Abstract
Objective:
Orthotopic liver transplantation (OLT) has become an established procedure for the treatment of pediatric patients with end-stage liver disease. Since starting our program in 1989, 422 pediatric OLTs have been performed using all techniques presently available. Analyzing our series, we have concluded that the year of transplantation is the most important prognostic factor in patient and graft survival in a multivariate analysis.
Methods:
From April 2001 to December 1, 2003, 18 whole organs (14%), 17 reduced-size organs (13%), 53 split organs (42%; 46 ex situ, 7 in situ), and 44 organs from living donors (33%) were transplanted into 115 patients (62 male and 53 female). One hundred twelve were primary liver transplants, 18 were retransplants, one third and one fourth liver transplants. Of the 132 OLTs, 26 were highly urgent (19.7%). The outcome of these 132 OLTs was retrospectively analyzed.
Results:
Of 132 consecutive pediatric liver transplants, no patients died within the 6 months posttransplantation. Overall, 3 recipients (2%) died during further follow-up, 1 child because of severe pneumonia 13 months after transplantation and the second recipient with unknown cause 7 months postoperatively, both with good functioning grafts after uneventful transplantation. The third had a recurrence of an unknown liver disease 9 months after transplantation. The 3-month and actual graft survival rates are 92% and 86%, respectively. Sixteen children (12%) had to undergo retransplantation, the causes of which were chronic rejection (3.8%), primary nonfunction (3.8%), primary poor function (PPF; 1.5%), and arterial thrombosis (3%). The biliary complication rate was 6%; arterial complications occurred in 8.3%; intestinal perforation was observed in 3%; and in 5%, postoperative bleeding required reoperation. The portal vein complication rate was 2%.
Conclusions:
Progress during the past 15 years has enabled us to perform pediatric liver transplantation with near perfect patient survival. Advances in posttransplant care of the recipients, technical refinements, standardization of surgery and monitoring, and adequate choice of the donor organ and transplantation technique enable these results, which mark a turning point at which immediate survival after transplantation will be considered the norm. The long-term treatment of the transplanted patient, with the aim of avoiding late graft loss and achieving optimal quality of life, will become the center of debate.
In 132 consecutive pediatric liver transplants using all available technical variants, no patients had died at 6 months posttransplantation. Three-month and actual graft survival rates are 92% and 86%, respectively. Surgically, pediatric liver transplantation is approaching perfection. Maintaining graft survival and quality of life are the challenges of the future.
Since the first successful liver transplant performed by T. Starzl in 1967, liver transplantation has evolved to become the treatment of choice for pediatric and adult patients with end-stage liver disease.1 Early short series of pediatric liver transplantation described patient survival rates of between 11% and 39%.1–6 The introduction of cyclosporine in 1979 by Calne et al7 led to a significant increase in patient survival to 74%.1,3,8 During the 1980s, better patient selection and the introduction of segmental liver grafts led to a further increase in patient and graft survival after transplantation.9–11 The procedure had changed from an heroic treatment modality in which simple patient survival could be considered a success to a standardized procedure in which most patients could survive. In the last 10 years, further developments have taken place in the field of pediatric liver transplantation, eg, improvements in immunosuppression and postoperative intensive care management, technical refinements, routine intraoperative Doppler ultrasound, and better patient selection, thus improving the early patient outcome in large pediatric transplant centers to more than 90%.12
A pediatric liver transplant program has been operational at the University of Hamburg since June 1989. Until December 2003, 422 pediatric liver transplants were performed in 356 recipients using all available technical variant grafts. As a result of the introduction of living related liver transplantation and split liver transplantation (SLT) in 1991 and 1993, respectively, we were able to reduce the waiting list mortality for pediatric recipients in recent years to less than 5%.
The start of the pediatric liver transplant program at Hamburg University was characterized by patient and graft survival rates between 50% and 70% and reoperation rates of more than 60%. Consequent improvements in surgical technique, patient selection, and pretransplant and posttransplant care led to an ongoing improvement in patient and graft survival.
The present study analyzes our last 132 consecutive pediatric liver transplants, in which no in-hospital death occurred, while applying all available techniques in pediatric liver transplantation, including retransplantations, to determine the most likely explanation for this encouraging improvement in outcome and to find future perspectives of improvements in pediatric liver transplantation.
PATIENTS AND METHODS
From 1984 to 2003, 1123 liver transplants were performed at the University Hospital Hamburg-Eppendorf. Of these 1123 procedures, 422 (38%) were in pediatric recipients (<16 years). In March 2001, we experienced the last in-hospital death after pediatric liver transplantation. Since then, all pediatric recipients of liver grafts, including retransplantations and high urgent transplants (UNOS I), were discharged from the hospital and survived the first 6 months after transplantation.
From April 2001 to December 1, 2003, 18 whole organs (14%), 17 reduced-size organs (13%), 53 split organs (42%; 46 ex situ, 7 in situ), and 44 organs from living donors (33%) were transplanted into 115 patients (62 male and 53 female). One hundred twelve were primary liver transplants, 18 first retransplants, one third and one fourth liver transplants. Of the 132 orthotopic liver transplantation (OLTs), 26 were UNOS I (19.7%). The outcome of these 132 consecutive pediatric liver transplants was retrospectively analyzed.
