Abstract
Objective:
To analyze the utility of quick intraoperative parathyroid hormone (PTH) measurement in the surgical management of primary hyperparathyroidism.
Background Data:
The use of intraoperative PTH monitoring is well established in the surgery of primary hyperparathyroidism. However, some false-negative predictions lead to unnecessary explorations; furthermore, surgeons are becoming increasingly dependent on hormone measurement for intraoperative decisions, which raises concerns about the cost-effectiveness of the method.
Methods:
A retrospective analysis of 268 neck explorations performed for primary hyperparathyroidism using intraoperative PTH monitoring from April 2001 to February 2003 was done. We used the criterion of “biologic recovery” of hyperfunctioning tissue, defined as a more than 50% decrease in PTH level from baseline value at 5 minutes after excision to predict the outcome of successful parathyroidectomy documented by normal postoperative serum calcium level. Additionally, we also sampled PTH at 10 minutes, 30 minutes, and the morning after surgery to compare the predictive value of delayed sampling. Patients were classified according to the prediction being concordant or discordant with the outcome. The data were analyzed using a 2 × 2 table construct for each of the sampling times, therefore providing sequential sensitivity, specificity, positive and negative predictive values, and overall accuracy of the predictions.
Results:
Concordance or overall accuracy of prediction (true positives and negatives) was obtained in 229 cases (85.4%), and discordance or failure of prediction (false positives and negatives) was obtained in 34 cases (12.7%) at T5. On analyzing the iPTH prediction at T10, T30, and D1 among the group of 33 false negatives, we found that 28 (10.4%) patients reached the concordance at 30 minutes, while by the first day 32 patients (12.3%) had achieved concordance. Thus, there was a progressive increase in sensitivity and overall accuracy, but more importantly, in the negative predictive value reaching 88.9% on the day after surgery.
Conclusions:
The method of sampling PTH intraoperatively at 5 minutes has a high positive predictive value (99.5%) but a low negative predictive value (19.5%), which can lead to unnecessary explorations and a delay in the operative procedure. The negative predictive value increases substantially at 30 minutes and is best on the day after surgery. We suggest giving up the intraoperative measurement of PTH to adopt the first day postoperative measurement of PTH as a predictor of successful parathyroidectomy.
Intraoperative parathyroid hormone monitoring has become a widely established procedure during surgery for primary hyperparathyroidism, and there is increasing dependence on biochemical confirmation of completion of surgery. This study was conducted to critically evaluate the reliability of quick intraoperative parathyroid hormone measurement for this purpose.
The rapid intraoperative determination of intact parathyroid hormone (iPTH) with a 2-site immunochemiluminometric assay (ICMA) has become widely established in the operation for primary hyperparathyroidism (HPT).1–8
This method allows both confirmation of complete removal of hypersecreting tissue and a real-time localization of the enlarged glands, facilitating the performance of unilateral or minimally invasive parathyroidectomy.9,10
However, surgeons are becoming increasingly dependent on the iPTH monitoring11 both for intraoperative decisions when the preoperative localization studies are negative or unclear and for validating unilateral cervical exploration when the preoperative localization techniques are unequivocal. Progressively more sophisticated and expensive techniques to increase the reliability of the assay have also decreased the cost-effectiveness of the method.10,12,13
The aim of our study was to critically evaluate the reliability of quick intraoperative measurement of PTH in the surgical management of primary hyperparathyroidism.
PATIENTS AND METHODS
From April 2001 to February 2003, 275 patients were operated on for primary HPT at the University Surgical Unit of Lille, France. Thirteen patients with inadequate data on intraoperative PTH values and 1 patient with a diagnosis of idiopathic hypercalciuria were not included in the study. Seven of those patients included had undergone parathyroid reinterventions for persistence of HPT; these were included as separate cases, making a total of 268 cases available for analysis. A total of 216 patients underwent both MIBI scintigraphy and neck ultrasonography, 24 patients only ultrasonography, 25 patients only scintigraphy, and 3 patients neither procedure.
