Abstract
Background:
The aim of this study was to assess the prognostic significance of nodal microinvolvement as well as the mode of spread in the early phase of lymphatic metastasis in patients with node-negative pancreatic ductal adenocarcinoma.
Methods:
Lymph nodes from 48 node-negative patients with R0 resected pancreatic ductal adenocarcinoma were sampled from 3 different compartments: 1) distal hepatoduodenal ligament, 2) superior-anterior compartment, and 3) posterior-inferior. Tissue sections of 148 lymph nodes classified as tumor free by routine histopathology were examined, using a sensitive immunohistochemical assay with the antiepithelial monoclonal antibody Ber-EP4 for tumor cell detection. With regard to histopathologic tumor staging and grading, 26 (54.2%) of the patients were staged as pT1/pT2, 22 (45.8%) as pT3/pT4, while 31 (64.6%) as G1/G2 and 17 (35.4%) patients as G3.
Of the 148 “tumor free” lymph nodes, 56 contained Ber-EP4–positive tumor cells. These 56 lymph nodes were from 28 of the 48 patients. The multivariate Cox regression analysis revealed the independent prognostic impact of nodal microinvolvement on relapse-free and overall survival.
Analysis by compartment, from which the lymph nodes were collected, revealed that overall survival time (P = 0.006) and time to local recurrence (P = 0.015) depend on the presence of nodal microinvolvement in the superior-anterior compartment.
Conclusions:
The influence of occult tumor cell dissemination in lymph nodes of patients with histologically proven pancreatic ductal adenocarcinoma supports the need for further tumor staging through immunohistochemistry. This could be a helpful tool in proper selection of patients for adjuvant chemotherapy.
The study deals with the incidence and the independent prognostic effect of nodal microinvolvement found in lymph nodes classified as “tumor free” (pN0) by conventional histopathology on relapse-free and overall survival in patients with pancreatic ductal adenocarcinoma. A distinct pattern in the early phase of lymphatic spread is identified.
Pancreatic ductal adenocarcinoma is a very aggressive carcinoma with a poor prognosis. According to its tumor incidence, it is ranked 11th but according to the mortality it is the fifth leading cause of deaths among all cancers.1 In Europe, this carcinoma type is responsible for almost 40,000 deaths per year.2 Although postoperative mortality has declined and rates of complete resection have improved considerably, reported rates of 5-year survival after potentially curative surgery are still below 25%.3,4 The prognosis for patients who undergo pancreatic resection has been shown to be determined by both the pathologic and molecular characteristics of the resected tumor. Up to now, the best pathologic predictors for survival after surgery are stage, grade, and resection margin status.4–10
Early metastatic relapse after complete resection of an apparently localized primary lesion indicates that disseminated tumor cells, undetectable by current methods, may already have been present at the time of surgery. Monoclonal antibodies against tumor-associated antigens or epithelial cell proteins can now be used to detect individual epithelial tumor cells in lymph nodes that are free of metastasis on routine histopathologic examination.11–14 The clinical significance of these immunohistochemical analyses is still controversial,15–23 and these assays have been rarely used in patients with pancreatic carcinoma.24–26 Recently, our group showed that immunohistochemical staining with monoclonal antibody Ber-EP4 is a sensitive and specific method for detecting isolated cells or clusters of cells in lymph nodes from patients with lung, esophageal, or pancreatic carcinomas.12,23,25
In view of the critical role of lymph-node metastasis in such patients,24 we studied the clinical implication of immunohistochemically identifiable tumor cells in lymph nodes of patients with resected pancreatic ductal adenocarcinoma and tumor-free resection margins on microscopical examination of the surgical specimen (R0). Our group of patients was homogeneous (only ductal adenocarcinoma), and none of the patients received adjuvant chemoradiation or chemotherapy. Therefore, this study was designed as a pure prognostic study, and the data collected reflect the prognostic influence of Ber-EP4-positive cells in lymph nodes of patients with pancreatic ductal adenocarcinoma.
MATERIALS AND METHODS
Patients and Study Design
The ethical committee of the chamber of physicians in Hamburg approved this study. Informed consent was obtained from all the patients before their inclusion in the study. Tumor samples and lymph nodes were collected from 487 patients with carcinomas of pancreas and periampullary region between April 1992 and November 2002. Of these, 171 (35.1%) patients presented with carcinomas of the papilla of Vater, 47 (9.7%) with carcinoma of the distal bile duct and 269 (55.2%) with pancreatic carcinoma. In this latter group of patients we had 49 (17.1%) patients with neuroendocrine tumors and 220 (45.2%) patients with pancreatic ductal adenocarcinoma.
