Abstract
Objective:
To determine whether the use of prophylactic antibiotics is effective in the prevention of postoperative wound infection after Lichtenstein open mesh inguinal hernia repair.
Summary Background Data:
A recent Cochrane meta-analysis (2003) concluded that “antibiotic prophylaxis for elective inguinal hernia repair cannot be firmly recommended or discarded.”
Methods:
Patients with a primary inguinal hernia scheduled for Lichtenstein repair were randomized to a preoperative single dose of 1.5 g intravenous cephalosporin or a placebo. Patients with recurrent hernias, immunosuppressive diseases, or allergies for the given antibiotic were excluded. Infection was defined using the Centers for Disease Control and Prevention criteria.
Results:
We included 1040 patients in the study between November 1998 and May 2003. According to the intention-to-treat principle, 1008 patients were analyzed. There were 8 infections (1.6%) in the antibiotic prophylaxis group and 9 (1.8%) in the placebo group (P = 0.82). There was 1 deep infection in the antibiotic prophylaxis group and 2 in the placebo group (P = 0.57). Statistical analysis showed an absolute risk reduction of 0.19% (95% confidence interval, −1.78%–1.40%) and a number needed to treat of 520 for the total number of infections. For deep infection, the absolute risk reduction is 0.20% (95% confidence interval, −0.87%–0.48%) with a number needed to treat of 508.
Conclusions:
A low percentage (1.7%) of wound infection after Lichtenstein open mesh inguinal (primary) hernia repair was found, and there was no difference between the antibiotic prophylaxis or placebo group. The results show that, in Lichtenstein inguinal primary hernia repair, antibiotic prophylaxis is not indicated in low-risk patients.
In a group of 1040 patients, a low percentage (1.7%) of wound infection after Lichtenstein hernia repair was found, and there was no difference between the antibiotic prophylaxis or placebo group. The results show that, in Lichtenstein inguinal primary hernia repair, antibiotic prophylaxis is not indicated in low-risk patients.
Mesh repair is, in many western countries, rapidly becoming the most popular technique for repair of inguinal hernia.1–6 Of the open mesh repair techniques, the Lichtenstein hernia repair is most frequently used. The Lichtenstein technique is a tension-free repair of the weakened inguinal floor using a polypropylene mesh.7 It is uncertain whether antibiotic prophylaxis is necessary as prevention against postoperative wound infections, which occur in 0% to 9% of inguinal hernia repairs.8 Especially when a foreign body like polypropylene mesh is involved, a deep infection should be prevented. Surgeons at the Lichtenstein Hernia Institute sprinkled bacitracin and polymyxin powder into the wound to prevent infection, but recently this strategy was abandoned.9 Few clinical trials have addressed this issue. One trial showed a significant (10-fold) decrease in wound infections with intravenous antibiotic prophylaxis in mesh repair10; 2 others did not.11,12 A Cochrane meta-analysis13 in 2003 concluded that “antibiotic prophylaxis for elective inguinal hernia repair cannot be firmly recommended or discarded” and “further studies are needed, particularly on the use of mesh repair.”
Since many randomized trials and meta-analyses have shown that mesh repair reduces the risk of hernia recurrence, the prosthetic repair is worldwide accepted as the gold standard in inguinal hernia repair.5,6,14–16 Both in the United States and Europe, more than 1 million inguinal hernia repairs are performed annually16; therefore, any improvement in their treatment could have a large medical and economic impact. Especially a reduction in the number of wound infections would have a great impact. Conversely, discarding the use of antibiotic prophylaxis in inguinal hernia repair could reduce the risks of toxic and allergic side effects, the possible development of bacterial resistance,17 or superinfections and reduced costs.
To assess if systemic antibiotic prophylaxis prevents wound infection in Lichtenstein inguinal hernia repair, a multicenter double-blind placebo controlled randomized trial was performed in the Netherlands.
PATIENTS AND METHODS
Three nonteaching and one teaching general hospital participated in this study. Surgical residents and surgeons in participating hospitals enrolled patients and performed the operations. The ethics committees of all hospitals approved the study and all patients gave informed consent.