There were 61 (22.8%) cases of multiglandular disease (MGD) and 207 (77.2%) of uniglandular disease (UGD). In UGD, the pathologic diagnosis was adenoma in 153, hyperplasia in 48, carcinoma in 4, and normal in 2 grossly enlarged glands (weight, 113 mg, 215 mg). Nine patients presented with primary HPT and chronic renal disease (3.4%), while 8 (3%) patients had familial disease (7 cases of type I multiple endocrine neoplasia (MEN) I, 1 case of non-MEN I familial HPT). One patient had associated Neurofibromatosis and 65 (24.3%) patients had an associated thyroid disease requiring combined thyroid surgery (44 total thyroidectomies and 21 lobo-isthmectomies).
Primary HPT was discovered incidentally at the time of thyroid surgery in 10 patients. In 9 patients, this was by routine preoperative calcium measurement; and in 1 case, an adenoma was discovered intraoperatively in a normocalcemic patient.
We performed 103 (38.4%) unilateral neck explorations (UNE) and 165 (61.6%) bilateral neck explorations (BNE), with 16 conversions. Unilateral neck approach was indicated when there was concordant localization of preoperative imaging, except in cases of goiter, MEN syndrome, and previous neck irradiation. UNE was considered successful when a single large gland weighing more than 60 mg was found accompanied by a significant drop in PTH value. Intraoperative PTH levels were measured with a rapid two-site IMCA (Nichols Advantage, Nichols Institute Diagnostics, Saint Clement, CA; normal range in our laboratory, 10–65 pg/mL).
The values of intraoperative PTH sampling were designated in the following manner. For each parathyroidectomy, the pre-incision value was T0, the pre-excision intraoperative value T1 and the values at 5, 10, and 30 minutes after removal of the enlarged parathyroid gland were T5, T10, and T30, with T5 sampling after completion of appropriate parathyroidectomy in patients with MGD. Serum PTH and calcium were measured the morning after surgery, with the PTH value designated D1. The additional blood sampling for PTH values at 30 minutes and day 1 was performed to confirm the validity of the standard 5- or 10-minute test and document the decay profile of postoperative PTH in our center.
The criterion of “biologic recovery” of hyperfunctioning parathyroid tissue was established, as defined in previous reports,4,8–12,14,15 by a decrease of more than 50% from the baseline value at 5 minutes. We considered the higher value between T0 and T1 as the baseline (T1 was used in cases where PTH levels increased at the time of dissection). All patients were followed postoperatively with serum calcium, phosphorus, and PTH measurement. The criterion of successful parathyroidectomy (the gold standard in this study) was defined as normal postoperative serum calcium and phosphorus measurements at last follow-up (range, 3 days to 22 months). Of 268 cases, 108 patients (40.3%) had the last follow-up in the early postoperative period (< 7 days), while 160 (59.7%) had a long-term follow-up, having been seen by the endocrinologist in the outpatient department.
Prediction of successful or failed parathyroidectomy by quick intraoperative measurement of PTH was evaluated by using the defined criterion of biologic recovery. Thus, a true positive was the correct prediction of success, a true negative was the correct prediction of failure, a false positive was the wrong prediction of success, and a false negative was the wrong prediction of failure. Five cases of doubtful interpretation including one case of metastatic parathyroid carcinoma were excluded from the analysis; all these cases had postoperative hypercalcemia but could not be clearly classified in any of these categories. The values from 263 cases were therefore available for final analysis. By constructing 2 × 2 tables comparing values at T5, T10, T30, and D1 separately with the gold standard of successful parathyroidectomy as defined above, the sensitivity, specificity, positive and negative predictive values, and overall accuracy of prediction were computed. For ease of understanding, we also presented the analysis in terms of concordance and discordance between the prediction of adequate biologic recovery by intraoperative PTH monitoring and the outcome of successful parathyroidectomy (Table 1). Overall accuracy is represented by concordance of the prediction with successful or failed outcome.
TABLE 1. Prediction Outcomes for Analysis
RESULTS
There was a total of 263 cases analyzed. The 4 possibilities of prediction outcomes are listed in Table 1.
