Abstract
Objective:
To evaluate the influence of the response to preoperative chemotherapy, especially tumor progression, on the outcome following resection of multiple colorectal liver metastases (CRM).
Summary Background Data:
Hepatic resection is the only treatment that currently offers a chance of long-term survival, although it is associated with a poor outcome in patients with multinodular CRM. Because of its better efficacy, chemotherapy is increasingly proposed as neoadjuvant treatment in such patients to allow or to facilitate the radicality of resection. However, little is known of the efficacy of such a strategy and the influence of the response to chemotherapy on the outcome of hepatic resection.
Methods:
We retrospectively analyzed the course of 131 consecutive patients who underwent liver resection for multiple (≥4) CRM after systemic chemotherapy between 1993 and 2000, representing 30% of all liver resections performed for CRM in our institution during that period.
Chemotherapy included mainly 5-fluorouracil, leucovorin, and either oxaliplatin or irinotecan for a mean of 9.8 courses (median, 9 courses). Patients were divided into 3 groups according to the type of response obtained to preoperative chemotherapy. All liver resections were performed with curative intent. We analyzed patient outcome in relation to response to preoperative chemotherapy.
Results:
There were 58 patients (44%) who underwent hepatectomy after an objective tumor response (group 1), 39 (30%) after tumor stabilization (group 2), and 34 (26%) after tumor progression (group 3). At the time of diagnosis, mean tumor size and number of metastases were similar in the 3 groups. No differences were observed regarding patient demographics, characteristics of the primary tumor, type of liver resection, and postoperative course. First line treatments were different between groups with a higher proportion of oxaliplatin- and/or irinotecan-based treatments in group 1 (P < 0.01). A higher number of lines of chemotherapy were used in group 2 (P = 0.002). Overall survival was 86%, 41%, and 28% at 1, 3, and 5 years, respectively. Five-year survival was much lower in group 3 compared with groups 1 and 2 (8% vs. 37% and 30%, respectively at 5 years, P < 0.0001). Disease-free survival was 3% compared with 21% and 20%, respectively (P = 0.02). In a multivariate analysis, tumor progression on chemotherapy (P < 0.0001), elevated preoperative serum CA 19–9 (P < 0.0001), number of resected metastases (P < 0.001), and the number of lines of chemotherapy (P < 0.04), but not the type of first line treatment, were independently associated with decreased survival.
Conclusions:
Liver resection is able to offer long-term survival to patients with multiple colorectal metastases provided that the metastatic disease is controlled by chemotherapy prior to surgery. Tumor progression before surgery is associated with a poor outcome, even after potentially curative hepatectomy. Tumor control before surgery is crucial to offer a chance of prolonged remission in patients with multiple metastases.
In patients with multiple colorectal liver metastases, tumor progression while on preoperative chemotherapy is associated with a poor outcome of liver surgery. Tumor control before liver resection is crucial to offer a chance of prolonged remission.
Hepatic resection is the only treatment that currently offers a chance of long-term survival in patients with colorectal metastases. It is associated with 5-year survival rates ranging from 25% to 41%.1–6 Among the prognostic factors affecting the outcome after liver resection, the number of metastases is one of the most commonly reported.2,3,5,7–11 At the time of diagnosis, it is also the major reason of unresectability. When liver resection is feasible, there is general agreement that patients with 4 or more metastatic nodules gain little benefit from liver resection,2,9–11 although some authors have found no effect of the number of lesions on prognosis.4,12 In our practice however, a high number of metastases has never been considered a contraindication to surgery provided that liver resection was potentially curative and that preoperative chemotherapy had been delivered to control or to downstage metastatic disease.
In recent years, great improvements in the effectiveness of chemotherapy have been achieved for metastatic colorectal cancer. Response rate observed with 5-fluorouracil (5-FU) and leucovorin have been significantly increased by the combination with oxaliplatin and/or irinotecan and by changes in drug delivery.13–17
These higher response rates have played a key role in improving the resectability of hepatic metastases, allowing 15% to 20% of patients with initially unresectable tumors to be secondarily resected with reported 5-year survival rates of 30% to 40%.18,19
Irrespective of their initial resectability, our attitude has been to manage these patients by a combination of preoperative chemotherapy and surgery with the objective to treat the metastatic disease through a combined systemic and local approach. The rationale of this policy has been recently supported by the better prognosis obtained with neoadjuvant chemotherapy and surgery, as compared with immediate surgery in patients with multinodular colorectal liver metastases.20 No attention was paid in this latter study to the influence of the response to preoperative chemotherapy on the outcome following hepatic resection. The aim of the present study was to evaluate the role of this factor for the outcome of patients having multiple (≥4) metastases who underwent liver resection.
PATIENTS AND METHODS
From February 1993 to January 2001, 441 consecutive patients underwent liver resection for CRM at our institution. Among these, 140 (32%) had at least 4 metastatic nodules, of whom 131 (30%) received systemic chemotherapy before liver surgery (Fig. 1). The rationale of this approach relied on the assumption that occult metastases could be frequent in this setting and that chemotherapy was likely to improve the radicality of liver resection and to optimize the otherwise poor outcome of these patients. The study therefore focused on the 131 patients who received preoperative chemotherapy.
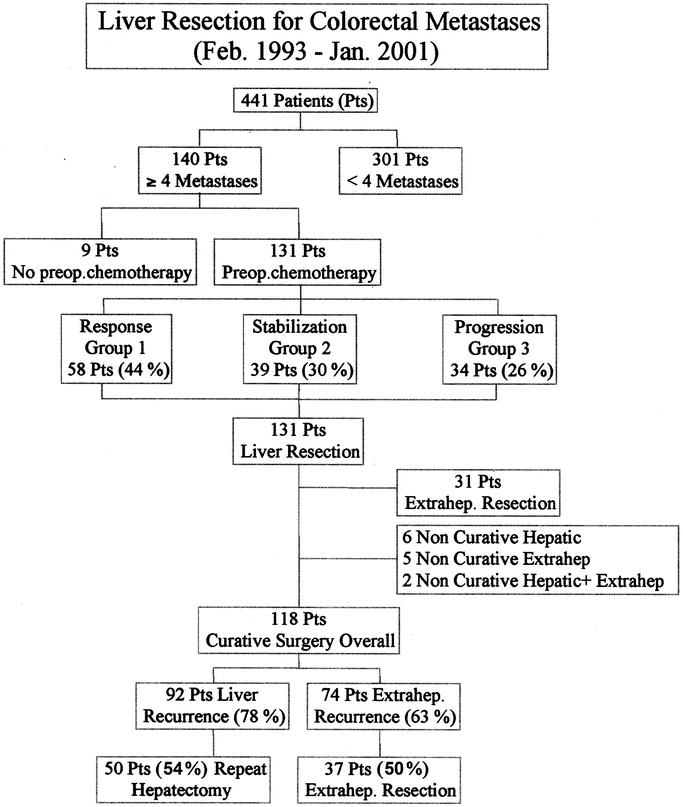
FIGURE 1. Selection and outcome of the population of the study.