Characteristics of the Patients
Patients with a primary uni- or bilateral inguinal hernia and an indication for Lichtenstein hernia repair were eligible for the study. Exclusion criteria were: age under 35, the need for antibiotics for a different reason, immunosuppressive disease (diabetes mellitus, malignancy, HIV) or medication (glucocorticoid therapy), allergy to the given antibiotic, recurrent hernia, or the inability to get an informed consent.
To get insight in a potential selection bias, all eligible patients in one of the 4 hospitals were registered.
Random Assignment to Treatment Groups
The patients were double-blinded randomly assigned to either intravenous placebo or antibiotic prophylaxis. A pharmacist carried out randomization according to a computer-generated list in blocks of 10 patients with stratification for each hospital.
Surgical Technique and Antibiotic Prophylaxis
The operations were performed either by a board certified surgeon or a (supervised) resident. In short, the groin of the patient was shaved just before or in the operating theater. A standard Lichtenstein hernia repair was performed as described by surgeons from the Lichtenstein Hernia Institute.7,9 Two surgeons with a special interest in hernia surgery educated the participating hospitals in the standard technique. A monofilament polypropylene flat mesh (Bard or Autosuture) was sutured in place with monofilament polypropylene suture (Prolene). Anesthesia and skin closure were not standardized.
The trial medication consisted of either 50 mL sterile saline (placebo) or 50 mL sterile saline with 1500 mg Cefuroxim (second generation cephalosporin). Cefuroxim was chosen because of its known activity against the causative pathogens in inguinal wound infection. The half-life time of this antibiotic is 1 to 2 hours; therefore, a single dose supplies therapeutic levels until a few (3–7) hours after wound closure. A pharmacist prepared the trial medication under laminar airflow condition, and it was packed in nontransparent material to exclude optical differences. The anesthesiologist administered the trial medication at the induction of anesthesia. The exact timing of administering was not standardized, thereby copying daily practice.
Data Collection and Follow-up
Data collection was standardized and performed by residents and surgeons in the participating hospitals. The patients were requested to return to the outpatient clinic at 1, 2, and 12 weeks for a standardized history taking and physical examination. In most cases, the surgeon who performed the operation did not perform the follow-up. In case of missing observations, the patients were contacted and a standardized telephone interview was performed.
Endpoints
The primary endpoint of the study was wound infection as defined by the Centers for Disease Control and Prevention criteria.18,19 In this definition, superficial infection occurs within 30 days after operation and involves only skin or subcutaneous tissue; deep infection involves fascial and muscle layers and, when related to an operation where an implant is used, may occur up to 1 year.
Statistical Analysis
The power of the trial (α = 0.05, β = 80%, 2-sided) was based on the assumption that antibiotic prophylaxis reduces the wound infection rate from 4% (average in literature) to 1%. The sample size calculated was 978 patients. Since we expected a dropout of 5%, we randomly allocated 1040 patients.
Data for all patients who were randomly assigned to a treatment group and underwent surgery were primarily analyzed on an intention-to-treat basis. A per-protocol analysis, which excluded patients with major protocol violations, was also performed. The third analysis performed was an as-treated analysis; that is, patients were assigned to a group based on whether they did actually get antibiotics or not. No interim analyses were performed. Continuous normally distributed data are expressed as median with 25% to 75% quartiles. χ2 or Fisher exact test tests were used to compare proportions. Multivariate analysis of various risk factors (when P < 0.10 in univariate analysis) for infection was performed with binary logistic regression analysis. For all analyses, the SPSS package was used. All analyses were made under the guidance of an epidemiologist.
RESULTS
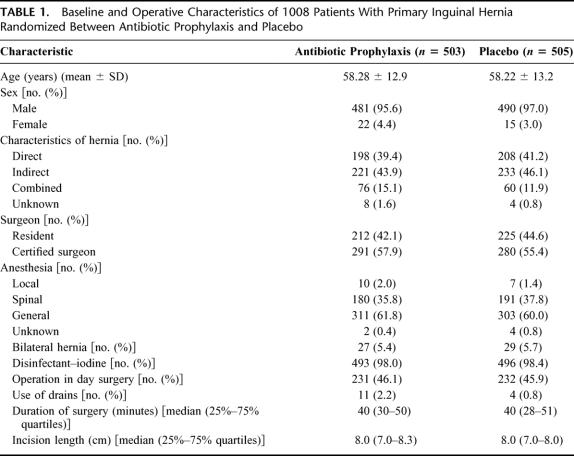
A total of 1040 patients were included in the study between November 1998 and May 2003. Twenty-five patients were not enrolled in the primary analysis: 12 patients withdrew informed consent, and 13 were eventually not operated. Another group of 19 patients with (in our view) an acceptable protocol violation (mainly age below 35 or presumed allergy) was included in the analysis. According to the intention-to-treat principle, 1008 patients were analyzed (Fig. 1). Randomization was successful: there were no significant differences in patient or operation characteristics (Table 1).

