Abstract
Objective:
To define whether laparoscopic gastric banding or laparoscopic Roux-en-Y gastric bypass represents the better approach to treat patients with morbid obesity.
Summary Background Data:
Two techniques, laparoscopic gastric bypass or gastric banding, are currently widely used to treat morbid obesity. Since both procedures offer certain advantages, a strong controversy exists as to which operation should be proposed to these patients. Therefore, data are urgently needed to identify the best therapy.
Methods:
Since randomized trials are most likely not feasible because of the highly different invasiveness and irreversibility of these procedures, a matched-pair design of a large prospectively collected database appears to be the best method. Therefore, we used our prospective database including 678 bariatric procedures performed at our institution since 1995. A total of 103 consecutive patients with laparoscopic gastric bypass were randomly matched to 103 patients with laparoscopic gastric banding according to age, body mass index, and gender.
Results:
Both groups were comparable regarding age, gender, body mass index, excessive weight, fat mass, and comorbidites such as diabetes, heart disease, and hypertension. Feasibility and safety: All gastric banding procedures were performed laparoscopically, and one gastric bypass operation had to be converted to an open procedure. Mean operating time was 145 minutes for gastric banding and 190 minutes for gastric bypass (P < 0.001). Hospital stay was 3.3 days for gastric banding and 8.4 days for gastric bypass. The incidence of early postoperative complications was not significantly different, but late complications were significantly more frequent in the gastric banding group (pouch dilatation). There was no mortality in both groups. Efficiency: Body mass index decreased from 48.0 to 36.8 kg/m2 in the gastric banding group and from 47.8 to 31.9 kg/m2 in the gastric bypass group within 2 years of surgery. These differences became significant from the first postoperative month until the end of the follow-up (24 months). The gastric bypass procedure achieved a significantly better reduction of comorbidities.
Conclusions:
Laparoscopic gastric banding and laparoscopic gastric bypass are feasible and safe. Pouch dilatations after gastric banding are responsible for more late complications compared with the gastric bypass. Laparoscopic gastric bypass offers a significant advantage regarding weight loss and reduction of comorbidities after surgery. Therefore, in our hands, laparoscopic Roux-en-Y gastric bypass appears to be the therapy of choice.
Laparoscopic gastric banding and Roux-en-Y gastric bypass are widely used for the treatment of morbid obesity. Which procedure should be used to treat morbid obesity is unclear. We found that both laparoscopic banding and Roux-en-Y gastric bypass are feasible and safe. However, laparoscopic Roux-en-Y gastric bypass provided better control of weight and reduction of comorbidities than laparoscopic banding.
Obesity surgery is currently one of the most frequent surgical procedures performed in the United States and Europe. The American Society of Bariatric Surgeons mentions 120,000 bariatric procedures for the year 2003, a figure corresponding to a 100% increase within a 2-year period (www.asbs.org). Over the past few years, many surgeons have learned to perform this type of surgery through a laparoscopic rather than an open approach; currently, the laparoscopic gastric banding and the laparoscopic Roux-en-Y gastric bypass are the most widely used bariatric procedures. However, which one of these 2 procedures should be preferred remains unknown. For example, gastric bypass is the procedure of choice in the United States, while most surgeons in Europe favor gastric banding.1,2 This discrepancy indicates that the choice of the procedure is driven by geographic factors and surgeon's skills rather than by medical evidence.3 Only very few centers have a large experience with both laparoscopic procedures; therefore, attempts to compare the 2 procedures have suffered from severe methodologic flaws with inadequate demographic data.4 The discussion is currently limited to the claim from the advocates of either laparoscopic procedure that their choice is valid because of their “good results.” Therefore, a well-designed comparative study is urgently needed.5
Both procedures are associated with advantages and disadvantages. The laparoscopic gastric banding represents the least invasive bariatric procedure with the potential of full reversibility.6 However, increasing experience with laparoscopic gastric banding has shown a high incidence of long-term failures and complications.7 For example, band erosion, band slippage, and esophageal dilatation occur between 15% and 58% of the cases.8–11 In contrast, while more invasive and requiring extra surgical skills, the laparoscopic gastric bypass may offer better weight loss but might be associated with a higher incidence of surgical complications with a longer learning curve.12–14
The ideal study design to identify the best procedure should be a prospective controlled randomized trial. Unfortunately, such study is likewise not feasible because of the important difference in the invasiveness and irreversibility of these procedures. Very few patients would give an informed consent for such a study. The next best strategy to compare these procedures is a matched-pair study design, including a large prospectively collected database. As laparoscopic gastric banding and laparoscopic gastric bypass have become routine at our institution since 1995, and as we have initiated a large database including all patients undergoing a bariatric procedure, we performed match-paired analysis to evaluate the safety, feasibility, and effectiveness of both approaches.