Postoperative Monitoring
In the early postoperative period, detailed analysis of laboratory data was performed: complete blood count (every 12 hours), arterial blood gases and electrolytes (every 12 hours), bilirubin in serum and drainage fluids (every 8 hours), creatinine, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, Factor V, γ-glutamyl transpeptidase, serum quick, PPT, antithrombin III, and albumin (every 8 hours). Microbiological diagnostic procedures were performed daily. CMV and EBV status (serology and PCR) were performed weekly.
Central venous pressure and arterial blood pressure were monitored continuously in the first postoperative days, and fluid monitoring was performed every 2 hours. Central venous pressure was maintained between 4 and 6 cm H2O and hemoglobin levels between 7 and 10 mg/dL. All children were examined intraoperatively immediately after reperfusion and after closure of the abdominal wall and postoperatively, twice daily, by using hepatic Duplex ultrasound.
Medication
Ampicillin (200 mg/kg/d), cefotaxime (100 mg/kg/d), and metronidazole (20 mg/kg/d) were administered for 5 days as prophylactic antibiotic therapy. All children received heparin (100–300 IE/kg/d) postoperatively to achieve a PTT between 50 and 70 seconds and were switched to aspirin (5 mg/kg 3 times per week) when an oral intake was possible. If necessary, fresh frozen plasma or antithrombin III concentrates (antithrombin III < 65%) were substituted to maintain adequate coagulation parameters. Platelets were kept above 80,000/μL in the first 24 hours, then above 50,000/μL.
To achieve early extubation, the cessation of sedative drugs was attempted as early as possible. The systemic antifungal agents fluconazole (3–6 mg/kg/d) or amphotericin (1 mg/kg/d) were given to patients with suspected or proven fungal infection.
Prophylaxis for CMV infection (hyperimmune CMV globulin) was performed for CMV-negative recipients of a CMV-positive donor. CMV disease was treated with ganciclovir (10 mg/kg/d) for at least 2 weeks.
Immunosuppression
The patients received CsA (Sandimmun Optoral; Novartis Pharma GmbH, Basel, Switzerland) and prednisolone (initial dose, 60 mg/m2) as primary immunosuppressive drugs. The monoclonal antibody basiliximab (Simulect; Novartis Pharma GmbH) was given in 2 single doses on day 1 and day 4 in addition to standard immunosuppression for all patients. Acute rejections were treated with a 3-day to 5-day course of intravenous methylprednisolone bolus therapy (10 mg/kg). Of the children, 18.9% were converted to tacrolimus during follow-up because of steroid-resistant rejection or histologically proven drug-induced parenchymal damage. None of the patients received OKT3.
Hepatic Doppler Flow Imaging
Ultrasound was performed with a commercial Duplex scanner (HDI 3000; ATL) using 3-MHz sector or 5-MHz curved array probes. Examinations were performed intraoperatively after reperfusion, on arrival in the intensive care unit (ICU), and further twice daily for the first 3 postoperative days. Furthermore, in critical cases, an additional check was made after wound closure in the operating room. All examinations, including the intraoperative ones, were performed by the same highly experienced radiologist (K.H.). The following parameters were considered normal for the transplanted organ.
Hepatic artery: hepatopetal flow, with a systolic flow maximum >30 cm/s, resistance index <0.85.
Portal vein: hepatopetal flow with curved flow pattern, velocity at least 10 cm/s.
Hepatic vein: biphasic or triphasic hepatofugal flow with independent flow velocity.
Definition of Postoperative Morbidity
Primary nonfunction (PNF) was defined as retransplantation within 10 days after implantation or death resulting from a nonfunctioning graft.
Primary poor function was defined as serum quick below 30% for more than 3 consecutive postoperative days with consequent substitution of fresh frozen plasma.
Rejection was defined when serum transaminase levels increased and signs of rejection were histologically seen.
Perioperative morbidity: in general, a liberal reoperation policy was introduced to detect and correct postoperative surgical morbidity. Biliary leakage was defined when bilirubin in the drainage doubled the value in serum. Every proven bile leak that persisted for more than 24 hours was surgically corrected. Bile duct stenosis leading to reoperation or radiologic intervention was also included in biliary morbidity. Arterial and portal complications were defined as any disturbance in arterial or portal flow that led to surgical or radiologic intervention or to retransplantation. All children with intestinal perforation and significant bleeding were taken back to the operating room.
Donor Selection
In the LDLT group, the grafts were harvested after informed consent of the donors and close medical, laboratory, and psychologic evaluation, according to our evaluation protocol described elsewhere.13
The selection of suitable cadaveric donors for liver splitting was performed by an experienced transplant surgeon during surgical exploration of the donor liver according to the following criteria: amount of fatty degeneration (<30%), age of the donor (<50), laboratory findings, cause of death, amount of catecholamines, length of stay in ICU (<5 days), and macroscopic appearance of the liver. Steatosis in split liver grafts was accepted as high as 30%.
