Abstract
The chimeric transcription factor E2a-Hlf is an oncoprotein associated with a subset of acute lymphoblastic leukemias of early B-lineage derivation. We employed a retroviral transduction-transplantation approach to evaluate the oncogenic effects of E2a-Hlf on murine B-cell progenitors harvested from adult bone marrow. Expression of E2a-Hlf induced short-lived clusters of primary hematopoietic cells but no long-term growth on preformed bone marrow stromal cell layers comprised of the AC6.21 cell line. Coexpression with Bcl-2, however, resulted in the sustained self-renewal of early preB-I cells that required stromal and interleukin-7 (IL-7) support for growth in vitro. Immortalized cells were unable to induce leukemias after transplantation into nonirradiated syngeneic hosts, unlike the leukemic properties and cytokine independence of preB-I cells transformed by p190Bcr-Abl under identical in vitro conditions. However, bone marrow cells expressing E2a-Hlf in combination with Bcl-2, but not E2a-Hlf alone, induced leukemias in irradiated recipients with long latencies, demonstrating both a requirement for suppression of apoptosis and the need for further secondary mutations in leukemia pathogenesis. Coexpression of IL-7 substituted for Bcl-2 to induce the in vitro growth of pre-B cells expressing E2a-Hlf, but leukemic conversion required additional abrogation of undefined stromal requirements and was associated with alterations in the Arf/Mdm2/p53 pathway. Thus, E2a-Hlf enhances the self-renewal of bone marrow B-cell progenitors without inciting a p53 tumor surveillance response or abrogating stromal and cytokine requirements for growth, which are nevertheless abrogated during progression to a leukemogenic phenotype.
B-lineage acute lymphoblastic leukemias (ALLs) are diverse in their biological and clinical manifestations, but they invariably reflect a major disruption in the orderly process by which B-cell progenitors differentiate from pluripotent stem cells within the bone marrow microenvironment (22). This maturation program is normally dependent upon interactions with stromal cells that help guide B-cell precursors through various checkpoints in the assembly and expression of immunoglobulin genes by providing survival and growth signals (1, 30). It is now clear that a wide variety of acquired genetic abnormalities can disrupt B-cell maturation and lead to leukemic transformation. However, little is known regarding how these genetic abnormalities subvert the normal programs of B-lineage development and alter the survival of normally apoptosis-prone progenitors. It is also unclear to what extent stromal cell-derived signals are required during the origin of leukemic B-cell precursors and whether eventual abrogation of stromal dependence plays a significant role in the pathogenesis of ALLs.
Many of the genetic abnormalities in B cell precursor ALLs are recurrent chromosomal translocations that create fusion genes encoding chimeric kinases or transcription factors (23). An uncommon but well-studied chimera is E2a-Hlf, a basic-leucine zipper DNA-binding protein that contains the heterologous E2a transactivation domains as a consequence of t(17;19) chromosomal translocations in a subset of pediatric ALLs (11, 14). Gene transfer and candidate target gene studies suggest an oncogenic role for E2a-Hlf through induction of apoptotic resistance. E2a-Hlf prevents apoptosis after cytokine withdrawal in interleukin-3 (IL-3)-dependent pro-B-cell lines, possibly through effects on several subordinate pathways (13, 15, 16). One of its candidate target genes, SLUG, also mediates apoptotic resistance and shares similarity with Ces1, which functions downstream of Ces2 to modulate survival of a rare neuronal cell population in Caenorhabditis elegans (17). Since Hlf itself shares limited similarity with Ces2, E2a-Hlf may inappropriately activate a conserved signaling pathway that both worms and B-cell precursors use for modulation of cell survival. An alternative oncogenic role for E2a-Hlf is suggested by studies in transgenic murine models where it appears to antagonize lymphoid differentiation in the course of inducing primarily T-lineage malignancies (9, 33). Due to the limitations of previously employed experimental systems, it remains unclear which of the several roles ascribed to E2a-Hlf are more relevant for B cell precursor leukemogenesis and how E2a-Hlf may disrupt the normal program of lymphopoiesis.
In this report we evaluated the oncogenic contributions of E2a-Hlf employing an in vitro culture system that recapitulates the origin of B-cell precursor ALL from a bone marrow stromal microenvironment. Under these conditions, E2a-Hlf required coexpression with Bcl-2, a general suppressor of apoptosis, to immortalize bone marrow pre-B cells, which maintained their stromal and cytokine requirements for growth. Abrogation of stroma and cytokine dependence was associated with leukemic progression, suggesting a multistep model for induction of B-cell precursor ALL initiated by E2a-Hlf.
MATERIALS AND METHODS
Design and production of retroviral constructs.
The MSCV-EH retroviral construct was made by inserting a 2-kb fragment of the E2A-HLF cDNA, containing its entire open reading frame (12), by blunt-end ligation into the EcoRI site of the retroviral vector MSCV-neo (8). MSCV-BIEH contained the human BCL2 and E2A-HLF cDNAs linked by an IRES element from the encephalomyocarditis virus (19). To create an in-frame IRES-E2A-HLF junction, PCR was used to replace the initiator ATG codon of E2A-HLF with the MscI site of the IRES element. The IRES and E2A-HLF fragments were joined at the MscI site by ligation, and the resulting IRES-E2A-HLF fragment was blunt-end cloned into the unique HpaI site of MSCV-neo. An 850-bp cDNA encoding human Bcl-2 (2) was then ligated into the upstream EcoRI site of MSCV-neo/IRES-E2A-HLF to yield MSCV-BiEH. To create the MSCV-neo/p190Bcr-Abl retrovirus, a 5-kb cDNA containing the BCR-ABL coding region was blunt-end ligated into the unique EcoRI site of MSCV-neo. MSCV-puro-IL-7 and MSCV-neo-IL-7 were constructed by ligating a 550-bp BamHI fragment containing the IL-7 open reading frame into the BglII site of MSCV-puromycin and MSCV-neo, respectively. The MSCV-neo IL-7-IRES-E2A-HLF construct was produced by blunt-end ligating the IRES-E2A-HLF fragment into the HpaI site of MSCV-neo-IL-7.
Retroviral stocks were produced by transfecting the Phoenix packaging cell line with 10 μg of purified plasmid DNA (27). Retrovirus-containing supernatants were collected 48 h later, filtered, and used for infection of primary bone marrow cells. Viral titers were determined by transfer of neomycin resistance to NIH 3T3 cells after infection with retroviral supernatants as described previously (21).
Isolation and transduction of B-cell progenitors.
In vitro bone marrow cultures were performed on monolayers of the stromal cell line AC-6.21 (34). To allow selection for transduced bone marrow cells, AC-6.21 cells were converted to neomycin and puromycin (or neomycin and hygromycin) resistance by retroviral transduction with MSCV-neo and MSCV-puro (or MSCV-hygro) vectors, respectively. Stromal cells were maintained in RPMI 1640-5% fetal calf serum and 5 × 10−5 M β-mercaptoethanol (R-5). On the day prior to bone marrow transduction, drug-resistant AC-6.21 cells were plated in six-well plates at a density of 5 × 105/well and then irradiated (3,000 rads) by using a gamma (137Cs) source. Primary bone marrow cells were isolated from 3.5-week-old BALB/c donor mice under sterile conditions. Bone marrow cells (4 × 105) were mixed with 1.0 ml of viral stock (ca. 107 PFU) supplemented with 4 μg of Polybrene/ml and spinoculated at 2,500 rpm for 2 h at 33°C. The spinoculated bone marrow cells were resuspended in R-5 medium, and equal numbers were plated into two replicate wells containing irradiated AC-6.21 stromal cells. Selection for drug-resistant colonies was initiated after 5 days. Cultures were fed weekly by carefully replacing half of the medium with fresh R-5 containing appropriate antibiotics (800 μg of G418/ml, and/or 1 μg of puromycin/ml, and/or 150 μg of hygromycin/ml). Growth of lymphoid cells overlying the stromal layer was assessed by using a manual hemocytometer after their removal by gentle pipetting.