The outcome as predicted by PTH values at T5, T10, T30, and D1 is listed in Table 2, which clearly shows a progressive shift of cases from false negative to true positive prediction with increasing delay in sampling. This reflects more cases meeting the criterion of biologic recovery in those who were finally cured of the disease. Only in 1 case, the PTH values at D1 had not yet reached the normal range; considering the slope of PTH, this patient had a discordance even at T30. The patient was however normocalcemic on long-term follow-up.
TABLE 2. No. of Cases in Prediction Outcomes According to Time of Sampling of Intraoperative PTH
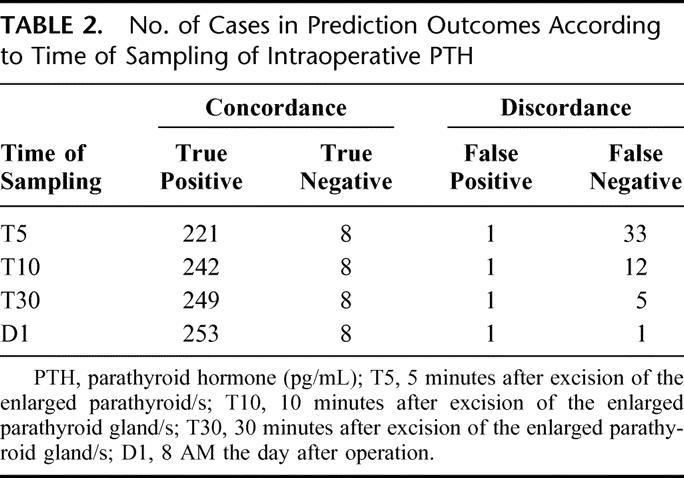
The above outcomes were analyzed by the 2 × 2 table construct to calculate the sensitivity, specificity, positive predictive value, negative predictive value, and overall accuracy according to the time of sampling, which are listed in Table 3.
TABLE 3. Prediction Outcome Measures According to Sampling Time of Intraoperative PTH
The effect of delayed sampling was to decrease the number of false predictions of failure (negative discordance or false negative) by the inadequate drop in PTH in these patients at 5 minutes. This led to a progressively increasing negative predictive value of intraoperative PTH assay from 19.5% at 5 minutes to 88.9% the next morning. The sensitivity and overall accuracy also increased correspondingly.
We compared the T5 group of 34 discordance (including mainly the 33 false-negative patients) with the 229 concordance cases to see if there was proportionately more MGD or renal insufficiency to see if they contributed to the phenomenon of high false-negative predictions as illustrated by Table 4.
TABLE 4. Percentage of Cases in Concordance and Discordance Groups (at T5) According to Type of Disease and Presence of Renal Failure
In the discordance group, there were 20.6% UNE and 79.4% BNE with 3 conversions; and in the concordance group, there were 40.6% UNE and 59.4% BNE with 13 conversions.
The T1 value was used as baseline in 32 (13.9%) concordant and 8 (23.5%) discordant cases where there had been an intraoperative rise above the pre-incision value.
Among the 34 discordance cases, 6 patients (17.6%) were operated for MGD, with resection of 2 to 4 parathyroid glands, all hyperplastic. In the concordance group, there were 55 cases (24%) of MGD.
There was only 1 false-positive case predicting cure where there was not. This patient was operated the first time with bilateral neck exploration and underwent resection of a left inferior intrathymic gland (weight, 113 mg), with exploration of 3 normal glands. The postoperative period was characterized by persistence of hypercalcemia and a right inferior uptake at 99mTc-MIBI scintigraphy. The patient underwent reoperation 8 months after the first surgical intervention, with resection of a right inferior hyperplastic gland and a supernumerary hyperplastic gland in the right lobe of the thyroid. At the second reoperation, the PTH decreased more than 50% at 5 minutes, with a concordance to the successful parathyroidectomy. The patient is still normocalcemic.
