Abstract
E-cadherin, an adhesive transmembrane protein of epithelial adherens junctions, forms two types of detergent-resistant dimers: adhesive dimers consisting of cadherin molecules derived from two neighboring cells and lateral dimers incorporating cadherins of the same cell. Both dimers depend on the integrity of the same residue, Trp156. While the relative amounts of these complexes are not certain, we show here that in epithelial A-431 cells, adhesive dimers may be a prevalent form. Inactivation of the calcium-binding sites, located between successive cadherin ectodomains, drastically reduced the amount of adhesive dimers and concomitantly increased the amount of lateral dimers. A similar interdependence of adhesive and lateral dimers was observed in digitonin-permeabilized cells. In these cells, adhesive dimers immediately disassembled after lowering the Ca2+ concentration below 0.1 mM. The disappearance of adhesive dimers was counterbalanced by an increase in Trp156-dependent lateral dimers. Increasing the calcium concentration to a normal level rapidly restored the original balance between adhesive and lateral dimers. We also present evidence that E-cadherin dimers in vivo have a short lifetime. These observations suggest that cadherin-mediated adhesion is based on the dynamic cycling of E-cadherin between monomeric and adhesive dimer states.
E-cadherin, a member of the classic cadherin family, drives homophilic Ca2+-dependent cell-cell adhesion in epithelial cells. This transmembrane protein is concentrated within adherens junctions where it mediates physical interactions between adjacent cells. Despite the important functions of classic cadherins in tissue integrity (reviewed in references 1, 3, 21, 26, and 27), the molecular mechanisms of cadherin-based adhesion remain largely unknown. Conflicting data have been published about the intercadherin interactions involved in cell-cell adhesion. In this work we extended our examination of lateral (or cis-) and adhesive (trans-) E-cadherin homodimers. Both types of dimers were proposed to be essential intermediates in the assembly of adherens junctions (9, 19).
The rod-like extracellular portion of classic cadherins consists of five homologous ectodomains (EC domains, which are numbered 1 to 5 starting from the N terminus). Successive EC domains are interconnected by three calcium ions (15). To mediate homophilic cell-cell adhesion, classic cadherins must form multimeric complexes in which cadherins align in an antiparallel fashion. However, the bulk of the E-cadherin molecules, which can be solubilized from cells using different extraction protocols, was found in a monomeric state. On the basis of this observation, it was proposed that cell-cell adhesion is mediated by low-affinity intercadherin interactions. By this assumption, stable cell-cell adhesion can be established only by large cadherin clusters (1, 7, 13). Nevertheless, evidence for the existence of stable cadherin dimers potentially important for cell-cell adhesion has been reported (reviewed in references 12 and 24).
Two different lateral dimers were demonstrated by structural studies. One type was observed in the crystals of an N-cadherin EC1 domain (20). The characteristic structural feature of this dimer is the reciprocal insertion of the conserved Trp156 residue (E-cadherin amino acid sequence numbering as in reference 6) into the hydrophobic pocket of the paired molecule. These lateral dimers mediated adhesive interactions through the surface containing His233 and Val235, which had been suggested to participate in adhesion by experiments using adhesion-blocking peptides (4, 17). None of these interactions, however, were detected in crystals of a cadherin fragment consisting of EC1 and EC2 domains (15, 18, 22). Instead, this fragment formed a lateral dimer through the Ca2+-binding region. The Trp156 residues within this dimer were either disordered or inserted into the hydrophobic pockets located in their own molecules. In addition, no Trp156-dependent lateral dimers were detected in experiments with the chimeric E-cadherin molecule ECADCOMP, consisting of the E-cadherin extracellular region and the assembly domain of the cartilage oligomeric matrix protein (18). This soluble protein formed oligomers in which adhesive but not lateral interactions were abolished by the Trp156 mutation. This observation implied that lateral Ca2+-dependent dimerization is involved in the activation of the Trp156-dependent adhesive interaction. Due to the low affinity of lateral interactions, this process was proposed to occur only in cadherin clusters (13). Data from experiments with Xenopus C-cadherin (5, 7) suggested that lateral interactions may play a role in cell-cell adhesion, but the biochemical features of the lateral dimers reported in these papers clearly differed from those formed through the Ca2+-binding region.
Coimmunoprecipitation experiments using protein extracts from epithelial cells demonstrated that E-cadherin is able to form stable Trp156-dependent adhesive dimers (9). Like E-cadherin-based adhesion, this type of dimerization was impaired either by depletion of Ca2+ ions, by deletion of the intracellular cadherin region, or by mutations of the Ca2+-binding sites or the Trp156. Thus, coimmunoprecipitation data confirm the role of the Trp156-dependent trans-interactions in cell-cell adhesion. Trp156-dependent adhesive dimers, however, were stable in solution even in the absence of calcium ions, which was not consistent with the model postulating that cadherin-based adhesion was mediated by calcium-dependent low-affinity interactions.
The only type of lateral cadherin interactions detected by the coimmunoprecipitation assay in cells cultured under standard conditions was Trp156 dependent (9, 19). This type of lateral dimerization was not observed in most in vitro experiments. Unfortunately, the presence of adhesive E-cadherin homodimers made it difficult to assess the amounts of lateral homodimers in our coimmunoprecipitation assay (9). The existence of Trp156-dependent lateral dimers was documented solely by the observation that the Ca2+-binding site mutant of E-cadherin (Ec1QNM) did not form adhesive homodimers and formed only lateral homodimers. Therefore, an extreme point of view is that lateral homodimers do not exist under normal culture conditions but rapidly form after the depletion of calcium ions (23). This assumption could explain why Trp156-dependent lateral interactions have been found only in the crystals obtained from the EC1 domain, which lacks Ca2+-binding sites (20). However, the available data cannot entirely exclude the alternative radical possibility that the adhesive dimers we detected are actually Trp156-dependent lateral dimers incorporating cadherins from closely opposed plasma membranes. If this is true, the amount of adhesive dimers can be expected to be negligible relative to the amount of lateral dimers. Thus, to understand the nature of Trp156-dependent adhesive dimers and their role in cell-cell adhesion, it was important to determine their relative abundance. The present work indicates that the amount of adhesive dimers in A-431 cells may exceed the amount of lateral dimers. We also show that both types of dimers are highly dynamic. Defects in the calcium-binding sites abolish adhesive but promote lateral Trp156-dependent dimer assembly. The dissociation of adhesive dimers accompanied by the immediate accumulation of lateral dimers were also detected in permeabilized cells after depletion of calcium ions. Taken together, the results reported here indicate that Trp156-dependent adhesive dimerization is a specific process and distinct from lateral dimerization. Our data allowed us to propose that the constant assembly of the short-lived adhesive dimers plays a critical function in the dynamic nature of cell-cell interactions.
MATERIALS AND METHODS
DNA constructs, cell culture, DNA transfection, and immunofluorescence microscopy.
