Abstract
Cdc55, a B-type regulatory subunit of protein phosphatase 2A, has been implicated in mitotic spindle checkpoint activity and maintenance of sister chromatid cohesion during metaphase. The spindle checkpoint is composed of two independent pathways, one leading to inhibition of the metaphase-to-anaphase transition by checkpoint proteins, including Mad2, and the other to inhibition of mitotic exit by Bub2. We show that Cdc55 is a negative regulator of mitotic exit. A cdc55 mutant, like a bub2 mutant, prematurely releases Cdc14 phosphatase from the nucleolus during spindle checkpoint activation, and premature exit from mitosis indirectly leads to loss of sister chromatid cohesion and inviability in nocodazole. The role of Cdc55 is separable from Bub2 and inhibits release of Cdc14 through a mechanism independent of the known negative regulators of mitotic exit. Epistasis experiments indicate Cdc55 acts either downstream or independent of the mitotic exit network kinase Cdc15. Interestingly, the B-type cyclin Clb2 is partially stable during premature activation of mitotic exit in a cdc55 mutant, indicating mitotic exit is incomplete.
INTRODUCTION
The critical task of mitosis is equal segregation of sister chromatids to daughter cells. Faithful chromosome transmission is required for maintenance of genomic integrity, and failures in this process lead to aneuploid states characteristic of cancer (Nowak et al., 2002). Checkpoints are mechanisms that inhibit cell division in the presence of damage, allowing time for repair and subsequent reentry into the cell cycle (Hartwell and Weinert, 1989). The spindle checkpoint arrests cells in mitosis when spindle function is inadequate for faithful chromosome segregation (Lew and Burke, 2003). This checkpoint can be induced by benzimidazole drugs, which cause microtubule depolymerization and compromised attachment to kinetochores. To arrest in mitosis, cells must stabilize sister chromatid cohesion to prevent premature separation of chromosomes and the mitotic cyclin Clb2 to maintain mitotic cyclin-dependent kinase activity (Lew and Burke, 2003). Maintenance of both cohesion and mitotic cyclin-dependent kinase (CDK) activity under these conditions requires the cell to stabilize proteins whose destruction is normally initiated by an E3 ubiquitin ligase known as the anaphase-promoting complex (APC) (Harper et al., 2002; Vodermaier, 2004).
The activity and substrate specificity of the APC is a key point of regulation in the cell cycle. Two distinct APC activators, Cdc20 and Cdh1, act at metaphase and mitotic exit respectively (Visintin et al., 1997). These proteins direct degradation of different, but partially overlapping, substrates to promote the irreversible passage through these cell cycle stages. APCCdc20 targets Pds1 “securin”, a protein required for metaphase sister chromatid cohesion, and the S-phase cyclin Clb5, while APCCdh1 targets Ase1, Cdc20 and others. Degradation of Clb2 is biphasic, with a subset of the protein being degraded by APCCdc20, and the remainder by APCCdh1 upon exit from mitosis (Yeong et al., 2000). Prevention of this directed proteolysis is a critical requirement for mitotic checkpoint arrest.
In response to spindle perturbations, two independent spindle checkpoint pathways are required to inhibit progression through metaphase and mitotic exit (Alexandru et al., 1999; Fraschini et al., 1999; Lew and Burke, 2003). Mad1, Mad2, Mad3, Bub1, Bub3 and Mps1 comprise a metaphase checkpoint that inhibits the metaphase to anaphase transition by stabilizing the APCCdc20 substrate Pds1, which binds to and inhibits the protease Esp1. During a normal mitosis Esp1 degrades Mcd1, a key member of the “cohesin” complex, allowing spindle forces to irreversibly separate sister chromatids (Uhlmann et al., 1999). This metaphase checkpoint requires Mad2, a direct inhibitor of Cdc20 (Hwang et al., 1998). Loss of Mad2 leads to failure in chromosome biorientation and elevated rates of chromosome loss following spindle damage (Lew and Burke, 2003).
The small GTPase Tem1 promotes mitotic exit and is inhibited by a two-component GTPase-activating protein (GAP) composed of Bub2 and Bfa1. Together the GAP inhibits mitotic exit in response to spindle damage by inactivating Tem1 and comprises the mitotic exit checkpoint (Bardin et al., 2000; Bloecher et al., 2000; Adames et al., 2001). Active Tem1 initiates a Ras-like GTPase signaling cascade known as the mitotic exit network or MEN (Shou et al., 1999). Lte1, a protein that localizes to the daughter cell cortex, normally comes in contact with and activates Tem1 to initiate the MEN pathway only when the daughter-bound spindle pole, to which Tem1 preferentially localizes, has been successfully delivered to the daughter cell (Jensen et al., 2002; Seshan et al., 2002).
The MEN promotes a series of downstream events, including release of the phosphatase Cdc14 from the nucleolus into the nucleus, a signature event in exit from mitosis. Released Cdc14 reverses the mitotic state by numerous downstream effects, one of which is activation of APCCdh1 to target Clb2 for destruction (Stegmeier and Amon, 2004). Therefore, inhibition of the MEN by Bub2 and Bfa1 leads to stabilization of the mitotic cyclin Clb2, preventing CDK activity from advancing into the subsequent G1 phase and resetting the cell cycle clock. An additional level of complexity in regulation of Cdc14 lies in a network known as fourteen early anaphase release (FEAR). Slk19, Spo12, Esp1, and Cdc5 are responsible for an early, transient release of Cdc14 from the nucleolus (D'Amours and Amon, 2004). This promotes completion of several events that occur between initiation of anaphase and exit from mitosis, including sub-threshold stimulation of MEN activity, but not full mitotic exit.
Cdc55 is one of two B-type subunits of protein phosphatase 2A in Saccharomyces cerevisiae. PP2A is a highly conserved serine/threonine phosphatase with roles in multiple cellular processes (Stark, 1996). Previous studies identified Cdc55 as a component of the spindle checkpoint in two independent screens for novel checkpoint mutants (Minshull et al., 1996; Wang and Burke, 1997). It was shown that Cdc55 was required for viability and maintenance of cohesion during spindle disruption by benzimidazoles but not for stabilization of the mitotic cyclin Clb2.
Our understanding of the spindle checkpoint has advanced dramatically since the initial identification of CDC55 as a checkpoint gene (Lew and Burke, 2003). This led us to reexamine the role of Cdc55 to determine whether it functioned in the metaphase or mitotic exit pathways of the spindle checkpoint. We examined Cdc55 in the context of the two known branches of the spindle checkpoint and show that Cdc55 is required to inhibit release of Cdc14 from the nucleolus in nocodazole, indicating a role in mitotic exit. After release of Cdc14, the anaphase inhibitor Pds1 was rapidly degraded, effectively bypassing the metaphase checkpoint and leading to failure in chromosome biorientation. We propose that Cdc55 is an inhibitor of the exit from mitosis that contributes to mitotic arrest by preventing premature release of Cdc14 from the nucleolus independently of the known spindle checkpoint pathways.
