,37 possible expansions of such Milan criteria have been proposed,38 even though the “slippery slope” of an increased risk of cancer recurrence has been pointed out.39
Living donation40,41 and in vivo splitting of cadaveric grafts42 have been considered to comply with the growing number of liver transplant candidates with cancer, together with procedures intended to achieve a pretransplant HCC down-staging. Among them, RFA is increasingly proposed as complementary or even alternative treatment to more conventional approaches11–14,16,22,34 such as transarterial chemoembolization or liver resection. In fact, 33% to 40% long-term patient survival and 3% to 10% tumor-free survival have been reported after RFA,43,44 with a complete necrosis rate as high as 58% in medium-large size HCC (ie, above 5 cm in diameter, with cases up to 9.5 cm), using updated equipment,26 association with hepatic artery balloon occlusion45,46 or chemoembolization.
Although the long-term results of RFA, as well as its claimed superiority over liver resection, are not supported by prospective randomized trials, a large mass of clinical experience and technical data has been collected in the last few years, up to the point of considering RFA as the first option to be applied for controlling HCC progression. The effectiveness of percutaneous therapies is widely accepted, and a consensus of experts has assigned to PEI and RFA the role of “curative” treatment of HCC.25 However, the final acceptance of RFA in the general treatment strategy against HCC still awaits confirmations on ideal assessment of posttreatment tumor necrosis, relationship with technology, application modalities, tumor characteristics, and operator experience.13,14,27,47,48
In current RFA applications, both technical performance and subsequent assessment of result are performed by the same specialty, and differently from any other treatment in oncology, there is a very rare cross-reference to pathology (as in surgical or medical oncology before/after tumor treatment/removal). In most studies, RFA is applied without previous biopsy, even though tumors of less than 2 cm with doubtful imaging and normal AFP warrant pretreatment histologic confirmation of HCC.24 Patients with small focal lesions needing a diagnostic biopsy before treatment are not a leftover category13,34 and in the present pretransplant series represented 12% of all nodules of HCC treated with RFA.
Even when ablative therapies are applied on histologically proven HCC, the results in terms of complete or partial tumor extinction are often debated, and very few studies have been designed to check at histology the precise results of RFA.11,15,16,22,28,49 On the contrary, assessment of response to RFA in the current practice mostly relies on careful imaging and AFP dosage during follow-up,17–19 without requirements for histologic confirmation of tumor persistence suspected at CT or NMR scan. In the present study, the effectiveness of RFA, in terms of complete destruction of HCC, has been confirmed at histologic analysis in 55% of the targeted nodules. The percentage of complete response to RFA increased to 63% when tumors did not exceed 3 cm in diameter, and in fact the size of HCC was confirmed as the only significant factor predicting complete extinction of neoplastic cells after RFA (Table 3) regardless of any other tumor characteristics and treatment modalities.
When the histologic response rate was compared with radiologic assessment (70% complete response rate), a nonsignificant trend toward a CT scan overestimation of RFA results was seen. Technical refinements in image acquisition, as well as the small size of the tumor nodules collected in the present series (medially 3 cm), accounts for such a high rate of success; in addiction, tumor necrosis was evaluated at a 6-month CT scan, namely, at a follow-up interval accepted as highly predictive for correct determination of post-RFA necrosis.27,50 A reliable and more independent forecast on post-RFA response will be certainly available in the near future through technical refinement in imaging (ie, tumor vascularity on NMR or contrast color Doppler) and with measurement of metabolic activity in the treated tumor by positron emission tomography (PET): until then, other approaches to the key question of response to treatment should be considered investigational.49
In the present prospective study, each tumor nodule was treated with a single session of RFA, and the histologic result became available only at transplantation, namely, at an unpredictable interval after treatment. Such condition allowed the calculation of a risk curve foreseeing a 59% and 70% tumor persistence rate 12 and 18 months after a single session of RFA on a small HCC (Fig. 2). Whether such quantitative analysis will be confirmed or denied by future larger trials, the qualitative aspects of the data support the fact that RFA, differently from resective surgery, does work as a tumor-debulking procedure whose effectiveness decreases over time and depends on the size of targeted HCC. On clinical grounds, that is proven by the high need for multiple RFA treatments in case of medium to large (>5 cm) HCC26,47 and by the widespread (although unquantified) practice of retreatment of already “ablated” small HCC whenever persistence of neoplastic tissue is suspected.13,45,50 Based on the results of the present study, the practice of repeated RFA could be even recommended at given intervals after the first treatment for prevention of tumor resumption even in apparently “silent” areas.
