Abstract
The Gα protein Gpa1 governs the cAMP-PKA signaling pathway and plays a central role in virulence and differentiation in the human fungal pathogen Cryptococcus neoformans, but the signals and receptors that trigger this pathway were unknown. We identified seven putative proteins that share identity with known G protein-coupled receptors (GPCRs). One protein, Gpr4, shares limited sequence identity with the Dictyostelium discoideum cAMP receptor cAR1 and the Aspergillus nidulans GPCR protein GprH and also shares structural similarity with the Saccharomyces cerevisiae receptor Gpr1. gpr4 mutants exhibited reduced capsule production and mating defects, similar to gpa1 mutants, and exogenous cAMP suppressed both gpr4 mutant phenotypes. Epistasis analysis provides further evidence that Gpr4 functions upstream of the Gα subunit Gpa1. Gpr4-Gpr4 homomeric interactions were observed in the yeast two-hybrid assay, and Gpr4 was shown to physically interact with Gpa1 in the split-ubiquitin system. A Gpr4::DsRED fusion protein was localized to the plasma membrane and methionine was found to trigger receptor internalization. The analysis of intracellular cAMP levels showed that gpr4 mutants still respond to glucose but not to certain amino acids, such as methionine. Amino acids might serve as ligands for Gpr4 and could contribute to engage the cAMP-PKA pathway. Activation of the cAMP-PKA pathway by glucose and amino acids represents a nutrient coincidence detection system shared in other pathogenic fungi.
INTRODUCTION
Cryptococcus neoformans is a basidiomycete human fungal pathogen that infects the CNS to cause meningoencephalitis (Casadevall and Perfect, 1998). The medical importance of C. neoformans has increased dramatically as a consequence of the acquired immune deficiency syndrome (AIDS) pandemic. Recent studies in C. neoformans have begun to delineate the signal transduction cascades that regulate morphological differentiation and virulence factor development. Many of these signaling cascades are conserved in other human and plant fungal pathogens. Among them, heterotrimeric G protein signaling has attracted considerable attention because of its conservation among organisms and its importance in fungal development and virulence (Lengeler et al., 2000).
Three G protein α subunits (Gpa1, Gpa2, Gpa3) and one Gβ subunit (Gpb1) have been identified in C. neoformans (Alspaugh et al., 1997; Wang and Heitman, 1999; Wang et al., 2000). Gpa1 governs the cAMP-protein kinase A (PKA) signaling together with the adenylyl cyclase associated protein Aca1 (Bahn et al., 2004) and plays a central role in the development of two major virulence factors, melanin and capsule, which are crucial for the pathogenicity of this organism (Alspaugh et al., 1997, 2002; D'Souza et al., 2001). Gpa1 also contributes to mating of C. neoformans. In contrast, the functions of Gpa2 and Gpa3 are less well established. The single Gβ subunit Gpb1 is required to mediate pheromone sensing during mating via the Cpk1 MAP kinase pathway that shares features conserved with the pheromone response pathway in Saccharomyces cerevisiae (Wang et al., 2000, 2002; Davidson et al., 2003).
G protein-coupled receptors (GPCRs) represent the largest family of transmembrane receptors responsible for transducing extracellular signals into intracellular responses and signal in response to stimuli as diverse as light, protons, Ca2+, odorants, amino acids, nucleotides, proteins, polypeptides, steroids, and fatty acids (Maller, 2003). GPCRs mediate myriad intracellular responses and thereby regulate cellular function via activation of G protein-dependent and -independent pathways. In mammalian cells, ∼720 GPCRs have been identified (Kostenis, 2004). Despite their importance in signaling regulation, only a few GPCRs other than pheromone receptors have been studied in any detail in most fungal systems (Xue et al., 1998; Lorenz et al., 2000; Han et al., 2004; Lemaire et al., 2004; Miwa et al., 2004; Maidan et al., 2005a, 2005b). Reported fungal GPCRs can be grouped into four groups (Han et al., 2004) including GPCRs similar to the following: group i: the pheromone sensing Ste2 and Ste3 receptors of S. cerevisiae; group ii, the glucose sensing Gpr1 receptor of S. cerevisiae; group iii, the proposed nutrient sensor Stm1 of Schizosaccharomyces pombe; and group iv, the cAMP receptors of Dictyostelium discoideum.
In S. cerevisiae, both the Gpa2-activated cAMP pathway and the Ste4/Ste18-activated pheromone response pathway use GPCRs as ligand sensors. In the mating pathway, two GPCRs, Ste2 and Ste3, sense pheromones produced by cells of the opposite mating type and activate the MAP kinase pathway (Versele et al., 2001; Dohlman, 2002). The S. cerevisiae pheromone receptors are well conserved in other fungi, including Schizosaccharomyces pombe (Tanaka et al., 1993), Aspergillus nidulans (Seo et al., 2004), and Neurospora crassa (Kim and Borkovich, 2004). Ste3 homologues have also been reported in basidiomycetes, such as Pra-1 in Ustilago maydis (Bolker et al., 1992). In C. neoformans, pheromone receptors expressed by α and a cells (Ste3α/Cprα and Ste3a/Cpra) have been identified; both are encoded by the mating type locus and are important for mating (Chung et al., 2002), and Cpra also may play a role in virulence (Chang et al., 2003). An additional pheromone receptor-like GPCR (Cpr2) is present in C. neoformans but its gene is not mating type specific and its functions are unknown.
In addition to the pheromone GPCRs, an unusual sugar sensing receptor (Gpr1) has also been identified in S. cerevisiae. Gpr1 senses glucose and sucrose and activates cAMP signaling through Gpa2 (Yun et al., 1997, 1998; Xue et al., 1998; Kraakman et al., 1999; Lorenz et al., 2000; Lemaire et al., 2004). Glucose-sensing receptor homologues have also been identified from several other yeasts. In Candida albicans, a homolog of the S. cerevisiae Gpr1 receptor also senses low concentrations of glucose and amino acids such as methionine to activate cAMP signaling (Miwa et al., 2004; Maidan et al., 2005a). In S. pombe, a related glucose sensing receptor, Git3, has been identified (Welton and Hoffman, 2000). Several GPCRs have also been reported that are important for morphological development in filamentous fungi. In A. nidulans, two pheromone receptors, GprA and GprB, and seven other putative GPCRs have been identified. One of these GPCRs, GprD, was found to function as a negative regulator of sexual development (Seo et al., 2004). Based on genome sequence analysis, nine potential GPCR genes have been identified in N. crassa (Galagan et al., 2003). In the slime mold D. discoideum, four cAMP signal receptors and three cAMP receptor-like GPCRs have been identified (Raisley et al., 2004). But it remains a mystery which proteins sense environmental signals to activate the cAMP signaling pathway in other fungal systems. We hypothesized that related GPCRs could function to activate cAMP signaling in C. neoformans.
