Abstract
Objective:
The aim of this study is to describe the experience with 100 TNF-based ILP for locally advanced melanoma and to determine prognostic factors for response, time to local progression, and survival.
Methods:
One hundred TNF-based ILPs were performed between 1991 and 2003 in 87 patients for whom local control by surgery of in-transit melanoma metastases was impossible. In total, 62 iliac, 33 femoral, and 5 axillary ILPs were performed in mild hyperthermic conditions with 2 to 4 mg of TNF and 10 to 13 mg of melphalan per liter of limb volume.
Results:
Overall response was 95%, with 69% complete response, 26% partial response, and 5% no change. Complete response rate differed significantly for patients with IIIA disease versus IIIAB and IV. Local and systemic toxicity was mild to moderate in almost all cases, with no treatment-related death and one treatment-related amputation. Five-year overall survival was 32%; local progression occurred in 55% after a median of 16 months. In complete response patients, 5-year survival was 42% with local progression in 52% at a median of 22 months. Response rate and survival were significantly influenced by stage of disease; (local progression free) survival was influenced by response rate.
Conclusions:
TNF-based ILP results in excellent response rates in this patient population with unfavorable characteristics. Response on ILP predicts outcome in patients and reflects aggressiveness of the tumor.
Isolated limb perfusions (ILP) have proven to be an effective regional treatment modality for in-transit metastasized melanoma. Tumor necrosis factor alpha (TNF) has lead to dramatic improvement of response rates, especially in patients with high tumor burden. One hundred TNF-based isolated limb perfusions for multiple in-transit melanoma metastases were performed in a single institution setting. Overall response was 95% with 69% CR rate. Stage of disease and CR were important indicators for survival and reflect the aggressiveness of the tumor.
In-transit metastases (IT-mets) occur in approximately 5% to 8% of patients with high-risk melanoma. The management of IT-mets remains a challenge because it is dictated by the biologic behavior of melanoma, especially in terms of number and size of the lesions.1 Simple wide surgical excision is often possible if IT-mets are limited in size and number, but fails when interval periods between new lesions are short, when numerous or bulky lesions are present, and when various treatment modalities, eg, radiotherapy, have preceded surgery. Apart from local control, melanoma is virtually refractory to all systemic treatments. Therefore, various locoregional approaches have been proposed and investigated. Isolated limb perfusion (ILP), developed in 1958 by Creech and Krementz,2 is the most effective regional treatment modality, because it achieves tissue concentrations in the affected limb of the chemotherapeutic agents that are more than 20 times higher than what can be achieved systemically.3 Melphalan (l-phenylalanine mustard [l-PAM], Alkeran®, Wellcome, London, UK) has been used as standard drug over the years because of its efficacy and toxicity profile.4 Melphalan-based ILP for melanoma IT-metastases is associated with complete response (CR) rates of 40% to 50% and overall response rates of 75% to 80%.1 Hyperthermia may increase the response rates somewhat, but at the cost of increased locoregional toxicity. Large melanoma lesions are difficult to eradicate because of poor and inhomogeneous drug uptake as with soft tissue sarcomas. Therefore, ILP programs with melphalan alone have been abandoned for treating irresectable soft tissue sarcomas.5 The application of tumor necrosis factor alpha (TNF)6 changed this situation dramatically because very large tumors were now observed to respond very well,7 which led to successful multicenter trials in Europe and the approval of TNF for the treatment of irresectable extremity soft tissue sarcomas.8
TNF has also been used increasingly in combination with melphalan for the treatment of melanoma IT-mets in ILP. An early report on TNF-based ILPs from four centers in Europe showed a significant increase in CR rate up to 90% compared with a 52% CR rate after ILPs in these centers with melphalan alone.9 The success of TNF in the treatment of large soft tissue sarcomas made the investigators aware early on that TNF also improved results in particular in melanoma patients with bulky lesions. Importantly, this clinical observation of efficacy in large tumors has been explained by observations in our laboratory in the experimental isolated perfusion setting in rats with advanced limb tumors. In contrast to the poor drug uptake of melphalan after an ILP with melphalan alone by these large tumors, it was shown that TNF increased the uptake of melphalan selectively in the tumor by a factor of 3 to 6 compared with ILP with melphalan alone.10
We report here on our experience with 100 TNF-based ILPs in patients with multiple melanoma IT-mets. We have analyzed the data in this large group to determine prognostic factors for response such as stage of disease, size and number of lesions, local recurrence-free interval, and efficacy after failing other treatments.
PATIENTS AND METHODS
Patients
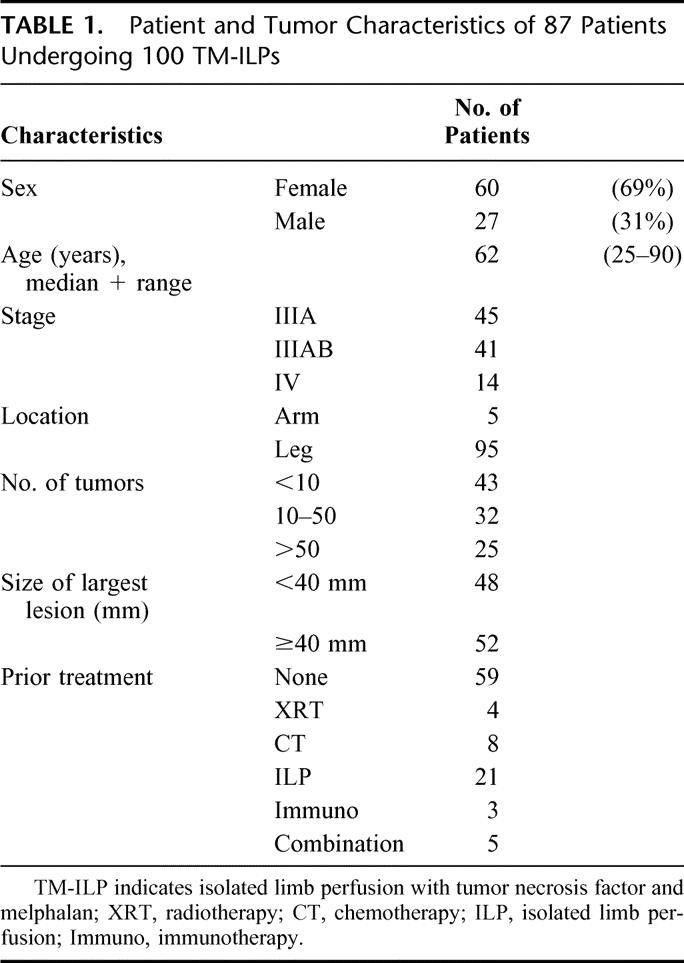
Between 1991 and 2003, 100 TNF and melphalan-ILPs were performed in 87 patients with multiple IT-mets in the limb. Demographic data, disease presentation at time of ILP, and ILP characteristics were obtained from a prospectively maintained database. Patients were staged according to the MD Anderson staging system11 and presented with stage IIIA disease in 45 cases, stage IIIAB in 41 cases, and stage IV in 14 cases. In all cases, local control by simple surgical excision was impossible due to bulky disease, which is illustrated by the size of the tumors (48 < 40 mm, 52 ≥ 40 mm) and the number of lesions (<10 lesions in 43 patients, 10–50 lesions in 32 patients, and >50 lesions in 25 patients). Besides surgical excision of respectable tumors, 59 patients did not undergo other treatment modalities prior to ILP, whereas 41 patients were previously treated with systemic chemotherapy, radiotherapy, ILP with any chemotherapeutic, immunotherapy, or a combination of the abovementioned modalities. Patient and tumor characteristics are summarized in Table 1.
