Abstract
Glu receptors are known to function as Glu-activated ion channels that mediate mostly excitatory neurotransmission in animals. Glu receptor–like genes have also been reported in higher plants, although their function is largely unknown. We have identified a rice (Oryza sativa) Glu receptor–like gene, designated GLR3.1, in which mutation by T-DNA insertion caused a short-root mutant phenotype. Histology and DNA synthesis analyses revealed that the mutant root meristematic activity is distorted and is accompanied by enhanced programmed cell death. Our results supply genetic evidence that a plant Glu receptor–like gene, rice GLR3.1, is essential for the maintenance of cell division and individual cell survival in the root apical meristem at the early seedling stage.
INTRODUCTION
Animal ionotropic glutamate receptors (iGluRs) function as Glu-activated ion channels that mediate most excitatory neurotransmission in the brain (Dingledine et al., 1999). The three pharmacologically defined classes of iGluRs were originally grouped according to their sequence homology and named after reasonably selective agonists: N-methyl-d-aspartate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, and kainate (Dingledine et al., 1999; Sprengel et al., 2001). However, the homologues of the iGluRs are also present in nonneuronal cells, and some have even been found in higher plants (Lam et al., 1998). A large family of 20 glutamate receptor–like genes (GLRs) has been identified and divided into three subgroups in Arabidopsis thaliana based on sequence similarity (Chiu et al., 2002). The identification of the putative iGluR genes in plants has given rise to the hypothesis that a primitive signaling mechanism involving excitatory amino acids or related small molecules existed before the divergence of plants and animals (Chiu et al., 2002). After the discovery of the plant GLRs, indirect evidence for the participation of those putative receptors in Glu-mediated calcium signaling was reported (Dennison and Spalding, 2000; Dubos et al., 2003). By means of molecular modeling and applying different iGluR agonists in combination with a pH-homeostasis mutant, det3, some characteristics of the N-methyl-d-aspartate family of iGluRs were reported in Arabidopsis (Dubos et al., 2003, 2005) and implicated in the redistribution of carbon (Dubos et al., 2005). The functional properties of these proteins and their roles in plant physiology and development, however, remain elusive because of a shortage of genetic studies. Transgenic plant studies have been used to examine the function of the family of GLR genes in plants. In Arabidopsis, overexpression of GLR3.2 showed that these genes may be involved in calcium homeostasis (Kim et al., 2001), and by an antisense approach, GLR1.1 was shown to be a regulator of abscisic acid biosynthesis (Kang et al., 2004) and carbon/nitrogen metabolism (Kang and Turano, 2003). Until now, despite many efforts, no visible phenotypes in plants have been linked to mutations in the GLRs.
Plant root development is controlled by internal programs and external factors and proceeds through controlled cell division and cell differentiation. Cell positional cues play a pivotal role in the development of different root components (van den Berg et al., 1995, 1997; Benfey and Scheres, 2000; Barlow, 2003). External signals, including light, temperature, and other nutrient conditions, can greatly influence plant growth and root development. It has been reported that Arabidopsis GLRs may mediate the transmission of light signals in whole plant assays in which the plants were treated with iGluR antagonists or agonists (Lam et al., 1998; Brenner et al., 2000). During the development of the nervous system in animals, cell–cell communication through Glu receptors plays an important role in regulating the proliferation, migration, and survival of neuronal progenitors and immature neurons (Dingledine et al., 1999; Ikonomidou et al., 1999). However, little is known about whether or not such signaling mechanisms could play a role in the communication between individual plant cells.
Here, we report the identification and characterization of a short-root mutant from our T-DNA insertional rice (Oryza sativa) library. Genetic and molecular analyses of the mutant indicate that a putative GLR gene was disrupted by the insertion of T-DNA. Sequence alignment of rice GLR3.1 with the 20 members of the Glu receptor–like family in Arabidopsis indicates that the disrupted rice GLR gene is closely related to clade III Arabidopsis GLRs; therefore, it is designated GLR3.1. Our results show that rice GLR3.1 is involved in cell proliferation and cell survival in the root apical meristem (RAM).
RESULTS
Isolation and Initial Characterization of a T-DNA Insertion Mutant of the Rice GLR3.1 Gene
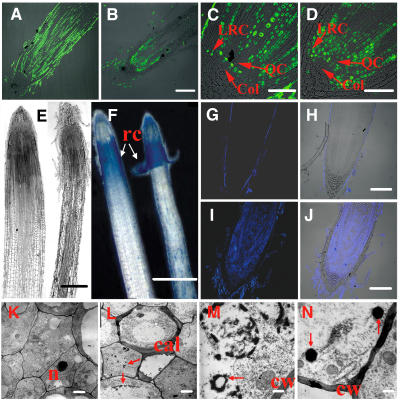
A rice mutant defective in root elongation under a room temperature regime (22 and 28°C, night and day) was isolated from a rice T-DNA insertion library (Chen et al., 2003). Compared with the wild type, the elongation of the primary, adventitious, and lateral roots of the mutant was severely inhibited at the early seedling stage during the first 2 weeks after germination (Figures 1A and 1B), and the diameter of the primary root apex of the mutant was reduced significantly (Figures 1D and 1E). However, the length of newly emergent roots of late vegetative plants after the seedling stage and during the reproductive phase was quite normal in mutant plants compared with the wild type. Histology analysis of 3-d-old seedling roots revealed that the number of cell layers in the lateral root cap was greater in the mutant compared with the wild type (Figure 1C), whereas the axial and radial patterns of root cell organization in the RAM were not affected in the mutant. However, radial expansion of root cells was inhibited in the mutant (Figures 1D to 1G), which results in the slender-root phenotype. The cell length in the root maturation zone of the mutant was similar to that of the wild type (Figures 1F and 1G).
Figure 1.
Root Phenotype of the Short-Root Mutant.
(A) Phenotypes of wild-type (Zhonghua 11; left) and mutant (right) rice in 7-d-old seedlings grown with a 22/28°C (night/day) regime.
(B) Lateral root lengths of the wild type (left) and mutant (right) of the same seedlings shown in (A).