In this study, we identified seven GPCR proteins and generated gene-deletion mutants for all of these GPCRs. Both phenotypic assays and biochemical analysis revealed that one GPCR, Gpr4, is important for capsule production and mating. This GPCR has a gene structure similar to S. cerevisiae Gpr1 and A. nidulans GprH. Gpr4 is physically and functionally associated with the G-protein α subunit Gpa1 and functions in sensing the amino acid methionine to activate cAMP-PKA signaling, in accord with recent studies on Gpr1 in C. albicans (Maidan et al., 2005a, 2005b).
MATERIALS AND METHODS
Strains, Media, and Growth Conditions
C. neoformans strains used in this study are listed in Table 1. gpr4 gpa1, gpr4 pkr1, gpr4 pka1, and gpr4 crg1 double deletion mutants were all generated by screening the progeny basidiospores from mating crosses of two individual mutants. ura5 auxotrophic mutants were generated by screening 5-FOA resistant yeast cells. Strains were grown at 30°C on yeast extract-peptone-dextrose (YPD) agar medium and synthetic (SD) medium. V8 medium (pH 5.0) was used for mating assays. Niger-seed medium was used to test for melanin production. Dulbecco's modified Eagle's (DME) medium for capsule production was prepared as previously described (Bahn et al., 2004). All other media preparations were followed as described previously (Granger et al., 1985; Alspaugh et al., 1997; Bahn et al., 2005).
Table 1.
Strains used in this study
| C. neoformans strains | Genotype | Source/reference |
|---|---|---|
| H99 | MATα | Perfect et al. (1993) |
| KN99a | MATa | Nielsen et al. (2003) |
| JEC21 | MATα serotype D | Moore and Edman (1993) |
| F99 | MATα ura5 | Wang et al. (2002) |
| YSB83 | MATα gpa1::NAT-STM#5 | Bahn et al. (2004) |
| YSB85 | MATagpa1::NEO | Bahn et al. (2004) |
| YSB42 | MATα cac1::NAT-STM#159 | Bahn et al. (2004) |
| YSB119 | MATα aca1::NAT-STM#43 ura5 ACA1-URA5 | Bahn et al. (2004) |
| YSB121 | MATaaca1::NEO ura5 ACA1-URA5 | Bahn et al. (2004) |
| JKH7 | MATα pka1::NAT-STM#191 ura5 | J. K. Hicks |
| CDX1 | MATα gpr1::NAT-STM#242 | This study |
| CDX3 | MATα gpr2::NEO | This study |
| CDX5 | MATα gpr3::NEO | This study |
| CDX6 | MATα gpr4::NEO | This study |
| CDX7 | MATα gpr4::NAT-STM#209 | This study |
| CDX9 | MATagpr4::NAT-STM#209 | This study |
| CDX10 | MATagpr5::NAT-STM#58 | This study |
| CDX12 | MATacpr2::NAT-STM#249 | This study |
| CDX14 | MATα gpr6::NAT-STM#218 | This study |
| CDX16 | MATα gpr7::NAT-STM#191 | This study |
| CDX18 | MATα gpr4::NEO gpr5::NAT-STM#58 | This study |
| CDX19 | MATagpr4::NEO gpr5::NAT-STM#58 | This study |
| CDX20 | MATα gpr4::NEO gpa1::NAT-STM#5 | This study |
| CDX21 | MATagpr4::NEO gpa1::NAT-STM#5 | This study |
| CDX24 | MATα gpr4::NAT-STM#209 pkr1::URA5 | This study |
| CDX25 | MATα gpr4::NAT-STM#209 pka1::URA5 | This study |
| CDX26 | MATα gpr4::NAT-STM#209 crg1::URA5 | This study |
| CDX27 | MATagpr4::NAT-STM#209 crg1::URA5 | This study |
| CDX8 | MATα gpr4::NAT-STM#209 ura5 | This study |
| CDX47 | MATα gpr4::NAT-STM#209 ura5 GPA1Q284L-URA5 | This study |
| CDX45 | MATagpr4::NAT-STM#209 GPR4-NEO | This study |
| CDX46 | MATα gpr4::NAT-STM#209 GPR4-NEO | This study |
| CDX78 | MATα gpr4::NAT-STM#209 GPR4-NEO ura5 | This study |
| CDX79 | MATα gpr4::NAT-STM#209 GPR4-NEO ura5 GPA1Q284L-URA5 | This study |
| CDX36 | MATα gpa1::NAT-STM#5 ura5 | This study |
| CDX37 | MATagpa1::NEO ura5 | This study |
| CDX38 | MATagpa1::NEO ura5 URA5 | This study |
| CDX40 | MATagpa1::NEO ura5 GPA1Q284L::FLAG-URA5 | This study |
| CDX42 | MATagpa1::NEO ura5 GPA1-URA5 | This study |
| CDX80 | MATα gpa1::NAT ura5 GPA1Q284L-URA5 | This study |
| CDX63 | MATα cac1::NAT-STM#159 ura5 GPA1Q284L::FLAG-URA5 | This study |
| CDX64 | MATα pka1::NAT-STM#191 ura5 GPA1Q284L::FLAG-URA5 | This study |
| CDX75 | MATα gpr4::NEO gpa1::NAT-STM#5 ura5 | This study |
| CDX76 | MATα gpr4::NEO gpa1::NAT-STM#5 ura5 GPA1Q284L-URA5 | This study |
| CDX77 | MATα ura5 GPA1Q284L-URA5 | This study |
| CDX70 | MATα gpr4::NAT-STM#209, serotype D | This study |
| CDX73 | MATα gpr4::NAT-STM#209 GPR4-DsRED-NEO, serotype D | This study |
Each NAT-STM# indicates the Natr marker with a unique signature tag. Unless labeled specifically, all strains are from the serotype A H99/KN99a background.
Disruption of the GPR4 Gene and Construction of a gpr4 + GPR4 Complemented Strain
The gpr4 null mutant was generated in the congenic C. neoformans serotype A MATα (H99) and MATa (KN99a) strains by overlap PCR as previously described (Davidson et al., 2002). The 5′ and 3′ regions of the GPR4 gene were amplified with primers JH12678/JH12679 (see Supplementary Table 1 for primer sequences) and JH12680/JH12681 from H99 or KN99 genomic DNA, whereas the dominant selectable markers (Natr or Neor) were amplified with the M13 primers (M13F/M13R) from plasmid pNATSTM#209 or pJAF1 (Fraser et al., 2003), respectively. The GPR4 gene replacement cassette was generated by overlap PCR with primers JH12678/JH12681, precipitated onto 600-μg gold microcarrier beads (0.8 μm; Bioworld, Dublin, OH) and biolistically transformed into strains H99 or KN99a as described previously (Davidson et al., 2000). Stable transformants were selected on YPD medium containing nourseothricin (100 mg/L) or G418 (200 mg/L). To screen for gpr4 mutants, diagnostic PCR was performed by analyzing the 5′ junction of the disrupted gpr4 alleles with primers JH12683/JH8994. Positive transformants identified by PCR screening were further confirmed by Southern blot.
