Abstract
Fibroblast growth factors play important roles in angiogenesis, but their functions in lymphangiogenesis remain poorly understood. The homeodomain transcription factor Prox1 is essential for development of the lymphatic system by specifying lymphatic endothelial cell (LEC) fate. Here, we identify fibroblast growth factor (FGF) receptor (FGFR)-3 as a novel Prox1 target gene. Ectopic overexpression of Prox1 in blood vascular endothelial cells up-regulates FGFR-3. Prox1 induces the expression of the IIIc isoform, which we also found to be the major isoform of FGFR-3 expressed in LECs. This transcriptional activation is mediated by a direct binding of Prox1 to newly identified Prox1-response elements in the FGFR-3 promoter. Consistently, FGFR-3 is up-regulated in Prox1-positive newly formed lymphatic vessels during embryogenesis and its lymphatic-specific expression is maintained throughout development. We also found that FGF-1 and FGF-2 promote proliferation, migration, and survival of cultured LECs without involvement of vascular endothelial cell growth factor receptor-3. We show that FGF-2 binds to low- and high-affinity receptors on LECs and is efficiently internalized and processed. Moreover, functional inhibition of FGFR-3 using small interfering RNA represses LEC proliferation. Together, these results indicate that FGFR-3 is an initial target of Prox1 during the lymphatic cell fate specification and that FGF signaling may play an important role in lymphatic vessel development.
INTRODUCTION
Fibroblast growth factor (FGF) signaling plays an important role in a broad range of biological processes of vascular endothelial cells, including proliferation, migration, survival, tubulogenesis, and differentiation (Javerzat et al., 2002). At least, 23 different FGFs and four FGF-receptors (FGFR-1 through FGFR-4) have been identified and characterized in vertebrates so far (Ornitz and Itoh, 2001; Javerzat et al., 2002). FGFRs belong to the receptor tyrosine kinase family and commonly consist of three extracellular immunoglobulin (Ig)-like domains, a single-pass transmembrane domain, and a split-tyrosine kinase domain. Alternative splicing generates a wide array of isoforms of FGFRs with distinct physical and biological characteristics (Dell and Williams, 1992; Ornitz, 2000; Hanneken, 2001; Groth and Lardelli, 2002; Terada et al., 2001; Wilkie et al., 2002). The most common variants, the IIIb or IIIc isoform, are formed by alternative splicing of the carboxy-terminal half of the third Ig domain of FGFR-1, -2, and -3 but not FGFR-4. The alternative splicing is regulated in a tissue-specific manner and also determines their binding specificity for various FGF ligands. In general, the IIIb isoforms of FGFRs are predominantly expressed by epithelial lineage cells, whereas the IIIc variants tend to be expressed in mesenchymal lineages (Alarid et al., 1994; Murgue et al., 1994; Orr-Urtreger et al., 1993; Yan et al., 1993). FGF ligands and their interacting receptor isoforms are often expressed in adjacent tissues.
The roles of FGFs in vascular development have been well characterized in the context of angiogenesis that is associated with tumor development, tissue repair, and embryogenesis (Bikfalvi et al., 1998; Javerzat et al., 2002; Auguste et al., 2003). FGF-2 was one of the first angiogenic factors identified for its potent activity on vascular endothelial cell proliferation (Shing et al., 1984). Recently, FGF-2 was reported to also induce lymphatic vessel growth in mouse cornea assay by promoting the secretion of the potent lymphangiogenic factor, vascular endothelial cell growth factor (VEGF)-C, by blood vascular endothelial cells (BECs) (Kubo et al., 2002; Chang et al., 2004). Moreover, systemic treatment with a blocking antibody against vascular endothelial cell growth factor receptor (VEGFR)-3, the major receptor for VEGF-C, reduced the FGF-2-induced corneal lymphangiogenesis (Kubo et al., 2002; Chang et al., 2004). These findings indicate that the effects of FGF-2 on lymphangiogenesis might be largely indirect through activation of the VEGF-C/VEGFR-3 signaling pathway.
The homeodomain transcriptional factor Prox1 was originally isolated because of its homology with the Drosophila Prospero protein (Oliver et al., 1993). Like Prospero, Prox1 plays an important role in cell fate decisions of diverse cell types and serves as a master regulator during embryonic development of the lymphatic vascular system (Wigle and Oliver, 1999; Hong et al., 2002; Wigle et al., 2002). On an inductive signal during early development, Prox1 is up-regulated in a subset of venous endothelial cells and reprograms their gene expression profile similar to that of lymphatic endothelial cells (LECs). The Prox1-positive venous endothelial cells then further differentiate to adopt lymphatic endothelial cell phenotypes and migrate out to form the primitive lymphatic vessels. Therefore, the Prox1-mediated cell fate reprogramming is the initial and essential step during lymphatic endothelial differentiation (Wigle and Oliver, 1999; Wigle et al., 2002). In addition, we and others have recently found that ectopic overexpression of Prox1 in cultured BECs isolated from human foreskin recapitulates the embryonic lymphatic reprogramming by down-regulating the BEC-specific genes and by up-regulating several lymphatic-specific genes (Hong et al., 2002; Petrova et al., 2002; Hirakawa et al., 2003). However, the molecular mechanisms underlying this lymphatic reprogramming are poorly understood. In this study, we present evidence demonstrating that Prox1 up-regulates the expression of FGFR-3 during lymphatic reprogramming and that FGF signaling through the up-regulated FGFR-3 plays an important role in the early lymphatic vascular system development.
MATERIALS AND METHODS
Isolation and Culturing of Cells
Primary human dermal LECs were isolated from neonatal human foreskins as described previously (Hirakawa et al., 2003). All experiments were performed using LECs in their early passages (less than passage number 4) because of reduced expression of FGFR-3 in LECs of high passage numbers (our unpublished data). Stably transfected rat myoblasts expressing human FGFR-3 IIIb or FGFR-3 IIIc were kind gifts from Dr. Daniel Podolsky (Massachusetts General Hospital, Charlestown, MA) (Kanai et al., 1997). Human embryonic kidney (HEK)293 cells were purchased from American Type Culture Collection (Manassas, VA).
Detection and Quantification of FGF Receptor Expression
Dual-labeled TaqMan probe-based real-time reverse transcription (RT)-PCRs were performed to quantify the expression of FGF receptors (Hong et al., 2002). The sequences of forward and reverse primers and dual-labeled probes are as follows: FGFR-1 (CTCCCGAGGCGGAACC, TGAGCTCGATCCTCCTTTTCA, FAM-CCACGCCGAGCGAGGGTCAG-TAMRA), FGFR-3 (GTCATGGAAAGCGTGGTGC, CCAAACTTGTTCTCCACGACG, FAM-TCGGACCGCGGCAACTACACC-TAMRA), and β-actin (TCACCGAGCGCGGCT, TAATGTCACGCACGATTTCCC, JOE-CAGCTTCACCACCACGGCCGAG-TAMRA). In addition, conventional RT-PCR was performed for FGFR-3 using forward and reverse primers (GACGGCACACCCTACGTTAC, GGATGCCTGCATACACACTG) that bind to the seventh and 10th exon of human FGFR-3, respectively, along with primers for β-actin (TGGGACGACATGGAGAAAAT, GAGGCGTACAGGGATAGCAC). An FGFR-3 cDNA clone (clone ID, 180447) from Invitrogen (Carlsbad, CA) was used as a probe for Northern blot analysis. RT-PCR analyses were performed at least three times with comparable results.