The construction of the expression plasmids coding for the E-cadherin with an internal deletion from His773 to Leu791 and tagged at the COOH terminus either by myc (Ec1M) or by Flag (Ec1F) epitopes, as well as mutants Ec1M-W156A and Ec1QNM, was described previously (9, 12). As reported, while not changing any known properties of E-cadherin (such as subcellular distribution, binding to catenins and to p120ctn, and mobility in sucrose gradient), the deletion of His773 to Leu791 completely abolished the binding of E-cadherin to anti-E-cadherin antibody C20820 (9). This unique feature of Ec1M and Ec1F allowed us to reveal physical interactions between E-cadherin molecules by the coimmunoprecipitation approach. Several new E-cadherin point mutants were produced using site-directed mutagenesis. Mutants Ec1M-Ca2/3, Ec1M-Ca3/4, and Ec1M-Ca4/5 contained mutations (N369A/D370A, N481A/D482A, and N589A/D590A, respectively) in the presumed calcium-binding sites located between the EC2/EC3, EC3/EC4, and EC4/EC5 domains. The mutants Ec1QNM-W156A, Ec1M-Ca2/3-W156A, and Ec1M-Ca3/4-W156A contained, in addition to mutations in calcium-binding sites, a point mutation (W156A), which affects the formation of any types of Trp156-dependent dimers. All mutants were constructed in the eukaryotic expression vector pRcCMV (Invitrogen, Carlsbad, Calif.) containing a neomycin resistance gene. Correct construction of the recombinant plasmids was verified by restriction endonuclease mapping and nucleotide sequencing of the entire regions derived from PCR.
Human epidermoid carcinoma A-431 cells were transfected, selected, grown, and examined by immunofluorescence microscopy as described previously (8, 9). The following mouse monoclonal antibodies were used: anti-E-cadherin, clone HECD-1 (Zymed Laboratories, San Francisco, Calif.); anti-myc (clone 9E10, provided by R. Kopan, Washington University Medical School, St. Louis, Mo.); anti-Flag M2 (Sigma, St. Louis, Mo.); and anti-E-cadherin C20820 (Transduction Laboratories, Lexington, Ky.).
Immunoprecipitation and sedimentation analysis.
For most immunoprecipitation experiments, 2 × 106 cells were cultured in a 10-cm-diameter tissue culture dish at 37°C for about 72 h. In coculture experiments, 6 × 106 cells producing myc- and Flag-tagged forms of E-cadherin were mixed in a 1:1 ratio and were cultured in a 10-cm-diameter dish for 16 h. Immunoprecipitation assay and sucrose gradient centrifugation were described previously (9). In brief, the confluent monolayer (approximately 107 cells) was washed and extracted at 4°C with 1.5 ml of immunoprecipitation lysis buffer (IP buffer) (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM dithiothreitol, 20 μM (4-amidinophenyl)-methanesulfonyl fluoride (APMSF), 2 mM EDTA, 1% Nonidet P-40 [NP-40]). The cells detached from the tissue culture dish after a few minutes in IP buffer and then were transferred into 1.5-ml microcentrifuge tubes and agitated for 10 min at 4°C. NP-40-insoluble material was removed by centrifugation at 100,000 × g for 1 h. The lysates were subjected to immunoprecipitation by subsequent incubations with a specific antibody (1 h, 1 μg/sample) and protein A-Sepharose (25-μl packed gel volume). Before the addition of sodium dodecyl sulfate (SDS) sample buffer (usually 40 μl of sample buffer was used for an immunoprecipitate obtained from one 10-cm-diameter dish), beads were washed five times with 1 ml of IP buffer. The duration of washing step did not change the yield of precipitated and coprecipitated proteins.
For sucrose gradient centrifugation, confluent monolayer cells from three 10-cm-diameter dishes were lysed with 2 ml of IP buffer. Lysates (1 ml) were precleaned by centrifugation at 100,000 × g for 1 h and then loaded on top of a 12-ml linear 5 to 20% (wt/wt) sucrose gradient prepared in IP buffer. Gradients were centrifuged at 200,000 × g for 17 h in a SW40Ti rotor (Beckman Instruments) at 4°C, fractionated from bottom to top into 12 fractions (1 ml each), and analyzed by coimmunoprecipitation. The following protein standards with known S values were centrifuged on replicate gradients: bovine serum albumin, 4.5S; immunoglobulin G, 7.5S; catalase, 11.35S; and apoferritin, 17S.
Biotinylation of cell surface proteins and metabolic labeling.
Confluent cultures in 10-cm-diameter tissue culture dishes were washed with ice-cold phosphate-buffered saline containing 0.5 mM CaCl2 (PBS-C). Then each plate was incubated at room temperature (RT) with 7 ml of 0.5 mg of Sulfo-NHS-LC-Biotin (Pierce, Rockford, Ill.) per ml in PBS-C for 10 min. The reaction was quenched by washing the cells with 1 M Tris-100 mM glycine buffer, pH 7.4. In some experiments, cells after biotinylation were dissociated into a single-cell suspension by 10-min treatment with 5 mM EDTA in PBS at 37°C and mixed with the same amount of unbiotinylated cells. In this case, cells were then cultivated for an additional 8 h. To analyze turnover of biotinylated E-cadherin, the cells after biotinylation were chased in the culture media for up to 16 h. After cultivation, cells were extracted in IP buffer and immunoprecipitated as described above. Biotinylated proteins were visualized with streptavidin-horseradish peroxidase (HRP) conjugate (Pierce) in conjunction with ECL (Boehringer Mannheim, Indianapolis, Ind.). The intensity of the biotin-derived signals was quantified using the NIH Image program, version 1.62. The samples were appropriately diluted to ensure the linear character of measurement.
For pulse-labeling experiments, confluent A-431 cells grown on 5-cm-diameter plates were first starved for 1 h in methionine- and cysteine-free Dulbecco modified Eagle medium supplemented with 10% dialyzed fetal calf serum. The cells were then biotinylated as described above and immediately afterward pulse-labeled with 0.25 mCi of [35S] methionine/cysteine (Amersham, Arlington Heights, Ill.) per plate in the same medium for 20 min. Plates were then washed with Dulbecco modified Eagle medium containing excess cold methionine and cysteine and chased in the regular medium for up to 3 h. After chase periods, cells were lysed in the IP buffer, as described above, and biotinylated proteins were precipitated with streptavidin-agarose (Sigma). Next, the proteins were eluted from the beads by incubating with 100 μl of the 1% SDS solution at 90°C. The eluted proteins were then diluted 15-fold in the IP buffer and immunoprecipitated with anti-E-cadherin C20820 antibody, as described above.
Adhesive dimer analysis in permeabilized cells.
Confluent monolayers of the cocultured cells in 10-cm-diameter tissue culture dishes were rinsed at RT in permeabilization buffer (PB buffer) (50 mM HEPES-KOH [pH 7.0], 100 mM KCl, 4 mM MgCl2, 20 μM APMSF) supplemented with 1 mM CaCl2. Cells were then incubated at RT for 2 min (unless indicated otherwise) in the same buffer containing a desirable concentration of digitonin (Calbiochem, La Jolla, Calif.) and CaCl2. In some experiments, the PB buffer used for permeabilization was gently replaced with a fresh PB buffer containing a different concentration of digitonin or CaCl2. After permeabilization, cells were either extracted in the IP buffer or were fixed for immunofluorescence microscopy.
RESULTS
Inactivation of calcium-binding sites specifically abolishes adhesive E-cadherin dimerization.