To construct the gpr4 complemented strains, H99 genomic DNA containing the entire GPR4 gene was isolated from a C. neoformans BAC library using a GPR4-specific probe amplified with primers JH13137/JH13138. The 5.2-kb XhoI-KpnI fragment containing the GPR4 gene was cloned into plasmid pJAF7 containing the NAT selectable marker (Fraser et al., 2003). The construct was linearized by KpnI digestion and transformed into a gpr4 mutant using biolistic transformation. The Gpr4 complemented strain was selected from transformants containing the full-length GPR4 gene.
Assays for Melanin and Capsule Production
Melanin production was assayed by inoculating C. neoformans strains into 2 ml YPD liquid medium and incubating overnight at 30°C. Five microliters of each overnight culture was placed on Niger-seed agar medium. The agar plates were incubated at 30 or 37°C for 2 d, and pigmentation of fungal colonies was assessed.
To examine capsule production, 5 μl of overnight cultures was inoculated onto DME agar medium and incubated at 30 or 37°C for 3 d. The capsule was visualized with India ink staining and observed with a 100× Zeiss Axioskop 2 equipped with an AxioCam MRM digital camera (Carl Zeiss, Thornwood, NY). Quantitative measurement of capsule size was performed as described previously (Zaragoza et al., 2003) by measuring the diameters of the capsule and the cell using Axio Vision 3.1 software (Carl Zeiss). The relative capsule diameter (100(Dw - Dc)/Dw, where Dw indicates the diameter of the cell plus capsule and Dc indicates the diameter of the cell only) was statistically compared between each mutant and wild-type strains by the Student's t test. p < 0.05 was considered significant.
Assays for Mating and Cell Fusion
C. neoformans cells of opposite mating type were homogenized and cocultured on V8 agar medium at 25°C in the dark for several days, and filamentation was examined under light microscopy.
Cell fusion efficiency was measured by mixing 2 × 106 cells of two strains. Culture suspension, 5 μl, was inoculated onto V8 agar medium (pH 5.0) and incubated for 24 h at room temperature in the dark. Four replicate plates were prepared for each of the three cell mixtures. Cells were then harvested and resuspended in 1 ml dH2O and 200 μl of the suspension (∼105 total cells) was plated onto YPD medium containing NAT and G418. The number of colonies on each plate was determined after incubation at 37°C for 3 d.
Generation of GPA1 Dominant Active Allele
The GPA1 dominant active allele (GPA1Q284L), corresponding to the GPA2Q300L mutant in S. cerevisiae, was amplified by overlap PCR using primers JH12497/JH12500 and JH12499/JH12498. The overlap PCR product was amplified with primers JH12497/JH12498, cloned into the integrating vector pRCD83 (Davidson et al., 2002), generating pDX10. This expression construct was transformed into wild-type and the gpa1, gpr4, gpr4 gpa1, and pka1 mutants to generate dominant active strains.
Construction of GFP::Gpr4 and Gpr4::DsRED Fluorescent Strains
The GPR4 cDNA was amplified from H99 first-strand cDNA with primers JH12961/JH12759 and cloned into plasmid pTH74 (Harashima and Heitman, 2002) between the SmaI and PstI sites, fused to the C-terminus of the GFP gene in the vector, under the control of the S. cerevisiae ADH1 promoter. The GFP::GPR4 fusion construct was transformed into the S. cerevisiae gpr1 mutant strain MLY232.
GPR4 genomic DNA was also amplified from H99 genomic DNA with primers JH15264/JH15307. The DsRED open reading frame was amplified from plasmid pDsRED2 with primers JH15305/JH15306. The GPR4::DsRED overlap PCR product was amplified with primers JH15264/JH15306 and cloned into plasmid pXL1 (Lin et al., unpublished results) between the FseI and PacI sites, under the control of the C. neoformans GPD1 promoter. The GPR4::DsRED fusion construct was transformed into the gpr4 mutant strain. Yeast cells were stained with the yeast vacuole membrane marker dye MDY-64 (Molecular Probes, Eugene, OR). Fluorescence was observed using a Zeiss Axioskop 2 fluorescent microscopy.
Assays for cAMP Production
cAMP assays were conducted as described previously (Bahn et al., 2004). Briefly, a single colony of each C. neoformans strain was inoculated into 10 ml of YPD liquid medium and incubated for 24 h at 30°C with shaking. Cells were collected and washed twice with dH2O, once with MES buffer (10 mM MES, 0.5 mM EDTA), and resuspended in 20 ml MES buffer. Diluted cells (15 ml; OD600 = 2.0) were incubated for 2 h at 30°C for glucose starvation. One milliliter of cells was filtered through a wet Millipore filter on a vacuum manifold (pore size, 0.45 μm, HVLP02500) for the 0 time point. Twenty percent glucose, 1.5 ml, was added to the remaining 14 ml of cell suspension. One milliliter was removed and filtered at 30 s, 1 min, and 3 min. At each time point, filters were immediately removed, placed into Petri-dishes containing 1 ml of formic acid (9.2 ml of 100% formic acid, 190.8 ml dH2O, and 50 ml butanol), and agitated for 1 h to lyse cells on a table-top orbital shaker. Cell suspensions were spun down and the supernatants were transferred to fresh tubes and lyophilized. Pellets were resuspended in 400 μl of assay buffer and 100 μl was used for each sample. cAMP concentrations were determined using the cAMP Biotrak Enzyme immunoassay (EIA) system (Amersham, Piscataway, NJ) and normalized to the wet weight of the cells.
Protein-Protein Interaction Assays Using the Yeast Two-hybrid and Split-ubiquitin Systems
Yeast two-hybrid interaction assays were performed as described (Osman, 2004). cDNAs of the GPR4 3rd cytoplasmic loop and C-terminal cytoplasmic tail were cloned into the bait vector pGAD424 and the prey vector pGBT9, respectively. The full-length GPA1 cDNA was cloned into plasmid pGBT9. All inserted cDNA sequences were confirmed by sequencing. Both bait constructs and prey constructs were cotransformed into yeast strain PJ69-4A. Transformants growing on medium lacking histidine were considered positive interactions. Positive interactions were further confirmed and quantified by β-galactosidase enzyme activity assays using chlorophenolred-β-d-galactopyranoside (CPRG; Calbiochem, San Diego, CA) as substrate as described previously (Idnurm and Heitman, 2005).