FIGURE 1. Trial profile of randomized controlled trial for antibiotic prophylaxis in Lichtenstein inguinal hernia repair. *One death because of operation-related complications.
TABLE 1. Baseline and Operative Characteristics of 1008 Patients With Primary Inguinal Hernia Randomized Between Antibiotic Prophylaxis and Placebo
In one of the 4 participating hospitals (OLVG), all eligible patients were registered. During 3 years of the study period, 625 patients were scheduled for Lichtenstein hernia repair, 483 were eligible, and 363 (75%) were eventually recruited in the study. Not included were 120 patients for the following reasons: 96 (20%) refused to participate and 24 (5%) were not asked to participate. These numbers could be slightly different for other hospitals.
Follow-up was not complete: 199 patients missed their third follow-up (12 weeks). Of this group, 195 (98%) could be contacted by telephone. The 4 patients (0.4%) lost to follow-up and 3 (0.3%) deceased patients had no indication of an occurring wound problem at their last visit to the outpatient clinic but did not contribute to the intention-to-treat analysis.
The number of wound infections was 8 (1.6%) in the antibiotic prophylaxis group and 9 (1.8%) in the placebo group (P = 0.82). There were 3 (0.3%) deep infections: 1 in the antibiotic prophylaxis group and 2 in the placebo group (P = 0.57). Statistical analysis showed an absolute risk reduction of 0.19% (95% confidence interval, −1.78%–1.40%) and a number needed to treat of 520 to prevent one infection. For the deep infection, the absolute risk reduction is 0.20% (95% confidence interval, −0.87%–0.48%) with a number needed to treat of 508 to prevent one infection.
Other postoperative infectious complications showed no significant differences between groups (Table 2). One patient died of pulmonary complications and a bleeding gastric ulcer.
TABLE 2. Postoperative Complications of 1008 Patients After Primary Inguinal Hernia Repair Randomized Between Antibiotic Prophylaxis and Placebo

For the per-protocol (antibiotic prophylaxis, 8 of 475 [1.7%]; and placebo, 8 of 472 [1.7%]) and as treated analysis (antibiotic prophylaxis, 9 of 540 [1.7%] and placebo: 8 of 480 [1.7%]) no significant differences were observed.
In the univariate analysis, sex (female, P < 0.01), bilateral hernia (P = 0.03), and age above 60 years (P = 0.02) were identified as risk factors for infection. Multivariate analysis of these factors together with operation not performed in day surgery (P = 0.06) and operation performed by a resident (P = 0.07) was performed. This analysis reached significance for sex (female, P < 0.01) as an independent risk factor for infection.
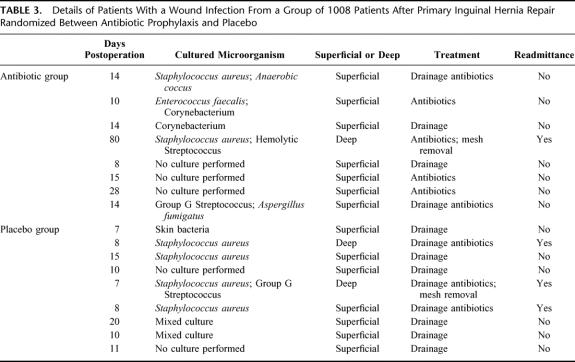
The details of the patients with a postoperative wound infection are displayed in Table 3. All 3 patients with deep wound infections had a culture with Staphylococcus aureus. One patient was treated with intravenous antibiotics and surgical drainage and recovered completely. Two other patients were treated with repeated courses of oral antibiotics and drainage of the wound. A persistent sinus necessitated removal of the mesh in both patients. Although recurrence was no endpoint of this study, we documented 6 (0.6%) recurrences.
