Abstract
Postembryonic organ formation in higher plants relies on the activity of stem cell niches in shoot and root meristems where differentiation of the resident cells is repressed by signals from surrounding cells. We searched for mutations affecting stem cell maintenance and isolated the semidominant l28 mutant, which displays premature termination of the shoot meristem and differentiation of the stem cells. Allele competition experiments suggest that l28 is a dominant-negative allele of the APETALA2 (AP2) gene, which previously has been implicated in floral patterning and seed development. Expression of both WUSCHEL (WUS) and CLAVATA3 (CLV3) genes, which regulate stem cell maintenance in the wild type, were disrupted in l28 shoot apices from early stages on. Unlike in floral patterning, AP2 mRNA is active in the center of the shoot meristem and acts via a mechanism independent of AGAMOUS, which is a repressor of WUS and stem cell maintenance in the floral meristem. Genetic analysis shows that termination of the primary shoot meristem in l28 mutants requires an active CLV signaling pathway, indicating that AP2 functions in stem cell maintenance by modifying the WUS-CLV3 feedback loop.
INTRODUCTION
Postembryonic organ formation in plants requires the maintenance of small stem cell pools in the shoot, floral, and root meristems (Weigel and Jürgens, 2002; Laux, 2003). Similar to animal stem cell systems, the meristems provide a microenvironment, the stem cell niche, in which differentiation of the resident cells is repressed, whereas daughter cells that leave this site undergo differentiation. In the Arabidopsis thaliana shoot meristem, the stem cells are located in the three outermost cell layers of the most central region of the shoot apex. The stem cells cannot be distinguished by their appearance from the surrounding meristem cells, and stem cell function can only be unambiguously identified by clonal studies (Stewart and Dermen, 1970). Expression of CLAVATA3 (CLV3) mRNA (Fletcher et al., 1999) that coincides with the presumed position of the apical stem cells provides an operational marker for stem cell identity in the shoot meristem.
Only a few mutants have been isolated that specifically fail to protect shoot meristem stem cells from differentiation. One of the genes identified from mutant studies encodes the homeodomain protein WUSCHEL (WUS). WUS is expressed in a domain underneath the stem cells termed the organizing center (OC) and is required and sufficient to maintain the overlying stem cells undifferentiated (Mayer et al., 1998; Schoof et al., 2000). The stem cells in turn signal back via the CLV3 peptide to restrict the size of the OC, thereby creating a negative feedback loop to dynamically control the size of the stem cell population (Brand et al., 2000; Schoof et al., 2000). CLV3 presumably acts as an extracellular ligand of the CLV1 receptor kinase complex, which eventually leads to downregulation of WUS transcription in the recipient cells (Clark et al., 1997; Stone et al., 1998; Trotochaud et al., 1999; Rojo et al., 2002; Lenhard and Laux, 2003). In floral meristems that are homologous to indeterminate shoot meristems, the AGAMOUS (AG) gene represses WUS expression late in flower development to terminate the stem cell niche after a limited number of organs have been formed (Lenhard et al., 2001; Lohmann et al., 2001).
The primary shoot meristem is formed during embryo development (Barton and Poethig, 1993). In Arabidopsis, the onset of WUS expression in precursor cells of the OC in the 16-cell embryo suggests that shoot meristem formation has been initiated already at this stage (Mayer et al., 1998). CLV3 expression is detected from late heart stage on between the outgrowing cotyledons (Fletcher et al., 1999). In bent cotyledon embryos, the primary shoot meristem becomes visible as a bulge of small cytoplasmic cells that after germination give rise to a rosette of leaves before an inflorescence stem is formed, carrying cauline leaves with axillary shoot meristems and bractless floral meristems.
Many aspects of the mechanisms that operate in the stem cell niches to maintain stem cells undifferentiated are not understood and might have escaped genetic analysis due to genetic redundancy. Therefore, we performed an extensive genetic screen to detect presumably rare dominant-negative mutations affecting stem cell maintenance. Here, we report the isolation and functional characterization of the semidominant l28 mutant that in the homozygous state is unable to keep stem cells undifferentiated similar to the wus mutant. We show that the premature termination of the shoot meristem in l28 mutants is caused by a mutation in the APETALA2 (AP2) gene that was previously identified as one of the components of the ABC model in floral patterning, where it represses AG (Bowman et al., 1991; Drews et al., 1991), in floral transition (Jofuku et al., 1994; Okamuro et al., 1997b), and in the control of seed size (Jofuku et al., 2005; Ohto et al., 2005).
In this article, we show that maintenance of the stem cell niche in the primary shoot meristem requires the activity of AP2 and redundant factor(s) via an AG independent mechanism that involves interaction with the WUS-CLV3 feedback loop.
RESULTS
The l28 Mutation Affects Shoot Apical Meristem Maintenance
The l28 mutant was identified in an ethyl methanesulfonate mutagenesis screen for Arabidopsis seedlings defective in shoot meristem development. In wild-type seedlings (Figure 1A), the shoot meristem successively forms a rosette of on average eight leaves and an inflorescence shoot carrying two to three cauline leaves with axillary side branches, and many flowers. By contrast, homozygous l28 mutant seedlings (genotyped by PCR after cloning of the gene; see below) displayed premature termination of the shoot meristem in an empty flat apex. We categorized the seedling phenotypes into three classes depending on the developmental stage when meristem termination occurred (Table 1).
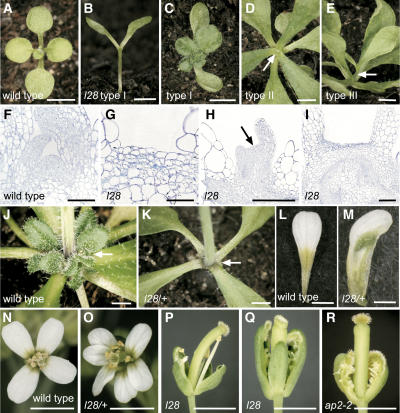
Figure 1.
Phenotypes of l28 Homozygous and Heterozygous Mutants.
(A) In 12-d-old wild-type seedlings, the shoot meristem has initiated rosette leaves.
(B) A 12-d-old l28 mutant in which the shoot meristem has terminated prematurely.
(C) A 20-d-old l28 seedling that has formed a few leaves from a transiently active adventitious meristem.
(D) and (E) The 24-d-old l28 plants that terminated shoot meristem activity after six leaves ([D]; arrow indicates terminated apex) or that terminated after forming a short stem ([E]; arrow).
(F) to (I) Histological sections stained with toluidine blue.
(F) In a 12-d-old wild-type seedling, the shoot meristem is characterized by small and densely stained cells.
(G) In a 12-d-old l28 mutant, the apical cells are larger and more vacuolated than in the wild type.
(H) In a 16-d-old l28 mutant, an adventitious meristem has been formed and already terminated (arrow).
(I) A 24-d-old l28 mutant. The cells of the enlarged flat apex have undergone differentiation.
(J) and (K) The axillary inflorescences that are initiated in the axils of the rosette leaves (arrows) in wild-type plants (J) are absent in l28 heterozygous plants (K).
(L) and (M) Second whorl petal of a wild-type flower (L) and chimeric petal (M) with patches of sepalloid green tissue of a l28/+ flower.
(N) to (R) Flowers from the wild type (N), l28/+ (O), homozygous l28 mutants ([P] and [Q]), and a flower from the ap2-2 mutant (R).
Bars = 5.0 mm in (A) to (E), (J), and (K), 50 μm in (F) to (I), and 2.0 mm in (L) to (R).