The split-ubiquitin system was also utilized to investigate the interaction between Gpr4 and Gpa1. Vectors and yeast strains were included in the DUALmembrane Kit 2 (Dualsystem Biotech, Zürich, Switzerland). GPR4 (full-length cDNA) and GPR481-840 (first transmembrane domain deletion) were cloned into pNCW (the C-terminal half of the ubiquitin Cub protein was fused to the N-terminus of Gpr4), or pCCW (Cub was fused to the C-terminus of Gpr4), respectively. GPA1, GPA2, and GPA3 full-length cDNAs were cloned into the pDL2XN vector (the mutated N-terminal half of ubiquitin NubG protein was fused to the G proteins C-termini). All cDNA sequences were confirmed by DNA sequencing. Cub and NubG fusion constructs were cotransformed into host yeast strain NMY32. Interaction was determined by the growth of yeast transformants on medium lacking histidine or adenine and also by measuring β-galactosidase activity.
Virulence Study
Yeast strains were grown at 30°C overnight and cultures were washed twice with 1× phosphate-buffered saline and resuspended at a final concentration of 2 × 106 CFU/ml. Groups of 10 female A/Jcr mice (NCI/Charles River Laboratories, Wilmington, MA) were intranasally infected with 105 yeast cells of each strain as previously described (Cox et al., 2000). Animals that appeared moribund or in pain were killed by CO2 inhalation. Survival data from the murine experiments were statistically analyzed between paired groups using the long-rank test using the PRISM program 4.0 (GraphPad Software, San Diego, CA. p < 0.05 were considered significant.
RESULTS
GPCR Proteins in C. neoformans
Capitalizing upon the now completed C. neoformans genome (Loftus et al., 2005), we identified over 60 putative proteins that have 7 transmembrane domains (TMs), a structural feature of GPCRs, based on a hidden Markov model (TM-HMM) program (Krogh et al., 2001; Han et al., 2004; see Supplementary Table 2). Among these proteins, seven proteins (Ste3α/Cprα, Cpr2, and Gpr1-5) share homology with GPCR proteins identified in other organisms (Table 2). Compared with the reported classification of fungal GPCRs (Han et al., 2004), Ste3α/Cprα (Chung et al., 2002), Ste3a/Cpra (Chang et al., 2003), and Cpr2 can be grouped into the pheromone receptor group i; Gpr1 shares some sequence identity to GprC in A. nidulans, but is grouped in the third group in the phylogenetic tree based on Clustal X (Thompson et al., 1997), indicating that it is more closely related to proteins in this group; Gpr2 and Gpr3 have sequence identity to Stm1 in S. pombe and belong to the third group iii; and Gpr4 and Gpr5 are more similar to the cAMP receptors in group iv (Figure 1A). Gene deletion mutants for these 7 GPCRs were generated. gpr4 and ste3α/cprα mutations produce phenotypes involved in virulence factor production or mating, whereas the other 5 GPCR deletion mutants have no obvious phenotype. Twenty-nine hypothetical proteins with 7 TMs have homology with proteins other than GPCRs; the other twenty-four hypothetical proteins with 7 TMs have no homology to any known protein sequence in GenBank (http://www.ncbi.nlm.nih.gov/), and these could represent additional GPCR candidates. Gene deletion mutants for two such genes (GPR6 and GPR7) have also been generated; however, no obvious phenotype was apparent.
Table 2.
GPCR genes in C. neoformans
| C. neoformans | A. nidulans | S. cerevisiae | S. pombe | D. discoideum |
|---|---|---|---|---|
| No homolog | GprA (AN2520.2) | Ste2 | Mam2 | |
| Cprα (163.m06349) | GprB (AN7743.2) | Ste3 | Map3 | |
| Cpr2 (186.m03862) | GprB (AN7743.2) | Ste3 | Map3 | |
| Gpr1 (164.m02000) | GprC (AN3765.2) | Gpr1 | Git3 | |
| No homolog | GprD (AN3387.2) | |||
| No homolog | GprE (AN9199.2) | |||
| Gpr2 (179.m00299) | GprF (not defined) | Stm1 | ||
| Gpr3 (186.m04059) | GprG (not defined) | Stm1 | ||
| Gpr4 (185.m02504) | GprH (AN8262.2) | cAR1 | ||
| Gpr5 (184.m04563) | GprI (AN8348.2) | cAR1 |
The GPCR sequences from A. nidulans were used in BLASTP searches of the C. neoformans annotated protein database. The GPCRs in A. nidulans were found by BLASTN searches using GPCRs from S. cerevisiae or S. pombe and the A. nidulans genome database (Han et al., 2004).
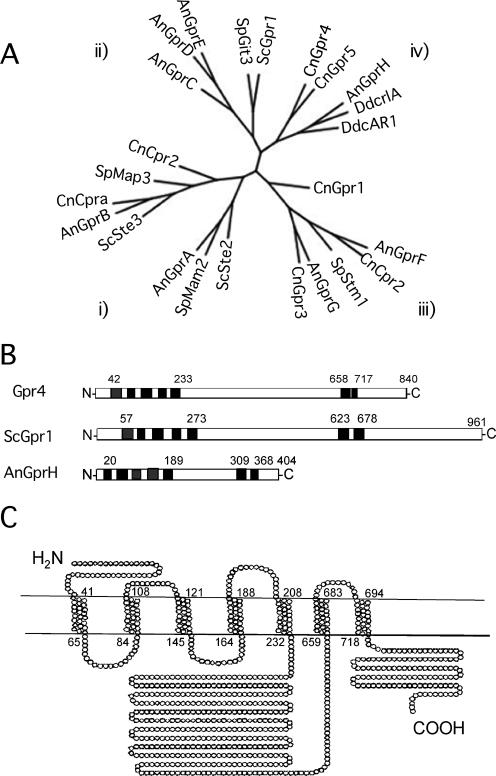
Figure 1.
Overall similarity between Gpr4 and other reported GPCRs and the predicted protein structure of Gpr4. (A) Phylogenetic tree of the fungal GPCR family. Classification of fungal GPCRs was carried out by analysis of GPCRs from C. neoformans (Cprα, Cpr2, Gpr1-5), A. nidulans (GprA-H), S. cerevisiae (Ste2, Ste3, Gpr1), S. pombe (Mam2, Map3, Git3, Stm1), and D. discoideum (cAR1, crlA) using Clustal X. The tree diagram was created with TreeView software. (B) The primary protein structures of Gpr4 in C. neoformans, Gpr1 in S. cerevisiae, and GprH in A. nidulans. ▪, transmembrane regions (TMs). Numbers indicate the beginning of the first TM, the end of TM-V, the beginning of TM-VI, and the end of TM-VII, as well as the total number of amino acids in each protein. (C) Proposed two-dimensional model of the transmembrane topology of Gpr4 in the plasma membrane. ○, amino acid residues. Amino acid residue numbers around the predicted seven TMs are indicated.