Immunofluorescence stainings were performed on frozen sections of 4% paraformaldehyde-fixed neonatal human foreskin sections or on E11.5 mouse embryo sections as described previously (Hong et al., 2002), using antibodies against human FGFR-3 (mAb 7661; R&D Systems, Minneapolis, MN), mouse FGFR-3 (mAb 710; R&D Systems), or LYVE-1 (Upstate Biotechnology, Charlottesville, VA). Secondary antibodies labeled with Alexa Fluor 488 or Alexa Fluor 594 (Invitrogen) were used to detect respective primary antibodies. Nuclei were counterstained with 20 μg/ml Hoechst bisbenzimide.
Construction of Mutant Prox1 and FGFR-3 Reporter Gene Luciferase Assays
To construct a mutant Prox1, two amino acid substitution mutations (N625A and R627A) were introduced into pcDNA/Prox1 (Hong et al., 2002) by using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA). DNA sequences of the primers used for the mutagenesis reaction are CTCATCAAGTGGTTTAGCgcTTTCgccGAGTTTTACTAC and CTGAATGTAGTAAAACTCggcGAAAgcGCTAAACCACTTG. The resulting product (pcDNA/MutProx1) was sequenced to confirm the base pair changes. The mouse FGFR-3 promoter-luciferase constructs were kindly provided by Dr. David Ornitz (Washington University School of Medicine, St. Louis, MO) (McEwen and Ornitz, 1998). Each luciferase construct was cotransfected into HEK293 cells in combination with pcDNA (Invitrogen), pcDNA/Prox1, or pcDNA/MutProx1. Forty-eight hours after transfection, 50 μl of the cell lysates was used to measure the activity of firefly luciferase using the Dual-Glo luciferase assay system (Promega, Madison, WI). Another 50 μl of the cell lysates was used to measure the protein concentration by using the Bio-Rad protein assay (Bio-Rad, Hercules, CA). Luciferase activity was normalized by the total protein amount. The assays were performed in triplicates in three independent experiments.
Electrophoretic Mobility Shift Assay
Purification of the glutathione S-transferase (GST)-Prox1 protein was performed as described previously (Belecky-Adams et al., 1997; Cui et al., 2004). The GST-Prox1 vector, a kind gift from Dr. M. Duncan (University of Delaware, Newark, DE) (Cui et al., 2004), expresses the C-terminal half of Prox1 (the homeodomain and prospero domains) fused to the GST protein. Rosetta bacterial cells (Novagen, San Diego, CA) were transformed with the GST-Prox1 vector or a control GST vector (pGEX-KG). Bacterial cell extracts were prepared using the BugBuster solution (Novagen). The GST and GST-Prox1 proteins were isolated by glutathione-Sepharose 4B beads (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Five micrograms of purified proteins were incubated in 10 mM Tris, pH 7.5, 10 mM MgCl2, 5 mM EDTA, pH 7.5, 10 mM dithiothreitol, 2% NP-40, 10% glycerol, 20% sucrose, 5 μg of bovine serum albumin (BSA), and 0.2 μg of poly(dI:dC) for 30 min at room temperature, together with 0.05 pmol of 32P-labeled probes (wild-type, ctgggctccCACGCCTCTgggaccgcccg; mutant, ctgggctccACTTAAGCTgggaccgcccg). The protein-DNA complex was separated in a 6% native polyacrylamide gel (30% polyacrylamide solution, 5× Tris borate-EDTA [TBE], 10% ammonium persulfate, N,N,N′,N′-tetramethylethylenediamine) in 0.5× TBE at 200 V for 1 h in an ice slurry, after a prerun in 0.5× TBE at 150 V at room temperature. For competition assays, 100-fold molar excess of the unlabeled probe was added to the incubation mixtures.
Cell Proliferation, Migration, Apoptosis Assays, and Functional Inhibition of FGFR-3
Recombinant human FGF-1 and FGF-2 were purchased from R&D Systems. For proliferation assays, 1500 LECs were seeded into a fibronectin-coated well of 96-well plates in complete growth medium (Hirakawa et al., 2003). After 24 h, cells were treated or not with FGFs (10 ng/ml) for 48 h in low serum medium (2% fetal bovine serum [FBS]) containing heparin (1 μg/ml). Cell proliferation was assessed by the 4-methylumbiliferyl heptanoate (MUH) fluorescence assay as described previously (Detmar et al., 1990). For migration assays, 24-well FluoroBlok inserts (8 μm pore size; Falcon; BD Biosciences Discovery Labware, Bedford, MA) were coated on the bottom side with 10 μg/ml fibronectin (BD Biosciences Discovery Labware) for 1 h and then by 100 μg/ml BSA (Sigma-Aldrich, St. Louis, MO) for 1 h. Endothelial basal medium (EBM) (750 μl) containing 0.2% BSA and heparin (1 μg/ml), supplemented with or without FGFs (10 ng/ml), was added to the bottom chambers. LECs (5 × 104) in serum-free EBM medium (Cambrex Bio Science Walkersville, Walkersville, MD) containing 0.2% BSA were added into each well. After 3 h, cells migrated onto the bottom side of the inserts were stained with calcein-AM (Invitrogen), and the fluorescence intensity was measured using the Victor2 fluorometer (PerkinElmer Life and Analytical Sciences, Boston, MA). For VEGFR-3 blocking experiments, LECs were preincubated with a control IgG or a rat anti-human VEGFR-3 blocking antibody (1 μg/ml) (kindly provided by Dr. Bronek Pytowsky, ImClone Systems, New York, NY) for 10 min. The serum-free EBM media in the bottom chambers contained VEGF-C (100 ng/ml; R&D Systems) or FGF (10 ng/ml). For apoptosis assays, 4000 LECs were seeded into a fibronectin-coated well of 96-well plates and cultured for 24 h. Cells were then incubated for 24 h in medium containing 0.1% BSA, 20% FBS, and 1 μg/ml heparin, with or without FGF-1 or FGF-2 at a concentration of 10 ng/ml. Cytoplasmic histone-associated DNA fragments generated by induction of cell death were quantified using the Cell Death Detection ELISA kit (Roche Diagnostics, Indianapolis, IN).
Functional inhibition of FGFR-3 was performed by transfecting cultured LECs (passage 2) with pooled small interfering RNAs (siRNAs) for FGFR-3 or siRNAs for the luciferase gene as a negative control by using (Amaxa, Cologne, Germany). The siRNA sequences are as follow (FGFR-3, CACGACCUGUACAUGAUCAdTdT, UGCACAACGUCACCUUUGAdTdT, and UGCACAACCUCGACUACUAdTdT; and Luciferase, CUUACGCUGAGUACUUCGAdTdt). Transfected cells were then plated into two 6-cm dishes. One dish was used to collect total RNAs to quantify the steady-state level of FGFR-3, and the other dish was used for cell proliferation assays. Proliferation assays were performed 24 h after transfection as described above.