MATERIALS AND METHODS
Yeast Media and Growth
Yeast media and growth conditions followed standard techniques. Nocodazole and benomyl stock solutions were 10 mg/ml in dimethyl sulfoxide (DMSO). Cell synchronization using α-factor synchronization has been described previously (Yellman and Burke, 2004). Benomyl (Dupont, Wilmington, DE) was added to YPD medium at 12 μg/ml in 1% DMSO. Nocodazole (Sigma-Aldrich, St. Louis, MO) was used at either 12 or 20 μg/ml in liquid YM-1 medium with 0.2% DMSO.
Yeast Strains and Transformation
All strains used in this study are listed in Table 1. All strains were isogenic with the reference strain 8058-4-4 and congenic with the A364a background. Mutations or alleles that were crossed into our strains were backcrossed at least 10 times by standard tetrad genetics to 8058-4-4 to ensure that strains were isogenic. Yeast transformations followed the lithium acetate method (Gietz and Woods, 2002). Epitope-tagged alleles PDS1-13MYC-HIS3MX6 and CDC14-13MYC-HIS3MX6 were constructed by PCR-mediated one-step gene replacements by the method of Longtine et al. (1998). Epitope-tagged alleles replaced the endogenous copies of the respective genes. We integrated PDS1-3HA-URA3 by endonuclease digestion of plasmid pOC52 with SacI and ClaI followed by transformation. We introduced CLB2-3FLAG-HIS3 into our strains by backcrossing. Deletion alleles were constructed by standard techniques (Longtine et al., 1998).
Table 1.
Yeast strains used in this study
| Name | Genotype |
|---|---|
| 8058-4-4 reference strain | MATabar1::loxP can1 cyh2 gal1 his3Δ1 leu2-3,112 trp1-289 ura3-52 |
| 8715-10-2 | his3:pHIS3-GFP-LacI-HIS3 leu2:256LacO-LEU2 PDS1-13MYC-HIS3MX6 |
| 8715-6-4 | mad2::loxP-kanMX-loxP his3:pHIS3-GFP-LacI-HIS3 leu2:256LacO-LEU2 PDS1-13MYC-HIS3MX6 |
| 8579-4-4 | bub2::kanMX4 his3:pHIS3-GFP-LacI-HIS3 leu2:256LacO-LEU2 PDS1-13MYC-HIS3MX6 |
| 8766-25-4 | cdc55::loxP-kanMX-loxP his3:pHIS3-GFP-LacI-HIS3 leu2:256LacO-LEU2 PDS1-13MYC-HIS3MX6 |
| 8778-12-4 | cdc55::loxP-kanMX-loxP his3:pHIS3-GFP-LacI-HIS3 leu2:256LacO-LEU2 PDS1-13MYC-HIS3MX6 |
| 8905-4-3 | CDC14-13MYC-HIS3MX6 CLB2-3FLAG-HIS3 PDS1-3HA-URA3 |
| 8905-1-3 | mad2::loxP-kanMX-loxP CDC14-13MYC-HIS3MX6 CLB2-3FLAG-HIS3 PDS1-3HA-URA3 |
| 8906-1-3 | bub2:kanMX4 CDC14-13MYC-HIS3MX6 CLB2-3FLAG-HIS3 PDS1-3HA-URA3 |
| 8866-30-2 | cdc55:loxP-kanMX-loxP CDC14-13MYC-HIS3MX6 CLB2-3FLAG-HIS3 PDS1-3HA-URA3 |
| 8933-1-3 | slk19::kanMX4 spo12::CaURA3MX4 |
| 8933-5-4 | slk19::kanMX4 spo12::CaURA3MX4 cdc55::loxP-natMX4-loxP |
| 8505-12-4 | lte1::kanMX4 |
| 8665-17-3 | cdc55::loxP-natMX4-loxP lte1::kanMX4 |
| 8487-7-4 | bub2::kanMX4 |
| 8694-2-4 | cdc55::loxP-kanMX-loxP |
| 8914-11-3 | cdc55::loxP-kanMX-loxP his3:pHIS3-GFP-LacI-HIS3 leu2:256LacO-LEU2 PDS1-13MYC-HIS3MX6 |
| 8827-13-4 | cdc15-2 his3:pHIS3-GFP-LacI-HIS3 leu2:256LacO-LEU2 PDS1-13MYC-HIS3MX6 |
| 8913-16-4 | mad2::loxP-kanMX-loxP cdc15-2 his3:pHIS3-GFP-LacI-HIS3 leu2:256LacO-LEU2 PDS1-13MYC-HIS3MX6 |
| 8827-23-4 | bub2::kanMX4 cdc15-2 his3:pHIS3-GFP-LacI-HIS3 leu2:256LacO-LEU2 PDS1-13MYC-HIS3MX6 |
| 8914-17-3 | cdc55::loxP-kanMX-loxP cdc15-2 his3:pHIS3-GFP-LacI-HIS3 leu2:256LacO-LEU2 PDS1-13MYC-HIS3MX6 |
| 8834-4-2 | bub2::kanMX4 cdc15-2 |
| 8660-1-4 | cdc55::loxP-kanMX-loxP cdc15-2 |
| 8426-9-4 | mad2::loxP-kanMX-loxP |
| 8862-10-2 | bub2::URA3 |
| 8615-1-4 | cdc55::loxP-natMX4-loxP |
| 8862-3-3 | bub2::URA3 mad2::loxP-kanMX-loxP |
| 8667-2-3 | cdc55::loxP-natMX4-loxP mad2::loxP-kanMX-loxP |
| 8818-9-3 | bub2::URA3 cdc55::loxP-natMX4-loxP |
| 8870-6-3 | CDC14-13MYC-HIS3MX6 PDS1-3HA-URA3 |
| 8870-2-4 | cdc55::loxP-kanMX-loxP CDC14-13MYC-HIS3MX6 PDS1-3HA-URA3 |
| 8993-25-3 | cdc15-2 CDC14-13MYC-HIS3MX6 PDS1-3HA-URA3 |
| 8993-2-3 | cdc55::loxP-kanMX-loxP cdc15-2 CDC14-13MYC-HIS3MX6 PDS1-3HA-URA3 |
All strains were generated during this study.
Green Fluorescent Protein (GFP) Labeling of Chromosome III
Labeling of chromosomes with GFP has been described previously (Straight et al., 1996). We integrated pAFS59 to insert the 256x array of Lac operators at the LEU2 locus, an ∼22-kb distance from the centromere of chromosome III. The plasmid pAFS135 integrated at HIS3 expressed GFP-LacI. Detection of the GFP-labeled chromosome has been described previously (Yellman and Burke, 2004).