Methylcellulose assays.
Stromal contact and IL-7 requirements for growth were evaluated by using methylcellulose assays. Lymphoid cells were removed from the underlying stromal layer by gentle pipetting and then suspended (at 2.5, 5, and 7.5 × 104 cells/well) in methylcellulose medium with or without exogenous IL-7 (StemCell Technologies, Vancouver, British Columbia, Canada). Colonies were enumerated by light microscopy. Colonies that grew in the presence of supplemental IL-7 were transferred to liquid medium containing RPMI 1640-10% fetal calf serum supplemented with 1 ng of murine IL-7 (R&D Systems, Minneapolis, Minn.)/ml and 5 × 10−5 M β-mercaptoethanol (R-10/7). Cytokine-dependent cells were maintained in R-10/7.
Leukemogenicity assays.
Syngeneic, nonirradiated 4-week-old BALB/c mice were used to evaluate the leukemogenic potential of immortalized cells from in vitro bone marrow cultures. Mice were injected intravenous (i.v.) with 106 cultured lymphoid cells, and development of leukemia was monitored by periodic assessment of peripheral blood cell counts and morphology. Blood smears were fixed and stained with May-Grunwald and counterstained with Giemsa (Fluka Chemika/Sigma-Aldrich, St. Louis, Mo.). To study the leukemogenic potential in vivo of freshly isolated hematopoietic cells transduced with E2A-HLF retroviral constructs, lethally irradiated BALB/c mice (two equal doses of 495 rads of X-irradiation) were injected i.v. with 106 bone marrow cells (in 0.1 ml of R-5) subjected to spinoculation as described above. Leukemogenic potential was assessed periodically by morphological staining of blood cells obtained from tail bleeds. Moribund animals were euthanized by CO2 inhalation and tissues were removed for in vitro culture, Western blots, Southern blots, fluorescence-activated cell sorting (FACS) analysis or histologic examination (after fixation in buffered formalin and staining with hematoxylin and eosin). Leukemic cells were plated in vitro in R-10 medium with or without IL-7 to assess growth and cytokine requirements. Transplantability of leukemias was analyzed by injecting 106 nucleated cells from primary diseased mice i.v. into five nonirradiated secondary BALB/c recipient mice. Secondary leukemias were also evaluated for phenotypes and in vitro dependence on IL-7 for growth. All animals were bred and maintained in the Stanford University Research Animal Facility. Irradiated animals were maintained on a regimen of double antibiotic water solution (polymyxin-B sulfate, 850 U/ml; neomycin, 10% final concentration) throughout the duration of the experiments.
DNA and protein analysis.
DNA was isolated from fresh cells or tissues, digested with appropriate restriction enzymes, and analyzed by Southern blot hybridization by using standard methods. Probes for the mouse IgH-JH4 and IgL-Jκ5 have described previously (3). Protein extracts were prepared from cells or tissues by boiling in 1× sample buffer for 10 min. The extracts were then sonicated and protein concentration determined by UV spectroscopy (i.e., the optical density at 280 nm). Total cellular proteins (30 to 50 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then analyzed by Western blotting with monoclonal antibodies specific for E2a (18), Abl or p53 (clone AB-7; Calbiochem, Boston, Mass.), murine IL-7 (PeproTech, Rocky Hill, N.J.), human Bcl-2 (Dako, Carpinteria, Calif.), p19ARF (Novus Biologicals, Littleton, Colo.), or Mdm2 (clone C-18; Santa Cruz Biotechnology, Santa Cruz, Calif.) as previously described (32). Immunoreactive proteins were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.). Antibody specific for mutant p53 (clone PAB 240; Neomarkers, Fremont, Calif.) was used for immunoprecipitation.
Fluorescence-activated flow cytometric analysis.
FACS analysis was performed as previously described (33) with antibodies purchased from Pharmingen Research Products (San Diego, Calif.). Specificities of antibodies were as follows: fluorescein isothiocyanate-conjugated S7 (anti-CD43), allophycocyanin-conjugated RA3-6B2 (anti-B220), phycoerythrin-conjugated M1/69 (anti-CD24/HSA), biotinylated 6C3/BP1 (anti-Ly51), phycoerythrin-conjugated DS-1 (anti-IgM, biotinylated RB6/8C5 (anti-Gr1), fluorescein isothiocyanate-conjugated M1/70 (anti-Mac1), and avidin-conjugated Texas red.
RESULTS
Altered in vitro growth of primary bone marrow cells transduced with E2A-HLF.
A retroviral transduction-transplantation approach was used to evaluate the effects of E2a-Hlf on the growth properties of primary B-cell progenitors and compared with those of p190Bcr-Abl (Fig. 1). Recombinant retroviruses that expressed the E2A-HLF (MSCV-EH) or BCR-ABL (MSCV-p190) cDNAs under transcriptional control of the long terminal repeat were used to infect primary bone marrow cells isolated from BALB/c donor mice. Transduced cells were plated on feeder layers consisting of the irradiated stromal cell line AC-6.21 and then selected for G418 resistance. Within 2 weeks of plating, distinct G418-resistant clusters of lymphoid cells were observed on the underlying stromal layer in cultures initiated by cells transduced with MSCV-p190 (data not shown). The individual cell clusters rapidly coalesced and grew to confluence by 2 to 3 weeks (Fig. 2B) similar to previous studies with primary stromal cell layers (25). Bone marrow cells infected with MSCV-E2A-HLF initially formed similar small clusters within the same time frame but then died with morphological features of apoptosis prior to expansion and coalescence of the colonies (Fig. 2A).
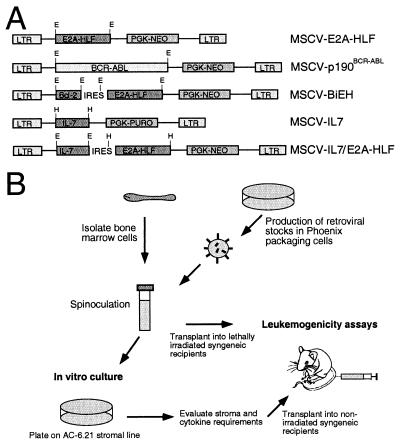
FIG. 1.
Design of retroviral constructs and experimental strategy for transduction-transplantation of primary bone marrow cells. (A) Schematic illustrations of the retroviral vectors employed. Restriction enzyme sites: E, EcoRI; H, HpaI. (B) Experimental scheme employed for transduction of B-cell precusors and their in vitro and in vivo characterization.