TABLE 1. Patient and Tumor Characteristics of 87 Patients Undergoing 100 TM-ILPs
Treatment
All patients underwent an ILP via the axillary (n = 5), iliac (n = 62), or femoral (n = 33) approach. The method of ILP is described in detail previously.8 In short, isolation of the limb is achieved by clamping and canulation of the major artery and vein, connection to an oxygenated extracorporeal circuit, ligation of collateral vessels, and application of a tourniquet proximal to the site of perfusion. Once tissue temperature has reached 38°C, recombinant TNF (Boehringer Ingelheim GmbH, Ingelheim/Rhein, Germany) is administered via the arterial line in a dose of 2 to 4 mg. Tissue temperatures are stabilized between 38°C and 39.5°C, and leakage monitoring is performed by using a precordial scintillation probe to detect leakage of radiolabelled albumin injected to the perfusion circuit.12 After 30 minutes, melphalan is added to the perfusate in a dose of 10 mg/L for leg and 13 mg/L for arm perfusions. In 23 ILPs performed between 1991 and 1994, interferon γ (IFN) was added to the schedule according to trial prescriptions consisting of the subcutaneous injection of 0.2 mg of IFN on days −2 and −1 prior to ILP and injection of 0.2 mg of IFN during the ILP procedure into the arterial line before administration of TNF. The median dose of melphalan was 89.2 mg (mean 94.5, range 39–140), the median dose of TNF was 4 mg (mean 3.69, range 2–4), and all 23 IFN-ILPs were performed with 0.2 mg of IFN. At the end of the perfusion period, a washout procedure using 2 to 4 L of a dextrane and/or electrolyte solution is performed. In patients undergoing an iliac perfusion, an iliac lymph node dissection is performed; an axillary lymph node dissection is performed in patients undergoing an axillary ILP. In patients with palpable nodal disease in the groin, an ilio-inguinal lymph node dissection is performed in the same operative session as the ILP but before executing the ILP.
Evaluation of Response and Toxicity
Acute local toxicity of the ILP procedure was classified according to Wieberdink et al in the following manner:13 (I) no reaction; (II) slight erythema or edema; (III) considerable erythema or edema with some blistering, slightly disturbed motility permissible; (IV) extensive epidermolysis or obvious damage to the deep tissues, causing definite functional disturbance and threatening or manifest compartmental syndrome; and (V) reaction that may necessitate amputation. Response evaluation was performed 2 to 4 and 8 weeks after ILP by clinical examination, and after that at 3-month regular intervals for the first 2 years and at longer intervals thereafter. Response rates were reported according to WHO criteria14 in which CR is the complete disappearance of all lesions with no new areas of disease appearing within the field of ILP. Partial response (PR) is defined as a reduction of 50% to 99% of the total tumor size; no change (NC) is recorded if <50% of the total tumor size responds.
Recurrence of tumor within the extremity after a CR, or progression of the lesions and the appearance of new lesions after a PR or after NC, is reported as local progression.
Statistical Evaluation
Overall survival (OS) and time to local/systemic progression (TTLP/TTSP) were defined as time from ILP to death, local progression, and systemic progression, respectively, and estimates were made according to the method of Kaplan and Meier.15 Disease-free survival is defined as the time from CR to local progression, systemic progression, or death, whichever occurs first. We evaluated the prognostic value of some baseline factors for these three endpoints (TTLP/TTSP and OS) with Cox regression. The hazard ratio belonging to each factor in Table 3 is defined as the hazard of the second category divided by the hazard in the first category. We also evaluated the prognostic value of some baseline factors on achievement of CR with logistic regression. The odds ratio of each factor is the odds of CR achievement in the second category divided by the odds of CR achievement in the first category. The prognostic factors that we included were the following: sex, age, site of tumor, stage of disease, size of largest lesion, and number of lesions. This list represents all previously reported studies on prognostic factors after ILP.16–21 After univariate analysis, all these factors were included in a multivariate model. We used a stepwise backward algorithm to exclude factors without prognostic value with a significance level of 5%. After obtaining the final model, we evaluated the additional prognostic value of CR after perfusion. However, it must be noted that a prognostic model with response on perfusion applies only to patients after perfusion, whereas the model without response on perfusion can also be applied to patients before perfusion. All tests were performed at a significance level of 5%.
