Abstract
Objectives:
The objective of this study was to evaluate the efficacy of nonwoven bioabsorbable nanofibrous membranes of poly(lactideco-glycolide) for prevention of postsurgery-induced abdominal adhesions.
Summary Background Data:
Recent reports indicated that current materials used for adhesion prevention have only limited success. Studies on other bioabsorbable materials using a new fabrication technique demonstrated the promising potential of generating an improved and inexpensive product that is suitable for a variety of surgical applications.
Methods:
All rats underwent a midline celiotomy. The cecum was identified and scored using an abrasive pad until serosal bleeding was noted on the anterior surface. A 1 × 1 cm2 of abdominal wall muscle was excised directly over the cecal wound. The celiotomy was then closed in 2 layers immediately (control) after a barrier was laid in between the cecum and the abdominal wall. All rats underwent a second celiotomy after 28 days to evaluate the extent of abdominal adhesions qualitatively and quantitatively.
Results:
Cecal adhesions were reduced from 78% in the control group to 50% in the group using biodegradable poly(lactide-co-glycolide) (PLGA) nonwoven nanofibrous membranes (n = 10, P = 0.2) and to 22% in the group using membranes containing PLGA and poly(ethylene glycol)/poly(D,L-lactide) (PEG-PLA) blends (n = 9, P = 0.03). Electrospinning method also enabled us to load an antibiotic drug Cefoxitin sodium (Mefoxin; Merck Inc., West Point, PA) with high efficacy. The electrospun PLGA/PEG-PLA membranes impregnated with 5 wt% cefoxitin sodium, which amounts to approximately 10% of the systemic daily dose typically taken after surgery in humans, completely prevented cecal adhesions (0%) in rats.
Conclusions:
Electrospun nonwoven bioabsorbable nanofibrous membranes of poly(lactide-co-glycolide) were effective to reduce adhesions at the site of injury using an objective rat model. The membrane acted as a physical barrier but with drug-delivery capability. The combined advantages of composition adjustment, drug-loading capability, and easy placement handling (relatively hydrophobic) make these membranes potentially successful candidates for further clinical evaluations.
Electrospun nonwoven bioabsorbable nanofibrous membranes of poly(lactide-coglycolide) were effective to reduce adhesions at the site of injury using an objective rat model. The membrane acted as a physical barrier but with drug delivery capability. The combined advantages of degradation adjustment, drug-loading capability, and easy placement handling make these membranes potentially successful candidates for further clinical evaluations.
In the last 10 years, as abdominal procedures are routinely practiced, adhesions have become a common cause of postoperative complications, which include small bowel obstruction, female infertility, chronic debilitating pain, and difficulty with future operations.1–4 For example, case studies have identified postoperative adhesions as being the cause for 54% to 74% of small bowel obstructions, in which 41% to 44% of these required additional surgical correction.5 Postoperative adhesions thus have a profound economic impact.6 It has been estimated that in the United States, the total annual cost of removing lower abdominal adhesions, including the surgical procedure itself, exceeds $2 billion in overall inpatient treatment charges, excluding recuperation and lost productivity. Hospitalizations for adhesion-related problems in Medicare patients alone cost over $700 million in 1996.
Trauma, foreign bodies, ischemia, and infections are major factors associated with the formation of postoperative adhesions. Prevention of adhesions can decrease the severity of complications. It can also ease the technical procedures for subsequent surgery. Prior methods to achieve the goal of antiadhesion included irrigation, the use of fibrinolytic agents such as anticoagulants, antiinflammatory agents, antibiotics, as well as the use of physical barriers. For example, Buckenmaier et al evaluated different antiadhesion agents using a rat model and found that tissue plasminogen activator, a fibrinolytic agent, was most successful in reducing the strength of adhesions, whereas physical barriers were the most successful in decreasing the actual incidence.7 The demonstrated barrier materials thus far included animal membranes, gold foils, mineral oil, silk, rubber, and Teflon sheets.8 However, these materials have had only limited success.