The fact that histologic response to RFA could be either partial or complete did not play a role in determining patient outcome after OLT, at least in the present study where the important bias of patient selection (ie, only HCC at early stage) should be acknowledged. However, the use of RFA for sustained down-staging of advanced HCC candidates to OLT should be further investigated, with possibly more applications and easier management than liver resection10,51 or chemoembolization.52
The condition of multifocality underlines any HCC arising in cirrhosis. However, patients can still be considered at early stage of diseases when carrying ≤3 nodules ≤3 cm of tumor, with the same chance of post-OLT survival of single HCC up to 5 or even 6.5 cm in size.32,38 Such “limited multifocal disease” emerged before transplantation in 18% of our patients while increased to 36% when the entire explanted liver was studied at the pathology table (Table 1). Small HCC <2 cm should be biopsied prior to any treatment,24 a recommendation that recognizes the risk of overtreatment of nontumoral lesion, with consequent overestimation of post-RFA results.
In this prospective study, RFA was confirmed as an effective bridge to liver transplantation11,28,49 since no rapid HCC progression, tumor seeding, or vascular invasion was observed during the pretransplant period. Safety was also confirmed in patients with a Child B-C cirrhosis33 since major complications occurred in 8% of our cases (Table 2) with only 1 (2%) experiencing a severe decline in liver function, a result that settles halfway between the 2.2% and 22% reported in other nontransplant settings.33,53,54 It is worth noting that in severe cirrhotics, the observed complication rate after RFA could even be considered a clinical amelioration with respect to chemoembolization, where posttreatment liver deterioration is more common.55
In contrast to microwave and laser-induced thermal ablation, newer RFA probes will achieve larger volumes of necrosis, with predictable extension of their applications in HCC. The employment of updated technologies in this study (up to 2003) has shown that destruction of small HCC can be achieved in a significant number of cases, although not always. The increased incidence of tumor resumption at progressively longer time intervals from RFA is likely to occur even more frequently after treatment of large tumor volumes or employing progressively shorter sessions of RFA. Such attempts need to be confirmed in carefully designed trials, considering the ultimate judgment of histology as the golden standard to pursue.
In summary, our data support RFA as a safe and effective treatment of small HCC, although size of tumor (>3 cm) and time from treatment (>1 year) are associated with a high risk of tumor persistence in the targeted nodule. The histologic analysis confirming RFA as the ultimate “debulking procedure” for HCC sets ablation procedures apart from any surgical removal of liver tumors, as for resection or transplantation. In the present prospective series, RFA was able to control tumor progression without additional complications and act as an effective bridge to transplantation, providing that the tumor was small in all candidates. Radiologic assessment remains the most common tool to score RFA results, although pre- and post-treatment histologic evaluation remains unique for assessing tumor stage and response to treatment.
RFA should not be considered as an independent therapy for liver tumors.13 Rather, combination with different therapies, including surgery, should be tested in prospective studies to establish solid multidisciplinary approaches for the treatment of HCC in cirrhosis.
ACKNOWLEDGMENTS
The authors thank the Italian Association for Cancer Research (AIRC) and the Nord Italia Transplant Procurement Agency (NITp) for their support. The editorial assistance of D. Guarneri and E. Bertocchi is greatly acknowledged.
Footnotes
Supported by AIRC (Italian Association for Cancer Research).
Reprints: Vincenzo Mazzaferro MD, Gastrointestinal Surgery and Liver Transplantation Unit, Istituto Nazionale Tumori, Via Venezian 1, 20133 Milan, Italy. E-mail: vincenzo.mazzaferro@istitutotumori.mi.it.