Our study population consisted of 48 patients with resectable pancreatic adenocarcinoma, who had undergone radical operation (pancreaticoduodenectomy) between April 1992 and November 2002, had tumor-free resection margins on microscopical examination of the surgical specimen (R0), and were histologically considered free of lymph node metastasis (pN0). Radical pancreatoduodenectomy as proposed by Pedrazzoli et al27 was performed, with only one exception: that lymph nodes on the left side of the superior mesenteric artery (no.14) were not removed. The median number of lymph nodes removed at the operation site was 16 (range, 8–32). Of all lymph nodes removed at the operation, 148 lymph nodes were screened by immunohistochemistry. Tumor stage and grade were classified according to the sixth edition of the tumor–node–metastasis (TNM) classification of the International Union against Cancer28 by investigators unaware of the immunohistochemical findings in the lymph nodes.
The follow-up was either performed by interviewing the patient's general practitioners or in our institution on an outpatient basis. The follow-up evaluations included a physical examination, abdominal ultrasonography, computed tomography of the abdomen, and studies of tumor markers, ie, carcinoembryonic antigen and CA 19–9. Of all 48 patients studied, it was possible to determine the vital status for 44 patients at the end of the time of the study. Four patients were excluded from the survival analysis because they were either censored or died within 90 days after surgery.
Tissue Preparation and Immunohistochemical Analysis
During lymphadenectomy, lymph nodes were systematically sampled from 3 compartments: 1) distal hepatoduodenal ligament, 2) superior-anterior compartment (common hepatic artery), and 3) posterior-inferior compartment (celiac trunk, superior mesenteric artery and interaortocaval lymph nodes). Each of the lymph node samples was divided into 2 parts. One part was embedded in paraffin for routine histopathologic staging and stained with hematoxylin and eosin; the other part and a representative sample of the primary tumor were snap-frozen in liquid nitrogen within 3 hours after their removal and stored at −80°C until use. A total of 148 lymph nodes were sampled from these 48 patients. Lymph nodes from patients without evidence of nodal metastasis on routine histopathologic examination were screened by immunohistochemical analysis with the antiepithelial-cell monoclonal antibody Ber-EP4 (IgG1; Dako, Hamburg, Germany) as described previously.13 Ber-EP4 is an antibody against 2 glycopolypeptides of 34 and 49 kDa on the surface and in the cytoplasm of all epithelial cells (except parietal cells, hepatocytes, and the superficial layers of squamous epithelium). The antibody does not react with mesenchymal tissue, including lymphoid tissue.14,20 Two cryostat sections of 5 to 6 μm thickness were cut at 3 different levels in each node and transferred onto glass slides treated with 3-triethoxysilylpropylamin (Merck, Darmstadt, Germany).
We have found that lymph nodes from 16 control patients with nonepithelial tumors or inflammatory diseases consistently stained negative. Sections of normal colon mucosa served as positive staining controls, and isotype-matched, irrelevant murine monoclonal antibodies served as negative controls (purified immunoglobulin mouse myeloma protein for IgG1; Sigma, Deisenhofen, Germany).
The antibody reaction was developed with the alkaline phosphatase-antialkaline phosphatase technique combined with the new fuchsine stain (Sena, Heidelberg, Germany) for the visualization reaction.21
The slides stained with hematoxylin and eosin and the immunostained slides were evaluated in a blinded fashion by 2 observers working independently (D.B., J.T.K.). For 95% of the slides, the observers’ evaluations were identical; the remaining slides were reevaluated, and consensus decisions were made. Minimal tumor cell involvement in a lymph node that was considered to be tumor-free by routine histopathologic staining was defined as the presence of 1 to 10 Ber-EP4–positive cells in the body of the node.
Statistical Analysis
We used the SPSS for Windows (Copyright SPSS Inc, 1999) for statistical analysis. Associations between categorical variables were assessed by Fisher exact test.
The Kaplan-Meier method29 was used to estimate the occurrence probability of an event, where the events considered were death, relapse, local recurrence, and observation of distant metastasis. Point and interval estimates of the survival rates at 60 months were calculated. For comparison purposes, log-rank tests and exact stratified log-rank tests were performed.
Cox proportional-hazards models30 were fitted for multivariate analysis.
Furthermore, significance statements refer to P values of 2-tailed tests that are less than 0.05. Since this analysis was intended to be explorative, no adjustment for multiple testing was carried out.
RESULTS
The material used in the study relates to 48 patients with R0 resection and histologically node-negative pancreatic ductal adenocarcinoma (Table 1). Median age was 60.5 years (range, 33–83 years), and 26 (54.2%) women and 22 (45.8%) men were included. For the immunohistochemical assay, monoclonal antibody Ber-EP4 was used to screen cryostat sections of all the primary tumors and 148 lymph nodes from the distal hepatoduodenal ligament, superior-anterior and posterior-inferior compartment, obtained from 48 patients. All these nodes were “tumor free” on conventional histopathologic analysis. For comparison purposes, in our study we used a cocktail of 2 anticytokeratin antibodies (AE1/AE3). The results obtained with this second antibody were comparable to those with Ber-EP4 (31 positive with AE1/AE3 vs. 28 with Ber-EP4; data not shown).