PATIENTS AND METHODS
More than 500 laparoscopic banding procedures and 153 laparoscopic proximal bypass operations were performed by 3 bariatric consultant surgeons at our institution between May 1995 and May 2003. Data for each patient were prospectively collected in our bariatric database. From this database, the first 50 patients with laparoscopic gastric bypass or laparoscopic gastric banding were excluded from the study to avoid a learning curve bias. The consecutive 103 patients who underwent a laparoscopic gastric bypass were randomly matched to 103 patients treated with a gastric banding operation. The patients were matched according to age, sex, and body mass index (BMI). Each patient was routinely thoroughly evaluated by a multidisciplinary team (nutritionist, endocrinologist, psychologist, and surgeon) using a standardized protocol.
The indication for obesity surgery was the same in both groups according to federal regulations.15 Inclusion criteria and indication for bariatric surgery were as follows: BMI > 40 kg/m2 or BMI > 35 kg/m2 with comorbidities, history of obesity more than 5 years, failed conservative treatment of more than 2 years, and age between 18 and 60 years. The choice for the type of surgery was based on the time when the operation was performed. In the first period of the study (from May 1995 until June 2000), we preferentially performed laparoscopic banding procedures as bariatric operation. Based on the growing evidence from the United States demonstrating the feasibility of laparoscopic gastric bypass, we progressively switched to this procedure after June 2000.
Operative Technique
The laparoscopic gastric banding, using a 10-cm Lap Band System (Bioenterics, Santa Barbara, CA), was performed as published before.16 The band was placed in a perigastric position creating a pouch of 15- to 25-mL size. The device was fixed with 4 to 6 gastrogastric stitches to avoid gastric slippage through the band. The reservoir was not filled until the fourth postoperative week.
The bypass procedure was performed like described by Wittgrove and Clark.17 The stomach was transsected creating a pouch of 25-mL size. The jejunum was transsected 50cm distally to the doudenojejunal flexure. A stapled side-to-side jejunojejunostomy was created with a Roux limb length of 150 cm. The Roux limb was positioned antecolic and the gastrojejunostomy was performed using a circular stapler (CEA 25 mm, Tyco, Mansfield, MA).
In the postoperative course, all patients got a contrast study of the esophagus and stomach after 1 day in case of a banding and after 3 days in case of a bypass procedure. Resumption of oral diet was started in absence of a leakage and if a prompt passage was documented by gastrographin follow-through. Patients were discharged as soon as sufficient oral fluid and soft food intake was possible.
Morbidity and mortality were reported up to 30 days after surgery as early complications and thereafter as late complications. The long-term follow-up was reported until the analysis of the data in January 2004. Postoperative assessment was performed after 1, 3, 6, 9, 12, 18, and 24 months. When patients disclosed signs of dysphagia in combination with insufficient weight loss, motility of the esophagus and the size of the gastric pouch were tested. The diagnosis of esophageal dysmotility was based on delayed clearance, regurgitation, dilatation, and pseudoachalasia in video fluoroscopy studies.
Comorbidities
Comorbidites were assessed before surgery and postoperatively up to 2 years or, if not possible, data from the last follow-up were recorded. Patients were considered having diabetes if receiving oral antidiabetic drugs or if the HbA1c was above 7%. Dyslipidemia was diagnosed on the basis of the use of statin or in presence of total cholesterin > 5 mmol/L or triglycerides > 2 mmol/L. Hypertension was defined as patients taking antihypertensive drugs or a systolic pressure > 130 mm Hg and a diastolic pressure > 95 mm Hg.
Statistical Analysis
Data were prospectively collected and stored in a database. Analysis was performed using standard software (SPSS 8.0 for Windows). To compare continuous variables between the 2 groups, the Mann-Whitney-U test was used. Categorical variable were compared using the χ2 test or when appropriate, Fischer′s exact test was applied. Results are expressed as mean ± SD, unless otherwise stated. A P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
Were Both Populations of Patients Comparable?