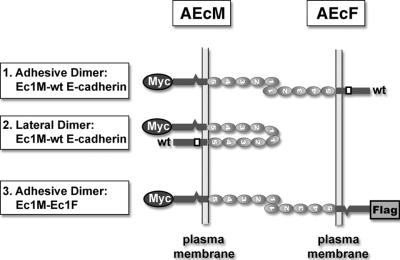
To monitor cadherin homodimerization, we have developed a mixed-culture coimmunoprecipitation assay (9) (Fig. 1). This approach utilizes cocultures of two clones of epithelial A-431 cells (AEcM and AEcF) expressing recombinant E-cadherin tagged C terminally either by myc (Ec1M) or by Flag (Ec1F) epitopes. In addition, both recombinant molecules lack the epitope for C20820 anti-E-cadherin monoclonal antibody. We demonstrated that in such cocultures, anti-myc antibody coimmunoprecipitated both Flag-tagged (Ec1F) and endogenous (C20820-reactive) forms of E-cadherin. Together with sucrose gradient analysis, our data showed that the coimmunoprecipitated Ec1F is derived from adhesive Ec1M-Ec1F cadherin dimers. The endogenous E-cadherin in the same immunoprecipitates can be derived from both adhesive and lateral dimers (Fig. 1). The replacement of the Trp156 with Ala (in the Ec1M-W156A mutant) or a defect of the Ca2+-binding site located between the EC1 and EC2 domains (in the Ec1QNM mutant) impaired adhesive dimerization (9). Notably, the Ec1QNM mutant, in contrast to Ec1M-W156A mutant, was still able to produce dimers with endogenous E-cadherin. This observation suggested that the inactivation of the calcium-binding site specifically affected the adhesive, but not the lateral, mode of dimerization.
FIG. 1.
Schematic representation of the mixed-culture coimmunoprecipitation assay. Two clones of A-431 cells expressing E-cadherin, tagged with either myc or Flag epitopes (AEcM and AEcF cells, respectively), were mixed and cocultivated overnight. Both tagged E-cadherin forms lack the epitope for the anti-E-cadherin antibody, C20820, in the intracellular segment of endogenous cadherin (indicated by an open box). The extracellular E-cadherin region consists of five repetitive ectodomains, which are indicated by numbers. Anti-myc immunoprecipitation of these cocultures results in coimmunoprecipitation of wild-type E-cadherin (wt) which can be derived from either Ec1M-E-cadherin adhesive (box 1), or Ec1M-E-cadherin lateral (box 2) dimers. In contrast, Ec1F can be derived only from adhesive Ec1M-Ec1F dimers (box 3).
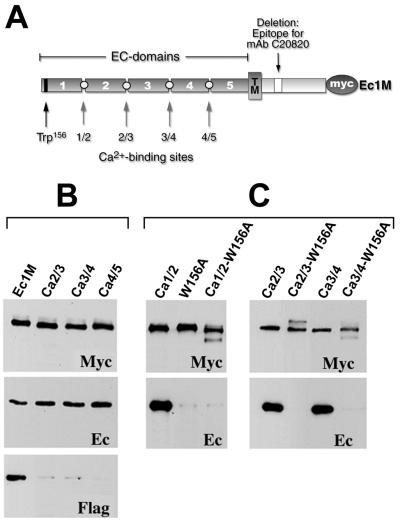
In order to determine whether the calcium-binding sites located between other EC domains of E-cadherin were also required for adhesive dimerization, three new Ec1M mutants (Ec1M-Ca2/3, Ec1M-Ca3/4, and Ec1M-Ca4/5) were constructed (Fig. 2A). They contained similar mutations converting the conserved Ca2+-binding motif DxND/E located at the EC2/EC3, EC3/EC4, or EC4/EC5 boundaries into DxAA. A-431 cell clones expressing these mutants at the same expression level as Ec1M in the AEcM cells were selected. As shown by surface biotinylation, all these mutants were efficiently delivered to the cell surface. Furthermore, these mutants induced a strong dominant-negative effect on cell-cell adhesion (not shown), similar to the effect which was described for the Ec1QNM mutant (9). Mixed-culture coimmunoprecipitation experiments with these cells showed that all three mutants similar to Ec1QNM associated with the same amounts of endogenous E-cadherin, but not with Ec1F (Fig. 2B). These data suggest that defects in any of the Ca2+-binding sites specifically affect the adhesive interaction of E-cadherin.
FIG. 2.
Schematic representation of calcium-binding mutants of Ec1M (A) and coimmunoprecipitation analysis of corresponding mutants for adhesive and lateral dimer formation (B and C). (A) The Myc-tagged form of E-cadherin, Ec1M, consists of five extracellular EC domains (only the number shown in the figure), transmembrane domain (TM), intracellular region, and myc epitope. The open box in the intracellular region indicates the deletion of the monoclonal antibody (mAb) C20820 epitope. The positions of the Trp156 residue and the presumed calcium-binding sites (1/2, 2/3, 3/4, and 4/5) which were mutated are indicated by arrows. Homogeneous cultures (C) or cocultures with AEcF (B) of A-431 cells producing Ec1M, Ec1M-Ca2/3 (Ca2/3), Ec1M-Ca3/4 (Ca3/4), Ec1M-Ca4/5 (Ca4/5), Ec1QNM (Ca1/2), Ec1M-W156A (W156A), Ec1QNM-W156A (Ca1/2-W156A), Ec1M-Ca2/3-W156A (Ca2/3-W156A), and Ec1M-Ca3/4-W156A (Ca3/4-W156A) were immunoprecipitated by an anti-myc antibody and probed for the presence of the immunoprecipitated myc-tagged proteins (Myc) or coimmunoprecipitated endogenous E-cadherin (Ec) or Ec1F (Flag) by Western blotting.
Previously we showed that the removal of calcium ions from the culture medium induced the formation of lateral dimers which were completely independent from the Trp156 residue (23). Therefore, we wished to determine whether the lateral dimers formed by the Ec1M mutants containing defects in their calcium-binding sites are Trp156 dependent. To study this, the Trp156Ala substitution was introduced into the mutants Ec1QNM, Ec1M-Ca2/3, and Ec1M-Ca3/4. The results presented in Fig. 2C show that this substitution completely abolished the association of the calcium-binding site mutants with endogenous E-cadherin. Taken together, these data show that inactivation of any single Ec1M Ca2+-binding site dramatically decreased its adhesive dimerization with Ec1F. Notably, none of these mutations changed its Trp156-dependent dimerization with endogenous E-cadherin.
The amounts of adhesive and lateral dimers in A-431 cells are in the same range.
Our finding that the inactivation of Ec1M Ca2+-binding sites selectively abolished its interaction with Ec1F, but not with endogenous E-cadherin, has two possible explanations. First, the amount of adhesive dimers may have been so small that their elimination does not detectably change the sum of adhesive and lateral dimers. Second, the defect in calcium-binding sites may have resulted in the formation of an abnormally large amount of lateral dimers, counterbalancing the loss of adhesive dimers. To distinguish between these two possibilities, we assessed the relative amounts of adhesive and lateral dimers.
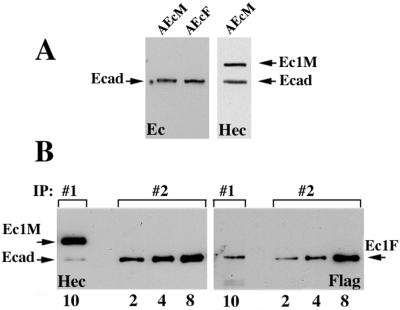
To this end, we compared the amounts of Ec1F in two immunoprecipitates: (i) in the anti-myc immunoprecipitate obtained from the coculture of AEcM and AEcF cells (IP#1) and (ii) in the anti-E-cadherin (monoclonal antibody C20820) immunoprecipitate obtained from the homogeneous culture of AEcF cells (IP#2). As described above, in IP#1, Ec1F was derived exclusively from adhesive dimers, while in IP#2, Ec1F was derived from both lateral and adhesive dimers (Table 1). To estimate the contribution of adhesive dimerization in IP#2 based on IP#1, the expression levels of all forms of E-cadherin must be determined. Western blot analysis of AEcF and AEcM cells with antibody C20820 showed that both cell clones expressed equal amounts of endogenous E-cadherin (Fig. 3A, left panel). Similar analysis of the AEcM cells using the HECD-1 antibody reacting with all forms of E-cadherin showed that these cells have the same amounts of Ec1M and endogenous E-cadherin (Fig. 3A, right panel). Therefore, the expression levels of endogenous E-cadherin in AEcF and AEcM cells, as well as Ec1M in AEcM cells, are equal. The amount of Ec1F cannot be estimated precisely. However, overexpression of recombinant E-cadherin was found to reduce the level of endogenous E-cadherin (16; also data not shown). Therefore, equal levels of endogenous E-cadherin in AEcM and AEcF cells suggest that the levels of Ec1M and Ec1F in these cells are also similar.