TABLE 3. Details of Patients With a Wound Infection From a Group of 1008 Patients After Primary Inguinal Hernia Repair Randomized Between Antibiotic Prophylaxis and Placebo
DISCUSSION
Both in the United States and Europe, more than 1 million inguinal hernia repairs are performed annually.16 The majority of these repairs are nowadays performed using a variety of mesh techniques of which the Lichtenstein “open flat mesh repair” is the most popular.1,3,4,16 Inguinal hernia repair is an elective clean operation, and the postoperative wound infection rate should be very low. Prophylaxis in clean operations has been shown of value in other areas of surgery such as trauma20 and vascular surgery,21,22 but in inguinal hernia repair its benefit remains uncertain.
In this large, randomized, placebo-controlled, double-blind trial analyzing wound infections after Lichtenstein hernia repair, there was no significant difference in the rate of wound infections between groups of patients receiving antibiotic prophylaxis (1.6%) or placebo (1.8%). This study was performed in general practice with a representative mix of general and teaching hospitals and surgical experience. In the Netherlands, there are no specialized hernia centers.
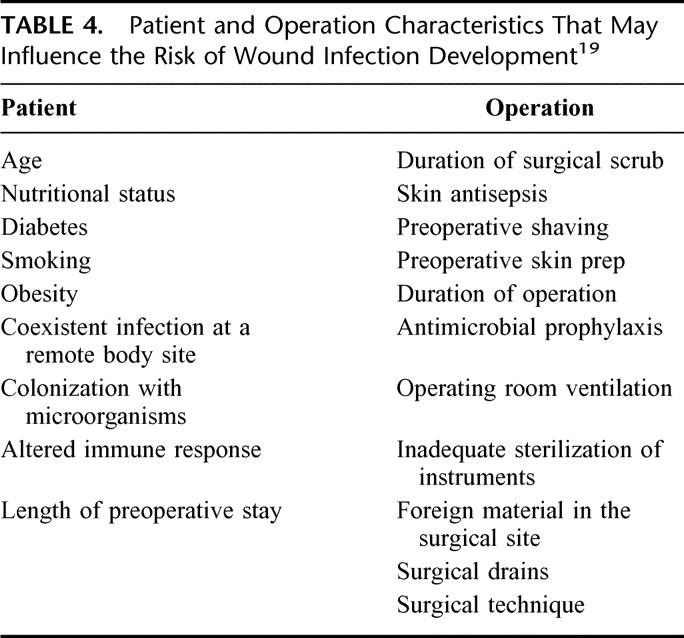
Overall infection rate was low (1.7%) compared with a similar trial of Yerdel et al10 (4.8%). The relatively low incidence of wound infection (1.8%) in our placebo group compared with the 9% in the study of Yerdel et al10 may be explained by patient and operation characteristics. Previous studies suggest that these factors influence the risk of wound infection19 (Table 4). Differences were the duration of operation (1.5 times longer in the Turkish study), more use of drains,23 and repeated aspiration of seromas that could cause secondary infections. In both studies, immunosuppressive disease was an exclusion criterion. In the Yerdel et al study10 of 280 patients, a significant (10-fold) reduction of wound infections (from 9% to 0.7%) was found. The number of deep infections, however, was also low and not significantly different from our study. Unfortunately, the study was prematurely stopped because of the high rate of wound infection. It is likely that the study was underpowered.
TABLE 4. Patient and Operation Characteristics That May Influence the Risk of Wound Infection Development19
A potential drawback of our study is the timing of administration of the antibiotic prophylaxis: 30 minutes before incision is difficult to organize in most hospitals. In theory, the optimal timing of the administration should be so that the bactericidal concentration is maximal in serum and tissues by the time the skin is incised.19,24 We chose a pragmatic approach, adhering to daily practice. Another drawback is the shortcoming of the follow-up at 3 months, since 20% was done by telephone. There might be an observational error, but these patients were told to come back if there was any complaint and they had no sign of infection at previous visits. It is unlikely that patients do not remember an infection and there is evidence that patients are accurate in determining when a wound is not infected.25,26 It can be assumed that especially a deep infection would be remembered; therefore, it is unlikely that this potential bias influences the final results. An explanation for the incomplete follow-up at 12 weeks might be that the study was not officially funded and that follow-up was performed during routine practice.
