Abstract
PDZ-GEF is a novel guanine nucleotide exchange factor for Rap1 GTPase. Here we isolated Drosophila melanogaster PDZ-GEF (dPDZ-GEF), which contains the all-conserved domains of mammalian and nematode PDZ-GEF including cyclic nucleotide monophosphate-binding, Ras exchange motif, PDZ, RA, and GEF domains. dPDZ-GEF loss-of-function mutants were defective in the development of various organs including eye, wing, and ovary. Many of these phenotypes are strikingly similar to the phenotype of the rolled mutant, implying that dPDZ-GEF functions upstream of the mitogen-activated protein (MAP) kinase pathway. Indeed, we found that dPDZ-GEF is specifically involved in photoreceptor cell differentiation, facilitating its neuronal fate via activation of the MAP kinase pathway. Rap1 was found to link dPDZ-GEF to the MAP kinase pathway; however, Ras was not involved in the regulation of the MAP kinase pathway by dPDZ-GEF and actually had an inhibitory function. The analyses of ovary development in dPDZ-GEF-deficient mutants also demonstrated another role of dPDZ-GEF independent of the MAP kinase signaling pathway. Collectively, our findings identify dPDZ-GEF as a novel upstream regulator of various morphogenetic pathways and demonstrate the presence of a novel, Ras-independent mechanism for activating the MAP kinase signaling pathway.
Ras superfamily small GTPases are molecular switches that regulate a variety of cellular processes including cell proliferation, differentiation, and apoptosis. They cycle between GDP-bound inactive forms and GTP-bound active forms, regulated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins. Various extracellular or intracellular signals indirectly regulate these GTPases by modulating the activities of their specific GEFs and GTPase-activating proteins, although the detailed mechanisms remain largely unknown (6).
Among the GTPases, Ras has been the most thoroughly characterized. It plays the role of a master switch in mitogenic signaling pathways including the mitogen-activated protein kinase (MAPK) and phosphatidylinositide-3 kinase pathways. Rap1 (also known as Roughened, Ras3, and Dras62B in Drosophila melanogaster) is another well-studied GTPase with a structure highly homologous to that of Ras. Rap1 was originally identified by its ability to reverse cellular transformation induced by oncogenic Ras (18). Rap1 is more than 50% identical to Ras and is thought to block the mitogenic activity of the cell by trapping the Ras effector C-Raf into an inactive complex, silencing MAPK activity (9, 14). However, many recent findings suggest that Rap1 could act as an activator of the MAPK pathway in other cellular contexts. For example, Rap1 was found to interact with and activate B-Raf (27), resulting in sustained MAPK activation (35, 36). These inconsistent observations obscure the relationship between Rap1 and MAPK, and thus, a deeper probing into the function of Rap1 is required in order to explain its seemingly contradictory functions within the MAPK signaling pathway.
Recently, the regulation of Rap1 activity has been extensively studied, and consequently, multiple Rap1-GEFs have been identified. They include smgGDS (23), C3G (12), Epac/cyclic AMP-GEF (11, 16), CalDAGGEF1 (17), and PDZ-GEF/RA-GEF/CNrasGEF/nRapGEP1 (10, 20, 26, 28). Among them, PDZ-GEF has attracted considerable attention because of its unique structural characteristics. PDZ-GEF contains multiple functional domains including cyclic nucleotide monophosphate-binding, Ras-exchange motif, PDZ, and Ras/Rap1-associating (RA) domains, as well as GEF catalytic domains.
Although several insightful findings pertaining to the functions of PDZ-GEF and other Rap1 GEFs have been made, they were based either on biochemical methods or on cell line-based overexpression studies, which have a limited in vivo relevance. Thus, we decided to define the physiological role of PDZ-GEF in the Drosophila system, which allows highly convenient genetic and histological studies. Using the dPDZ-GEF mutant fly, the first genetic model for a Rap1 GEF, we have elucidated dPDZ-GEF/Rap1-specific signaling in context of the MAPK signaling pathway.
MATERIALS AND METHODS
Fly strains.
dPDZ-GEF1 (l(2)k13720), Rase1b, DSorLH110, phl11, rl1, gmr-GAL4, rhoAA69, and other stocks used for mitotic recombination analyses and P-element excisions were obtained from the Bloomington stock center (Bloomington, Ind.). Raprv(R)B1 and UAS-Raswt were kind gifts from Gerald Rubin (University of California—Berkeley). dPDZ-GEF2 (EP(2)388) were provided by Exelixis Inc. All Drosophila stocks were maintained with standard cornmeal-yeast-agar medium at 25°C, except during escapee searching at 18°C and transheterozygote phenotype scoring at 30°C.
Northern blot analysis.
Total RNAs, extracted by the easy-Blue system (Intron, Seoul, Korea), were separated by electrophoresis on denaturing formaldehyde agarose gels in MOPS (morpholinepropanesulfonic acid) buffer, transferred onto a nylon membrane, and successively hybridized with nick-translated 32P-labeled cDNA probes for dPDZ-GEF. Hybridized probes were visualized by autoradiography.
Analysis of eye phenotypes.
To obtain the eye-specific mitotic clone, the dPDZ-GEF1 (l(2)K13720) allele was recombined onto the FRT40A y+ chromosome, to generate the y w; dPDZ-GEF1 FRT40A/CyO line. Females from this line were crossed to males of either y w ey-flp; FRT40A or y w; gmr-hid FRT40A/CyO; ey-GAL4 UAS-flp line.
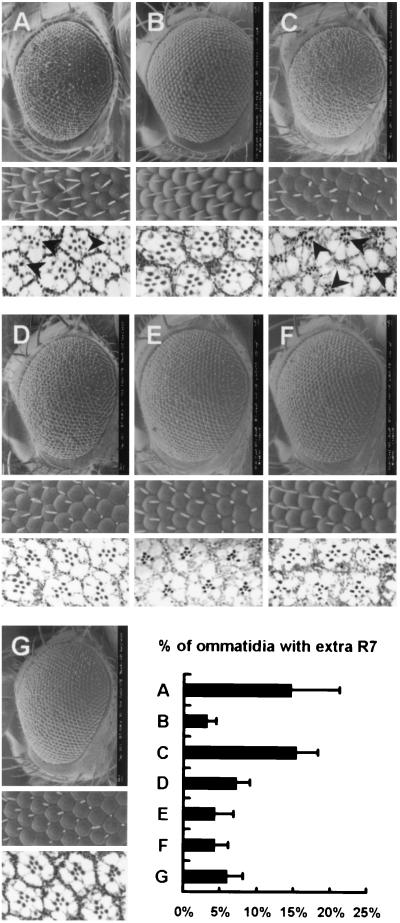
The genotypes of the control eyes shown in Fig. 5 are as follows: UAS-Raswt/+ and gmr-GAL4/UAS-Raswt for Fig. 5B, Rase1b/+ and gmr-GAL4/+;Rase1b/+ for Fig. 5C, Rrv(R)B1/+ and gmr-GAL4/+;Rrv(R)B1/+ for Fig. 5D, phl11/+ and phl11/+;gmr-GAL4/+ for Fig. 5E, dSorLH110/+ and dSorLH110/+;gmr-GAL4/+ for Fig. 5F, rl1/+ and gmr-GAL4/rl1 for Fig. 5G. All of these were analyzed by scanning electron microscopy (SEM) and tangential section, and each eye was shown to be normal with intact photoreceptor numbers.