Surgical Technique
Donor Operation
The procedure for obtaining in situ split liver grafts was entirely comparable with that for living donation and described elsewhere.13,14
To increase the acceptance of liver splitting within the transplant community, the common trunks of the hepatic artery and portal vein remained to the right if the graft was primarily allocated to an adult recipient and to the left if a child was the first recipient.
The majority of our split liver procedures have been performed ex situ because of a change in the Eurotransplant allocation system. Parenchymal transection was performed ex situ by the sharp knife technique as introduced by Daniel Azoulay from Paris.
Recipient Operation
During hepatectomy, the recipient's hepatic artery and portal vein were kept as long as possible to allow vascular anastomosis without the use of interpositioning grafts. For implantation of the left lateral graft, the recipient's hepatic vein confluence of the left and middle hepatic vein was preserved and enlarged by a longitudinal ventral incision of the vena cava. Then, the left hepatic vein of the graft was anastomosed end-to-side to the caval vein. Portal vein anastomosis was performed mainly in an end-to-end fashion by using the recipient's portal vein bifurcation. Arterial anastomosis was performed with the aid of magnification loops (4×) mainly end-to-end between the left hepatic artery of the graft and the hepatic artery of the recipient without interpositioning grafts. Biliary reconstruction was performed by Roux-en-Y hepaticojejunostomy, occasionally as an end-to-end anastomosis. The diameter of the jejunal opening was limited to that of the bile ducts at the hilar plate. The parts of the hilar plate that did not carry segmental bile ducts were closed by fine sutures (7/0) to avoid leaking from small accessory bile ducts. The hepatico-jejunostomy was performed with interrupted absorbable monofilament sutures (7/0) with the aid of magnification loops (4×).
Statistics
Statistical analysis of patient and graft survival was performed according to the Kaplan-Meier method, calculated from the time of transplantation until December 2003. Comparison of patient survival in different groups was performed using the log-rank test. Categorical and continuous variables were compared using the Fisher exact test and the Mann-Whitney U test, respectively. A P value less than 0.05 was considered statistically significant.
RESULTS
The leading indications for OLT were biliary atresia (32%), acute liver failure (9%), and cryptogenic liver cirrhosis (8%; Table 1). Pediatric recipients with a median age of 1 year (range, 9 days–15 years) and a median weight and height of 9.4 kg (3–62) and 77 cm (38–175), respectively, were transplanted. Twenty-six children (19.7%) had a high urgent status.
TABLE 1. Indications for Liver Transplantation

The median weight of the transplanted grafts was 348 g (106–1900), and the median cold ischemic time was 492 minutes (±189). The median warm ischemic time was 30 minutes (±12; Table 2). The left lateral lobe (segments 2 and 3) was used in 87 (66%) transplants, the left lobe (segments 1–4 or 2–4) in 19 (14%), the right lobe (segments 5–8) in 1 (0.8%), and the right extended lobe (segments 1, 4–8) in 7 (5%) transplants. The median postoperative ICU stay of the patients was 5 days (1–32).
TABLE 2. Characteristics of Recipients and Grafts
One hundred thirty-two pediatric liver transplants using all techniques were performed with a 6-month patient survival rate of 100% (Fig. 1). Three children died after hospital discharge (98% actual patient survival rate; Table 5), 2 of whom had good liver function. The causes were recurrence of unknown liver disease 9 months after SLT, severe pneumonia 13 months after reduced-size liver transplantation (RED), and unknown cause 7 months after SLT. To evaluate the progress and evolution of our pediatric liver transplant program, we retrospectively analyzed the 1-year and 3-year patient survival rates according to the Kaplan-Meier method in 4 different periods. The 1-year and 3-year patient survival rates increased significantly from 73% and 67% between 1991 and 1994, to 85% and 74% between 1995 and 1997, to 91% and 90% between 1998 and 2000, and to 98% and 97% between April 2001 and December 2003. The patients transplanted in the last period had a statistically significant higher survival rate compared with the previous periods (log-rank P < 0.05; Fig. 2).
FIGURE 1. Patient versus graft survival after 132 consecutive pediatric liver transplants between April 2001 and December 2003.
TABLE 5. Reasons for Graft Loss After 132 Consecutive Pediatric Liver Transplants
FIGURE 2. Patient survival in different periods (period 1, 1991–1994, n = 70; period 2, 1995–1997, n = 83; period 3: 1998–2000, n = 87; period 4, 2001–2003, n = 121) after pediatric liver transplantation at the University of Hamburg.
Between April 2001 and December 2003, 44 living related liver transplants were performed, with an actual patient survival rate of 100%. The 3-month and actual patient survival rates after 53 split liver transplants were 100% and 96%, respectively. Fourteen patients (26%) from the split group were of a high urgent status (UNOS I).