Since there is no benefit in the use of antibiotic prophylaxis for inguinal hernia repair in low-risk patients, its use is not cost-effective. Because of an unknown impact on bacterial resistance,17 the use of routine antibiotic prophylaxis in primary inguinal hernia repair should be discouraged. The cost benefit for 1 patient is relatively limited (13.54 euro).27 However, because of the large number of inguinal hernia repairs performed in low-risk patients (estimated 70% of all hernias), discarding the use of antibiotic prophylaxis will save around 10 million euro in the United States and Europe.
In contrast, if a wound infection occurs, it has been postulated that there is an increase in the recurrence rate,28,29 but this was in particular when nonmesh techniques were performed.
A major problem occurs when the mesh is infected. Several studies reported late-onset of mesh infection or chronic groin sepsis30,31 eventually leading to complete mesh removal. In this study, 3 deep infections are reported. In all, Staphylococcus aureus was cultured, resulting in mesh removal in 2 patients (0.2%).
In this trial, performed in general practice, a low wound infection rate (1.7%) after Lichtenstein inguinal (primary) hernia repair was found. The results show that, in Lichtenstein inguinal primary hernia repair, antibiotic prophylaxis is not indicated in low-risk patients.
ACKNOWLEDGMENTS
The authors thank the surgeons, residents, and others at Ziekenhuis Amstelveen, Ziekenhuis Hilversum, Onze Lieve Vrouwe Gasthuis and West-Fries Gasthuis for their help with this study and Dr. G.J. Weverling, for statistical support.
Discussions
Dr. Sitges-Serra: The administration of antibiotics in surgical patients is a matter of concern whenever there is no evidence that its benefits outweigh its drawbacks. The latter can be summarized by the “triple E”: ecological impact on the patient's flora, encouraging selection of naturally resistant and mutated microorganisms; adverse effects related to their toxicity and allergenic potential, and, finally, its economic impact, which poses a significant economical burden to our health systems. Antibiotic prophylaxis for Lichtenstein's hernioplasty is a controversial issue.
This multicenter randomized double-blind clinical trial provides hard data against antibiotic prophylaxis in elective inguinal hernia mesh repair. The study was carefully planned and conducted, and I see no major methodological flaws. The inclusion criteria and the sample size imply that the results of this trial can be applied in most settings to most patients undergoing primary inguinal hernia mesh repair.
My criticisms to the study design are that I would have also included patients younger than 35 years. Secondly, I would have stratified patients in low- and high-risk groups. Finally, I would have recorded the body mass index, since, in my view (Badía JM, et al. Br J Surg. 1995;82:479), obesity is one of the major limitations of parenteral antibiotic prophylaxis. The key to the interpretation of Dr. Aufenacker's paper lies in the results his team has achieved in the control group showing that excellent surgical outcomes can be achieved by surgeons operating in appropriate settings with a careful technique. In these circumstances, antibiotic prophylaxis adds nothing and should be discouraged because its potential benefits are outweighed by the “triple E.”
Whether antibiotic prophylaxis may be beneficial when surgical care is suboptimal is still unknown but may be the case. A recent paper on the same topic (Yerdel MA, et al. Ann Surg. 2001;233:26) reports an unacceptable 9% infection rate in the control group, which was reduced to 0.7% by prophylaxis with ampicillin-sulbactam. In low-risk, clean surgery, lasting for less than 1 hour, the prevalence of infection for any control group should not be above 2%, and this can be achieved by careful surgery alone, making the administration of antibiotics unnecessary.
I have 2 questions: after this study, would you please tell us whether there may exist a high-risk group of patients that may benefit from antibiotic prophylaxis? And were the bacteria recovered in the cases of wound infection resistant to prophylactic antibiotics?
Dr. Aufenacker: Thank you, Dr. Sitges-Serra, for your valuable comments and questions.
Regarding the age, stratification in high- and low-risk, and body mass index, looking back I would really like to have included these points myself, as they are all very important. But for the patients who are at risk of a wound infection, the only tool we have is the univariate analysis we performed, which shows that long operation time, double-sided hernia, and age above 60 years were nonindependent risk factors.
So if you have a patient who has a high risk, for example, diabetes, extreme obesity, or HIV, a double-sided hernia, a long operation, you still should give antibiotics. We do not know whether it is necessary because there is no evidence so far, but you can imagine that in these circumstances it might have a function. All bacteria cultured in infected wounds were sensitive to the given prophylaxis.