Among the 221 true-positive cases, 13 patients were normocalcemic with elevated values of PTH on follow-up (6 cases had an associated deficiency of vitamin D), while the other 208 were normocalcemic with normal values of PTH. In the presence of normocalcemia, a postoperative PTH value up to 4 times normal may be found on follow-up of cured cases as reported earlier.16–20 The range of the preoperative PTH values was between 118 and 1680 (in 30% of these patients being more than 700). In all except 1 of these cases, the drop in PTH was very significant, with a decrease of more than 60% at 5 minutes. Besides, in 70% of these cases, the D1 value of PTH was less than 80.
There were 8 true-negative cases of persistent hyperparathyroidism. Six cases were reoperated: 5 in the immediate postoperative period and 1 patient 3 months after initial surgery. Of these, 4 underwent a unilateral neck dissection at the first operation, with a subsequent bilateral neck dissection and a concordant iPTH prediction of success. Two cases have been on regular follow-up for pauci-symptomatic persistent HPT.
DISCUSSION
From our results, when using T5 or T10 as the sampling times, the method has low negative predictive values of 19.5% and 40% (33 and 12 false negatives, respectively). These false-negative cases would be considered not cured by the measured iPTH level, when actually cured of the disease. However, at 30 minutes, there were only 5 false negatives (negative predictive value, 61.5%); the situation further improved by the D1 value yielding only one false-negative case (negative predictive value, 88.9%). Therefore, 97% of false-negative cases (32 of 33) become true positive or achieve concordance at D1. This progressive improvement in accuracy of prediction reflects a prolonged decay profile of PTH in some cases, not explained by confounding factors like renal failure and MGD. Chronic renal failure had comparable rates of 5.9% among discordance and 3.1% in the concordance group, this being supported by published study.21 There were also comparable rates of MGD in the group of discordance(17.6%) versus concordance (24%). This observation contrasts with those of other authors who report limits of the test in multiglandular disease.22,23 Thus, unnecessary further exploration or conversion may result when using the standard 5- or 10-minute samplings as an indicator of biologic recovery. In our series, the number of conversions remains lower than the negative predictions because the surgeon additionally used his experience in interpreting preoperative imaging and intraoperative findings to guide the decision to convert.
An increased rate of bilateral neck explorations was noted in the discordant group, although this did not translate into proportionately more MGD and may reflect the preoperative indication for bilateral exploration being different in the groups.
A normal PTH value was achieved at D1 in 97.1% of the discordance group, while we have found the same in 95.6% of the concordance group. The supra-normal values of PTH at D1 in the concordance group could be explained in patients with highly elevated baseline values of PTH (30% more than 700). Indeed, all the cases reported of supra-normal values of PTH at D1 had a more than 60% decrease from the baseline level at 5 minutes, with a subsequent slow decrease of PTH values, which can be explained by the variability of PTH half-life, as well demonstrated by Libutti et al.24 So, even if we consider a normal PTH (10–65 pg/mL) and not just the significant drop in PTH required for concordance at D1 as the criterion of biologic recovery, we have a 94% concordance or overall accuracy rate. Our results are in keeping with those of Agarwal et al, who have proposed the same-day routine PTH testing instead of intraoperative quick PTH testing to improve significantly the cost-effectiveness of the procedure.12
In the discordance group, 94.3% of patients attained normocalcemia along with a normal value of PTH in the postoperative period (vs. 93.7% of the concordance group). These results agree with those reported in literature.17–22,25,26
CONCLUSION
The low negative predictive value of the standard 5- or 10-minute samplings can lead to unnecessary conversion or further exploration. The negative predictive value of the method can be increased by sampling the PTH at times greater than 10 minutes up to 30 minutes, but this necessitates lengthening the operative procedure with its consequences. To maximize the reliability and cost-effectiveness of the method, we suggest giving up the intraoperative measurement of PTH in favor of adopting the first postoperative day measurement of PTH as an indicator of biologic recovery (88.9% negative predictive value and 99.2% overall accuracy) to predict successful parathyroidectomy.
Discussions
Dr. Hellman: Thank you, Dr. Mozzon, for your presentation, and the challenge of the intraoperative PTH measurements, which recently have become popular among endocrine surgeons. I acknowledge your scrutiny of the outcome of this method. What we need to ask ourselves is: what is the goal of the intraoperative PTH measurements? Do we want our patients to be normocalcemic after the operation to reduce the risk for cardiovascular disease and other complications and/or do we want to avoid reoperation for multiglandular disease?