Recently, scientists have developed absorbable antiadhesion barriers that can protect tissue when in use but dissolve when they are no longer needed.9–14 Such products, approved by the U.S. Food and Drug Administration (FDA), included INTERCEED made by Johnson & Johnson and Seprafilm made by Genzyme Corporation. INTERCEED is a satin-like fabric made from oxidized regenerated cellulose. Seprafilm is made from carboxymethylcellulose and hyaluronic acid. Both of them dissolve and form gel-like barriers after placement at the potential site of adhesion formation. Studies showed that the formation of postoperative adhesions could be reduced by these barriers. However, the efficacies of INTERCEED may be significantly reduced in the presence of blood.7–9 Another product, PRECLUDE, produced by W. L. Gore, is made of Gore-Tex, a version of Teflon (polytetrafluoroethylene). Although this product has been available on the market for almost 10 years, it is not bioabsorbable and its usefulness is limited by the need for suturing and later removal.
Although newer products have been attempted to fulfill the criteria for adhesion prevention, none can completely prevent adhesions in all situations. Thus, there remains a need for an improved and inexpensive product that is effective in a variety of surgical applications for a greater number of patients. In this study, we have examined the use of a novel nanostructured barrier made by the electrospinning technique, which uses electrostatic forces to fabricate nonwoven mats of nanofibers (diameters from submicron to 10s of nanometers). The materials used were biocompatible and biodegradable poly(lactide-co-glycolide) (PLGA) copolymers, which have been used in many FDA-approved implant devices such as suture fibers. Figure 1A shows a typical scan electron microscopy (SEM) image of electrospun PLGA barrier. The morphology and the fiber diameter can be controlled by the electrospinning processing parameters.15 The unique morphology of electrospun membranes and the relatively hydrophobic property provide a comfortable texture and easy handling ability for the electrospun membranes during surgery. Figure 1B demonstrates the placement of the electrospun PLGA barrier at the surgical site between the defects on abdominal wall and cecum of the test animal. The wetted membrane is not sticky, has the desired flexibility, and can be easily moved around.
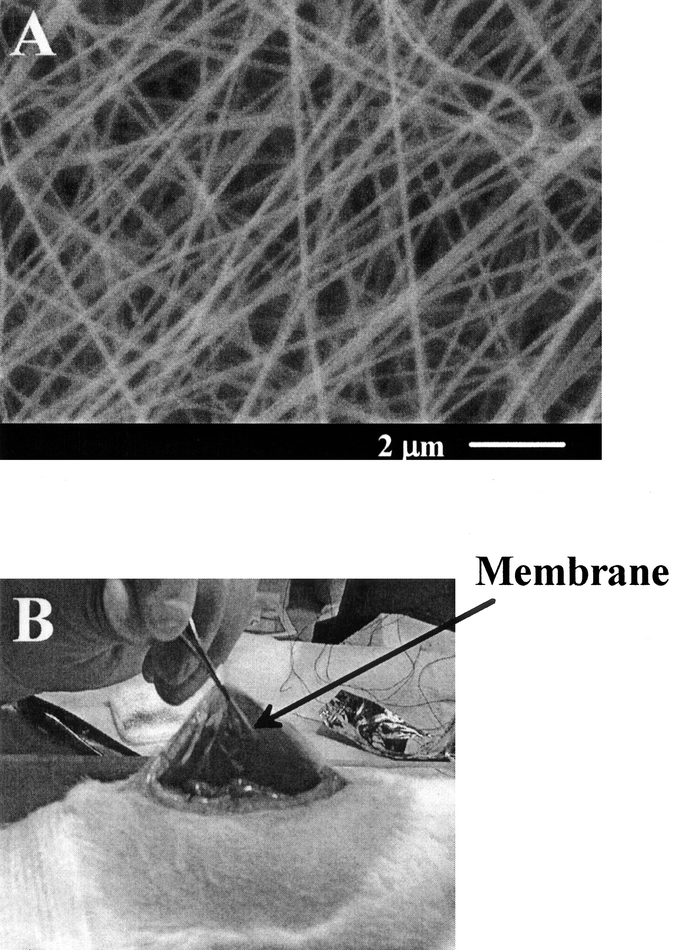
FIGURE 1. A: Scanning electron microscopic images of nonwoven nanofibrous membrane fabricated by electrospinning processing using a 30 wt% poly(lactide-coglycolide) (PLGA) in N,N-dimethyl formamide (DMF) solution containing 5 wt% of cefoxitin sodium (Mefoxin); and B: placement of electrospun membrane between the defects on abdominal wall and cecum.