(C) Longitudinal sections of wild-type (left) and mutant (right) root tips of 3-d-old seedlings. More cell layers in the lateral root cap were found in the mutant. To keep the root cap intact, we fixed and embedded samples using Steedman's wax under soft conditions.
(D) and (E) Transverse sections of the wild type (D) and the mutant (E) in 3-d-old seedlings. The images were taken at 100 μm away from the root tips.
(F) and (G) Longitudinal sections of wild-type (F) and mutant (G) maturation zones.
Bars = 2 cm (A), 2 mm (B), and 40 μm ([C] to [G]).
A BLAST analysis of the flanking sequences, amplified by thermal asymmetric interlaced (TAIL) PCR (Liu et al., 1995), of the T-DNA insertion site in the mutant revealed that a Glu receptor–like gene, designated GLR3.1, was disrupted by a T-DNA insertion on chromosome 4. Comparison of the flanking sequences with the genomic DNA and two EST sequences of GLR3.1 (D47824 and AU032687; http://www.ncbi.nlm.mih.gov) revealed that a T-DNA fragment of ∼5.7 kb was inserted into the first intron of the 5′ untranslated region of GLR3.1 (Figure 2A). DNA gel blot analysis using a DNA probe spanning the inserted site indicates that GLR3.1 is a single-copy gene in rice (Figure 2B). The probe hybridized with two DNA fragments in the mutant with the expected molecular weight, but with only one fragment in the wild type (Figure 2B). The cosegregation of the T-DNA insert and the short-root phenotype was confirmed by DNA gel blot analysis of EcoRI-digested DNA of 38 T2 plants derived from a heterozygous mutant line (Figure 2C). All mutant phenotype defects were restored by transforming a 7.6-kb segment of genomic DNA, including the GLR3.1 coding region, ∼1.6 kb of upstream sequence, and 1.4 kb of downstream sequence, further confirming that the short-root phenotype is caused by a loss of function in GLR3.1 (Figure 2D). The restoration of GLR3.1 expression in the transgenic lines was verified by RT-PCR, and an ∼105-kD protein corresponding to the expected size for GLR3.1 was revealed by protein gel blot analysis (Figure 2E).
Figure 2.
Cloning of the Rice GLR3.1 Gene.
(A) T-DNA insertional sites and genomic structure of rice GLR3.1.
(B) DNA gel blot analysis of genomic DNA using a DNA fragment spanning the T-DNA insertional site as a probe. The genomic DNA from the wild type and the GLR3.1 mutant were digested with EcoRI (CE, TE) and HindIII (CH, TH), respectively.
(C) Cosegregation analysis of T-DNA insertion and root phenotype. Genomic DNA of progeny from a heterozygous line was digested by EcoRI and hybridized to the same probe as in (A). Top, progeny with the wild-type phenotype. Bottom, progeny with the short-root phenotype. The EcoRI bands corresponding to the T-DNA insertion allele (T) and the wild-type allele (W) are marked at right.
(D) Rice seedlings of the wild type (a), the GLR3.1 mutant (b), and the complemented GLR3.1 mutant (c).
(E) Protein gel blot using GLR3.1 monoclonal antibody to detect the protein in cells from whole seedlings of the wild type, the GLR3.1 mutant, and the complemented GLR3.1 mutant (top; WB), and RT-PCR analysis of the wild type, the GLR3.1 mutant, and the complemented GLR3.1 mutant (middle; RT). The mRNA level of the actin gene (AT) is shown at bottom as a control.
Rice GLR3.1 Encodes a Typical Glu Receptor–Like Protein
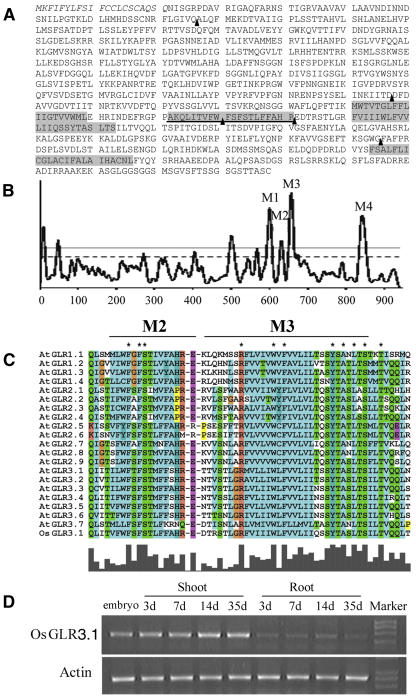
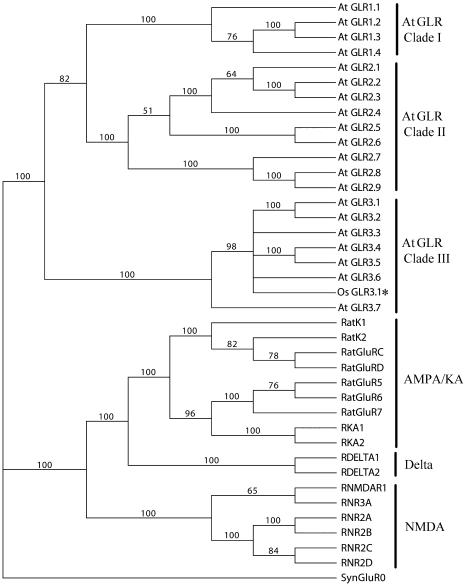
The rice GLR3.1 cDNA includes an open reading frame encoding a membrane protein of 938 amino acid residues with a predicted molecular mass of 103 kD. The GLR3.1 protein contains all of the signature domains of animal ionotropic GluRs, including three transmembrane domains (M1, M3, and M4), one reentrant membrane domain (M2) (Figures 3A and 3B), and the putative ligand binding domains (GlnH1 and GlnH2). A distinctive exon–intron structure was found in the region encoding the pore-forming domain (M2) characteristic of kainite subtypes of animal iGluRs (Figure 3A). A similar result has been reported in all members of the clade III genes of the Arabidopsis GLR family (Davenport, 2002). Alignment analysis showed a high similarity between rice GLR3.1 and 20 members of the Arabidopsis GLR family, especially in the M3 transmembrane domain (Figure 3C). In the rice GLR3.1 protein sequence, we have identified all of the 37 invariant amino acid residues that are absolutely conserved in the Arabidopsis GLR family (Chiu et al., 2002) (data not shown). Phylogenetic analysis of rice GLR3.1 with the entire GLR family from Arabidopsis and the entire iGluR family from rat indicates that GLR3.1 is evolutionarily close to the clade III Arabidopsis GLRs (Figure 4).