FIG. 5.
Genetic interactions between dPDZ-GEF and components of Ras1, Rap1, and the MAPK pathway. Scanning electron micrographs of the whole eye, magnified ommatidia, and tangential sections from the flies of the following genetic backgrounds are presented. (A) gmr-GAL4 dPDZ-GEF2/+; (B) gmr-GAL4 dPDZ-GEF2/UAS-Raswt; (C) gmr-GAL4 dPDZ-GEF2/+; Rase1b/+; (D) gmr-GAL4 dPDZ-GEF2/+; Raprv(R)B1/+; (E) phl11/+; gmr-GAL4 dPDZ-GEF2/+; (F) dSorLH110/+; gmr-GAL4 dPDZ-GEF2/+; (G) gmr-GAL4 dPDZ-GEF2/rl1. Percentile proportions of the ommatidia with extra photoreceptor cells in each genetic background were determined by scoring more than four eyes (∼500 ommatidia) per experiment and are presented as a bar graph. The control eyes of each genotype whose gmr-GAL4 dPDZ-GEF2 chromosome is patched into a gmr-GAL4 or + chromosome displayed a wild-type phenotype. Detailed genotypes of control flies are described in Materials and Methods. The arrowheads in the figures indicate the ommatidia with extra photoreceptor cells.
For eye section experiments, fly heads were fixed with 4% formaldehyde in 0.1 M sodium phosphate buffer (pH = 7.2) overnight, dehydrated with a graded acetone series, and embedded in Spurr medium (Sigma). Compound eyes were sectioned tangentially with a width of 0.5 to 1 μm and stained with 1% toluidine blue and 1% sodium borax. Scanning electron micrograph images were obtained by LEO 1455VP in a variable-pressure secondary electron mode.
Analysis of ovaries.
For phalloidin and Hoechst staining, ovaries were fixed for 20 min in 9.4% formaldehyde in phosphate-buffered saline and stained with phalloidin-tetramethyl rhodamine isocyanate (Sigma) overnight at 4°C. After being washed, the same samples were stained with Hoechst 33258 for 4 min. The stained ovaries were examined with an LSM510 laser confocal microscope (Carl Zeiss). Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) analyses were performed as described previously (8). In situ hybridization experiments were performed with a digoxigenin-labeled RNA probe of the same fragment used for the Northern blot analysis (Fig. 1B).
FIG. 1.
Characterization of dPDZ-GEF. (A) Schematic representation of the comparison between Drosophila and human PDZ-GEFs. Amino acid sequence identity is displayed as a percentage. (B) Expression of dPDZ-GEF in different developmental stages of Drosophila. E, embryo; 1st, first instar larva; 2nd, second instar larva; 3rd, third instar larva; P, pupa; F, adult female; M, adult male. A 600-bp fragment encompassing the central portion of dPDZ-GEF coding region was used as a probe. Arrowheads indicate 5.5- and 7.5-kb dPDZ-GEF message bands. 28S rRNA was shown as a control (bottom panel). (C) dPDZ-GEF transcription is dramatically abolished in the dPDZ-GEF1 mutant. The quantities of dPDZ-GEF transcripts were visualized by RT-PCR experiments from the following samples: cloned dPDZ-GEF cDNA, adult Drosophila brain cDNA library, dPDZ-GEF1/CyO Act-GFP (+/−) larvae, dPDZ-GEF1/dPDZ-GEF1 (−/−) larvae, and genomic DNA; rp49 transcripts were used as a control. Green fluorescent protein selection was performed under the fluorescent microscope at 30 h after egg laying to separate heterozygotes from homozygotes. (D) Genes near dPDZ-GEF are unaffected in the dPDZ-GEF1 mutant. The expression profiles of dPDZ-GEF, Pez, Cpr, and CG9490, which are all the identified and/or predicted genes within 20 kb upstream and downstream of the dPDZ-GEF1 insertion, were examined by RT-PCR in the heterozygotic (+/−) or homozygotic (−/−) presence of the dPDZ-GEF1 allele. (E) Structure of the dPDZ-GEF locus. Open boxes indicate the coding sequence of the dPDZ-GEF gene, and breaks between the boxes indicate introns. The triangle represents the P-element insertion. dPDZ-GEF1 and dPDZ-GEF2 are dPDZ-GEF mutant alleles l(2)k13720 and EP(2)388, respectively.
X-Gal staining of eye discs.
For 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining, eye discs were fixed in 4% formaldehyde in phosphate-buffered saline for 30 min, washed, submerged in the standard X-Gal staining solution [X-Gal, 3.1 mM K4Fe(CN)6, 3.1 mM K3Fe(CN)6, 1 mM MgCl2, 150 mM NaCl, 10 mM Na2HPO4, 10 mM NaH2PO4, 0.3% Triton X-100], and incubated overnight at 37°C.
RESULTS
Identification of Drosophila PDZ-GEF and its mutants.
Previously, a putative Drosophila PDZ-GEF gene (CG9491, GenBank accession number AE003613) was annotated by computational analyses of the Drosophila genome (1). To obtain the full-length gene for this candidate PDZ-GEF gene, we performed reverse transcription (RT)-PCR using the genomic sequences flanking CG9491 as primers. The identified putative Drosophila PDZ-GEF sequence was identical to that of CG9491, containing six exons and 1,552 amino acids. By BLAST search, we determined that this is the only orthologue of the PDZ-GEF in Drosophila and named this gene Drosophila PDZ-GEF (dPDZ-GEF). dPDZ-GEF was 52 and 45% identical in amino acid sequence to human PDZ-GEF and Caenorhabditis elegans PDZ-GEF, respectively, and contained all the conserved domains including cyclic nucleotide monophosphate binding, Ras exchange motif, PDZ, RA, and GEF domains (Fig. 1A). To determine the expression pattern of dPDZ-GEF during various developmental stages, we performed Northern blot analyses. As shown in Fig. 1B, dPDZ-GEF messages were detected at 7.5 and 5.5 kb, possibly caused by alternative splicing (28, 30). Both dPDZ-GEF messages were expressed in all developmental stages (Fig. 1B).