TABLE 1.
Possible versus detected adhesive and lateral dimers
| Dimer type | Dimer produced froma:
|
|
|---|---|---|
| IP#1 | IP#2 | |
| Adhesive | Ec1M-Ec1Fb | Ec-Ec1Fc |
| Ec1M-Ec | Ec-Ec | |
| Ec-Ec1F | Ec1F-Ec1F | |
| Ec-Ec | ||
| Lateral | None detected | Ec-Ec1Fc |
| Ec-Ec | ||
| Ec1F-Ec1F | ||
IP#1, anti-myc immunoprecipitate from AEcM/AEcF coculture; IP#2, anti-Ec (endogenous E-cadherin) immunoprecipitate from AEcF culture. For IP#1, only dimers present on the surface of AEcM cells are shown. The dimeric species which were detected in the immunoprecipitate by anti-Flag staining of the Western blots are shown in bold type.
If all E-cadherin forms are expressed at the same level, then this type of dimers composes 25% of all adhesive dimers.
If all E-cadherin forms are equally present, then these dimers compose 50% of all dimeric forms.
FIG. 3.
Relative amounts of the adhesive and lateral dimers in A-431 cells. (A) Confluent AEcM and AEcF cells were extracted with IP buffer, adjusted to the same protein concentration, and analyzed by immunoblotting with two different anti-E-cadherin antibodies, C20820 (Ec) or HECD-1 (Hec). Note that the amounts of endogenous E-cadherin (Ecad) in both cell lines were equal; the amounts of Ec1M (Ec1M) and endogenous cadherin in AEcM cells were also equal. (B) Ten microliters of the myc immunoprecipitate (IP:#1) obtained from AEcM/AEcF coculture and 2, 4, and 8 μl of the anti-E-cadherin (monoclonal antibody C20820) immunoprecipitate (IP:#2) obtained from a homogeneous AEcF culture were analyzed with HECD-1 (Hec) and anti-Flag (Flag) antibodies (the number of microliters of immunoprecipitate is shown below the blots). In IP#1, Ec1F was derived exclusively from adhesive dimers, while in IP#2, it was derived from both lateral and adhesive dimers. The staining with the HECD-1 antibody shows that 10 μl of IP#1 and 8 μl of IP#2 contain equal amounts of immunoprecipitated components, Ec1M (Ec1M) in IP#1 and E-cadherin (E-cad) in IP#2. Staining the identical blot with anti-Flag antibody shows that the amount of coimmunoprecipitated Ec1F in 10 μl of IP#1 is about four times less than in 8 μl of IP#2.
The comparison of Ec1F signal in both immunoprecipitates is shown in Fig. 3B. Both immunoprecipitates were stained with the HECD-1 antibody (Fig. 3B, left panel). Only the directly immunoprecipitated forms, Ec1M (in IP#1) and endogenous E-cadherin (in IP#2), are visible under this staining condition. This staining shows that the amount of Ec1M in 10 μl of the first immunoprecipitate was the same as the amount of E-cadherin in 8 μl of the second immunoprecipitate. The parallel samples were analyzed for the presence of the coimmunoprecipitated Ec1F (Fig. 3B, right panel). After correction for the differences in protein concentrations, these data showed that IP#1 (in which Ec1F was derived from the adhesive dimers only) contains approximately 20% of Ec1F relative to IP#2.
These data allowed us to conclude that the adhesive dimers appear to be more abundant than lateral dimers. This is suggested by two additional considerations. First, considering that in a totally random AEcM/AEcF coculture, each AEcM cell interacts with equal numbers of AEcF and AEcM cells, one may conclude that approximately 40% of the E-cadherin homodimers detected in our coimmunoprecipitation assay were derived from adhesive dimers. This assumption was strengthened by immunofluorescence microscopic examination of the AEcM/AEcF cocultures used for coimmunoprecipitation. It showed that in fact, among direct neighbors of a given AEcM cell, the number of AEcM cells was approximately two times more than that of AEcF cells (not shown). Second, as indicated in Table 1, if the amounts of all forms of E-cadherin in our cells are the same, the amount of the detected adhesive dimers (Ec1M-Ec1F) in IP#1 is four times less than the actual amount of adhesive dimers. The amount of adhesive dimers detected in IP#2 (E-cad-Ec1F) is only two times less than their total amount. Thus, at these conditions, the percentage of adhesive dimers must again be doubled.
Of course, the amounts of Ec1F in both immunoprecipitates reliably reflect the abundance of the adhesive and lateral dimers if all E-cadherin molecules (tagged and untagged) form lateral and adhesion dimers equally well with each other. Indeed, sucrose gradient centrifugation did not reveal any differences in dimerization efficiency of different E-cadherin forms (9). It was also shown that C-terminal tags did not change the known properties of classic cadherins (2, 9, 19). Also, cell-to-cell variations in Ec1M or Ec1F expression in the AEcM and AEcF cell clones were not detected by immunofluorescence microscopy. Therefore, while approximate, our data show that under standard culture conditions the amount of the immunoprecipitated adhesive dimers is significant and even exceeds the amount of lateral dimers. These data strongly support the hypothesis that the impairment of Ca2+-binding sites in E-cadherin results in the activation of lateral and reduction of adhesive Trp156-dependent dimerization.
Assembly and disassembly of adhesive dimers in semi-intact A-431 cells.