Gpr4 Is Important for Capsule Formation and Mating via the cAMP-PKA Pathway
Among the GPCRs identified, Gpr4 was found to play an important role in capsule production and mating. Gpr4 is a novel GPCR and does not share overall sequence identity with the S. cerevisiae Gpr1 receptor but rather has homology with the A. nidulans GprH GPCR protein (Han et al., 2004) and the D. dicoideum cARl cAMP receptor (Pupillo et al., 1992; Raisley et al., 2004) and thus can be grouped into receptor group iv (Table 2; Figure 1B). Gpr4 contains 840 amino acids and has a large third cytoplasmic loop and C-terminal cytoplasmic tail, similar to Gpr1 of S. cerevisiae (Figure 1, B and C). Therefore, we hypothesized that Gpr4 might function as a receptor for cAMP signaling and proceeded to compare the phenotypes of gpr4 and gpa1 mutants.
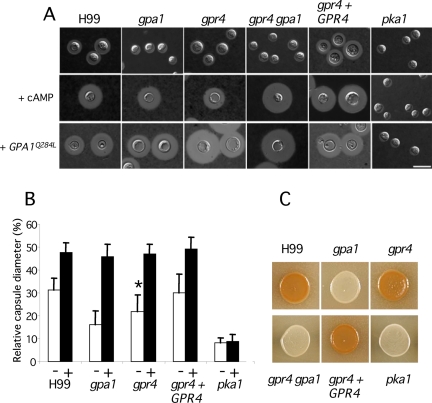
When incubated on DME medium, C. neoformans produces a polysaccharide capsule that surrounds and protects the cell. gpr4 mutants exhibit reduced capsule size, a phenotype similar to that of gpa1 mutants (Figure 2A). The average relative size of the capsule produced by gpr4 mutant cells was reduced by 30% compared with the wild-type isogenic strain H99 (Figure 2B). This reduction is statistically significant (p < 0.01) based on a Student's t-test. In contrast to gpa1 mutants, melanin production was not altered in gpr4 mutants (Figure 2C). Thus, Gpr4 could be one of several receptors coupled to Gpa1 that activate the cAMP signaling pathway, or the loss of Gpr4 could result in partial constitutive active of Gpa1 sufficient to drive melanin but not capsule production.
Figure 2.
Gpr4 is important for capsule formation but not melanin production. (A) Capsule production was visualized by India ink staining of wild-type strain H99, gpa1 (YSB83), gpr4 (CDX6), gpr4 gpa1 (CDX20), and pka1 (JKH7) mutant strains, and the gpr4 + GPR4 complemented strain (CDX46) after growth on DME medium for 3 d at 37°C (top row). Capsule production by the same series of yeast strains was examined in the presence of 10 mM cAMP (middle row) or expressing the GPA1 dominant active allele (GPA1Q284L; bottom row) grown under the same conditions. Bar, 10 μm. (B) Quantitative measurements of the relative capsule diameter under standard conditions without cAMP (□) or with 10 mM cAMP (▪). A total of 50 cells were measured for each strain, and error bars indicate the SD of the mean. t-test was performed for the statistical significance of the relative capsule sizes between H99 and gpr4 mutants (* p < 0.01). (C) Melanin produced by the wild-type strain H99, gpa1 (YSB83), gpr4 (CDX6), gpa1 gpr4 (CDX20), and pka1 (JKH7) mutant strains, and the gpr4 + GPR4 complemented strain (CDX46) was photographed after cells were grown on Niger-seed agar for 36 h at 37°C.
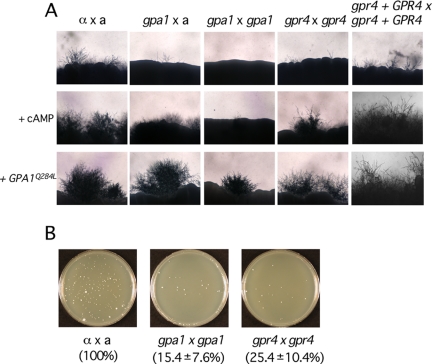
In addition to a defect in capsule production, gpr4 mutants also exhibit a mating defect (Figure 3). During unilateral mating between gpr4 mutants and a wild-type strain of opposite mating type, no obvious mating defect was observed. But in a bilateral gpr4 × gpr4 cross, a significant mating defect with reduced filamentation was apparent (Figure 3A). To further define whether the mating defect observed in gpr4 mutants results from defects in cell fusion, mating filamentation, or both, cell fusion assays were performed. gpr4 mutants were less efficient in cell fusion (25.4%), similar to gpa1 mutants (15.4%), indicating that the gpr4 deletion results in a modest defect in cell fusion (Figure 3B). When the wild-type GPR4 gene was reintroduced, both mutant phenotypes were complemented and wild-type levels of capsule production and mating were restored. The addition of 1 mM cAMP to DME medium restored capsule production in the gpa1 and gpr4 mutant strains, providing further evidence that both Gpr4 and Gpa1 act via the cAMP-PKA pathway (Figure 2). The mating defects of both gpa1 and gpr4 mutants were also suppressed by 1 mM cAMP in V8 agar medium, further implicating Gpr4 in a functional role in the cAMP-PKA pathway (Figure 3).
Figure 3.
gpr4 mutants exhibit a bilateral mating defect. (A) The following strains were cocultured on V8 agar medium (pH 5.0) for 6 d in the dark at room temperature: Top row: H99 × KN99a, α gpa1 (YSB83) × KN99a, α gpa1 (YSB83) × a gpa1 (YSB85), α gpr4 (CDX6) × a gpr4 (CDX9), and α gpr4 + GPR4 (CDX46) × a gpr4 + GPR4 (CDX45). The same series of yeast strains was also cocultured under the same conditions in the presence of 1 mM cAMP (middle row) or expressing the GPA1 dominant active allele (GPA1Q284L) in α cells (bottom row). (B) Cell fusion assays were performed with wild-type strains α (YSB119) × a (YSB121), α gpa1 (YSB83) × a gpa1 (YSB85), and α gpr4 (CDX6) × a gpr4 (CDX9). In each experiment, the percentage of cell fusion products relative to the α × a mixture (100%) was calculated by averaging results from duplicate plates from three independent experiments and calculating the SD of the mean.