To understand the physiological roles of dPDZ-GEF, we have isolated flies containing mutations in the dPDZ-GEF locus for genetic analyses. While searching the Berkeley Drosophila Genome Project P-element database, we found two P-element insertion mutants, l(2)k13720 and EP(2)388, with insertion at the 5′ upstream region of the dPDZ-GEF gene (designated here dPDZ-GEF1 and dPDZ-GEF2, respectively, Fig. 1E). While dPDZ-GEF2 showed no visible phenotype, dPDZ-GEF1 homozygous flies showed lethality, with a reduced hatching rate below 50% (data not shown). We expected that P-element insertion at the 5′ flanking region of dPDZ-GEF in dPDZ-GEF1 would prevent dPDZ-GEF expression. As predicted, we found that the expression of dPDZ-GEF was almost completely abolished in the dPDZ-GEF1 homozygous larvae (Fig. 1C) while the nearby genes were unaffected (Fig. 1D).
To further prove that the dPDZ-GEF1 lethality is specifically due to the P-element insertion and to exclude the possibility that this lethality is due to a second-site mutation in the same chromosome arm bearing the dPDZ-GEF1 mutation, we attempted to rescue the phenotype of dPDZ-GEF1 by precisely excising out the existing P{lacW} element from the insertion locus. By mobilizing the P-element by use of the delta2-3 line, we obtained a considerable number of revertants, which displayed no abnormal effects on viability or fertility (data not shown). These revertants could also fully complement the dPDZ-GEF1 allele (data not shown), and we named this allele dPDZ-GEFrv-1. Thus, we could conclude that the dPDZ-GEF1 is a reliable loss-of-function allele of the dPDZ-GEF gene.
dPDZ-GEF is required for normal eye development.
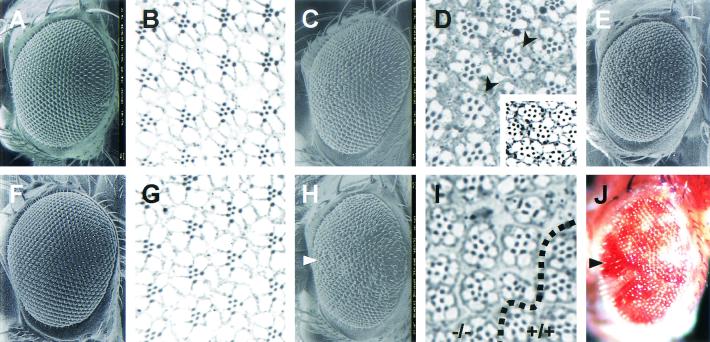
To study the physiological role of dPDZ-GEF, we attempted to use transallelic conditions to obtain a hypomorphic phenotype of the dPDZ-GEF gene. dPDZ-GEF1/dPDZ-GEF2 flies, which have decreased dPDZ-GEF expression, showed a slight rough eye phenotype (Fig. 2C and E), with some missing photoreceptor cells (Fig. 2D). Because the precisely excised allele dPDZ-GEFrv-1 did not show any phenotype in the eye morphology and photoreceptor cell number (Fig. 2F and G), we hypothesized that dPDZ-GEF is involved in the eye development process. To verify this hypothesis, dPDZ-GEF loss-of-function clones were induced in the Drosophila eye by ey-flp mitotic recombination technique (25) as briefly described in Materials and Methods. The dPDZ-GEF1 homozygotic ommatidia, indicated as red eye pigment by mini-white P-element marker, displayed a sunken and disarrayed morphology (Fig. 2H and J). Further tangential sections revealed the loss of many photoreceptor cells in this area (Fig. 2I, −/− area).
FIG. 2.
dPDZ-GEF is required for normal eye development. Flies of the following genotype were examined by SEM and tangential section: w1118 (A and B); dPDZ-GEF1/dPDZ-GEF2 (C, D, and E); dPDZ-GEFrv-1/dPDZ-GEFrv-1 (F and G); y w ey-flp/y w; dPDZ-GEF1 FRT40A/FRT40A (H and I). Shown in panel E and in the insert in panel D are flies of the same genotype as those shown in panel C and in the upper portion of panel D, grown at 30°C for a more severe phenotype. The dPDZ-GEFrv-1 allele (F and G) was obtained by precise excision of dPDZ-GEF1 allele. Shown in panel J is the same eye as that in panel H, photographed by light microscopy. Arrows in panels H and J indicate the same area containing dPDZ-GEF1 homozygotic clones. The red pigment indicates the existence of the P-element, which causes a loss of function of dPDZ-GEF in that area. The dotted line in panel I indicates the boundary between mutant (−/−, upper left part) and normal (+/+, lower right part) ommatidia.
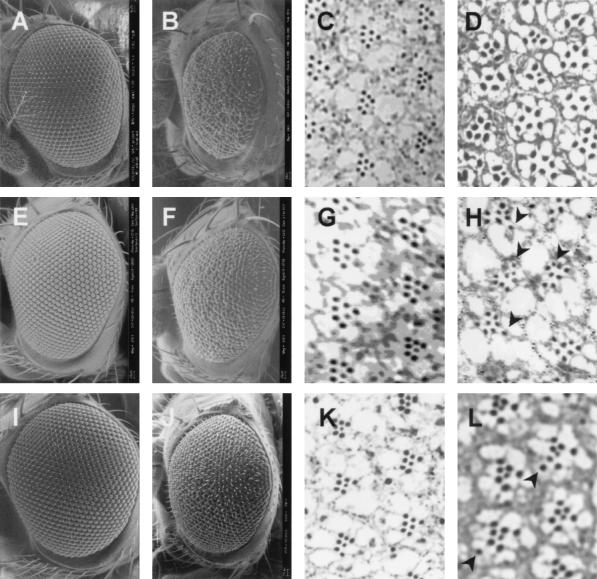
In addition, the dPDZ-GEF1 homozygotic eye, which was generated by ey-flp and gmr-hid technique (31), also showed a small and rough eye phenotype (Fig. 3B) compared to the control eye (Fig. 3A). Subsequent tangential sections of the dPDZ-GEF loss-of-function eye revealed that photoreceptor cell numbers were greatly reduced, to between 3 and 6 (Fig. 3D). Furthermore, some ommatidia were fused together to form a larger cluster.