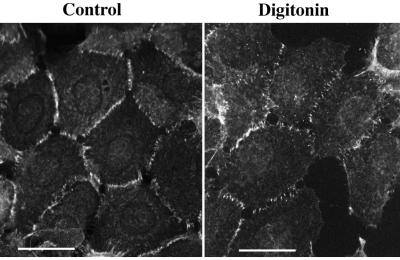
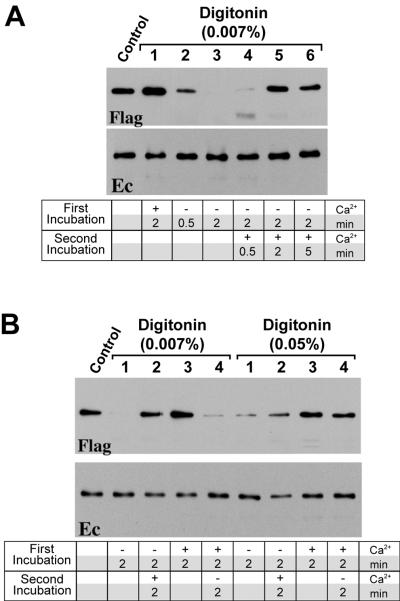
The dependence of adhesive and lateral dimerization on the integrity of the calcium-binding sites suggests that removal of calcium ions from the cell culture medium could also shift the mode of cadherin dimerization from the adhesive to the lateral state. Such a change in intercadherin interactions, however, is difficult to monitor in living cells, since the calcium-free conditions induced Trp156-independent lateral cadherin dimerization (23). In search of conditions which would specifically affect the formation of such Trp156-independent cadherin dimers, we examined cadherin dimerization in permeabilized A-431 cells. The AEcM/AEcF cocultures were permeabilized with 0.007% digitonin in the presence or absence of Ca2+ ions, followed by immunoprecipitation with an anti-myc antibody. As shown in Fig. 4, permeabilized cells retained their normal morphology and E-cadherin distribution independently of calcium concentration. Under this condition, more than 90% of cells were permeabilized as determined by cell labeling with fluorescein isothiocyanate-phalloidin (not shown). Cell permeabilization in the presence of calcium ions did not change Ec1F coimmunoprecipitation. However, incubating the cells for 2 min in the PB buffer without calcium ions completely abolished adhesive dimerization (Fig. 5A, lane 3). The amount of coimmunoprecipitated endogenous E-cadherin remained unchanged. These data indicate that adhesive dimers in permeabilized cells are much more sensitive to the lack of calcium ions than adhesive dimers in intact cells. In the latter, the complete disappearance of the dimers was evident only 10 to 15 min after removing of calcium ions (not shown). The high level of coimmunoprecipitated endogenous E-cadherin in permeabilized cells suggests that calcium removal immediately induced the rapid formation of lateral dimers. Importantly, the significant change in the balance between adhesive and lateral dimers was not accompanied by any detectable changes in the distribution of E-cadherin, as observed by immunofluorescence microscopy (Fig. 4).
FIG. 4.
Immunofluorescence microscopy of AEcM cells stained with anti-myc antibody. Before the cells were stained, they were either fixed with acetone-methanol (Control) or incubated for 2 min in digitonin-containing (0.007%) low-calcium PB buffer (Digitonin) before fixation. Bars, 40 μm.
FIG. 5.
Assembly and disassembly of adhesive dimers in permeabilized cells. (A) Coimmunoprecipitation of AEcM/AEcF cocultures with an anti-myc antibody were performed under standard conditions (control) or after incubation of cells in PB buffer with 0.007% digitonin for various times in the presence or absence of calcium as follows: 2 min with buffer containing 1 mM Ca2+ (lane 1); 30 s without Ca2+ (lane 2); 2 min without Ca2+ (lane 3); 2 min without Ca2+ and then 30 s with 1 mM Ca2+ (lane 4); 2 min without Ca2+ and then 2 min with 1 mM Ca2+ (lane 5); 2 min without Ca2+ and then 5 min with 1 mM Ca2+ (lane 6). (B) Comparison of assembly and disassembly of adhesive dimers at digitonin concentrations of 0.007 or 0.05%. Before lysis, cells were incubated with PB buffer for various times in the presence or absence of calcium as follows: 2 min without Ca2+ (lane 1); 2 min without Ca2+ and then 2 min with 1 mM Ca2+ (lane 2); 2 min with Ca2+ (lane 3); 2 min with Ca2+ and then 2 min without Ca2+ (lane 4).
In order to examine the nature of lateral dimers detected in the permeabilized cells in Ca2+-free conditions, we performed experiments with cells expressing the Ec1M-W156A mutant. This mutant is unable to form any dimers under standard culture condition but forms lateral dimers after the removal of calcium (23). However, this mutant did not form dimers with endogenous E-cadherin (not shown) when cells were permeabilized for 2 min with low-Ca2+ buffer. These data suggested that the lateral dimers, assembled in permeabilized cells after Ca2+ removal, were Trp156 dependent.
Next, we studied whether adhesive dimers could be reconstituted after raising the calcium concentration to the normal concentration. Figure 5A (lane 5) shows that the addition of 1 mM calcium for 2 min to cells which were previously permeabilized in the low-Ca2+ buffer nearly restored the amount of adhesive dimers. Notably, there was no significant effect on the amount of coimmunoprecipitated endogenous E-cadherin. The opposite experiment (Fig. 5B) showed that adhesive dimers present in cells permeabilized with calcium-containing buffer were rapidly disassembled by removing calcium ions. Thus, adhesive dimers were reversibly assembled or disassembled in the semi-intact A-431 cells by shifting the calcium concentration in a time course on the order of several minutes.
To determine whether digitonin level influences the disassembly of adhesive dimers, the AEcM/AEcF cocultures were permeabilized in the presence of calcium and different digitonin concentrations. Afterward, cell samples were either directly lysed or incubated for an additional 2 min with low-Ca2+ buffer containing the same digitonin concentration (Fig. 5B and data not shown). This experiment showed that concentrations of digitonin of 0.015% or higher (concentrations up to 0.1% were tested) abolished the dissociation of adhesive dimers, while the cellular distribution of E-cadherin or its total amount in the immunoprecipitates were not changed. Furthermore, a digitonin concentration of 0.015% abolished disassembly of adhesive dimers during permeabilization in low-calcium buffer (Fig. 5B). Thus, some cellular activity sensitive to high concentrations of digitonin was required for both dissociation and assembly of adhesive dimers.
Assembly and disassembly of adhesive dimers occur at the same calcium concentration in intact and permeabilized cells.
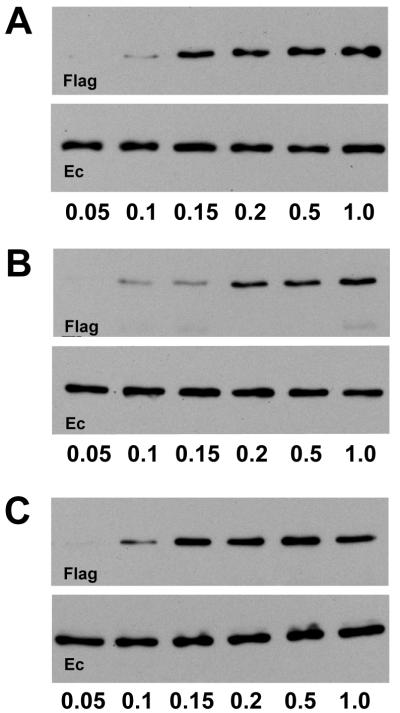
The disassembly of adhesive dimers in the permeabilized cells in low-calcium buffer could be caused, in theory, by an increase of cytosolic calcium concentration above the 1 μM concentration that activates calcium-dependent signaling pathways. To examine this possibility, we compared the Ca2+ concentration dependence on the adhesive dimer disassembly in permeabilized and in intact cells. Before anti-myc immunoprecipitation, the AEcM/AEcF cocultures were incubated for 2 min in PB buffer containing different Ca2+ concentrations. These experiments showed that adhesive dimers were not detected below a Ca2+ concentration of 0.1 mM (Fig. 6A). The 0.1 mM threshold level of adhesive dimer dissociation makes involvement of intracellular calcium signaling in the dissociation process unlikely, since this threshold would not change the calcium-binding state of the signaling cytosolic proteins. To determine the extracellular Ca2+ concentration, which is critical for the dissociation of adhesive dimers in intact cells, AEcM/AEcF cocultures were exposed for 10 min to different levels of Ca2+ in Hanks balanced solution. Figure 6B shows that the same 0.1 mM Ca2+ threshold was also critical for the intact cells. Below this concentration, adhesive dimers gradually disappeared. Furthermore, when the calcium concentration was decreased below 0.1 mM, the morphology of intact cells changed and the dimers dissociated (not shown). We also studied the requirement of Ca2+ ions for the assembly of adhesive dimers after they had dissociated in the low-calcium PB buffer (Fig. 6C). Again, the assembly proceeded only at a Ca2+ concentration above the 0.1 mM threshold. The equal Ca2+ dependence of the adhesive E-cadherin interactions in the intact and permeabilized cells strongly suggests that, despite different kinetics, the assembly or disassembly of adhesive dimers follow the same basic mechanisms in both types of cells.