Patients and Tumor Characteristics
Patient and tumor characteristics are summarized in Table 1. There were 76 men and 55 women with a median age of 59.5 years (range, 32–78 years). Liver metastases were synchronous in 97 cases (74%), bilobar in 116 cases (89%) with a median number of 5 (range, 4–17) and a median largest diameter of 38 mm (range, 10–160 mm) (Table 1). Extrahepatic metastases were detected either preoperatively or at the time of liver resection in 31 patients (24%). All extrahepatic sites were technically resectable either sequentially or at the time of liver resection. Sites of extrahepatic tumor were: lungs (n = 11), hepatic or celiac lymph nodes (n = 7), peritoneum (n = 5), site of the primary tumor (n = 2), ovaries (n = 2), vagina (n = 1), uterus (n = 1), prostate (n = 1), and small bowel (n = 1).
TABLE 1. Patient and Tumor Characteristics and Preoperative Chemotherapy
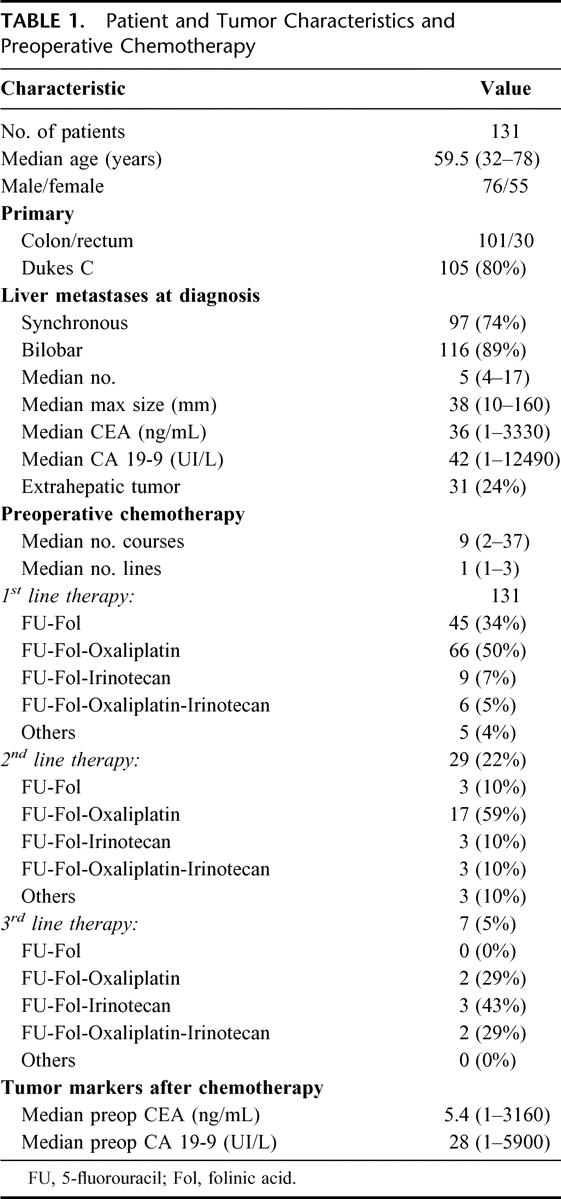
Preoperative Management
All patients received preoperative chemotherapy, mainly 5-FU and leucovorin, combined with either oxaliplatin or irinotecan or both. The treatment was delivered for a median number of 9 courses (range, 2–37 courses) with chronomodulated infusion in 50% of cases, as previously described.14,16 A total of 101 patients (77%) received a single line of chemotherapy, 23 (18%) had 2 lines, and 7 (5%) received 3 lines of chemotherapy. The regimens of each line are detailed in Table 1. The objective was different according to the initial resectability of metastases:
For unresectable patients, chemotherapy was the only means to convert them into resectable ones, through downstaging of the tumors. Chemotherapy in these circumstances was continued as long as the resectability of metastases was not obtained.
For resectable patients, the first objective of chemotherapy was to provide a time interval before surgery, to better assess the tumor biology, to treat potentially occult disease, and to avoid surgery in those patients with rapidly progressive disease as a result of primary resistance to chemotherapy. A second objective in these resectable patients was to achieve cytoreduction both to limit the extent of liver resection, and thus the operative and postoperative morbidity, and to facilitate the radicality of resection.
In all cases, imaging studies were routinely done every 3 to 4 courses of chemotherapy and reviewed by a multidisciplinary team, including medical oncologists, radiologists, and surgeons at oncosurgical staff meetings, to evaluate the tolerance and response to treatment, the need to switch the protocol of chemotherapy in case of tumor progression, and to assess the possibility of radical liver surgery.
Evaluation of Response to Chemotherapy
Response to chemotherapy was evaluated from serial imaging studies (abdominopelvic and chest CT scan, abdominopelvic ultrasound) and was based on tumor diameter changes according to the World Health Organization criteria (WHO).21 Response was defined as 50% or more decrease in total tumor size of the lesions, ie, of the sum of the products of the perpendicular diameters of all measurable lesions. Stabilization was defined as a less than 50% decrease or a less than 25% increase in total tumor size. Progression was defined as a 25% or more increase in total tumor size and/or the appearance of new lesions at any site. Serum carcinoembryonic antigen (CEA) and carbohydrate antigen (CA 19–9) levels were routinely measured. When more than one treatment regimen was used in the same patient, it was the response to the last regimen used preoperatively that was considered for the study.