REFERENCES
- 1.Mc Gahan JP, Browning PD, Brock JM, et al. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25:267–270. [DOI] [PubMed] [Google Scholar]
- 2.Rossi S, Fornari F, Pathies C, et al. Thermal lesions induced by 480KHz localized current field in guinea pig and pig liver. Tumori. 1990;76:54–57. [DOI] [PubMed] [Google Scholar]
- 3.Rossi S, Fornari F, Buscarini L. Percutaneous ultrasound-guided radiofrequency electrocautery for the treatment of small hepatocellular carcinoma. J Interv Radiol. 1993;8:97–103. [Google Scholar]
- 4.Curley SA, Izzo F. Laparoscopic radiofrequency [editorial]. Ann Surg Oncol. 2000;7:78–79. [DOI] [PubMed] [Google Scholar]
- 5.Montorsi M, Santambrogio R, Bianchi P, et al. Radiofrequency interstitial thermal ablation of hepatocellular carcinoma in liver cirrhosis: role of the laparoscopic approach. Surg Endosc. 2001;15:141–145. [DOI] [PubMed] [Google Scholar]
- 6.Wood TF, Rose DM, Chung M, et al. Radiofrequency ablation of 21 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol. 2000;7:593–600. [DOI] [PubMed] [Google Scholar]
- 7.Livraghi T, Goldberg SN, Lazzaroni S, et al. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. [DOI] [PubMed] [Google Scholar]
- 8.Maluf D, Fisher A, Maroney T, et al. Non-resective ablation and liver transplantation in patients with cirrhosis and hepatocellular carcinoma (HCC): safety and efficacy. Am J Transpl. 2003;3:312–317. [DOI] [PubMed] [Google Scholar]
- 9.Lau WY, Leung TWT, Yu SCH, et al. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llovet JM, Mas X, Aponte JJ, et al. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulvirenti A, Garbagnati A, Regalia E, et al. Experience with radiofrequency ablation of small hepatocellular carcinomas before liver transplantation. Transplant Proc. 2001;33:1516–1517. [DOI] [PubMed] [Google Scholar]
- 12.Wong LL. Current status of liver transplantation for hepatocellular cancer. Am J Surg. 2002;183:309–316. [DOI] [PubMed] [Google Scholar]
- 13.Ng KKC, Lam CM, Poon RTP, et al. Thermal ablative therapy for malignant liver tumors: a critical appraisal. J Gastroenterol Hepatol. 2003;18:616–629. [DOI] [PubMed] [Google Scholar]
- 14.Curley SA. Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol. 2003;10:338–347. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg SN, Gazelle GC, Compton CC, et al. Treatment of intra-hepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88:2452–2463. [PubMed] [Google Scholar]
- 16.Morimoto M, Sugimori K, Shirato K, et al. Treatment of hepatocellular carcinoma with radiofrequency ablation: radiologic-histologic correlation during follow-up periods. Hepatology. 2002;5:1467–1475. [DOI] [PubMed] [Google Scholar]
- 17.Dromain C, de Baere T, Elias D, et al. Hepatic tumors treated with percutaneous radio-frequency ablation: CT and MR imaging follow up. Radiology. 2002;223:255–262. [DOI] [PubMed] [Google Scholar]
- 18.Meloni MF, Goldberg SN, Livraghi T, et al. Hepatocellular carcinoma treated with radiofrequency ablation: comparison of pulse inversion contrast-enhanced harmonic sonography, contrast-enhanced power Doppler sonography, and helical CT. Am J Roentgenol. 2001;177:735–380. [DOI] [PubMed] [Google Scholar]
- 19.Sironi S, Livraghi T, Meloni F, et al. Small hepatocellular carcinoma treated with percutaneous RF ablation: MR imaging follow up. Am J Roentgenol. 1999;173:1225–1229. [DOI] [PubMed] [Google Scholar]
- 20.Chopra S, Dodd III GD, Chintapalli KN, et al. Tumor recurrence after radiofrequency thermal ablation of hepatic tumors: spectrum of findings on dual-phase contrast-enhanced CT. Am J Roentgenol. 2001;177:381–387. [DOI] [PubMed] [Google Scholar]
- 21.Lim HK, Choi D, Lee WJ, et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001;221:447–454. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman AL, Wu SS, Obaid AK, et al. Histologic evaluation and treatment outcome after sequential radiofrequency ablation and hepatic resection for primary and metastatic tumors. Am Surg. 2002;68:1038–1043. [PubMed] [Google Scholar]
- 23.Pugh RNH, Murray-Lyon IM, Dawson JL, et al. Transaction of the esophagus for bleeding esophageal varices. Br J Surg. 1973;60:646–654. [DOI] [PubMed] [Google Scholar]
- 24.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL Conference. J Hepatol. 2001;35:421–430. [DOI] [PubMed] [Google Scholar]
- 25.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. [DOI] [PubMed] [Google Scholar]
- 26.Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–768. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. Am J Roentgenol. 2000;174:323–331. [DOI] [PubMed] [Google Scholar]
- 28.Kosari K, Coad JE, Humar A, et al. Thermal fixation: the histopathology of hepatocellular cancer after radiofrequency ablation in liver transplantation. J Gastroint Surg 2003;7:259. [Google Scholar]
- 29.Liang KY, Zeger SL. Longitudinal data analysis using general linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 30.SAS Institute. SAS/STAT User's Guide. Cary, NC; 1990.