Table 1.
l28 Gene Dosage Effects on Primary Shoot Meristem Termination
| Distribution of Phenotypes
|
Type I Early Stop
|
Type II: Shoot Meristem Termination after n Leaves (%)
|
Type III Late Stop
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 from Cross | n | WT (%)a | FP (%)b | Stop (%)c | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| l28/+ × wild type | 1418 | 49.8 | 50.1 | 0.1 | 50 | 50 | ||||||||
| l28/+ × l28/+ | 3260 | 25.9 | 49.8 | 24.3 | 12 | 4 | 4 | 2 | 13 | 18 | 22 | 9 | 1 | 16 |
| l28/+ × ap2-1 | 2154 | 49.1 | 44.1 | 6.8 | 8 | 35 | 31 | 10 | 0 | 17 | ||||
| l28/+ × ap2-2 | 2163 | 50.6 | 8.8 | 40.6 | 7 | 1 | 2 | 2 | 23 | 25 | 21 | 3 | 16 | |
WT, indistinguishable from the wild type.
FP, floral phenotype indistinguishable from l28 heterozygous plants.
Stop, shoot meristem termination at the seedling stage.
Type I seedlings (12%) showed the most severe phenotype with only two cotyledons but no shoot meristem at 12 d after germination (Figure 1B). Approximately 1 week later, a number of leaves would form (Figure 1C) before a flat apex appeared. This suggests that these plants do not form a functional primary (embryo derived) shoot meristem, whereas they are able to give rise to transiently active adventitious meristems. Inflorescences, however, were never formed. Type II seedlings (73%) gave rise to between one and eight rosette leaves indistinguishable from wild-type plants before the shoot meristem terminated in a flat apex (Figure 1D, arrow). Type III seedlings (16%) gave rise to a rosette of leaves and a short inflorescence stem before meristem termination occurred (Figure 1E, arrow). The inflorescence stem carried a few cauline leaves but, with rare exceptions (see below), no flowers. Unlike the shoot meristem, the root meristem of homozygous l28 mutants of all three types appeared normal (data not shown).
In order to reveal the cellular basis of the premature meristem termination, we studied histological sections of homozygous l28 mutants. In wild-type plants, the shoot meristem can be recognized as a convex structure that is organized in distinct layers and contains small, cytoplasmically rich cells (Figure 1F). By contrast, terminated apices of homozygous l28 plants were flat and contained larger and more vacuolated cells when compared with the wild type, suggesting that they have undergone differentiation (Figures 1G to 1I). Notably, this phenotype was indistinguishable from the wus-1 mutant (Laux et al., 1996), which is defective in stem cell maintenance. Complementation analysis demonstrated that l28 was not allelic to wus-1 (data not shown), indicating that it defined a novel genetic locus involved in stem cell regulation.
In contrast with homozygotes, heterozygous l28/+ plants had an indeterminate shoot meristem and gave rise to flowering shoots. The number of secondary meristems in the axils of the rosette leaves, however, was reduced in comparison with the wild type (Figures 1J and 1K; Table 2). Only in a scarce minority of l28/+ plants (2/712) did the shoot meristem terminate prematurely in a flat apex similar to homozygous mutants. Thus, shoot meristem development was affected by the l28 mutation in a semidominant manner.
Table 2.
l28 Gene Dosage Effects on Rosette Leaves and Axillary Inflorescences
| Genotype | n | Rosette Leaves | Axillary Inflorescences |
|---|---|---|---|
| AP2/AP2/AP2 | 30 | 7.7 ± 0.6 | 4.1 ± 0.5 |
| l28/AP2/AP2 | 32 | 6.3 ± 0.6 | 1.3 ± 0.8 |
| AP2/AP2 | 37 | 7.5 ± 0.7 | 4.6 ± 0.5 |
| l28/AP2 | 50 | 5.3 ± 0.7 | 1.3 ± 0.7 |
| l28/ap2-1 | 52 | 4.6 ± 0.6 | 0.9 ± 0.7 |
| l28/ap2-2 | 70 | 4.5 ± 0.8 | 0.3 ± 0.4 |
| ap2-1/ap2-1 | 30 | 7.2 ± 0.6 | 4.2 ± 0.5 |
| ap2-2/ap2-2 | 35 | 7.1 ± 0.6 | 4.0 ± 0.4 |
| clv3-2/clv3-2 | 18 | 7.9 ± 0.7 | 4.7 ± 0.8 |
| l28/+/clv3-2/clv3-2 | 19 | 7.7 ± 0.7 | 0.3 ± 0.5 |
| l28/l28 clv3-2/clv3-2 | 10 | 6.9 ± 1.0 | 0.0 ± 0.0 |
In addition to the shoot meristem, flower development was also affected by the l28 mutation. Wild-type flowers consist of four whorls of organs from outermost to inner: four sepals, four petals, six stamens, and a gynoecium of two fused carpels (Figure 1N). Only three out of 800 homozygous l28 mutants formed flowering inflorescences, allowing us to study mutant flower development. The first five flowers formed on each l28 inflorescence had reduced organ numbers in the second and third whorls (Figure 1P). In later formed flowers, organs in whorls two and three were completely absent, and carpelloid organs instead of sepals were present in the first whorl (Figure 1Q). The few inflorescences that did not form any axillary meristems eventually terminated in a mass of carpels (data not shown). Notably, the floral defects and the increasing severity during inflorescence development resembled those of strong ap2 mutants (Figure 1R) (Bowman et al., 1989, 1991).
Flowers of l28/+ plants displayed weaker defects: namely, variable organ numbers, an increased number of petals (Figure 1O), and chimeric organs in whorls two and three (Figure 1M). In addition, the gynoecia elongated prematurely and protruded from the flower bud (data not shown).
Taken together, the l28 mutation affects two separate aspects of plant development: maintenance of undifferentiated cells in the shoot meristem and floral patterning. In this article, we focus on the function of l28 in shoot meristem maintenance.
Positional Cloning of l28
The l28 mutation was mapped to a 9.3-kb interval on chromosome 4 between derived cleaved-amplified polymorphic sequence (dCAPS) markers TWC11 and TWC7 (Figure 2A; see Methods for details). This region contained the promoter and most of the coding sequence of the floral organ identity gene APETALA2 (AP2). We therefore compared the genomic sequences of AP2 in the wild type and in l28 and identified a single-base exchange in the coding sequence of l28 (Figure 2B). AP2 encodes one of the founding members of the large AP2/ERF family of putative transcription factors that are marked by the presence of at least one AP2 domain, which is presumably involved in DNA binding (Okamuro et al., 1997a; Allen et al., 1998; Nole-Wilson and Krizek, 2000; Magnani et al., 2004; Nole-Wilson et al., 2005). Within this family, AP2 defines a subgroup of 15 proteins that contain two copies of the AP2 domain. The nucleotide change from G to A at position 433 in the l28 allele causes a change of Glu residue 145 to Lys in the first of the two AP2 domains of AP2 (Figure 2B).
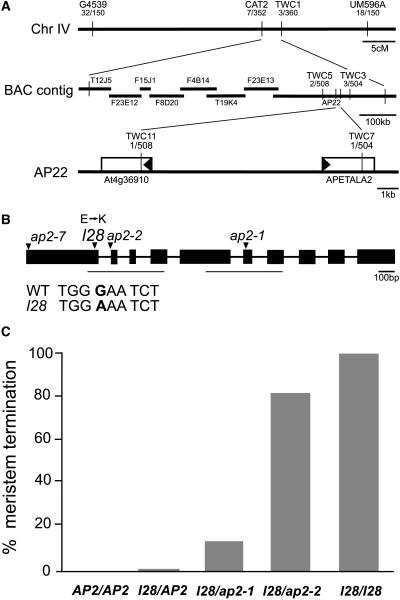
Figure 2.
Cloning of l28 and the Gene Dosage Experiment.