Selection Criteria for Liver Resection
Patients were eligible for liver resection when the following conditions were met: 1) no comorbid condition precluded a major hepatic resection; 2) all tumoral liver disease was amenable to resection and ablative treatment if any, while leaving at least 30% of nontumoral liver parenchyma; 3) recurrence of the primary tumor was excluded by colonoscopy; and 4) no unresectable extrahepatic disease was detected by preoperative CT scan of abdomen, pelvis, and chest. When potentially resectable, extrahepatic tumor was not considered a contraindication to sequential surgery.
The time interval between the last chemotherapy and hepatic surgery was usually 2 to 4 weeks to minimize the risk of tumor progression.
Operative Technique
The policy of liver resection attempted a radical resection by anatomic or wedge resection, sparing the highest amount of liver parenchyma possible but providing a tumor-free margin of ≥1 cm whenever possible. All procedures routinely used intraoperative ultrasound, ultrasonic dissector, argon beam, and bipolar coagulation forceps to reduce intraoperative blood loss. Cryosurgery and/or radiofrequency devices were used in combination with conventional surgery to treat nonresectable remnant lesions, thus permitting to extend the indications of liver resection to patients who otherwise would not have been candidates for surgery.
Postoperative Management
Patients had planned follow-up at 1 month and then every 4 months with evaluation of tumor markers (CEA and CA 19–9), liver function tests, and hepatic ultrasound. Abdominal and chest CT scan were performed every 8 months. In case of resectable extrahepatic metastases, they were resected usually 2 to 3 months after hepatic surgery, using systemic chemotherapy between operations, to prevent tumor progression.
To decrease the risk of recurrence in these patients, systemic chemotherapy was continued postoperatively for 6 to 8 courses.
Design of the Study
Patients were divided into 3 groups according to the response to preoperative chemotherapy (response, stabilization, or progression). Liver resection either in one or in 2 stages was performed only when potentially curative. Outcome was analyzed in relation to the response to preoperative chemotherapy.
Statistics
Comparison of the main characteristics of the primary tumor, of liver metastases, of chemotherapy, and of liver resection within the 3 groups. An univariate analysis was performed with survival and disease-free survival as endpoints for 35 items concerning patients characteristics (age, gender), data of the primary tumor (location, stage, lymph node invasion, adjuvant chemotherapy, time interval between colectomy and hepatectomy), preoperative chemotherapy (number of courses, number of lines, chronomodulated infusion, type of regimen, response to chemotherapy), liver metastases (synchronous, bilobarity, number, size, resectability, serum CEA, serum CA 19–9, metastatic pedicular lymph nodes), concomitant extrahepatic disease (location, curative resection), and liver resection (preoperative serum levels of CEA and CA 19–9, portal embolization, 2-stage procedure, combined radiofrequency or cryotherapy, major hepatectomy, anatomic, curative, number and size of metastases on the specimen, blood units transfused, duration of hospital stay). Overall and disease-free survival probabilities were calculated using the Kaplan-Meier method, and data were compared by the log-rank test. A multivariate analysis using a Cox model was performed for all factors emerging as determinant for overall and disease-free survival in the univariate analysis.
RESULTS
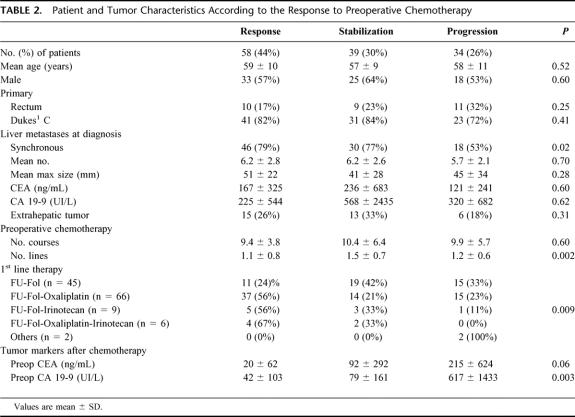
Liver resection was performed after an objective tumor response in 58 patients (44%) (group 1), after tumor stabilization in 39 patients (30%) (group 2), and after tumor progression in 34 patients (26%) (group 3) (Fig. 1). At the time of diagnosis, mean tumor size and mean number of metastases were similar between groups (Table 2). However, there were less synchronous metastases in group 3 (53%) than in groups 1 and 2 (79% and 77%, respectively, P = 0.02). No difference was noted between groups for patient characteristics. The chemotherapy used was similar between groups regarding the number of courses and the proportion of chronomodulated infusion. First line treatment was different between groups with a higher proportion of oxaliplatin and/or irinotecan based treatments in group 1 (P = 0.009) and a higher number of lines of chemotherapy in group 2 (P = 0.002). Although similar between groups at the time of diagnosis and decreased overall by chemotherapy, tumor marker (CEA, CA 19–9) levels were significantly higher preoperatively in group 3, especially for CA 19–9 (P = 0.003) (Table 2).
TABLE 2. Patient and Tumor Characteristics According to the Response to Preoperative Chemotherapy
Liver Resection
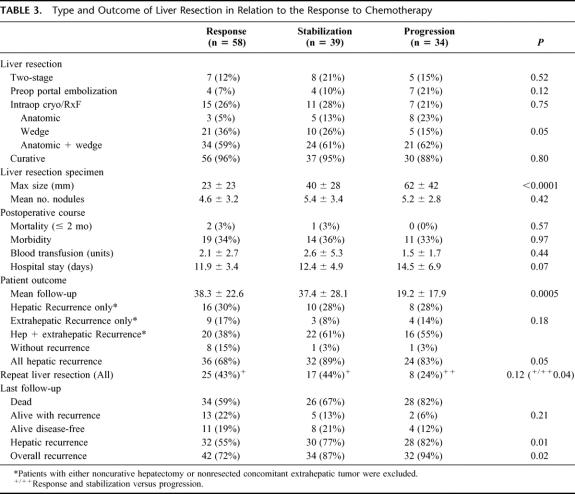
The type of liver resection was similar between groups with the exception that there were more anatomic resections in group 3 (23%) than in groups 1 and 2 (5% and 13%, respectively, P = 0.05) (Table 3). A total of 127 procedures (97%) were major hepatectomies (≥3 segments resected) and 123 patients (94%) had a potentially curative hepatectomy.