TABLE 1. Characteristics of Patients and Tumors
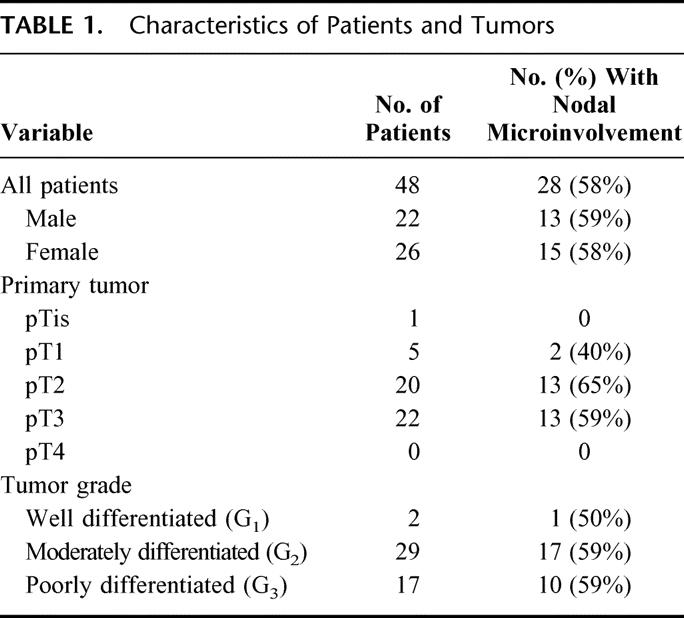
Table 1 shows the characteristics of patients and tumors. In 28 of 48 patients (58.3%), Ber-EP4 positive cells were found in lymph nodes. These single Ber-EP4-positive cells or cell clusters were found in the sinuses, the lymphoid interstitium, or both in 56 (37.8%) of the 148 lymph nodes. The identification of Ber-EP4-positive cells did not depend on tumor stage (pT1/pT2 15 of 26 vs. pT3/pT4 13 of 22; P = 0.51) or tumor grade (G1/G2 18 of 31 vs. G3 10 of 17; P = 0.49).
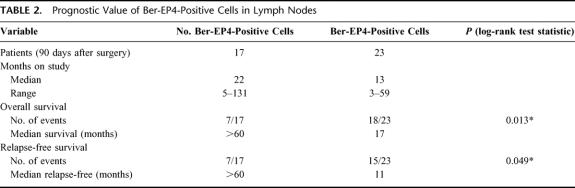
The presence of Ber-EP4-positive tumor cells in lymph nodes was significantly associated with a shorter relapse-free and overall survival time (Table 2). The rate of relapse-free survival at 18 months was 56% in patients without nodal microinvolvement, compared with only 21% in patients with. Furthermore, the probability of a relapse appeared to depend on the presence of nodal microinvolvement (P = 0.049). Similar data were obtained for local recurrence-free survival (P = 0.017) and overall survival (P = 0.013). For distant metastasis-free survival, the differences were of borderline significance (P = 0.067). There were 3 patients with local recurrence, 3 with distant metastasis, and 1 patient with both. However, the median distant metastasis-free survival time of patients without nodal microinvolvement was more than 60 months, compared with only 17 months in patients with nodal microinvolvement (Table 2). Similar median rates for events-free survival were obtained for relapse-free (not yet reached [NYR] 60+ vs. 11), local recurrence-free (NYR 60+ vs. 11), and overall survival time (NYR 60+ vs. 17) (Table 2).
TABLE 2. Prognostic Value of Ber-EP4-Positive Cells in Lymph Nodes
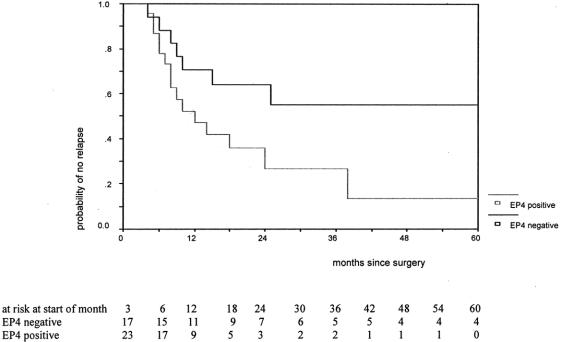
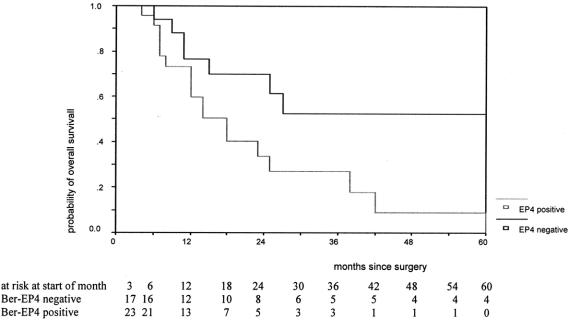
The prognostic influence of Ber-EP4 positive cells in lymph nodes can be gathered from the figures showing the Kaplan-Meyer relapse-free and overall survival curves on time to relapse (P = 0.049; Fig. 1) and overall survival (P = 0.013; Fig. 2). They clearly demonstrate that the probability of 5-year relapse-free and overall survival in patients without nodal microinvolvement was approximately 60%. On the contrary, in patients where no metastasis was found by conventional histopathology but nodal microinvolvement was identified by immunohistochemistry, the 5-year probabilities of relapse-free and overall survival were drastically lower (approximately 10%).