Figure 3.
Characterization of Rice GLR3.1.
(A) Deduced amino acid sequence of GLR3.1. The signal peptide is indicated in italic type; transmembrane domains (M1, M2, M3, and M4) are shaded; and the underlined region is the pore-forming domain (M2). The arrowheads indicate the positions of the exon–intron junctions identified in the genomic clone compared with the cDNA sequence. A distinctive exon–intron structure exists in the M2 domain.
(B) Hydropathy plot analysis of GLR3.1 with the program TMpred (http://www.ch.embnet.org/software/TMPRED_form.html).
(C) Alignment of the M2 and M3 domains in rice GLR3.1 with Arabidopsis GLRs. Rice GLR3.1 is shown at bottom, and the absolutely conserved residues are marked with asterisks.
(D) RT-PCR analysis of rice GLR3.1 expression at different developmental stages of rice seedlings.
Figure 4.
Phylogenetic Tree Generated from Parsimony Analysis of Amino Acid Sequences of Rat iGluRs, Arabidopsis GLRs, and Rice GLR3.1 with synGluR0 as the Outgroup.
Rice GLR3.1 marked by the asterisk. The numbers at the nodes are bootstrap values. Rice GLR3.1 was most similar to clade III of the GLRs. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; KA, kainate; NMDA, N-methyl-d-aspartate.
The RT-PCR mRNA expression data established that transcription of all 20 members of the Arabidopsis GLR gene family could be detected in roots, although expression profiles differed (Chiu et al., 2002). The expression of rice GLR3.1 remained at a very low level and was not detected by in situ, RNA gel blot, or promoter–β-glucuronidase assays. The β-glucuronidase activity of transgenic lines could be detected only under abiotic stresses or other severe treatments (J. Li and P. Wu, unpublished data), which supports the conclusion that rice GLR3.1 is expressed in vivo, although at low abundance. The expression of GLR3.1 was demonstrated in both root and shoot via an RT-PCR assay (Figure 3D), although there is no obvious mutant phenotype in the aerial part.
Rice GLR3.1 Could Form a Homomultimeric Complex but Was Retained in the Endoplasmic Reticulum in HEK Cells
In animals, communication between neurons occurs at highly specialized sites of cell–cell contact formed between a presynaptic nerve terminal and a postsynaptic neuron, termed synapses (Kittler and Moss, 2001). Ionotropic GluRs are concentrated at the postsynaptic density of excitatory synapses and mediate most excitatory neurotransmission (Dingledine et al., 1999). The intracellular trafficking and surface delivery of Glu receptors play an essential role in the regulation of synaptic activity and development (Kittler and Moss, 2001), which are likely to be tightly controlled processes requiring the proper folding and assembly of constituent subunits. Only fully assembled, functional receptors can be localized to the plasma membrane (Ren et al., 2003a).
Understanding the cell trafficking and localization of GLRs is important to characterizing their physiological function in plants. Most GLRs are predicted by computer-based programs to be targeted to the secretory pathway. However, less is known about plant GLR processing in the secretory pathway and subcellular localization, because most efforts to express the green fluorescent protein (GFP)–GLR fusions in plant cells have been unsuccessful (Davenport, 2002). In our case, an N-terminal signal peptide was found in GLR3.1 by PSORT analysis (Nakai and Kanehisa, 1992) (Figure 3A). Repeated attempts to test whether GLR3.1 is indeed involved in the secretory pathway by examining the trafficking of GFP fusions with GLR3.1 in plant cells were unsuccessful. We then fused enhanced GFP (EGFP) to the C terminus of GLR3.1 and expressed this construct in human HEK293 cells. The GLR3.1-EGFP fusion protein did not reach the plasma membrane (data not shown) and was instead retained in the endoplasmic reticulum (ER), where it colocalized with an ER marker, protein disulfide isomerase (Figures 5A to 5C).
Figure 5.
Rice GLR3.1 Is Retained in the ER and Forms a Homomultimeric Complex in HEK293 Cells.
(A) to (C) GLR3.1-EGFP colocalizes with protein disulfide isomerase (PDI), a marker for ER compartments. Bar = 10 μm.
(D) Acceptor photobleaching of cells cotransfected with PCMV:GLR3.1-ECFP and PCMV:GLR3.1-EYFP.
(E) Acceptor photobleaching of cells transfected with PCMV:ECFP-EYFP.
(F) Acceptor photobleaching of cells cotransfected with PCMV:ECFP and PCMV:EYFP.
In (D) to (F), black squares show the bleached zones. The left panels show the ECFP (donor) channel before bleaching, and the right panels show the same channel after bleaching. Bars = 10 μm.
An important property of the animal Glu receptors is that they function as multimeric transmembrane proteins (Dingledine et al., 1999). To determine whether GLR3.1 is also capable of multimerization, a fluorescence resonance energy transfer (FRET) analysis was performed fusing GLR3.1 with enhanced yellow fluorescent protein (EYFP) and enhanced cyan fluorescent protein (ECFP). After the acceptor (EYFP fusion) was photobleached, significant increases in the fluorescence of the donor (ECFP fusion) was observed in HEK293 cells cotransfected with PCMV:GLR3.1-ECFP and PCMV:GLR3.1-EYFP (Figure 5D). This effect was similar to that seen in positive control cells transfected with PCMV:ECFP-EYFP (Figure 5E). In the negative control with cells cotransfected with PCMV:ECFP and PCMV:EYFP, there was no difference in the observed fluorescence of the donor CFP after photobleaching of the acceptor YFP (Figure 5F). Quantitatively, the FRET efficiency was 11.36 ± 1.37 (n = 16) for the cells coexpressing GLR3.1 fusion constructs. The values of the positive and negative controls were 15.93 ± 0.84 (n = 21) and 1.28 ± 0.89 (n = 22), respectively.