FIG. 3.
dPDZ-GEF is required for photoreceptor cell differentiation. Eyes from the following genetic backgrounds were analyzed by SEM (A, B, E, F, I, and J) and tangential section (C, D, G, H, K, and L): y w; FRT40A/gmr-hid FRT40A; ey-GAL4 UAS-flp/+ (A and C); y w; dPDZ-GEF1 FRT40A/gmr-hid FRT40A; ey-GAL4 UAS-flp/+ (B and D); gmr-GAL4/+ (F and H); gmr-GAL4/dPDZ-GEF2 (I and K); sev-GAL4/+ (E and G); sev-GAL4/dPDZ-GEF2 (J and L). The arrowheads in panels H and L indicate the ommatidia with extra photoreceptor cells.
Next, we further studied the function of dPDZ-GEF through eye-specific overexpression. Fortunately, the insertion site and direction of the EP-element of the dPDZ-GEF2 (EP(2)388) mutant (Fig. 1E) makes it possible to induce dPDZ-GEF expression by using a tissue-specific GAL4 driver. The eye overexpressing dPDZ-GEF induced by the gmr-GAL4 driver, which directs expression of the gene in the developing eye, showed a rough eye phenotype with some fused clusters of individual ommatidia, without a reduction in total size (Fig. 3F) compared to the control eye (Fig. 3E). Tangential sections of the dPDZ-GEF-overexpressing eye revealed that photoreceptor cell numbers were increased in some ommatidia (Fig. 3H) compared to the control (Fig. 3G). Similar dPDZ-GEF-induced eye phenotypes were also observed by using the sev-GAL4 driver (Fig. 3J and L), which induces a weaker expression than the gmr-GAL4 driver, confirming that these phenotypes are caused by dPDZ-GEF activity, not by GAL4 line-specific side effects.
From the observations shown in Fig. 2 and 3, we concluded that dPDZ-GEF plays important roles in eye development and photoreceptor cell specification in Drosophila.
dPDZ-GEF1 homozygous escapees display a rolled phenotype.
While conducting experiments for determining the lethal phase in dPDZ-GEF1 homozygotes, we found that there is some fluctuation in lethality, possibly due to temperature variations, implying that this mutant is temperature sensitive. Indeed, we were able to obtain the escapees of the lethal phenotype of dPDZ-GEF1 homozygotes by large-scale culture of the flies at a low temperature (18°C). We were able to find more than 40 escapees at a low frequency (∼0.01%) between dPDZ-GEF1/CyO parents, distinguished by the Cy dominant marker.
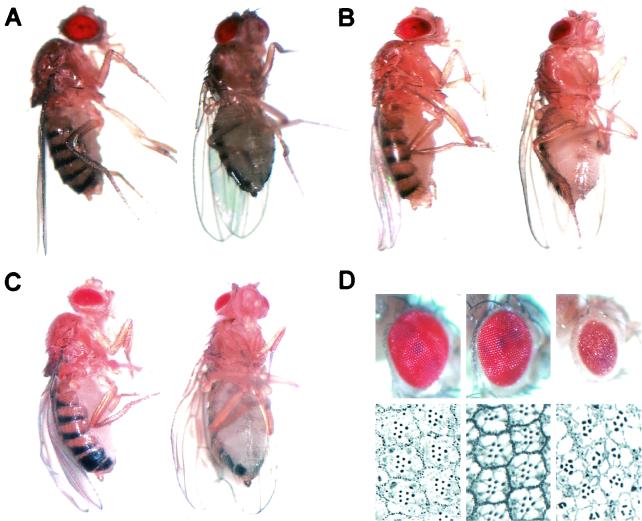
These dPDZ-GEF1 homozygous mutants displayed striking features. The wing edges were rolled downward with frayed margins (Fig. 4C; compare with Fig. 4A), and the eyes became small and rough compared to those of the wild-type flies (Fig. 4D, left panels), a phenotype identical to the loss-of-function eye made through mitotic clone analyses (Fig. 2H through J and 3B and D). We sectioned each eye and could observe the loss of photoreceptor cells in the dPDZ-GEF1 homozygous mutant (Fig. 4D, right panels). These phenotypes are highly similar to the one already described in the rolled mutant, the Drosophila MAPK mutant (Fig. 4B and middle panels of Fig. 4D; see also reference 4 for more-severe phenotypes). This phenotypical relationship suggests that dPDZ-GEF and Drosophila MAPK are involved in the same signaling pathway.
FIG. 4.
The phenotypes of dPDZ-GEF1 homozygous escapees. Rolled and frayed wing blades were visible in lateral and ventral views of rolled1 (B) and dPDZ-GEF1 (C) homozygous mutants but were not observed in Canton-S wild-type flies (A). (D) Surface pictures (top) and sections (bottom) of the eyes from the Canton-S (left), rolled1/rolled1 (middle), and dPDZ-GEF1/dPDZ-GEF1 (right) flies.
However, although rolled mutants usually have gaps in their wing veins, particularly L4, the wing vein pattern was not affected in dPDZ-GEF1 escapees, even though they displayed severe phenotypes for many other aspects. To further examine the effect of dPDZ-GEF in vein formation, we drove dPDZ-GEF overexpression by dpp, ptc, and MS1096 GAL4 drivers, which cause strong gene expression specifically in the wing. As each of these dPDZ-GEF-overexpressing flies showed no apparent change in the vein pattern (data not shown), we concluded that dPDZ-GEF is not directly linked to vein development. Because many other upstream components have been shown to regulate MAPK in vein formation, such as Ras (29), Sos (32), and C3G (15), we hypothesize that dPDZ-GEF does not make a significant contribution to wing vein formation.
Ras is not a functional target of dPDZ-GEF.
As dPDZ-GEF was required for photoreceptor cell fate determination and as the dPDZ-GEF loss-of-function mutants displayed a phenotype similar to that of the rolled mutant, it appeared highly likely that dPDZ-GEF lies upstream of the conventional Ras-MAPK pathway. Therefore, we decided to examine the relationship between Ras and dPDZ-GEF. We speculated that dPDZ-GEF may enhance the activity of wild-type Ras by stimulating its GDP-GTP exchange activity, resulting in a more severely disrupted eye.