Binding and Internalization of 125I-FGF-2
FGF-2 was labeled with 125I-Na using iodogen (Pierce Chemical, Rockford, IL) as a coupling agent according to the manufacturer's instructions. The specific activity of 125I-FGF-2 was 150,000 cpm/ng. FGF-2 binding to high- and low-affinity sites was investigated as described previously (Moscatelli, 1987). Cells were seeded at 2.5 × 105/cm2 and were cultured in complete medium in 3.5-cm diameter dishes for 2 d. Cells were washed twice with ice-cold phosphate-buffered saline (PBS) and were incubated with the indicated concentrations of 125I-FGF-2 in DMEM containing 20 mM HEPES, pH 7.4, and 0.15% gelatin for 2 h at 4°C. Cells were then washed three times with ice-cold PBS. 125I-FGF-2 was dissociated from its cellular low-affinity binding sites by two 20-s washes with ice-cold 20 mM HEPES, pH 7.4, 2 M NaCl, and from its high-affinity sites by two 20-s washes with ice-cold 20 mM NaAc, pH 4.0, 2 M NaCl. Bound 125I-FGF-2 was quantified using a Kontron MR 250 gamma-counter (Saint-Quentin-Yvelines, France). Nonspecific binding was determined by incubating LECs in separate dishes with 125I-FGF-2 and a 100-fold excess of unlabeled ligand. Specific binding was determined by subtracting nonspecific binding from total binding. Experiments were done in duplicates and repeated twice with comparable results. Internalization experiments were performed as described previously (Perollet et al., 1998). Cells in 3.5-cm-diameter dishes were incubated with 10 ng/ml 125I-FGF-2 and shifted to 37°C. After the specified time points (0-24 h), cells were washed three times with PBS and twice for 20 s with 20 mM HEPES, pH 7.4, containing 2 M NaCl and twice for 20 s with ice-cold 20 mM NaAc, pH 4.0, containing 2 M NaCl, to remove cell surface-associated radioactivity. Cells were then extracted with 5% Triton X-100, 2% SDS in PBS, pH 7.4, and internalized 125I-FGF-2 was quantified by radioactive counting in a Kontron MR 250 gamma-counter. Experiments were done in duplicates and repeated twice.
RESULTS
Ectopic Expression of Prox1 in Primary Blood Vascular Endothelial Cells (BECs) Up-Regulates FGFR-3
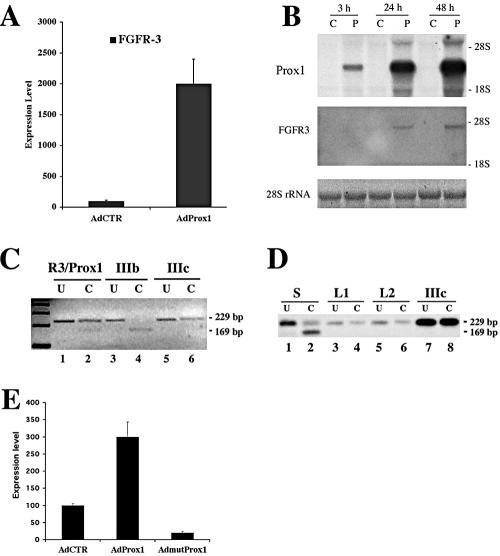
We and others have previously reported that ectopic expression of Prox1 in BECs led to up-regulation of several LEC-specific genes (Hong et al., 2002; Petrova et al., 2002). Detailed microarray analyses further indicate that expression of FGFR-3 is also regulated by the expression of Prox1 in BECs. Real-time RT-PCR analyses confirmed that Prox1 increased FGFR-3 expression by 20-fold (Figure 1A). The Prox1-mediated up-regulation of FGFR-3 was further confirmed by Northern blot analysis by using total RNAs harvested 3, 24, and 48 h after transduction of BECs with an adenovirus expressing Prox1 (Figure 1B).
Figure 1.
Prox1 up-regulates FGFR-3 expression. (A) The steadystate level of FGFR-3 mRNA was increased by 20-fold when Prox1 is ectopically overexpressed in BECs. FGFR-3 expression level was measured by real-time RT-PCR in BECs after transduced with control (AdCTR) or Prox1 (AdProx1)-adenovirus. Data were normalized by β-actin mRNA levels and expressed as percentage of the control virus-infected cells (means ± SD). (B) Up-regulation of FGFR-3 mRNA expression by Prox1 was confirmed by Northern blot analysis of total RNAs obtained from BECs infected with control (C) or Prox1 (P) adenovirus for 3, 24, or 48 h. (C) Prox1 induces expression of the IIIc isoform of FGFR-3, as determined by a diagnostic ApaI restriction analysis of RT-PCR product (229 base pairs) amplified from BECs transduced with Prox1 adenovirus for 24 h (R3/Prox1). As controls, RT-PCR products from FGFR-3 IIIb- or IIIc-expressing cell lines were digested in parallel. Only the product from the IIIb isoform was digested to yield a 169-base pair fragment. (D) Cultured LECs exclusively express the IIIc isoform of FGFR-3. RT-PCR products of unpurified cell mixture (S) from human neonatal foreskins, two independent batches of LECs (L1 and L2) and FGFR-3 IIIc-expressing control cells (IIIc) were subjected to the diagnostic ApaI restriction analysis. Although the product from the cell mixture contains both ApaI-sensitive and -resistant fragments, those of LECs and of FGFR-3 IIIc control cells (IIIc) were resistant to the digestion, indicating that the IIIc is the dominant isoform of FGFR-3 in LECs. U, undigested; C, digested with ApaI. (E) Cultured lymphatic endothelial cells were transduced with adenovirus expressing the wild-type (AdProx1) or mutant (AdmutProx1) Prox1, or with control adenovirus (AdCTR). After 2 d, the expression level of FGFR-3 was determined using real-time RT-PCR.
To determine which of the two major FGFR-3 isoforms (IIIb and IIIc) was up-regulated by Prox1, RT-PCR was performed by using primers designed to yield a 235-base pairs product containing an ApaI site from the IIIb isoform, or a 229-base pair fragment without an ApaI site from the IIIc isoform. As controls for the analyses, we also used stably transfected myoblast cells that selectively express either human FGFR-3 IIIb or IIIc isoform (Kanai et al., 1997). As expected, RT-PCR analysis yielded an ApaI-sensitive 235-base pair product from the IIIb-expressing control cells, and an ApaI-insensitive 229-base pair fragment from the IIIc-expressing cells (Figure 1C). The same analysis amplified an ApaI-insensitive 229-base pair product from BECs infected with the Prox1-adenovirus (Figure 1C). This indicates that Prox1 predominantly up-regulates the expression of the FGFR-3 IIIc isoform in vascular endothelial cells. Furthermore, RT-PCR of RNA obtained from primary lymphatic endothelial cells generated the ApaI-insensitive 229-base pair product, whereas unpurified cell mixtures isolated from human foreskins yielded products of both ApaI-sensitive and insensitive fragments (Figure 1D). These data indicate that the FGFR-3 IIIc isoform is the major variant present in LECs and that Prox1 selectively up-regulates the IIIc isoform of FGFR-3.