Dr. O'Connors: I have a question that concerns the informed consent: if I was a prospective participant in the trial and I had been told about the trial study and the 8% infection rate and the placebo, and even more having been told about the Cochrane meta-analysis and that there is a fourfold difference between treatment and placebo, I would not have given consent. Were your patients informed about the context in which this trial was being conducted.
Dr. Aufenacker: When the trial started in 1998, the study results mentioned today were not reported. The informed consent, however, consists of the hypothesis we had for a reduction of 4% toward 1%, but also included the side effects of antibiotics. Twenty percent of the patients did not want to participate, mainly patients with a company of their own, who felt safer with the use of antibiotics. The results of the Yerdel study and the Cochrane analysis were not included in a revised informed consent later.
Dr. Clavien: Congratulations for this excellent randomized study including more than 1000 patients. I also had the opportunity to look at the full paper. I have 4 questions. First, as you had a very low infection rate overall, I wonder whether the study ended up somewhat underpowered. Did you include enough patients to detect a difference? Second, did you standardize the type of mesh used among the different surgeons or centers? Third, it is not clear in the manuscript whether prophylactic antibiotics were consistently administered at the same time in each patient. This could be an important bias as inadequate administration may mask protective effects of antibiotics. Finally, you commented that you had more infections in women. Was the same operation performed in both genders?
Dr. Aufenacker: Thank you very much for your important questions. To start with the last one, the operation was the same in women, and I have no good explanation for the difference. But the data are clear.
With regard to the timing of antibiotics, we did not standardize this because in daily practice there is also a large variation in the timing, at least in the hospitals that participated in the study. Many times, antibiotic prophylaxis was provided when thinking about it. Generally, the patient is already in the operating theatre during the anesthesia introduction, even though we know that it is better to give it half an hour beforehand.
The question about the mesh: there were 2 types of mesh used: a Bard mesh and an Autosuture mesh, and both were monofilament polypropylene flat mesh. It was only the label that was different and not the mesh itself.
Footnotes
Reprints: Maarten Simons, MD, Department of Surgery, Onze Lieve Vrouwe Gasthuis, Eerste Oosterparkstraat 279, 1091 HA Amsterdam, The Netherlands. E-mail: mpsimons@worldonline.nl.
REFERENCES
- 1.Bay-Nielsen M, Kehlet M, Strand L, et al. Quality assessment of 26304 herniorrhaphies in Denmark: a prospective nationwide study. Lancet. 2001;358:1124–1128. [DOI] [PubMed] [Google Scholar]
- 2.Hair A, Duffy K, McLean J, et al. Groin hernia repair in Scotland. Br J Surg. 2000;87:1722–1726. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson E, Haapaniemi S, Gruber G, et al. Methods of repair and risk for reoperation in Swedish hernia surgery from 1992 to 1996. Br J Surg. 1998;85:1686–1691. [DOI] [PubMed] [Google Scholar]
- 4.Nyhus LM, Alani A, O'Dwyer PJ, et al. The problem: how to treat a hernia. In: Schumpelick V, Nyhus LM, eds. Meshes: Benefits and Risks, 1st ed. Berlin: Springer-Verlag, 2004:3–30. [Google Scholar]
- 5.EU Hernia Trialists Collaboration. Mesh compared with non-mesh methods of open groin hernia repair: systematic review of randomized controlled trials. Br J Surg. 2000;87:854–859. [DOI] [PubMed] [Google Scholar]
- 6.EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002;235:322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lichtenstein IL, Shulman AG, Amid PK, et al. The tension-free hernioplasty. Am J Surg. 1989;157:188–193. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson BM. Complications of open groin hernia repair. Surg Clin North Am. 2003;83:1255–1278. [DOI] [PubMed] [Google Scholar]
- 9.Shulman AG. Changes in technique of primary inguinal hernioplasty since 1984. In: The Lichtenstein Hernia Repairs, and How to Do Them Right!, 1st ed. Wagner Design, 1996:49.