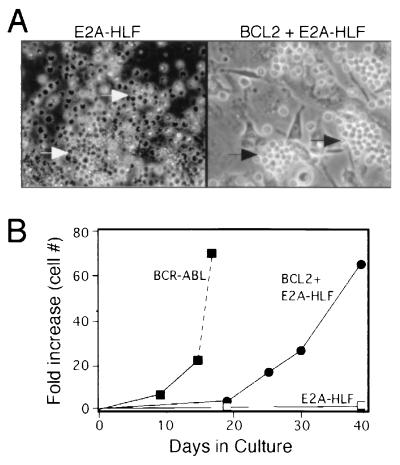
FIG. 2.
Effects of E2a-Hlf compared with p190Bcr-Abl on the in vitro growth of primary bone marrow progenitors. (A) Typical colonies arising on the underlying stromal layer in primary bone marrow cultures 2 weeks after initiation with cells transduced by MSCV-E2A-HLF (left) and MSCV-BiEH (right). Clusters of viable and apopotic cells are indicated by black and white arrows, respectively. (B) Effects of BCR-ABL and E2A-HLF ± BCL2 on the in vitro growth of hematopoietic cells. Each point represents the fold increase in number (mean of triplicate determinations) of cells adherent to the underlying AC-6.21 stromal layer.
To prevent cell death that appeared to be triggered by E2a-Hlf under these culture conditions, we coexpressed BCL2 with E2A-HLF as a bicistronic transcript by using an internal ribosome entry site (IRES) element in the MSCV vector (MSCV-BiEH in Fig. 1A). Cells transduced with MSCV-BiEH formed clusters on the stromal layer within 2 weeks (Fig. 2A), continued to expand, and grew to confluence within 4 to 5 weeks. Growth was less robust than that observed in the BCR-ABL cultures, but overall cell numbers increased at least 50-fold by 40 days in culture (contrasted with a 65-fold increase at day 18 for the BCR-ABL cultures) (Fig. 2B). Cultures initiated with the MSCV vector alone or MSCV-BCL2 did not result in formation of G418-resistant cell clusters on the underlying stromal layer. Both BiEH- and p190-initiated cultures could be maintained indefinitely (>30 population doublings) by serial passage of overlying cells onto fresh layers of AC-6.21 stromal cells. High-level expression of human Bcl-2 and E2a-Hlf was observed in the MSCV-BiEH initiated cells and p190Bcr-Abl in the MSCV-p190 cells, respectively, by Western blot analyses (Fig. 3A). Therefore, coexpression of E2a-Hlf and Bcl-2 appeared to be capable of immortalizing primary bone marrow cells in vitro.
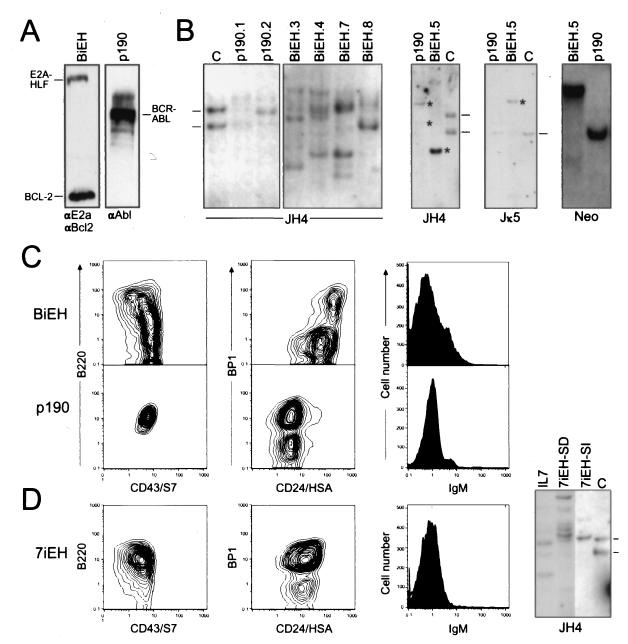
FIG. 3.
Transgene expression, genotypes, and phenotypes of transduced cells. (A) Western blot analysis of total cellular proteins isolated from cells growing in bone marrow cultures initiated with BiEH (left lane) or p190 (right lane). Antibodies are indicated below the gel lanes. (B) Southern blot analysis of genomic DNA isolated from transduced cells. Sources of DNA are indicated above the respective gel lanes. Identities of DNA probes are denoted below the panels. DNA was digested with XbaI for Ig gene analyses. Retroviral integration patterns were analyzed by using BamHI, which cleaves the proviruses once, allowing detection of DNA fragments whose size varies with the site of integration. (C) Flow cytometric analysis of surface antigen expression by cells growing in representative bone marrow cultures transduced by BiEH (upper row) or p190 (lower row). (D) Flow cytometric and Southern blot analyses for bone marrow cultures initiated by cells transduced with 7iEH.
Primary bone marrow cells immortalized by E2A-HLF + BCL2 are stroma- and IL-7-dependent B-cell progenitors.
Cells from eight independently initiated BiEH cultures and four p190 cultures expressed the pan-B-cell marker CD45R/B220, indicating derivation from the B-lymphoid lineage (representative cases shown in Fig. 3C). Primary BiEH cultures appeared somewhat heterogeneous in phenotypic composition. The predominant cellular population expressed S7(CD43) and HSA(CD24) but lacked BP1 and surface IgM. Most cells in the p190 cultures displayed a similar phenotype with expression of BP1. By Southern blot analyses, cells from BiEH primary cultures displayed several rearranged IgH genes and germ line Igκ genes (Fig. 3B and Table 1, BiEH.3-SD and BiEH.4-SD), suggesting an oligoclonal population of B lineage progenitors. In contrast, the cells in p190 primary cultures displayed one or two clonal IgH gene rearrangements, germ line Igκ genes and single proviral integrations.
TABLE 1.
Characteristics of immortalized cells
| Cell line | Phenotype | Genotypea | Growth requirements
|
Syngeneic, nonirradiated recipients
|
||
|---|---|---|---|---|---|---|
| IL-7 | AC-6.21 stroma | Leukemiab | Latency (wk) | |||
| BiEH.3-SD | B220+ CD43+ CD24+ BP1− IgM− | IgHM, IgLG | + | + | − | NAc |
| BiEH.4-SD | B220+ CD43+ CD24+ BP1− IgM− | IgHM, IgLG | + | + | − | NA |
| BiEH.1-SI | B220+ CD43+ CD24+ BP1− IgM− | IgHR, IgLR | + | − | − | NA |
| BiEH.2-SI | B220+ CD43+ CD24+ BP1+ IgM+ | IgHR, IgLR | + | − | − | NA |
| BiEH.3-SI | B220+ CD43+ CD24+ BP1− IgM− | IgHR, IgLG | + | − | − | NA |
| BiEH.4-SI | B220+ CD43+ CD24+ BP1− IgM− | IgHR, IgLG | + | − | − | NA |
| BiEH.5 | B220+ CD43+ CD24+ BP1+ IgMlo | IgHR, IgLR | − | − | + | 3-4 |
| p190.1 | B220+ CD43+ CD24+ BP1+ IgM− | IgHR, IgLG | − | − | + | 2-3 |
| 7iEH-SD | B220+ CD43+ CD24+ BP1+ IgM− | IgHM, IgLG | − | + | − | NA |
| 7iEH-SI | B220+ CD43+ CD24+ BP1+ IgM− | IgHR, IgLG | − | − | + | 2-3 |
IgHM, multiple immunoglobulin heavy-chain gene rearrangements; IgHR, one or two immunoglobulin heavy-chain gene rearrangements; IgLG, germ line immunoglobulin light-chain gene configuration.