However, surprisingly, coexpression of dPDZ-GEF and wild-type Ras resulted in a rather normal eye, with a normal ommatidia array and photoreceptor structure (Fig. 5B). To confirm this, we used the loss-of-function mutant of Ras (Rase1b) to reduce the dosage of the endogenous Ras gene. At one-half dosage background of Ras, the dPDZ-GEF-induced rough eye phenotype and increase of photoreceptor cells are not reduced (Fig. 5C). This is in stark contrast to similar experiments in which Ras dosage reduction resulted in a phenotype rescue in Son of sevenless (Sos) (5) or dC3G (15) gain-of-function mutants. Thus, we came to the unexpected conclusion that Ras is not a functional target of dPDZ-GEF but rather an inhibitor of dPDZ-GEF signaling.
Rap1 is a functional target of dPDZ-GEF.
Next, we examined whether Rap1 is a downstream target of dPDZ-GEF. As described, Rap1 is a Ras-family GTPase of considerable importance in photoreceptor cell development. We used the Raprv(R)B1 allele, a loss-of-function mutant of Rap1, to examine whether there is a functional interaction between PDZ-GEF and Rap1 in Drosophila (13). The number of increased photoreceptors due to dPDZ-GEF overexpression is reduced compared to that of the wild type when one copy of Rap1 is removed (Fig. 5D). Thus, we were able to conclude that the rough eye phenotype caused by dPDZ-GEF is accomplished by Rap1 activation. This is consistent with numerous recent reports (10, 20, 21, 26, 30) that mammalian PDZ-GEF is a Rap1-specific GEF.
MAPK is a downstream target of the dPDZ-GEF signaling pathway.
The phenotype similarity between the dPDZ-GEF mutants and the rolled mutant (Fig. 4) prompted us to further investigate whether the dPDZ-GEF mutant eye phenotype is mediated by MAPK signaling components. Interestingly, the rough eye phenotype and irregular ommatidia array, as well as the increased photoreceptor number caused by dPDZ-GEF overexpression, were strongly suppressed by D-Raf (Fig. 5E), dMEK (Fig. 5F), or dMAPK (Fig. 5G) dosage reduction. We quantified this genetic interaction by counting and calculating the percentage of ommatidia having extra photoreceptor cells for each eye (Fig. 5, lower right panel). These results strongly support that dPDZ-GEF modulates eye development through the MAPK signaling cascade.
rhomboid expression is strongly induced by dPDZ-GEF.
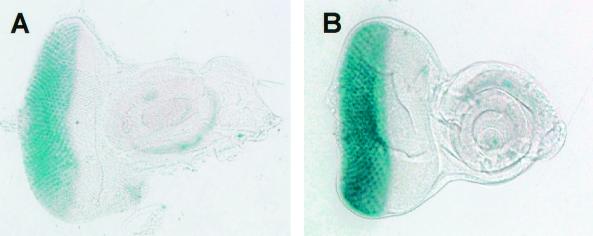
The transcriptional regulation of rhomboid is important in the establishment of EGF receptor-dependent MAPK activation (24). Furthermore, rhomboid is a transcriptional target of the MAPK pathway (22). To further confirm whether dPDZ-GEF activates MAPK, we examined rhomboid expression in the eye under a gmr-GAL4-driven dPDZ-GEF overexpression background. As expected, we were able to detect a much stronger expression of rhomboid in the eye disk overexpressing dPDZ-GEF (Fig. 6B) than in the control eye disk (Fig. 6A) by analyzing the activities of the rhoAA69 enhancer trap reporter.
FIG. 6.
Enhanced rhomboid expression in dPDZ-GEF-overexpressing eye. Enhancer trap lacZ reporter AA69 reveals the expression of rhomboid. (A) Wild-type eye disk (+/+; rhoAA69/+); (B) dPDZ-GEF-overexpressing eye disk (gmr-GAL4 dPDZ-GEF2/+; rhoAA69/+). The two disks were developed under the same conditions and time, as indicated in Materials and Methods.
Ovary degeneration and sterility in dPDZ-GEF1 null females.
We observed that every dPDZ-GEF1 homozygous female exhibited sterility, with no egg laying. To understand this phenomenon, wild-type and dPDZ-GEF1 mutant ovarioles were isolated by dissection and visualized by staining with Hoechst 33258 (Fig. 7A, B, and G) and tetramethyl rhodamine isocyanate-labeled phalloidin (Fig. 7C and D). In contrast to wild-type ovarioles (Fig. 7A and C), we noticed that only a few dPDZ-GEF1 mutant ovarioles survived beyond stage 9, the rest displaying significant degeneration instead (Fig. 7B and D). After dissecting a considerable number (over 10) of female escapees, we observed degeneration in the egg chamber accompanied by nuclei undergoing fragmentation (Fig. 7G). Consistently, TUNEL in situ cell death assays also showed that extensive apoptosis occurred in the degenerated egg chamber (Fig. 7F).
FIG. 7.
Degeneration of ovarioles in the dPDZ-GEF1 mutant female. Ovaries from heterozygous (A, C, and E) and homozygous (B, D, F, and G) dPDZ-GEF1 mutants were stained with Hoechst 33258 (A, B, and G) and phalloidin (C and D). The ovaries of the same genotypes were also examined by TUNEL apoptosis assay (E and F). The degenerating ovarioles of homozygous mutants displayed fragmentation of nuclei (B and G) and a shrunken and crumpled morphology (D), with extensive apoptosis signals (F). RNA in situ hybridization of wild-type ovaries (H through K) showed that dPDZ-GEF expression first appears at stage 8 of ovariole development and continues to a later stage (H through J). (K) RNA in situ signals in the stage 10 egg chamber following longer staining.
RNA in situ experiments also revealed that dPDZ-GEF transcripts are robustly expressed in the nurse cells (Fig. 7H and J) and follicle cells (Fig. 7K) of developing ovarioles. Because the degeneration in the egg chambers (Fig. 7B, D, F, and G) of dPDZ-GEF mutants is observed in a locus and timing similar to dPDZ-GEF expression in wild-type egg chambers, we could conclude that the dPDZ-GEF gene is required for ovary development, possibly by maintaining Rap1 activity, which has previously been shown to be required for ovarian survival (3).
DISCUSSION
dPDZ-GEF and Rap1.
Recent findings have suggested that PDZ-GEF specifically activates Rap1 GTPase by stimulating GTP-GDP exchange (10, 20, 21, 30). Our genetic epistasis analyses showed that Rap1, but not Ras, is the in vivo target of dPDZ-GEF during Drosophila eye development. Similar experiments completed for dC3G, another Rap1-GEF, showed that both Ras and Rap1 are the downstream targets of dC3G (15). Therefore, dPDZ-GEF is the first Drosophila GEF that acts specifically on Rap1 GTPase.