To determine whether Prox1 is necessary to maintain the expression of FGFR-3 in LECs, we ectopically expressed a mutant Prox1 in cultured lymphatic endothelial cells through the adenovirus gene transfer. The mutant Prox1 protein has two amino-acid substitution mutations in its DNA binding domain and does not display any transcriptional activity (see below). We found that when expressed in LECs, the mutant Prox1 was able to decrease the expression level of FGFR-3 by fourfold, whereas the wild-type Prox1 up-regulated FGFR-3 by threefold (Figure 1E). These findings indicate that the mutant Prox1, serving as a dominant negative mutant, may compete with the endogenous Prox1 in LECs and that Prox1 function is necessary to maintain the expression of FGFR-3.
Prox1 Binds to the FGFR-3 Promoter and Activates Its Transcription
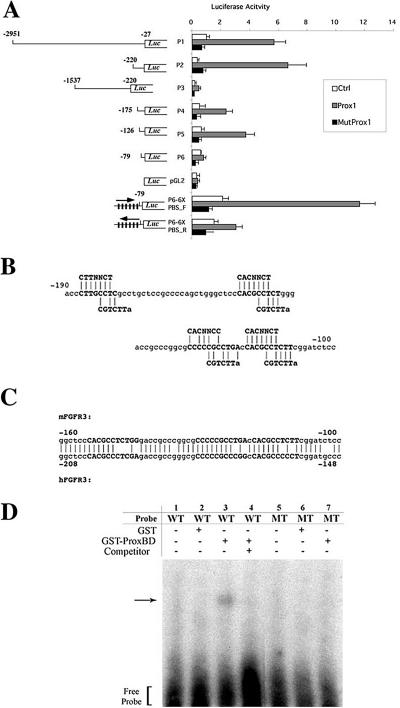
To study the molecular mechanism underlying the Prox1-mediated up-regulation of FGFR-3, we performed promoter-reporter assays using FGFR-3 promoter-luciferase constructs, which have been characterized previously (McEwen and Ornitz, 1998). A 3-kb promoter fragment was sufficient to mediate transcriptional activation of the firefly luciferase reporter (P1) by Prox1 (Figure 2A). The Prox1-mediated activation was still maintained even after deleting most of the promoter region to -220 nt upstream of the FGFR-3 transcriptional initiation site (P2), but removal of the proximal 220 base pairs of the promoter sequence abrogated the activation by Prox1 (P3). The Prox1-mediated activation progressively decreased with deletions to -175 and -126 nt and then was abolished by deletion to -79 nt (P4-P6). These data indicate the presence of putative Prox1 response elements (PRE) between -220 and -79 nt of the FGFR-3 promoter.
Figure 2.
Prox1 binds to the FGFR-3 promoter to up-regulate its transcription. (A) The FGFR-3 promoter-luciferase (Luc) reporter constructs (P1-P6) and an empty control vector (pGL2) were tested for their luciferase activity in the presence of a vector control (Ctrl), a Prox1-expressing vector (Prox1), or a mutant Prox1-expressing vector (MutProx1). P6-6XPBS contains six-tandem repeats of the Prox1 binding site (PBS, CACGCCTCT) in the P6 construct in the forward- (P6-6XPBS_F) or reverse (P6-6XPBS_R) orientation. Numbers indicate relative locations from the transcriptional initiation site (McEwen and Ornitz, 1998). Data are shown as means ± SD (B) Sequence analysis of the mouse FGFR-3 promoter region revealed four putative Prox1 binding sites. Two previously reported Prospero consensus sequences, C(A/T)(C/T)NNC(T/C) and CGTCTT(A) (Hassan et al., 1997; Cook et al., 2003), are shown above and below the putative Prox1 binding sites (bold), respectively. (C) The proximal three putative Prox1 binding sequences (bold) are conserved between the mouse and human FGFR-3 promoters. (D) Gel electrophoresis mobility shift assays showed that the purified GST-Prox1 fusion protein (GST-ProxBD) but not the GST protein alone binds to the putative Prox1 binding sequences found in the FGFR-3 promoter. The GST or GST-Prox1 proteins were incubated with 32P-labeled a wild-type (WT) or a mutant probe (MT). Arrow indicates a slow migrating complex of GST-Prox1 and the wild-type probe (lane 3). Excessive amount of unlabeled wild-type probe (Competitor) competes for the interaction between GST-Prox1 and the labeled wild-type probe (lane 4).
To further determine whether the Prox1-mediated activation of FGFR-3 is dependent on DNA-protein interaction, we introduced two amino acid substitution mutations (N625A and R627A) into the third helix of the homeodomain region that is involved in DNA binding of Prospero, the Drosophila homologue of Prox1 (Ryter et al., 2002). Prospero and Prox1 share a high amino acid identity in their DNA binding domains (Hong and Detmar, 2003). The Prox1 protein with the two substitution mutations (MutProx1) completely lost its transcriptional activity (Figure 2A). These findings indicate that a direct DNA-protein interaction is necessary for the Prox1-mediated up-regulation of FGFR-3.
Identification of the Putative Prox1 Binding Sites in the FGFR-3 Promoter
Previous reports had identified two seemingly different consensus sequences [C(a/t)(c/t)NNC(t/c) and (T)AAGACG] as putative Prospero binding sites (Hassan et al., 1997; Cook et al., 2003). Interestingly, we found four putative Prox1 binding sites, composed of the two partially overlapping Prospero consensus sites, between -190 and -100 nt of the mouse FGFR-3 promoter (Figure 2B). The proximal three putative Prox1 binding sequences are highly conserved between the mouse and human FGFR-3 promoters (Figure 2C). To investigate whether these sequence motifs serve as Prox1 binding sites, we performed gel electrophoresis mobility shift assays using a GST-Prox1 fusion protein (Belecky-Adams et al., 1997; Cui et al., 2004). Purified GST-Prox1 fusion protein efficiently bound to a probe containing the putative Prox1 site in the FGFR-3 promoter (Figure 2D). However, the fusion protein did not bind to a mutant probe whose putative Prox1 site was replaced with random nucleotides. Interaction of the GST-Prox1 protein with the labeled wild-type probe was competed out by addition of excessive unlabeled wild-type probe, and the GST protein alone did not interact with either probe (Figure 2D). These data demonstrate that Prox1 bind to the putative Prox1 site present in the FGFR-3 promoter.
We next investigated whether the Prox1 binding site identified in the FGFR-3 promoter is sufficient to mediate transcriptional activation of the reporter gene. We introduced six tandem repeats of the Prox1 binding sequences (PBS, CACGCCTCT) into the P6 construct in the forward or the reverse orientation (P6-6XPBS_F and P6-6XPBS_R) (Figure 2A). The P6 construct was shown to be unable to mediate any transcriptional activation by Prox1. However, introduction of six repeats of the putative Prox1 binding sites in the forward orientation (P6-6XPBS_F) reinstated transcriptional activity to the P6 construct by wild type but not by the mutant Prox1 (Figure 2A). However, when the repeats were introduced in the reverse orientation (P6-6XPBS_R), only marginal activation was observed. These findings indicate that the nine-nucleotide sequence (CACGCCTCT) present in the FGFR-3 promoter is necessary and sufficient to mediate transcriptional activation by Prox1.