TABLE 3. Type and Outcome of Liver Resection in Relation to the Response to Chemotherapy
Overall operative mortality rate within 2 months was 2.3% (3 of 131) and related to postoperative liver failure (1 patient), myocardial infarction (1 patient), and rectal bleeding related to radiation (1 patient). Postoperative morbidity was similar between groups (Table 3).
Mean duration of hospitalization was longer in group 3 (14.5 days) than in groups 1 and 2 (11.9 and 12.4 days, respectively), but the difference did not reach significance (P = 0.07). While the number of metastases on the liver specimen was similar between groups, the size of the largest lesion was greater in group 3 (diameter, 62 mm compared with 23 or 40 mm in groups 1 or 2, respectively, P < 0.0001).
Resection of Concomitant Extrahepatic Metastases
Among the 31 patients with concomitant extrahepatic sites, 24 ultimately underwent resection of the extrahepatic site (74%). The ratio between actual and planned resections of extrahepatic metastases was as follows: 7 of 11 pulmonary resections, 5 of 7 lymphadectomies (hepatic pedicle and/or celiac nodes), 5 of 5 peritoneal resections, 2 of 2 ovariectomies, 2 of 2 colorectal resections, 1 of 1 partial resection of the small intestine, 1 of 1 vaginal resection, 1 of 1 hysterectomy, and 0 of 1 prostate resection. When both hepatic and extrahepatic sites were considered, surgery achieved to be curative for 118 of the 131 patients of the study (90%) (Fig. 1).
Outcome
After a mean follow-up of 33.1 months (range, 1–115 months; median, 27 months), 92 patients suffered from hepatic recurrence (78%) among the 118 patients who had undergone curative surgery. Liver recurrence was isolated in 34 patients (29%) and associated with extrahepatic recurrence in 58 patients (49%). Overall, recurrences were less in group 1 (68%) than in groups 2 and 3 (89% and 83%, respectively, P = 0.05). Fifty of the 92 patients with hepatic recurrence had a repeat hepatectomy (54%). Repeat liver resection was more frequently performed in patients of groups 1 and 2 (43% and 44%) than in group 3 (24%) (P = 0.04). Overall, a total of 184 hepatectomies were performed in 131 patients.
A total of 124 patients were free of extrahepatic disease after surgery (7 patients could not undergo the planned resection of their extrahepatic tumor). Among these, 74 developed extrahepatic recurrence (60%). Of these latter 74 patients, 37 (50%) underwent one or more reoperations for extrahepatic recurrence. The total number of surgical procedures was 67. Six of these 37 patients (16%) were ultimately free of tumor. Overall 90 resections of extrahepatic disease were performed.
At the last follow-up, hepatic recurrence rate was 69%, significantly higher in group 3 (82%) than in group 1 and 2 (55% and 77%, P = 0.01). Overall recurrence rate was 82%, still higher in group 3 (94%) than in groups 1 and 2 (72% and 87%, P = 0.02).
Survival
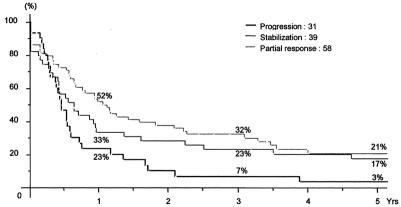
Overall survival of the 131 patients was 86%, 41%, and 28% at 1, 3, and 5 years, respectively, with a median survival of 30 months (Fig. 2). It was much lower for patients of group 3 (8% at 5 years) than for those of groups 1 and 2 (37% and 30%, respectively, P < 0.0001) (Fig. 3). Similarly, disease-free survival was different between groups (3% vs. 21% and 17% at 5 years, respectively, P = 0.02) (Fig. 4).
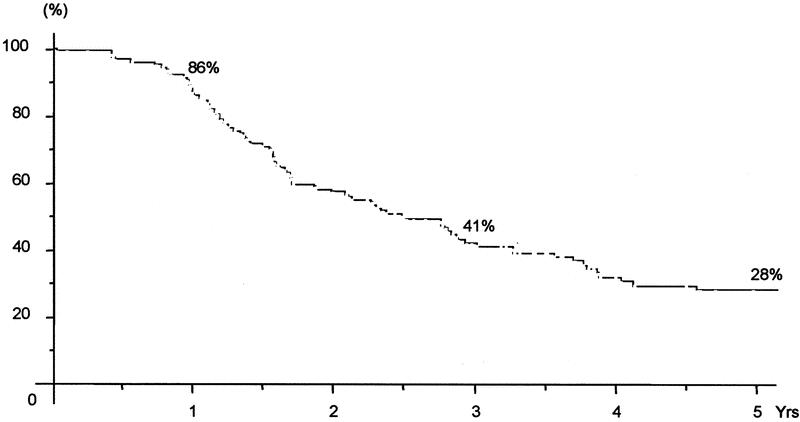
FIGURE 2. Overall survival of all patients who underwent liver resection for ≥4 metastases after chemotherapy.

FIGURE 3. Overall survival in relation to the response to preoperative chemotherapy.
FIGURE 4. Disease-free survival in relation to the response to preoperative chemotherapy.