FIGURE 1. Time to relapse by presence of Ber-EP4 cells for patients alive after the end of the third month after surgery (P = 0.049 with log-rank test). EP4 negative, negative for lymph node microinvolvement; EP4 positive, positive for lymph node microinvolvement.
FIGURE 2. Survival function by presence of Ber-EP4 cells for patients alive after the end of the third month after surgery (P = 0.013 with log-rank test). EP4 negative, negative for lymph node microinvolvement; EP4 positive, positive for lymph node microinvolvement.
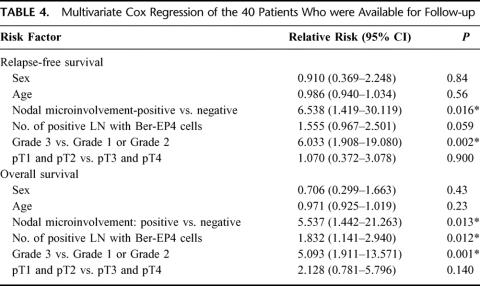
Since the univariate analysis (Table 3) revealed the influence of the nodal microinvolvement, the number of lymph nodes affected with disseminated tumor cells and the histologic grading of the tumor on both clinical end points, we also performed the multivariate Cox regression analysis. This showed that the detection of nodal microinvolvement had a strong independent prognostic importance for disease-free and overall survival (relative risk, 6.53 and 5.53, respectively) (Table 4). As expected, the histopathologic grade (G grading) was also a strong independent prognostic factor for disease-free and overall survival (relative risk, 6.03 and 5.09, respectively) (Table 4). The number of lymph nodes with Ber-EP4-positive cells was an independent prognostic factor for overall (relative risk, 1.83) but was only of borderline significance for relapse-free survival (Table 4). Age, sex, and primary tumor stage had no independent prognostic influence on both clinical end points (Table 4).
TABLE 3. Univariate Influence of Various Factors on the Event's Occurrence After the Third Month
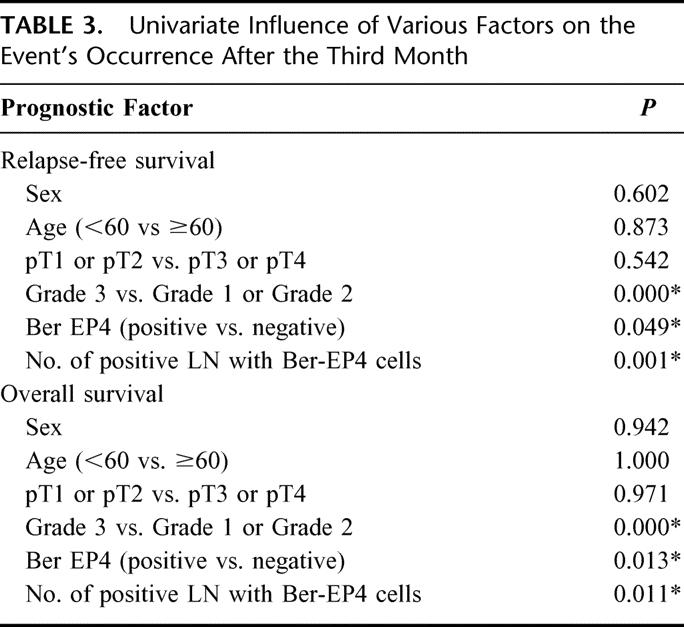
TABLE 4. Multivariate Cox Regression of the 40 Patients Who were Available for Follow-up
The frequency of distribution of nodal microinvolvement in the 3 compartments was not significantly different. The percentages were 41.8% for the distal hepatoduodenal ligament, 42.5% for the superior-anterior compartment, and 37.8% for the posterior-inferior compartment.
The multivariate Cox regression analysis for stratification of affected lymph nodes with nodal microinvolvement in the compartments revealed an independent prognostic effect of the presence of nodal microinvolvement in the superior-anterior compartment (common hepatic artery) on overall survival (relative risk, 2.510; 95% confidence interval, 1.037–6.075; P = 0.041).
Immunostaining showed tumor cells in 1 lymph-node compartment in 19 patients and in 2 or more compartments in 9 patients. This stratification did not yield any significant prognostic information (data not shown).
DISCUSSION
Lymph node metastasis identified by histopathologic examination is the most important prognostic factor in patients with pancreatic ductal adenocarcinoma.3–7 Our study provides evidence that even isolated tumor cells or small clusters of cells, which can be detected in lymph nodes by immunohistochemical analysis, are also independent prognostic factors in pancreatic ductal adenocarcinoma staged as node negative.