Protein Immunoblotting
Protein samples were collected as described by Kushnirov (2000). We normalized total protein amounts by cell number. Proteins were resolved using standard SDS-PAGE. We transferred protein to polyvinylidene difluoride membrane with a semidry transfer apparatus run at 20 V for 1-1.25 h. All antibodies were diluted into phosphate-buffered saline (PBS) containing 3% nonfat milk. Epitopes were detected using monoclonal 9E10 anti-Myc and 12CA5 anti-hemagglutinin (HA) antibodies (University of Virginia Hybridoma Facility, Charlottesville, VA) and the M2 monoclonal anti-FLAG antibody (Sigma-Aldrich). Primary antibody incubations were as follows. For detection of Myc, we used a 1:20,000 dilution of 0.5 mg/ml 9E10 monoclonal antibody (mAb) stock solution, incubated overnight at 4°C, followed by 1-2 h at room temperature. For anti-HA immunoblotting, we used a 1:6000 dilution of a 0.5 mg/ml 12CA5 mAb stock solution, incubating the blots at 4°C overnight, followed by 1-2 h at room temperature. For anti-FLAG blots, we used the M2 antibody at 1:12,500, incubated for 90 min at room temperature. Blots were washed three times in PBS for 5 min before incubation in secondary antibody. To detect β-tubulin, we used a rabbit polyclonal anti-peptide antibody (a gift from Doug Koshland, Carnegie Institution of Washington, Washington, D.C.). Secondary antibodies were horseradish peroxidase-conjugated anti-mouse and anti-rabbit (Jackson ImmunoResearch Laboratories, West Grove, PA) used at 1:20,000 for 45-60 min at room temperature. Quantitation of Clb2-3FLAG immunoblots was done from scanned blots using ImageQuant software (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom).
Microscopy and Immunofluorescence
Microscopy and immunofluorescence has been described previously (Yellman and Burke, 2004).
Flow Cytometry
Flow cytometry has been described previously (Yellman and Burke, 2004). Flow cytometry analysis was performed at the University of Virginia core fluorescence-activated cell sorting facility.
RESULTS
Timing of Mitotic Chromosome Segregation in the cdc55Δ Mutant Is Normal
CDC55 was identified in two independent screens as a member of the spindle checkpoint required for metaphase arrest and maintenance of sister chromatid cohesion when cells are grown in the presence of nocodazole (Minshull et al., 1996; Wang and Burke, 1997). It is not known whether Cdc55 has a role in the regulation of mitosis in an unperturbed cell cycle. Deleting either MAD2, a component of the metaphase checkpoint, or BUB2, a mitotic exit checkpoint gene, has little effect on the timing of mitotic events during growth in rich medium, but both pathways are required to arrest cells in response to spindle disruption by benzimidazoles (Lew and Burke, 2003). We tested whether deletion of CDC55 affected the kinetics of chromosome segregation in an unperturbed cell cycle. We synchronized wild-type, mad2Δ, bub2Δ, and cdc55Δ cells in G1 phase with mating pheromone α-factor and released them into the cell cycle into rich medium. We added α-factor after 90 min to arrest them in the subsequent G1 phase, limiting the experiment to a single cell cycle. We used budding index and flow cytometry to monitor cell cycle progression, GFP chromosome tagging to assay the physical association of sister chromatids, and Pds1-13Myc to follow degradation of the anaphase inhibitor Pds1 (Yamamoto et al., 1996).
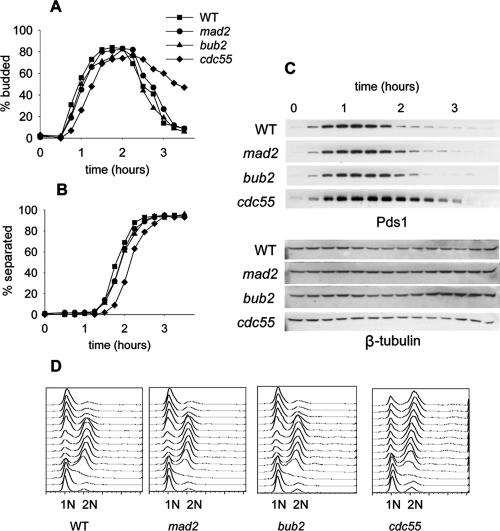
Cell cycle progression was slightly delayed in the cdc55Δ mutant early in the cell cycle as indicated by a 20- to 30-min delay in bud emergence (Figure 1A). The cdc55Δ mutant had a pronounced delay in G2/M as determined by flow cytometry (Figure 1D), consistent with previous reports indicating a role for Cdc55 in cytokinesis (Healy et al., 1991). Pds1 degradation and separation of sister chromatids were slightly delayed in cdc55Δ mutants, but the extent of the delay was equivalent to the delay in bud emergence (Figure 1, B and C). Therefore, the mitotic events of Pds1 degradation and sister chromatid separation proceeded with kinetics similar to wild-type, mad2Δ, and bub2Δ cells. The loss of Cdc55 did not disrupt the timing of mitosis in an unperturbed cell cycle.
Figure 1.
Mitosis in Cdc55Δ and checkpoint mutants in a normal cell cycle. Wild-type (8715-10-2), mad2Δ (8715-6-4), bub2Δ (8579-4-4), and cdc55Δ (8766-25-4) cells were synchronized with α-factor, released into the cell cycle in YM-1 medium, and sampled at 15-min intervals. Cells were limited to a single cell cycle by addition of α-factor 100 min after release. Symbols for all graphs are as indicated in A. (A) Timing of bud emergence, determined by phase contrast microscopy. (B) Timing of sister chromatid separation at LEU2, determined by GFP fluorescence microscopy. (C) Immunoblots of PDS1-13Myc levels to assay the onset of anaphase. (D) Flow cytometry of DNA content to determine the kinetics of replication and cell division.
Cdc55 Is Required to Inhibit Late Mitotic Events but Also Fails in Chromosome Biorientation
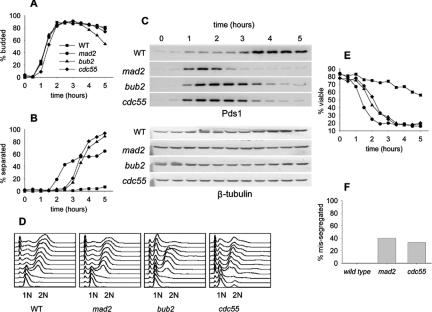
Mad2 and Bub2 have clearly defined roles in spindle checkpoint inhibition of mitosis. To better define the role of Cdc55 in the checkpoint response, we compared the kinetics of Pds1 degradation and loss of sister chromatid cohesion in wild-type, mad2Δ, bub2Δ, and cdc55Δ mutants released synchronously into medium containing nocodazole. A comparison of bud emergence and flow cytometry profiles indicated that entry into the cell cycle was synchronous (Figure 2, A and D). Pds1 was stable, and sister chromatids remained cohesive in wild-type cells treated with nocodazole (Figure 2, B and C). Pds1 was degraded in the mad2Δ mutant with kinetics similar to that of wild-type cells in a normal cell cycle as described previously (Biggins and Murray, 2001). Pds1 degradation and loss of sister chromatid cohesion occurred 90 min later in the bub2Δ mutant compared with the mad2Δ mutant. The cdc55Δ mutant degraded Pds1 and lost sister chromatid cohesion with kinetics similar to that of bub2Δ (Figure 2, B and C). This suggested that the checkpoint role of Cdc55 was not direct inhibition of APCCdc20 as it is for Mad2. The failure of cdc55Δ cells to arrest in response to nocodazole resembled bub2Δ, suggesting that Cdc55 had a role in inhibiting mitotic exit when the spindle checkpoint was activated. We assayed cell viability to determine when cells respond to nocodazole as an independent measure of timing in the cell cycle. We synchronized cells with α-factor, released them into the cell cycle in the presence of nocodazole, and plated samples over time to determine viability (Figure 2E). Viability fell earliest in the cell cycle in the mad2Δ mutant and later in cdc55Δ and bub2Δ.
Figure 2.