That is, 106 cells injected i.v.
NA, not applicable.
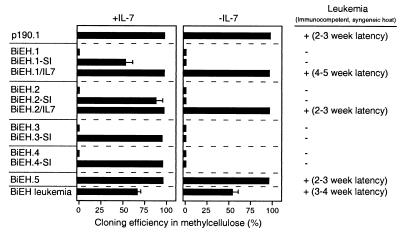
Stromal contact and cytokine requirements were evaluated by plating immortalized cells in methylcellulose in the presence or absence of IL-7. p190-transduced cells formed colonies with high plating efficiencies in methylcellulose (Fig. 4) in the absence of exogenous IL-7 and readily adapted to growth in liquid medium. This contrasted with BiEH cells, which typically displayed plating efficiencies of <0.1% in methylcellulose even in the presence of exogenous IL-7 (Fig. 4, BiEH.1 to BiEH.4). The surface phenotypes, Ig gene configurations, and stromal and IL-7 dependence of BiEH-transduced cells were consistent with maturational arrest at an early pre-B-I stage of differentiation (fraction B in reference 7). Conversely, p190-transduced cells, which also displayed a pre-B-I phenotype (fraction C), were IL-7 and stroma independent. Since pre-B-I cells are normally dependent on IL-7 and stroma (7), one effect of p190, but not E2a-Hlf, was to abrogate these requirements.
FIG. 4.
Stroma and IL-7 dependence correlated with leukemogenic potential of E2A-HLF/BCL2-transduced B-cell progenitors. Cells were cultured in methylcellulose with or without exogenous IL-7 as indicated at the top. Identities of various transduced cell populations, cell lines, and leukemias are indicated at the left. Cloning efficiencies were determined by examination under light microscopy at day 14. Leukemogenic potentials and latencies in immunocompetent, syngeneic recipients are indicated to the right.
Progenitor B cells immortalized by E2A-HLF + BCL2 do not induce leukemias in nonirradiated hosts.
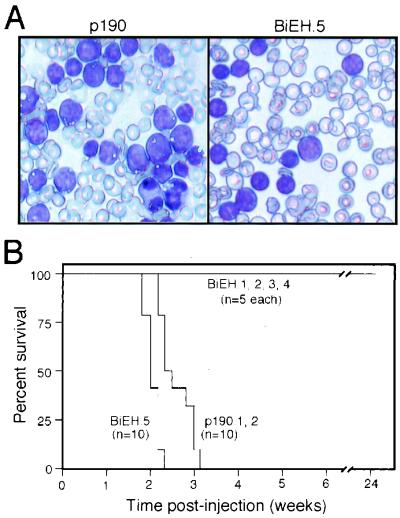
The leukemogenic potentials of BiEH and p190 immortalized pre-B cells were tested in syngeneic, nonirradiated BALB/c recipients. Mice injected with BiEH-transduced cells (BIEH.1, BIEH.2, BIEH.3, and BIEH.4) did not develop leukemias over an observation period of at least 6 months (Fig. 5). In contrast, p190-transduced cells (lines p190.1 and p190.2) were highly efficient in inducing leukemias (Fig. 5), with all recipients succumbing between 15 and 22 days posttransplant with tachypnea and hepatosplenomegaly. Peripheral blood showed numerous cells with blast-like morphology (Fig. 5A), which also infiltrated the bone marrow and extramedullary sites.
FIG. 5.
Development of leukemia in mice transplanted with E2A-HLF/BCL2 or p190Bcr-Abl immortalized cells. (A) Wright-Giemsa-stained preparations of peripheral blood showing leukemia cells with morphological features of lymphoid blasts in animals injected with p190 (left) or BiEH (right) transduced cells. (B) Survival of mice transplanted with cells immortalized by various retroviral constructs. A total of 106 cells from the indicated cell lines were injected into immunocompetent, syngeneic BALB/c mice. Numbers of mice in each cohort are denoted in parentheses.
Acquisition of stoma and IL-7 independence is associated with leukemogenicity of E2A-HLF + BCL2-transduced cells.
The potential relationship of the contrasting leukemic potentials of p190- and BiEH-immortalized cells with their different IL-7 and stromal requirements was further investigated. Subclones of BiEH-immortalized cells that no longer required stromal contact were isolated by adapting the cells from four cultures (BiEH.1 to BiEH.4) to growth in liquid medium supplemented with murine IL-7. These sublines were clonal based on Ig gene configurations, and all but one (BiEH.2-SI) retained a preB-I phenotype (Table 1). Subsequent plating in methylcellulose showed that the sublines were capable of forming colonies (55 to 95% cloning efficiencies) in the presence but not absence of IL-7 (Fig. 4). Attempts to derive IL-7-independent sublines by transfer of BiEH cells to liquid medium supplemented with 10% serum were less successful but yielded one line (BiEH.5) that was not dependent on IL-7. BiEH.5 cells displayed a high clonogenic potential in methylcellulose without exogenous IL-7 (Fig. 4) and displayed an atypical B-lineage phenotype (B220+ S7− BP1+ IgMlo) and genotype (Fig. 3B) that did not appear representative of normal B-lineage precursors.
The leukemogenic potentials of the stroma-independent (BiEH.1-SI, BiEH.2-SI, BiEH.3-SI, and BiEH.4-SI) and IL-7-independent (BiEH.5) cells were tested in syngeneic, nonirradiated recipients. BiEH.5 cells induced fatal leukemias with a latency of 2 to 3 weeks (Fig. 4 and 5). The pathological and cytologic features of the leukemias were comparable to those described above for p190-associated disease. In contrast, the stroma-independent, IL-7-dependent lines (BiEH.1-SI, BiEH.2-SI, BiEH.3-SI, and BiEH.4-SI) were nonleukemogenic under these conditions (Fig. 4 and 5B).
To address whether leukemogenicity required abrogation of the IL-7 cytokine requirement, stroma-independent cells were transduced with a retroviral construct expressing murine IL-7 under control of the MSCV LTR (Fig. 1A). After selection of stable transductants for resistance to puromycin, the resulting cells (BiEH.1-IL-7 and BiEH.2-IL-7) expressed exogenous IL-7 that was released into the culture medium (not shown). These lines displayed high clonogenic potentials in methylcellulose lacking added IL-7 (Fig. 4) and grew in liquid medium containing serum in contrast to their respective parental lines. Both lines induced rapidly fatal leukemias in recipient mice (Fig. 4). The disease latencies, pathology, and cytology were comparable to those observed in animals with BiEH.5 and p190 disease (Fig. 4 and data not shown). Taken together, our observations strongly suggested that abrogation of IL-7 dependence was a requirement for induction of aggressive leukemias by cells immortalized through the combined actions of E2A-HLF + BCL2.
Coexpression of IL-7 with E2a-Hlf is not sufficient to induce pre-B-cell leukemias.