Remarkably, 10 of 17 (58.8%) patients who were found to be free of nodal tumor involvement by both histopathologic and immunohistochemical analysis survived the observation period without recurrence. In contrast, only 5 of 23 (21.7%) patients in whom no lymph node metastases were found by conventional histopathology but who had immunostained tumor cells in their lymph nodes had survived the observation time without recurrence.
Residual tumor cells may explain this surprising finding, a potential source of subsequent metastatic relapse. This hypothesis can be confirmed by the fact that prognosis of patients was strongly influenced by the number of lymph nodes affected with disseminated tumor cells. It is also obvious that, compared with patients without nodal microinvolvement, those with such cells in lymph nodes have a shorter median time to tumor relapse, as well as a shorter median overall survival. Therefore, the identification of Ber-EP4-positive tumor cells can serve as highly predictive indicator for tumor relapse.
According to Paget's seed-and-soil hypothesis, the environment into which the cells have been seeded influences the growth of disseminated tumor cells into overt metastasis. The presence of growth factors in the local environment and the capacity of isolated tumor cells to respond to them may determine the pattern of relapse.31
As shown by multivariate Cox regression analysis, the Ber-EP4-positive cells found in “tumor free” pN0 nodes, as well as the histologic grading of the tumors had an independent prognostic effect on mean relapse-free and cancer-related survivals. This supports the need for a refined tumor staging by immunohistochemistry to assess nodal microinvolvement. Surprisingly, the primary tumor stage (pTx)3,6,7 had no independent prognostic effect on outcomes of both end points, probably due to selection of patients (R0 resections32 and pN0 patients), stressing the importance of locally aggressive surgery.
The identification of Ber-EP4-positive cells in the superior-anterior compartment had an independent prognostic value for overall survival rates, while for the relapse-free survival the significance was only of borderline significance (data not shown). Still, this should be taken with caution due to the rather small number of patients and lymph nodes in these groups. Ohta et al33 and Kayahara et al34 proposed the posterior pancreaticoduodenal lymph nodes as the first regional drainage site for primary tumors in the pancreatic head. Our results showed that there is no difference in frequency of nodal microinvolvement in between the compartments.
Recently, our group showed that immunohistochemical analysis with monoclonal antibody Ber-EP4 is a sensitive and specific method for detecting isolated lung, esophageal, or pancreatic cancer cells or clusters of cells in lymph nodes.12,23,25 There are still controversial data in the literature about the value of this antibody. Recently, Rosenberg et al35 used 3 different antibodies (CEA, CK20, and Ber-EP4) for immunohistochemical analysis of lymph nodes in colon carcinoma. In their study, the monoclonal antibody Ber-EP4 had a lower sensitivity and the same specificity as an anticytokeratin 20 antibody. However, the difference in sensitivity was based on only 1 patient. In our study, we performed additional staining with a cocktail of 2 anticytokeratin antibodies (AE1/AE3) and the results were comparable to those obtained with Ber-EP4. We conclude that immunohistochemistry with Ber-EP4 is a well-established standard for detection of nodal microinvolvement. In addition, CEA is also expressed in normal lymph nodes36 and cytokeratin 20 can be expressed in normal leukocytes.37 Furthermore, the results from our study provide evidence that examination with Ber-EP4 is sufficient.
A major issue that has to be addressed is the sampling error, which might be influenced by the number of lymph nodes assessed by immunohistochemistry, the number of lymph node sections, and the level of these sections within the lymph node. In our present study, 6 sections were cut from 3 different levels of each lymph node. Analyzing more than 3 sections, however, would not be routinely feasible, and the positive correlation between the result of our assay and clinical outcome indicates that examining 3 lymph node levels is sufficient.
Our group of patients was homogeneous (only ductal adenocarcinoma and R0 resections), and no patient had received adjuvant chemoradiation or chemotherapy. Therefore, this study was designed as a pure prognostic study, and the data collected reflect the prognostic influence of Ber-EP4-positive cells in lymph nodes. Our data indicate that immunohistochemical assessment of lymph nodes can be used to refine the staging system for pancreatic ductal adenocarcinoma and might help us to identify patients who will not be cured by surgery alone. Our results support the need for routine use of this method to refine the staging of the patients with pancreatic ductal adenocarcinoma.
The value of adjuvant chemotherapy in patients with small primary tumors who are thought to have no nodal involvement is unknown, but patients with a minimal amount of residual tumor may respond better to such therapy than patients with more advanced disease. In contrast, patients who were found to be free of nodal tumor involvement by both histopathologic and immunohistochemical analysis had an excellent prognosis (5-year survival of almost 60% and median survival of more than 60 months) and might be spared of adjuvant chemoradiation and/or chemotherapy, which has severe side effects, as shown by the study of the ESPAC1.38 Still, this needs to be investigated in future trials using immunohistochemical assays for stratification of treated and untreated patients.
CONCLUSION
The prognostic influence of occult tumor cell dissemination in lymph nodes of patients with histologically confirmed pancreatic ductal adenocarcinoma supports the need for a refined tumor staging through immunohistochemistry. This refined detection could be a helpful tool in appropriate selection of patients for adjuvant therapy in future clinical trials.38
ACKNOWLEDGMENTS
The authors thank Michael Bubenheim, PhD, from the Institute of Medical Biometry and Epidemiology–University Medical Centre of Hamburg-Eppendorf, for his help in the statistical analysis.