Response of Cdc55Δ and checkpoint mutants to nocodazole to assay metaphase arrest. Wild-type (8715-10-2), mad2Δ (8715-6-4), bub2Δ (8579-4-4), and cdc55Δ (8778-12-4) cells were synchronized with α-factor, released into YM-1 medium containing 12 μg/ml nocodazole, and sampled at 30-min intervals for a total of 5 h. Cells were limited to a single cell cycle by addition of α-factor at 100 min after release. Symbols for all graphs are as indicated in A. (A) Bud morphology, determined by phase contrast microscopy. (B) Timing of sister chromatid separation at LEU2, determined by GFP fluorescence microscopy. Each time point contains data from 100 cells. (C) Immunoblots of PDS1-13Myc levels to assay the onset of anaphase. (D) Flow cytometry of DNA content to determine the kinetics of replication and cell division. (E) Viability after α-factor block and release into medium containing 15 μg/ml nocodazole at 30°C, as determined by colony-forming ability when returned to YPD agar plates. (F) Segregation fidelity was determined in the same α-factor block and release experiment at 30°C described for Figure 2E. Cells were released into medium containing 15 μg/ml nocodazole, removed at the 2-h point, washed, and returned to liquid medium lacking nocodazole for 1 h. Cells were fixed, and GFP and 4,6-diamidino-2-phenylindole (DAPI) were visualized. Missegregation is the percentage cells that have undergone full nuclear division into mother and daughter cells but have segregated both copies of GFP-labeled chromosome 3 into the same nucleus.
To determine the efficacy of chromosome segregation, we removed cells from nocodazole and grew them for 1 h, during which time they recovered and completed anaphase. Cdc55Δ cells segregated the GFP-labeled chromosome in an asymmetric manner, with close to 30% of cells segregating two copies of the chromosome to one spindle pole (Figure 2F). This phenotype resembled mad2Δ, whereas wild-type cells were able to accurately segregate the chromosome. Asymmetric segregation is characteristic of cells that fail to properly biorient sister chromatids on the mitotic spindle before segregation.
Cdc55 Is a Negative Regulator of Mitotic Exit and Inhibits a Subset of Events
We used a simple genetic test to determine whether Cdc55 regulated the exit from mitosis. An lte1 spo12 double mutant is inviable (synthetic lethal) because cells lack two independent activators of the exit from mitosis. The lethality can be suppressed by eliminating a negative regulator such as Bub2 or Bfa1 (Stegmeier et al., 2002). We constructed a diploid strain that was heterozygous for lte1::G418, spo12::URA3, and cdc55::NAT, sporulated the diploid, and dissected 35 tetrads to generate all combinations of the deletion mutants. We recovered 20 tetrads that were either directly scored as tetratype for lte1::G418 and spo12::URA3 (four viable spores) or inferred to be tetratype for lte1::G418 and spo12::URA3 from the segregation of markers in the three viable spores. Twelve of the tetrads had three viable spores, and in every case the inviable spore was lte1::G418, spo12::URA3 and CDC55, as expected for synthetic lethality of lte1 spo12. Eight of the tetrads gave four viable spores, and in seven cases the spore that was lte1::G418 spo12::URA3 was also cdc55::NAT. A chi-square test of significance showed that we recovered equal proportions of lte1::G418 spo12::URA3 CDC55 and lte1::G418 spo12::URA3 cdc55::NAT (p < 0.05). Deletion of CDC55 suppressed the synthetic lethality of lte1 spo12, suggesting that Cdc55 was a negative regulator of the exit from mitosis.
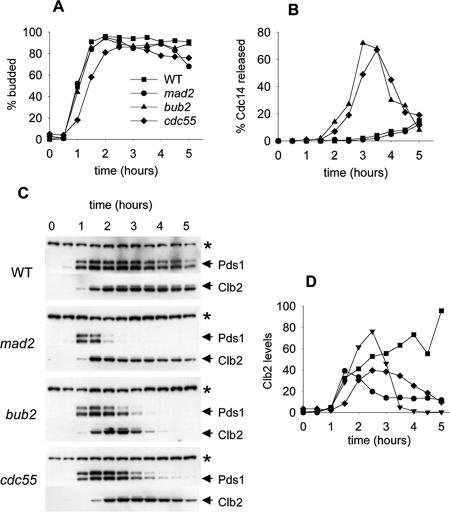
We assayed two other indicators of mitotic exit, release of Cdc14 from the nucleolus and degradation of Clb2. In addition, we included Pds1-3HA to establish the timing of loss of sister chromatid cohesion relative to these events. Cdc14 is sequestered in the nucleolus and released upon activation of the MEN (Stegmeier and Amon, 2004). Nucleolar localization and release of Cdc14 is a simple assay for MEN activation. The relocalization of Cdc14 promotes reversal of the mitotic state, including activation of the Cdh1-dependent form of the APC by dephosphorylation of Cdh1. APCCdh1 then targets specific substrates, including Clb2, for ubiquitin-dependent proteolysis, promoting the transition from mitosis to G1. We synchronized cells in α-factor and released them into medium containing nocodazole, limiting the cells to one cell cycle by readdition of pheromone. Bud emergence data indicated that there was synchronous entry into the cell cycle, with cdc55Δ slightly delayed as shown in Figure 3A. We assayed Cdc14-13Myc localization by immunofluorescence to determine the timing of Cdc14 release in the mutants. Examples of Cdc14-13Myc immunofluorescence in the unreleased (wild-type) and released (cdc55Δ cells) states can be seen in Figure 5B. Cdc14 remained nucleolar in both the wild-type and mad2Δ control strains for the duration of the experiment, demonstrating that these strains were able to prevent mitotic exit (Figure 3B). In contrast, both bub2Δ and cdc55Δ efficiently released Cdc14 from the nucleolus, indicating potent stimulation of this event in both mutants. The pattern of Pds1-3HA degradation was consistent with our previous data (Figures 2C and 3C). In both the bub2Δ and cdc55Δ mutants, Cdc14 release began just before Pds1 degradation, consistent with the interpretation that Cdc14 activity was the indirect cause of this degradation. We concluded that cdc55 mutants were sensitive to benzimidazoles such as benomyl and nocodazole because of inability to restrain Cdc14 release. We also assayed Clb2 protein levels to determine whether degradation of Clb2 correlated with Cdc14 release. During normal exit from mitosis Clb2 degradation is biphasic, with a subset of the protein being degraded by APCCdc20 at the transition to anaphase and the remainder by APCCdh1 during mitotic exit (Yeong et al., 2000). We found that Clb2 was partially stable in the cdc55Δ mutant, despite the release of Cdc14 from the nucleolus (Figure 3, C and D). A subset of Clb2 protein remained stable in cdc55Δ and mad2Δ mutants, whereas the protein was fully degraded in the bub2Δ mutant. Therefore, Cdc14 release and Clb2 degradation, two key indicators of mitotic exit, were uncoupled in the cdc55Δ mutant.
Figure 3.