In multivariate analysis of patient survival after pediatric liver transplant in 422 recipients from 1984 until 2003 using Cox regression, the period of transplantation turned out to be the strongest prognostic factor for the recipient (1991–1994, P = 0.0001; 1995–1997, P = 0.012; 1998–2000, P = 0.005; 2001–2003, P = 0.051). UNOS status and type of graft were not significant prognostic factors for patient survival (UNOS: status 1, P = 0.180; status 2, P = 0.434; status 3, P = 0.954; status 4, P = 0.555; LRT, P = 0.651; SLT, P = 0.591; RED, P = 0.775; WLT, P = 0.408), nor were the cold ischemic time (<6 hours, P = 0.445; 7–12 hours, P = 0.526; 13–18 hours, P = 0.488; >18 hours, P = 0.751), donor age (<10 years, P = 0.802; 10–20 years, P = 0.658; 21–40 years, P = 0.896; 41–55 years, P = 0.451; >55 years, P = 0.561), and recipient age (<2 years, P = 0.055; 2–10 years, P = 0.062; 11–16 years, P = 0.072).
The 3-month and actual graft survival rates after 132 liver transplants were 92% and 86%, respectively (Fig. 1). The 1-year and 3-year graft survival rates increased slowly from 63% and 56% in the first period (1991–1994) to 70% and 61% in the second period (1995–1997; P = 0.627). In the third period (1998–2000), 3-month and actual graft survival rates significantly increased to 84% and 79%, respectively (P = 0.0039). Three-month and actual graft survival rates were 86% and 80% in period 4 (April 2001–December 2003), not significantly different from those in period 3 (P = 0.9242).
The actual and 3-month graft survival rates after 44 LRTs were 96% and 98% and after 53 SLTs 85% and 94%, respectively. After whole organ transplantation, the actual and 3-month graft survival rates were both 83.3%. Transplantation of reduced-size grafts was followed by actual and 3-month survival rates of only 64.7% and 76.5%, respectively. Graft survival according to the different types of grafts used during April 2001 and December 2003 showed a significantly higher rate in the living related versus reduced-size group (P = 0.0053) but was not significant compared with the split group (Fig. 3).
FIGURE 3. Graft survival according to the different types of grafts used during April 2001 and December 2003. LR, n = 44; split, n = 53; OLT, n = 18; RED, n = 17. Log-rank test: LR versus split, P = 0.2244; LR versus OLT, P = 0.135; LR versus RED, P = 0.0053
After 132 liver transplants, 5 patients (3.8%) developed PNF and had to undergo retransplantation (Table 5). Analyzing the PNF rate after pediatric liver transplantation in Hamburg, we had a peak of 11% in 1998 and a minimum of zero in 2000 (Fig. 4).
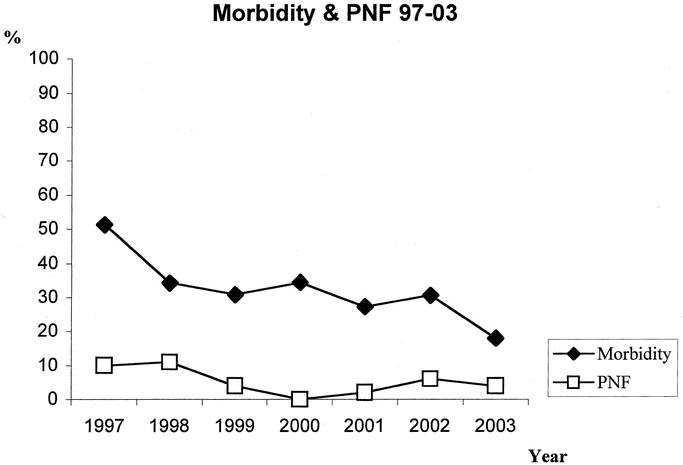
FIGURE 4. Evolution of perioperative morbidity and PNF from 1997 until 2003.
Eight patients (6%) developed biliary complications (5 cut surface, 2 leaks from anastomosis, and 1 late anastomotic stricture). Arterial complications occurred in 11 patients (8.3%). Bowel perforation was observed in 4 patients (3%). Seven patients (5%) had to be reoperated because of postoperative bleeding. Three (2%) portal vein complications (2 patients developed a thrombosis and 1 had a kink in the portal vein) led to surgical intervention. In 29%, acute rejection occurred during the hospital course.
After 44 LRTs, biliary complications occurred in 2 patients (4.6%), arterial and portal complications in 2 (4.6%) and 1 patient (2.3%), respectively, bowel perforation in 1 patient (2.3%), and postoperative bleeding in 3 patients (6.8%). Twenty-five percent of the LRT group had acute cellular rejection. After 53 SLTs, 3 patients (5.7%) were reoperated because of biliary complications, 4 (7.5%) and 2 patients (3.8%) because of arterial and portal complications, respectively, 2 patients (3.8%) because of intestinal perforation, and 1 patient (1.9%) because of postoperative bleeding. Thirty-two percent of the split group developed acute rejection.
During follow-up, we analyzed the surgical morbidity after discharge from the hospital; 3.8% were taken back to the operating room because of abdominal complications. Three children developed cholestasis and consequently had a revision of the Roux-en-Y hepaticojejunostomy 3 and 4 months after transplantation. In 1 case, a bile duct end-to-end anastomosis had to be converted to a Roux-en-Y hepaticojejunostomy because of persistent cholestasis in the 4th postoperative month. One child was reoperated on 3 months after liver transplantation because of a cholascos, necessitating intravenous nutrition.