- 31.MathSoft. S-Plus User's Manual: S-Plus 2000. Seattle, WA; 1999.
- 32.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 33.Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radiofrequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. [DOI] [PubMed] [Google Scholar]
- 34.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bismuth H, Majno P, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999;19:311–322. [DOI] [PubMed] [Google Scholar]
- 36.Vivarelli M, Bellusci R, Cucchetti A, et al. A. Low recurrence rate of hepatocellular carcinoma after liver transplantation: better patient selection or lower immunosuppression? Transplantation. 2002;74:1746–1751. [DOI] [PubMed] [Google Scholar]
- 37.Keeffe EB. Liver transplantation: current status and novel approaches to liver replacement. Gastroenterology. 2001;120:749–762. [DOI] [PubMed] [Google Scholar]
- 38.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. [DOI] [PubMed] [Google Scholar]
- 39.Eng SC, Kowdley KV. Expansion criteria for liver transplantation in HCC: a slippery slope? Gastroenterology. 2002;122:579–582. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T, Tanaka K, Kiuchi T, et al. Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation. 2002;73:1896–1903. [DOI] [PubMed] [Google Scholar]
- 41.Hashikura Y, Kawasaki S, Terada M, et al. Long term results of living-related donor liver graft transplantation: a single center analysis of 110 patients. Ann Surg. 2001;72:95–99. [DOI] [PubMed] [Google Scholar]
- 42.Rogiers X, Malago M, Gawad K, et al. In situ splitting of cadaveric livers: the ultimate expansion of a limited donor pool. Ann Surg. 1996;224:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buscarini L, Buscarini E, Di Stasi M, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long term results. Eur Radiol. 2001;11:914–921. [DOI] [PubMed] [Google Scholar]
- 44.Rossi S, Di Stasi M, Buscarini E, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. Am J Roentgenol. 2001;174:759–768. [DOI] [PubMed] [Google Scholar]
- 45.Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119–126. [DOI] [PubMed] [Google Scholar]
- 46.Yamasaki T, Kurokawa F, Shirahashi H, et al. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow: comparison with standard percutaneous radiofrequency ablation therapy. Cancer. 2002;95:2353–2360. [DOI] [PubMed] [Google Scholar]
- 47.Bleicher RJ, Allegra DP, Nora DT, et al. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol. 2003;10:52–58. [DOI] [PubMed] [Google Scholar]
- 48.Erce C, Parks RW. Interstitial ablative techniques for hepatic tumors. Br J Surg. 2003;90:272–289. [DOI] [PubMed] [Google Scholar]
- 49.Fontana RJ, Hamidullah H, Nghiem H, et al. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: a safe and effective bridge to liver transplantation. Liver Transpl. 2002;8:1165–1174. [DOI] [PubMed] [Google Scholar]
- 50.Cioni D, Lencioni R, Rossi S, et al. Radiofrequency thermal ablation of hepatocellular carcinoma: using contrast-enhanced harmonic power Doppler sonography to assess treatment outcome. Am J Roentgenol. 2001;177:783–788. [DOI] [PubMed] [Google Scholar]
- 51.Majno PE, Sarasin FP, Mentha G, et al. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology. 2000;31:1019–1021. [DOI] [PubMed] [Google Scholar]
- 52.Spreafico C, Marchianò A, Regalia E, et al. Chemoembolization of hepatocellular carcinoma in patients who undergo liver transplantation. Radiology. 1994;192:687–690. [DOI] [PubMed] [Google Scholar]
- 53.Llovet J, Villana R, Brù C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. [DOI] [PubMed] [Google Scholar]
- 54.Curley SA, Izzo F, Ellis LM, et al. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graziadei IW, Sandmueller H, Waldenberger P, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads excellent outcome. Liver Transplant. 2003;9:557–563. [DOI] [PubMed] [Google Scholar]