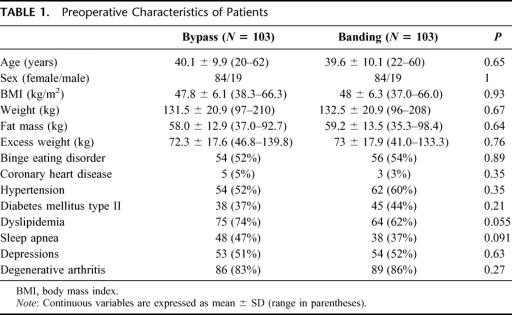
As a consequence to the matching process, the 2 groups were comparable regarding age, sex, and BMI before surgery (Table 1). The mean age for bypass patients was 40.1 years compared with 39.6 years in the banding group, body mass index was 47.8 versus 48.0 kg/m2, respectively. Mean body weight (131.5 vs. 132.5 kg) as well as fat mass (58.0 vs. 59.2 kg) and excessive weight (72.3 kg vs. 73.0 kg) were comparable. Possible factors influencing operative risk and postoperative morbidity were present in both groups with comparable incidence. The metabolic syndrome with hypertension and diabetes mellitus type II occurred with the same frequency in the 2 groups; however, dyslipidemia and sleep apnea syndrome were more frequent in the bypass group but not significantly different (74% vs. 62%, P = 0.055; and 47% vs. 37%, P = 0.09, respectively). The mean follow-up was 17.6 ± 8.3 months for the bypass group and 41.9 ± 21.4 months for the gastric banding group.
TABLE 1. Preoperative Characteristics of Patients
Was the Outcome Within the Groups Influenced by the Timing of Surgery?
Since the period of recruitment for laparoscopic banding procedure was longer than for laparoscopic gastric bypass, we performed a subanalysis of the first 30 versus the last 30 patients in the banding group to test whether homogeneous surgical treatment was offered to the patients. The reduction of weight and incidence of complications were not significantly different at each time point between the 2 subgroups. The excessive weight loss within 24 months was 40.2% in the initial group versus 47.0% in the latter group (P = 0.14). The period of recruitment in the gastric bypass group was only 3 years, providing a uniform surgical treatment.
Was the Laparoscopic Approach Feasible in Both Procedures?
There was one conversion to open surgery in the bypass group because of a staple device malfunction, whereas in the banding group no conversion was necessary (Table 2). The operation time was significantly longer for the bypass procedure than for the gastric banding (190 vs. 145 minutes; P < 0.001). The hospital stay was also significantly longer for the bypass procedure (8.4 vs. 3.3 days; P < 0.001).
TABLE 2. Operation Time, Conversion Rate, and Hospital Stay
Were the Complication Rates and Types of Complication Different Between the Two Procedures?
There was zero mortality in either group. Morbidity was reported as early complications within 30 days after surgery and late complications thereafter. To compare the severity of the complications, rates of reinterventions were reported and divided further to surgical reoperation and endoscopic dilatation.
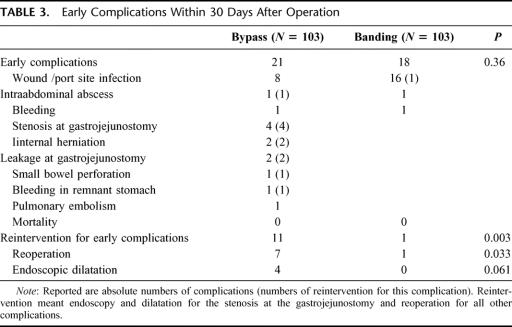
Early Complications
Early complications (Table 3) accounted for 21 cases in the bypass group and 18 in the banding group (P = 0.36). Reinterventions, including endoscopic dilatation, due to early complications were performed in 11 cases in the bypass group and once in the banding group (P = 0.003). Surgical reoperations were indicated in 7 bypass patients and in 1 banding patient (P = 0.033).
TABLE 3. Early Complications Within 30 Days After Operation
Early complications in the bypass group were mainly wound infections at the trocar site where the circular stapler was inserted. These local infections were routinely treated with opening of the wound at bedside without further morbidity. Two patients with an internal hernia and 2 with a leakage at the gastrojejunostomy required an urgent reoperation. One patient had an iatrogenic perforation of the small bowel, which was detected 2 days after bypass surgery requiring reoperation. One patient had a bleeding from the staple line into the remnant stomach and had to be reoperated. One patient with an intra-abdominal abscess formation had a CT-guided drainage. Four patients with an early anastomotic stenosis had a balloon dilatation of the gastrojejunostomy.