The rate of posttransplant infectious complications was low. Incidences of CMV infection, PTLD, and EBV infection were 11%, 0.7%, and 14.1%, respectively.
Analyzing the perioperative morbidity throughout the years at our center, we were able to decrease the perioperative morbidity from 51% to 18%. In 1997, almost half of the patients were taken back to the operating room, and in 2003, 18% were reoperated because of surgical complications (Fig. 4).
The median age of the 53 cadaveric donors (35 male, 18 female) was 28 years (range, 15–54), with a median weight and height of 75 kg (range, 35–98) and 175 cm (range, 133–198). The median ICU stay was 4 days (range, 1–15) and median serum sodium was 145 mmol/L (range, 126–161). Details of the characteristics of the living and cadaveric donors are listed in Table 3.
TABLE 3. Donor Characteristics

The retransplantation rate was 12%, with the causes chronic rejection (3.8%), PPF (1.5%), PNF (3.8%), and arterial thrombosis (3%). Of these 16 retransplants, 6 were transplanted with split-liver grafts, 5 reduced-size organs, 3 whole organs, and 2 grafts from living donors as primary liver transplantation.
Five children had to undergo retransplantation because of chronic rejection. The earliest retransplantation was 3 months after primary LTX (SLT), and additionally, the explanted graft showed signs of malignancy (B-cell lymphoma). The latest retransplantation because of chronic rejection was 2 years after primary LTX (SLT).
DISCUSSION
Since the first pediatric liver transplant at University of Hamburg, the face of this procedure has totally changed from an experimental one to routine case management. In the early years, the development of pediatric liver transplantation was hampered by a severe shortage of size-matched grafts, especially for small infants. To overcome this severe organ shortage, living related and in situ SLTs were introduced. The following years were used to improve patient selection, timing of transplantation, and surgical technique, and to introduce routine intraoperative and postoperative flow measurement by Doppler ultrasound and postoperative care. All these factors contributed to the continuous improvement in outcome, which ends in the present series of transplantations without hospital mortality. It is this that prompted this analysis of the learning curve.
The last in-hospital death after pediatric liver transplantation in our center occurred in March 2001. Since then, 157 consecutive pediatric liver transplants have been performed without in-hospital mortality. To achieve a minimal follow-up of 6 months, we defined the study period from April 2001 to December 1, 2003. We lost 3 patients during follow-up, 2 of them with good functioning grafts, 1 because of a recurrence of the unknown primary liver disease. These deaths underline the need for follow-up care of the recipients in close collaboration with the primary transplant center and a careful diagnosis of the underlying disease to enable strategies to avoid disease recurrence. To our knowledge, there is no published series with more than 100 recipients with an actual 6-month patient survival rate of 100%. Borenstein et al15 from Toronto published patient and graft survival rates of 100% after 13 living donor liver transplants in children. Between July 1993 and April 2002, the group from Brussels performed 235 primary liver transplants with a 5-year recipient survival rate of 85% in the cadaveric group (n =1 35) and 92% in the LDLT group (n = 100).16 After 138 split-liver transplants in 124 patients, Gridelli et al17 described a 2-year patient survival rate of 93%. The group from Kings College in London published an actual 6-month patient survival rate of 96.2% after 80 consecutive pediatric liver transplants.18
The patient survival rate in this study was not significantly different among the different types of grafts. During the study period, we tried to serve the children only with very good quality grafts (donor age <50 years, fatty change <30%, stay on ICU below 5 days, near normal liver enzymes) if the status of the recipient allowed selection of the cadaveric graft. Consequently, tendentially younger cadaveric donors with lower risk factors (less fatty degeneration, shorter ICU stay, better laboratory results) were used. On the other hand, this resulted in an increased need for living related liver transplants. The majority of our transplanted grafts were derived from splitting adult cadaveric livers (n = 53). Because patient and graft survival after SLT is shown not to be significantly lower compared with that after LDLT,16,17,19,20 we considered SLT as the first-line modality, which was first suggested by the Bismuth group.21 Living donor liver transplantation was performed if the parents accepted only LDLT or the child deteriorated during the waiting time.
UNOS status I is a well recognized prognostic factor for patient survival. Twenty percent of our recipients were high-urgent. Out of this group, 2 patients died during follow-up, and 2 had to be retransplanted, both because of PNF of the graft. In multivariate analysis of prognostic factors in our whole series of pediatric liver transplantation, the UNOS status did not significantly influence patient survival.
Twelve percent (n = 16) of the recipients were less than 5 kg in body weight, and all are doing well with good liver function. One neonate had to be retransplanted because of PNF. In a recent review, the patient and graft survivals of this subgroup were 57% and 38%, respectively.22–24 We already observed no disadvantage concerning mortality in children with very low body weight of less than 10 kg when compared with older graft recipients.25 This might be mainly a result of growing experience of the whole staff including surgeons, pediatricians, and the intensive care physicians and nurses. Goss et al26 reported remarkably better patient survival of infants younger than 1 year when transplanted after 1993 in contrast with those receiving an OLT between 1984 and 1993 (84% versus 64%), highlighting the influence of the center's learning curve. This factor was especially evident in children younger than 1 year.26
The significantly higher patient survival rate in Hamburg throughout the years was not sustained by a significantly higher graft survival. This is one of the future challenges, in addition to a decrease in the chronic rejection rate and graft loss during follow-up.