We investigated whether forced expression of IL-7 could substitute for Bcl-2 in collaborating with E2a-Hlf to immortalize pre-B-I cells and facilitate development of acute leukemias. Primary bone marrow cells were transduced with MSCV constructs expressing either IL-7 or IL-7-IRES-E2A-HLF (7iEH) or the control IL-7+BCL2 and plated on AC-6.21 stromal cells. Robust growth under these conditions was observed for cells transduced with these constructs. Phenotype analyses showed that the cultures were predominantly comprised of pre-B-I cells (Table 1) with some heterogeneity in cell compositions (Fig. 3D). Southern blot analyses showed multiple IgH gene rearrangements in the absence of IgL rearrangements consistent with an oligoclonal pre-B-I population (Fig. 3D and Table 1). Despite their vigorous in vitro growth, however, cells expressing IL-7 alone, IL-7+BCL2, or IL-7 + E2A-HLF were incapable of inducing leukemias in syngeneic, immunocompetent mice over an observation period in excess of 6 months. Thus, IL-7 could substitute for Bcl-2 to allow the in vitro growth of pre-B cells expressing E2a-Hlf, but the combined actions of IL-7 and E2a-Hlf were insufficient for leukemic conversion.
Cells expressing IL-7 + E2A-HLF preferred growth on stroma but could be adapted to growth in liquid culture without stroma. Pre-B cells expressing ectopic IL-7+BCL2, or IL-7 alone, in contrast, did not grow in the absence of stroma. In liquid culture, IL-7/E2A-HLF cells (7iEH-SI) maintained a pre-B-I phenotype. However, FACS analysis showed a more homogenous expression of surface antigens, and Southern blot analyses revealed a single IgH gene rearrangement (Fig. 3D), indicating that they had undergone a clonal selection in the process of adapting to growth in the absence of stroma. Injection of these cells into syngeneic, nonirradiated mice resulted in the rapid induction of fatal leukemias in all recipients within 3 weeks. Taken together, these results suggested that coexpression of IL-7 and E2a-Hlf was not sufficient for induction of pre-B-cell leukemias due to the requirement for yet an additional event that appeared to correlate with stroma independence.
Stromal independence is associated with p53 pathway alterations.
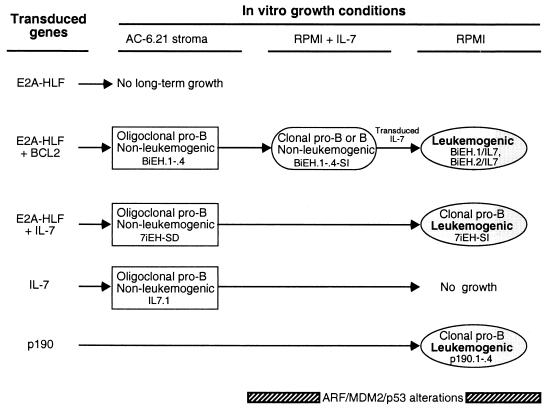
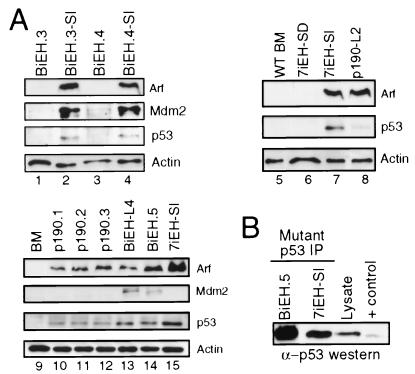
We investigated whether the clonal selections occurring at different points in the evolution of E2a-Hlf-associated leukemias in vitro (summarized in Fig. 6) may involve inactivation of the p53 apoptosis and tumor surveillance pathway. Western blot analyses of Arf, Mdm2, and p53 expression were performed on various cell lines that differed in their stroma and cytokine requirements and leukemogenic potentials. Stroma-dependent cells immortalized by E2a-Hlf in combination with Bcl2 (BiEH.3 and BiEH.4) lacked expression of all three proteins above background levels. However, clonal sublines that had been selected for growth in the absence of stroma (BiEH.3-SI and BiEH.4-SI) expressed abundant Arf and Mdm2 (Fig. 7A, compare lanes 1 and 3 with lanes 2 and 4). Similarly, stroma-dependent cells immortalized by E2a-Hlf + IL-7 (7iEH-SD) lacked detectable Arf, Mdm2, or p53, whereas a stroma-independent subclone (7iEH-SI) expressed mutated p53 that reacts with a conformation-dependent monoclonal antibody (Fig. 7A, lanes 6 and 7, and Fig. 7B). Immortalized cells expressing abundant Arf and Mdm2 (e.g., BiEH.3-SI and BiEH.4-SI) were not leukemogenic if they retained a requirement for exogenous IL-7. Cells capable of inducing leukemias with short latencies in syngeneic hosts, however, harbored detectable alterations in the Arf/Mdm2/p53 pathway, evidenced by expression of high level Arf and/or Mdm2 and mutant p53 (7iEH-SI and BiEH.5) (Fig. 7). Leukemias arising de novo after transplantation of cells freshly transduced with E2a-Hlf + Bcl2 (see below) also expressed abundant Arf and Mdm2 (e.g., BiEH-L4 in Fig. 7A, lane 13). Cells immortalized by p190 expressed Arf and p53 (Fig. 7A, lanes 10 to 12), although p53 mutant status was not determined. Taken together, these observations indicated that alterations of the Arf/Mdm2/p53 pathway were a consistent feature of E2a-Hlf associated leukemogenesis and typically arose in vitro during selection for stroma-independent growth.
FIG. 6.
Summary of growth alterations induced by various genes transduced into primary B-cell progenitors.
FIG. 7.
p53 pathway alterations correlate with stroma-independent growth of B-cell progenitors immmortalized by E2a-Hlf. (A) Western blot analysis of total cellular proteins isolated from cells growing in bone marrow cultures (identities indicated above gel lanes). Antibodies are indicated to the right of gel lanes. (B) Western blot analysis was performed with a sheep anti-p53 serum on immune complexes resulting from immunoprecipitation with a monoclonal antibody that recognizes an epitope exposed by conformational changes associated with a subset of p53 missense mutations (6).
Leukemic conversion of freshly isolated B cells transduced by E2A-HLF + BCL2.
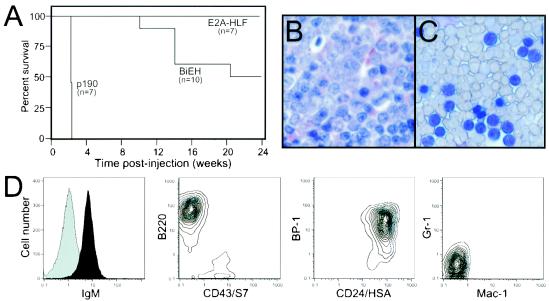
The leukemogenic potentials of transduced bone marrow cells were also tested in lethally irradiated recipients. BALB/c mice were transplanted with 106 bone marrow cells after infection with MSCV-E2A-HLF or MSCV-BiEH. Similar transplants were performed with bone marrow cells infected by MSCV-p190 or MSCV as positive and negative controls, respectively. All mice transplanted with MSCV-p190 developed lymphoid leukemias within 21 to 22 days (Fig. 8A and Table 2). Disease was characterized by high numbers of clonal B220+ IgM− blasts in the peripheral blood and extensive extramedullary disease (Table 2). In comparison, 5 of 10 animals (50%) transplanted with MSCV-BiEH transduced cells developed lymphoid leukemias with latencies of 70 to 110 days (Fig. 8A to C). None of the animals transplanted with cells tranduced with MSCV-E2A-HLF or MSCV developed disease over an observation period of 6 months or greater. Elevated white blood cell counts and abnormal increases in the ratio of lymphoid to myeloid cells were detected in all leukemic animals. At least 90% of mononuclear cells in the peripheral blood had features of blasts (Fig. 8C) and displayed an immature B-lineage phenotype (B220+ IgMlo BP1+ HSA+ Mac1− Gr1−) (Fig. 8D). Histologic analysis of spleen, liver, and bone marrow tissues of diseased animals revealed infiltration by large numbers of immature lymphoid cells (Fig. 8B and data not shown). Upon explantation, leukemic cells were observed to not require IL-7 for in vitro growth (Fig. 4 and Table 2). BiEH-induced leukemias were transplantable to secondary syngeneic, immunocompetent recipients, which succumbed to disease with shortened latencies compared to that observed in primary animals (Table 2). The secondary leukemias expressed similar surface phenotypes as the primary tumors and displayed no stromal or IL-7 requirements (data not shown). Therefore, E2a-Hlf in combination with Bcl-2, but not alone, was capable of inducing leukemias after long latencies in irradiated recipients, thus confirming the requirement for the suppression of apoptosis in leukemia pathogenesis.