Rap1 was originally thought to inhibit the Ras-MAPK signaling pathway (18), and subsequent studies mainly focused on how Rap1 inhibits the Ras-MAPK pathway (9, 14). However, recent studies on Rap1 action have hinted that Rap1 may have other roles besides inhibiting Ras. Both Ras and Rap1 exhibited mitogenic effects in the Swiss 3T3 cell line (37), and most signals that activate Ras also stimulate Rap1 activation (39). These results suggested that Rap1 plays a Ras-like role in cell growth regulation. In support of this, B-Raf has been suggested to be a specific effector for Rap1. B-Raf can bind to and is activated by Rap1 in vitro (27), and Rap1 was shown to play an important role in the cyclic AMP- or NGF-stimulated activation of B-Raf, which leads to the activation of MAPK (35, 36).
In concert with these findings, the view on the in vivo function of Drosophila Rap1 has also been changed. The Roughened mutation, which causes a reduction in photoreceptor cell number, was originally regarded as a hyperactivated Rap1 allele that inhibits the Ras-dependent MAPK pathway (13). However, genetic analyses revealed that this allele cannot be regarded as a simple hypermorph (19), and Raprv(R)B1, a loss-of-function allele of Rap1, showed no phenotype related to the expected hyperactivation of the Ras-MAPK pathway (3). Furthermore, Rap1 overexpression by gmr-GAL4 caused increases in photoreceptor cell number with defects in eye morphogenesis (15), implicating that Rap1 is rather a positive regulator of photoreceptor cell differentiation. This conclusion is also consistent with the original observation that the Rap1rv(R)B1/Rap1Roughened genotype reduces the photoreceptor cell number more severely than does +/Rap1Roughened (13).
As shown in Fig. 3F, overexpression of dPDZ-GEF also displayed a rough eye phenotype, in a manner similar to that of Rap1 overexpression, with an increase in photoreceptor cell number (Fig. 3H), and this rough eye phenotype was suppressed by reduction of Rap1 activity (Fig. 5D). These results suggest that dPDZ-GEF plays a crucial role in Rap1-mediated ommatidium formation and eye development. The phenotypic similarity between loss-of-function mutants of dPDZ-GEF and Rap1 in ovariole development (Fig. 7) further confirms this connection in the other cellular processes besides photoreceptor cell fate determination.
Although dPDZ-GEF-deficient ommatidia survived with only partial defects in photoreceptor structure (Fig. 3B and D and Fig. 4D, right panels), Rap1-deficient ommatidia did not survive to adult stage, leaving only a scar in their location (3). This implies that another activator, possibly dC3G or other unidentified GEFs, could maintain the minimal activity of Rap1 in the dPDZ-GEF1 homozygotic eye. In addition, suppression of the dPDZ-GEF-induced rough eye phenotype by Rap1 dosage reduction was weaker than those induced by the dosage reductions of downstream components such as D-Raf, dMEK, and dMAPK (Fig. 5). This suggests that dPDZ-GEF could activate another target, possibly another GTPase, to affect the activity of D-Raf and the MAPK signaling pathway. It has been reported that in the mammalian system PDZ-GEF was also able to activate another Rap-subfamily GTPase, Rap2 (10, 30). Indeed, a homologue of Rap2 exists in Drosophila and is involved in eye development (33), although its loss-of-function mutation has not yet been reported. However, we must be aware of the possibility that the Drosophila version of PDZ-GEF may not behave exactly in the same way as mammalian PDZ-GEF. Nonetheless, Rap2 should be investigated as a possible downstream target of dPDZ-GEF.
dPDZ-GEF, a novel MAPK regulator.
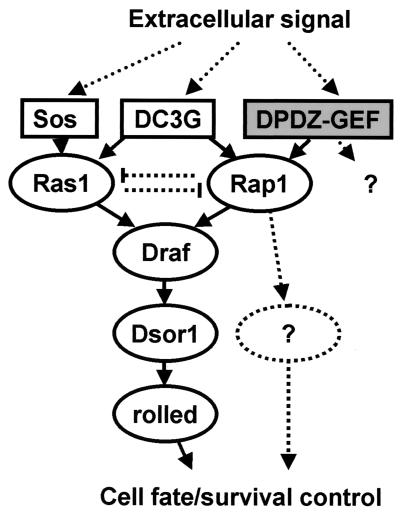
Our findings that dPDZ-GEF is phenotypically related to rolled and is also involved in photoreceptor cell differentiation implicate that dPDZ-GEF plays an important role in the MAPK signaling pathway. As shown in Fig. 5, dPDZ-GEF involves the activities of the well-known downstream components of the MAPK pathway D-Raf, the product of pole hole, dMEK, the product of Drosophila Suppressor of Ras1, and dMAPK, the product of rolled. Thus, in addition to the traditional Ras-MAPK signaling pathway for photoreceptor cell determination, we now propose the presence of a Ras-independent and dPDZ-GEF/Rap1-dependent MAPK signaling pathway, where dPDZ-GEF/Rap1 signaling molecules functionally substitute Sos/Ras1. The idea that Rap1 and Ras1 share and compete for the same effector molecule(s) has been proposed since the discovery of Rap1 (15, 18, 38); however, no further studies have addressed this issue. In this report, we convincingly demonstrated that the dPDZ-GEF/Rap1 signaling pathway and the Sos/Ras signaling pathway converge at D-Raf, the only orthologue of mammalian Rafs in Drosophila (Fig. 8). While a recent review has cautiously suggested that Rap1-dependent signaling may be involved in MAPK signaling in Drosophila (7), our results clearly demonstrate that dPDZ-GEF and Rap1 are involved in the MAPK signaling pathway, at least in photoreceptor cell fate determination.
FIG. 8.
Model showing the role of dPDZ-GEF in the MAPK pathway. Various extracellular signals instigate the MAPK-related signaling cascade. Three GEFs, i.e., Sos, dC3G, and the newly discovered dPDZ-GEF, are involved in this pathway. dPDZ-GEF is the first genetically revealed GEF that is specific for Rap1. The dPDZ-GEF/Rap1 component shares the effector signaling pathway with the Sos/Ras component, and there are likely other effectors yet to be found. In this complex network, dPDZ-GEF controls cell fate and survival as an upstream regulatory component.