Our results clearly indicate that rice GLR3.1 fusion proteins enter the secretory pathway and form homomultimers, but the homomultimeric GLR3.1 receptors are retained in the ER in this heterologous expression system.
Rice GLR3.1 Is Critical for the Mitotic Activity of the RAM and Root Cell Survival
To further characterize the cells in the RAM of the mutant, bromodeoxyuridine (BrdU) incorporation was used to monitor the mitotic activity of cells in the root tip. The immunofluorescence signals mark the cells that are in the active S-phase of the cell cycle during the labeling period (Kerk et al., 2000). In maize (Zea mays) and rice, the quiescent center (QC), a central region of the root tip with reduced mitotic activity, consists of 800 to 1200 cells and is surrounded by meristematic cells, which initiate the growth of the emerging root and the root cap. Such a pattern was observed in the wild type (Figure 6C), but an overall lower level of incorporated BrdU was observed in the RAM of the mutants of 3-d-old seedlings (Figures 6A and 6B). However, we noted a significant immunofluorescence signal in the cells of the QC region and the initial cells of the lateral root cap of the mutant lines (Figures 6C and 6D). The enhanced cell division in the QC region correlates with a diminution in QC size in the mutant (Figures 6C and 6D). In addition, premature cell differentiation coupled with reduced mitotic activity in the RAM of the mutant occurred (Figures 1C, 6A, and 6B). It is well known that the QC maintains stem cells by suppressing the differentiation of initial stem cells and defines the stem cell niche (Aida et al., 2004). The laser ablation of QC cells can lead to an arrest of cell division and increased differentiation of the initial cells (van den Berg et al., 1997). This diminution in QC size observed in the GLR3.1 mutant could lead to a general reduction of cell division and a distortion of cell differentiation in the mutant meristem.
Figure 6.
Cell Division and Programmed Cell Death Were Investigated in the RAM of the GLR3.1 Mutant.
Cell division activity is shown in longitudinal sections of the root apex of 3-d-old seedlings supplied with 10 μM BrdU for 24 h. Immunofluorescence identifies nuclei in which BrdU was incorporated during DNA synthesis.
(A) Root apex of the wild type.
(B) Root apex of the mutant.
(C) Enlargement of (A). Col, columella; LRC, lateral root cap; QC, quiescent center.
(D) Enlargement of (B).
(E) Longitudinal sections of wild-type (left) and mutant (right) root tips of 7-d-old seedlings.
(F) Evans blue staining of the roots of wild-type and GLR3.1 3-d-old seedlings. Blue spots show the dead cells. rc, root cap.
(G) to (J) Root tips stained using the terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) method.
(G) Fluorescence (stained by TUNEL) image of a wild-type root tip.
(H) Overlap of fluorescence and photo images of a wild-type root tip.
(I) Fluorescence (stained by TUNEL) image of a mutant root tip.
(J) Overlap of fluorescence and photo images of a mutant root tip.
(K) Electron micrograph of cells in the meristem of a wild-type root. n, nucleus.
(L) to (N) Electron micrographs of cells undergoing programmed cell death in mutants.
(L) Callose deposition and chromatin condensation (arrows) at the early stages of programmed cell death. cal, callose.
(M) Chromatin condensation in the later stages of programmed cell death. A nuclear membrane (arrow) has become fragmented and discontinuous. cw, cell wall.
(N) Formation of apoptosis-like bodies (arrows).
Bars = 100 μm ([A], [B], and [G] to [J]), 50 μm ([C] and [D]), 200 μm (E), 500 μm (F), 1 μm ([K] and [L]), and 200 nm ([M] and [N]).
The enhanced BrdU incorporation (Figures 6A to 6D) could account for the fact that a greater number of cell layers exist in the lateral root cap of the GLR3.1 mutant compared with the wild type (Figure 1C). By contrast, a similar division pattern of columella was found in the wild type and the mutant (Figures 5C and 5D). In Arabidopsis, the primary root cap is composed of cells derived from two different origins. The lateral root cap is generated from the same set of initial cells as the epidermis, and the columella has its own initial cells (Benfey and Scheres, 2000; Sievers et al., 2002). Our results indicate that GLR3.1 has disparate effects on cells of differing origins in root cap development.
In animals, Glu receptors are believed to be involved in neuronal cell death for pathological conditions or central nervous system development (Dingledine et al., 1999). Either too much or too little Glu receptor activity is harmful and can result in neuronal cell death (Hardingham and Bading, 2003). In addition to disorders of cell proliferation and differentiation, obvious cell death was also observed, along with an excess number of cells coming off at the root tip of 7-d-old seedlings of the GLR3.1 mutant (Figure 6E). By staining the 3-d-old seedling roots with Evans blue, which preferentially enters and stains dead cells with a completely permeable plasma membrane (Gaff and Okong'O-Ogola, 1971), cell death was observed in the root cap of both wild-type and mutant plants. However, there was comparatively greater cell death in the elongation zone of the mutant seedlings (Figure 6F). To determine whether the cell death resulted from programmed cell death, we performed a TUNEL assay in root tips of 1-d-old seedlings. Nuclear DNA fragmentation was detected in situ throughout the RAM in the mutant, whereas it was restricted to the root cap cells in the wild type (Figures 6G to 6J). The cellular features of programmed cell death were also noted, such as nuclear DNA fragmentation, nuclear shrinkage, chromatin condensation, and callose deposition in the RAM of the mutant lines of 3-d-old seedlings by transmission electron microscopy (Figures 6K to 6N). Together, these results demonstrate that the rice GLR3.1 gene mutation results in excessive programmed cell death accompanied by disordered cell proliferation in root development. They suggest that rice GLR3.1 could be a key factor in maintaining the normal function of the RAM and preserving the balance between cellular proliferation and cell death during the early development of the plant root.