Early complications in the banding group were mainly wound infections at the port site, of which one required an early reoperation. One patient had an intra-abdominal abscess formation, which could be treated conservatively with antibiotics as well.
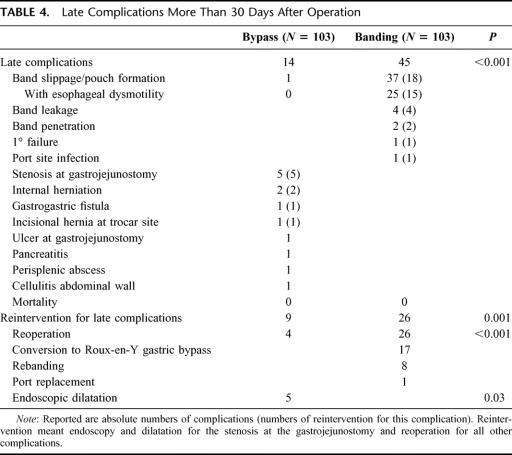
Late Complications
Late complications (Table 4) occurred in 14 patients in the bypass group and in 45 in banding group. Reinterventions were undertaken in 9 bypass patients and 26 banding patients (P = 0.001). All 26 banding patients required surgery, while surgery was necessary in only 4 of 9 patients treated with gastric bypass (P < 0.001).
TABLE 4. Late Complications More Than 30 Days After Operation
Late complications in the bypass group were mainly stenosis at the gastrojejunal anastomosis in 5 cases, which could be treated by endoscopic dilatation. Two patients had an internal herniation, 1 an incisional hernia, and 1 a gastrogastric fistula between the proximal pouch and the remnant stomach. All 4 patients needed a surgical reoperation.
Late complications in the banding group were mainly pouch complications in 37 cases (concentric pouch formation >60 mL with insufficient restriction and weight gain or eccentric pouch formation and slippage with obstruction and need for urgent reoperation), of which 18 underwent a reoperation (rebanding or conversion to bypass). Of the 37 patients with pouch complications, 25 patients presented additional esophageal dysmotility. Seventeen patients with banding were converted to a bypass, whereas no patient with a bypass was switched to another procedure. Four patients had their band replaced due to leakage of the band system, 3 patients due to secondary failure with pouch dilatation and weight gain, and 1 required a band repositioning for primary band failure.
Which Procedure Was Associated With the Better Control of Weight Loss and Better Control of Comorbidities?
Weight loss was significantly different between both groups in favor of the bypass procedure. Already after 1 month and thereafter at every time point of follow-up, 3, 6, 9, 12, 18, and 24 months, BMI values differed significantly between the groups (P < 0.02, range <0.001–0.012). The mean BMI decreased in the bypass group from 47.8 to 31.9 kg/m2, whereas in the banding group the decrease was from 48.0 to 36.8 kg/m2 (Fig. 1). Regarding the excessive weight loss, one could observe similar findings with a significant difference at every time point of follow-up favoring the bypass procedure (Fig. 2). The excessive weight loss after 24 months was 54.0% in the bypass group versus 42.1% in the banding group (P < 0.05, range <0.001–0.043). After 12 months, body composition was routinely measured with an impedance analysis showing a mean fat mass loss of 38.8% for the bypass group versus 26.6% for the banding patients (P < 0.001).
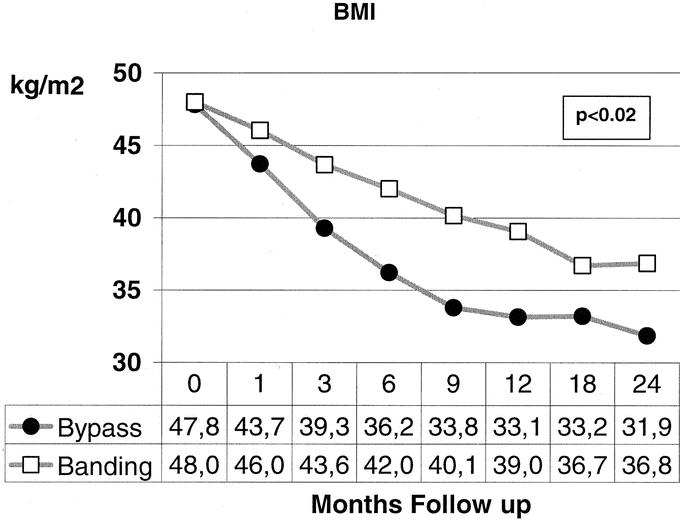
FIGURE 1. The decrease of body mass index (BMI) points was significantly different between the 2 procedures at all time postoperative points during follow-up.