Some studies showed that the use of antibiotics by oral administration16 or in systemic injection could slow down the adhesion formation and thus reduce the severity of the adhesions in a rat model.17 However, the efficacy of antibiotic peritoneal lavage for antiadhesions is still controversial. For instance, other reports, using different rat models, indicated that antibiotics in intraabdominal irrigation fluid could actually cause the adhesion formation and should not be used as a single agent for adhesion prevention.18 Recent studies using germ-free rats by Bothin et al have raised an interesting issue if the antibiotic treatment would affect intraabdominal adhesion formation.19–21 However, it is not the purpose of this work to resolve this issue. Instead, we have attempted to demonstrate a new kind of antiadhesion system having the combined properties of a physical barrier (PLGA nonwoven nanofibrous membranes) and a rate-controlled drug (antibiotics) delivery system at the injured site. In this study, cefoxitin sodium (Mefoxin; Merck Inc., West Point, PA) was chosen as a model antibiotic drug because it has been shown to be effective against the strains of gastrointestinal organisms, including Escherichia coli and Bacteroides fragilis, which are known to cause intraabdominal infections.22
MATERIALS AND METHODS
Poly (D,L-lactide-co-glycolide) (LA/GA molecular ratio: 75/25), PLGA, with an inherent viscosity of 0.55–0.75 dL/g, was purchased from Birmingham Polymers, Inc. (Birmingham, AL). Poly(ethylene glycol)-poly(D,L-lactide) (PEG-PLA) diblock copolymer (5k-5k) was synthesized from D,L-lactide and methoxy poly(ethylene glycol) by ring-opening polymerization using the procedure described elsewhere.23 Cefoxitin sodium (Mefoxin), purchased directly from Merck Inc., was mixed slowly with the polymer solution in appropriate amounts to form the polymer/drug solutions with a weight ratio of 95:5. Nonwoven nanofibrous membranes were fabricated from polymer solutions (with and without drug) using the electrospinning process described elsewhere.15 The morphology of electrospun membranes was examined using scanning electron microscopy (SEM) (JEOL JSM5300). The γ-irradiation method, with a total dosage of 25 kGy, was used as the terminal sterilization process of the electrospun membranes for in vivo animal tests.
Animal studies were carried out at the Division of Laboratory Animal Resources at Stony Brook University in a facility that has animal care and use programs accredited by AAALAC International and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The chosen animal model, based on the objective rat model developed by Buckenmaier et al,7 is as follows: 300–450 g male Sprague-Dawley rats were used. They were individually housed and given food and water with libitum addition both pre- and postoperatively. Anesthesia was produced using an 80 mg/kg intramuscular ketamine and 10 mg/kg xylazine injection into the right hindleg before the celiotomy. Euthanasia was produced using inhaled isoflurane. All rats underwent a midline celiotomy, and the cecum was identified and scored using an abrasive pad until serosal bleeding was noted on the anterior surface. A 1 × 1 cm2 of abdominal wall muscle was excised directly over the cecal wound. The celiotomy was then closed in 2 layers immediately (control) after a barrier (2 cm × 2 cm × 250 μm, approximately 20 mg) was laid in between the cecum and the abdominal wall or after an antibiotic-impregnated barrier was placed in the aforementioned area.
All rats underwent a second celiotomy after 28 days by surgeons, who were blind to the group assignment, to evaluate the extent of abdominal adhesions qualitatively and quantitatively under the following guidelines:
The general overall health of the test animals indicated by their weight gain after the initial surgery.
The incidence ratio of adhesions recorded with the number of animals with adhesions divided by the total number of animals in the group.