Discussions
Dr. Obertop: Thank you that I may discuss this interesting paper, and I thank the group of Dr. Jakob Izbicki for sending me the manuscript in advance.
The occurrence of micrometastases in lymph nodes has been shown by this group in a number of tumors using this anti-epithelial monoclonal antibody as a marker. And now the prognostic significance of lymph node micrometastases has been clearly shown in patients after R0 resection for pancreatic cancer with negative (N0) nodes on routine histology.
My question deals with the type of resection and extent of lymph node dissection that is done. You could have used the terms standard, radical, and extended radical as proposed by Pedrazolli et al in 1999 (Dig Surg) using the classification of the Japanese Pancreatic Society. You used your own terms of 3 lymph node compartments: 1) hepatoduodenal ligament; 2) superior (hepatic artery);) posterior compartment.
In my opinion, compartment 1 and 2 are the same (all 12 nodes). Can you explain whether, and if so, what kind of lymph node dissection you performed? How come the yield was so low (only 3 nodes/patient or 1 node/station). And how this, in my opinion artificial compartment hepatic artery, can be prognostic as sentinel node.
Dr. Izbicki: Thank you, Dr. Obertop, for the insightful comments and questions. Let me address first the type of dissection we performed. Radical pancreatoduodenectomy as proposed by Pedrazzoli et al was performed, with the exception that neither the lymph nodes on the left side of the superior mesenteric artery were removed (LN 14) nor the lymph nodes around the inferior mesenteric artery. The median number of lymph nodes removed at the operation site was 16 with a range of 8 to 32.
We used this immunohistochemical assay as a screening method; therefore, we sampled 1 lymph node per compartment from the compartments already mentioned. Our compartment 1 (distal hepatoduodenal ligament) includes LNs 12a2, 12p2, and 12b2 as proposed by the classification of the Japanese Pancreatic Society, compartment 2 (superior-anterior) includes LNs 8a, 8p, 17a, and 17b, while compartment 3 (inferior-posterior) includes LNs 9, 14a, 14b, 14c, 14v, 15, 16a1, and 16a2. Therefore, compartments 1 and 2 are not the same!
Dr. Howard: The authors are to be congratulated on their demonstration of lymphatic micrometastases in phase pN0, pancreatic cancer, generally considered as early stage. The almost inevitable recurrence of the cancer following resection of this “early phase” tumor clearly supports their findings. At the time of resection, micrometastases are almost always present beyond the lines of resection. Is there a place for intralymphatic chemotherapy? Although the anatomy of the lymphatic draining the area of the pancreas is complex and perhaps incompletely defined, might intralymphatic administration of chemotherapeutic agents be of conceivable value? Pilot studies, involving intralymphatic injection at the foot and collection of lymph from the thoracic duct in the neck, indicate that small molecular weight substances leak out of the lymphatics between these 2 points, but a substance of larger molecular weight, such as albumin, will largely transit the lymphatics between the foot and the neck. Would the anatomic complexity of the lymphatics in the retroperitoneal area contraindicate an experimental approach to such infusion of a chemotherapeutic agent?
Dr. Izbicki: Thank you, Dr. Howard, for the thoughtful comment. We do not have our own data on the intralymphatic administration of chemotherapeutic agents. However, this is an interesting approach you address to tackle the problem of nodal microinvolvement.
Dr. Jeekel: The title suggests that you investigated the mode of spread, but it does not seem that you did. The level of positive nodes did not correlate with recurrence. Do you not think that you need larger groups to prove this? Does it mean that there is not a sentinel node mechanism, and is there no level where you have an indication for a palliative nonoperative approach?
Dr. Izbicki: What we have examined was the mode of early lymphatic spread in the 3 compartments. We completely agree that nodal microinvolvement was an indicator of generalization of the disease and not a predictor of local recurrence. This has also been shown in other tumor entities, eg, esophageal carcinoma.
In our opinion, it is too early to conclude that identification of nodal microinvolvement in some of the compartments is a criterion for denying a patient a potentially curative operation. Up to now, the data from studies dealing with sentinel lymph node metastasis in pancreatic carcinoma are controversial. Even if such a mechanism exists, from the surgical point of view, we would face difficulties in discriminating patients without nodal involvement on frozen sections (pN0) but with microinvolvement from those pN0 patients with microinvolvement.
Dr. Pedrazolli: The presence of minimal residual disease (MRD) in lymph nodes may correspond to an elevated number of neoplastic cells scattered within the body. Did you try to correlate the presence of MRD in lymph nodes with the postoperative levels of CA 19–9? The site where it is easier to find the possible MRD is the bone marrow. Did you try to correlate the presence of MRD in lymph nodes with the presence of tumor cell in bone marrow?