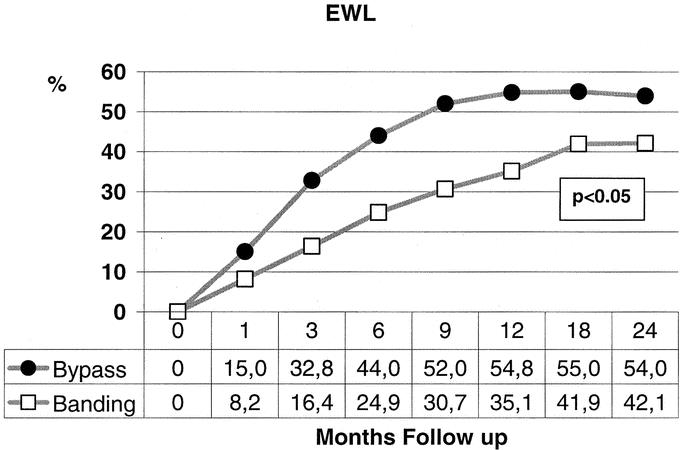
FIGURE 2. The excessive weight loss (EWL) was significantly different during all postoperative time points of follow-up between the 2 procedures.
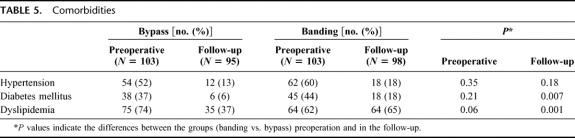
The prevalence of comorbidities, such as arterial hypertension, diabetes mellitus Type II, and dyslipidemia, was comparable in the 2 groups before surgery (Table 5). The frequency of all these comorbidities decreased in the follow-up period, with one exception of dyslipidemia in the banding patients. The prevalence of hypertension dropped from 52% to 13% in the bypass group, and from 60% to 18% in the banding group. Diabetes declined from 37% to 6%, and 44% to 18%, respectively, leading to a significantly lower frequency in the bypass patients (P = 0.007) in the follow-up. Dyslipidemia was as well significantly lower in the bypass group after follow-up, than in the banding group (P = 0.001).
TABLE 5. Comorbidities
DISCUSSION
In this single center study, we found that both gastric bypass and gastric banding are feasible and safe through a laparoscopic approach with a zero mortality. Early complications requiring surgical reoperation were more frequent after laparoscopic gastric bypass. In contrast, pouch dilatations after gastric banding were responsible for most late complications in this series, often requiring late reoperations. In addition, laparoscopic gastric bypass was more effective in controlling weight loss and reduction of comorbidities.
The quality of a study comparing 2 different surgical therapies relies in the similarity of the patient demographics between the groups and the constancy of a surgical team at a high volume center.18 To meet these requirements, we chose a matched-pair methodology in the setting of a single center study in an institution with more than 100 bariatric procedures per year. By matching for age, sex, and BMI, we established a homogeneous patient demographic between the groups with minimal selection bias. The treatment by one single team involving only 3 surgeons additionally provided an equal standard of care throughout the study. To eliminate a potential bias within the banding group caused by the longer period of recruitment, we compared the outcome of early versus lately operated banding patients. We could not find any difference in this subanalysis, which might be explained by the well-standardized technique of this procedure.
To our knowledge, there is only one other large study available comparing laparoscopic gastric banding versus the laparoscopic gastric bypass. In this report, 805 laparoscopic gastric bandings performed in Europe were compared with 456 laparoscopic gastric bypass performed in the United States.19 This analysis suffers from methodologic deficiency mainly because the 2 groups were highly inhomogeneous, leading to inconclusive comparison. There were major differences between both groups as patients were from different parts of the world with different eating habits, genetic profiles, and cultural influence. Patients were also treated by distinct surgeons and physicians from both sides of the Atlantic depending on the procedure performed.
Our data show that both procedures are feasible laparoscopically with a zero mortality rate and a low conversion rate to an open operation. The longer operation time and hospital stay are often quoted as a disadvantage of gastric bypass procedure.20 This observation is also confirmed in our study since operation time and hospital stay were significantly longer for the laparoscopic Roux-en-Y gastric bypass than for the banding procedures. However, laparoscopic banding was associated with higher late complication and failure rates leading to rehospitalization and eventually to conversion to a bypass. In contrast, the laparoscopic gastric bypass procedures were associated with increased early complication rates, often during the first hospital stay. Therefore, the comparison of the primary hospital stay has to be interpreted with caution.