Expression of FGFR-3 in Developing Lymphatic Vessels of Mouse Embryo and of Human Skin
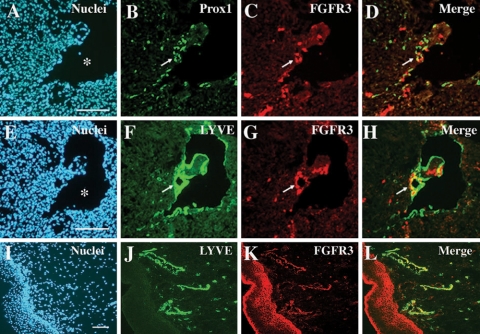
We next investigated whether FGFR-3 is expressed in the lymphatically differentiating endothelial cells during mouse embryogenesis. In agreement with our in vitro results, many of the Prox1-positive lymphatically differentiating endothelial cells were positively stained for FGFR-3 in E11.5 mouse embryos (Figure 3, A-D). Furthermore, double immunofluorescent stainings for the lymphatic-specific marker LYVE-1 and for FGFR-3 showed that FGFR-3 was strongly and specifically expressed in the newly formed LYVE-1-positive lymphatic vessels (Figure 3, E-H) but not in developing blood vessels (cardinal vein) (Figure 3, C and G). Furthermore, double stainings of human neonatal foreskin for LYVE-1 and FGFR-3 revealed that the lymphatic specific expression of FGFR-3 is also maintained after embryonic development (Figure 3, I-L).
Figure 3.
FGFR-3 expression in lymphatic endothelial cells during and after embryonic development. Adjacent mouse embryo sections (E11.5) were stained for Prox1 (green) and FGFR-3 (red) (A-D) and for LYVE-1 (green) and FGFR-3 (red) (E-H). Lymphatically differentiating budding endothelial cells and resident endothelial cells in a newly formed lymphatic vessel are costained positively for Prox1 and for FGFR-3 (D). Similarly, LYVE-1-positive lymphatic endothelial cells express FGFR-3 (H). Arrows indicate a newly formed lymphatic vessel (B-D and F-H). A human neonatal foreskin section was costained for LYVE-1 and FGFR-3 (I-L). Asterisk, cardinal vein; bar, 100 μm.
Signaling through FGFR-3 Promotes LEC Proliferation
To further evaluate the biological role of FGFR-3-mediated signaling, we inhibited expression of FGFR-3 in LECs using siRNAs and studied the effects on cell proliferation. Real-time RT-PCR analyses revealed that transfection of FGFR-3 siRNAs into LECs decreased the steady-state level of FGFR-3 by 50-fold, whereas the expression of FGFR-1 was not altered (Figure 4A). Notably, knockdown of FGFR-3 resulted in a significant inhibition of proliferation of LECs by 30-40% (Figure 4B). However, the FGF-2-induced proliferation of LECs was largely unaffected (≈2-fold) with or without inhibition of FGFR-3. This may be due to the presence of other functional FGF receptors (FGFR-1, -2, and -4) that may be activated by FGF-2. Together, these data demonstrate that the FGFR-3-mediated signaling plays an important role in proliferation of LECs.
Figure 4.
FGFR-3 mediates proliferation signaling of LECs. (A) siRNAs against FGFR-3 significantly reduced the steady-state levels of FGFR-3 but not of FGFR-1. (B) Knockdown of FGFR-3 inhibits proliferation of LECs in the presence or absence of FGF-2 and its cofactor heparin. Experiments were performed in triplicate twice, and LECs with a passage number 2 were used. siCTR, control siRNA for luciferase; siFGFR-3, siRNAs for FGFR-3; *p < 0.05, **p < 0.01, and ***p < 0.001.
FGF-2 Binds Directly to Low- and High-Affinity Receptors in LECs and Subsequently Internalized for Degradation
We next investigated whether FGF ligands physically interact with FGF receptors present in lymphatic endothelial cells. LECs were incubated with increasing amounts of 125I-FGF-2, and levels of binding to the low- and high-affinity sites were determined. 125I-FGF-2 was bound in a concentration-dependent manner to LECs, but binding was not fully saturable for the low-affinity binding sites (Figure 5A). For low-affinity binding (proteoglycans), a Kd of 1 nM and 400,000 binding sites/cell were determined (Figure 5A). For high-affinity binding sites (receptors), maximum binding was detected between 4 and 6 ng/ml 125I-FGF-2 (Figure 5B). Scatchard analysis revealed high-affinity binding (Kd of 72 pM) and ∼5300 binding sites/cell (Figure 5B). These values are similar to those found on vascular endothelial cells (Moscatelli, 1987). We then determined internalization of 125I-FGF-2 in LECs. Between 1 and 4 h, the internalization rate was 0.046 ng/h/105 cells (Figure 5C). This value progressively decreased between 4 and 8 h (0.01 ng/h/105 cells) and 8-12 h (0.004 ng/h/105 cells), indicating that FGF-2 internalization progressively slows down with time. After 1 h of internalization, a fragment of 15 kDa (together with the 18-kDa band) was detected (Figure 5D). At 1.5 and 2 h, two additional fragments of 10 and 8 kDa occurred, and their amounts increased with time. Maximum degradation was observed between 12 and 24 h. Interestingly, at 24 h, 18-kDa FGF-2 was still present in significant amounts in LECs. Together, our biochemical study provides detailed information on binding kinetics of FGF-2 to its receptors and subsequent internalization and degradation patterns of the ligand in lymphatic endothelial cells, which are largely comparable with those of vascular endothelial cells as described previously (Bikfalvi et al., 1989).
Figure 5.
Binding, internalization and degradation of FGF-2 in LECs. Concentration dependence of 125I-FGF-2 binding to low-affinity sites (A) and high-affinity receptors (B). Cells were incubated with increasing concentrations of 125I-FGF-2, and the specific binding was determined as described in Materials and Methods. Scatchard plots are shown in insets. (C) Internalization of 125I-FGF-2 was determined by incubating cells with 10 ng/ml 125I-FGF-2 at 37°C for specified time intervals. (D) After internalization, solubilized cell extracts were run on a 15% SDS-PAGE gel, dried and processed for autoradiography (PhosphorImager) to visualize the degradation profile. Time (hours) after incubation and molecular mass of the degraded products are shown. The data are representative for two independent experiments performed in duplicates.
FGF Signaling Regulates Migration, Proliferation, and Apoptosis of Cultured Primary Lymphatic Endothelial Cells
We next investigated the effects of two specific FGF ligands on migration, proliferation, and apoptosis of primary human LECs. Treatment with recombinant human FGF-1 and FGF-2 significantly enhanced migration and proliferation of LECs (Figure 6, A and B). Furthermore, both FGF ligands protected LECs from apoptosis induced by serum depletion (Figure 6C). A previous in vivo study in mouse corneas indicated that FGF-2 might indirectly promote lymphangiogenesis through activation of the VEGF-C/VEGFR-3 pathway (Kubo et al., 2002). To determine whether FGF-2 can stimulate LEC migration in vitro directly or indirectly, we studied the effect of FGF-2 in the presence or absence of an anti-VEGFR-3 blocking antibody. Both VEGF-C and FGF-2 stimulated the migration of LECs at a comparable level (Figure 6D). However, neutralization of VEGFR-3 abrogated the enhanced migration of LECs by VEGF-C but not by FGF-2, indicating that FGF-2 can function independently of the VEGF-C/VEGFR-3 pathway in vitro.