Dr. Izbicki: Thank you, Dr. Pedrazzoli, for these interesting questions. Unfortunately, we did not correlate the presence of nodal microinvolvement with the postoperative levels of CA19-9. This should be part of the upcoming study in which we would also present the results we have for correlation of the nodal microinvolvement and the affection of bone marrow.
Dr. Eggermont: Congratulations on a nice study. I have 2 questions. First, it is not surprising that expanding the workup of lymph nodes by immunohistochemistry, in this case Ber-Ep-4, will increase the detection of micrometastases. Was Ber-Ep-4 the only marker you worked with or did your pathologist compare its sensitivity to other markers? I ask this since in a recent similar study in colon carcinoma Ber-Ep-4 was also evaluated, and compared to 2 other markers, each of which outperformed BE-Ep-4.
Second, lymph node metastases are indicators of prognosis rather than governors of prognosis and reflect in general widespread micrometastatic disease. I am therefore sceptical about the prognostic value of 1 lymph node compartment versus the other. Did you stratify in your analysis for the different compartments and did you correct the P value requirements for multiple comparisons?
Dr. Izbicki: Thank you, Dr. Eggermont, for your valuable comments and questions. I completely agree with you that it is not a surprise to detect micrometastases using immunohistochemistry. As already shown by our former studies, the monoclonal antibody Ber-EP4 that we used is well established for detection of nodal microinvolvement. Nevertheless, in our present study for comparison, we also used a cocktail of 2 monoclonal anti-cytokeratin antibodies (AE1/AE3) and the results were comparable to those obtained with Ber-EP4.
I completely agree that lymph node metastases are indicators of generalization of the disease. The finding that the superior-anterior compartment influences the overall survival was surprising for us too, and we also think that this needs to be confirmed in future studies, maybe in a multicentric study.
At the end, let me address the statistics. Since the statistical analysis was intended to be explorative and not confirmative, no adjustment for multiple testing had to be carried out.
Footnotes
Supported in part by the “Werner Otto Stiftung” and “Hamburger Krebsgesellschaft e. V.”
Reprints: Jakob R. Izbicki, MD, FACS, Department of General, Visceral and Thoracic Surgery, University Medical Centre of Hamburg-Eppendorf, Martinistraβe 52, 20246 Hamburg, Germany. E-mail: izbicki@uke.uni-hamburg.de.
REFERENCES
- 1.Greenlee RT, Hill-Harmon M, Muzzay T, et al. Cancer statistics. CA Cancer J Clin. 2001;51:15–36. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Muir CS, Whelan SL, et al. Cancer Incidence in Five Continents, vol. 6 [IARC Scientific Publications No. 120]. Lyon, France: International Agency for Research on Cancer, 1992. [Google Scholar]
- 3.Brik D, Fortnagel G, Formentini A, et al. Small carcinoma of the pancreas: factors of prognostic relevance. J Hepatobiliary Pancreat Surg. 1998;23:234–240. [DOI] [PubMed] [Google Scholar]
- 4.Mosca F, Giulianotti PC, Balestracci T, et al. Long-term survival in pancreatic cancer: pylorus-preserving vs. Whipple pancreatoduodenectomy. Surgery. 1997;122:553–566. [DOI] [PubMed] [Google Scholar]
- 5.Trede M, Schwall G, Saeger H-D, et al. Survival after pancreatoduodenectomy: 118 consecutive resections without an operative mortality. Ann Surg. 1990;211:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geer RJ, Brennan MF. Prognostic indicators of survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. [DOI] [PubMed] [Google Scholar]
- 7.Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas: is it really improving? Ann Surg. 1995;221:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo C, Abrams R, Grochow L, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma. Postoperative adjuvant chemoradiation improves survival: a prospective, single institution experience. Ann Surg. 1997;225:621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allema JH, Reinders ME, Vangulik TM, et al. Prognostic factors for survival after pancreaticoduodenectomy for patients with carcinoma of the pancreatic head region. Cancer. 1995;75:2069–2076. [DOI] [PubMed] [Google Scholar]
- 10.Allison DC, Piantadosi S, Hruban RH, et al. DNA content and other factors associated with ten-year survival after resection of pancreatic carcinoma. J Surg Oncol. 1998;67:151–159. [DOI] [PubMed] [Google Scholar]
- 11.Byrne J, Waldron R, McAvinchey D, et al. The use of monoclonal antibodies for the histopathological detection of mammary axillary micrometastasis. Eur J Surg Oncol. 1987;13:409–411. [PubMed] [Google Scholar]
- 12.Passlick B, Izbicki JR, Kubuschok B, et al. Immunohistochemical assessment of individual tumor cells in lymph nodes of patients with nonsmall-cell lung cancer. J Clin Oncol. 1994;12:1827–1832. [DOI] [PubMed] [Google Scholar]