However, the requirement of dPDZ-GEF in the developing egg chamber also suggests the possibility of MAPK-independent dPDZ-GEF/Rap1 activity. The ovaries of dPDZ-GEF loss-of-function mutants are degenerated (Fig. 7B, D, F, and G) in a timing similar to that which occurs when the dPDZ-GEF transcription normally starts (Fig. 7H). The consistent observation that the ovaries deficient for Rap1 are degenerated along with apoptotic features (3) also supports the importance of the dPDZ-GEF/Rap1 component for normal ovarian development. However, although the canonical MAPK signaling pathway is highly involved in the regulation of survival and/or proliferation in somatic cells, ovarioles deficient for D-Raf and dMEK develop normally except for Torso pathway defects (2, 34). From these observations, we could deduce that the ovary degeneration phenotype caused by the loss of dPDZ-GEF/Rap1 activity is not due to the silencing in MAPK signaling activity. Therefore, dPDZ-GEF/Rap1 may have unknown effectors other than Raf and the MAPK signaling pathway (Fig. 8).
Another novel finding is that Ras1 can inversely inhibit Rap1-dependent signaling (Fig. 5C). Considering our findings demonstrating that Rap1 and Ras share effector molecule(s), it could be expected that overexpression of either Ras or Rap1 inhibits the other by competing for effector molecule(s). Previous reports have shown the inhibition of Ras-mediated MAPK activation by Rap1 (9, 14); we have demonstrated for the first time that Ras1 is also able to suppress the Rap1-dependent signaling pathway.
In summary, our findings demonstrate that dPDZ-GEF is highly involved in the MAPK signaling pathway, specifically in photoreceptor cell development, while the analysis of wing vein patterns and ovary developments also showed that dPDZ-GEF has other roles that are independent of the MAPK signaling pathway. Further research is required on dPDZ-GEF as an important upstream regulator of the MAPK pathway and modulator of morphogenesis.
Acknowledgments
We thank J. M. Jeung and J. H. Song in KBSI for their technical assistance. We are also grateful to anonymous reviewers for their constructive suggestions to improve this paper.
This research was supported by the Ministry of Science and Technology of Korea.
J.H.L. and K.S.C. contributed equally to this work.
REFERENCES
- 1.Adams, M. D., S. E. Celniker, R. A. Holt, C. A. Evans, J. D. Gocayne, P. G. Amanatides, S. E. Scherer, P. W. Li, R. A. Hoskins, R. F. Galle, et al. 2000. The genome sequence of Drosophila melanogaster. Science 287:2185-2195. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosio, L., A. P. Mahowald, and N. Perrimon. 1989. l(1)pole hole is required maternally for pattern formation in the terminal regions of the embryo. Development 106:145-158. [DOI] [PubMed] [Google Scholar]
- 3.Asha, H., N. D. de Ruiter, M. G. Wang, and I. K. Hariharan. 1999. The Rap1 GTPase functions as a regulator of morphogenesis in vivo. EMBO J. 18:605-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biggs, W. H., III, K. H. Zavitz, B. Dickson, A. van der Straten, D. Brunner, E. Hafen, and S. L. Zipursky. 1994. The Drosophila rolled locus encodes a MAP kinase required in the sevenless signal transduction pathway. EMBO J. 13:1628-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonfini, L., C. A. Karlovich, C. Dasgupta, and U. Banerjee. 1992. The Son of sevenless gene product: a putative activator of Ras. Science 255:603-606. [DOI] [PubMed] [Google Scholar]
- 6.Bos, J. L. 1998. All in the family? New insights and questions regarding interconnectivity of Ras, Rap1 and Ral. EMBO J. 17:6776-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos, J. L., J. de Rooij, and K. A. Reedquist. 2001. Rap1 signaling: adhering to new models. Nat. Rev. Mol. Cell. Biol. 2:369-377. [DOI] [PubMed] [Google Scholar]
- 8.Cho, K. S., J. H. Lee, S. Kim, D. Kim, H. Koh, J. Lee, C. Kim, J. Kim, and J. Chung. 2001. Drosophila phosphoinositide-dependent kinase-1 regulates apoptosis and growth via the phosphoinositide 3-kinase-dependent signaling pathway. Proc. Natl. Acad. Sci. USA 98:6144-6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook, S. J., B. Rubinfeld, I. Albert, and F. McCormick. 1993. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 12:3475-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Rooij, J., N. M. Boenink, M. van Triest, R. H. Cool, A. Wittinghofer, and J. L. Bos. 1999. PDZ-GEF1, a guanine nucleotide exchange factor specific for Rap1 and Rap2. J. Biol. Chem. 274:38125-38130. [DOI] [PubMed] [Google Scholar]
- 11.de Rooij, J., F. J. Zwartkruis, M. H. Verheijen, R. H. Cool, S. M. Nijman, A. Wittinghofer, and J. L. Bos. 1998. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474-477. [DOI] [PubMed] [Google Scholar]
- 12.Gotoh, T., S. Hattori, S. Nakamura, H. Kitayama, M. Noda, Y. Takai, K. Kaibuchi, H. Matsui, O. Hatase, H. Takahashi, et al. 1995. Identification of Rap1 as a target for the Crk SH3 domain-binding guanine nucleotide-releasing factor C3G. Mol. Cell. Biol. 15:6746-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hariharan, I. K., R. W. Carthew, and G. M. Rubin. 1991. The Drosophila roughened mutation: activation of a rap homolog disrupts eye development and interferes with cell determination. Cell 67:717-722. [DOI] [PubMed] [Google Scholar]
- 14.Hu, C. D., K. Kariya, G. Kotani, M. Shirouzu, S. Yokoyama, and T. Kataoka. 1997. Coassociation of Rap1A and Ha-Ras with Raf-1 N-terminal region interferes with ras-dependent activation of Raf-1. J. Biol. Chem. 272:11702-11705. [DOI] [PubMed] [Google Scholar]