(A) Genetic mapping of l28 to BAC AP22 on chromosome 4. The markers used for mapping are shown as well as the number of recombination breakpoints between l28 and these markers. The l28 mutation was mapped to a 9.3-kb interval that included the AP2 gene.
(B) Genomic organization of AP2. The sites of the l28, ap2-1, ap2-2, and ap2-7 mutations are shown. The sequences of the two AP2 domains are underlined.
(C) l28 gene dosage experiment. The frequency of the shoot meristem termination by l28 is enhanced by reduction of AP2 wild type activity in a dosage-dependent manner.
Transformation of a genomic DNA fragment containing the l28 mutation into wild-type plants caused similar defects as observed in l28/+ mutants in the T1 generation, and segregating plants in the T2 generation terminated shoot meristem development similar to homozygous l28 mutants (see Supplemental Table 1 online). Conversely, transformation of a wild-type AP2 genomic DNA rescued shoot meristem development in homozygous l28 mutants (see Supplemental Table 1 online). This indicates that the identified mutation in the AP2 gene causes the l28 phenotype.
l28 Suggests a Novel Role of AP2 in Stem Cell Maintenance
Previous studies of recessive loss-of-function mutants had not revealed a function of AP2 in shoot meristem maintenance. This posed the question whether the l28 mutation uncovers a yet unknown function of AP2 or whether the mutated protein interferes with a process not affected by the wild-type protein. To address this question, we investigated whether wild-type and mutant proteins compete for the same target(s) in allele dosage experiments.
First, we reduced wild-type AP2 activity from l28 heterozygous plants by crossing l28/+ plants to homozygous ap2-1 and ap2-2 mutants. The ap2-1 mutation results in an amino acid exchange and phenotypically is a weak loss-of-function allele, whereas the ap2-2 mutation renders no detectable protein and is a putative null allele (Bowman et al., 1989; Chen, 2004). The frequency of primary shoot meristem termination increased dramatically with decreasing AP2 activity from 0.3% in l28/AP2 plants to 13.4% in l28/ap2-1 plants and to 82.3% in l28/ap2-2 plants (Figure 2C; plants genotyped by PCR). In addition, the average number of leaves formed before the shoot meristem terminated declined with reduction of AP2 activity (Table 1), indicating that the more the l28 allele is in excess of wild-type AP2, the earlier in development the primary shoot meristem terminates. Note that the frequency of meristem termination in l28/ap2-2 plants was still significantly lower than that of l28 homozygotes (99.7%). This indicates that the l28 protein acts in a dosage-dependent manner in the absence of the wild-type AP2 protein, suggesting competition between l28 and factor(s) redundant to AP2.
The remaining fraction of plants within each genotype that did not terminate the shoot meristem resembled l28/+ plants and displayed decreasing axillary meristem formation the more AP2 activity was reduced (Table 2).
In a complementary experiment, we increased the number of wild-type AP2 gene copies by crossing l28/AP2 plants to a tetraploid Arabidopsis line to generate triploid F1 plants. l28/AP2/AP2 plants showed a significantly weaker phenotype than l28/AP2 plants in that they formed more rosette leaves (Table 2). In addition, transformation of the wild-type AP2 gene into l28/+ plants also alleviated the mutant defects (data not shown).
Together, these results indicate that the l28 phenotype is enhanced by reduction and alleviated by increase of AP2 wild-type activity in a dosage-dependent manner, suggesting that l28 and AP2 proteins compete for the same target or an interacting protein.
Shoot Meristem Size Is Reduced in l28
Since the l28 mutation interfered with wild-type AP2 activity, we carefully examined ap2 loss-of-function mutants for yet undetected effects on shoot meristem development. First, we analyzed the shape and the size of the shoot meristem in mature embryos. The shape of the mutant shoot meristems was convex as in the wild type (Figures 3A and 3B). However, shoot meristem size measured by the number of epidermal cells across the apical dome was significantly reduced in ap2-1 (9.0 cells), ap2-2 (8.3 cells), and l28 (8.1 cells) mature embryos in comparison with the wild type (11.2 cells; Figure 3G). This reduction in size was specific for the shoot meristem and contrasts with the enlargement of all other organs in the ap2 embryo compared with the wild type (see Supplemental Figure 1 online; Jofuku et al., 2005; Ohto et al., 2005).
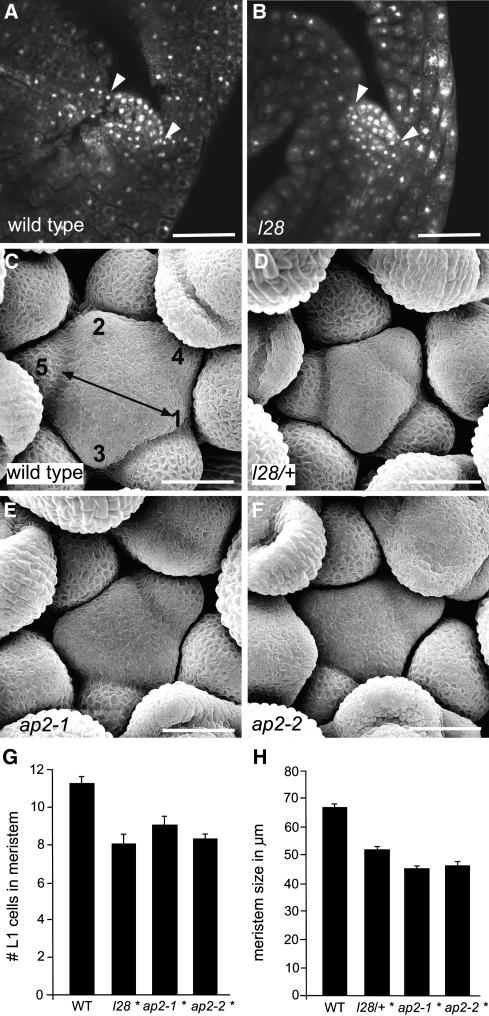
Figure 3.
Meristem Size Is Reduced in ap2 Mutants.
(A) and (B) Confocal laser scanning microscopy of propidium iodide–stained embryos. Wild-type (A) and l28 homozygous mutant (B) embryos. The arrowheads indicate the size of the embryonic shoot meristem as measured by the number of L1 cells.
(C) to (F) Scanning electron micrographs of shoot apices. In (C), young flower primordia are numbered successively from 1 to 5, and the double-headed arrow indicates the distance used to measure the size of the inflorescence meristem. Bars = 50 μm (A) to (F).
(G) and (H) Average embryonic meristem size (G) and average inflorescence meristem size (H). At least 10 plants of each genotype were analyzed. Asterisk indicates statistically significantly different from the wild type (Student's t test, P < 0.01).
We next measured the size of inflorescence meristems by scanning electron microscopy as described previously (Lenhard and Laux, 2003). The size of the shoot meristem was reduced on average by 23% in l28 heterozygous plants in comparison with the wild type (Figures 3C to 3F and 3H). Surprisingly, ap2-1 and ap2-2 mutants displayed an even more pronounced reduction of up to ∼30% compared with the wild type, even though these mutants display indeterminate shoot meristem activity. By contrast, root meristem size in these mutants was the same as in the wild type, showing that this effect was specific for the shoot meristem (see Supplemental Figure 1 online).
Thus, recessive loss-of-function ap2 mutations and the l28 mutation both result in a size decrease of the shoot meristem at the embryonic and inflorescence stages. However, since stem cell maintenance is only affected in the l28 mutant, the reduced meristem size per se appears not to be the cause of premature meristem termination.