The 3-month and actual graft survival rates after 132 consecutive liver transplants were 92% and 86%, respectively. The 3 main reasons for graft loss were PNF (n = 5), arterial thrombosis (n = 4), and chronic rejection (n = 5). No PNF occurred after LDLT, confirming the superiority of grafts from living donors regarding PNF. The reasons for PNF in the cadaveric groups were intraoperative hypoxia caused by tubus displacement after reperfusion and, in each case, fatty changes in the graft. The other 3 failed organs came from 2 pediatric and 1 adult donor. Causes of death in these donors were drowning of a 1-year-old donor and traumatic brain injury in the 3-year-old and 25-year-old donors. It remains a challenge to identify the cadaveric donor who may present a higher risk of PNF. Caution must be taken in cases of drowning and in donors of a very young age (<5 years).
Actual graft survival was not significantly different among the LDLT, split, and WLT groups. Only the RED group resulted in significantly lower graft survival. In the era of liver splitting, graft reduction was performed only in the event of inferior liver quality and partially traumatized livers. Using traumatized livers is known to result in inferior graft survival27 and fatty changes to the liver.28,29
Perioperative morbidity after pediatric liver transplantation mainly includes arterial, portal, and biliary complications, intestinal perforation, and bleeding. The incidence of arterial thrombosis ranges from 4% to 26%26,30–33 and remains a major cause for graft loss. Various risk factors and the etiology of hepatic artery thrombosis have been discussed in the literature, and especially small children less than 3 years old weighing less than 15 kg have been reported to have a high incidence of arterial thrombosis.33 In our series, 4 children (3%) had to undergo retransplantation because of hepatic artery thrombosis. In the last 2 cases, trauma grafts were size-reduced and the macroscopic traumatic right graft discarded. The arterial reconstructions were very difficult in these cases, with various revisions because of intrahepatic injuries of the left hepatic artery. At our center, the routine Doppler-ultrasound examination performed by the same highly experienced radiologist during operation, before and immediately after closure of the abdominal wall, and in the ICU has led, over the years, to a decrease in the rate of hepatic artery thrombosis. Biliary complications including bile leaks and anastomotic strictures have historically been a major problem of partial liver grafts. An initial series of SLT in the early 1990s had a biliary complication rate of 20% to 25%.34,35 Technical advances, including biliary reconstruction without using stents and refinement of the surgical technique, significantly lowered the incidence of anastomotic strictures and bile leaks. In this series, the rate of biliary complications (6%) after 132 liver transplants, using all techniques, was similar to those after SLT (5.7%) and LRT (4.6%). The most dramatic decrease in our series was seen in the postoperative bowel perforation rate (3%), with the last patient operated because of a small colon perforation in July 2002. In other centers, the intestinal perforation rate was between 5% and 14% after pediatric liver transplantation.26,36
Portal vein complications were most often associated with cryopreserved vein conduits for portal anastomosis, with a complication rate as high as 6%.37 In our series, 2 patients (1.5%) developed a portal vein thrombosis, and 1 patient (0.8%) had a kink in the portal vein, all diagnosed at the postoperative Doppler-ultrasound examination with consequent surgical intervention. None of these patients had a cryopreserved conduit for portal anastomosis. Analyzing our data throughout the years, we successfully decreased the perioperative morbidity from 51% to 18%, which is definitely one of the vital factors for increasing patient and graft survival after pediatric liver transplantation.
Compared with other international centers, we used comparably low immunosuppression in our pediatric liver graft recipients, resulting in a low incidence of acute rejection. Our main focus in the future lies in preventing chronic graft rejection. However, the incidence of 3.8% is acceptable and represents a rising problem in our patient population. It is a challenge to identify predictive surrogate markers for chronic graft rejection, and future analysis must show whether there is an effect of different immunosuppressive protocols.
In multivariate analysis of the most recognized factors in pediatric liver transplantation, the year of transplantation turned out to be the strongest significant factor in predicting patient survival, thus underlining the learning curve in our center. The age of the recipient, the cold ischemic time, the type of graft used, and UNOS status and pretransplant diagnosis did not predict patient survival. Goss et al26 found in a multivariate analysis also using the Cox regression model that the era of transplantation, age of the recipient, and number of allografts performed significantly predicted patient survival rates.
In summary, in our experience, the factors that contributed most to the results of this series were the following:
(1) Adequate choice of the splitting technique
(2) Technique of graft implantation
(3) Timely decision for retransplantation
(4) Standardized posttransplant care, including closed follow-up of the hepatic flow
(5) Choice of the donor
CONCLUSIONS
These very good immediate results are a sign that liver transplantation has reached the threshold of a new era where survival of the patient is considered the rule. It is to be expected that, similar to a few decades ago in renal transplantation, attention will now be focused more on long-term graft and patient survival. Recurrence of disease, side effects of immunosuppression, diagnosis and prevention of chronic rejection, and quality of life are some of the topics that will dominate our concerns in the years to come. It is crucial for transplant surgeons not to miss this development and to seek and organize actively the interdisciplinary approach necessary to tackle the challenges ahead. The major problem of transplantation, the shortage of organs, is of particular importance in light of the almost 100% survival chances after transplantation. Although this is still somewhat bearable in the pediatric population with its low pretransplant mortality, it may put the patient and the surgeon under enormous pressure once similar survival results are reached for adult recipients, who face a high waiting list mortality.