Regarding the last issue, we need to already preoperatively identify patients with risk for multiglandular disease, most often due to renal dysfunction. Among your patients, you had some with primary HTP but somewhat unknown levels of renal function and others you say have primary HTP but also chronic renal failure. You need to clarify this further. What was the renal status of your patients? How many had mild renal insufficiency? This could potentially affect your data and results significantly. In conjunction with that, what was the vitamin D status of your patients?
The second issue is: how did you measure PTH? It is not clearly stated. You have a prolonged half-life with some assays, especially in patients with renal insufficiency. Among your patients, there may have been some in whom you could have anticipated multiglandular disease (renal dysfunction, low vitamin D levels); and in my opinion, you should exclude them from your study, since you are scrutinizing the need for intraoperative PTH in primary HPT, and what you are trying to find is the risk of missing multiglandular disease by avoiding intraoperative PTH but still do a minimally invasive approach.
Dr. Mozzon: In our series of patients with PTH, we have included patients with mild renal insufficiency, ie, creatinine level less than twice the upper normal range. We could observe a 5.9% rate of renal insufficiency in the concordance group versus a 3.1% rate in the discordance group. Mild renal insufficiency therefore does not affect the quick intraoperative PTH measurements.
Dr. Proye: Primary HPT with mild renal insufficiency is part of the full spectrum of the disease. The problem is absolutely different when you have a patient on maintenance hemodialysis with secondary HPT. In this setting, intraoperative PTH level decrease reflects not the secretion but the clearance of PTH. Half-life of PTH increases from 2.2 to 6.6 minutes. We have demonstrated with Lokey (Surgery. 2000;128:1029) that in this special case a drop of only 50% at 20 minutes is diagnostic of cure. You point out the interesting problem of vitamin D deficiency, a very common occurrence, especially in HPT patients indeed. It has been studied extensively in our material and does not affect the slope of intraoperative PTH decay.
Dr. Hamberger: It is a very interesting study; it is really focused on problems of interpretation of intraoperative PTH. Sometimes the discussion is more focused on whether you cure the PTH level or the patient. However, I cannot agree on that we should aim at measurements during the first day because I think that we are more and more going into doing this as a day surgery, and then we cannot have the patient in the hospital at the next morning; that would not be cost-effective. Probably the most cost-effective would be not to measure PTH at all.
However, I would like to have your comments on moving toward day surgery. Furthermore, I would like your comment on that most endocrine centers would make use of the 10-minute level, which might give better results than when using the 5-minute level.
Dr Mozzon: Our intention is not to discuss the issue of various economic health systems in different countries. In France and in many other countries, it is not a problem to keep the patient overnight. They rather ask for it and feel more secure and the surgeon also. Anyway, we have demonstrated that the overall accuracy of the method increases from 85% at 5 minutes to 93% at 10 minutes and 99% on the morning after surgery.
Dr. Fernandez-Cruz: I want to congratulate for your excellent paper and presentation, and I also think that we recognized that Dr. Proye has the largest experience in the field of parathyroid surgery in the world. My question is: did the presence of nodular goiter have any influence on the concordance and discordance of PTH results? Also, how did the unilateral or bilateral approach influence the results?
Dr. Mozzon: Thank you for your comments. In our population, there were 65 patients, ie, 24% with associated thyroid disease, 44 underwent a total thyroidectomy and 21 a thyroid lobectomy. There was no significant difference between concordances or discordances in thyroidectomized versus nonthyroidectomized patients. The same is true for unilateral versus bilateral approach. We were expecting more discordances in the group of multiglandular disease versus uniglandular diseases, but amazingly the contrary was observed (Table 4). Or if we express the results otherwise, the method yields a discordance rate of 10% in multiglandular versus 13.5% in uniglandular disease.