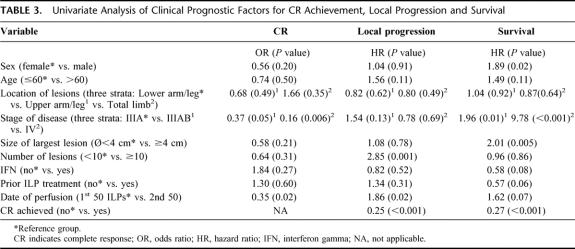
TABLE 3. Univariate Analysis of Clinical Prognostic Factors for CR Achievement, Local Progression and Survival
RESULTS
Sixty female and 27 male patients with a median age of 62 years (mean 61, range 25–90) had multiple IT-mets in the upper (n = 5) or lower (n = 95) extremity. A melphalan-ILP was performed to achieve local control in all 87 patients; 26 ILPs were performed in patients who had undergone one or multiple ILPs previously during the course of their disease either in our institution (n = 13, melphalan-ILPs) or in the referring hospital (n = 13). In total, 100 TNF-based ILPs ± IFN were performed in our institution.
Leakage and Toxicity
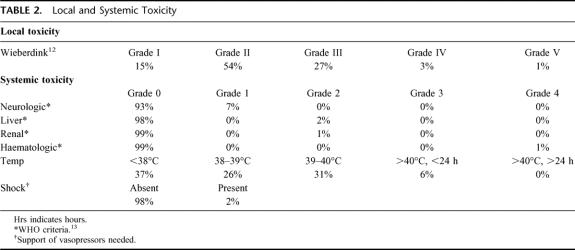
There was no or minor leakage of the drugs in 93 ILPs (median leakage 0%). Eight ILPs had leakage percentages of 10% to 32%. Toxicity in these 8 cases was limited to transient hypotension in 2 patients for which vasopressor support was given; in 1 patient, a grade IV leucopenia was observed that lasted only for 1 day and that did not need any type of intervention. Local toxicity after ILP was mild to moderate (Wieberdink grade II−III) in 81%. A Wieberdink I (no reaction) was seen after 15 procedures and in 3 patients a grade IV local toxicity occurred, with the need of performing a fasciotomy in 1 patient. One patient with IT-mets in the lower leg experienced extensive rhabdomyolysis of the upper leg, necessitating an amputation. This reaction was classified as Wieberdink grade V (Table 2).
TABLE 2. Local and Systemic Toxicity
Response Rates and Limb Function
The overall response rate was 95%, with 69 CRs (69%), 26 PRs (26%), and 5 NCs (5%). The proportion of patients reaching CR presenting with stage IIIA disease (82%) differed from those presenting with stage IIIAB (63%) and stage IV disease (43%) (Table 3). These differences showed a significant correlation between CR and stage of disease (IIIA vs. IIIAB, P = 0.053; IIIA vs. IV, P = 0.004; IIIAB vs. IV, P = 0.184). Limb function was assessed in all 87 patients and was unaffected with respect to standard daily activities in 84 of them. One case of moderate function loss was recorded, and 2 amputations had to be performed—1 because of a Wieberdink grade V local toxicity (see above) and 1 because of severe arteriosclerosis, which required a below-knee amputation more than 1 year after the ILP.
Local Progression
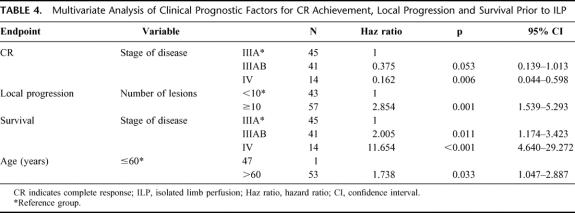
Local progression occurred after 55 ILPs (55%), at a median time of 16 months. Median TTLP of patients after CR was 22 months versus 6 months for patients after PR or NC (P < 0.001) (Fig. 1). There was more rapid progression of disease in patients with a high (≥10) number of IT-mets (P = <0.001), consistent in both uni- and multivariate analysis (Tables 3 and 4, Fig. 2). No difference in TTLP was observed for stage IIIA versus stage IIIAB patients (P = 0.12).
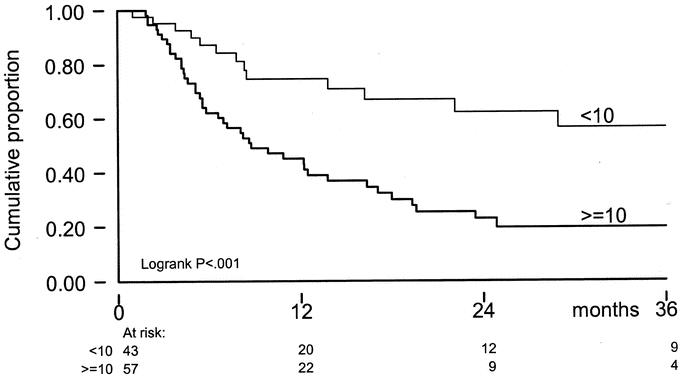
FIGURE 1. TTLP by response on ILP; x-axis: time in months; y-axis: cumulative proportion.
TABLE 4. Multivariate Analysis of Clinical Prognostic Factors for CR Achievement, Local Progression and Survival Prior to ILP
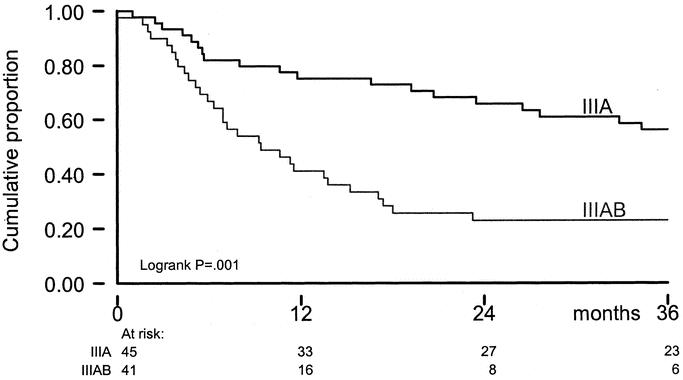
FIGURE 2. TTLP by number of lesions; x-axis: time in months; y-axis: cumulative proportion.