DISCUSSION
Characteristics of Rice GLR3.1
We have shown that rice GLR3.1, a Glu receptor–like gene, serves an important function in maintaining normal cell division activity and cell survival during rice seedling root development. The deduced amino acid sequence indicates that the GLR3.1 gene encodes a typical Glu receptor–like protein with close sequence and structural similarities to animal iGluRs. The extracellular and cytoplasmic segments of iGluRs are thought to be available for ligand recognition, cytoplasmic modification, or interactions between the receptor and cytoplasmic proteins. The channel pore, which is formed by three membrane-spanning domains and one reentrant membrane loop, defines channel properties such as ion selectivity, permeability, and conductance. In the channel pore, the M2 loop is the structural basis for the ion selectivity filter (Dingledine et al., 1999). The identification of a distinctive exon–intron structure, which was found in the kainite subgroup of iGluRs and in clade III plant GLRs, indicates that the structure of the M2 loop might have emerged before the divergence of animals and plants.
Although plant GLRs are significantly similar to animal iGluRs, their ability to function as channels has not been demonstrated successfully in either Xenopus oocytes or HEK293 cells, the most commonly used heterologous expression systems for iGluRs. Our efforts also encountered difficulties in recording the current by patch clamp in HEK293 cells (data not shown). However, we found that rice GLR3.1 fusion proteins clearly enter the secretory pathway and form homomultimers, but in our experiments the homomultimeric GLR3.1 receptors were retained in the ER in HEK293 cells. The failure of GLR3.1 in HEK293 cells to reach their proper surface destination could explain the absence of recorded membrane current activity. Our study of rice GLR3.1 suggests that successful plasma membrane targeting of the protein is a critical prerequisite for the future characterization of the channel properties of plant GLR in a heterologous expression system.
In most cases, the functional iGluRs are composed of heteromeric multimers and do not appear to function in the homomultimeric forms (Dingledine et al., 1999). It is well known that the ER is the primary checkpoint in animals to prevent the improperly assembled Glu receptor complexes from reaching the cell surface at synapses (Ren et al., 2003a, 2003b). The ER retention of GLR3.1 could result from assembly deficiency attributable to the absence of a native cofactor in HEK293 cells. To screen for such a cofactor, coexpression of GLR3.1 with other rice GLR-like proteins using a heterologous expression system may reveal the critical missing link. In addition to the sequence signal for multimer assembly in the ER, other protein trafficking signals exist in the C terminus of iGluRs to regulate their surface expression (Ren et al., 2003a, 2003b; Yan et al., 2004). It would be interesting to test for the presence of any such motif in rice GLR3.1 that mediates trafficking and surface expression. Considering the evolutionary distance between higher plants and mammals, the utility of a heterologous mammalian expression system for plant ion channels may not be a viable option. Therefore, developing a plant cell expression system to analyze plant GLR function may be the best alternative.
Rice GLR3.1 Plays a Role in the Maintenance of Cell Proliferation and Cell Death in Root Development
Plant root development is a well-coordinated and highly ordered process. As one of the most tractable systems for the study of plant organogenesis, the plant root has been valuable for studying the control of radial patterning, specification of epidermal cell types, placement of the root organizing center, and other fundamental processes (Benfey and Scheres, 2000). As one of the most important organs for sensing environmental signals, the root may have a set of rapid reaction systems to coordinate its development with the environment. The GLR-type candidate channels may be a logical participant in plants' ability to detect environmental cues. Our results indicate that rice GLR3.1, a Glu receptor–like gene, is involved in root development as a regulator of cell proliferation and cell death in the root apex.
In the BrdU incorporation test, rice GLR3.1 has differential effects on root cap cells with distinct origins. The root cap plays an important role in protecting the RAM as well as in sensing variations in environmental and development cues (Sievers et al., 2002). For example, aluminum toxicity was known to significantly interfere with border cell development by inhibiting mitosis in the root cap meristem (Pan et al., 2004). It was implied that the response of root cap cells to aluminum toxicity is initiated by efflux of a Glu-like ligand and subsequent activation of some plant GLRs (Sivaguru et al., 2003). In the BrdU incorporation test, the disruption of GLR3.1 correlated with enhanced cell division in the outer portion of the lateral root cap but not the columella in the central portion, which leads to more cell layers in the lateral root cap and more cell shedding in the root cap of the mutant. The observation that both aluminum and Glu could stimulate radial expansion (Sivaguru et al., 2003) is also consistent with the slender-root phenotype observed in rice GLR3.1 T-DNA mutant seedlings.
In addition to affecting lateral root cap development and root radial expansion, root elongation was inhibited in the mutant, in contrast with the reported inhibitory effect of Glu and aluminum treatments (Sivaguru et al., 2003). It is possible that in mutant roots, defects in the root tip (such as active division in the QC region, enhanced cell differentiation, and enhanced cell death) somehow affect the elongation of the root as a result of a coordinated regulation mechanism. Indeed, more recent evidence indicates that the root cap plays a very important role in other parts of root development. Plants with root caps genetically ablated by toxin protein have normal aerial parts but short, prematurely differentiated roots with enhanced division activity of QC cells compared with wild-type plants (Tsugeki and Fedoroff, 1999). In the rice GLR3.1 mutant, disorder of root cap development, diminution of QC size, and reduction in root meristematic activity were observed. These results suggest that root development is a highly coordinated process involving the QC, root cap, and meristem. Although it is not clear which one of those effects is the primary manifestation of the GLR3.1 defect, it appears most likely that the loss of GLR3.1 function disrupts the cell–cell signaling that plays an essential role in maintaining and coordinating the normal function of the RAM in rice root development.
It has long been recognized that the balance between cell proliferation and cell death plays a key role in the early development of the central nervous system (Ross, 1996), and recent studies have implicated Glu and Glu receptor involvement in those processes (Ghiani et al., 1999; Copani et al., 2001). During plant root development, programmed cell death has been observed in many morphogenetic processes, such as aerenchyma formation, root cap cell production, and shedding processes (Levine et al., 1996; Samuilov et al., 2000). Similar to its role in the central nervous system, programmed cell death is a necessary process in the normal development and functioning of plant roots. The disruption of GLR3.1 results in disordered cell division and differentiation coupled with enhanced programmed cell death in rice root development, suggesting that plant GLRs may have functions similar to those of their animal homologues.