AP2 Is Expressed in Immature Tissues
Since our results indicate a novel function of AP2 in the shoot meristem as early as in embryos, we analyzed AP2 expression throughout shoot meristem development by in situ hybridization. We detected uniform AP2 expression throughout the embryo from the globular stage on (Figures 4A to 4C). Before this stage, no signal over background was observed (data not shown). After germination, AP2 mRNA was expressed in inflorescence and floral meristems (Figure 4E) consistent with previous studies (Jofuku et al., 1994). Since gene expression in differentiated cells cannot easily be detected by in situ hybridization due to the small fraction of cytoplasm in the vacuolated cells, we fused 5 kb of the AP2 upstream sequence to the β-glucuronidase (GUS) coding region. This promoter fragment provided GUS expression in embryos and in shoot meristems indistinguishable from the AP2 mRNA pattern and recapitulated the l28 phenotype when fused to the l28 coding region (see Supplemental Table 1 online). In addition to the embryo and the shoot meristem, we detected strong GUS expression in leaf primordia and in young leaves, floral organs, stem, and pedicels (data not shown) but declining levels of expression as organs underwent maturation (Figure 4G).
Figure 4.
Expression Pattern of AP2.
(A) to (F) In situ hybridization using AP2 antisense probe ([A] to [C] and [E]) and sense control ([D] and [F]). Signal is detected as reddish color. Background staining independent of the in situ procedure is marked (asterisks). Globular stage embryo (A), heart stage embryo (B), mature embryo ([C] and [D]), inflorescence meristems ([E] and [F]). Bars = 20 μm in (A) and (B) and 50 μm in (C) to (F).
(G) A 14-d-old AP2:GUS seedling. Bar = 5.0 mm.
Thus, AP2 is strongly expressed in all immature tissues from early embryo stages on, whereas expression levels decrease as cells undergo differentiation in maturing organs.
The l28 Mutation Represses WUS and CLV3 Expression in the Shoot Meristem Stem Cell Niche
The striking similarity of l28 and wus seedling phenotypes raised the question of whether AP2 acts together with the WUS-CLV3 pathway in stem cell regulation. To this end, we investigated the expression of WUS and CLV3 in l28 mutant embryos.
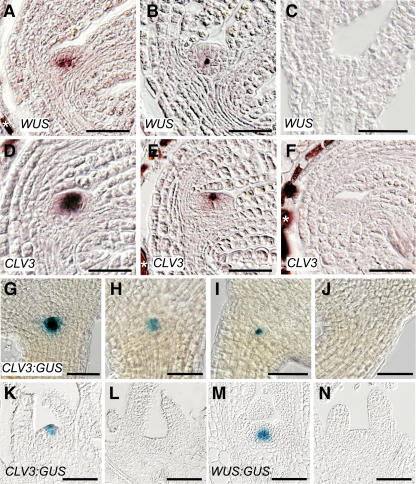
In segregating bent cotyledon embryos from an l28/+ mother plant, we detected WUS expression in the subepidermal precursor cells of the OC indistinguishable from the wild type in 42% (n = 81) (Figure 5A), reduced expression in 48% (Figure 5B), and no expression in 10% (Figure 5C) of all embryos. Similarly, we detected CLV3 mRNA expression at the stem cell position indistinguishable from the wild type (Figure 5D) but also embryos where CLV3 expression was reduced to one or two cells or completely absent (Figures 5E and 5F). In this case, a GUS reporter gene was available (Lenhard et al., 2002) that is correctly expressed during embryo development (A. Sarkar and T. Laux, unpublished data) and thus allowed a detailed segregation analysis. The staining efficiency of dissected embryos in the wild type was 90%, and we corrected the observed frequencies accordingly. Thirty-four percent of the bent cotyledon embryos (n = 290) from an l28/+ mother plant stained in a wild-type pattern (Figure 5G), 51% displayed weaker staining intensities (Figure 5H) or a reduction of the CLV3 expression domain to one or two cells (Figure 5I), and 15% did not show CLV3:GUS expression at all (Figure 5J). A similar distribution was observed in torpedo stage and mature embryos (data not shown).
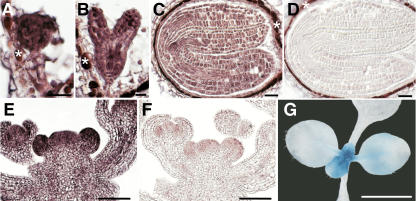
Figure 5.
The l28 Mutation Affects Gene Expression in the Stem Cell Niche.
(A) to (F) In situ hybridization in bent-cotyledon-stage embryos in the progeny of an l28/+ plant. Signal is detected as reddish color. Background staining independent of the in situ procedure is marked (asterisk). WUS expression is shown in (A) to (C): wild-type-like expression (A) and reduced (B) or absent (C) expression. CLV3 expression is shown in (D) to (F): wild-type-like expression (D) and reduced (E) or absent (F) expression.
(G) to (J) GUS-stained embryos from a CLV3:GUS l28/+ plant. Wild-type-like expression (G) and embryos with reduced ([H] and [I]) or no (J) CLV3 expression.
(K) and (L) CLV3:GUS expression in 12-d-old seedlings.
(K) l28 mutant with convex apex and wild-type-like expression.
(L) Mutant in which the apex has lost its dome-like structure and shows no expression.
(M) and (N) WUS:GUS expression in 12-d-old mutants.
(M) l28 mutant with wild-type meristem and wild-type-like expression.
(N) Mutant seedling with a terminated apex and no expression.
Bars = 50 μm.
The segregation ratios suggests that a large part of embryos homozygous for the l28 mutation did not express WUS and CLV3 and that most heterozygous embryos expressed both genes in a reduced domain.
We then analyzed expression of CLV3:GUS and WUS:GUS reporter genes (Lenhard et al., 2002) in histological sections of 12-d-old homozygous l28 seedlings (genotyped by PCR). At this stage, ∼10% of the seedlings had not yet terminated shoot meristem activity and still displayed a convex apex, and we detected wild-type-like expression of both reporter genes (Figures 5K and 5M). By contrast, the remaining seedlings displayed a flat apex, and we did not detect GUS staining for either of the two reporter genes in the majority of these seedlings (Figures 5L and 5N). Occasionally we observed weak expression in seedlings with flat apices (data not shown). We assume that these represent apices that terminated just prior to tissue harvest and still showed weak staining due to the stability of the GUS protein. In situ detection of ubiquitin mRNA (UBQ4) as a positive control in terminated l28 apices indicated that downregulation of CLV3 and WUS expression was not due to a general transcriptional inactivity in the apex (data not shown).
Taken together, embryonic expression of both WUS and CLV3 genes is reduced or abolished by the l28 mutation apparently in a dosage-dependent manner as early as at the torpedo embryo stage. Consistent with the variability of shoot meristem termination in homozygous l28 mutants, loss of WUS and CLV3 expression occurred at different times in development between individual embryos.
AP2 Functions Independently of AG in Shoot Meristem Stem Cell Maintenance
AP2 acts as a repressor of AG expression in the floral meristem (Drews et al., 1991), and AG represses WUS expression late in floral development (Lenhard et al., 2001; Lohmann et al., 2001). This raised the possibility that the repression of WUS and the termination of the shoot meristem in l28 mutants were caused via ectopic expression of AG. To test this hypothesis, we analyzed shoot meristem development in a segregating progeny of a l28/+ ag-1/+ mother plant (see Supplemental Table 2 online). We found that both l28/l28 ag-1/ag-1 and l28/l28 ag-1/+ double mutants prematurely terminated the shoot meristem in a flat apex indistinguishable from homozygous l28 mutants, indicating that reduction of AG activity from homozygous l28 mutants did not affect shoot meristem termination. Therefore, in shoot meristem stem cell maintenance, AP2 acts in a mechanism independent of AG.
clv Mutations Rescue Shoot Meristem Development in l28
To examine whether AP2 genetically interacts with the WUS-CLV3 pathway in the regulation of the shoot meristem stem cell niche, we analyzed double mutant combinations. Mutations in genes of the CLV signaling pathway result in a gradual increase of the WUS expression domain and in turn in a progressively enlarging shoot meristem size (Clark et al., 1993, 1995). We analyzed the progeny (genotyped by PCR) of plants homozygous for the strong loss-of-function alleles clv3-2 or clv1-10 and heterozygous for l28. We obtained analogous results with both mutants and will concentrate on the results for clv3-2.
clv3-2 single mutants displayed a progressively enlarging shoot meristem and supernumerary floral organs, most notably stamens and carpels (Figures 6E and 6F) (Clark et al., 1995).