The quality of adhesions on the previously abraded cecal side with a numeric score of 0–3 (0 = no adhesion, 1 = thin and filmy, 2 = significant and filmy, and 3 = severe with fibrosis).
Altogether, 9 groups of animals were used in this study with 10 to 12 animals in each group. These groups were divided into 3 categories: 1) control, electrospun membrane of PLGA, electrospun membrane of PLGA/PEG-PLA blend (85:15 weight ratio); 2) control, electrospun membrane of PLGA, electrospun membrane of PLGA with 5 wt% cefoxitin sodium; and 3) control, electrospun membrane of PLGA/PEG-PLA, electrospun membrane of PLGA/PEG-PLA with 5 wt% cefoxitin sodium. The purpose of this division is explained later.
Statistical Analysis
The incidence of animals with adhesions and the scores of adhesions were compared between treated and control groups using the Fisher exact test. A P value <0.05 was considered significant.
RESULTS
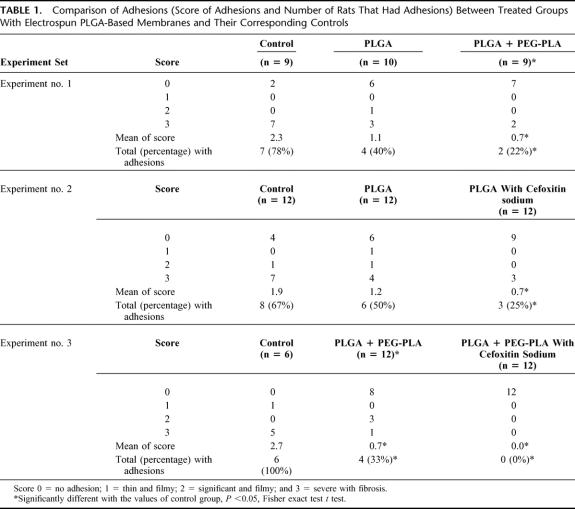
In vivo animal tests were carried out to compare the performance of 2 based materials without medication: PLGA and PLGA/PEG-PLA polymer blend (85:15 by weight). The addition of PEG-PLA was used to modify the hydrophilicity of the electrospun membrane. Results of abdominal adhesions for this comparison (experiment no. 1) are presented in Table 1. It was noted that the incidence of animals with adhesions was 78% for the control group, 40% for the group with PLGA membranes, and 22% for the group with PLGA/PEG-PLA membranes. The relatively ineffective performance of the PLGA membrane was the result of the substantial shrinkage incurred by the body fluid.24 In contrast, the electrospun PLGA/PEG-PLA membrane remained dimensionally stable in the body. The membrane of PLGA/PEG-PLA blend exhibited a significant difference from the control group (P < 0.05). This indicated that by improving dimensional stability of the membrane under in vivo conditions through adjustment of hydrophilicity, the antiadhesion properties were significantly improved.
TABLE 1. Comparison of Adhesions (Score of Adhesions and Number of Rats That Had Adhesions) Between Treated Groups With Electrospun PLGA-Based Membranes and Their Corresponding Controls
The electrospinning process enabled us to add secondary agents (eg, antibiotics) in the barrier membrane. By placing a medicated membrane at the injured site, the embedded medication can be delivered locally at a controlled dose and rate without systemic side effects. To test the efficacy of electrospun PLGA membranes with cefoxitin sodium, 2 sets of animal tests were carried out with the same compositions as discussed earlier: the electrospun PLGA membranes with and without cefoxitin sodium and the electrospun PLGA/PEG-PLA membranes with and without the drug. We intentionally selected the PLGA membrane because of its shrinkage problem, which allowed us to use the PLGA membrane as a drug delivery vehicle and to evaluate the efficacy of cefoxitin sodium in good confidence. We, however, did not carry out the study of cefoxitin sodium alone as an antiadhesion agent. In Table 1, experiment no. 2, it is seen that the group with PLGA membrane alone was not able to provide a significant difference from the control group (results were similar to the second group in experiment no. 1), but the group with the membrane containing antibiotics showed significant difference from the control group (P < 0.05). In other words, the efficacy to prevent adhesions was significantly improved with the addition of cefoxitin sodium, despite that the barrier properties were not optimized.