Dr. Proye: Comments have been made about 5 minutes, 10 minutes, and next day measurement. If there is just one message to be given to the surgeon not specialized in parathyroid surgery, that is the extreme variability of the half-life of PTH. It can vary more than ninefold from 25 seconds to 4 minutes (range, 1.55 ± 0.88 minutes) in the absence of renal insufficiency as demonstrated by Libutti (Surgery. 1999;126–145). So that underscores the relativity of the value of this measurement and also of the discussion.
Footnotes
Reprints: Charles A.G. Proye, MD, Department of General & Endocrine Surgery, Hospital C. Huriez, rue Michel Polonovski, 59037 Lille, France. E-mail: c-proye@chru-lille.fr.
REFERENCES
- 1.Carneiro DM, Irvin GL. New point-of-care intraoperative parathyroid hormone assay for intraoperative guidance in parathyroidectomy. World J Surg. 2002;26:1074–1077. [DOI] [PubMed] [Google Scholar]
- 2.Carneiro DM, Irvin GL. Late parathyroid function after successful parathyroidectomy guided by intraoperative hormone assay (QPTH) compared with the standard bilateral neck exploration. Surgery. 2000;128:925–929. [DOI] [PubMed] [Google Scholar]
- 3.Boggs JE, Irvin GL, Carneiro DM, et al. The evolution of parathyroidectomy failures. Surgery. 1999;126:998–1002. [PubMed] [Google Scholar]
- 4.Inabnet WB 3rd, Dakin GF, Haber RS, et al. Targeted parathyroidectomy in the era of intraoperative parathormone monitoring. World J Surg. 2002;26:921–925. [DOI] [PubMed] [Google Scholar]
- 5.Alexander HR Jr, Chen CC, Shawker T, et al. Role of preoperative localization and intraoperative localization maneuvers including intraoperative PTH assay determination for patients with persistent or recurrent hyperparathyroidism. J Bone Miner Res. 2002;17(suppl 2):N133–N140. [PubMed] [Google Scholar]
- 6.Sokoll LJ, Drew H, Udelsman R. Intraoperative parathormone analysis: a study of 200 consecutive cases. Clin Chem. 2000;46:1662–1668. [PubMed] [Google Scholar]
- 7.Johnson LR, Doherty G, Lairmore T, et al. Evaluation of the performance and clinical impact of a rapid intraoperative parathyroid hormone assay in conjunction with pre-operative imaging and concise parathyroidectomy. Clin Chem. 2001;47:919–925. [PubMed] [Google Scholar]
- 8.Vignali E, Picone A, Materazzi G, et al. A quick intraoperative parathyroid hormone assay in the surgical management of patients with primary hyperparathyroidism: a study of 206 consecutive cases. Eur J Endocrinol. 2002;146:783–738. [DOI] [PubMed] [Google Scholar]
- 9.Hallfeldt KK, Trupka A, Gallwas J, et al. Minimally invasive video-assisted parathyroidectomy and intraoperative parathyroid hormone monitoring: the first 36 cases and some pitfalls. Surg Endosc. 2002;16:1759–1763. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal G, Barakate MS, Robinson B, et al. Intraoperative quick parathyroid hormone versus same-day parathyroid hormone testing for minimally invasive parathyroidectomy: a cost-effectiveness study. Surgery. 2001;130:963–970. [DOI] [PubMed] [Google Scholar]
- 11.Mandell DL, Genden EM, Mechanick JI, et al. The influence of intraoperative parathyroid hormone monitoring on the surgical management of hyperparathyroidism. Arch Otolaryngol Head Neck Surg. 2001;127:821–827. [PubMed] [Google Scholar]
- 12.Jaskowiak NT, Sugg SL, Helke J, et al. Pitfalls of intraoperative quick parathyroid hormone monitoring and gamma probe localization in surgery for primary hyperparathyroidism. Arch Surg. 2002;137:659–668. [DOI] [PubMed] [Google Scholar]
- 13.Miura D, Wada N, Arici C, et al. Does intra-operative quick PTH assay improve the results of parathyroidectomy? World J Surg. 2002;26:926. [DOI] [PubMed] [Google Scholar]