FIG. 8.
Leukemogenic transformation of primary bone marrow cells transduced with E2A-HLF/BCL2 or p190BCR-ABL. (A) Survival of mice transplanted with cells immortalized by various retroviral constructs. A total of 106 transduced whole bone marrow cells were transplanted into lethally irradiated, syngeneic BALB/c mice. Numbers of mice in each cohort are denoted in parentheses. (B and C) Bone marrow biopsy and peripheral blood, respectively, showing leukemia cells with morphological features of lymphoid blasts in animals transplanted with BiEH-transduced cells. (D) Flow cytometric analysis of surface antigen expression by leukemia cells from mice transplanted with BiEH-transduced bone marrow.
TABLE 2.
Characteristics of BiEH and p190Bcr-Abl leukemias
| Leukemia | Latency (days) | Phenotype | Splenomegaly | % Blasts in PB | IL-7 dependence | Secondary leukemiaa [no. with leukemia/ no. injected (latency in days)] |
|---|---|---|---|---|---|---|
| BiEH-L1 | 100 | B220+ CD43− BP1+ IgMlo | + | >90 | − | 5/5 (21) |
| BiEH-L2 | 70 | B220+ CD43− BP1+ IgMlo | + | >90 | − | ND |
| BiEH-L3 | 105 | B220+ CD43− BP1+ IgMlo | + | >90 | − | ND |
| BiEH-L4 | 110 | B220+ CD43− BP1+ IgMlo | + | >90 | − | 5/5 (30-40) |
| P190-L1 | 21 | B220+ CD43+ BP1+ IgM− | + | >90 | − | 5/5 (17) |
| P190-L2 | 22 | B220+ CD43+ BP1+ IgM− | + | >90 | − | ND |
| P190-L3 | 22 | B220+ CD43+ BP1+ IgM− | + | >90 | − | ND |
| P190-L4 | 22 | B220+ CD43+ BP1+ IgM− | + | >90 | − | ND |
That is, 106 cells were injected i.v. into nonirradiated syngeneic recipients. ND, not determined.
DISCUSSION
Chimeric transcription factors are commonly created by chromosomal translocations in ALL, but they have proven to be particularly resistant to yielding representative experimental models of B-cell precursor ALL following forced expression in vitro or in vivo. In the current studies, we employed an in vitro experimental system to recapitulate the origin of B cell precursor ALL from a bone marrow stromal microenvironment to evaluate the oncogenic contributions of E2a-Hlf. The AC-6.21 stromal cell line was used since it has been shown in previous studies to support the long-term growth and differentiation of primary B-cell progenitors in vitro (35).
E2a-Hlf collaborates with Bcl-2 to immortalize bone marrow B-cell progenitors.
E2a-Hlf sustained the long-term in vitro growth of B-cell progenitors when coexpressed with Bcl-2. This was evident as developmental arrest and unlimited self-renewal of progenitors at the preB-I stage of differentiation comparable in phenotype to human leukemias bearing translocations of the HLF gene. The requirement for coexpressed Bcl-2 was unexpected, since E2a-Hlf itself has reported antiapoptotic properties (15). However, the survival of primary pre-B-I cells in vitro (the present study) and B cells in vivo (33) does not appear to be measurably enhanced by E2a-Hlf despite its ability to inhibit death associated with IL-3 deprivation of pro-B-cell lines in vitro (15). Thus, E2a-Hlf may be relatively selective in its antiapoptotic effects, which appear to be mediated at least in part through downstream perturbations of the IL-3 signaling pathway (13). In our cultures, IL-3 was unable to substitute for IL-7, the major cytokine associated with pre-B-I cell growth and survival in normal bone marrow microenvironments (1). Furthermore, increased apoptotic sensitivity, not resistance, of thymocytes has been reported in E2A-HLF transgenic mice (9), which develop profound thymic hypoplasias (9, 33).
In the present studies, coexpressed Bcl-2 was required for both long-term growth in vitro and induction of leukemias after transplantation of transduced cells. The requirement for Bcl-2 does not appear to reflect a need to suppress a tumor surveillance response to E2a-Hlf, since immortalized progenitors growing on stroma do not display activation of the p53 apoptosis pathway (see below). It is likely that the high apoptotic sensitivity of normal B-cell progenitors to even minimal alterations in their maturation programs constitutes a barrier to initiation of ALL by chimeric oncoproteins, as suggested by previous transgenic studies (3, 32). Coexpressed Bcl-2 may suppress apoptosis that is initiated by a developmental checkpoint that is triggered by E2a-Hlf under our experimental conditions. Abundant endogenous Bcl-2 levels in human cell lines harboring E2a-Hlf chromosomal translocations (K. S. Smith and M. L. Cleary, unpublished observations) raise the possibility of a similar Bcl-2 requirement in human leukemias, although the molecular mechanisms sustaining these Bcl-2 levels are not known.
B-cell progenitors immortalized by E2a-Hlf lack mutations in the Arf/Mdm2/p53 pathway, which subsequently arise during leukemic progression.
Immortalization of murine B-cell progenitors by E2a-Hlf occurred in the absence of apparent mutations in the Arf/Mdm2/p53 pathway. This contrasts with murine fibroblast immortalization, which is invariably associated with perturbations of this pathway (20). Although Arf-deficient B-cell progenitors are immortal (28), our results clearly indicate that murine B-cell progenitors can be immortalized by an alternative route in the absence of apparent lesions in this pathway. Similar to Arf-null pre-B cells, primary B-cell progenitors immortalized by the combined actions of E2a-Hlf and Bcl-2 are nonleukemogenic and retained stromal and cytokine requirements for in vitro growth and survival. This resembles the supportive needs of normal B-cell progenitors, which differentiate and proliferate in close contact with bone marrow stromal cells (30). Our studies support the thesis that the primary effect of E2a-Hlf is to impair the differentiation of B-cell progenitors, arresting them in a proliferative state. Whereas normally this may result in programmed cell death, coexpression of Bcl-2 apparently prevents these arrested cells from undergoing apoptosis.