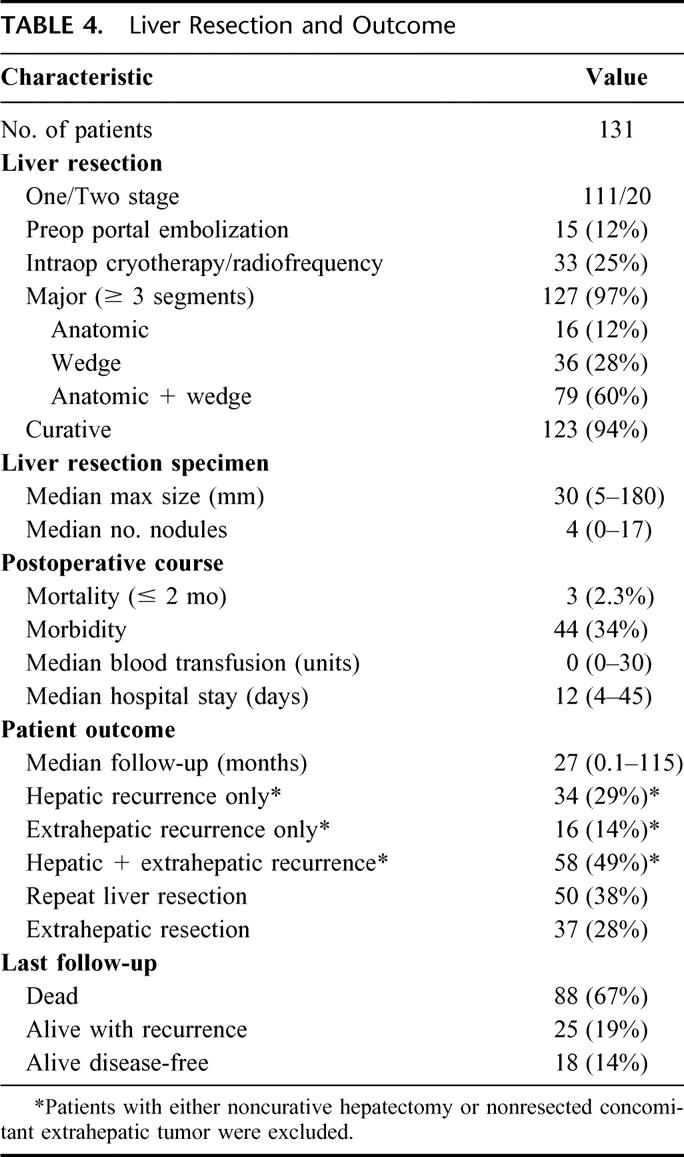
At last follow-up, 88 patients had died (67%) and 43 were alive (33%), 18 of whom were disease-free (14%) and 25 were alive with disease (19%) (hepatic disease only, 5 patients; extrahepatic disease only, 2 patients; and both, 18 patients) (Table 4).
TABLE 4. Liver Resection and Outcome
Prognostic Factors of Survival
At univariate analysis, 4 factors were significantly associated with decreased survival (Table 5): Tumor progression (P < 0.0001), elevated preoperative serum CA 19–9 (P < 0.0001), number of resected metastases (P = 0.01), maximum tumor diameter (P = 0.009). The type of first line chemotherapy had no significant impact on survival, while the number of lines of treatment had a nonsignificant impact (P = 0.09).
TABLE 5. Prognostic Factors of Survival
At multivariate analysis, 4 factors were significantly associated with decreased survival (Table 5): tumor progression (P < 0.0001), elevated preoperative serum CA 19–9 (P < 0.0001), number of resected metastases (P < 0.001), number of lines of chemotherapy (P = 0.04). All but the latter factor were also significant for disease-free survival (Table 5).
DISCUSSION
This study shows that liver resection combined with pre and postoperative chemotherapy offers the possibility of long-term survival to patients with multiple colorectal metastases. This benefit can be obtained, only when metastatic disease is controlled by chemotherapy prior to liver surgery. Tumor progression while on preoperative chemotherapy is associated with poor outcome, even when hepatectomy is potentially curative. Therefore, disease control before surgery is crucial to prolonged disease-free survival.
Although usually associated with a poor outcome, liver resection for multinodular metastases was used as a model to test the influence of response to neoadjuvant chemotherapy on outcome. In the 1980s, the presence of more than 3 lesions was considered a contraindication to liver surgery.9,22 More recently, this dogma was overcome because better results were obtained with rising expertise in liver surgery, decreasing operative mortality, and higher efficacy of chemotherapy.23,24 However, there is still no agreement on the real benefit to operate such patients and on the strategy to adopt for an optimized outcome.
In our practice, the number of metastases has never been a contraindication to surgery provided that a complete resection was technically possible, leaving at least 30% of nontumoral liver parenchyma behind, to prevent the risk of postoperative liver failure. Five-year survival after liver resection was 28% in our patients. This rate compares favorably to other surgical series reporting no 3-year survivors at all9 or more recently, a 23% 5-year survival rate.23,25 The median survival of 30 months in our study population also compares favorably to that of 20 months observed with the currently most effective chemotherapy regimens alone.15,26 However, the overall survival rate was lower than the 51% 5-year survival of our patients resected for less than 4 metastases during the same period (data not shown). Nevertheless, as long-term survival is almost impossible to obtain with the sole use of chemotherapy,1,27 liver resection is the only means to offer these patients a real chance of long-term remission.
Owing to the extent of the disease within the liver and sometimes outside, these patients are at high risk for occult metastases and for recurrence after surgery. Therefore, our policy has been to combine surgery with pre and postoperative chemotherapy. We previously showed, in patients with multiple liver metastases of colorectal cancer, that neoadjuvant chemotherapy before hepatectomy was associated with improved survival and less extended hepatectomies compared with immediate surgery.20 However, the influence of the response to chemotherapy remained to be evaluated. Response to neoadjuvant chemotherapy has been associated with improved outcome following surgery in breast cancer,28 esophageal cancer,29 and in soft tissue sarcomas.30 In hepatocellular carcinoma, preoperative transarterial chemoembolization was associated with improved outcome through the positive effect of tumor downstaging or tumor necrosis.31 Similarly, in colorectal liver metastases, it was recently reported that patients whose disease did not progress while they were receiving neoadjuvant chemotherapy experienced improved survival after liver resection as compared with patients who did not receive chemotherapy.32 These latter results were restricted to the setting of synchronous metastases, and the outcome was not specifically related to the type of response on chemotherapy. By focusing on a homogeneous group of patients with multiple (≥4) colorectal metastases, our study demonstrated not only that response to preoperative chemotherapy was a key prognostic indicator of survival after resection in such patients but that tumor progression while on chemotherapy could represent a contraindication to surgery. The type of first line chemotherapy played an important role as evidenced by the fact that oxaliplatin or irinotecan-based treatments had better response rates (56%) than only 5-FU based regimens (24%). While having similar patient and tumor characteristics initially and identical pre and intraoperative management, patients whose metastases responded to or were stabilized with chemotherapy had a lower incidence of tumor recurrence and a better survival than those who developed tumor progression. This latter group of patients had only an 8% overall survival rate at 5 years. This low survival puts into question the utility of liver resection in this situation and may have different implications for the therapeutic strategy adopted by medical oncologists and by surgeons. For oncologists, such results mean that the best timing for referring a patient to a surgeon is when chemotherapy is having a positive effect and not when the disease “escapes” to treatment. For surgeons sometimes more focused on resectability criteria, such results mean that the radicality of the procedure is necessary but not sufficient to achieve long-term survival in such patients.