METHODS
Plant Material, Growth Conditions, and Mutant Screening
A japonica rice (Oryza sativa) variety, Zhonghua 11, was used in our experiments. All plants were grown in half-strength rice culture solution (Yoshida et al., 1971) in growth chambers (MC1000 system; Snijders) at temperature regimes of 28/22°C (day/night) and 70% humidity under a 12-h photoperiod (15,000 lux).
Microscopic Analysis
For microscopic analysis, root tips were fixed and embedded in Spurr resin. Semithin sections were stained in methylene blue. The samples were rinsed with distilled water and visualized with a Zeiss Axiovert 200 microscope with a color charge-coupled device camera (Zeiss). For electron microscopic analysis, root tips of 3-d-old seedlings were fixed and embedded in Spurr resin. Ultrathin sections were stained with uranyl acetate and observed with a JEM-1230 electron microscope (JEOL).
Gene Cloning and Complementation Test
The flanking sequences of the insertional T-DNA fragment in the mutant were determined using TAIL-PCR as described (Liu et al., 1995). The products of TAIL-PCR were cloned into T vector and then sequenced (MegaBACE; Amersham Pharmacia). The sequences obtained were analyzed using the BLAST Web server (http://www.ncbi.nlm.nih.gov/blast/). Then we amplified the full-length cDNA of rice GLR3.1 using primers 5′-TTCACACGGGCTGCTAGTTCTTC-3′ and 5′-ATATAAGCTTGGCATGAAGCGGTGGTGCTG-3′ (the original TTA corresponding to a stop codon was replaced by TTG) using high-fidelity Platinum Pfx DNA polymerase (Invitrogen). The products were cloned into T vector and confirmed by sequencing. For complementation assays of the GLR3.1 mutant, a 7.6-kb genomic DNA fragment containing GLR3.1 flanked by 1.6 kb of upstream sequence and 1.4 kb of downstream sequence was amplified and inserted into pCambia2300. The construct was confirmed by sequencing and used to transform homozygous GLR3.1 mutants using a biolistics (particle gun) method (Bio-Rad).
Antibody Production and Protein Blot
The primer pairs (5′-ATTACATATGTACCAGTACTCCCGCC-3′ and 5′-ATTTCTCGAGGCATGAAGCGGTGGT-3′) were used to amplify the C-terminal 81–amino acid polypeptide coding sequence of GLR1. The PCR products were cloned into the NdeI and XhoI sites of pET29b (Novagen). The resulting plasmid was transformed into Escherichia coli BL21 (DE3) (Novagen), and recombinant 81–amino acid polypeptide fused with the 6×His tag was purified by nickel-nitrilotriacetic acid agarose resin (Qiagen) according to the manufacturer's instruction. The purified polypeptide was used to produce mouse monoclonal antibodies as described (Harlow and Lane, 1988).
Crude membrane proteins were prepared from 7-d-old seedlings of wild-type, mutant, and complemented mutant lines as described (Rogers et al., 1991). The extracted proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Amresco). The membrane was first probed with the anti-GLR1 monoclonal antibody (1:4000 dilution) and then with an anti-mouse alkaline phosphatase–conjugated secondary antibody (1:3000). The antibody conjugates were detected in the reaction buffer (100 mM NaCl, 5 mM MgCl2, and 100 mM Tris-Cl, pH 9.5) containing 1 mg/mL nitroblue tetrazolium and 0.5 mg/mL 5-bromo-4-chloro-3-indolyl phosphate, and the reaction was stopped with 1 mM EDTA.
Sequence Alignment and Phylogenetic Analysis
The amino acid sequences of rice GLR3.1, the Arabidopsis thaliana GLR gene family, and the Rat iGluR family were aligned by ClustalX (Thompson et al., 1997) using three separate sets of alignment parameters that produced preferable alignments (gap opening cost = 10, 15, 20; gap extension cost = 1.0; amino acid substitution matrix = Blosum 30). These alignments were examined for alignment-ambiguous regions, and the regions of alignment instability were culled from the matrix to ensure validity of the analysis as described (Chiu et al., 2002). Phylogenetic analysis was conducted using PAUP 4.0* (Swofford, 2002). The phylogenetic tree was constructed using the neighbor-joining method (Saitou and Nei, 1987) with Synechocystis PCC6803 GluR0 (accession number BAA17851) as the outgroup. Bootstrap analysis (bootstrap method NJ, 100 replicates) (Felsenstein, 1985) was performed to measure the robustness of all nodes. All other sequences (except rice GLR3.1) were obtained from the Ligand-Gated Ion Channel database (http://www.ebi.ac.uk/compneur-srv/LGICdb/) (Le Novere and Changeux, 2001). See Supplemental Table 1 online for the full sequence alignment.
BrdU Incorporation
To observe the cell division activity of the root, germinated seeds were cultured for 3 d, then 10 μM BrdU (Sigma-Aldrich) was added and the plants were incubated for a further 24 h. The root tips of the plants were then excised, fixed, and embedded using Steedman's wax and sectioned as described (Vitha et al., 2000).
Immunofluorescence Staining
A modification of the procedure described by Kerk et al. (2000) was used to detect incorporated BrdU. The sections were hydrolyzed in 1 n HCl for 1.5 h at 37°C, then neutralized by immersion in 0.1 M borate buffer, pH 8.5, washed with PBS, and incubated with anti-BrdU antibody (Sigma-Aldrich) for 2 h. Then the slides were labeled with rabbit anti-mouse IgG(H+L)–Alexa Fluor 488 (Molecular Probes) and observed with the laser scanning system LSM 510 (Zeiss).
Cell Death Assay
Cell death was examined using Evans blue, a compound that enters dead cells easily as a result of their freely permeable plasma membranes (Gaff and Okong'O-Ogola, 1971). The root tips of 3-d-old seedlings were submerged in 20 mL of 1% (w/v) Evans blue water solution for 10 min, washed with distilled water for 2 h, and photographed. For in situ detection of DNA fragmentation, the Steedman's wax sections of the root tips of 1-d-old seedlings were analyzed using a TUNEL assay as described by Wang et al. (1996). In this assay, the 1 nM digoxigenin-11-dUTP was replaced by 1 nM BrdU. The labeled sections were detected as described above.