Figure 6.
l28 Double Mutant Analysis.
(A) to (C) Inflorescences and flower of a wild-type plant.
(D) to (F) Plants that are homozygous for clv3-2 and wild-type for AP2 display supernumerary flowers (D), an enlarged shoot meristem (E), and supernumerary floral organs (F).
(G) to (I) clv3-2 mutants heterozygous for l28 resemble clv3 plants but additionally have flowers with chimeric floral organs ([I]; arrowhead).
(J) to (L) The primary shoot meristem of clv3-2 l28 double mutants does not terminate as in l28 but enlarges as in the clv3 single mutant. No side branches are formed in the axils of the cauline leaves (J). The flowers lack second whorl organs or have sepaloid organs in the second whorl (L).
(M) Dosage-dependent effect of wus-1 on the stage of meristem termination in heterozygous and homozygous l28 mutants: Type I, termination before any true leaves have formed; Type II, termination after some true leaves have formed; Type III, termination after a short stem has formed.
Arrows in (A), (D), (G), and (J) indicate leaf axils. Black arrowheads in (B), (E), (H), and (K) indicate the shoot meristem. Bars = 3.0 mm.
All clv3-2 l28 doubly homozygous seedlings possessed an indeterminate primary shoot meristem that progressively enlarged and formed a fasciated inflorescence indistinguishable from clv3 single mutants (Figures 6J to 6L). However, double mutants did not form any side branches in the axils of rosette or cauline leaves, similar to l28 mutants alone (cf. Figures 6D and 6J, Table 2). Thus, the clv3-2 mutation suppressed the premature termination of the embryonically formed primary shoot meristem of l28 homozygous seedlings but did not rescue postembryonic axillary meristem formation.
All clv3-2 l28/+ plants formed an enlarged primary shoot meristem like clv single mutants (Figures 6G to 6I). However, similar to l28/+ heterozygotes alone, these plants rarely formed side meristems in the axils of most rosette leaves (Table 2).
The majority of double mutants between l28 and the intermediate clv3-1 allele (Clark et al., 1995) were indistinguishable from l28 clv3-2 double mutants. However, in contrast with the latter, a minority (2.2%, n = 179) of the progeny of clv3-1 l28/+ plants displayed premature shoot meristem termination, indicating that this allele rescued the l28 phenotype less efficiently than the strong clv3-2 allele.
Taken together, these results indicate that strong clv3 and clv1 mutations rescue primary shoot meristem formation in l28. By contrast, the effects of l28 on postembryonically formed axillary shoot meristem formation were not rescued, indicating that AP2 has additional affects on leaf axil development prior to the establishment of the WUS-CLV3 feedback loop.
AP2 and WUS Act in the Same Process in Stem Cell Maintenance
To study the functional relationship between WUS and AP2 in stem cell specification, we analyzed genetic combinations of the putative null allele wus-1 and l28 in a segregating progeny of a wus-1/+ l28/+ transheterozygous plant. All genotypes were determined by dCAPS-PCR (see Methods). Five-day-old wus-1 seedlings (Laux et al., 1996) are indistinguishable from the Type I l28 seedlings with regard to the lack of a shoot meristem, absence of CLV3 expression, and the structure of a flat apex of differentiated cells. However, they differ in leaf formation: wus-1 seedlings initiate the first two leaves similar to the wild type, albeit delayed, in agreement with the view that the stem cell niche is not required for the formation of the first organs but only for sustained organ formation (Laux et al., 1996). By contrast, Type I l28 apices only form adventitious leaves in the axils of the cotyledons at ∼14 d after germination (see Figure 1C). This suggests that both mutations disrupt the stem cell niche in the center of the apex in a similar way, whereas only l28 additionally affects initial leaf formation, consistent with the broader expression domain of AP2 in comparison with WUS.
Gradual reduction of WUS activity in a homozygous l28 background resulted in an increasing severity of shoot meristem termination, with frequencies of the earliest termination (Type I) being 12% in l28 single mutants, 50% in l28 wus-1/+, and 100% in l28 wus-1 double mutant seedlings (Figure 6M). This indicates that transient shoot meristem activity in homozygous l28 mutants before termination occurs is dependent on WUS activity in a dosage-dependent manner.
Approximately one-third of l28/+ wus-1/+ plants also resulted in premature termination of the primary shoot meristem after formation of a few leaves, which was not observed in plants heterozygous for either mutation alone (Figure 6M). Subsequently, these plants were able to form flowering adventitious shoots indistinguishable from inflorescences of l28/+ heterozygotes alone. The remaining two-thirds of the l28/+ wus-1/+ plants did not terminate the shoot meristem prematurely but developed similar to l28/+ heterozygotes alone.
Taken together, l28 wus-1 genetic combinations indicate that l28 and WUS affect the same process of shoot meristem maintenance. We did not detect any interaction in combinations of wus-1 and the loss-of-function alleles ap2-1 and ap2-2 (see Supplemental Table 3 online), indicating that plants with reduced WUS activity are sensitive to l28 but not to recessive alleles.
Different Domains of the Shoot Meristem Affect the Stem Cell Niche
In the floral meristem, translation of AP2 mRNA is repressed in the central whorls (Chen, 2004). To address whether AP2 is functional in the central cells of the primary shoot meristem and in which cells of the shoot meristem l28 affects stem cell maintenance, we analyzed the effects of l28 expression in different domains of the shoot apex. All lines analyzed carried one copy of a transgene expressing the synthetic transcription factor LhG4 from different promoters and one copy of a pOp:l28 transgene that is activated by LhG4 (Moore et al., 1998).
Expression of l28 in the stem cell region (CLV3≫l28, the double arrowheads denote a two-component expression construct; see Methods) resulted in premature shoot meristem termination in a flat apex similar to homozygous l28 mutants (Types II and III; Figure 7, Table 3), consistent with l28 mRNA being translated in the central cells of the shoot meristem. Expression of l28 in the inner cells of the shoot meristem that express the receptor for CLV3 (CLV1≫l28) and in the L1 cells of the shoot apex (ATML1≫l28) gave similar results (Figure 7, Table 3). Occasionally seedlings formed a terminal flower (Figure 7E) or gave rise to an inflorescence stem with several flowers before the inflorescence meristem terminated. The flowers on these inflorescences resembled those of l28/+ plants (data not shown). Notably, expression of l28 in the stem cell region from the CLV3 promoter resulted on average in earlier termination of the primary shoot meristem than expression from any other promoter (Table 3). Nevertheless, even in this case we did not observe the most severe Type I phenotype that we found in homozygous l28 mutants.
Figure 7.
Ectopic Expression of l28 in Subdomains of the Shoot Apex.
(A) Expression domains of used transgenes.
(B) and (C) Expression of l28 under the CLV3 promoter results in premature termination of the shoot meristem after a few leaves have formed (Type II).
(D) and (E) When driven by the CLV1 promoter, the shoot meristem terminates after formation of several leaves (D) or production of a terminal flower (arrowhead in [E]).
(F) and (G) ATML1≫l28 plants terminate the primary shoot meristem and display curled leaves (F).
(H) and (I) In presumably doubly homozygous WUS≫l28 F2 plants, the inflorescence meristem is terminated after the formation of a few flowers. (I) is a close-up of the plant shown in (H).