FIG. 6.
Dependence of the disassembly (A and B) and assembly (C) of adhesive dimers on Ca2+ ions in intact cells (B) and permeabilized cells (A and C). (A) AEcM/AEcF cocultures were incubated for 2 min in PB buffer with 0.007% digitonin and different calcium concentrations (micromolar calcium concentrations indicated below the blots) and then subjected to coimmunoprecipitation with an anti-myc antibody. (B) Intact AEcM/AEcF cocultures before coimmunoprecipitation were incubated for 10 min at different calcium concentrations. (C) Cocultures, before coimmunoprecipitation, were preincubated for 2 min in PB buffer with 0.007% digitonin and without Ca2+ ions and then allowed to assemble dimers in the same buffer with different calcium concentrations. The immunoprecipitates were analyzed with an anti-Flag (Flag) or an anti-E-cadherin C20820 (Ec) antibody.
The half-life of cadherin dimers may be very short.
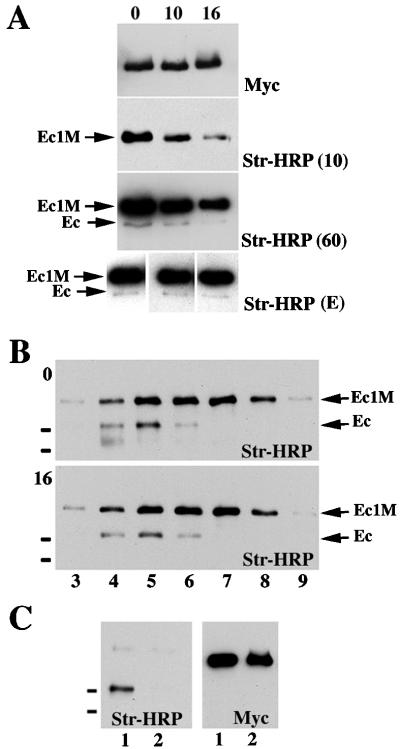
Although adhesive dimers are stable and calcium independent in solution, they are extremely sensitive to calcium concentration changes in intact and semi-intact cells. This paradox can be explained by the possibility that adhesive dimers in living cells are dynamic structures and the removal of calcium ions specifically blocks their assembly by inhibiting an important intermediate step in this process. Thus, we next studied the dynamic behavior of E-cadherin dimers in A-431 cells. First, we assessed the metabolic stability of monomeric and dimeric forms of Ec1M. Surface proteins of AEcM cells were biotinylated, and then the cells were chased for different time periods (up to 16 h) before anti-myc immunoprecipitation. The immunoprecipitates were analyzed by Western blotting using an anti-myc antibody or a HRP-streptavidin conjugate. The quantification of the biotin-derived signal in Ec1M and endogenous E-cadherin bands showed that the half-lives of both immunoprecipitated (Ec1M) and coimmunoprecipitated (endogenous E-cadherin) proteins were about the same (Fig. 7A). The same rates of degradation for both E-cadherin forms were also found in experiments where cell lysates were fractionated by sucrose gradient centrifugation (Fig. 7B).
FIG. 7.
Metabolic stability of the dimeric and monomeric forms of E-cadherin. (A) Surface proteins of AEcM cells grown in 5-cm-diameter dishes were biotinylated and then chased in regular media. The chase times (in hours) are indicated above the blots. Cells were immunoprecipitated with an excess of anti-myc antibody, and the immunoprecipitates were adjusted to 60 μl with SDS-polyacrylamide gel electrophoresis sample buffer. Five microliters of each immunoprecipitate was loaded. The blots were probed either with streptavidin-HRP (Str-HRP) or anti-myc antibody (Myc). Three different exposure times of the blot developed by Str-HRP are shown. After an exposure time of 10 s [Str-HRP (10)], only the Ec1M protein was visualized, allowing us to estimate the half-life of the monomeric E-cadherin. A longer, 60-s exposure [Str-HRP (60)] visualized coimmunoprecipitated endogenous cadherin (Ec). The blots at the bottom of panel A [Str-HRP (E)] show blots of different exposure times equilibrated on the Ec1M signals, which allowed us to demonstrate that the ratio of Ec1M to endogenous cadherin did not change during the chase periods. Panel B is identical to panel A except that cell lysates before anti-myc immunoprecipitation were subjected to sucrose gradient centrifugation either immediately after the biotinylation (blot 0) or after the 16-h chase (blot 16). The exposure time of blot 0 was shorter than that of blot 16, showing that during the 16-h chase the Ec1M/E-cadherin ratio did not change. Note that immunoprecipitation of Ec1M leads to coimmunoprecipitation of the endogenous E-cadherin (Ec) only in fractions 4 to 6. (C) Surface-biotinylated A-431 cells were dissociated by EGTA and either cocultured for additional 8 h with AEcM cells (lane 1) or cultivated separately and combined after lysis (lane 2). The latter served as a control, showing the absence of interactions between cadherin molecules in solution. The small black bars on the left of the blots indicate the positions of molecular mass markers of 116 and 97.4 kDa.
This approach cannot distinguish adhesive dimers from lateral dimers. Similar half-life values for the immunoprecipitated Ec1M and coimmunoprecipitated E-cadherin indicate that either both monomeric and dimeric cadherin forms have the same turnover rate, or cadherin dimers are highly dynamic. In the latter case, the degraded biotin-labeled dimers are replaced with new ones assembled from the pool of biotin-labeled monomers. To examine this hypothesis, we studied whether biotinylated E-cadherin was able to form adhesive dimers. The surface-biotinylated wild-type A-431 cells were dissociated by EDTA and mixed with nonbiotinylated AEcM cells. In a control experiment, the same amounts of biotinylated A-431 and nonbiotinylated AEcM cells were cultivated separately and were mixed only after lysis (Fig. 7C). This experiment showed the specific incorporation of biotinylated E-cadherin molecules into adhesive dimers.
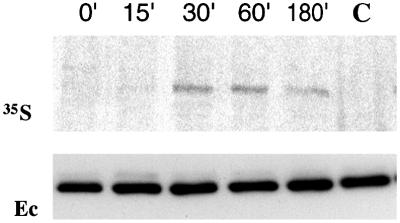
Finally, to determine the kinetics of cadherin dimer assembly, pulse-chase labeling experiments with prebiotinylated cells were performed. A-431 cells were surface biotinylated and then pulse-labeled with [35S]methionine/cysteine. This subsequent labeling produced two separate 35S- and biotin-labeled pools of proteins. At different chase periods, biotinylated proteins were pulled down by streptavidin-agarose, eluted by SDS, and then immunoprecipitated with an anti-E-cadherin antibody (Fig. 8). Because E-cadherin does not interact with any other biotinylated proteins (not shown), radioactively labeled E-cadherin in this experiment derives exclusively from dimers containing both biotin- and 35S-labeled cadherin molecules. This experiment showed that the amount of such dimers reached a maximum very rapidly, within a 30-min chase period. The absence of the 35S-labeled E-cadherin in the control immunoprecipitate (Fig. 8, lane C) showed that differently labeled forms of E-cadherin do not associate after cell lysis. The observation that the amount of pulse-labeled E-cadherin in the dimers with biotinylated counterparts rapidly reached a maximum supports the idea that the assembly and disassembly of cadherin homodimers are highly dynamic processes.