Figure 6.
Stimulatory effects of fibroblast growth factors on proliferation, migration, and survival of LECs. (A) Migration of LECs was promoted by FGF-1 and FGF-2. Cells were allowed to migrate toward fibronectin in serum-free media containing FGF-1 or FGF-2 (10 ng/ml) in the presence of heparin (1 μg/ml). Numbers of migrated cells were quantified by fluorescence assay. (B) FGF-1 and FGF-2 stimulated LEC proliferation. LECs were treated with or without FGFs for 48 h. Increase in cell numbers was determined using the MUH fluorescence assay. (C) FGF-1 and -2 (10 ng/ml) inhibit LEC apoptosis induced by serum depletion for 24 h. Addition of 20% serum but not of heparin alone prevented LEC apoptosis. Data are expressed in percentage of BSA control and are shown as means ± SD. (D) FGF-2 directly promoted LEC migration independently from VEGFR-3 activation. LEC migration was stimulated by VEGF-C (100 ng/ml) or FGF-2 (10 ng/ml), but the enhanced migration by VEGF-C was abrogated by addition of an anti-VEGFR-3 blocking antibody. *p < 0.05, **p < 0.01, and ***p < 0.001.
DISCUSSION
The homeodomain protein Prox1 plays an essential role in the lymphatic system development during embryogenesis as a master regulator that induces lymphatic lineage-specific differentiation (Wigle and Oliver, 1999; Hong et al., 2002; Petrova et al., 2002; Wigle et al., 2002; Hong and Detmar, 2003). Furthermore, the LEC lineage specification occurring during embryogenesis can be post-developmentally recapitulated when Prox1 is ectopically expressed in neonatal BECs (Hong et al., 2002; Petrova et al., 2002; Hong and Detmar, 2003). However, the molecular mechanisms underlying the cell fate decision controlled by Prox1 remained to be studied. In this report, we identified FGFR-3 as an initial Prox1 target gene during the early lymphatic system development. This up-regulation is mediated at the transcriptional level by a direct binding of Prox1 to the specific sequence elements in the FGFR-3 promoter. Consistently, FGFR-3 is strongly expressed in the vein-derived lymphatically differentiating endothelial cells and in postdevelopmental lymphatic vessels in neonatal human foreskins. We also found that FGFR-3 plays an important role in mediating proliferating signals of LECs. Furthermore, our biochemical study demonstrated that FGF-2 bind to the low- and high-affinity receptors in LEC to promote migration, proliferation, and cell survival of LECs independently of the VEGF-C/VEGFR-3 signal pathway.
Lymphatic endothelial cells are derived from venous endothelial cells that are of mesodermal origin. Our finding that Prox1 specifically up-regulates the IIIc variant of FGFR-3, the major isoform in LEC, is consistent with previous studies that the IIIc forms of FGF receptors (FGFR-1 to -3) are mainly expressed by the mesenchymal lineage cells (Orr-Urtreger et al., 1993; Yan et al., 1993; Alarid et al., 1994). Interestingly, FGF receptors and their splicing variants exhibit strikingly distinct binding affinities to different FGF ligands (Ornitz et al., 1992; Powers et al., 2000; Ornitz and Itoh, 2001). As an example, the FGFR-3 IIIb isoform interacts with FGF-1 but not with FGF-2, FGF-4, or FGF-6, whereas the IIIc isoform is activated by all of these ligands to promote fibroblast proliferation (Ornitz et al., 1996; Kanai et al., 1997). Furthermore, FGFR-3 IIIc also displays a high-affinity to FGF-8, FGF-17, and FGF-18 (Xu et al., 1999; Xu et al., 2000; Liu et al., 2002). Given these facts and our findings presented here, up-regulation of FGFR-3 IIIc by Prox1 in the LEC-specific manner may be essential for mediating proliferation signals for the lymphatic system development, which may be distinct from signals for the blood vascular system development. This notion of differential proliferation signal is highly conceivable because only a subset of endothelial cells in the developing vein needs to be activated to proliferate and migrate out to form initial lymphatic vessels during embryogenesis. Therefore, FGFR-3 may be one of the major players in the molecular mechanism responsible for the LEC differentiation and subsequent lymphatic system development. Furthermore, the expression and maintenance of an additional FGF receptor may be also advantageous for the function of the lymphatic system. Because the lymphatic system plays essential roles in various aspects of the immune system, FGFR-3 may be important for cross talk between LECs and immune cells. It will be interesting to study the role of FGFR-3 during tissue repair, inflammation, and tumor development and metastasis.
We found that interaction of Prox1 with a specific DNA sequence element in the FGFR-3 promoter was necessary for the Prox1-mediated transcriptional activation of FGFR-3. The Prox1 binding sequences found in the FGFR-3 promoter consist of two overlapping consensus binding sequences of Prospero (Hassan et al., 1997; Cook et al., 2003). These sequence motifs, conserved between the mouse and human FGFR-3 genes, form a complex with purified GST-Prox1 protein and were sufficient to reinstate the Prox1-mediated transcriptional activation to a nonactivating reporter vector. Previously, functional interactions of Prox1 with other transcriptional regulators were reported in the developing lens. The sequence-specific Six3 repressor antagonizes the Prox1 activation of the γ-crystallin promoter (Lengler et al., 2001). Similarly, Pax-6 occupies a specific sequence motif and prevents Prox1-mediated activation of the βB1-crystallin gene in chicken lens epithelial cells, whereas Prox1 binds to the same site to activate the gene in lens fiber cells (Cui et al., 2004). In contrast, Prox1 was shown to function as a corepressor of Ff1b, the Zebra fish homologue of mammalian steroidogenic factor-1 by a direct protein-protein interaction during embryonic development of the interrenal primordium (Liu et al., 2003). It remains unknown whether these Prox1 interacting partners also play a role in the development of the lymphatic system. Because Prox1 activates some genes but represses others in lymphatically differentiating endothelial cells, it will be important to characterize transcriptional factors involved in this regulation during lymphatic development.
The VEGF-C/VEGFR-3 signaling was shown to play an essential role in the development of the lymphatic system (Karkkainen et al., 2004). Promotion of lymphangiogenesis by FGF-2 in mouse corneas was suggested to be mediated through up-regulation of VEGF-C by stromal cells, and FGF-2-induced corneal lymphangiogenesis was abrogated by a neutralizing antibody against VEGFR-3, the major receptor for VEGF-C (Kubo et al., 2002; Chang et al., 2004). In contrast, we found specific expression of FGFR-3 in LECs in vitro and in vivo and direct binding of FGF-2 to low- and high-affinity receptors in LECs. In addition, we found that FGF-1 and FGF-2 can enhance migration, proliferation, and survival of LECs and that the FGF-2-mediated activation of LEC migration is not dependent on the function of VEGFR-3. These results clearly indicate that these FGF ligands directly bind to their receptors in LEC and exert a direct role in lymphatic vessel formation. Nonetheless, our data do not rule out an indirect activation of FGF ligands through VEGFR-3 because our experiments involved only purified LECs but not accompanying other stromal cells, the proposed source of VEGF-C (Kubo et al., 2002; Chang et al., 2004). Therefore, FGF ligands may exert their functions in multiple manners depending on the tissue microenvironment. Our finding that LECs expressed an additional FGF receptor is of particular interest because a recent study showed that lymphangiogenesis occurred at a low dosage of FGF-2 (12.5 ng), a concentration that did not induce accompanying angiogenesis in the mouse cornea assay (Chang et al., 2004). Therefore, it is conceivable that LECs may be more sensitive to FGF-2 stimulation than BECs because of expression of additional FGF receptors.