Gpr4 Functions Upstream of the Gα Protein Gpa1
To further corroborate a functional relationship between Gpr4 and Gpa1, gpr4 gpa1 double mutants were generated. A MATa gpr4 mutant was crossed with a MATα gpa1 mutant. Single basidiospores were isolated and gpr4 gpa1 double mutants were identified by double dominant marker (Natr and Neor) selection. Analysis of capsule formation, melanin production, and mating of gpr4 gpa1 double mutants supports the hypothesis that Gpr4 functions upstream of Gpa1. gpr4 gpa1 mutants exhibited identical phenotypes with gpa1 mutants, consistent with models in which Gpr4 functions upstream of Gpa1 (Figure 2A). However, because gpa1 mutant phenotypes are more severe than those of gpr4 mutants, the possibility that Gpr4 and Gpa1 signal in different pathways could not be excluded.
To address this caveat, additional epistasis tests were performed. In S. cerevisiae, the GPA2Q300L mutation produces a dominant active form of Gpa2 (Sprang, 1997; Harashima and Heitman, 2002). The corresponding Q284L point mutation was introduced into C. neoformans Gpa1 using overlap PCR. Wild type, gpa1, and gpr4 mutant ura5 auxotrophic strains were transformed with this dominant active GPA1 allele. Expression of the GPA1Q284L allele in the wild-type strain produced enlarged capsules in capsule induction conditions, indicating that this dominant active form of Gpa1 is functional. Expression of the GPA1Q284L dominant active allele suppressed both the gpa1 and the gpr4 mutant defects, restoring both capsule production (Figure 2A) and mating (Figure 3A) to a level similar to the wild-type strain H99 expressing GPA1Q284L and supporting models in which the Gpr4 receptor functions upstream of the Gα subunit Gpa1.
Gpr4 Forms Homodimers and Interacts with Gpa1
To assess the potential for physical interactions between Gpr4 and Gpa1, conventional yeast two-hybrid interaction assays were performed with Gpa1 and Gpr4. Because Gpr4 is a membrane-bound protein, we made several truncated Gpr4 constructs by using gene fragments encoding portions of the receptor located in the cytosol, including the third cytoplasmic loop (GPR4233-658 and GPR4384-658) and the C-terminal cytoplasmic tail (GPR4696-840, GPR4718-840, GPR4754-840, and GPR4781-840). No interaction was observed between any of these fragments and Gpa1 (or Gpa2 or Gpa3). However, we did find that the 3rd cytoplasmic loop of Gpr4 could interact with itself or with the C-terminal tail (Figure 4A), indicating that the Gpr4 receptor may form an oligomeric structure, similar to reports with other GPCRs (Overton et al., 2003; Bai, 2004; Park et al., 2004; Ladds et al., 2005).
Figure 4.
The GPCR Gpr4 interacts with itself and the Gα protein Gpa1. (A) Dimerization of Gpr4. Yeast two-hybrid interaction assays were performed using portions of the Gpr4 protein, including the 3rd cytoplasmic loop (Gpr4233-658 and Gpr4394-658), and C-terminal cytoplasmic tail (Gpr4696-840, Gpr4718-840, Gpr4754-840, and Gpr4781-840) as baits, and full-length Gpa1, Gpr4233-658, and Gpr4394-658 as preys. An interaction between the C-terminal 120 amino acids of the S. cerevisiae Gpr1 receptor (ScGpr1841-961) with ScGpa2 served as a positive control, and Gpr4233-658, Gpr4394-658 with the empty vector pGBT9 served as negative controls. Yeast transformants were grown on selective medium lacking histidine as serial dilutions (1:1, 1:10, and 1:100). β-galactosidase activity assays (see Materials and Methods) were performed to further verify the interactions. The portions of Gpr4 analyzed are indicated schematically in blue, and the TMs of Gpr4 are marked in black. (B) Physical interactions between Gpr4 and Gpa1 in the split-ubiquitin system. The C-terminal half of ubiquitin (Cub) was fused to the N-terminus (Cub::Gpr4) or C-terminus (Gpr4::Cub) of the full-length Gpr4 cDNA or to the N-terminus of Gpr4 without TM-I (Cub::Gpr473-840). The N-terminal half of ubiquitin (NubG) was fused to the C-terminus of full-length Gpa1 (Gpa1::NubG), Gpa2 (Gpa2::NubG), and Gpa3 (Gpa3::NubG). Gpr4::Cub interaction with the control vector pAI-Alg5 served as a control to ensure the correct topology of the Gpr4:Cub fusion protein, Gpr4::Cub interaction with the empty vector pDL2-Alg5 served as a negative control, and the pCCW-Alg5 interaction with pAI-Alg5 served as a positive control for the assay. Yeast transformants contained both a Cub fusion and a NubG fusion construct and were grown on selective medium lacking histidine or adenine after serial dilution (1:1, 1:10, and 1:100). β-galactosidase activity assays were performed to further verify the interaction. A schematic of interactions between Gpr4::Cub, Cub::Gpr4, or Cub::Gpr473-840 and Gpa1 are presented at the top of B.
To further explore the potential for interactions between Gpr4 and Gpa1, the split-ubiquitin system was used. The split-ubiquitin system has been developed to assess interactions between membrane proteins (Johnsson and Varshavsky, 1994; Stagljar et al., 1998; Iyer et al., 2005). In this system, interactions between membrane-bound proteins can be detected by the release of an artificial transcription factor consisting of protein A, LexA, and VP16, which then activates nuclear reporter genes (HIS3, ADE2, and LACZ) resulting in expression of His3, Ade2, and β-galactosidase.
Three versions of Gpr4 were tested in the split-ubiquitin system. A Cub::GPR4 fusion plasmid was generated by fusing the C-terminal half of ubiquitin (Cub) to the N-terminus of full-length GPR4 cDNA, which resulted in an 8TM structural fusion protein based on topology prediction programs TMpred (Ikeda et al., 2002) and HMMTOP (Tusnady and Simon, 2001; Figure 4B). A GPR4::Cub plasmid was constructed by fusing Cub to the C-terminus of full-length GPR4 cDNA. A Cub::GPR473-840 plasmid was generated by fusing Cub to the N-terminus of Gpr4 lacking the first TM domain sequence to ensure the N-terminus of the fusion protein is in the cytosol (Figure 4B). All these fusion alleles were able to interact with protein expressed from the control construct pAI-Alg5 (expresses the native N-terminal half of ubiquitin) and grew on selective medium, indicating that the Cub fusion proteins were localized inside the cell and ubiquitin was successfully reconstituted. None of these fusion alleles showed interaction with the negative control pDL2-Alg5, indicating that they were not self-activated. G protein constructs were generated by fusing full-length Gpa1, Gpa2, and Gpa3 with the mutated N-terminal half of ubiquitin (NubG), respectively. Transformants coexpressing Gpr4::Cub and Gpa1::NubG grew on medium lacking histidine or adenine and produced robust β-galactosidase enzyme activity, indicating that Gpr4 interacts with Gpa1 directly. Gpr4 did not interact with Gpa2 or Gpa3 in this assay (Figure 4B).