Exit from mitosis in Cdc55Δ and checkpoint mutants in nocodazole. Wild-type (8905-4-3), mad2Δ (8905-1-3), bub2Δ (8906-1-3), and cdc55Δ (8866-30-2) cells were synchronized with α-factor, released into YM-1 medium containing 12 μg/ml nocodazole, and sampled at 30-min intervals for a total of 5 h. Cells were limited to a single cell cycle by addition of α-factor 100 min after release. Symbols for all graphs are as indicated in A. (A) Bud morphology, determined by phase contrast microscopy. (B) Timing of Cdc14-13Myc release from the nucleolus, determined by immunofluorescence microscopy. Each time point contains data from at least 100 cells. (C) Immunoblots to assay the stability of PDS1-3HA and Clb2-3FLAG proteins. (D) Quantitation of Clb2 levels from scanned immunoblots.
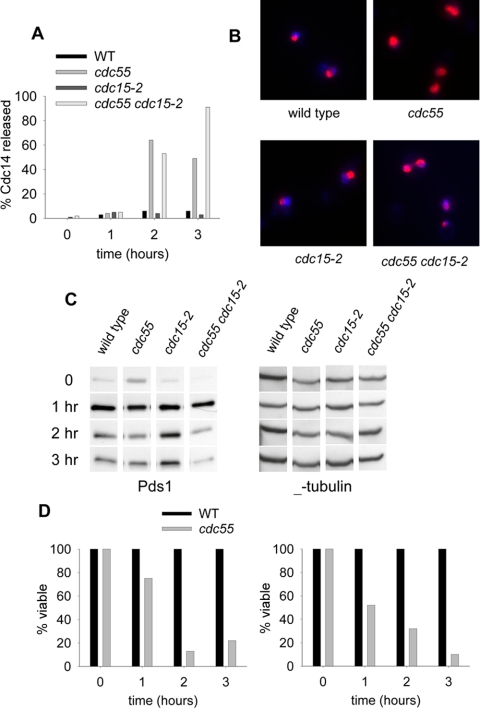
Figure 5.
Mitotic arrest phenotypes during MEN block by cdc15-2. Cells were synchronized with α-factor in YM-1 medium at 23°C. The water bath temperature was then raised to 34°C to allow a gradual temperature shift. Cells were washed twice with water, released into YM-1 medium containing 20 μg/ml nocodazole and incubated in a 35°C water bath for 3 h. Samples were collected at the 0-, 1-, 2-, and 3-h time points. (A) Percentage of cells that have released Cdc14 from the nucleolus as determined by Cdc14-13Myc immunofluorescence and DAPI staining. Strains were wild type (8870-6-3), cdc55Δ (8870-2-4), cdc15-2 (8993-25-3), and cdc55Δ cdc15-2 (8993-2-3). (B) Cdc14-13Myc immunofluorescence of cells from the 2-h time point. (C) Pds1-13Myc protein levels during the MEN block. Strains were wild type (8715-10-2), cdc55Δ (8914-11-3), cdc15-2 (8827-13-4), and cdc55Δ cdc15-2 (8914-17-3). (D) Viability during the MEN block. The same cells sampled for B were removed and plated onto YPD plates at the permissive temperature of 23°C to determine the number of colonies formed. Viability data were normalized to wild-type and cdc15-2 levels, respectively.
Inhibition of Mitotic Exit by Cdc55 Is Independent of Bub2
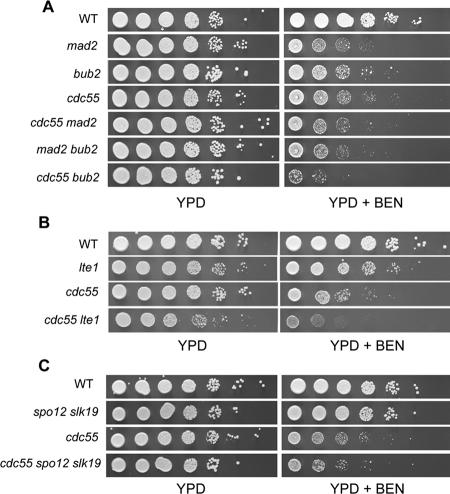
We performed epistasis analysis of benomyl sensitivity with mad2Δ, bub2Δ, and cdc55Δ mutants to determine whether Cdc55 inhibited mitotic exit through Bub2 or by a separate mechanism. The mad2Δ cdc55Δ double mutant showed a level of benomyl sensitivity similar to either mad2Δ or cdc55Δ single mutants. In contrast the bub2Δ cdc55Δ double mutant was more benomyl sensitive than either single mutant (Figure 4A). We conclude that Bub2 and Cdc55 inhibit the exit from mitosis by separate mechanisms but that Mad2 and Cdc55 checkpoint functions overlap.
Figure 4.
(A) Benomyl sensitivity and epistasis. (A) cdc55Δ mutant in combination with deletions of the checkpoint genes MAD2 and BUB2. Stationary phase cultures were normalized to equal densities (1 × 107 cells/ml) and spotted in a 10-fold serial dilution series on YPD (rich medium) or YPD plus 12 μg/ml benomyl. Plates were incubated at 25°C. Strains were wild-type (8058-4-4), mad2Δ (8426-9-4), bub2Δ (8862-10-2), cdc55Δ (8615-1-4), mad2Δ cdc55Δ (8667-2-3), and bub2Δ cdc55Δ (8818-9-3). (B) Deletion of LTE1 in combination with cdc55Δ. Strains were wild type (8058-4-4), cdc55Δ (8615-1-4), lte1Δ (8505-12-4), and cdc55Δ lte1Δ (8665-17-3). (C) Deletion of the FEAR components SLK19 and SPO12 in combination with cdc55Δ. Strains were wild-type (8058-4-4), cdc55Δ (8615-1-4), spo12Δ slk19Δ (8933-1-3), and cdc55Δ spo12Δ slk19Δ (8933-5-4).
Cdc55 Acts Either Downstream of Lte1 in the MEN, Downstream of Spo12 and Slk19 in the FEAR Pathway, or in an Unidentified Pathway
Additional mechanisms can contribute positively to mitotic exit. Lte1, a putative guanidine exchange factor, stimulates Tem1 to activate the MEN, and the FEAR network stimulates the MEN by releasing Cdc14 from the nucleolus in early anaphase. Because the cdc55Δ mutant cannot inhibit mitotic exit appropriately in nocodazole, we wanted to know whether inappropriate stimulation of exit by either Lte1 or FEAR was the cause.
Lte1 is maintained on the bud cortex of the daughter cell and prevented from migrating into the mother cell by factors acting at the bud neck (Barral et al., 2000; Castillon et al., 2003). Cdc55 has been shown to localize to the septin ring surrounding the bud neck (Gentry and Hallberg, 2002). If the role of Cdc55 were to restrict Lte1 to the daughter cell, then a loss of Cdc55 could lead to inappropriate exit from mitosis. We constructed a cdc55Δ lte1Δ double mutant and assayed the ability of the mutant to restrain mitotic exit by determining the benomyl sensitivity. If cdc55Δ mutants are benomyl sensitive because of hyperactive Lte1, the benomyl sensitivity of cdc55Δ should be suppressed by lte1Δ. The cdc55Δ lte1Δ did not have lower benomyl sensitivity and was even slightly more sensitive to benomyl that the cdc55Δ single mutant (Figure 4B). We concluded that the benomyl sensitivity of cdc55Δ was not because of MEN activation upstream of Lte1.