- 14.Trupka A, Hallfeldt K, Horn K, et al. Intraoperative monitoring of intact parathyroid hormone (iPTH) in surgery of primary hyperparathyroidism with a new rapid test. Chirurgia. 2001;72:578–583. [DOI] [PubMed] [Google Scholar]
- 15.Hallfeldt K, Trupka A, Gallwas J, et al. Intraoperative monitoring of intact parathyroid hormone during surgery for primary hyperparathyroidism. Zentralbl Chir. 2002;127:448–452. [DOI] [PubMed] [Google Scholar]
- 16.Proye C, Minuto M. Primary hyperparathyroidism: successful parathyroidectomy and persistent elevated intact PTH. Il Giornale Chirurgia. 2000;21:125. [PubMed] [Google Scholar]
- 17.Duh Q-Y, Arnaud CD, Levin KE, et al. Parathyroid hormone: before and after parathyroidectomy. Surgery. 1986;100:1021–1031. [PubMed] [Google Scholar]
- 18.Lundgren E, Rastad J, Ridefelt P, et al. Long-term effects of parathyroid operation on serum calcium and parathyroid hormone valued in sporadic primary hyperparathyroidism. Surgery. 1992;112:1123–1129. [PubMed] [Google Scholar]
- 19.Bergenfelz A, Valdemarsson S, Tibblin S. Persistent elevated serum levels of intact parathyroid hormone after operation for sporadic parathyroid adenoma: evidence of detrimental effects of severe parathyroid disease. Surgery. 1996;119:624–633. [DOI] [PubMed] [Google Scholar]
- 20.Mandal AK, Udelsman R. Secondary hyperparathyroidism is an expected consequence of parathyroidectomy of primary hyperparathyroidism: a prospective study. Surgery. 1998;124:1021–1027. [DOI] [PubMed] [Google Scholar]
- 21.Proye CA, Goropoulos A, Franz C, et al. Usefulness and limits of quick intra-operative measurements of intact (1–84) parathyroid hormone in the surgical management of hyperparathyroidism: sequential measurements in patients with multiglandular disease. Surgery. 1991;110:1035–1042. [PubMed] [Google Scholar]
- 22.Weber CJ, Ritchie JC. Retrospective analysis of sequential changes in serum intact parathyroid hormone levels during conventional parathyroid exploration. Surgery. 1999;126:1139–1143. [DOI] [PubMed] [Google Scholar]
- 23.Gauger PG, Agarwal G, England BG, et al. Intraoperative parathyroid hormone monitoring fails to detect double parathyroid adenomas: a 2-institution experience. Surgery. 2001;130:1005–1010. [DOI] [PubMed] [Google Scholar]
- 24.Libutti SK, Alexander HR, Sampson ML, et al. Kinetic analysis of the rapid intraoperative parathyroid hormone assay in patients during operation for hyperparathyroidism. Surgery. 1999;126:1145–1150. [DOI] [PubMed] [Google Scholar]
- 25.Lowney JK, Weber B, Johnson S, et al. Minimal incision parathyroidectomy: cure cosmesis and cost. World J Surg. 2000;24:1442–1445. [DOI] [PubMed] [Google Scholar]
- 26.Starr FL, De Cresce R, Prinz CA. Use of intraoperative parathyroid hormone measurement does not improve success of bilateral neck exploration for hyperparathyroidism. Arch Surg. 2001;136:536. [DOI] [PubMed] [Google Scholar]
- 27.Denizot A, Pucini M, Chagnaud, et al. Normocalcemia with elevated parathyroid hormone levels after surgical treatment of primary hyperparathyroidism. Am J Surg. 2001;182:15–19. [DOI] [PubMed] [Google Scholar]
- 28.Debruyne F, Delaere P, Vander Poorten V. Postoperative course of serum parathyroid hormone and calcium after surgery for primary hyperparathyroidism. Acta Otorhinolaryngol Belg. 2001;55:153–157. [PubMed] [Google Scholar]
- 29.Debruyne F, Delaere P, Ostyn F, et al. Daily follow up of serum parathyroid hormone and calcium after surgery for primary hyperparathyroidism. J Otolaryngol. 1999;28:305–308. [PubMed] [Google Scholar]