Gpr4 Is Membrane Localized and Rapidly Internalized in Rich Medium
A GFP::GPR4 fusion allele was expressed in S. cerevisiae and found to target GFP to the plasma membrane. The GFP::GPR4 expression plasmid was transformed into the S. cerevisiae gpr1 deletion strain MLY232. By direct fluorescence microscopy, cells containing the GFP::GPR4 expression plasmid exhibited a robust GFP signal localized exclusively on the cell membrane, whereas cells containing the control GFP vector had fluorescence throughout the cell, indicating that the GFP::GPR4 fusion construct is expressed and that Gpr4 localizes to the plasma membrane (Figure 5A).
Figure 5.
Gpr4 is membrane localized and rapidly internalized in response to rich medium or methionine. (A) A GFP::GPR4 fusion construct was expressed in the S. cerevisiae gpr1 mutant strain MLY232. Control transformants (pADH1-GFP) expressed GFP alone. (B) A GPR4::DsRED fusion construct was expressed in the C. neoformans gpr4 mutant strain (CDX70), and cells were grown in minimal medium (YNB, 2% glucose, no amino acid) or rich medium YPD. Cells were stained with the vacuole membrane marker dye MDY-64 and the fluorescent signals were monitored by direct fluorescence microscopy. Red channel fluorescence, green channel fluorescence, and their superimposition, as well as the corresponding light microscopy images are presented. (C) Gpr4 internalization was induced by methionine or YPgly medium (glycerol as carbon source). Cells were grown in 5 ml YNB and incubated overnight at 30°C. Cells were collected and resuspended into 2 ml fresh YNB medium and incubated for 2 h. Cells were then collected and resuspended in YNB, YNB plus 10 mM methionine, or YPgly medium. Gpr4 localization was examined by direct fluorescence microscopy at 0-, 30-, and 60-min time points. Left panel, DIC images; right panel, red fluorescence images. Bars, (A-C) 5 μm.
A GPR4::DsRED construct was also generated and expressed in C. neoformans. When this expression allele was transformed into a gpr4 mutant strain, robust red fluorescence was observed on the plasma membrane of cells grown in minimal medium without amino acids (YNB). When cells were shifted to grow in YPD or YPgly medium, within 30 min the majority of cells exhibited fluorescence in the cytosol, especially concentrated in the vacuole, which was visualized with the vacuole membrane marker dye MDY-64 (Figure 5, B and C). These results suggest that the Gpr4::DsRed receptor fusion is properly expressed and localized to the cell membrane and that YP medium contains components that can serve as ligands for Gpr4 and trigger its internalization, which is one key step in GPCR trafficking and recycling. We also tested the possibility of the involvement of glucose in Gpr4 internalization by adding 2% glucose to cells grown in YNgly medium, and no clear fluorescence localization change was observed. Together with the result that Gpr4 is localized to the plasma membrane when grown in YNB medium containing 2% glucose, we conclude that glucose is not an important factor for Gpr4 trafficking.
Amino Acids But Not Glucose May Function as Ligands for the Gpr4 Receptor
In S. cerevisiae, Gpr1 senses glucose and sucrose and activates cAMP signaling (Lemaire et al., 2004). To identify potential Gpr4 ligands, we first measured cAMP production in response to glucose added to starved cells. As expected, neither gpa1 nor adenylyl cyclase (cac1) mutants responded to glucose re-addition (Figure 6). But surprisingly, these cAMP assays consistently showed that gpr4 mutants still respond to glucose, although there may be a very modest reduction compared with wild type (Figure 6). This result indicates that Gpr4 is not a major glucose sensor, and other receptors may be involved in glucose sensing and Gpa1 activation. The possibility that other carbon sources may function as potential agonists for Gpr4 and activate downstream G protein signaling was also examined. cAMP assays showed that neither H99 nor gpr4 mutants responded to sucrose, indicating sucrose cannot activate cAMP signaling in C. neoformans (Supplementary Figure 1), and thus different mechanisms may be involved in cAMP pathway activation in S. cerevisiae and C. neoformans. Taking advantage of the interaction between Gpr4 and Gpa1 in the split-ubiquitin system, we cultured this yeast strain (Gpr4::Cub with Gpa1::NubG) on media with different carbon sources and measured β-galactosidase activity to quantify the interaction. If any of these carbon sources were to activate Gpr4, the degree of the interaction between Gpr4 and Gpa1 might be reduced. We failed to detect significant differences in the interactions between Gpr4 and Gpa1 in response to glucose, galactose, mannose, fructose, or maltose (unpublished data). We concluded that Gpr4 is not a major receptor for carbon sources.
Figure 6.
Gpa1 but not Gpr4 is required for cAMP production in response to glucose. The wild-type strain H99, and gpa1 (RSB83), gpr4 (CDX6), and cac1 (YSB42) mutants were starved for glucose for 2 h. One milliliter of cell suspension for each strain was extracted and cAMP levels were measured at the indicated time points after glucose re-adding. Each data point and error bar indicates the SD of the mean for three independent experiments.
Recently, several reports indicated that in C. albicans, Ras proteins but not Gpr1 are critical for glucose sensing (Leberer et al., 2001; Maidan et al., 2005a). However, Gpr1 signaling can be activated by amino acids such as methionine and alanine (Maidan et al., 2005a, 2005b). To address the hypothesis that Gpr4 may also function as an amino acid sensor, cAMP assays were performed by adding different amino acids and measuring the production of cAMP. Fifteen amino acids were divided into three groups and amino acid mixtures for these three groups of amino acids were generated. Group A contained isoleucine, valine, histidine, and leucine; group B contained lysine, methionine, phenylalanine, tryptophan, alanine, and tyrosine; and group C contained serine, glutamic acid, arginine, threonine, and aspartate. Only group B resulted in an altered cAMP level between the isogenic GPR4 wild-type strain H99 and a gpr4 mutant (Figure 7A). Several amino acids in this group were tested individually for affects on cAMP production, and methionine was found to induce cAMP accumulation in H99 but not the gpr4 mutant in our study (Figure 7B). cAMP production was induced in both strains by tryptophan (unpublished data). However, neither wild-type nor the gpr4 mutant responded to alanine (Figure 7B), which differs from Gpr1 activation in C. albicans (Maidan et al., 2005a). Taking advantage of the interaction between Gpr4 and Gpa1 in the split-ubiquitin system, we also cultured this yeast strain (Gpr4::Cub with Gpa1::NubG) on media with or without methionine and measured β-galactosidase activity to quantify the interaction. We observed an approximately twofold reduction of β-galactosidase activity for the samples grown on medium with methionine (average 15.5 U/ml) compared with that grown on medium without methionine (average, 30.3 U/ml), furthering support our hypothesis that Gpr4 is involved in methionine sensing.