Arrows indicate prematurely terminated shoot apex. Bars = 5.0 mm, except (G), which is 1.0 mm.
Table 3.
l28 Expression in Subdomains of the Shoot Meristem
| Shoot Meristem Termination after n Leaves (%)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I n | Type II
|
Type III Late Stop | ||||||||||
| Transgenes | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Terminal Flower | No Termination | ||
| CLV3≫l28 | 31 | 23 | 42 | 13 | 19 | 3 | 0 | 0 | 0 | |||
| CLV1≫l28 | 55 | 2 | 2 | 16 | 9 | 42 | 27 | 2 | ||||
| ATML1≫l28 | 17 | 12 | 18 | 29 | 23 | 6 | 0 | 6 | 6 | |||
| WUS≫l28 | 35 | 0 | 0 | 100 | ||||||||
Developmental time point of plants showing a meristem arrest (in percent) in plants ectopically expressing l28. F1 plants from a cross between the activator line and the pOp:l28 line were analyzed.
In contrast with the activator lines mentioned above, expression of l28 in the OC under the control of the WUS promoter did not give a shoot meristem phenotype in plants that were heterozygous for both WUS:LhG4 and pOp:l28 (Table 3). We therefore analyzed F2 plants and found that in one-sixteenth of the progeny the inflorescence meristem prematurely terminated in a flat apex after the formation of a few flowers (Figures 7H and 7I). This segregation ratio suggests that only the plants that were homozygous for both constructs prematurely terminated shoot meristem development. The relatively weak effects correlate with weaker expression levels conferred by the WUS:LhG4 activator line in comparison with the other activator lines used in this experiment (see Supplemental Figure 2 online).
In control experiments, the effects of l28 expression in all meristem regions were alleviated by coexpression of wild-type AP2 in the respective subdomains, suggesting competitive interference between both activities. Transgenic plants with a mutant version of l28 with all three reading frames disrupted were aphenotypic, demonstrating that the effects were not caused by cosuppression (data not shown).
In conclusion, l28 and AP2 mRNA were active in the central region of the shoot meristem, and the effects of l28 expression in different subdomains of the shoot meristem suggest that AP2 or a factor(s) regulated by it affect the stem cell niche via non-cell-autonomous mechanisms.
DISCUSSION
Continuous organ formation from the shoot meristem during the postembryonic life of higher plants depends on the maintenance of undifferentiated stem cells in specific microenvironments, named stem cell niches (Weigel and Jürgens, 2002; Laux, 2003). Here, we identify a novel regulatory pathway in shoot meristem stem cell maintenance defined by AP2 function.
Stem Cell Maintenance Is Regulated by AP2
AP2 was originally identified as a floral homeotic gene encoding A-function of the ABC model of organ identity specification (Bowman et al., 1989, 1991). Recently, it has also been implicated in floral transition and control of seed size (Okamuro et al., 1997b; Jofuku et al., 2005; Ohto et al., 2005). Here, we show that l28 and l28/+ plants have a number of phenotypes in common with ap2 loss-of-function alleles, such as early flowering, mispecified floral organs, and a reduction in shoot meristem size. By contrast, premature termination of the shoot meristem and differentiation of the stem cells is only observed in the l28 allele. Wild-type AP2 is expressed in the shoot meristem at all stages, consistent with an as yet unknown function there. Our gene dosage experiments indicate that wild-type AP2 activity competes with l28 activity. A plausible interpretation of these data is that l28 acts as a dominant-negative allele of AP2 and thus identifies a novel pathway in shoot meristem development. Definite proof of this model has to await the identification of potential redundant factors that appear to mask AP2 function in the shoot meristem.
The l28 mutation changes a highly conserved negatively charged Glu residue into a positively charged Lys residue within the first of the two putative DNA binding domains of AP2. A possible model is therefore that the mutant protein competes with wild-type AP2 and redundant factor(s) for interactors in a protein complex but compromises its binding to DNA targets. Alternatively, the mutant protein may still be able to bind to AP2 target genes but fail to interact with cofactors required for transcriptional regulation. Stochastic variations in the balance between mutant and redundant wild-type proteins might account for the differences when meristem termination occurs in individual homozygous l28 mutants.
AP2 Functions to Maintain the Stem Cell Niche in the Arabidopsis Shoot Meristem
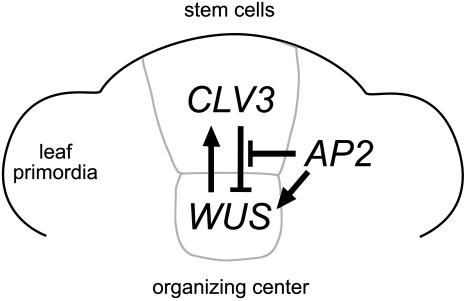
How does AP2 regulate stem cells? Our results indicate that termination of the primary shoot meristem in l28 mutants is not caused by a shortage of cells but by the failure to specify the undifferentiated state of stem cells. WUS and CLV3 expression is disrupted in l28 mutants from early embryo stages on, and l28 primary shoot meristems terminate in flat apices where the cells have undergone partial differentiation indistinguishable from wus seedlings. Both CLV3 and AG function as repressors of WUS and stem cell maintenance. However, unlike in floral patterning, AP2 functions in shoot meristem stem cell maintenance by a mechanism independent of AG. By contrast, termination of the shoot meristem by l28 requires an active CLV3 gene. Therefore, a plausible interpretation of these data is that AP2 or a factor(s) regulated by it affect stem cell maintenance in the primary shoot meristem by negatively regulating the CLV signaling pathway (Figure 8).
Figure 8.
Model for AP2 Action in the Stem Cell Niche of the Primary Shoot Meristem.
AP2 could promote stem cell maintenance either by negatively regulating CLV signaling (blunt-ended bars) or by independently antagonizing its effects (arrows).
According to this model, dominant inhibition of AP2 activity and redundant factors in l28 mutants would result in upregulated CLV signaling, which causes enhanced repression of WUS and in turn downregulation of CLV3 expression. Consequently, l28 would have no effect on the stem cell niche in a clv1 or clv3 mutant background. Since we never observed an increase of CLV3 expression in sectioned apices of l28 mutants, as expected if AP2 negatively regulated CLV3 promoter activity, AP2 appears to affect CLV signaling downstream of CLV3 transcription.
Alternatively, AP2 and CLV3 could act antagonistically but independently (Figure 8). However, such a mechanism would require the absence of CLV3 signaling to have a far greater effect on WUS expression than the inhibition of AP2 in clv3 l28 double mutants to explain not only the rescue of primary shoot meristem formation but also meristem enlargement similar to clv3 single mutants.
Our results suggest that AP2 can affect the stem cell niche from different regions of the shoot meristem. It is thus possible that this pathway serves to integrate information from surrounding shoot meristem regions to tune the activity of the stem cell niche. The future identification of target genes of AP2 will facilitate exposure of the precise molecular mechanism underlying its function in stem cell control.
Redundancy of AP2 Function in Stem Cell Regulation
The factors that mask AP2 function in stem cell maintenance in recessive hypomorphic alleles are yet to be identified. Our ongoing genetic studies suggest that the two closest homologs, TOE3 and TOE1 (Aukerman and Sakai, 2003), do not act redundantly to AP2 in stem cell maintenance (T. Würschum and T. Laux, unpublished data). Some members of the AP2/ERF family have been associated with the regulation of cell differentiation (Boutilier et al., 2002; Kirch et al., 2003). Among these, the DORNRÖSCHEN gene affects shoot meristem development but appears to have opposite and more pleiotropic effects compared with AP2 (Kirch et al., 2003). Intriguingly, PLETHORA1 and PLETHORA2 genes have been found to play an important role in regulating the stem cell niche of the Arabidopsis root meristem and to act redundantly (Aida et al., 2004). Together with the evidence presented here, these findings suggest that some members of the AP2 subfamily represent conserved units in the regulation of stem cell niches.