We were able to isolate subpopulations of immortalized cells that no longer required stromal contact by selective passage through methylcellulose containing high exogenous levels of IL-7. The resulting stroma-independent cells were clonal and invariably harbored alterations in the p53 pathway, as evidenced by mutations of p53 itself and/or increased Arf and Mdm2 expression, markers of downstream perturbations in the pathway (5, 31). This suggests a mechanism for limiting the growth of progenitors immortalized by E2a-Hlf that may be analogous to the tumor surveillance responses to other oncoproteins, such as c-Myc, whose forced expression induces apoptosis in low serum but not normal growth conditions (10). Thus, within the context of a stromal microenvironment, E2a-Hlf enhances the self-renewal of B-cell progenitors without initiating a p53-dependent surveillance response. However, inactivating alterations of the INK4A/ARF-MDM2-P53 axis were associated with leukemic progression in our model system and are commonly observed in pediatric ALLs (4, 26), including most t(17;19) cell lines and leukemias, which contain deletions or methylations that silence the INK4A/ARF gene (24). MDM2 overexpression is also observed in a subset of pediatric ALLs and has been linked to poor response to therapy and early relapse (36), a feature of leukemias expressing E2a-Hlf.
Leukemic progression of immortalized B-cell progenitors is associated with abrogation of stromal and cytokine requirements.
The cytokine IL-7 is crucial for B-cell development, serving multiple roles that are not alleviated by forced expression of E2a-Hlf. This contrasts with the properties of cells transduced by p190Bcr-Abl, which lack similar stromal and cytokine requirements for growth in vitro. Bcr-Abl, a cytoplasmic kinase, is known to perturb multiple signaling pathways, including PI-3 kinase, ras and Jak/STAT pathways (37), which likely accounts for its potent ability to abrogate the cytokine requirements of primary B-cell progenitors. Furthermore, cells freshly transduced with p190 rapidly induced leukemias when transplanted into nonirradiated recipients, whereas BiEH-transduced cells induced leukemias only in irradiated syngeneic recipients. This, as well as the increased latency and reduced frequency, suggests that BiEH immortalized cells require a supportive microenvironmental niche to initiate a multistep neoplastic process in vivo. By comparison to p190, E2a-Hlf appears to mediate a more limited subset of the requirements for autonomous growth and leukemogenesis. As a consequence, the stromal microenvironment may serve a relatively greater role in nurturing the growth and survival of “preleukemic” B-cell precursors that express this fusion oncogene.
In spite of the need for IL-7 to sustain the growth of immortalized B-cell precursors in vitro, abrogation of IL-7 dependence was necessary for induction of leukemias by cells coexpressing E2a-Hlf and Bcl-2. Several observations support this conclusion. First, regardless of their method of establishment, cells that required exogenous IL-7 or stromal support for in vitro growth were consistently nonleukemogenic in syngeneic recipients. Second, immortalized cells adapted to grow without stroma were nonleukemogenic until programmed to express autocrine IL-7. Third, growth in RPMI medium lacking IL-7 was invariably associated with a rapidly leukemogenic phenotype. Fourth, leukemias that arose after long latency after transplantation of freshly transduced cells displayed cytokine-independent growth upon explantation and were rapidly leukemogenic in secondary recipients. Taken together with the lack of spontaneously arising IL-7-independent pro-B cells in vitro, these data strongly suggest that in our model system IL-7 dependence is a major rate-limiting step in leukemogenesis.
Our experimental model for B-cell precursor ALL reveals dichotomous roles for IL-7. It is essential for growth and survival of immortalized cells, but their dependence on exogenous IL-7 limits progression to a full leukemogenic phenotype. Thus, mutations that constitutively activate the IL-7 signal transduction pathway could provide a possible mechanism for satisfying both roles. To test this hypothesis, we coexpressed IL-7 and observed that it substitutes for Bcl-2 and collaborates with E2a-Hlf to immortalize B-cell precursors in vitro on stromal layers to fulfill both survival and cytokine requirements. Nevertheless, cells expressing E2a-Hlf and autocrine IL-7 underwent crisis and clonal outgrowth of a subpopulation with mutation of p53 upon withdrawal from stromal support. Notably, this was associated with progression to a leukemogenic phenotype in syngeneic, immunocompetent recipients. Thus, abrogation of IL-7 dependence is an important, albeit not sole, requirement for leukemogenic progression. Human cell lines representative of pediatric ALL, including three with the t(17;19) chromosomal translocation, generally lack cytokine requirements for in vitro growth. Although their reduced cytokine requirements may have been selected for during explantation, activating mutations in signal transduction pathways have been reported in a subset of primary ALL cells (29).
In summary, E2a-Hlf enhances the self-renewal of B-cell progenitors that retain their dependence on stromal and cytokine support in vitro and maintain an intact p53 pathway. This requires coexpression of Bcl-2 and is not sufficient for leukemogenesis. Our observations implicate perturbations of stromal and cytokine (IL-7) dependence pathways to collaborate with E2a-Hlf for progression to aggressive ALL from immortalized B-cell precursors. Mutations that disrupt these pathways have been reported previously in human B-cell progenitor leukemias, providing tentative support for the relevance of this multistep model for human leukemia pathogenesis.
Acknowledgments
We thank Cita Nicolas for expert technical assistance; Jay Bream for providing the murine IL-7 cDNA; Joe Lipsick for the IRES clone; Paul Ayton for helpful conversations; Linda Boxer, Dean Felsher, and Steve Hunger for comments on the manuscript; and Phil Verzola for photographic support.
These studies were supported by funds from the NIH (CA42971) and the Children's Health Initiative Innovations Fund.