Systemic Progression
During follow-up, systemic progression occurred after 71 ILPs. Median time to TTSP was 14 months and differed significantly between stage IIIA (55 months) and stage IIIAB (9 months, P < 0.001) patients (Fig. 3). Other prognostic factors for systemic progression included sex (P < 0.001), size of the largest lesion (P < 0.001), prior ILP treatment (P = 0.003), and ILP response (P < 0.001). Prognostic factors for systemic progression were consistent with prognostic factors for survival.
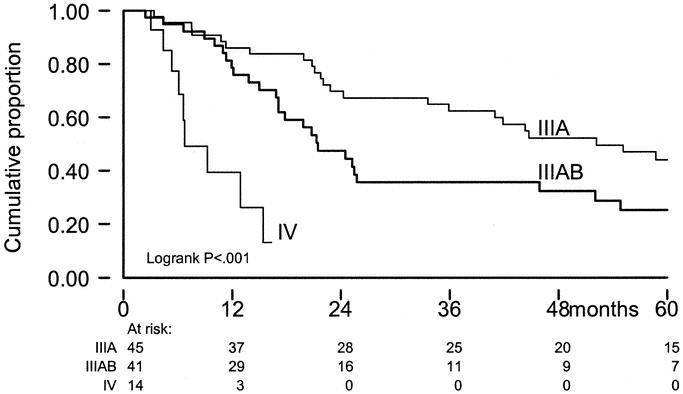
FIGURE 3. TTSP by stage of disease; x-axis: time in months; y-axis: cumulative proportion; IIIA = stage IIIA disease; IIIAB = stage IIIAB disease; IV = stage IV disease.
Survival
The overall actuarial 5-year survival rate was 32% (±5 SE); median survival was 25 months. Survival was influenced by stage of disease, sex, size of the largest tumor, and previous ILP-treatment (Table 3). After multivariate analysis, stage of disease, and age of the patient remained prognostic variables for survival (Table 4, Fig. 4). Disease-free survival was estimated for stage IIIA/IIIAB patients. At 3 years, disease-free survival was 16% and was significantly different between stage IIIA (32%) and IIIAB (0%, P = 0.008) patients.
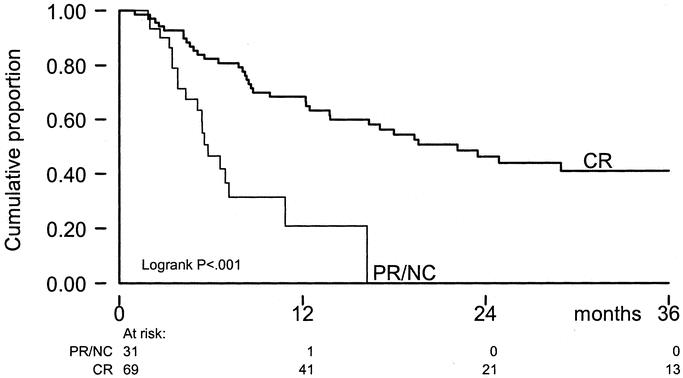
FIGURE 4. OS by stage of disease; x-axis: time in months; y-axis: cumulative proportion; IIIA = stage IIIA disease; IIIAB = stage IIIAB disease; IV = stage IV disease.
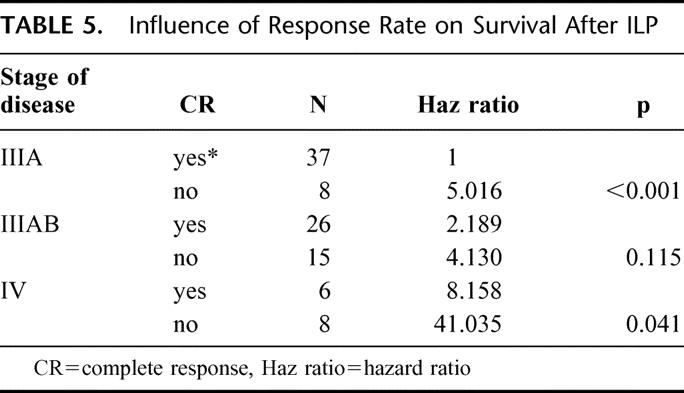
When the achievement of CR after ILP was added to the prognostic factor analysis in the multivariate model, it was shown to be of significance in TTLP (P < 0.001) without influencing the prognostic value of number of lesions (P = 0.001). For survival, the achievement of CR is of significant prognostic value, reducing the impact of age of the patient. Five-year OS for patients with a CR after ILP was 42% versus 5% for PR/NC-patients (P = <0.001) (Fig. 5). Because stage of disease itself is a prognostic factor for response, the effect of response was evaluated for each stage of disease (Table 5) and remained significant, especially in stage IIIA patients.
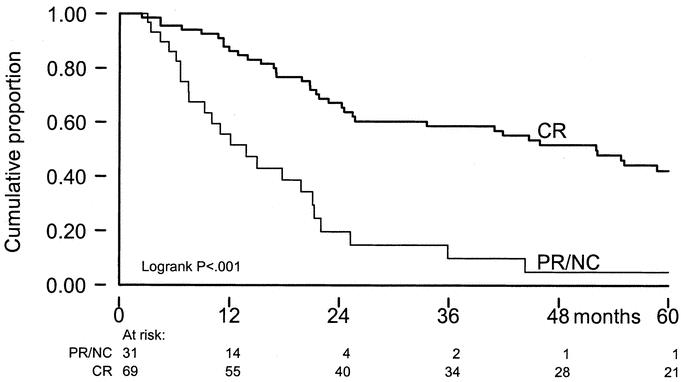
FIGURE 5. OS by response on ILP; x-axis: time in months; y-axis: cumulative proportion.
TABLE 5. Influence of Response Rate on Survival After ILP
Influence of IFN, Previous ILP Treatment, and Tumor Bulk
IFN-γ: No significant difference in CR-rate was found between patients receiving a melphalan-ILP with or without IFN (78% vs. 66% respectively, P = 0.274). Neither could an additive effect of IFN be detected for TTLP (P = 0.521) or for survival corrected for stage of disease (P = 0.149).