METHODS
Plant Growth, Mutant Lines, and Mapping
All plants were in the Landsberg erecta (Ler) ecotype, which was also used as the wild-type control. Growth conditions have been described previously (Laux et al., 1996). The l28 mutant was identified in the M2 of ethyl methanesulfonate–mutagenized wild-type plants and subsequently backcrossed three times to wild-type Ler before further analysis. clv1-4, clv1-10, clv3-1, and clv3-2 (Clark et al., 1993, 1995), wus-1 (Laux et al., 1996), stm-5 (Endrizzi et al., 1996), and ap2-1 and ap2-2 mutants have been described (Bowman et al., 1989, 1991).
The l28 mutation was mapped using plants with a wild-type-like phenotype in the F2 from a cross of an l28/+ plant with the Columbia ecotype. The initial mapping was done using CAPS markers from The Arabidopsis Information Resource (http://www.arabidopsis.org). The dCAPS markers used for fine mapping of the l28 mutation were generated based on the information available by CEREON (Jander et al., 2002). Details about primer sequences and polymorphisms are available upon request.
Histology, GUS Staining, and in Situ Hybridization
Preparation of histological sections from LR-White embedded material (Laux et al., 1996), GUS staining (Schoof et al., 2000), and in situ hybridization were previously described (Mayer et al., 1998).
For the AP2 riboprobe, the C-terminal part of AP2 lacking both AP2 domains was amplified from a Ler cDNA library using primers AP2 FOR (5′-GCCGAGTCATCAGGGAATCCTAC-3′) and AP2 Op REV (5′-GCAAGATCTAAGCCGATGATTAATGAAATGACC-3′) and subcloned into pGEM-T (Promega) to yield TW44. For the antisense probe, TW44 was linearized with SalI and transcribed with T7 RNA polymerase (Promega) using a digoxigenin-labeling kit (Roche Diagnostics); for the sense probe, TW44 was linearized with NcoI and transcribed with SP6 RNA polymerase (Promega).
Construction of Transgenes and Plant Transformation
To generate the AP2:AP2 and AP2:l28 constructs, the AP2 and l28 genomic regions were amplified using primers AP2 FOR (5′-GTCGACGGAGAATCATACCAGAGGATTGAAGTTTG-3′) and AP2 REV (5′-CCAATGTTAGATCTAGGAAACCATGTGTATACC-3′) and subcloned into pGEM-T (Promega) to yield TW28 and TW29. Both TW28 and TW29 were sequenced to exclude amplification errors. As promoter, 5 kb of upstream sequence was amplified using primers AP2 Prom FOR (5′-GTCGACTCCTTACTATATTTGTACATTAACGTGTTCC-3′) and AP2 Prom REV (5′-GTCGACCCTCAAGGTTAGAAGCTAAATAAGAGAAACC-3′) and subcloned into pGEM-T to yield TW26. The promoter was excised from TW26 by digestion with SalI and cloned into TW28 and TW29 each digested with SalI/CIAP to yield TW35 and TW37. TW35 and TW37 were digested with NcoI/T4 and SacI, and the resulting AP2:AP2 and AP2:l28 inserts were cloned into pBarM (Lenhard et al., 2002) to yield TW51 and TW54.
For the AP2:GUS reporter, the same 5 kb were amplified using primers that introduced PstI restriction sites AP2 Pd FOR (5′-CCTGAGTAATCATTTGTCCTGCAGCCATATC-3′) and AP2 Pd REV (5′-GTTCAATCTGCAGTCCTCTGGTATGATTCTC-3′). The resulting PCR product was digested with PstI and cloned into the PstI site of mL255 to yield TW49.
For all misexpression studies, we used the pOpL two-component system where the synthetic transcription factor LhG4 is expressed under the control of the promoter of interest (Moore et al., 1998). The gene to be misexpressed is under the control of the pOp promoter, which is specifically activated by LhG4. In every case, transgenic lines carrying either the activator or the target construct alone did not show a phenotype. The phenotypes that are described were only observed when both the activator and the target were brought together in one plant by crossing. Both the pOp:l28 and the pOp:AP2 lines used in these experiments carried a linked pOp:GUS to monitor the expression of the target construct.
For the pOp:AP2 and pOp:l28 constructs, TW28 and TW29 were digested with SalI and BglII, and the resulting AP2 and l28 inserts were cloned into mL204 (Lenhard and Laux, 2003) digested with XhoI and BamHI to yield TW40 and TW42. For the negative control constructs pOp:nsAP2 and pOp:nsl28, an analogous fusion was created using cDNAs that contained stop codons in all three reading frames.
The WUS:LhG4, CLV1:LhG4, CLV3:LhG4, and ATML1:LhG4 driver lines have been described (Schoof et al., 2000; Lenhard et al., 2001; Lenhard and Laux, 2003).
All plasmids were introduced into Agrobacterium tumefaciens strain GV3101 (pMP90; Koncz and Schell, 1986) by electroporation and transformed into Ler wild-type plants by the floral dip method (Clough and Bent, 1998).
PCR-Based Genotyping
Plants were genotyped for the l28 allele by dCAPS (Neff et al., 1998) using primers l28 FOR (5′-GGGCTCACTGGTATGGTGTTAAGT-3′) and l28 REV (5′-TAAAGATATGAGATTATTACCAAATATGGAATT-3′) at an annealing temperature of 53°C. Digestion of the PCR products with EcoRI produces two bands of 189 and 33 bp from the product of the wild-type allele, while the product from the l28 allele is 222 bp and is not digested. For the wus-1 allele, the primers WUS1 FOR (5′-TAGTATGGTCTGGATTCTGGAATC-3′) and WUS1 REV (5′-TATTTGTATTAATGAATTATAGTTTGATACGTA-3′) were used. Digestion with BsaBI gives a 193-bp wild-type fragment and 165- and 28-bp fragments for the wus-1 allele.
Shoot Meristem Size Measurements
Confocal analysis of embryos (Running et al., 1995), scanning electron microscopy, and inflorescence meristem size measurements were done as previously described (Lenhard and Laux, 2003).
Accession Numbers
Arabidopsis Genome Initiative locus identifiers are as follows: AP2 (At4g36920), TOE1 (At2g28550), and TOE3 (At5g67180).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table 1. Transformation of AP2 and l28 Genomic DNA.
Supplemental Table 2. l28 ag-1 Double Mutant Analysis.
Supplemental Table 3. ap2 wus-1 Double Mutant Analysis.
Supplemental Figure 1. Comparison of Hypocotyl and Root Size between l28 and the Wild Type.
Supplemental Figure 2. Expression Strength of the Activator Lines.
Supplementary Material
Acknowledgments
We thank Michael Lenhard and the members of the Laux lab for critical comments. This project was funded by the Deutsche Forschungsgemeinschaft as part of the SFB592 (T.L.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Thomas Laux (laux@biologie.uni-freiburg.de).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.038398.