To determine whether inappropriate FEAR activity was the cause of mitotic exit in cdc55Δ cells that were challenged with benomyl, we deleted the genes encoding Spo12 and Slk19, two positively acting members of FEAR, to make a spo12Δ slk19Δ cdc55Δ triple mutant. Loss of Spo12 and Slk19 specifically eliminates FEAR (D'Amours and Amon, 2004). The spo12Δ slk19Δ double mutant had viability and benomyl resistance comparable with wild type (Figure 4C). The spo12Δ slk19Δ cdc55Δ triple mutant was no less sensitive to benomyl than cdc55Δ alone, suggesting that activation of these FEAR components was not the reason for benomyl sensitivity in cdc55Δ. Together, our data show that the role of Cdc55 in mitotic arrest, as measured by nocodazole-induced lethality, must lie downstream of the MEN activator Lte1 or the FEAR activators Spo12 and Slk19 or in some unidentified pathway.
The MEN Mutant cdc15-2 Does Not Block Mitotic Exit in cdc55Δ Cells
We reasoned that if the lethality of cdc55Δ cells in the presence of benzimidazoles was because of inappropriate activation of the MEN, then it should be suppressed by blocking the MEN pathway. Cdc15 kinase acts in the MEN and cdc15-2 is a temperature-sensitive allele that reversibly blocks the pathway (Futcher, 1999; Lee et al., 2001). We assayed Cdc14 localization, Pds1 stability, and nocodazole-induced lethality of wild-type and cdc55Δ cells and the corresponding double mutants with cdc15-2 at restrictive temperature. Synchronous populations of cells were released from α-factor arrest into nocodazole at 35°C to inactivate Cdc15 and sampled for 3 h. Samples were removed at 1-h intervals to determine Cdc14-13Myc localization and Pds1-3HA levels and plated at 23°C in the absence of nocodazole to determine viability by colony formation. The immunofluorescence images show the localization of Cdc14-13Myc at the 2-h time point in each strain (Figure 5B). Wild-type cells maintained Cdc14-13Myc in the nucleolus and remained largely viable (Figure 5; our unpublished data). The viability was normalized to wild type. Partial degradation of Pds1-3HA occurred in wild-type cells, probably reflecting the reduced efficacy of nocodazole at the elevated temperature (Figure 5C). The cdc55Δ mutant released Cdc14-13Myc, partially degraded Pds1-3HA, and lost viability during exposure to nocodazole, indicating failure to arrest (Figure 5). Blocking the MEN with the cdc15-2 allele caused cells to maintain Cdc14 in the nucleolus, stabilize Pds1, and maintain viability in nocodazole. The viability data were normalized to cdc15-2. In contrast, the cdc55Δ cdc15-2 double mutant cells released Cdc14-13Myc from the nucleolus, degraded Pds1-3HA, and lost viability. We concluded that premature mitotic exit in cdc55Δ occurred because of events that were either downstream of Cdc15 in the MEN or in a separate pathway.
DISCUSSION
We showed that Cdc55 was required for inhibiting release of Cdc14 from the nucleolus when the spindle checkpoint was activated. Degradation of the anaphase inhibitor Pds1 was preceded by Cdc14 release from the nucleolus in cdc55 cells treated with nocodazole. Surprisingly, Cdc55 was required for chromosome biorientation after spindle disruption, a role normally ascribed to the metaphase spindle checkpoint. Deleting CDC55 suppressed the synthetic lethality of an lte1 spo12 double mutant, suggesting that Cdc55 is a negative regulator of mitotic exit. Although cdc55 cells prematurely released Cdc14 in nocodazole, this was accompanied by only partial Clb2 degradation, indicating exit from mitosis was incomplete. Genetic data showed that Cdc55 acted downstream of Lte1 in the MEN or downstream of Spo12 and Slk19 in the FEAR pathway to stimulate the release of Cdc14. Cdc14 release, Pds1 degradation, or lethality of cdc55 in nocodazole was not suppressed by a mutation in the gene for the MEN component Cdc15. Therefore, Cdc55 acted after the Cdc15 step in mitotic exit or in a separate pathway.
Previous studies of Cdc55 in S. cerevisiae have described roles in a variety of processes, including cell morphology, cytokinesis, and entry into mitosis (Healy et al., 1991; Lin and Arndt, 1995). We confirmed these observations and found that a cdc55 mutant delayed bud emergence, indicating multiple roles in the cell cycle. However, we detected no effect on the kinetics of mitosis in an unperturbed cell cycle. The inhibitory role of Cdc55 became evident during treatment with nocodazole, which activates the spindle checkpoint. The complexity of the spindle checkpoint was not appreciated at the time that a checkpoint role was first proposed for Cdc55 (Minshull et al., 1996; Wang and Burke, 1997). The mislocalization of Cdc14 in cdc55 cells, treated with nocodazole, in addition to sister chromatid separation and Pds1 degradation seem paradoxical because the metaphase-to-anaphase transition is regulated by Mad2-dependent inhibition of APCCdc20, which is intact in a cdc55 mutant. We propose that cdc55 mutants (and bub2 mutants) bypassed Cdc20 inhibition as a consequence of the release of Cdc14 from the nucleolus, leading to Pds1 degradation and premature sister chromatid separation.
The metaphase checkpoint is capable of responding to two different types of defects, loss of microtubule occupancy at the kinetochore or decreased tension across the kinetochores (Lew and Burke, 2003). Recent models of the tension-sensing branch of the metaphase checkpoint propose that it acts through Mad2 to inhibit APCCdc20 and hold cells at metaphase to ensure biorientation of sister chromatids (Stern and Murray, 2001; Indjeian et al., 2005). Failure in chromosome biorientation is an important assay that identifies mutants lacking the tension component of the spindle checkpoint. Our results suggest an alternative interpretation. We found that both cdc55 and mad2 cells taken from nocodazole and returned to permissive conditions missegregated a single labeled chromosome, indicating failure to properly biorient paired sister chromatids before losing cohesion. We propose that cdc55 mutants failed in biorientation upon recovery from nocodazole because of premature release of Cdc14 followed by degradation of Pds1 and sister chromatids separation. Future studies of the tension-sensing branch of the spindle checkpoint must account for the possibility that failure to biorient chromosomes may be because of misregulation of Cdc14 localization.
Two independent measures, Cdc14 release in cdc55 cells treated with nocodazole and suppression of synthetic lethality of spo12 lte1 by cdc55 triple mutant show that Cdc55 is an inhibitor of mitotic exit. However, some Clb2 was stabilized in cdc55 cells, indicating that full mitotic exit had not occurred as it had in bub2 cells. Cdc14 release from the nucleolus at the exit from mitosis requires the protein kinase Cdc15, a member of the MEN pathway (Stegmeier and Amon, 2004). The failure of cdc15-2 arrest to prevent Cdc14 release, Pds1 degradation, and benzimidazole-sensitivity in the cdc55 mutant indicated that Cdc55 acted either downstream of the Cdc15 step in mitotic exit or in a separate pathway. Epistasis experiments such as these do not allow us to distinguish between these alternatives.