Prior ILP: Prior ILP treatment, a typical indication for a repeat ILP with TNF, had no effect on CR rate (P = 0.601) or TTLP (P = 0.312). The 26 patients who received multiple ILPs had a 5-year survival of 44% versus 28% for patients receiving a single treatment (P = 0.059).
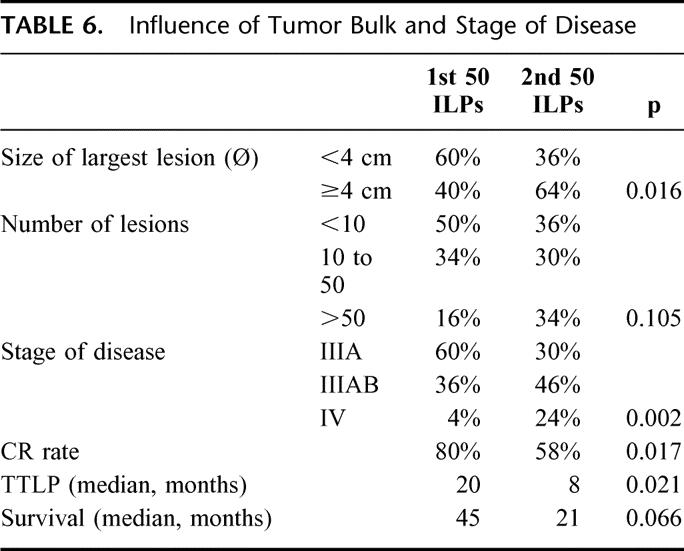
Tumor bulk: Because the experience with TNF-based ILP in soft tissue sarcomas clearly indicated that the addition of TNF induced impressive responses in large tumors in contrast to the experience with melphalan alone, we gradually changed our inclusion criteria for a TNF-based ILP and decided only to offer this treatment to patients with bulky disease or to patients who had failed previous perfusion(s). For this reason, we retrospectively analyzed the results of the first 50 ILPs versus the last 50. The tumor burden and stage of disease of the latter group differed significantly from the first (Table 6). Both CR rate (80% vs. 58%; P = 0.017) and TTLP (20 vs. 8 months; P = 0.021) were significantly lower in the last 50 ILPs. Survival was also shorter in the latter group (45 vs. 21 months), but this difference just failed to reach statistical significance (P = 0.066).
TABLE 6. Influence of Tumor Bulk and Stage of Disease
DISCUSSION
We report here on 100 TNF-based ILPs in melanoma patients out of a total of 350 TNF-based ILPs that we performed in our institution during the period from 1991 though July 2003. In patients with melanoma IT-mets, the procedure is associated with a close to 100% response rate in a patient population that has significantly shifted towards more bulky disease and pretreatments over the years. This patient population is considerably less likely to respond to treatment than the patient population of “all patients that present with IT-mets” that we used to elect for a melphalan-only ILP in the past, even when IT-mets were limited in number and size. This single institution experience further underscores that in the expert setting, the procedure is very safe. TNF can be used in high doses without a single case of treatment-related mortality; there were no cases of grade 4 systemic toxicity except for a single case in which significant leakage occurred and leukocytes dropped below 1.0 for a single day. In such a setting, there is no need for other than standard cardiovascular monitoring peroperatively, no need for standard use of vasopressors, or for standardly having patients in the intensive care unit postoperatively.22
The present series shows a CR rate of 69%, which is within the range of CR rates reported in previously published smaller series.6,18,23–25 We identified stage of disease as a prognostic factor for response with the most prominent difference in IIIA versus IIIAB/IV patients, which is in line with the literature on melphalan-based ILP.16–19 This probably reflects a difference in aggressiveness of melanoma biology in these patient categories, further exemplified by significant differences in survival. This hypothesis is sustained by the observation that during the course of melanoma progression, the ability of melanoma cells to express so-called death receptors diminishes.26 TNF receptor and TNF-related apoptosis inducing ligand, 2 examples of these receptors, play key roles in the acquired resistance of melanoma cells to undergo TNF-mediated apoptosis; whereas early-phase melanoma appears to use TNF pathways to undergo programmed cell death, late-phase melanoma does not.27 Response to treatment therefore is an indicator of the tumor phenotype, which itself is of influence on patient survival.
Potentially, it may also reflect a difference in immunocompetence between these patient categories that differ greatly because of the absence or presence of lymph node metastases and/or visceral metastases. In our laboratory, we showed an attenuation of tumor response to ILP in leukocyte-depleted rats,28 which corresponds well to the report on the role of an early and rapid invasion of tumor lesions after a TNF-based ILP by neutrophils.29 Furthermore, we reported in our observation of tumor lesions in patients after ILP that eosinophils and macrophages (“melanophages”) play an important role in the delayed-type reaction to melphalan-ILP.30
Results of ILP treatment are often presented in overall response rates. Our prognostic factor analysis shows that especially the CR rate is of imminent importance, because it significantly affects both TTLP and survival. In fact, the marked difference in TTLP between CR and PR/NC patients identifies the period that the patient is without any need of locoregional treatment. We therefore specifically analyzed the group of patients with IIIA/IIIAB disease and a PR after ILP, because these patients progress rapidly in the limb and therefore might need repeat locoregional treatment. Of these 23 patients, however, only 5 patients lived for more than 9 months without presenting distant metastases, and they received additional treatment (among which, 2 repeat ILPs). This indicates that response on ILP selects those patients with more aggressive disease, which is sustained by the prognostic influence of response rate on OS, especially when corrected for stage of disease. The 42% 5-year survival rate in CR patients, which make up the large majority of patients (69%), is quite substantial and compares favorably with most commonly reported survival rates for this patient category (23–47%).31 This underscores the importance of the treatment and shows that it is a worthwhile intervention.