References
- Aida, M., Beis, D., Heidstra, R., Willemsen, V., Blilou, I., Galinha, C., Nussaume, L., Noh, Y.S., Amasino, R., and Scheres, B. (2004). The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119 109–120. [DOI] [PubMed] [Google Scholar]
- Allen, M.D., Yamasaki, K., Ohme-Takagi, M., Tateno, M., and Suzuki, M. (1998). A novel mode of DNA recognition by a beta-sheet revealed by the solution structure of the GCC-box binding domain in complex with DNA. EMBO J. 17 5484–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukerman, M.J., and Sakai, H. (2003). Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15 2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton, M.K., and Poethig, R.S. (1993). Formation of the shoot apical meristem in Arabidopsis thaliana: An analysis of development in the wild type and in the shoot meristemless mutant. Development 119 823–831. [Google Scholar]
- Boutilier, K., Offringa, R., Sharma, V.K., Kieft, H., Ouellet, T., Zhang, L., Hattori, J., Liu, C.M., van Lammeren, A.A., Miki, B.L., Custers, J.B., and van Lookeren Campagne, M.M. (2002). Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, J.L., Smyth, D.R., and Meyerowitz, E.M. (1989). Genes directing flower development in Arabidopsis. Plant Cell 1 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, J.L., Smyth, D.R., and Meyerowitz, E.M. (1991). Genetic interactions among floral homeotic genes of Arabidopsis. Development 112 1–20. [DOI] [PubMed] [Google Scholar]
- Brand, U., Fletcher, J.C., Hobe, M., Meyerowitz, E.M., and Simon, R. (2000). Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289 617–619. [DOI] [PubMed] [Google Scholar]
- Chen, X. (2004). A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303 2022–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S.E., Running, M.P., and Meyerowitz, E.M. (1993). CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119 397–418. [DOI] [PubMed] [Google Scholar]
- Clark, S.E., Running, M.P., and Meyerowitz, E.M. (1995). CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121 2057–2067. [Google Scholar]
- Clark, S.E., Williams, R.W., and Meyerowitz, E.M. (1997). The CLAVATA1 gene encodes a putative receptor-kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89 575–585. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Drews, G.N., Bowman, J.L., and Meyerowitz, E.M. (1991). Negative regulation of the Arabidopsis homeotic gene AGAMOUS by the APETALA2 product. Cell 65 991–1002. [DOI] [PubMed] [Google Scholar]
- Endrizzi, K., Moussian, B., Haecker, A., Levin, J., and Laux, T. (1996). The SHOOTMERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and floral meristems and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE. Plant J. 10 967–979. [DOI] [PubMed] [Google Scholar]
- Fletcher, J.C., Brand, U., Running, M.P., Simon, R., and Meyerowitz, E.M. (1999). Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283 1911–1914. [DOI] [PubMed] [Google Scholar]
- Jander, G., Norris, S.R., Rounsley, S.D., Bush, D.F., Levin, I.M., and Last, R.L. (2002). Arabidopsis map-based cloning in the post-genome era. Plant Physiol. 129 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofuku, K.D., Boer, B.G., Montagu, M.V., and Okamuro, J.K. (1994). Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6 1211–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofuku, K.D., Omidyar, P.K., Gee, Z., and Okamuro, J.K. (2005). Control of seed mass and seed yield by the floral homeotic gene APETALA2. Proc. Natl. Acad. Sci. USA 102 3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirch, T., Simon, R., Grunewald, M., and Werr, W. (2003). The DORNROSCHEN/ENHANCER OF SHOOT REGENERATION1 gene of Arabidopsis acts in the control of meristem cell fate and lateral organ development. Plant Cell 15 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz, C., and Schell, J. (1986). The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel Agrobacterium binary vector. Mol. Gen. Genet. 204 383–396. [Google Scholar]
- Laux, T. (2003). The stem cell concept in plants: A matter of debate. Cell 113 281–283. [DOI] [PubMed] [Google Scholar]
- Laux, T., Mayer, K.F.X., Berger, J., and Jürgens, G. (1996). The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122 87–96. [DOI] [PubMed] [Google Scholar]
- Lenhard, M., Bohnert, A., Jürgens, G., and Laux, T. (2001). Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 105 805–814. [DOI] [PubMed] [Google Scholar]
- Lenhard, M., Jürgens, G., and Laux, T. (2002). The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development 129 3195–3206. [DOI] [PubMed] [Google Scholar]
- Lenhard, M., and Laux, T. (2003). Stem cell homeostasis in the Arabidopsis shoot meristem is regulated by intercellular movement of CLAVATA3 and its sequestration by CLAVATA1. Development 130 3163–3173. [DOI] [PubMed] [Google Scholar]
- Lohmann, J., Huong, R., Hobe, M., Busch, M., Parcy, F., Simon, R., and Weigel, D. (2001). A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105 793–803. [DOI] [PubMed] [Google Scholar]
- Magnani, E., Sjolander, K., and Hake, S. (2004). From endonucleases to transcription factors: Evolution of the AP2 DNA binding domain in plants. Plant Cell 16 2265–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, K.F.X., Schoof, H., Haecker, A., Lenhard, M., Jürgens, G., and Laux, T. (1998). Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95 805–815. [DOI] [PubMed] [Google Scholar]
- Moore, I., Galweiler, L., Grosskopf, D., Schell, J., and Palme, K. (1998). A transcription activation system for regulated gene expression in transgenic plants. Proc. Natl. Acad. Sci. USA 95 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff, M., Neff, J., Chory, J., and Pepper, A. (1998). dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: Experimental applications in Arabidopsis thaliana genetics. Plant J. 14 387–392. [DOI] [PubMed] [Google Scholar]
- Nole-Wilson, S., and Krizek, B.A. (2000). DNA binding properties of the Arabidopsis floral development protein AINTEGUMENTA. Nucleic Acids Res. 28 4076–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nole-Wilson, S., Tranby, T.L., and Krizek, B.A. (2005). AINTEGUMENTA-like (AIL) genes are expressed in young tissues and may specify meristematic or division-competent states. Plant Mol. Biol. 57 613–628. [DOI] [PubMed] [Google Scholar]
- Ohto, M.A., Fischer, R.L., Goldberg, R.B., Nakamura, K., and Harada, J.J. (2005). Control of seed mass by APETALA2. Proc. Natl. Acad. Sci. USA 102 3123–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamuro, J.K., Caster, B., Villarroel, R., Van Montagu, M., and Jofuku, K.D. (1997. a). The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc. Natl. Acad. Sci. USA 94 7076–7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamuro, J.K., Szeto, W., Lotys-Prass, C., and Jofuku, K.D. (1997. b). Photo and hormonal control of meristem identity in the Arabidopsis flower mutants apetala2 and apetala1. Plant Cell 9 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo, E., Sharma, V.K., Kovaleva, V., Raikhel, N.V., and Fletcher, J.C. (2002). CLV3 is localized to the extracellular space, where it activates the Arabidopsis CLAVATA stem cell signaling pathway. Plant Cell 14 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Running, M.P., Clark, S.E., and Meyerowitz, E.M. (1995). Confocal microscopy of the shoot apex. In Methods in Cell Biology: Plant Cell Biology, D.W. Galbraith, D.P. Burque, and H.J. Bohnert, eds (San Diego, CA: Academic Press), pp. 215–227. [DOI] [PubMed]
- Schoof, H., Lenhard, M., Haecker, A., Mayer, K.F.X., Jürgens, G., and Laux, T. (2000). The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100 635–644. [DOI] [PubMed] [Google Scholar]
- Stewart, R.N., and Dermen, H. (1970). Determination of number and mitotic activity of shoot apical initial cells by analysis of mericlinal chimeras. Am. J. Bot. 57 816–826. [Google Scholar]
- Stone, J.M., Trotochaud, A.E., Walker, J.C., and Clark, S.E. (1998). Control of meristem development by CLAVATA1 receptor kinase and kinase- associated protein phosphatase interactions. Plant Physiol. 117 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotochaud, A.E., Hao, T., Wu, G., Yang, Z., and Clark, S.E. (1999). The CLAVATA1 receptor-like kinase requires CLAVATA3 for its assembly into a signaling complex that includes KAPP and a Rho-related protein. Plant Cell 11 393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel, D., and Jürgens, G. (2002). Stem cells that make stems. Nature 415 751–754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.