Despite the release of Cdc14 from the nucleolus and degradation of Pds1 in nocodazole-treated cdc55 cells, at least a portion of Clb2, an important substrate of APCCdh1, remained stable. In addition, Cdc20, an APCCdh1 substrate, remained stable in cdc55 mutants during exposure to nocodazole, indicating that APCCdh1 was inactive (our unpublished data). The stability of these two APCCdh1 substrates and the instability of Pds1 suggested that only APCCdc20 was active in the cdc55 mutant in nocodazole. This was consistent with the genetic data that the mad2 cdc55 double mutant was no more benomyl sensitive than either single mutant, because both Mad2 and Cdc55 inhibited APCCdc20 during mitotic arrest.
We propose two models for the role of Cdc55 in the spindle checkpoint. The first is that Cdc55 acts in the MEN downstream of Cdc15 to inhibit Cdc14 release, but it has a separate role in promoting APCCdh1 activation. It is possible that the phosphorylation status of APCCdh1 substrates is important for degradation by APCCdh1. The second model is that Cdc55 acts downstream of the known FEAR components to inhibit Cdc14 release. It is known that FEAR-dependent release of Cdc14 does not lead to sustained exit from mitosis, consistent with partial stabilization of Clb2 in cdc55 (D'Amours and Amon, 2004). This model is attractive because it would explain the independence of Cdc55 and Bub2 in regulating mitotic exit and because a recent report has shown that Cdc14 is released from its inhibitory binding partner Net1 by FEAR-activated CDK phosphorylation during mitosis (Azzam et al., 2004). Net1 is also an in vitro target of phosphorylation by the polo-like kinase Cdc5, which promotes disassembly of recombinant Cdc14-Net1 complexes (Loughrey et al., 2002). In our model, PP2A, acting through Cdc55, would reverse phosphorylation of Net1 to inhibit Cdc14 release during mitosis. This model explains the checkpoint nature of Cdc55 phenotypes, because maintaining the cell in mitosis is the key function of mitotic checkpoint arrest, and explains why loss of Cdc55 cannot be suppressed by deleting the known upstream activators of FEAR. Although we did not test any of the other components of PP2A, previous data show that the other subunits of the enzyme are required for spindle checkpoint arrest, implicating the enzyme complex in regulation of mitotic exit (Wang and Burke, 1997).
Our data differ from a recent study that also identified Cdc55 as a negative regulator of mitotic exit (Wang and Ng, 2006). The authors suggest that Cdc55, through PP2A, dephosphorylates the MEN activator Tem1 to prevent mitotic exit. Our data indicate that the effect of Cdc55 is either downstream or independent of both Tem1 and Cdc15. In addition, our finding that Clb2 protein is partially stabilized in the cdc55 mutant indicates that regardless of misregulation of Tem1, MEN activity did not reach the level required for full mitotic exit. In our experiments, the only clear indicator of mitotic exit was the release of Cdc14 from the nucleolus. For this reason, we conclude that Cdc55 inhibits only a subset of mitotic exit events. It is possible that Cdc55 has roles in both Tem1 and FEAR regulation, and this is not unreasonable given that Cdc55 encodes a subunit of PP2A and has multiple targets within the cell.
A mutation in Par1, a B′-type subunit of PP2A of Schiz-zosaccharomyces pombe suppresses a temperature-sensitive mutation in spg1, the S. pombe homologue of S. cerevisiae Tem1 (Le Goff et al., 2001). Therefore, Par1 plays a role in initiation of cell division through the septin initiation network, a network analogous to the MEN (Simanis, 2003). Together with our results, this suggests that PP2A regulation is a conserved feature of the exit from mitosis. There is evidence that Cdc55 regulates mitotic exit in a cell cycle in which the checkpoint is not active. Loss of Cdc55, similar to loss of Bub2, is able to bypass the mitotic arrest of a cdc20-1 mutant (Tavormina and Burke, 1998). Furthermore, we show in this study that cdc55 suppressed the synthetic lethality of a spo12 lte1 double mutant. This is evidence that Cdc55 has a function, apart from checkpoint response, and is likely to act during every cell cycle. This is an exciting observation suggesting that there are roles of Cdc55 and potential targets of PP2A in mitosis that remain to be identified and that the regulation will be more complex and interesting than previously thought.
Acknowledgments
We thank Orna Cohen-Fix, Doug Koshland, Vinny Guacci, Andrew Murray, Aaron Straight, and Andy Hoyt for strains, plasmids, and reagents. We thank Todd Stukenberg for helpful discussion and for critically reading the manuscript. This work was supported by National Institutes of Health Grant GM-40334 and from the Cell and Molecular Biology Training Grant, University of Virginia.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-04-0336) on November 28, 2005.
Abbreviations used: APC, anaphase-promoting complex; FEAR, fourteen early anaphase release; GAP, GTPase-activating protein; MEN, mitotic exit network.
References
- Adames, N. R., Oberle, J. R., and Cooper, J. A. (2001). The surveillance mechanism of the spindle position checkpoint in yeast. J. Cell Biol. 153, 159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandru, G., Zachariae, W., Schleiffer, A., and Nasmyth, K. (1999). Sister chromatid separation and chromosome re-duplication are regulated by different mechanisms in response to spindle damage. EMBO J. 18, 2707-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam, R., Chen, S. L., Shou, W., Mah, A. S., Alexandru, G., Nasmyth, K., Annan, R. S., Carr, S. A., and Deshaies, R. J. (2004). Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science 305, 516-519. [DOI] [PubMed] [Google Scholar]
- Bardin, A. J., Visintin, R., and Amon, A. (2000). A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell 102, 21-31. [DOI] [PubMed] [Google Scholar]
- Barral, Y., Mermall, V., Mooseker, M. S., and Snyder, M. (2000). Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell 5, 841-851. [DOI] [PubMed] [Google Scholar]
- Biggins, S., and Murray, A. W. (2001). The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 15, 3118-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloecher, A., Venturi, G. M., and Tatchell, K. (2000). Anaphase spindle position is monitored by the BUB2 checkpoint. Nat. Cell Biol. 2, 556-558. [DOI] [PubMed] [Google Scholar]
- Castillon, G. A., Adames, N. R., Rosello, C. H., Seidel, H. S., Longtine, M. S., Cooper, J. A., and Heil-Chapdelaine, R. A. (2003). Septins have a dual role in controlling mitotic exit in budding yeast. Curr. Biol. 13, 654-658. [DOI] [PubMed] [Google Scholar]
- D'Amours, D., and Amon, A. (2004). At the interface between signaling and executing anaphase-Cdc14 and the FEAR network. Genes Dev. 18, 2581-2595. [DOI] [PubMed] [Google Scholar]