In a separate study, a similar trend toward the improved efficacy by incorporating cefoxitin sodium in electrospun PLGA/PEG-PLA membranes was also seen. In Table 1, experiment no. 3, it is noted that electrospun PLGA/PEG-PLA barriers significantly reduced defect–cecum adhesions compared with the control group (results were similar to the last group in experiment no. 1), whereas the membranes impregnated with cefoxitin sodium completely prevented adhesions (0%). These results confirm that the local delivery of antibiotics in the barrier significantly improves the efficacy for prevention of postsurgical adhesions. The mean score of the cecal adhesions provided additional support of this conclusion. Both groups of PLGA/PEG-PLA membranes alone and PLGA/PEG-PLA membranes with cefoxitin sodium showed significant differences from the control group (P < 0.05).
The average weight gains in the test animals after 28 days were also recorded as an indicator of postoperative recovery. The average weight gains were slightly higher in all treated groups than the control groups (data not shown).
DISCUSSION
Adhesions are abnormal depositions of fibrous tissue caused by a multitude of sources: abdominal trauma, operations, foreign bodies, and irradiation, just to name a few.25–27 The end result is inflammation of the peritoneum and resultant fibrinous exudates that organize into adhesional bands. A variety of techniques and agents have been proposed for the prevention of postoperative adhesions. Among these, physical barriers are probably the most successful means to decrease the actual incidence.8 Recent advances in adhesion prevention have led to a greater desire to develop an “ideal” barrier.25 In this study, we have evaluated the ability of biodegradable nonwoven nanofibrous membranes (based on PLGA and blends) to reduce postsurgical adhesions by using an objective rat model. We have examined 2 common causes of adhesion formation in the surgical animal: operative injury and foreign bodies. The base materials have been used in many FDA-approved implant devices such as suture fibers.
The group with PLGA barrier alone did not show statistically significant difference from the control group. This is possibly because the hydrophobic nature of PLGA caused the nanofiber scaffold to shrink in the animal body. The reduced membrane size decreased the covered area of the injured site and thus compromised the efficacy of the barrier properties. The shrinkage problem was overcome by the addition of 15 wt% of hydrophilic PEG-PLA diblock copolymer in the base material. Because electrospun PLGA/PEG-PLA membranes maintained a good dimensional stability in the body, in vivo study demonstrated that both frequency of adhesions and adhesion score of the group treated with the PLGA/PEG-PLA barrier were significantly improved. The electrospun membranes have overcome several shortcomings in commercial products. One problem of the commercial barrier films is that they tend to be very sticky on contact with bodily fluids or saline solution. The relatively hydrophobic nature of electrospun PLGA-based membranes makes them much easier to handle during surgery. They also provide a soft and comfortable texture for routine handling because of their unique nonwoven nanofibrous structure.
It was interesting to note that, despite the shrinkage problem in the electrospun PLGA membrane, the medicated barriers (containing cefoxitin sodium) exhibited excellent results for the reduction of adhesions. In the case of PLGA/PEG-PLA electrospun membranes containing cefoxitin sodium, cecal adhesions were completely prevented. Although the role of antibiotics used in intraperitoneal irrigation fluid or in systemic injection for the reduction of adhesions is still uncertain,16–19 our results clearly indicate that the local delivery of antibiotics (cefoxitin sodium) at the injured site from the barrier scaffold significantly improves the efficacy of antiadhesion.