TABLE 4. Outcome After 132 Consecutive Pediatric Liver Transplants
Discussions
Dr. Neuhaus: Thank you very much, Dr. Broering, for this presentation. I congratulate you and Dr. Rogiers for this excellent series. I have the pleasure to comment on your paper. It looks to me like the first series of Professor Trede of Mannheim, when he presented 120 pancreatic head resections without mortality. It was I think, the first time in the world that this occurred and it was a milestone.
When I did presentations on outcome of liver transplantations, I always mentioned that there are factors related to the donor, the indications, and there is the surgical technique, the intensive care, immuno suppression and different kinds of disease. This is similar in the paediatric population.
My first question therefore is what of all these reasons is the most important in your opinion to provide these excellent results?
Secondly, as I could see on your slide, most of the donors are below the age of 15. For the paediatric population nowadays you use very few living donors. The split livers maybe come from the young, excellent donors and in comparison the adult recipients receive nowadays from donors of 62 –80 years of age. My question is, are these good results a question of excellent donors from which you can select out of a larger the pool of the young transplant donor population?
Dr. Broering: Thank you very much, Professor Neuhaus, for these comments. The answer to the first question is that there is not one factor alone that contributes to these results. Paediatric liver transplantation is a multidisciplinary approach and you need an experienced intensive care unit, an anaesthesiologist, as well as an experienced pediatric liver transplantation surgeon.
About the second question: of course we choose only young donors for split liver transplantation and the choice of the graft is also a very important factor to get these results and we have to continue to choose these young donors. Maybe in the future we have to be more selective to improve further the graft survival, but this we have to balance with the availability of donors for the paediatric population.
Dr. Rogiers: I just want to make an additional comment. The main reason why we wanted to present this paper is because indeed, it might be an important milestone in the history of liver transplantation. In a certain sense, we are now reaching the point that kidney transplantation reached fifteen years ago, when suddenly the results of surgery were so good that the whole attention goes in the direction of the long term treatment. Somehow, we as surgeons have to be careful that this part of liver transplantation is not being taken progressively from us now.
So, we have to look at ways of actively collaborating in multidisciplinary discussions with our hepatologists and not letting the whole initiative to them.
Footnotes
Reprints: Dieter C. Broering, University Hospital Eppendorf, Department of Hepatobiliary Surgery, Martinistr. 52, 20246 Hamburg, Germany. E-mail: broering@uke.uni-hamburg.de.
REFERENCES
- 1.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Koep LJ, Schroter GP, et al. Liver replacement for pediatric patients. Pediatrics. 1979;63:825–829. [PMC free article] [PubMed] [Google Scholar]
- 3.Bismuth H. Liver transplantation: the Paul Brousse experience. Transplant Proc. 1988;20(suppl 1):486–489. [PubMed] [Google Scholar]
- 4.Gartner JC Jr, Zitelli BJ, Malatack JJ, et al. Orthotopic liver transplantation in children: two-year experience with 47 patients. Pediatrics. 1984;74:140–145. [PMC free article] [PubMed] [Google Scholar]
- 5.Pichlmayr R, Brolsch C, Wonigeit K, et al. Experiences with liver transplantation in Hannover. Hepatology. 1984;4(suppl 1):56S–60S. [DOI] [PubMed] [Google Scholar]
- 6.Scharschmidt BF. Human liver transplantation: analysis of data on 540 patients from four centers. Hepatology. 1984;4(suppl 1):95S–101S. [DOI] [PubMed] [Google Scholar]
- 7.Calne RY, Rolles K, White DJ, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033–1036. [DOI] [PubMed] [Google Scholar]
- 8.Iwatsuki S, Starzl TE, Todo S, et al. Experience in 1,000 liver transplants under cyclosporine-steroid therapy: a survival report. Transplant Proc. 1988;20(suppl 1):498–504. [PMC free article] [PubMed] [Google Scholar]
- 9.Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery. 1984;95:367–370. [PubMed] [Google Scholar]
- 10.Broelsch CE, Emond JC, Thistlethwaite JR, et al. Liver transplantation with reduced-size donor organs. Transplantation. 1988;45:519–524. [DOI] [PubMed] [Google Scholar]
- 11.Emond JC, Whitington PF, Thistlethwaite JR, et al. Reduced-size orthotopic liver transplantation: use in the management of children with chronic liver disease. Hepatology. 1989;10:867–872. [DOI] [PubMed] [Google Scholar]