- 13.Raymond WA, Leong AL. Immunoperoxidase staining in the detection of lymph node metastasis in stage I breast cancer. Pathology. 1989;21:11–15. [DOI] [PubMed] [Google Scholar]
- 14.Latza U, Niedobitek G, Schwarting R, et al. Ber-EP4: new monoclonal antibody which distinguishes epithelia from mesothelia. J Clin Pathol. 1990;43:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bussolati G, Gugliotta P, Morra I, et al. The immunohistochemical detection of lymph node metastasis from infiltrating lobular carcinoma of the breast. Br J Cancer. 1986;54:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen ZL, Perez S, Holmes CE, et al. Frequency and distribution of occult micrometastasis in lymph nodes of patients with non-small-cell lung carcinoma. J Natl Cancer Inst. 1993;85:493–498. [DOI] [PubMed] [Google Scholar]
- 17.de Mascarel I, Bonichon F, Coindre JM, et al. Prognostic significance of breast cancer axillary lymph node micro-metastasis assessed by two special techniques: reevaluation with longer follow-up. Br J Cancer. 1992;66:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martini N, Flehinger BJ, Zaman MB, et al. Results of resection in non-oat cell carcinoma of the lung with mediastinal lymph node metastasis. Ann Surg. 1983;198:386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casson AG, Rusch VW, Ginsberg RJ, et al. Lymph node mapping of esophageal cancer. Ann Thorac Surg. 1994;58:1569–1570. [DOI] [PubMed] [Google Scholar]
- 20.Momburg F, Moldenhauer G, Hammerling GJ, et al. Immunohistochemical study of the expression of a Mr 34,000 human epithelium-specific surface glycoprotein in normal and malignant tissues. Cancer Res. 1987;47:2883–2891. [PubMed] [Google Scholar]
- 21.Pantel K, Schlimok G, Angstwurm M, et al. Methodological analysis of immunocytochemical screening for disseminated epithelial tumor cells in bone marrow. J Hematother. 1994;3:165–173. [DOI] [PubMed] [Google Scholar]
- 22.Kasper M, Stosiek P, Typlt H, et al. Histological evaluation of three new monoclonal anti-cytokeratin antibodies. 1. Normal tissues. Eur J Cancer Clin Oncol. 1987;23:137–147. [DOI] [PubMed] [Google Scholar]
- 23.Izbicki JR, Hosch SB, Pantel K, et al. Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med. 1997;337:1188–1194. [DOI] [PubMed] [Google Scholar]
- 24.Kanemitsu K, Hiraoka T, Tsuji T, et al. Implication of micrometastasis of lymph nodes in patients with extended operation for pancreatic cancer. Pancreas. 2003;26:315–321. [DOI] [PubMed] [Google Scholar]
- 25.Hosch SB, Knoefel WT, Mety S, et al. Early lymphatic tumor cell dissemination in pancreatic cancer: frequency and prognostic significance. Pancreas. 1997;15:154–159. [DOI] [PubMed] [Google Scholar]
- 26.Vogel I, Kruger U, Marxsen J, et al. Disseminated tumor cells in pancreatic cancer patients detected by immunocytology: a new prognostic factor. Clin Cancer Res. 1999;5:593–595. [PubMed] [Google Scholar]
- 27.Pedrazzoli S, Beger HG, Obertop H, et al. A surgical and pathological based classification of resective treatment of pancreatic cancer. Dig Surg. 1999;16:337–345. [DOI] [PubMed] [Google Scholar]
- 28.Hermanek P, Sobin LH. TNM Classification of Malignant Tumours, 4th ed. New York: Springer, 1992. [Google Scholar]
- 29.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. New York: Springer, 1997. [Google Scholar]
- 30.Cox DR. Regression models and life tables [with discussion]. J R Statist Soc. 1972;34:187–220. [Google Scholar]
- 31.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1989;1:571–573. [PubMed] [Google Scholar]
- 32.Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohta T, Kitagawa H, Kayahara M, et al. Sentinel lymph node navigation surgery for pancreatic head cancers. Oncol Report. 2003;10:315–319. [PubMed] [Google Scholar]
- 34.Kayahara M, Nagakawa T, Ohta T, et al. Analysis of paraaortic lymph node involvement in pancreatic carcinoma: a significant indication for surgery? Cancer. 1999;85:583–590. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg R, Friederichs J, Gertler R, et al. Prognostic evaluation and review of immunohistochemically detected disseminated tumor cells in peritumoral lymph nodes of patients with pN0 colorectal cancer. Int J Colorectal Dis. 2004;19:430–437. [DOI] [PubMed] [Google Scholar]
- 36.Bostik PJ, Chatterjee S, Chi DD, et al. Limitations of specific reverse-transcriptase polymerase chain reaction markers in detection of metastasis in the lymph nodes and blood of breast cancer patients. J Clin Oncol. 1998;16:2632–2640. [DOI] [PubMed] [Google Scholar]
- 37.Jung R, Petersen K, Kruger W, et al. Detection of micrometastasis by cytokeratin 20 RT-PCR is limited due to stable background transcription in granulocytes. Br J Cancer. 1999;81:870–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotheraphy and chemotherapy in resectable pancreatic carcinoma: a randomised controlled trial. Lancet. 2001;358:1576–1585. [DOI] [PubMed] [Google Scholar]