Medicated electrospun membranes exhibited a “burst” drug release profile (data not shown) as a result of the large surface to volume ratio of such membranes. The burst mode may represent an ideal release profile for the prevention of surgically induced adhesions because most infections are related to contaminations introduced at the time of surgery when the skin barrier is violated. One major disadvantage in antibiotic peritoneal lavage is that antibiotics in saline solution can be absorbed systemically and quickly metabolized by the body. In contrast, the release period of embedded antibiotics in electrospun PLGA barriers can be controlled from several hours to 1 week, thus maintaining an antibiotic presence during this timeframe when adhesions are thought to develop. The dual effects of physical barrier and drug interactions are crucial for achieving the excellent efficacy of antiadhesions. The dose of cefoxitin sodium in the 5 wt% medicated membranes (approximately 20 mg in mass) for rats (approximately 350 g) is equivalent to approximately 10% of the typical daily dose given to adults (1 g/70 kg for 2 doses) after surgery. Although cefoxitin sodium locally released from the polymer membranes was effective to reduce the frequency and the severity of adhesions, the exact dose and the delivery profile have not been optimized. It is conceivable that even a smaller dose of cefoxitin sodium is needed for site-specific delivery, which will be a subject of future study.
Our current findings raise 2 interesting possibilities for the role of antibiotics in the prevention of postsurgical adhesions: 1) Adhesion is caused by contamination, whereby cefoxitin sodium eliminates the infections at the contaminated sites and thus reduces the incidence of adhesion. 2) Cefoxitin sodium directly limits the incidence of adhesion, even in the absence of contamination. Oncel et al recently pointed out that the effect of antibiotics on antiadhesions might be secondary. The primary effect was the reduction in the frequency and duration of infections.17 It is conceivable that the release of antibiotics from the medicated barrier results in a decrease in the local bacterial counts, thereby reducing the local inflammatory response at the wounded site and reducing the overall formation of fibrotic bands. However, the exact effect of antibiotics on the prevention of adhesions is still not clear and deserves further systematic study.
ACKNOWLEDGMENTS
Financial support of this work was provided by the Center for Biotechnology at Stony Brook, a National Institutes of Health-SBIR grant (GM63283-02) administered by the Stonybrook Technology and Applied Research, Inc., and the SUNY-SPIR program.
Footnotes
Supported by a National Institutes of Health-SBIR grant (GM63283-02) administered by the Stonybrook Technology and Applied Research, Inc., Center of Biotechnology at New York, and the SUNY-SPIR program.
Reprints: Professor Benjamin Chu, Department of Chemistry, State University of New York, Stony Brook, NY 11794-3400. E-mail: bchu@notes.cc.sunysb.edu.
REFERENCES
- 1.Menzies D. Peritoneal adhesions: incidence, cause and prevention. Surg Annu. 1992;24:27–45. [PubMed] [Google Scholar]
- 2.di Zerega GS. Contemporary adhesion prevention. Fertil Steril. 1994;61:219–235. [DOI] [PubMed] [Google Scholar]
- 3.Holtz G. Prevention and management of peritoneal adhesions. Fertil Steril. 1984;41:497–507. [DOI] [PubMed] [Google Scholar]
- 4.Ellis H. The causes and prevention of intestinal adhesions. Br J Surg. 1982;69:241–243. [DOI] [PubMed] [Google Scholar]
- 5.Menzies D. Postoperative Adhesions: their treatment and relevance in clinical practice. Ann R Coll Surg Engl. 1993;75:147–153. [PMC free article] [PubMed] [Google Scholar]
- 6.Ray NF, Larseb JW Jr, Stillman RJ, et al. Economic impact of hospitalizations for lower abdominal adhesiolysis in the United States in 1988. Surg Gynecol Obstet. 1993;176:271–276. [PubMed] [Google Scholar]