The type of complications was different between the 2 groups. Banding patients suffered from pouch complications and band leakages, which could not occur in the bypass group. Other specific complications, such as internal herniation and anastomotic stenosis, were seen only in the bypass group. Severe complications requiring reoperations occurred early in the gastric bypass group, whereas they occurred late in the banding group. One possible explanation might be that the early complications in the bypass group were typically related to surgical flaws during the operation. This hypothesis is supported by numerous reports about the learning curve of laparoscopic gastric bypass and the reduction of postoperative complications with increasing experience.21,22 In contrast, the higher incidence of late complications and the need for conversion to another procedure in the gastric banding group might point toward the limitation of this purely restrictive procedure in the long-term.23,24
Of note, esophagus dysmotility, as a late complication, was seen only after gastric banding. In severe cases, esophagus dysmotility leads to pseudoachalasia of the esophagus, as we have reported earlier.25 These patients are prone to show lack of satiety and poor compliance with dietary instruction. They can use their lower esophagus as additional space for food, resulting in insufficient weight loss. Esophagus dysmotility led to a high rate of reoperation in our study with secondary conversion from gastric banding to gastric bypass, exposing the patient to an additional operative risk. Conversion to an alternate bariatric procedure was not necessary in the gastric bypass group. This phenomenon is well known from the vertical banded gastroplasty procedure where also conversion to a gastric bypass relieves the symptoms of esophagus decompensation.26 The reason for this complication after gastric banding may be related to the mode of action, since both gastric banding and vertical banded gastroplasty are purely restrictive bariatric procedures. In contrast, the gastric bypass acts through different means, including restrictive malabsorptive and hormonal mechanisms.27
Laparoscopic gastric banding and gastric bypass procedures resulted in a stable weight loss. The gastric bypass procedure was more effective regarding weight loss than the gastric banding operation. However, the absolute amount of weight loss as the most important outcome variable to measure to compare surgical bariatric interventions has been questioned.3 The reduction of comorbidities might be more important to demonstrate long-term success of a bariatric procedure. Therefore, we included comorbidity data in our analysis to compare the effectiveness of the 2 procedures. We found that weight loss in the gastric bypass group paralleled the resolution of comorbidities such as diabetes and dyslipidemia. The gastric banding procedure, however, was less effective in controlling comorbidities. Of note, the dislipidemia was reduced only in the gastric bypass group but remained unchanged in the banding group. This observation might be explained again through the different mode of action as the gastric bypass procedure leads to a moderate malabsorption where increased fat consumption can result in uncomfortable steatorrhea. In contrast, fat consumption in the purely restrictive gastric banding procedure eases the food intake by greasing the passage through the narrow gastric outlet.
CONCLUSION
We demonstrate that both laparoscopic Roux-en-Y gastric bypass and laparoscopic gastric bandings are feasible and safe procedures. The higher incidence of early complications in the gastric bypass group is outweighed by a significantly higher rate of late complications in the banding group. The banding procedure is associated with a higher rate of reoperations requiring eventually conversion to a bypass procedure in many patients. Moreover, laparoscopic gastric bypass offers a significant advantage regarding weight loss and reduction of comorbidities after surgery. Therefore, in our hands, laparoscopic Roux-en-Y gastric bypass is superior to laparoscopic gastric banding.
Discussions
Dr. M. Büchler: I would like to thank the Society to give me the opportunity to comment on this paper. Dr. Weber and your colleagues should be congratulated on an excellent presentation. You have shown that zero mortality is possible to treat such a difficult patient population as morbidly obese patients. I have 3 questions. First, you mentioned that a randomized trial comparing banding versus bypass is not feasible. I would like to challenge this statement and ask you why such a study is not possible. Second, you conclude that the bypass is superior to the banding procedure, although you describe a higher incidence of early reoperations and a longer operation time and hospital stay in the bypass group. This does not look superior to me. My third comment is why not consider a stepwise procedure with the simple operation first (banding) with the use of the bypass approach only as rescue therapy for failed banding. This might be a better strategy considering invasiveness and safety, surgery, and importantly cost.