The potential risk to miss the possibility of resection in patients initially resectable, because of tumor progression on chemotherapy, could be used to argue against neoadjuvant chemotherapy. In our view, the risk was minimized by 1) repeating evaluation frequently (every 3 courses), allowing a change of treatment if necessary; and 2) the efficacy of current regimens of chemotherapy achieving either a stabilization or a response in more than 80% of patients.13–17 Accordingly, treating resectable patients with neoadjuvant chemotherapy did not lead to unresectable. metastases, during the period of the study, as observed by others.32
The question remains whether the response to chemotherapy simply identified patients who have a predetermined favorable prognosis or whether the response was able to modify the course of the disease. Supporting the second hypothesis, progression was clearly identified as an independent factor of outcome on multivariate analysis. Also, the possibility of 5-year survival in initially unresectable patients switched to resectability by chemotherapy,18,19 compared with the absence of any long-term survivor without liver surgery,1,27 argues for the possibility that the course of the disease could be altered by an aggressive strategy. The course of the disease was not only influenced by the response to neoadjuvant chemotherapy combined with liver surgery. In addition, resection of extrahepatic metastases, postoperative chemotherapy, as well as repeat hepatic or extrahepatic surgery were critical to obtain long-term benefit.
CONCLUSION
Response to neoadjuvant chemotherapy plays a key role in the potential benefit offered by liver resection to patients with multiple metastases. The poor results obtained by surgery in patients with tumor progression suggest that control of the disease with a modern combination regimen is preferable to immediate surgery. Overall, a good survival benefit could only be obtained through an aggressive treatment of hepatic and extrahepatic disease.
Discussions
Dr. Margreiter: This is certainly an important piece of work, which may have implications on future therapeutic strategies in patients with colorectal liver metastases. This retrospective analysis contains, however, a few weak points such as the number of different chemotherapy protocols. According to Table 1 in the manuscript, only 120 of 131 patients received chemotherapy. Since most of them were given FU-Fol or FU-Fol + oxaliplatinum, results would be more meaningful if patients were stratified according to these 2 regimens. Furthermore, 31 patients with extrahepatic tumor manifestations were included, but only 23 were operated. What were the reasons for not operating on 8 patients and how did that influence the outcome?
There were 2 categories of patients: category I patients were primarily inoperable and were given neoadjuvant chemotherapy to downstage the tumors and make them resectable. Other patients were primarily resectable and received chemotherapy to better assess tumor biology. It would be interesting to know how many patients were in each category and how the number of treatment cycles was determined in patients who were already operable before treatment.
Thirty-three patients were obviously not curatively resectable, and cryotherapy or radiofrequency ablation had to be applied. Was that known beforehand or did it come as a surprise during surgery? A total of 15 patients underwent portal embolization before surgery in order to augment the remaining liver segments. It has been asserted that this principle cannot work under cytotoxic chemotherapy. What was the result of portalembolization with regard to augmentation of the liver volume in this cohort of patients?
I would agree with the authors' conclusion that patients with progression of their metastatic liver disease under polychemotherapy should not be resected, since other authors have reported better survival using only chemotherapy.
Finally, I would like to congratulate the authors on their outstanding work.
Dr. Adam: Many thanks for these very interesting comments. With regard to your first question regarding chemotherapy, of course we have been operating patients after different protocols of chemotherapy. As we often act as a second or tertiary center, it is difficult in practice to obtain from first medical oncologists the same use of chemotherapy. Nevertheless, I think that the important message remains that, whichever is the chemotherapy delivered, tumor progression while on chemotherapy is a factor of poor prognosis and could be a relative contraindication to liver surgery. Concerning the presence of extrahepatic disease, our policy is not to consider it as an absolute contraindication to sequential surgery provided that the global strategy is potentially curative. However, this objective was not achieved for 7 of the 31 patients with extrahepatic metastases, mainly because tumor progression after liver resection or because of the appearance of new tumor localizations. On an intention-to-treat basis, those patients were included in the series despite their poor ultimate prognosis compared to that of patients who achieved to undergo a sequential resection. With regard to the initial resectability of patients, we deliberately made the choice not to deal with it. As you know, criteria of resectability may vary from one group to another, and within the same group with the evolution of time. Therefore, it could be a subjective criterion, and we have preferred to use an objective selection criterion such as a number of metastases of 4 and more. Concerning your last question about portal embolization, I agree with you that early chemotherapy could inhibit contralateral hypertrophy. For this reason, we wait at least 3 weeks after portal embolization to do chemotherapy.
Dr. Jaeck: Dr. Adam, congratulations for this nice study. You underlined very important data and particularly concerning the group of patients with disease progression. Indeed, the problem is to try to identify the patients who will progress and for the oncologists not to treat them too long until they may become nonresectable. How can you detect the patients who will progress? Do you think that tumor markers or proliferation index (such as Ki-67) can be useful to detect these patients? Furthermore, in case of synchronous colorectal liver metastases, do you believe that simultaneous resection of the primary tumor and of the liver metastases, in selected cases, could sometimes avoid such progression?
Dr. Adam: Thank you, Dr. Jaeck. Your first question is very important: how to identify those patients who will progress while on chemotherapy. In the current practice, we have no clear predictive factors available, but gene expression will probably allow us, in the near future, to predict the response to each type of chemotherapy. In any case, this raises the fact that the management of these patients should be multidisciplinary. The surgeon should be involved early in the process of treatment of these patients, to be part of the decision, and to avoid that they may be referred only when they progress on chemotherapy. However, as a tertiary center, we receive sometimes these patients when they have yet received 1 or 2 lines of chemotherapy, and we have to take the decision whether to do or not to do liver surgery when they are still resectable. In this setting, the result of the study shows clearly that we should not operate on patients who progress provided that a new chemotherapy regimen is able to control the tumor process. With regard to the policy adopted for synchronous metastases, I think really that both colorectal and hepatic resections may be done at the same time when the extent of the tumor is limited in the liver. But here we are referring on patients with at least 4 metastases, and I would not advocate to do any combined liver and colorectal surgery at the same time, since we will miss the selection process operated by neoadjuvant chemotherapy, to know if we should or not propose surgery, with an objective of long-term benefit.