Patient Selection
Since the introduction of TNF in the ILP setting, it has been discussed which patient subgroup would profit the most from addition of this cytokine.32 The early experience of four European centers showed that the treatment of all patients with IT-mets, irrespective of number of tumors or size of the lesions, increased the complete response rate from 52% to 91%.9 Yet the notion that the clearest benefit was seen in the patients population with bulky tumors that previously was known to be very poorly responsive to melphalan-based ILP was already obvious through our experiences in the multicenter trials in sarcoma patients since 1991, and it affected over the years the patient selection in our institution. We managed patients with small tumors and a small number of tumors increasingly by repeated excisions and vaccine protocols, and we offered TNF-based ILP more and more exclusively to patients with a high tumor burden. In this patient category with highly unfavorable characteristics, analyzed separately as the second 50 ILPs in our series, CR rate was still 58%. This is still superior to the response rate in the historical group of patients treated with melphalan alone without such unfavorable patient selection,9 and it is similar to observations made in the United States. In an interim analysis of a randomized trial by Fraker et al, TNF-based ILP was shown to be of significant benefit in patients with a high tumor load, increasing the CR rate from 19% for melphalan-ILP to 58% in melphalan(+IFN)-ILP.33 Moreover, Rossi et al reported recently in a series of 20 melanoma patients with a high tumor burden a CR rate of 70% after TNF-based ILP.34 We demonstrate in this series of patients that the median TTLP of 16 months exceeds the median of TTSP of 14 months in the overall patient population. It underscores that systemic metastases present frequently in stage IIIA and IIIAB populations and that an ILP should be reserved for patients who run out of simple management options such as excision of a few lesions or participation in nontoxic vaccination protocols for (multiple) small lesions that do not present clear local morbidity. Those patients without local morbidity that still progress systemically can thus be spared the ILP intervention. Moreover, in case these patients respond to a vaccine, they most likely will do so also at systemic disease sites at the cost of little toxicity and no surgical intervention. This policy shift has occurred over the 12-year period described here and has led to a patient population with significantly more advanced disease locally in the second bracket of 50 ILPs (after 1996). It is important to realize that even in this patient population, the CR rate of 58% is still higher than the 52% CR rate in our previous experience with melphalan alone in patients with clearly less extensive disease.9
A second indication for melphalan-ILP is failure after previous ILP-therapy.35 In our recent study, 26 ILPs were performed for recurrences in the limb after previous ILP treatment (13 ILPs after prior ILP elsewhere, 13 repeat melphalan-ILPs in our institution). The overall response rate of repeat ILP was 96% (CR 73%, PR 23%, NC 3%). This did not differ from the primary ILPs in our series, and no increased toxicity was observed, as opposed to previous reports on the outcome of repeat melphalan-based ILP.36 This observation underscores the efficacy of a TNF-based ILP in the repeat-ILP setting and thereby its indication.
Toxicity
Local toxicity after TNF-based ILP in our series was moderate to severe (grade III-IV) in 31 ILPs (31%), which compares with reported percentages in TNF or melphalan-based ILP.37 A reintervention by amputation or fasciotomy occurred in only two patients. Local toxicity is reported to be directly correlated with the incidence of long-term morbidity (tissue fibrosis, muscle atrophy, and limb malfunction).38 In our patient population, however, the rate of limb function loss was markedly low, even in the 27 patients older than 75 years and in the 26 patients with multiple ILP treatments.
Systemic toxicity is directly correlated with leakage of the chemotherapeutics to the systemic circulation.8,22,39,40 In the present situation of leakage-free ILPs, the systemic toxicity is mostly limited to fever in the first 24 hours postoperatively, which can be easily avoided by the immediate postoperative application of indomethacin and/or paracetamol. Furthermore, patients commonly show a period of 6 to 10 hours of slightly elevated circulation due to a drop in peripheral resistance, which is compensated by a mild tachycardia and a small drop in blood pressure, easily managed by a generous intravenous fluid infusion policy, and does not require the standard use of vasopressors. The feared systemic adverse reaction to TNF—a systemic inflammatory response syndrome with a major drop in blood pressure that requires the administration of vasopressors—was not observed in any of our patients. Only in two patients, both with substantial leakage during the ILP, did the blood pressure drop lead to the administration of vasopressors at a mild dose of 3 to 6 μg/kg for a maximum duration of 36 hours. The absence of any major toxicity is due to a number of factors: first, adequate leakage control in virtually all patients; second, and this is of crucial importance, we have a policy of ample hydration of the patient during ILP and for the first 12 to 24 hours after ILP. This policy assures that all our patients have adequate diuresis to keep the period of high circulating TNF levels post-ILP as short as possible.22 Eight ILPs in our series had a leakage percentage above 10% (up to 32%), but in only one patient did this lead to a systemic reaction by elevated liver enzymes, elevated urea, and leucopenia, which are all easily managed with normal conservative measures. We do not use and therefore do not advocate the standard use of Swann-Ganz catheters, vasopressors, or postoperative stay in the intensive care. In our experience, neither systemic nor local toxicity is significantly enhanced in TNF-based ILP compared with melphalan-based ILPs.
In conclusion, our results demonstrate the very high efficacy of TNF-based ILP in melanoma patients both in terms of local control of disease and of survival. Outcome is influenced by stage of disease, reflecting the aggressiveness of the melanoma. Complete response to ILP selects within each stage of disease those patients with relatively favorable characteristics. In our experience, the use of TNF increases the CR rate, especially in patients with a high tumor burden and in those having failed previous therapy. Local and systemic safety profile of the TNF-based ILP is so good that in the expert setting, a TNF-based ILP does not need a standard approach that differs from a melphalan-based ILP. The procedure should be considered in all cases of limb-threatening tumors or in situations where simple surgical procedures to obtain local control fail. Currently, the procedure is the most efficacious one to obtain local control and achieve limb salvage in such conditions.41
Discussion
Prof. Borel Rinkes: I have a question on the mechanism. How does TNF + melphalan act, what mechanism is involved that causes this synergistic antitumor effect?