Dr. M. Weber: Thank you, Professor Büchler, for your comments and insights into the study. I expected your first question since we initially thoroughly considered such a randomized trial. We, as others, did not initiate such study for 2 main reasons. First, we believe that patient acceptance would be very low to accept a randomization between a simple and easily reversible procedure versus extensive surgery. Currently, many patients are very well informed about bariatric procedures, and on an increasing manner chose their surgeon based on their choice or belief of the procedure they wish to have. Second, such a randomized study would be either too early or too late, too early because of an inherent bias linked to the learning curve associated with a complex procedure such as bypass or too late because patients are fully aware of the safety and effectiveness of the bypass in experienced hands. These aspects convinced us that a sound matched study was the best method to address the question on which procedure is best. Regarding your second question, it is correct that we had more early reoperations in the bypass group, but the late and total reoperation rates were much higher in the banding group with the need for conversion to a bypass in a number of cases. Additionally, the risk of redo surgery was not taken into account in our study, which would make the banding procedure even less attractive. Of importance in our conclusion was the observation that weight loss and reduction of comorbidities were significantly better in the bypass group. Finally, a stepwise procedure would put many patients at risk for 2 operations, with the need for a much more difficult bypass procedure in the redo setting after banding.
Dr. M. Büchler: You mentioned a complication rate in the banding group, which seems higher than reported elsewhere. Could this high complication rate in the banding group explain the superior outcome of the bypass procedure?
Dr. M. Weber: Our reported complication rate is well within the limits of other publications on gastric banding. The most common complication in our series was band slippage and pouch formation with 37% and a reoperation rate of 26%; a recent report quoted even a reoperation rate of 58% after a median follow-up of 7 years.
Dr. A.G. Johnson: Did the patients who underwent a bypass procedure as redo surgery after banding enjoy the same favorable weight loss as those primarily receiving a bypass?
Dr. M. Weber: In a paper presented last year in the ESA meeting in Paris, published in the December 2003 issue of Annals of Surgery, we demonstrated that patients with a secondary bypass enjoyed a further average weight loss from BMI 42 to 31 kg/m2, whereas those treated by a re-banding as rescue procedure did not.
Dr. Ch. Russell: My question is whether the 6 patients who continued to have diabetes after a Roux-en-Y bypass were type 1 diabetics or whether other particular characteristics were present in these patients. I also would like to know whether those patients whose lipidemia was reduced were those whose diabetes was markedly improved postoperatively.
Dr. M. Weber: We had only type 2 diabetic patients in this series. The reduction of both diabetes and hyperlipidemia was highly significant only in the bypass group. We did not perform further metabolic studies. However, I would like to speculate that the purely restrictive nature of the banding procedure will not prevent absorption of fatty food which slips easily through the narrow band. In contrast, fatty food leads to steatorrhea after gastric bypass with a moderate malabsorptive effect. This might explain the better effect of the bypass procedure on hyperlipidemia.
Dr. J. Wong: Did this study change your policy to recommend the type of surgical procedure to treat morbid obesity in your patients?
Dr. M. Weber: Yes, as a result of this analysis, we consider the bypass procedure as the treatment of choice. However, there are undoubtedly patients who would best benefit from banding or other bariatric procedures such as the mainly malabsorptive one, including the biliopancreatic diversion or duodenal switch with gastric sleeve resection. Currently, no well-documented selection criteria are available to tailor patients toward the most appropriate bariatric procedure.
Footnotes
Reprints: Markus Weber, MD, Division of Visceral and Transplantation Surgery, University Hospital Zürich, Rämistrasse 100, 8091 Zurich, Switzerland. E-mail: markus.weber@usz.ch.