Dr. Senninger: I take the liberty of challenging your conclusions on 2 points. First, you say these patients with progressive disease under chemotherapy should not be operated on and you present good reasons for that. But tell me, which chemotherapy study, considering the patient is resisting chemotherapeutic effects, shows a 1-year survival of 63%, I am not aware of any. The second thing is, you are aware of Prof. Scheele's results, he showed, and I wonder why you did not show us these results, that the most important thing is the R0 situation no matter how many metastasis there are. If we have the R0 and maybe even R1 situation with a new modern modality of resection, maybe you do not need 10 mm. Why do you have such different conclusions?
If we can resect the disease, we would go for it, we would not do extended hepatectomy in an 84-year-old patient with 5 colorectal metastases, of course not, there are limits. But we would push it in a younger patient.
Dr. Adam: Thank you for your comment. Really, I think there is no difference at all, between the philosophy of Dr. Scheele and ours. Maybe the interpretation is different. I agree with you that these patients are offered a 28% 5-year survival overall. So, as a general policy, we should do our best to operate on them. The problem is that when we subdivide these patients into those who respond (or are stabilized) and those who “escape” to chemotherapy, the outcome is very different. Among 38 patients operated with progressive disease, only 2 are presently alive at 5 years after surgery. So, we should question our policy to operate on all patients, based on the sole criterion of resectability. As you say, it is possible that surgery in “progressing” patients could give better results at short-term than chemotherapy alone. But all depends on the objective planned for each patient and on the potential to treat him with a chemotherapy regimen that could reverse the initial resistance of the tumoral process. In this connection, once more, a discussion with the medical oncologist may help us to take an appropriate decision. With regard to R0 resection, I agree with you that this should be the objective of the surgeon. We even advocate doing R1 resections (zero margin of resection) rather than no surgery at all, when resection of the tumor is macroscopically complete. In this situation, we have currently a 5-year survival of 34% in our global series of resected patients. So, the goal for the surgeon should be to remove the tumor, even if it is not possible to obtain a margin of security.
Dr. Neuhaus: Dr. Adam, let me first say that I really admire your big series with these excellent results and your thoughtful analysis. One can, of course, debate whether all of these questions can be answered within a retrospective analysis, at least some of the questions would need a prospective randomized trail.
For patients with irresectable metastasis, chemotherapy including oxaliplatin or irinotecan is of course useful and can render some patients resectable. The really important question is whether the resectable patient should have chemotherapy before surgery. Under chemotherapy, some patients will have progress and become irresectable; in other cases, we just don't know whether neoadjuvant chemotherapy may improve survival after resection.
Our experience is that most patients with so-called unresectable metastases, who have been submitted to chemotherapy and then after response to this treatment were referred to our centre for resection, have also been resectable before their chemotherapy. But the functional capacity of the remaining part of the liver after resection can be critical, so that extended resections are not possible anymore. This might be a disadvantage for the patient. Please comment on this issue and the ongoing studies in the EORTC.
In experimental and also already in clinical studies, it was shown that tumor cells in the liver grow very rapidly after two thirds resection of the liver. The growth rate of the tumor cells is almost similar as under immunosuppression. In one clinical study, using right portal vein ligation as a preliminary step before liver resection, none of the patients survived 5 years. Again, the induction of growth factors by portal vein ligation appears to promote tumor growths. Please comment also on these aspects.
Dr. Adam: Thank you, Dr. Neuhaus. Our objective in the present study was not to assess the benefit of chemotherapy compared to immediate surgery nor to deal with patients deemed initially resectable who could have missed the possibility to be resected because of ineffective chemotherapy. Therefore, although very important to consider, these aspects were not explored. We focused the study on patients resected for at least 4 liver metastases. In the current clinical practice, the vast majority of these patients are primarily referred to medical oncologists and treated by systemic chemotherapy. Whether some of them could be advantageously treated by immediate surgery is possible. However, a previous study of our group showed that chemotherapy followed by surgery gave better survival than primary resection, in patients with multiple metastases. As you know, a randomized multicenter study is currently ongoing to assess the usefulness of preoperative chemotherapy in resectable patients, whatever their number of metastases. Up to the expected results of this study, I would agree with you that for patients with 1 or 2 nodules appearing a long time after colorectal resection, immediate surgery could be more appropriate. But this is not my opinion for patients with 4 metastasis and more, should these metastases still be resectable. In this situation, the risk to miss occult liver metastases is high and chemotherapy could 1) have an effect on microscopic lesions and 2) at least provide a longer follow-up from the diagnosis of metastases, to take an adequate decision. With regard to tumor growth induced by repeat resections or by portal embolization, we have observed such evolution in few cases of portal embolization or 2-stage hepatectomies, and we try to prevent now this event by treating contralateral lesions by radiofrequency and systemic chemotherapy. We have not such experience with repeat liver resections, probably because conversely to the 2 other situations, no evident liver tumor is present in the remnant liver.
Dr. Clavien: Dr. Adam, congratulations for this thorough analysis in probably so far the largest reported series of patients with multiple colorectal metastases to the liver. I had the opportunity to look in detail at the manuscript prior to the meeting. The central message that responders to chemotherapy should be presented to the liver surgeon is important. Hopefully, this will be known and applied by our colleague oncologists, as these patients are typically in their hands. I have 3 questions. First, how many patients also received an adjuvant chemotherapy regimen after surgery, and how may have such therapy influenced the outcome? The second question relates to the observation that you performed significantly more anatomic, ie, larger, resection in patients with disease progression than those who responded to chemotherapy. May this suggest a bias with better outcome in those patients displaying less extensive disease? The third question is in line with Dr. Jaeck's question: could the initial histology and degree of differentiation of the colorectal or liver tumor have predicted the response to chemotherapy and, thereby, be a useful first step to predict the response to chemotherapy?