Dr. Eggermont: The synergy is based on at least two separate effects, an immediate effect and a late effect. Moreover, the synergy is observed in the treatment of large tumors, tumors that because of their pathophysiology do not respond to chemotherapy alone because of impaired drug uptake, even in the ILP setting. Our laboratory program has really elucidated a number of these crucial data. Originally, it was thought that the eradication of the tumor-associated vasculature by the TNF + melphalan combination was the main event, as had been demonstrated clearly by the angiograms pre- and post-ILP in patients. However, it was only in the laboratory that we were able to show that the early and immediate event may be even more important. The immediate effect of TNF on the tumor is a change in the pathophysiology of the perfusion of the tumor. The TNF opens up nonfunctional vessels, and this vasodilation diminishes the shunting of blood flow around the tumor and enhances the blood flow through the tumor and slows it down. This leads to a much more homogenous perfusion of the tumor and enhances thereby drug uptake 5- to 6-fold, as we have demonstrated. This is an enormous difference, and such an intensification of drug concentrations in the tumor can never ever be achieved by any type of intensification of systemic chemotherapy. With systemic chemotherapy, you can intensify 1.2 to 1.3 times, and then you will need stem cell support. So in the TNF + melphalan ILP setting, this pharmacological enhancement of drug uptake will lead to massive tumor cell kill, and the later effects are characterized by the tumor-selective eradication of the tumor vasculature, which adds to the tumor necrosis. On slides you can see the shedding and destruction of the endothelial cell lining of the vessels; it only happens in the tumor-associated vessels. This leads to thrombosis and it leads vasodestruction. Tumor vessels are much more sensitive to the TNF, not only by up-regulation of the TNF receptors, but also by the lack of anchor mechanisms like the alphaVbeta3 and therefore they do not have the survival signals as opposed by the normal lining of the normal vasculature. It is this selective expression that gives you the window of opportunity for your therapeutic effect.
Dr. Borel Rinkes: Is TNF + melphalan better than melphalan alone in vitro culture systems?
Dr. Eggermont: Only about one third of the tumor cells lines are sensitive to TNF in vitro. So apparently the major effects are indirect effects mediated in vivo by host mechanisms. So to study these mechanisms, you need to work in vivo. For in vitro models, you need at least sophisticated matrix models, and in these the TNF + chemotherapy combination shows the same type of efficacy enhancement that you see in vivo. In cell cultures you will miss those effects because it is by interactive host-mediated mechanisms and it is not only by direct mechanisms.
Footnotes
Reprints: Alexander M.M. Eggermont, MD, PhD, Department of Surgical Oncology, Daniel den Hoed Cancer Center, PO Box 5201 3008, Rotterdam, Netherlands. E-mail: a.m.m.eggermont@erasmusmc.nl.
REFERENCES
- 1.Eggermont AM. Treatment of melanoma in-transit metastases confined to the limb. Cancer Surv. 1996;26:335–349. [PubMed] [Google Scholar]
- 2.Creech O Jr, Krementz ET, Ryan RF, Winblad JN. Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg. 1958;148:616–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benckhuijsen C, Kroon BB, van Geel AN, Wieberdink J. Regional perfusion treatment with melphalan for melanoma in a limb: an evaluation of drug kinetics. Eur J Surg Oncol. 1988;14:157–163. [PubMed] [Google Scholar]
- 4.Thompson JF, Gianoutsos MP. Isolated limb perfusion for melanoma: effectiveness and toxicity of cisplatin compared with that of melphalan and other drugs. World J Surg. 1992;16:227–233. [DOI] [PubMed] [Google Scholar]
- 5.Klaase JM, Kroon BB, Benckhuijsen C, et al. Results of regional isolation perfusion with cytostatics in patients with soft tissue tumors of the extremities. Cancer. 1989;64:616–621. [DOI] [PubMed] [Google Scholar]
- 6.Lienard D, Ewalenko P, Delmotte JJ, et al. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992;10:52–60. [DOI] [PubMed] [Google Scholar]
- 7.Eggermont AM, Schraffordt Koops H, Lienard D, et al. Isolated limb perfusion with high-dose tumor necrosis factor-alpha in combination with interferon-gamma and melphalan for nonresectable extremity soft tissue sarcomas: a multicenter trial. J Clin Oncol. 1996;14:2653–2665. [DOI] [PubMed] [Google Scholar]
- 8.Eggermont AM, Schraffordt Koops H, Klausner JM, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg. 1996;224:756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lejeune F, Lienard D, Eggermont A, et al. Rationale for using TNF alpha and chemotherapy in regional therapy of melanoma. J Cell Biochem. 1994;56:52–61. [DOI] [PubMed] [Google Scholar]
- 10.de Wilt JH, ten Hagen TL, de Boeck G, et al. Tumor necrosis factor alpha increases melphalan concentration in tumor tissue after isolated limb perfusion. Br J Cancer. 2000;82:1000–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith J. Histopathology and Biological Behavior of Melanoma, Neoplasms of the Skin and Malignant Melanomas. Chicago: Year Book Medical Publishers; 1976. [Google Scholar]
- 12.Klaase JM, Kroon BB, van Geel AN, et al. Systemic leakage during isolated limb perfusion for melanoma. Br J Surg. 1993;80:1124–1126. [DOI] [PubMed] [Google Scholar]
- 13.Wieberdink J, Benckhuysen C, Braat RP, et al. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18:905–910. [DOI] [PubMed] [Google Scholar]
- 14.WHO. WHO Handbook for Reporting Results of Cancer Treatment. Geneva: World Health Organisation; 1979. [Google Scholar]
- 15.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Di Filippo F, Calabro A, Giannarelli D, et al. Prognostic variables in recurrent limb melanoma treated with hyperthermic antiblastic perfusion. Cancer. 1989;63:2551–2561. [DOI] [PubMed] [Google Scholar]