FGFR-3 has been previously shown to be essential for various developmental processes such as bone morphogenesis, inner ear development, and alveogenesis in the lung (Weinstein et al., 1998; Ornitz and Marie, 2002). Because we found that FGFR-3 is a target gene of Prox1 and that Prox1 specifies lymphatic endothelial cell fate, we investigated whether FGFR-3 mediates an inductive signal for lymphatic differentiation and found that knockdown of FGFR-3 mRNA significantly inhibited LEC proliferation. This suggests that the receptor may play an important role in mediating cell proliferation during lymphatic system development. Our preliminary study indicates that the FGFR-3 null mice developed apparently normal lymphatic capillaries in the skin. We believe that this is most likely because of functional complementation by other FGF receptors. This notion of functional cooperation among FGF receptors is further supported by a study of the FGFR-3 and FGFR-4 double knockout mice (Weinstein et al., 1998). Homozygous fgfr-3-/-fgfr-4-/- mutant mice displayed abnormal alveogenesis during lung development, a phenotype that was not present in single knockout mutants, suggesting that the two FGF receptors function together to direct normal lung development. It will be of great interest to evaluate lymphatic vessel development in the fgfr-3-/-fgfr-4-/- mutant mice. Furthermore, mice lacking FGF-18 display a similar mutant phenotype in bone morphogenesis as FGFR-3 null mice, defining FGF-18 as a physiological ligand for FGFR-3 during bone development (Liu et al., 2002). It will be also interesting to see whether FGF-18 single or FGFR-3/FGF-18 double knockout mice develop a normally functioning lymphatic system.
Acknowledgments
We thank Drs. D. M. Ornitz (FGFR-3 promoter constructs), M. K. Duncan (GST-Prox1 fusion vector), and D. Podolsky (rat myoblast expressing FGFR-3 IIIb or IIIc) for the generous gifts. This work was supported by National Institutes of Health Grants CA-69184, CA-86410, and CA-92644, American Cancer Society Research Project Grant 99-23901 (to M. D.), the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co. Agreement (to M. D.), grants from the “Association de la Recherche sur le Cancer,” the “Conseil Regional d'Aquitaine,” The “Institute National de la Santé et de la Recherche Medicale” (Institut National de la Santé et de la Recherche Médicale), and the “Ministere de la Recherche” (to A. B.), and a grant from the Max Kade Foundation (to R. K.). The cost of publication was supported by a startup fund from Department of Surgery/Norris Comprehensive Cancer Center of University of Southern California (to Y. H.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-04-0368) on November 16, 2005.
Abbreviations used: BEC, blood vascular endothelial cell; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; LEC, lymphatic vascular endothelial cell; VEGF, vascular endothelial cell growth factor; VEGFR, vascular endothelial cell growth factor receptor.
References
- Alarid, E. T., Rubin, J. S., Young, P., Chedid, M., Ron, D., Aaronson, S. A., and Cunha, G. R. (1994). Keratinocyte growth factor functions in epithelial induction during seminal vesicle development. Proc. Natl. Acad. Sci. USA 91, 1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auguste, P., Javerzat, S., and Bikfalvi, A. (2003). Regulation of vascular development by fibroblast growth factors. Cell Tissue Res. 314, 157-166. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams, T., Tomarev, S., Li, H. S., Ploder, L., McInnes, R. R., Sundin, O., and Adler, R. (1997). Pax-6, Prox 1, and Chx10 homeobox gene expression correlates with phenotypic fate of retinal precursor cells. Investig. Ophthalmol. Vis. Sci. 38, 1293-1303. [PubMed] [Google Scholar]
- Bikfalvi, A., Dupuy, E., Inyang, A. L., Fayein, N., Leseche, G., Courtois, Y., and Tobelem, G. (1989). Binding, internalization, and degradation of basic fibroblast growth factor in human microvascular endothelial cells. Exp. Cell Res. 181, 75-84. [DOI] [PubMed] [Google Scholar]
- Bikfalvi, A., Savona, C., Perollet, C., and Javerzat, S. (1998). New insights in the biology of fibroblast growth factor-2. Angiogenesis 1, 155-173. [DOI] [PubMed] [Google Scholar]
- Chang, L. K., Garcia-Cardena, G., Farnebo, F., Fannon, M., Chen, E. J., Butterfield, C., Moses, M. A., Mulligan, R. C., Folkman, J., and Kaipainen, A. (2004). Dose-dependent response of FGF-2 for lymphangiogenesis. Proc. Natl. Acad. Sci. USA 101, 11658-11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, T., Pichaud, F., Sonneville, R., Papatsenko, D., and Desplan, C. (2003). Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila. Dev. Cell 4, 853-864. [DOI] [PubMed] [Google Scholar]
- Cui, W., Tomarev, S. I., Piatigorsky, J., Chepelinsky, A. B., and Duncan, M. K. (2004). Mafs, Prox1, and Pax6 can regulate chicken betaB1-crystallin gene expression. J. Biol. Chem. 279, 11088-11095. [DOI] [PubMed] [Google Scholar]
- Dell, K. R., and Williams, L. T. (1992). A novel form of fibroblast growth factor receptor 2. Alternative splicing of the third Ig-like domain confers ligand binding specificity. J. Biol. Chem. 267, 21225-21229. [PubMed] [Google Scholar]
- Detmar, M., Imcke, E., Ruszczak, Z., and Orfanos, C. E. (1990). Effects of recombinant tumor necrosis factor-alpha on cultured microvascular endothelial cells derived from human dermis. J. Investig. Dermatol. 95, 219S-222S [DOI] [PubMed] [Google Scholar]
- Groth, C., and Lardelli, M. (2002). The structure and function of vertebrate fibroblast growth factor receptor 1. Int. J. Dev. Biol. 46, 393-400. [PubMed] [Google Scholar]
- Hanneken, A. (2001). Structural characterization of the circulating soluble FGF receptors reveals multiple isoforms generated by secretion and ectodomain shedding. FEBS Lett. 489, 176-181. [DOI] [PubMed] [Google Scholar]
- Hassan, B., Li, L., Bremer, K. A., Chang, W., Pinsonneault, J., and Vaessin, H. (1997). Prospero is a panneural transcription factor that modulates homeodomain protein activity. Proc. Natl. Acad. Sci. USA 94, 10991-10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa, S., Hong, Y. K., Harvey, N., Schacht, V., Matsuda, K., Libermann, T., and Detmar, M. (2003). Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am. J. Pathol. 162, 575-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, Y. K., and Detmar, M. (2003). Prox1, master regulator of the lymphatic vasculature phenotype. Cell Tissue Res. 314, 85-92. [DOI] [PubMed] [Google Scholar]