REFERENCES
- 1.Schirmer BD. Laparoscopic bariatric surgery. Surg Clin North Am. 2000;80:1253–1267. [DOI] [PubMed] [Google Scholar]
- 2.Cottam DR, Mattar SG, Schauer PR. Laparoscopic era of operations for morbid obesity. Arch Surg. 2003;138:367–375. [DOI] [PubMed] [Google Scholar]
- 3.Gentileschi P, Gagner M. The author replies. Evidence-based medicine: open and laparoscopic surgery. Surg Endosc. 2003;17:667. [DOI] [PubMed] [Google Scholar]
- 4.De Maria. Invited commentary to “Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding: a comparative study of 1200 cases”. J Am Coll Surg. 2003;197:545–547. [DOI] [PubMed] [Google Scholar]
- 5.Kellum JM. Gastric banding [Editorial]. Ann Surg. 2003;237:17–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belachew M, Legrand M, Defechereux T, et al. Laparoscopic adjustable silicone gastric banding in the treatment of morbid obesity: a preliminary report. Surg Endosc. 1994;8:1354–1356. [DOI] [PubMed] [Google Scholar]
- 7.Miller K, Hell E. Laparoscopic surgical concepts of morbid obesity. Langenbecks Arch Surg. 2003;388:375–384. [DOI] [PubMed] [Google Scholar]
- 8.Suter M, Bettschard V, Giusti V, et al. A 3-year experience with laparoscopic gastric banding for obesity. Surg Endosc. 2000;14:532–536. [DOI] [PubMed] [Google Scholar]
- 9.Niville E, Dams A. Late pouch dilatation after laparoscopic adjustable gastric and eosophagogastric banding: incidence, treatment and outcome. Obes Surg. 1999;9:381–384. [DOI] [PubMed] [Google Scholar]
- 10.Holeczy P, Novak P, Kralova A. 30% complications with adjustable gastric banding: what did we do wrong? Obes Surg. 2001;11:748–751. [DOI] [PubMed] [Google Scholar]
- 11.Gustavsson S, Westling A. Laparoscopic adjustable gastric banding: complications and side effects responsible for the poor long-term outcome. Semin Laparosc Surg. 2002;9:115–124. [PubMed] [Google Scholar]
- 12.Nguyen N, Rivers R, Wolfe BM. Factors associated with operative outcomes in laparoscopic gastric bypass. J Am Coll Surg. 2003;197:548–557. [DOI] [PubMed] [Google Scholar]
- 13.Podnos YD, Jimenez JC, Wilson SE, et al. Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg. 2003;138:957–961. [DOI] [PubMed] [Google Scholar]
- 14.DeMaria EJ, Sugerman HJ, Meador JG. High failure rate after laparoscopic adjustable silicone gastric banding for treatment of morbid obesity. Ann Surg. 2001;233:809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bundesgesetz über die Krankenversicherung der Schweizerischen Eidgenossenschaft. Anhang 1 des Artikel 1 der Leistungsverordnung. January 1, 2000. Available at: http://www.bsv.admin.ch/blind/aktuell/presse/2001/d/01070902.pdf.
- 16.Kuzmak LI. A review of seven years’ experience with silicone gastric banding. Obes Surg. 1991;1:403–408. [DOI] [PubMed] [Google Scholar]
- 17.Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg. 1994;4:353–357. [DOI] [PubMed] [Google Scholar]
- 18.Courcoulas A, Schuchert M, Gatti G, et al. The relationship of surgeon and hospital volume to outcome after gastric bypass surgery in Pennsylvania: a 3-year summary. Surgery. 2003;134:613–623. [DOI] [PubMed] [Google Scholar]
- 19.Biertho L, Steffen R, Ricklin T, et al. Laparoscopic gastric bypass versus laparoscopic adjustable banding: a comparative study of 1200 cases. J Am Coll Surg. 2003;197:536–547. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen NT, Rivers R, Wolfe BM. Factors associated with operative outcomes in laparoscopic gastric bypass. J Am Coll Surg. 2003;197:548–555 ; discussion 555–557. [DOI] [PubMed]
- 21.Oliak D, Ballantyne H, Weber P, et al. Laparoscopic Roux-en-Y gastric bypass: defining the learning curve. Surg Endosc. 2003;17:405–408. [DOI] [PubMed] [Google Scholar]
- 22.Champion JK, Williams M. Small bowel obstruction and internal hernias after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2003;13:596–600. [DOI] [PubMed] [Google Scholar]
- 23.Weber M, Muller MK, Michel JM, et al. Laparoscopic Roux-en-Y gastric bypass, but not rebanding, should be proposed as rescue procedure for patients with failed laparoscopic gastric banding. Ann Surg. 2003;238:827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suter M. Laparoscopic band repositioning for pouch dilatation/slippage after gastric banding: disappointing results. Obes Surg. 2001;11:507–512. [DOI] [PubMed] [Google Scholar]
- 25.Wiesner W, Hauser M, Schob O, et al. Pseudo-achalasia following laparoscopically placed adjustable gastric banding. Obes Surg. 2001;11:513–518. [DOI] [PubMed] [Google Scholar]
- 26.Balsiger B, Murr MM, Mai J, et al. Gastroesophageal reflux after intact vertical banded gastroplasty: correction by conversion to Roux-en-Y gastric bypass. J Gastrointest Surg. 2000;4:276–281. [DOI] [PubMed] [Google Scholar]
- 27.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. [DOI] [PubMed] [Google Scholar]