REFERENCES
- 1.Carsetti, R. 2000. The development of B cells in the bone marrow is controlled by the balance between cell-autonomous mechanisms and signals from the microenvironment. J. Exp. Med. 191:5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cleary, M. L., S. D. Smith, and J. Sklar. 1986. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell 47:19-28. [DOI] [PubMed] [Google Scholar]
- 3.Dedera, D. A., E. K. Waller, D. P. LeBrun, A. Sen-Majumbar, M. E. Stevens, G. S. Barsh, and M. L. Cleary. 1993. Chimeric homeobox gene E2A-PBX1 induces proliferation, apoptosis, and malignant lymphomas in transgenic mice. Cell 74:833-843. [DOI] [PubMed] [Google Scholar]
- 4.Drexler, H. G. 1998. Review of alterations of the cyclin-dependent kinase inhibitor INK4 family genes p15, p16, p18, and p19 in human leukemia-lymphoma cells. Leukemia 12:845-859. [DOI] [PubMed] [Google Scholar]
- 5.Eischen, C. M., J. D. Weber, M. F. Roussel, C. J. Sherr, and J. L. Cleveland. 1999. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 13:2658-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gannon, J. V., R. Greaves, R. Iggo, and D. P. Lane. 1990. Activating mutations in p53 produce a common conformational effect: a monoclonal antibody specific for the mutant form. EMBO J. 9:1595-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardy, R. R., C. E., Carmack, S. A. Shinton, J. D. Kemp, and K. Hayakawa. 1991. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 173:1213-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawley, R. G., F. H. L. Lieu, A. Z. C. Fong, and T. S. Hawley. 1994. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1:136-138. [PubMed] [Google Scholar]
- 9.Honda, H., T. Inaba, T. Suzuki, H. Oda, Y. Ebihara, K. Tsuiji, T. Nakahata, T. Ishikawa, Y. Yazaki, and H. Hirai. 1999. Expression of E2A-HLF chimeric protein induced T-cell apoptosis, B-cell maturation arrest, and development of acute lymphoblastic leukemia. Blood 93:2780-2790. [PubMed] [Google Scholar]
- 10.Hueber, A. O., and G. I. Evan. 1998. Traps to catch unwary oncogenes. Trends Genet. 14:364-367. [DOI] [PubMed] [Google Scholar]
- 11.Hunger, S. P., K. Ohyashiki, K. Toyama, and M. L. Cleary. 1992. Hlf, a novel hepatic bZIP protein, shows altered DNA binding properties following fusion to E2A in t(17;19) acute lymphoblastic leukemia. Genes Dev. 6:1608-1620. [DOI] [PubMed] [Google Scholar]
- 12.Hunger, S. P., R. Brown, and M. L. Cleary. 1994. DNA-binding and transcriptional regulatory properties of hepatic leukemia factor (HLF) and the t(17;19) acute lymphoblastic leukemia chimera E2A-HLF. Mol. Cell. Biol. 14:5986-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikushima, S., T. Inukai, T. Inaba, S. D. Nimer, J. L. Cleveland, and A. T. Look. 1997. Pivotal role for the NFIL3/E4BP4 transcription factor in interleukin 3-mediated survival of pro-B lymphocytes. Proc. Natl. Acad. Sci. USA 94:2609-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inaba, T., W. M. Roberts, L. H. Shapiro, K. W. Jolly, S. C. Raimondi, S. D. Smith, and A. T. Look. 1992. Fusion of the leucine zipper gene HLF to the E2A gene in human acute B-lineage leukemia. Science 257:531-534. [DOI] [PubMed] [Google Scholar]
- 15.Inaba, T., T. Inukai, T. Yoshihara, H. Seyschab, R. A. Ashmun, C. E. Canman, S. J. Laken, M. B. Kastan, and A. T. Look. 1996. Reversal of apoptosis by the leukemia-associated E2A-HLF chimaeric transcription factor. Nature 382:541-544. [DOI] [PubMed] [Google Scholar]
- 16.Inukai, T., T. Inaba, S. Ikushima, and A. T. Look. 1998. The AD1 and AD2 transactivation domains of E2A are essential for the anti-apoptotic activity of the chimeric oncoprotein E2A-HLF. Mol. Cell. Biol. 18:6035-6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inukai, T., A. Inoue, H. Kurosawa, K. Goi, T. Shinjyo, K. Ozawa, M. Mao, T. Inaba, and A. T. Look. 1999. SLUG, a ces-1-related zinc finger transcription factor gene with antiapoptotic activity, is a downstream target of the E2A-HLF oncoprotein. Mol. Cell 4:343-352. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs, Y., C. Vierra, and C. Nelson. 1993. E2A expression, nuclear localization, and in vivo formation of DNA- and non-DNA-binding species during B-cell development. Mol. Cell. Biol. 13:7321-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang, S. K., and E. Wimmer. 1990. Cap-independent translation of encephalomyocarditis virus RNA: structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev. 4:1560-1572. [DOI] [PubMed] [Google Scholar]
- 20.Kamijo, T., F. Zindy, M. F. Roussel, D. E. Quelle, J. R. Downing, R. A. Ashmun, G., Grosveld, and C. J. Sherr. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91:649-659. [DOI] [PubMed] [Google Scholar]
- 21.Lavau, C., J. M. Heard, O. Danos, and A. Dejean. 1996. Retroviral vectors for the transduction of the PML-RARα fusion product of acute promyelocytic leukemia. Exp. Hematol. 24:544-551. [PubMed] [Google Scholar]
- 22.LeBien, T. W. 2000. Fates of human B-cell precursors. Blood 96:9-23. [PubMed] [Google Scholar]
- 23.Look, A. T. 1997. Oncogenic transcription factors in the human acute leukemias. Science 278:1059-1064. [DOI] [PubMed] [Google Scholar]
- 24.Maloney, K. W., L. McGavran, L. F. Odom, and S. P. Hunger. 1998. Different patterns of homozygous p16INK4A and p15INK4B deletions in childhood acute lymphoblastic leukemias containing distinct E2A translocations. Leukemia 12:1417-1421. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin, J., E. Chianese, and O. N. Witte. 1989. Alternative forms of the BCR-ABL oncogene have quantitatively different potencies for stimulation of immature lymphoid cells. Mol. Cell. Biol. 9:1866-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohnishi, H., R. Hanada, K. Horibe, T. Hongo, M. Kawamura, S. Naritaka, F. Bessho, M. Yanagisawa, T. Nobori, S. Yamamori, and Y. Hayashi. 1996. Homozygous deletions of p16/MTS1 and p15/MTS2 genes are frequent in t(1;19)-negative but not in t(1;19)-positive B precursor acute lymphoblastic leukemia in childhood. Leukemia 10:1104-1110. [PubMed] [Google Scholar]
- 27.Pear, W., G. Nolan, M. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randle, D. H., F. Zindy, C. J. Sherr, and M. F. Roussel. 2001. Differential effects of p19(Arf) and p16(Ink4a) loss on senescence of murine bone marrow-derived preB cells and macrophages. Proc. Natl. Acad. Sci. USA 98:9654-9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodenhuis, S., J. L. Bos, R. M. Slater, H. Behrendt, M. van't Veer, and L. A. Smets. 1986. Absence of oncogene amplifications and occasional activation of N-ras in lymphoblastic leukemia of childhood. Blood 67:1698-1704. [PubMed] [Google Scholar]
- 30.Rolink, A., and F. Melchers. 1996. B-cell development in the mouse. Immunol. Lett. 54:157-161. [DOI] [PubMed] [Google Scholar]
- 31.Scherr, J., and J. D. Weber. 2000. The ARF/p53 pathway. Curr. Opin. Genet. Dev. 10:94-99. [DOI] [PubMed] [Google Scholar]
- 32.Smith, K. S., Y. Jacobs, C. P. Chang, and M. L. Cleary. 1997. Chimeric oncoprotein E2a-Pbx1 induces apoptosis of hematopoietic cells by a p53-independent mechanism that is suppressed by Bcl-2. Oncogene 14:2917-2926. [DOI] [PubMed] [Google Scholar]
- 33.Smith, K. S., J. W. Rhee, L. Naumovski, and M. L. Cleary. 1999. Disrupted differentiation and oncogenic transformation of lymphoid progenitors in E2A-HLF transgenic mice. Mol. Cell. Biol. 19:4443-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitlock, C. A., G. F. Tidmarsh, C. Muller-Sieburg, and I. L. Weissman. 1987. Bone marrow stromal cell lines with lymphopoietic activity express high levels of a pre-B neoplasia-associated molecule. Cell 48:1009-1021. [DOI] [PubMed] [Google Scholar]
- 35.Whitlock, C. A., and C. Muller-Sieburg. 1997. Long-term B-lymphoid cultures from murine bone marrow: establishment and cloning by using stromal cell line AC 6.21. Methods Mol. Biol. 75:231-248. [DOI] [PubMed] [Google Scholar]
- 36.Zhou, M., L. Gu, T. C. Abshire, A. Homans, A. L. Billett, A. M. Yeager, and H. W. Findley. 2000. Incidence and prognostic significance of MDM2 oncoprotein overexpression in relapsed childhood acute lymphoblastic leukemia. Leukemia 14:61-67. [DOI] [PubMed] [Google Scholar]
- 37.Zou, X., and K. Calame. 1999. Signaling pathways activated by oncogenic forms of Abl tyrosine kinase. J. Biol. Chem. 274:18141-18144. [DOI] [PubMed] [Google Scholar]