Construction of Fusion Vectors and Detection of FRET via Acceptor Photobleaching
To generate an in-frame fusion of GLR3.1 and EGFP, the BglII restriction sites were introduced at both ends of the coding sequences for GLR3.1. The resulting DNA fragment was placed upstream of EGFP in the mammalian expression vector pEGFP-N1 (Clontech). The BglII fragment was then excised from PCMV:GLR3.1-GFP and cloned into pECFP-N1 and pEYFP-N1 (Clontech) to transfect HEK293 cells for FRET analysis. The detection of FRET via acceptor photobleaching of overnight cell cultures was performed as described (Karpova et al., 2003). Fluorescence signals were collected with an LSM 510 confocal microscope (Zeiss). Photobleaching in selective regions was performed at 514 nm, with a mean reduction in YFP emissions to <15% using the software-controlled LSM 510 confocal microscope. Images were acquired before and after photobleaching. Effective FRET efficiency (EF) between CFP (donor) and YFP (acceptor) was quantified with acceptor photobleaching methods using the formula EF = (A1 − A0) × 100/A0, where A0 and A1 represent CFP emission with 458-nm excitation in the photobleached region before and after photobleaching with 514-nm excitation, respectively.
Accession Number
Rice GLR3.1 sequence data from this article can be found in the GenBank data library under accession number DQ305408.
Supplemental Data
The following material is available in the online version of this article.
Supplemental Table 1. Full Alignment of GLRs.
Supplementary Material
Acknowledgments
This work was supported by the Key Basic Research Special Foundation of China (2005CB120901), the Special Program of Rice Functional Genomics of China, the National Natural Science Foundation of China, the Science and Technology Bureau of Zhejiang Province, and the National Institute of Biological Sciences at Beijing. We thank Jianhong Luo for assistance with the FRET assays and Heven Sze for critical reading of the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instruction for Authors (www.plantcell.org) is: Ping Wu (clspwu@zju.edu.cn).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.037713.
References
- Aida, M., Beis, D., Heidstra, R., Willemsen, V., Blilou, I., Galinha, C., Nussaume, L., Noh, Y.S., Amasino, R., and Scheres, B. (2004). The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119 109–120. [DOI] [PubMed] [Google Scholar]
- Barlow, W. (2003). The root cap: Cell dynamics, cell differentiation and cap function. J. Plant Growth Regul. 21 261–286. [Google Scholar]
- Benfey, P.N., and Scheres, B. (2000). Root development. Curr. Biol. 10 R813–R815. [DOI] [PubMed] [Google Scholar]
- Brenner, E.D., Martinez-Barboza, N., Clark, A.P., Liang, Q.S., Stevenson, D.W., and Coruzzi, G.M. (2000). Arabidopsis mutants resistant to S(+)-β-methyl-α,β-diaminopropionic acid, a cycad-derived glutamate receptor agonist. Plant Physiol. 124 1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S., Jin, W., Wang, M., Zhang, F., Zhou, J., Jia, Q., Wu, Y., Liu, F., and Wu, P. (2003). Distribution and characterization of over 1000 T-DNA tags in rice genome. Plant J. 36 105–113. [DOI] [PubMed] [Google Scholar]
- Chiu, J.C., Brenner, E.D., DeSalle, R., Nitabach, M.N., Holmes, T.C., and Coruzzi, G.M. (2002). Phylogenetic and expression analysis of the glutamate-receptor-like gene family in Arabidopsis thaliana. Mol. Biol. Evol. 19 1066–1082. [DOI] [PubMed] [Google Scholar]
- Copani, A., Uberti, D., Sortino, M.A., Bruno, V., Nicoletti, F., and Memo, M. (2001). Activation of cell-cycle-associated proteins in neuronal death: A mandatory or dispensable path? Trends Neurosci. 24 25–31. [DOI] [PubMed] [Google Scholar]
- Davenport, R. (2002). Glutamate receptors in plants. Ann. Bot. 90 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison, K.L., and Spalding, E.P. (2000). Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol. 124 1511–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine, R., Borges, K., Bowie, D., and Traynelis, S.F. (1999). The glutamate receptor ion channels. Pharmacol. Rev. 51 7–61. [PubMed] [Google Scholar]
- Dubos, C., Huggins, D., Grant, G.H., Knight, M.R., and Campbell, M.M. (2003). A role for glycine in the gating of plant NMDA-like receptors. Plant J. 35 800–810. [DOI] [PubMed] [Google Scholar]
- Dubos, C., Willment, J., Huggins, D., Grant, G.H., and Campbell, M.M. (2005). Kanamycin reveals the role played by glutamate receptors in shaping plant resource allocation. Plant J. 43 348–355. [DOI] [PubMed] [Google Scholar]
- Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39 783–791. [DOI] [PubMed] [Google Scholar]
- Gaff, D.F., and Okong'O-Ogola, O. (1971). The use of nonpermeating pigments for testing the survival of cells. J. Exp. Bot. 22 756–758. [Google Scholar]
- Ghiani, C.A., Eisen, A.M., Yuan, X., DePinho, R.A., McBain, C.J., and Gallo, V. (1999). Neurotransmitter receptor activation triggers p27(Kip1)and p21(CIP1) accumulation and G1 cell cycle arrest in oligodendrocyte progenitors. Development 126 1077–1090. [DOI] [PubMed] [Google Scholar]
- Hardingham, G.E., and Bading, H. (2003). The yin and yang of NMDA receptor signalling. Trends Neurosci. 26 81–89. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1988). Antibodies: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Ikonomidou, C., Bosch, F., Miksa, M., Bittigau, P., Vockler, J., Dikranian, K., Tenkova, T.I., Stefovska, V., Turski, L., and Olney, J.W. (1999). Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283 70–74. [DOI] [PubMed] [Google Scholar]
- Kang, J., Mehta, S., and Turano, F.J. (2004). The putative glutamate receptor 1.1 (AtGLR1.1) in Arabidopsis thaliana regulates abscisic acid biosynthesis and signaling to control development and water loss. Plant Cell Physiol. 45 1380–1389. [DOI] [PubMed] [Google Scholar]