FIG. 8.
Fast incorporation of 35S-labeled E-cadherin into dimers. A-431 cells were biotinylated and then pulse-labeled with [35S]methionine/cysteine. Next, the cells were chased for 15, 30, 60, or 180 min (chase times in minutes are indicated above the blots). After extraction and precipitation by streptavidin-biotin agarose, proteins were eluted from the beads and then immunoprecipitated with an E-cadherin-specific antibody. The autoradiogram (35S) of the immunoprecipitates shows that the incorporation of the 35S-labeled E-cadherin into dimers containing a biotinylated form of the same protein reached a plateau after 30 min. Staining the same blot with E-cadherin antibody (Ec) demonstrated that all precipitates contained the same amounts of E-cadherin. In the control experiment (lane C), lysates obtained from biotinylated and metabolically labeled cells (after 30-min chase), were mixed and processed as described above. No E-cadherin-derived signal was detected in the autoradiogram.
DISCUSSION
In the past decade, evidence has emerged for the participation of classic cadherins in different kinds of homophilic interactions. At least two types of lateral (Ca2+- and Trp156-dependent) and two types of adhesive (His233/Val235- and Trp156-dependent) interactions were revealed by different approaches (reviewed in references 12 and 24). In all of these cases, the interactions are mediated by the EC1 or EC1/EC2 domains of the extracellular cadherin region. It is assumed that lateral dimerization activates an adhesive interaction, and at least one of these interactions is weak, so adhesion can proceed only in cadherin clusters. Another type of low-affinity intercadherin adhesive interaction was described by surface force measurement (14) and by bead aggregation assay (7). These experiments suggest that intercellular adhesion is established by entirely overlapping extracellular cadherin regions. Whether only one unique type or a cascade of different interactions mediate cadherin-based adhesion is not yet clear.
In this paper we further characterized the Trp156-dependent adhesive and lateral homodimerization of E-cadherin, the only intercadherin interactions strong enough to be detected in living cells by the coimmunoprecipitation assay (9). Similar to cadherin-based adhesion, adhesive dimerization depends on the binding of E-cadherin to calcium ions or catenins and on the integrity of the Trp156 residue. Furthermore, the specificity of adhesive dimerization exemplified in cadherin-based adhesion is governed by the amino-terminal domain (11). The importance of Trp156-dependent interactions in the formation of trans-cadherin complexes was also shown by electron microscopy (18).
While ample circumstantial evidence suggests that adhesive Trp156-dependent dimers are structural components of intercellular junctions, there is still room for other interpretations. For example, one may argue that these dimers in fact represent a rare and nonspecific event of lateral Trp156-dependent interaction between two E-cadherin molecules exposed on the surfaces of tightly adjacent cells. However, in this work we present strong evidence that the amount of adhesive dimers exceeds the amount of lateral dimers. Additionally, adhesive dimerization was selectively abolished by mutations of the Ca2+-binding sites. Taken together, these observations indicate that adhesive dimers are products of specific interactions and are unlikely to be an artificial subtype of the lateral dimers. Another skeptical point of view is that the formation of adhesive dimers is a consequence but not a reason for cell-cell adhesion. In the present work, we describe new features of the adhesive dimers supporting their role in cell-cell adhesion. These features may explain how these dimers, stable and calcium insensitive in solution, can mediate dynamic and Ca2+-dependent cell-cell adhesion.
In this work we showed that A-431 cells, permeabilized by 0.007% digitonin in low-calcium buffer, did not dissociate cell-cell contacts or change subcellular distribution of E-cadherin. The cell shape stability of the permeabilized cells is apparently due to the inhibition of cell contraction, possibly caused by the depletion of intracellular factors required for this process. On the other hand, cell permeabilization in low-calcium buffer induced complete dissociation of adhesive dimers. The dissociation of the dimers occurs at calcium concentrations below 0.1 mM, i.e., exactly at the same concentration threshold as the dissociation of adhesive dimers and intercellular contacts in intact cells. Adhesive dimers, which have been dissociated by low calcium, could be rapidly reconstituted by raising the calcium level above 0.1 mM. The reconstitution took only 2 min and had the same dependence on digitonin concentration as the dissociation. Thus, the adhesive dimers can be rapidly assembled and disassembled in permeabilized cells by switching the calcium concentration around the 0.1 mM level.
While the dependence on Ca2+ of adhesive interaction in intact and permeabilized cells was very similar, the time course of adhesive dimer disassembly was significantly longer in the intact cells. In a typical experiment, the complete dissociation of the adhesive dimers in standard culture took about 10 min, whereas in permeabilized cells the same effect was completed in just 2 min. We cannot entirely exclude the possibility that the mechanisms of adhesive dimer dissociation in both cases are different. However, the most obvious explanation for this difference is that it is due to the greater rate of calcium diffusion in permeabilized cell cultures.
Notably, the total amount of cadherin dimers, which was estimated by staining the anti-myc immunoprecipitates with an anti-E-cadherin antibody, did not change after permeabilization of cells in low-calcium buffer. Apparently, lateral dimers immediately counterbalanced the loss of adhesive dimers. The lateral dimers, formed in permeabilized cells in low calcium, are Trp156 dependent. The fast assembly of the lateral dimers and simultaneous disassembly of adhesive dimers suggest a dynamic relationship between these two processes. The simplest interpretation of this effect is that the incorrect conformation of the E-cadherin ectodomain, induced by the lack of calcium ions, impedes the assembly of adhesive dimers but does not affect their dissociation rate. Instead of forming adhesive dimers, an incorrectly folded E-cadherin extracellular region efficiently participates in lateral interactions. This point of view is also consistent with the observation that the inactivation of any single calcium-binding site within the extracellular cadherin region led to the elimination of adhesive interactions without significant changes in the total amount of E-cadherin dimers.
Several control experiments presented in this paper and elsewhere (9, 19) demonstrate that the E-cadherin dimers detected by coimmunoprecipitation assay are not produced in solution. Moreover, they do not dissociate upon recentrifugation (9), and their yield is not affected by the duration of immunoprecipitation, numbers of washing steps, or the presence of 0.2% SDS in the lysis IP buffer (data not shown). The absence of association or dissociation of dimers in vitro and their dynamic behavior in permeabilized cells suggest a specific mechanism responsible for Trp156-dependent dimerization.
The same mechanism involved in the formation of highly dynamic adhesive dimers could function in living cells. To strengthen this hypothesis, we approximated the half-life of E-cadherin dimers in vivo. Surface biotinylation showed that the labeled dimer-derived E-cadherin degraded with exactly the same half-life (about 5 h [data not shown]) as monomeric E-cadherin. A similar half-life value for E-cadherin was obtained by pulse-chase labeling experiments for MDCK cells (25). One possible interpretation of our results is that the half-life of E-cadherin dimers could be exactly the same as that of monomers. In this case, once formed, the dimers persist on the cell surface until decaying together with the bulk of E-cadherin. This idea cannot be reconciled, however, with the dimerization kinetics of newly synthesized E-cadherin. We showed that as early as 30 min after pulse-labeling, 35S-labeled E-cadherin associates with its cell-surface-biotinylated counterpart and the amount of such double-labeled dimers did not increase during further cultivation. Previously, it was shown that it takes E-cadherin about 30 min to be delivered to the plasma membrane (10). Thus, E-cadherin assembles into dimers almost immediately after reaching the cell surface. Furthermore, the fact that the peak of double-labeled dimers is observed at this point suggests that the equilibrium between the assembly of such dimers and their disassembly is reached very fast. This observation implies that there is a specific mechanism responsible for either dissociation or selective degradation of the E-cadherin dimers. The latter possibility seems to be unlikely, since the Ec1M-W156A mutant unable to form dimers has exactly the same half-life as Ec1M (data not shown). In either way, the same apparent half-lives of dimeric and monomeric forms of E-cadherin as well as the very fast E-cadherin dimerization are consistent with the highly dynamic nature of E-cadherin dimers.