- 10.Yerdel MA, Akin EB, Dolalan S, et al. Effect of single-dose prophylactic ampicillin and sulbactam on wound infection after tension-free inguinal hernia repair with polypropylene mesh. Ann Surg. 2001;233:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morales R, Carmona A, Pagán A, et al. Utility of antibiotic prophylaxis in reducing wound infection in inguinal or femoral hernia repair using polypropylene mesh. Cir Esp. 2000;67:51–59. [Google Scholar]
- 12.Gilbert AI, Felton LL. Infection in inguinal hernia repair considering biomaterials and antibiotics. Surg Gynecol Obstet. 1993;177:126–130. [PubMed] [Google Scholar]
- 13.Sanchez-Manuel FJ, Seco-Gil JL. Antibiotic prophylaxis for hernia repair (Cochrane Review). In: The Cochrane Library, Issue 2. Oxford: Update software, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Vrijland WW, van den Tol MP, Luijendijk RW, et al. Randomized clinical trial of non-mesh versus mesh repair of primary inguinal hernia. Br J Surg. 2002;89:293–297. [DOI] [PubMed] [Google Scholar]
- 15.Nordin P, Bartelmess P, Jansson C, et al. Randomized trial of Lichtenstein versus Shouldice hernia repair in general surgical practice. Br J Surg. 2002;89:45–49. [DOI] [PubMed] [Google Scholar]
- 16.Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83:1045–1051. [DOI] [PubMed] [Google Scholar]
- 17.Waldvogel FA, Vaudaux PE, Pittet D, et al. Perioperative antibiotic prophylaxis of wound and foreign body infections: microbial factors affecting efficacy. Rev Infect Dis. 1991;13(suppl):782–789. [DOI] [PubMed] [Google Scholar]
- 18.Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992;20:271–274. [DOI] [PubMed] [Google Scholar]
- 19.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention or surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999;20:247–280. [DOI] [PubMed] [Google Scholar]
- 20.Boxma H, Broekhuizen T, Patka P, et al. Randomised controlled trial of single-dose antibiotic prophylaxis in surgical treatment of closed fractures: the Dutch Trauma Trial. Lancet. 1996;347:1133–1137. [DOI] [PubMed] [Google Scholar]
- 21.Pitt HA, Postier RG, MacGowan AW, et al. Prophylactic antibiotics in vascular surgery: topical, systemic or both? Ann Surg. 1980;192:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DaCosta A, Kirkorian G, Cuccherat M, et al. Antibiotic prophylaxis for permanent pacemaker implantation, a meta-analysis. Circulation. 1998;97:1796–1801. [DOI] [PubMed] [Google Scholar]
- 23.Simchen E, Rozin R, Wax Y. The Israeli study of surgical infection of drains and the risk of wound infection in operation for hernia. Surg Gynecol Obstet. 1990;170:331–337. [PubMed] [Google Scholar]
- 24.Classen DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical wound infection. N Engl J Med. 1992;326:281–286. [DOI] [PubMed] [Google Scholar]
- 25.Whitby M, McLaws M-L, Collopy B, et al. Post-discharge surveillance: can patients reliably diagnose wound infections? J Hosp Infect. 2002;55:154–160. [DOI] [PubMed] [Google Scholar]
- 26.Reilly JS, Baird D, Hill R. The importance of definitions and methods in surgical wound infection audit. J Hosp Infect. 2001;47:64–66. [DOI] [PubMed] [Google Scholar]
- 27.Source: Z-index database of Koninklijke Nederlandse Maatschappij ter bevordering der Pharmacie (KNMP), The Hague, The Netherlands. http://www.knmp.nl [in Dutch]. Accessed December 2003.
- 28.Glassow F. Is postoperative wound infection following simple inguinal herniorrhaphy a predisposing cause for recurrent hernia? Can J Surg. 1964;91:870–871. [PMC free article] [PubMed] [Google Scholar]
- 29.Meyers RN, Shearburn EW. The problem of recurrent inguinal hernia. Surg Clin North Am. 1973;53:555–558. [DOI] [PubMed] [Google Scholar]
- 30.Mann DV, Prout J, Havranek E, et al. Late-onset deep prosthetic infection following mesh repair of inguinal hernia. Am J Surg. 1998;176:12–14. [DOI] [PubMed] [Google Scholar]
- 31.Taylor SG, O'Dwyer PJ. Chronic groin sepsis following tension-free inguinal hernioplasty. Br J Surg. 1999;86:562–565. [DOI] [PubMed] [Google Scholar]