- 15.Ishimaru, S., R. Williams, E. Clark, H. Hanafusa, and U. Gaul. 1999. Activation of the Drosophila C3G leads to cell fate changes and overproliferation during development, mediated by the RAS-MAPK pathway and RAP1. EMBO J. 18:145-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawasaki, H., G. M. Springett, N. Mochizuki, S. Toki, M. Nakaya, M. Matsuda, D. E. Housman, and A. M. Graybiel. 1998. A family of cAMP-binding proteins that directly activate Rap1. Science 282:2275-2279. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki, H., G. M. Springett, S. Toki, J. J. Canales, P. Harlan, J. P. Blumenstiel, E. J. Chen, I. A. Bany, N. Mochizuki, A. Ashbacher, M. Matsuda, D. E. Housman, and A. M. Graybiel. 1998. A Rap guanine nucleotide exchange factor enriched highly in the basal ganglia. Proc. Natl. Acad. Sci. USA 95:13278-13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitayama, H., Y. Sugimoto, T. Matsuzaki, Y. Ikawa, and M. Noda. 1989. A ras-related gene with transformation suppressor activity. Cell 56:77-84. [DOI] [PubMed] [Google Scholar]
- 19.Li, Q., I. K. Hariharan, F. Chen, Y. Huang, and J. A. Fischer. 1997. Genetic interactions with Rap1 and Ras1 reveal a second function for the fat facets deubiquitinating enzyme in Drosophila eye development. Proc. Natl. Acad. Sci. USA 94:12515-12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao, Y., K. Kariya, C. D. Hu, M. Shibatohge, M. Goshima, T. Okada, Y. Watari, X. Gao, T. G. Jin, Y. Yamawaki-Kataoka, and T. Kataoka. 1999. RA-GEF, a novel Rap1A guanine nucleotide exchange factor containing a Ras/Rap1A-associating domain, is conserved between nematode and humans. J. Biol. Chem. 274:37815-37820. [DOI] [PubMed] [Google Scholar]
- 21.Liao, Y., T. Satoh, X. Gao, T. G. Jin, C. D. Hu, and T. Kataoka. 2001. RA-GEF-1, a guanine nucleotide exchange factor for Rap1, is activated by translocation induced by association with Rap1-GTP and enhances Rap1-dependent B-Raf activation. J. Biol. Chem. 276:28478-28483. [DOI] [PubMed] [Google Scholar]
- 22.Martin-Blanco, E., F. Roch, E. Noll, A. Baonza, J. B. Duffy, and N. Perrimon. 1999. A temporal switch in DER signaling controls the specification and differentiation of veins and interveins in the Drosophila wing. Development 126:5739-5747. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno, T., K. Kaibuchi, T. Yamamoto, M. Kawamura, T. Sakoda, H. Fujioka, Y. Matsuura, and Y. Takai. 1991. A stimulatory GDP/GTP exchange protein for smg p21 is active on the post-translationally processed form of c-Ki-ras p21 and rhoA p21. Proc. Natl. Acad. Sci. USA 88:6442-6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nambu, J. R., R. G. Franks, S. Hu, and S. T. Crews. 1990. The single-minded gene of Drosophila is required for the expression of genes important for the development of CNS midline cells. Cell 63:63-75. [DOI] [PubMed] [Google Scholar]
- 25.Newsome, T. P., B. Asling, and B. J. Dickson. 2000. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127:851-860. [DOI] [PubMed] [Google Scholar]
- 26.Ohtsuka, T., Y. Hata, N. Ide, T. Yasuda, E. Inoue, T. Inoue, A. Mizoguchi, and Y. Takai. 1999. nRap GEP: a novel neural GDP/GTP exchange protein for rap1 small G protein that interacts with synaptic scaffolding molecule (S-SCAM). Biochem. Biophys. Res. Commun. 265:38-44. [DOI] [PubMed] [Google Scholar]
- 27.Ohtsuka, T., K. Shimizu, B. Yamamori, S. Kuroda, and Y. Takai. 1996. Activation of brain B-Raf protein kinase by Rap1B small GTP-binding protein. J. Biol. Chem. 271:1258-1261. [DOI] [PubMed] [Google Scholar]
- 28.Pham, N., I. Cheglakov, C. A. Koch, C. L. de Hoog, M. F. Moran, and D. Rotin. 2000. The guanine nucleotide exchange factor CNrasGEF activates ras in response to cAMP and cGMP. Curr. Biol. 10:555-558. [DOI] [PubMed] [Google Scholar]
- 29.Prober, D. A., and B. A. Edgar. 2000. Ras1 promotes cellular growth in the Drosophila wing. Cell 100:435-446. [DOI] [PubMed] [Google Scholar]
- 30.Rebhun, J. F., A. F. Castro, and L. A. Quilliam. 2000. Identification of guanine nucleotide exchange factors (GEFs) for the Rap1 GTPase. Regulation of MR-GEF by M-Ras-GTP interaction. J. Biol. Chem. 275:34901-34908. [DOI] [PubMed] [Google Scholar]
- 31.Stowers, R. S., and T. L. Schwarz. 1999. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics 152:1631-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taguchi, A., K. Sawamoto, and H. Okano. 2000. Mutations modulating the argos-regulated signaling pathway in Drosophila eye development. Genetics 154:1639-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toba, G., T. Ohsako, N. Miyata, T. Ohtsuka, K. H. Seong, and T. Aigaki. 1999. The gene search system. A method for efficient detection and rapid molecular identification of genes in Drosophila melanogaster. Genetics 151:725-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuda, L., Y. H. Inoue, M. A. Yoo, M. Mizuno, M. Hata, Y. M. Lim, T. Adachi-Yamada, H. Ryo, Y. Masamune, and Y. Nishida. 1993. A protein kinase similar to MAP kinase activator acts downstream of the raf kinase in Drosophila. Cell 72:407-414. [DOI] [PubMed] [Google Scholar]
- 35.Vossler, M. R., H. Yao, R. D. York, M. G. Pan, C. S. Rim, and P. J. Stork. 1997. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell 89:73-82. [DOI] [PubMed] [Google Scholar]
- 36.York, R. D., H. Yao, T. Dillon, C. L. Ellig, S. P. Eckert, E. W. McCleskey, and P. J. Stork. 1998. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature 392:622-626. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida, Y., M. Kawata, Y. Miura, T. Musha, T. Sasaki, A. Kikuchi, and Y. Takai. 1992. Microinjection of smg/rap1/Krev-1 p21 into Swiss 3T3 cells induces DNA synthesis and morphological changes. Mol. Cell. Biol. 12:3407-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwartkruis, F. J., and J. L. Bos. 1999. Ras and Rap1: two highly related small GTPases with distinct function. Exp. Cell Res. 253:157-165. [DOI] [PubMed] [Google Scholar]
- 39.Zwartkruis, F. J., R. M. Wolthuis, N. M. Nabben, B. Franke, and J. L. Bos. 1998. Extracellular signal-regulated activation of Rap1 fails to interfere in Ras effector signalling. EMBO J. 17:5905-5912. [DOI] [PMC free article] [PubMed] [Google Scholar]