- 7.Buckenmaier CC, Pusateri AE, Harris RA, et al. Comparison of anti-adhesive treatments using an objective rat model. Am Surg. 1999;65:274–281. [PubMed] [Google Scholar]
- 8.Wiseman DM. Polymers for the prevention of surgical adhesions. In: Domb AJ, eds. Polymeric Site-Specific Pharmacotherapy. Chichester: John Wiley and Sons; 1994:369–421. [Google Scholar]
- 9.Wiseman DM, Gottlick-Larkowski L, Kamp L. Effect of different barriers of oxidized regenerated cellulose (ORC) on cecal and sidewall adhesions in the presence and absence of bleeding. J Invest Surg. 1999;12:141–146. [DOI] [PubMed] [Google Scholar]
- 10.Burns JW, Colt MJ, Burgees LS, et al. Preclinical evaluation of Seprafilm bioresorbable membrane. Eur J Surg Suppl. 1997;577:40–48. [PubMed] [Google Scholar]
- 11.De Iaco PA, Muzzupapa G, Bigon E, et al. Efficacy of a hyaluronan derivative gel in postsurgical adhesion prevention in the presence of inadequate hemostasis. Surgery. 2001;130:60–64. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda S, Se N, Iwata H, et al. Evaluation of the anti-adhesion potential of UV cross-linked gelatin films in a rat abdominal model. Biomaterials. 2002;23:2901–2908. [DOI] [PubMed] [Google Scholar]
- 13.Burns JW, Burgess L, Skinner K, et al. A hyaluronate based gel for the prevention of post-surgical adhesions: evaluation in two animal species. Fertil Steril. 1996;66:814–821. [PubMed] [Google Scholar]
- 14.Johns DB, Kiorpes TC, Rodgers KE, et al. Reduction of adhesion formation by postoperative administration of ionically cross-linked hyaluronic acid. Fertil Steril. 1997;68:37–42. [DOI] [PubMed] [Google Scholar]
- 15.Zong XH, Kim KS, Fang DF, et al. Structure and process relationship of electrospun bioabsorbable nanofibrous membranes. Polymer. 2002;16:4403–4412. [Google Scholar]
- 16.Bothin C, Midtvedt T, Perbeck L. Orally delivered antibiotics which lower bacterial numbers decrease experimental intra-abdominal adhesions. Langernbecks Arch Surg. 2003;388:112–115. [DOI] [PubMed] [Google Scholar]
- 17.Oncel M, Kurt N, Remzi FH, et al. The effectiveness of systemic antibiotics in preventing postoperative, intra-abdominal adhesions in an animal model. J Surg Res. 2001;101:52–55. [DOI] [PubMed] [Google Scholar]
- 18.Gutmann JN, Penzias AS, Diamond MP. Adhesion in reproductive surgery. In: Wallach EE, Zaccur HA, eds. Reproductive Medicine and Surgery. St. Louis: Mosby; 1995:681–693. [Google Scholar]
- 19.Bothin C, Midtvedt T. The role of the gastrointestinal micro flora in post surgical adhesion formation—a study in germfree rats. Eur Surg Res. 1992;24:309–312. [DOI] [PubMed] [Google Scholar]
- 20.Bothin C, Okada M, Midtvedt T. Postsurgical adhesion formation in germfree and ex-germfree rats—a study using three scoring scales. J Invest Surg. 1999;12:147–150. [DOI] [PubMed] [Google Scholar]
- 21.Bothin C, Okada M, Midtvedt T, et al. The intestinal flora influence adhesion formation around surgical anastomoses. Br J Surg. 2001;88:143–145. [DOI] [PubMed] [Google Scholar]
- 22.Tzianabos AO, Cisneros RL, Gershkovich J, et al. Effect of surgical adhesion reduction devices on the propagation of experimental intra-abdominal infection. Arch Surg. 1999;134:1254–1259. [DOI] [PubMed] [Google Scholar]
- 23.Kim KS, Chung S, Chin IJ, et al. Crystallization behavior of biodegradable amphiphilic poly(ethylene glycol)-poly(L-lactide) block copolymers. J Appl Polymer Sci. 1999;72:341–348. [Google Scholar]
- 24.Zong XH, Ran SF, Kim KS, et al. Structure and morphology changes during in vitro degradation of electrospun poly (glycolide-co-lactide) nanofibrous membrane. Biomacromolecules. 2003;4:416–423. [DOI] [PubMed] [Google Scholar]
- 25.Thompson J. Pathogenesis and prevention of adhesion formation. Dig Surg. 1998;15:153–157. [DOI] [PubMed] [Google Scholar]
- 26.Luijendijk RW, de Lange DCD, Wauters CCAP, et al. Foreign material in postoperative adhesions. Ann Surg. 1996;223:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liakakos T, Thomakos N, Fine PM, et al. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Dig Surg. 2001;18:260–273. [DOI] [PubMed] [Google Scholar]