- Hong, Y. K., Harvey, N., Noh, Y. H., Schacht, V., Hirakawa, S., Detmar, M., and Oliver, G. (2002). Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 225, 351-357. [DOI] [PubMed] [Google Scholar]
- Javerzat, S., Auguste, P., and Bikfalvi, A. (2002). The role of fibroblast growth factors in vascular development. Trends Mol. Med. 8, 483-489. [DOI] [PubMed] [Google Scholar]
- Kanai, M., Goke, M., Tsunekawa, S., and Podolsky, D. K. (1997). Signal transduction pathway of human fibroblast growth factor receptor 3. Identification of a novel 66-kDa phosphoprotein. J. Biol. Chem. 272, 6621-6628. [DOI] [PubMed] [Google Scholar]
- Karkkainen, M. J., et al. (2004). Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74-80. [DOI] [PubMed] [Google Scholar]
- Kubo, H., Cao, R., Brakenhielm, E., Makinen, T., Cao, Y., and Alitalo, K. (2002). Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc. Natl. Acad. Sci. USA 99, 8868-8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengler, J., Krausz, E., Tomarev, S., Prescott, A., Quinlan, R. A., and Graw, J. (2001). Antagonistic action of Six3 and Prox1 at the gamma-crystallin promoter. Nucleic Acids Res. 29, 515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. W., Gao, W., Teh, H. L., Tan, J. H., and Chan, W. K. (2003). Prox1 is a novel coregulator of Ff1b and is involved in the embryonic development of the zebra fish interrenal primordium. Mol. Cell. Biol. 23, 7243-7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z., Xu, J., Colvin, J. S., and Ornitz, D. M. (2002). Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 16, 859-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, D. G., and Ornitz, D. M. (1998). Regulation of the fibroblast growth factor receptor 3 promoter and intron I enhancer by Sp1 family transcription factors. J. Biol. Chem. 273, 5349-5357. [DOI] [PubMed] [Google Scholar]
- Moscatelli, D. (1987). High and low affinity binding sites for basic fibroblast growth factor on cultured cells: absence of a role for low affinity binding in the stimulation of plasminogen activator production by bovine capillary endothelial cells. J. Cell. Physiol. 131, 123-130. [DOI] [PubMed] [Google Scholar]
- Murgue, B., Tsunekawa, S., Rosenberg, I., deBeaumont, M., and Podolsky, D. K. (1994). Identification of a novel variant form of fibroblast growth factor receptor 3 (FGFR3 IIIb) in human colonic epithelium. Cancer Res. 54, 5206-5211. [PubMed] [Google Scholar]
- Oliver, G., Sosa-Pineda, B., Geisendorf, S., Spana, E. P., Doe, C. Q., and Gruss, P. (1993). Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech. Dev. 44, 3-16. [DOI] [PubMed] [Google Scholar]
- Ornitz, D. M. (2000). FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays 22, 108-112. [DOI] [PubMed] [Google Scholar]
- Ornitz, D. M., and Itoh, N. (2001). Fibroblast growth factors. Genome Biol. 2, REVIEWS3005. [DOI] [PMC free article] [PubMed]
- Ornitz, D. M., and Marie, P. J. (2002). FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 16, 1446-1465. [DOI] [PubMed] [Google Scholar]
- Ornitz, D. M., Xu, J., Colvin, J. S., McEwen, D. G., MacArthur, C. A., Coulier, F., Gao, G., and Goldfarb, M. (1996). Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 271, 15292-15297. [DOI] [PubMed] [Google Scholar]
- Ornitz, D. M., Yayon, A., Flanagan, J. G., Svahn, C. M., Levi, E., and Leder, P. (1992). Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol. Cell. Biol. 12, 240-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Urtreger, A., Bedford, M. T., Burakova, T., Arman, E., Zimmer, Y., Yayon, A., Givol, D., and Lonai, P. (1993). Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2). Dev. Biol. 158, 475-486. [DOI] [PubMed] [Google Scholar]
- Perollet, C., Han, Z. C., Savona, C., Caen, J. P., and Bikfalvi, A. (1998). Platelet factor 4 modulates fibroblast growth factor 2 (FGF-2) activity and inhibits FGF-2 dimerization. Blood 91, 3289-3299. [PubMed] [Google Scholar]
- Petrova, T. V., Makinen, T., Makela, T. P., Saarela, J., Virtanen, I., Ferrell, R. E., Finegold, D. N., Kerjaschki, D., Yla-Herttuala, S., and Alitalo, K. (2002). Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 21, 4593-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, C. J., McLeskey, S. W., and Wellstein, A. (2000). Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 7, 165-197. [DOI] [PubMed] [Google Scholar]
- Ryter, J. M., Doe, C. Q., and Matthews, B. W. (2002). Structure of the DNA binding region of prospero reveals a novel homeo-prospero domain. Structure 10, 1541-1549. [DOI] [PubMed] [Google Scholar]
- Shing, Y., Folkman, J., Sullivan, R., Butterfield, C., Murray, J., and Klagsbrun, M. (1984). Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science 223, 1296-1299. [DOI] [PubMed] [Google Scholar]
- Terada, M., Shimizu, A., Sato, N., Miyakaze, S. I., Katayama, H., and Kurokawa-Seo, M. (2001). Fibroblast growth factor receptor 3 lacking the Ig IIIb and transmembrane domains secreted from human squamous cell carcinoma DJM-1 binds to FGFs. Mol. Cell. Biol. Res Commun. 4, 365-373. [DOI] [PubMed] [Google Scholar]
- Weinstein, M., Xu, X., Ohyama, K., and Deng, C. X. (1998). FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development 125, 3615-3623. [DOI] [PubMed] [Google Scholar]
- Wigle, J. T., Harvey, N., Detmar, M., Lagutina, I., Grosveld, G., Gunn, M. D., Jackson, D. G., and Oliver, G. (2002). An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 21, 1505-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle, J. T., and Oliver, G. (1999). Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769-778. [DOI] [PubMed] [Google Scholar]
- Wilkie, A. O., Patey, S. J., Kan, S. H., van den Ouweland, A. M., and Hamel, B. C. (2002). FGFs, their receptors, and human limb malformations: clinical and molecular correlations. Am. J. Med. Genet. 112, 266-278. [DOI] [PubMed] [Google Scholar]
- Xu, J., Lawshe, A., MacArthur, C. A., and Ornitz, D. M. (1999). Genomic structure, mapping, activity and expression of fibroblast growth factor 17. Mech. Dev. 83, 165-178. [DOI] [PubMed] [Google Scholar]
- Xu, J., Liu, Z., and Ornitz, D. M. (2000). Temporal and spatial gradients of Fgf8 and Fgf17 regulate proliferation and differentiation of midline cerebellar structures. Development 127, 1833-1843. [DOI] [PubMed] [Google Scholar]
- Yan, G., Fukabori, Y., McBride, G., Nikolaropolous, S., and McKeehan, W. L. (1993). Exon switching and activation of stromal and embryonic fibroblast growth factor (FGF)-FGF receptor genes in prostate epithelial cells accompany stromal independence and malignancy. Mol. Cell. Biol. 13, 4513-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]