Dr. Adam: Thank you, Dr. Clavien, for your comment and your questions. All our patients were treated with adjuvant chemotherapy after liver resection since we assume that, because of multinodularity, they were exposed to a high postoperative risk of recurrence. The same regimen was used in case of preoperative response, while a different one was chosen in case of tumoral progression. While I have no proof that adjuvant chemotherapy was useful in case of preoperative tumor progression, my conviction is that it was certainly useful in patients who responded. More anatomic resections were performed in the group who progressed. This was probably related to the more advanced disease in this group, compared to the group with either response or stabilization. With reference to the histologic patterns of the tumor likely to be used as prognostic factors before liver resection, I would agree that probably in the near future, molecular biology markers will give us the possibility to foresee what will be the response to chemotherapy and probably define a sort of identity card of the tumor reflecting its aggressiveness. By this way, we will possibly avoid the need of a selection process by chemotherapy.
Dr. Bismuth: I will like to stress one point. In 1996, we described the resectability of irresectable colorectal liver metastases after chemotherapy. This has modified the attitude toward this form of liver metastases and now oncologists are treating patients with this disease and send some of them after successful treatment to the surgeon for liver resection.
Today what we say is about the time of the referral. If the chemotherapy is maintained too long, it could happen that the treatment becomes inefficient with increase of size of the tumors and the patient is sent to the surgeon for resection, at a moment when he escapes to the treatment. What we show now is that it is not a recommended approach: often in our experience, the oncologist continues to treat the patient even when the tumor has become resectable (if he knows that). In his opinion, he has to continue the chemotherapy to achieve the maximum effect; unfortunately, the tumor often escapes from the treatment. We think that it is the wrong attitude.
It is much better to do surgery as soon as the tumor has become resectable. That means practically that the patient has to be followed from the beginning of the treatment by the surgeon and the oncologist to make a quick decision on the date of surgery.
Footnotes
Reprints: René Adam, MD, PhD, Centre Hépato-Biliaire, Hopital Paul Brousse 14, Av PV Couturier, 94800 Villejuif, France. E-mail: rene.adam@pbr.ap-hop-paris.fr.
REFERENCES
- 1.Stangl R, Altendorf-Hofmann A, Charnley RM, et al. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–1410. [DOI] [PubMed] [Google Scholar]
- 2.Hughes KS, Simon R, Soughorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection. Surgery. 1988;103:278–288. [PMC free article] [PubMed] [Google Scholar]
- 3.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection based on 1588 patients. Assoc Fr Chirurgie Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 4.Scheele J, Strangl R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. [DOI] [PubMed] [Google Scholar]
- 5.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adam R, Pascal G, Azoulay D, et al. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238:871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cady B, Jenkins RL, Steele GD Jr, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227:566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheele J, Stangl R, Altendorf-Hofmann A, et al. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991;110:13–29. [PubMed] [Google Scholar]
- 9.Ekberg H, Tranberg KG, Andersson R, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–731. [DOI] [PubMed] [Google Scholar]
- 10.Iwatsuki S, Dvorchik I, Madariaga JR, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Registry of Hepatic Metastases. Resection of the liver for colorectal carcinoma metastases: a multiinstitutional study of indications for resection. Surgery. 1988;103:278–288. [PMC free article] [PubMed] [Google Scholar]
- 12.Bakalakos EA, Kim JA, Young DC, et al. Determinants of survival following hepatic resection for metastatic colorectal cancer. World J Surg. 1998;22:399–404. [DOI] [PubMed] [Google Scholar]
- 13.Levi F, Zidani R, Brienza S, et al. A multicenter evaluation of intensified, ambulatory, chronomodulated chemotherapy with oxaliplatin, 5-fluorouracil, and leucovorin as initial treatment of patients with metastatic colorectal carcinoma: International Organization for Cancer Chronotherapy. Cancer. 1999;85:2532–2540. [DOI] [PubMed] [Google Scholar]
- 14.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. [DOI] [PubMed] [Google Scholar]
- 15.Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147. [DOI] [PubMed] [Google Scholar]
- 16.Rougier P, Van Cutsem E, Bajetta E, et al. Randomized trial of irinotecan vs fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet. 1998;35:1407–1412. [DOI] [PubMed] [Google Scholar]
- 17.Douillard J-Y, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet. 2000;355:1041–1047. [DOI] [PubMed] [Google Scholar]
- 18.Bismuth H, Adam R, Levi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adam R, Avisar E, Ariche A, et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal liver metastases. Ann Surg Oncol. 2001;8:347–353. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Adam R, Shimada H, et al. Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. Br J Surg. 2003;90:963–969. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Handbook for Reporting Results of Cancer Treatment [WHO Offset Publication No. 48]. Geneva: World Health Organization, 1979. [Google Scholar]
- 22.Steele G Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer. Ann Surg. 1989;210:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber SM, Jarnagin WR, DeMatteo RP, et al. Survival after resection of multiple hepatic colorectal metastases. Ann Surg Oncol. 2000;7:643–650. [DOI] [PubMed] [Google Scholar]
- 24.Adam R. The importance of visceral metastasectomy in colorectal cancer. Ann Oncol. 2000;11:29–36. [DOI] [PubMed] [Google Scholar]
- 25.Figueras J, Valls C, Rafecas A, et al. Resection rate and effect of chemotherapy on survival after surgery for colorectal liver metastases. Br J Surg. 2001;88:980–985. [DOI] [PubMed] [Google Scholar]
- 26.Tournigand C, Louvet C, Quinaux E, et al. FOLFIRI followed by FOLFOX versus FOLFOX followed by FOLFIRI in metastatic colorectal cancer (MCRC): final results of a phase III study [Abstract]. Proc Am Soc Clin Oncol. 2001;20:494. [Google Scholar]
- 27.Hobday TJ, Kugler JW, Mahoney MR, et al. Long term survivors of metastatic colorectal cancer treated with chemotherapy only: a North Central Cancer Treatment Group review. J Clin Oncol. 2002;20:4574–4580.12454115 [Google Scholar]
- 28.Chollet P, Amat S, Cure H, et al. Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer. 2002;86:1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabira Y, Yasunaga M, Sakaguchi T, et al. Predicting initial recurrence pattern of esophageal cancer after neoadjuvant chemotherapy. Hepato-Gastroenterology. 2000;47:1315–1318. [PubMed] [Google Scholar]
- 30.Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203–3209. [DOI] [PubMed] [Google Scholar]
- 31.Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial Lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen PJ, Kemeny N, Jarnagin W, et al. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg. 2003;7:109–117. [DOI] [PubMed] [Google Scholar]