In summary, the features of adhesive dimers we report suggest a new working model of how adhesive dimers mediate cell-cell adhesion. In this model, E-cadherin molecules in adherens junctions continually form adhesive dimers which then quickly dissociate. The lateral dimers can be an intermediate step in the assembly of adhesive dimers. The short lifetime of individual adhesive dimers and their low level relative to the level of monomeric E-cadherin may establish, in theory, highly dynamic adhesive structures. The collapse of the rod-like conformation of the extracellular E-cadherin region, induced by the depletion of calcium ions or by defects in the calcium-binding sites, attenuates the formation of adhesive dimers and results in an unusually high level of lateral dimers. The mechanisms underlying the assembly and disassembly of adhesive dimers and the role of the lateral dimers in cell-cell adhesion remain to be explored.
Acknowledgments
J. Klingelhöfer and O. Y. Laur contributed equally to this work.
We thank G. Goldberg and C. Rowley (Washington University, St. Louis, Mo.), and A. Ljubimov (Cedars-Sinai Medical Center, Los-Angeles, Calif.) for valuable discussions.
This work has been supported in part by National Institutes of Health grants AR44016-04 and AR45254-01.
REFERENCES
- 1.Adams, C. I., and W. J. Nelson. 1998. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr. Opin. Cell Biol. 10:572-577. [DOI] [PubMed] [Google Scholar]
- 2.Adams, C. L., Y. T. Chen, S. J. Smith, and W. J. Nelson. 1998. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J. Cell Biol. 142:1105-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behrens, J. 1999. Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev. 18:15-30. [DOI] [PubMed] [Google Scholar]
- 4.Blaschuk, O. W., R. Sullivan, S. David, and Y. Pouliot. 1990. Identification of a cadherin cell adhesion recognition sequence. Dev. Biol. 139:227-229. [DOI] [PubMed] [Google Scholar]
- 5.Brieher, W. M., A. S. Yap, and B. M. Gumbiner. 1996. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J. Cell Biol. 135:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bussemakers, M. J., A. van Bokhoven, S. G. Mees, R. Kemler, and J. A. Schalken. 1993. Molecular cloning and characterization of the human E-cadherin cDNA. Mol. Biol. Rep. 17:123-128. [DOI] [PubMed] [Google Scholar]
- 7.Chappuis-Flament, S., E. Wong, L. D. Hicks, C. M. Kay, and B. M. Gumbiner. 2001. Multiple cadherin extracellular repeats mediate homophilic binding and adhesion. J. Cell Biol. 154:231-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chitaev, N. A., and S. M. Troyanovsky. 1997. Direct Ca2+-dependent heterophilic interaction between desmosomal cadherins, desmoglein and desmocollin, contributes to cell-cell adhesion. J. Cell Biol. 138:193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chitaev, N. A., and S. M. Troyanovsky. 1998. Adhesive but not lateral E-cadherin complexes require calcium and catenins for their formation. J. Cell Biol. 142:837-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinck, L., I. S. Näthke, J. Papkoff, and W. J. Nelson. 1994. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J. Cell Biol. 125:1327-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klingelhöfer, J., R. B. Troyanovsky, O. Y. Laur, and S. M. Troyanovsky. 2000. Amino-terminal domain of classic cadherins determines the specificity of the adhesive interactions. J. Cell Sci. 113:2829-2836. [DOI] [PubMed] [Google Scholar]
- 12.Koch, A. W., D. Bozic, O. Pertz, and J. Engel. 1999. Homophilic adhesion by cadherins. Curr. Opin. Struct. Biol. 9:275-281. [DOI] [PubMed] [Google Scholar]
- 13.Kusumi, A., K. Suzuki, and K. Koyasako. 1999. Mobility and cytoskeletal interactions of cell adhesion receptors. Curr. Opin. Cell Biol. 11:582-590. [DOI] [PubMed] [Google Scholar]
- 14.Leckband, D., and S. Sivasankar. 2000. Mechanism of homophilic cadherin adhesion. Curr. Opin. Cell Biol. 12:587-592. [DOI] [PubMed] [Google Scholar]
- 15.Nagar, B., M. Overduin, M. Ikura, and J. M. Rini. 1996. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature 380:360-364. [DOI] [PubMed] [Google Scholar]
- 16.Nieman, M. T., J. B. Kim, K. R. Johnson, and M. J. Wheelock. 1999. Mechanism of extracellular domain-deleted dominant negative cadherins. J. Cell Sci. 112:1621-1632. [DOI] [PubMed] [Google Scholar]
- 17.Noë, V., J. Willems, J. Vandekerckhove, F. V. Roy, E. Bruyneel, and M. Mareel. 1999. Inhibition of adhesion and induction of epithelial cell invasion by HAV-containing E-cadherin-specific peptides. J. Cell Sci. 112:127-135. [DOI] [PubMed] [Google Scholar]
- 18.Pertz, O., D. Bozic, A. W. Koch, C. Fauser, A. Brancaccio, and J. Engel. 1999. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J. 18:1738-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan, W.-S., H. Tanaka, G. R. Phillips, K. Arndt, M. Yoshida, D. R. Colman, and L. Shapiro. 2000. Functional cis-heterodimers of N- and R-cadherins. J. Cell Biol. 148:579-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro, L., A. M. Fannon, P. D. Kwong, A. Thompson, M. S. Lehmann, G. Grubel, J.-F. Legrand, J. Als-Neilsen, D. R. Colman, and W. A. Hendrickson. 1995. Structural basis of cell-cell adhesion by cadherins. Nature 374:327-337. [DOI] [PubMed] [Google Scholar]
- 21.Takeichi, M. 1995. Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 7:619-627. [DOI] [PubMed] [Google Scholar]
- 22.Tamura, K., W.-S. Shan, W. A. Hendrickson, D. R. Colman, and L. Shapiro. 1998. Structure-function analysis of cell adhesion by neural (N-) cadherin. Neuron 20:1153-1163. [DOI] [PubMed] [Google Scholar]
- 23.Troyanovsky, R. B., J. Klingelhöfer, and S. M. Troyanovsky. 1999. Removal of calcium ions triggers a novel type of intercadherin interaction. J. Cell Sci. 112:4379-4387. [DOI] [PubMed] [Google Scholar]
- 24.Troyanovsky, S. M. 1999. Mechanisms of cell-cell adhesion complex assembly. Curr. Opin. Cell Biol. 11:561-566. [DOI] [PubMed] [Google Scholar]
- 25.Troxell, M. L., Y.-T. Chen, N. Cobb, W. J. Nelson, and J. A. Marrs. 1999. Cadherin function in junctional complex rearrangement and posttranslational control of cadherin expression. Am. J. Physiol. 276:C404-C418. [DOI] [PubMed]
- 26.Vleminckx, K., and R. Kemler. 1999. Cadherins and tissue formation: integrating adhesion and signaling. Bioessays 21:211-220. [DOI] [PubMed] [Google Scholar]
- 27.Yap, A. S., W. M. Brieher, and B. M. Gumbiner. 1997. Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 13:119-146. [DOI] [PubMed] [Google Scholar]