- Kang, J., and Turano, F.J. (2003). The putative glutamate receptor 1.1 (AtGLR1.1) functions as a regulator of carbon and nitrogen metabolism in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 100 6872–6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova, T.S., Baumann, C.T., He, L., Wu, X., Grammer, A., Lipsky, P., Hager, G.L., and McNally, J.G. (2003). Fluorescence resonance energy transfer from cyan to yellow fluorescent protein detected by acceptor photobleaching using confocal microscopy and a single laser. J. Microsc. 209 56–70. [DOI] [PubMed] [Google Scholar]
- Kerk, N.M., Jiang, K., and Feldman, L.J. (2000). Auxin metabolism in the root apical meristem. Plant Physiol. 122 925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.A., Kwak, J.M., Jae, S.K., Wang, M.H., and Nam, H.G. (2001). Overexpression of the AtGluR2 gene encoding an Arabidopsis homolog of mammalian glutamate receptors impairs calcium utilization and sensitivity to ionic stress in transgenic plants. Plant Cell Physiol. 42 74–84. [DOI] [PubMed] [Google Scholar]
- Kittler, J.T., and Moss, S.J. (2001). Neurotransmitter receptor trafficking and the regulation of synaptic strength. Traffic 2 437–448. [DOI] [PubMed] [Google Scholar]
- Lam, H.M., Chiu, J., Hsieh, M.H., Meisel, L., Oliveira, I.C., Shin, M., and Coruzzi, G. (1998). Glutamate-receptor genes in plants. Nature 396 125–126. [DOI] [PubMed] [Google Scholar]
- Le Novere, N., and Changeux, J.P. (2001). LGICdb: the ligand-gated ion channel database. Nucleic Acids Res. 29 294–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, A., Pennell, R.I., Alvarez, M.E., Palmer, R., and Lamb, C. (1996). Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr. Biol. 6 427–437. [DOI] [PubMed] [Google Scholar]
- Liu, Y.G., Mitsukawa, N., Oosumi, T., and Whittier, R.F. (1995). Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 8 457–463. [DOI] [PubMed] [Google Scholar]
- Nakai, K., and Kanehisa, M. (1992). A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics 14 897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, J.W., Ye, D., Wang, L.L., Hua, J., Zhao, G.F., Pan, W.H., Han, N., and Zhu, M.Y. (2004). Root border cell development is a temperature-insensitive and Al-sensitive process in barley. Plant Cell Physiol. 45 751–760. [DOI] [PubMed] [Google Scholar]
- Ren, Z., Riley, N.J., Garcia, E.P., Sanders, J.M., Swanson, G.T., and Marshall, J. (2003. a). Multiple trafficking signals regulate kainate receptor KA2 subunit surface expression. J. Neurosci. 23 6608–6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, Z., Riley, N.J., Needleman, L.A., Sanders, J.M., Swanson, G.T., and Marshall, J. (2003. b). Cell surface expression of GluR5 kainate receptors is regulated by an endoplasmic reticulum retention signal. J. Biol. Chem. 278 52700–52709. [DOI] [PubMed] [Google Scholar]
- Rogers, S.W., Hughes, T.E., Hollmann, M., Gasic, G.P., Deneris, E.S., and Heinemann, S. (1991). The characterization and localization of the glutamate receptor subunit GluR1 in the rat brain. J. Neurosci. 11 2713–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, M.E. (1996). Cell division and the nervous system: Regulating the cycle from neural differentiation to death. Trends Neurosci. 19 62–68. [DOI] [PubMed] [Google Scholar]
- Saitou, N., and Nei, M. (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4 406–425. [DOI] [PubMed] [Google Scholar]
- Samuilov, V.D., Oleskin, A.V., and Lagunova, E.M. (2000). Programmed cell death. Biochemistry (Mosc.) 65 873–887. [PubMed] [Google Scholar]
- Sievers, A., Braun, M., Monshausen, G.B., Waisel, Y., Eshel, A., and Kafkafi, U. (2002). The root cap: Structure and function. In Plant Roots: The Hidden Half, 3rd ed. (New York: Marcel Dekker), pp. 33–47.
- Sivaguru, M., Pike, S., Gassmann, W., and Baskin, T.I. (2003). Aluminum rapidly depolymerizes cortical microtubules and depolarizes the plasma membrane: Evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol. 44 667–675. [DOI] [PubMed] [Google Scholar]
- Sprengel, R., Aronoff, R., Volkner, M., Schmitt, B., Mosbach, R., and Kuner, T. (2001). Glutamate receptor channel signatures. Trends Pharmacol. Sci. 22 7–10. [DOI] [PubMed] [Google Scholar]
- Swofford, D. (2002). Phylogenetic Analysis Using Parsimony (& Other Methods), Version 4. (Sunderland, MA: Sinauer Associates).
- Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F., and Higgins, D.G. (1997). The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugeki, R., and Fedoroff, N.V. (1999). Genetic ablation of root cap cells in Arabidopsis. Proc. Natl. Acad. Sci. USA 96 12941–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg, C., Willemsen, V., Hage, W., Weisbeek, P., and Scheres, B. (1995). Cell fate in the Arabidopsis root meristem determined by directional signalling. Nature 378 62–65. [DOI] [PubMed] [Google Scholar]
- van den Berg, C., Willemsen, V., Hendriks, G., Weisbeek, P., and Scheres, B. (1997). Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 390 287–289. [DOI] [PubMed] [Google Scholar]
- Vitha, S., Baluska, F., Jasik, J., Volkmann, D., Barlow, P., Staiger, C.J., Baluska, F., Volkmann, D., and Barlow, P.W. (2000). Steedman's wax for F-actin visualization. In Actin: A Dynamic Framework for Multiple Plant Cell Functions, C.J. Staiger, F. Baluska, D. Volkmann, and P. Barlow, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 619–636.
- Wang, H., Li, J., Bostock, R.M., and Gilchrist, D.G. (1996). Apoptosis: A functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, S., Sanders, J.M., Xu, J., Zhu, Y., Contractor, A., and Swanson, G.T. (2004). A C-terminal determinant of GluR6 kainate receptor trafficking. J. Neurosci. 24 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, S., Forno, D.A., and Cock, J.H. (1971). Laboratory Manual for Physiological Studies of Rice. (Manila, The Philippines: International Rice Research Institute).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.