- Fraschini, R., Formenti, E., Lucchini, G., and Piatti, S. (1999). Budding yeast Bub2 is localized at spindle pole bodies and activates the mitotic checkpoint via a different pathway from Mad2. J. Cell Biol. 145, 979-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futcher, B. (1999). Cell cycle synchronization. Methods Cell Sci. 21, 79-86. [DOI] [PubMed] [Google Scholar]
- Gentry, M. S., and Hallberg, R. L. (2002). Localization of Saccharomyces cerevisiae protein phosphatase 2A subunits throughout mitotic cell cycle. Mol. Biol. Cell 13, 3477-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz, R. D., and Woods, R. A. (2002). Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350, 87-96. [DOI] [PubMed] [Google Scholar]
- Harper, J. W., Burton, J. L., and Solomon, M. J. (2002). The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 16, 2179-2206. [DOI] [PubMed] [Google Scholar]
- Hartwell, L. H., and Weinert, T. A. (1989). Checkpoints: controls that ensure the order of cell cycle events. Science 246, 629-634. [DOI] [PubMed] [Google Scholar]
- Healy, A. M., Zolnierowicz, S., Stapleton, A. E., Goebl, M., DePaoli-Roach, A. A., and Pringle, J. R. (1991). CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol. Cell Biol. 11, 5767-5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, L. H., Lau, L. F., Smith, D. L., Mistrot, C. A., Hardwick, K. G., Hwang, E. S., Amon, A., and Murray, A. W. (1998). Budding yeast Cdc 20, a target of the spindle checkpoint. Science 279, 1041-1044. [DOI] [PubMed] [Google Scholar]
- Indjeian, V. B., Stern, B. M., and Murray, A. W. (2005). The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science 307, 130-133. [DOI] [PubMed] [Google Scholar]
- Jensen, S., Geymonat, M., Johnson, A. L., Segal, M., and Johnston, L. H. (2002). Spatial regulation of the guanine nucleotide exchange factor Lte1 in Saccharomyces cerevisiae. J. Cell Sci. 115, 4977-4991. [DOI] [PubMed] [Google Scholar]
- Kushnirov, V. V. (2000). Rapid and reliable protein extraction from yeast. Yeast 16, 857-860. [DOI] [PubMed] [Google Scholar]
- Le Goff, X., Buvelot, S., Salimova, E., Guerry, F., Schmidt, S., Cueille, N., Cano, E., and Simanis, V. (2001). The protein phosphatase 2A B'-regulatory subunit par1p is implicated in regulation of the S. pombe septation initiation network. FEBS Lett. 508, 136-142. [DOI] [PubMed] [Google Scholar]
- Lee, S. E., Frenz, L. M., Wells, N. J., Johnson, A. L., and Johnston, L. H. (2001). Order of function of the budding-yeast mitotic exit-network proteins Tem1, Cdc15, Mob1, Dbf2, and Cdc5. Curr. Biol. 11, 784-788. [DOI] [PubMed] [Google Scholar]
- Lew, D. J., and Burke, D. J. (2003). The spindle assembly and spindle position checkpoints. Annu. Rev. Genet. 37, 251-282. [DOI] [PubMed] [Google Scholar]
- Lin, F. C., and Arndt, K. T. (1995). The role of Saccharomyces cerevisiae type 2A phosphatase in the actin cytoskeleton and in entry into mitosis. EMBO J. 14, 2745-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M. S., McKenzie, A., III, Demarini, D. J., Shah, N. G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J. R. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953-961. [DOI] [PubMed] [Google Scholar]
- Loughrey, C. S., Huddleston, M. J., Shou, W., Deshaies, R. J., Annan, R. S., Carr, S. A. (2002). Mass spectrometry-based methods for phosphorylation site mapping of hyperphosphorylated proteins applied to Net1, a regulator of exit from mitosis in yeast. Mol. Cell Proteomics 1, 186-196. [DOI] [PubMed] [Google Scholar]
- Minshull, J., Straight, A., Rudner, A. D., Dernburg, A. F., Belmont, A., and Murray, A. W. (1996). Protein phosphatase 2A regulates MPF activity and sister chromatid cohesion in budding yeast. Curr. Biol. 6, 1609-1620. [DOI] [PubMed] [Google Scholar]
- Nowak, M. A., Komarova, N. L., Sengupta, A., Jallepalli, P. V., Shih, I., Vogelstein, B., and Lengauer, C. (2002). The role of chromosomal instability in tumor initiation. Proc. Natl. Acad. Sci. USA 99, 16226-16231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshan, A., Bardin, A. J., and Amon, A. (2002). Control of Lte1 localization by cell polarity determinants and Cdc14. Curr. Biol. 12, 2098-2110. [DOI] [PubMed] [Google Scholar]
- Shou, W., Seol, J. H., Shevchenko, A., Baskerville, C., Moazed, D., Chen, Z. W., Jang, J., Shevchenko, A., Charbonneau, H., and Deshaies, R. J. (1999). Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell 97, 233-244. [DOI] [PubMed] [Google Scholar]
- Simanis, V. (2003). Events at the end of mitosis in the budding and fission yeasts. J. Cell Sci. 116, 4263-4275. [DOI] [PubMed] [Google Scholar]
- Stark, M. J. (1996). Yeast protein serine/threonine phosphatases: multiple roles and diverse regulation. Yeast 12, 1647-1675. [DOI] [PubMed] [Google Scholar]
- Stegmeier, F., and Amon, A. (2004). Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu. Rev. Genet. 38, 203-232. [DOI] [PubMed] [Google Scholar]
- Stegmeier, F., Visintin, R., and Amon, A. (2002). Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell 108, 207-220. [DOI] [PubMed] [Google Scholar]
- Stern, B. M., and Murray, A. W. (2001). Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr. Biol. 11, 1462-1467. [DOI] [PubMed] [Google Scholar]
- Straight, A. F., Belmont, A. S., Robinett, C. C., and Murray, A. W. (1996). GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr. Biol. 6, 1599-1608. [DOI] [PubMed] [Google Scholar]
- Tavormina, P. A., and Burke, D. J. (1998). Cell cycle arrest in cdc20 mutants of Saccharomyces cerevisiae is independent of Ndc10p and kinetochore function but requires a subset of spindle checkpoint genes. Genetics 148, 1701-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann, F., Lottspeich, F., and Nasmyth, K. (1999). Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400, 37-42. [DOI] [PubMed] [Google Scholar]
- Visintin, R., Prinz, S., and Amon, A. (1997). CDC20 and CDH 1, a family of substrate-specific activators of APC-dependent proteolysis. Science 278, 460-463. [DOI] [PubMed] [Google Scholar]
- Vodermaier, H. C. (2004). APC/C and SCF: controlling each other and the cell cycle. Curr. Biol. 14, R787-R796. [DOI] [PubMed] [Google Scholar]
- Wang, Y., and Burke, D. J. (1997). Cdc55p, the B-type regulatory subunit of protein phosphatase 2A, has multiple functions in mitosis and is required for the kinetochore/spindle checkpoint in Saccharomyces cerevisiae. Mol. Cell Biol. 17, 620-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., and Ng, T. Y. (2006). Phosphatase 2A negatively regulates mitotic exit in Saccharomyces cerevisiae. Mol. Biol. Cell 17, 80-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, A., Guacci, V., and Koshland, D. (1996). Pds1p, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s). J. Cell Biol. 133, 99-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellman, C. M., and Burke, D. J. (2004). Assaying the spindle checkpoint in the budding yeast Saccharomyces cerevisiae. Methods Mol. Biol. 280, 275-290. [DOI] [PubMed] [Google Scholar]
- Yeong, F. M., Lim, H. H., Padmashree, C. G., and Surana, U. (2000). Exit from mitosis in budding yeast: biphasic inactivation of the Cdc28-Clb2 mitotic kinase and the role of Cdc20. Mol. Cell 5, 501-511. [DOI] [PubMed] [Google Scholar]