- 12.Otte JB. History of pediatric liver transplantation: where are we coming from? where do we stand? Pediatr Transplant. 2002;6:378–387. [DOI] [PubMed] [Google Scholar]
- 13.Broering DC, Sterneck M, Rogiers X. Living donor liver transplantation. J Hepatol. 2003;38(suppl 1):S119–S135. [DOI] [PubMed] [Google Scholar]
- 14.Rogiers X, Bismuth H, Busutttil R, et al. Split Liver Transplantation. Darmstad, Germany: Steinkoff Verlag; 2002. [Google Scholar]
- 15.Borenstein S, Diamond IR, Grant DR, et al. Outcome of pediatric live-donor liver transplantation—the Toronto experience. J Pediatr Surg. 2003;38:668–671. [DOI] [PubMed] [Google Scholar]
- 16.Otte JB. Donor complications and outcomes in live-liver transplantation. Transplantation. 2003;75:1625–1626. [DOI] [PubMed] [Google Scholar]
- 17.Gridelli B, Spada M, Petz W, et al. Split-liver transplantation eliminates the need for living-donor liver transplantation in children with end-stage cholestatic liver disease. Transplantation. 2003;75:1197–1203. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande RR, Bowles MJ, Vilca-Melendez H, et al. Results of split liver transplantation in children. Ann Surg. 2002;236:248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farmer DG, Yersiz H, Ghobrial RM, et al. Early graft function after pediatric liver transplantation: comparison between in situ split liver grafts and living-related liver grafts. Transplantation. 2001;72:1795–1802. [DOI] [PubMed] [Google Scholar]
- 20.Broering DC, Mueller L, Ganschow R, et al. Is there still a need for living-related liver transplantation in children? Ann Surg. 2001;234:713–721; discussion 721–722. [DOI] [PMC free article] [PubMed]
- 21.Azoulay D, Astarcioglu I, Bismuth H, et al. Split-liver transplantation: the Paul Brousse policy. Ann Surg. 1996;224:737–746; discussion 746–748. [DOI] [PMC free article] [PubMed]
- 22.Cacciarelli TV, Esquivel CO, Moore DH, et al. Factors affecting survival after orthotopic liver transplantation in infants. Transplantation. 1997;64:242–248. [DOI] [PubMed] [Google Scholar]
- 23.Sundaram SS, Alonso EM, Whitington PF. Liver transplantation in neonates. Liver Transpl. 2003;9:783–788. [DOI] [PubMed] [Google Scholar]
- 24.Woodle ES, Millis JM, So SK, et al. Liver transplantation in the first three months of life. Transplantation. 1998;66:606–609. [DOI] [PubMed] [Google Scholar]
- 25.Ganschow R, Nolkemper D, Helmke K, et al. Intensive care management after pediatric liver transplantation: a single-center experience. Pediatr Transplant. 2000;4:273–279. [DOI] [PubMed] [Google Scholar]
- 26.Goss JA, Shackleton CR, McDiarmid SV, et al. Long-term results of pediatric liver transplantation: an analysis of 569 transplants. Ann Surg. 1998;228:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broering DC, Gundlach M, Bockhorn M, et al. Transplantation of traumatized livers: is it safe? Transplant Proc. 1999;31:540. [DOI] [PubMed] [Google Scholar]
- 28.Strasberg SM, Howard TK, Molmenti EP, et al. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829–838. [DOI] [PubMed] [Google Scholar]
- 29.D'Alessandro AM, Kalayoglu M, Sollinger HW, et al. The predictive value of donor liver biopsies on the development of primary nonfunction after orthotopic liver transplantation. Transplant Proc. 1991;23:1536–1537. [PubMed] [Google Scholar]
- 30.Blumhardt G, Ringe B, Lauchart W, et al. Vascular problems in liver transplantation. Transplant Proc. 1987;19:2412. [PubMed] [Google Scholar]
- 31.Tzakis AG, Gordon RD, Shaw BW Jr, et al. Clinical presentation of hepatic artery thrombosis after liver transplantation in the cyclosporine era. Transplantation. 1985;40:667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rela M, Muiesan P, Bhatnagar V, et al. Hepatic artery thrombosis after liver transplantation in children under 5 years of age. Transplantation. 1996;61:1355–1357. [DOI] [PubMed] [Google Scholar]
- 33.Tan KC, Yandza T, de Hemptinne B, et al. Hepatic artery thrombosis in pediatric liver transplantation. J Pediatr Surg. 1988;23:927–930. [DOI] [PubMed] [Google Scholar]
- 34.Emond JC, Whitington PF, Thistlethwaite JR, et al. Transplantation of two patients with one liver: analysis of a preliminary experience with “split-liver” grafting. Ann Surg. 1990;212:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houssin D, Boillot O, Soubrane O, et al. Controlled liver splitting for transplantation in two recipients: technique, results and perspectives. Br J Surg. 1993;80:75–80. [DOI] [PubMed] [Google Scholar]
- 36.Yamanaka J, Lynch SV, Ong TH, et al. Posttransplant gastrointestinal perforation in pediatric liver transplantation. J Pediatr Surg. 1994;29:635–638. [DOI] [PubMed] [Google Scholar]
- 37.Buell JF, Funaki B, Cronin DC, et al. Long-term venous complications after full-size and segmental pediatric liver transplantation. Ann Surg. 2002;236:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]