- 17.Klaase JM, Kroon BB, van Geel AN, et al. Prognostic factors for tumor response and limb recurrence-free interval in patients with advanced melanoma of the limbs treated with regional isolated perfusion with melphalan. Surgery. 1994;115:39–45. [PubMed] [Google Scholar]
- 18.Lienard D, Eggermont AM, Koops HS, et al. Isolated limb perfusion with tumor necrosis factor-alpha and melphalan with or without interferon-gamma for the treatment of in-transit melanoma metastases: a multicentre randomized phase II study. Melanoma Res. 1999;9:491–502. [DOI] [PubMed] [Google Scholar]
- 19.Vrouenraets BC, Hart GA, Eggermont AM, et al. Relation between limb toxicity and treatment outcomes after isolated limb perfusion for recurrent melanoma. J Am Coll Surg. 1999;188:522–530. [DOI] [PubMed] [Google Scholar]
- 20.Zogakis TG, Bartlett DL, Libutti SK, et al. Factors affecting survival after complete response to isolated limb perfusion in patients with in-transit melanoma. Ann Surg Oncol. 2001;8:771–778. [DOI] [PubMed] [Google Scholar]
- 21.Noorda EM, Vrouenraets BC, Nieweg OE, et al. Prognostic factors for survival after isolated limb perfusion for malignant melanoma. Eur J Surg Oncol. 2003;29:916–921. [DOI] [PubMed] [Google Scholar]
- 22.Stam TC, Swaak AJ, de Vries MR, et al. Systemic toxicity and cytokine/acute phase protein levels in patients after isolated limb perfusion with tumor necrosis factor-alpha complicated by high leakage. Ann Surg Oncol. 2000;7:268–275. [DOI] [PubMed] [Google Scholar]
- 23.Lejeune FJ, Lienard D, Leyvraz S, Mirimanoff RO. Regional therapy of melanoma. Eur J Cancer. 1993;29A:606–612. [DOI] [PubMed] [Google Scholar]
- 24.Vaglini M, Belli F, Ammatuna M, et al. Treatment of primary or relapsing limb cancer by isolation perfusion with high-dose alpha-tumor necrosis factor, gamma-interferon, and melphalan. Cancer. 1994;73:483–492. [DOI] [PubMed] [Google Scholar]
- 25.Fraker DL, Alexander HR, Andrich M, Rosenberg SA. Treatment of patients with melanoma of the extremity using hyperthermic isolated limb perfusion with melphalan, tumor necrosis factor, and interferon gamma: results of a tumor necrosis factor dose-escalation study. J Clin Oncol. 1996;14:479–489. [DOI] [PubMed] [Google Scholar]
- 26.Soengas MS, Capodieci P, Polsky D, et al. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–211. [DOI] [PubMed] [Google Scholar]
- 27.Ivanov VN, Bhoumik A, Ronai Z. Death receptors and melanoma resistance to apoptosis. Oncogene. 2003;22:3152–3161. [DOI] [PubMed] [Google Scholar]
- 28.Manusama ER, Nooijen PT, Stavast J, et al. Assessment of the role of neutrophils on the antitumor effect of TNFalpha in an in vivo isolated limb perfusion model in sarcoma-bearing brown Norway rats. J Surg Res. 1998;78:169–175. [DOI] [PubMed] [Google Scholar]
- 29.Renard N, Lienard D, Lespagnard L, et al. Early endothelium activation and polymorphonuclear cell invasion precede specific necrosis of human melanoma and sarcoma treated by intravascular high-dose tumor necrosis factor alpha (rTNF alpha). Int J Cancer. 1994;57:656–663. [DOI] [PubMed] [Google Scholar]
- 30.Nooijen PT, Eggermont AM, Schalkwijk L, et al. Complete response of melanoma-in-transit metastasis after isolated limb perfusion with tumor necrosis factor alpha and melphalan without massive tumor necrosis: a clinical and histopathological study of the delayed-type reaction pattern. Cancer Res. 1998;58:4880–4887. [PubMed] [Google Scholar]
- 31.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. [DOI] [PubMed] [Google Scholar]
- 32.de Wilt JH, Thompson JF. Is there a role for isolated limb perfusion with tumor necrosis factor in patients with melanoma? Ann Surg Oncol. 2004;11:119–121. [DOI] [PubMed] [Google Scholar]
- 33.Fraker DL, Alexander HR, Ross M, et al. A phase III trial of isolated limb perfusion for extremity melanoma comparing melphalan alone versus melphalan plus tumor necrosis factor (TNF) plus interferon gamma (IFN). Ann Surg Oncol. 2002;9:S8. [Google Scholar]
- 34.Rossi CR, Foletto M, Mocellin S, et al. Hyperthermic isolated limb perfusion with low-dose tumor necrosis factor-alpha and melphalan for bulky in-transit melanoma metastases. Ann Surg Oncol. 2004;11:173–177. [DOI] [PubMed] [Google Scholar]
- 35.Bartlett DL, Ma G, Alexander HR, et al. Isolated limb reperfusion with tumor necrosis factor and melphalan in patients with extremity melanoma after failure of isolated limb perfusion with chemotherapeutics. Cancer. 1997;80:2084–2090. [DOI] [PubMed] [Google Scholar]
- 36.Klop WM, Vrouenraets BC, van Geel BN, et al. Repeat isolated limb perfusion with melphalan for recurrent melanoma of the limbs. J Am Coll Surg. 1996;182:467–472. [PubMed] [Google Scholar]
- 37.Vrouenraets BC, Eggermont AM, Hart AA, et al. Regional toxicity after isolated limb perfusion with melphalan and tumor necrosis factor-alpha versus toxicity after melphalan alone. Eur J Surg Oncol. 2001;27:390–395. [DOI] [PubMed] [Google Scholar]
- 38.Vrouenraets BC, Klaase JM, Kroon BB, et al. Long-term morbidity after regional isolated perfusion with melphalan for melanoma of the limbs. The influence of acute regional toxic reactions. Arch Surg. 1995;130:43–47. [DOI] [PubMed] [Google Scholar]
- 39.Swaak AJ, Lienard D, Schraffordt Koops H, et al. Effects of recombinant tumor necrosis factor (rTNF-alpha) in cancer. Observations on the acute phase protein reaction and immunoglobulin synthesis after high dose recombinant TNF-alpha administration in isolated limb perfusions in cancer patients. Eur J Clin Invest. 1993;23:812–818. [DOI] [PubMed] [Google Scholar]
- 40.Thom AK, Alexander HR, Andrich MP, et al. Cytokine levels and systemic toxicity in patients undergoing isolated limb perfusion with high-dose tumor necrosis factor, interferon gamma, and melphalan. J Clin Oncol. 1995;13:264–273. [DOI] [PubMed] [Google Scholar]
- 41.Eggermont AM, de Wilt JH, ten Hagen TL. Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol. 2003;4:429–437. [DOI] [PubMed] [Google Scholar]