Figure 7.
Gpr4 is required for cAMP induction in response to methionine. (A) Fifteen amino acids were divided into three groups (groups A-C) and each mixture was added to amino acid starved cells of H99 (⋄), the gpr4 mutant strain CDX6 (▴), or the gpa1 mutant strain YSB83 (□) and cAMP levels were assayed. (B) Methionine, alanine, and methionine + glucose were tested individually by re-adding each to starved cells and conducting cAMP assays. All data presented is representative of three independent experiments.
Because the cAMP accumulation pattern showed some modest differences in responses to glucose induction between the gpr4 mutant and wild type, we hypothesized that cAMP pathway activation might be affected by both glucose and amino acids through Gpr4. cAMP assays were performed using a glucose and methionine mixture as the inducer, and cAMP accumulation in the wild-type strain H99 attained a higher level (average 1.64-fold increase after 30 s of induction) than either glucose or methionine alone, which is statistically significant in a Student's t test (p < 0.05), indicating that C. neoformans can sense both glucose and methionine to activate the cAMP-PKA pathway (Figure 7B).
Gpr4 Is Rapidly Internalized in Response to Amino Acids
GPCR internalization is one key step in GPCR trafficking, which is a fundamental biological process and a conserved mechanism leading to GPCR signaling desensitization. Briefly, when ligands bind to a GPCR receptor, the GPCR changes conformation and can be phosphorylated by GPCR kinases, releasing its interactions with G proteins and allowing internalization. We can visualize this internalization process by fusing a GPCR to a fluorescent tag such as GFP or DsRed and verify the ligand of a specific GPCR by testing the ability of the compound to trigger the internalization of the corresponding GPCR. To further elucidate the potential biological role of amino acids on Gpr4 activation, we tested whether Gpr4 trafficking would respond to different amino acids. Red fluorescence can be observed on the plasma membrane when a strain expressing the Gpr4::DsRED fusion protein (strain CDX75) is grown in minimal medium lacking amino acids. Within 30 min after addition of 10 mM methionine, red fluorescence was observed in endocytic vesicles or vacuoles, indicating that the Gpr4 protein has been internalized (Figure 5C). We also tested several other amino acids, including alanine, but no clear fluorescence internalization was observed. These results further confirm that methionine could function as a ligand for the Gpr4 receptor.
Methionine Stimulates Filamentation during Mating through Gpr4
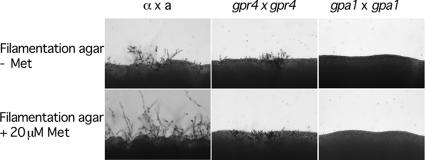
We used defined filamentation agar medium (amino acid free) to further investigate the biological role of methionine on mating. Without any amino acid, mating crosses between wild-type strains produced robust filament growth after 6-d incubation. gpr4 mutants produced less and shorter filaments, indicating a mating defect. By adding 20 μM methionine, a significant stimulation of filamentous growth could be observed from the mixture of wild-type strains, but not from the mixture of gpr4 or gpa1 mutants, indicating that neither gpr4 nor gpa1 mutants respond to methionine (Figure 8). We also test additional concentrations (50 μM, 100 μM, 1 mM, and 10 mM) and observed an inhibition effect on filamentation during mating when the methionine concentration was higher than 1 mM. This result further confirms that methionine plays a role in mating via the Gpr4, Gpa1-activated cAMP pathway in C. neoformans in a dose-dependent manner.
Figure 8.
Gpr4 is required for methionine-induced mating stimulation. The following strains were cocultured on filamentation agar medium for 7 d in the dark at room temperature without (top row) or with 20 μM methionine (bottom row): H99 × KN99a, α gpr4 (CDX6) × a gpr4 (CDX9) and α gpa1 (YSB83) × a gpa1 (YSB85).
Gpr4 Is Not Essential for Virulence
The involvement of Gpr4 in capsule production and cAMP signaling prompted us to investigate its possible role in virulence using a murine inhalation model of systemic C. neoformans infection (Figure 9). Female A/Jcr mice were intranasally inoculated with 105 yeast cells, and animals were monitored twice daily. As previously demonstrated, all mice infected with the wild-type strain H99 survived between 18 and 26 d after infection, whereas mice infected with the attenuated gpa1 mutant survived for more than 60 d (Alspaugh et al., 1997). Surprisingly, mice infected with the gpr4 mutant exhibited mortality similar to those infected with the wild-type H99 strain, indicating that Gpr4 is not critical for virulence of C. neoformans. Because the GPCR Gpr5 shares sequence identity with Gpr4, we generated a gpr4 gpr5 double mutant strain and its virulence was also evaluated. No significant mortality difference was observed compared with H99 and the gpr4 mutant, indicating that Gpr4 and Gpr5 are not functionally redundant (Figure 9).
Figure 9.
The GPCR Gpr4 is not essential for virulence. Female A/Jcr mice were intranasally inoculated with 105 cells of the following strains: H99, gpa1 mutant (YSB83), gpr4 mutant (CDX6), gpr4 gpr5 mutant (CDX18), and gpr4 + GPR4 complemented strain (CDX46). Animals were monitored for clinical signs of cryptococcal infection and sacrificed at predetermined clinical end points that predict imminent mortality.
DISCUSSION
The cAMP pathway is well conserved from mammalian cells to microbes such as fungi. In C. neoformans, the cAMP pathway plays a central role in pathogenicity. In this pathway, the Gα protein Gpa1 and the cyclase associated protein Aca1 activate adenylyl cyclase (Cac1) and stimulate the production of cAMP, which binds to the regulatory subunit of PKA (Pkr1), and releases the PKA catalytic subunits (Pka1/2). Although many components in this pathway have been characterized in several model fungal systems, little is known as to how most fungi sense extracellular signals to activate cAMP signaling. The Gpr1 receptor in S. cerevisiae is the best characterized nutrient-sensing GPCR system. Gpr1 senses glucose and sucrose and functions as the receptor for the cAMP pathway (Xue et al., 1998; Yun et al., 1998; Lemaire et al., 2004). In this study, we investigated the GPCR Gpr4 as a potential receptor for cAMP signaling in C. neoformans. This protein drew our attention because it has structural similarity with Gpr1 in S. cerevisiae, and it also shares sequence identity with the cAMP receptor in D. discoideum. Gpr4 has a very long cytoplasmic loop (encompassing more than half of the total protein) between TM-V and TM-VI, but unlike the large 3rd cytoplasmic loop in the S. cerevisiae Gpr1 receptor, which contains a long asparagine residue (N) repeat, no conserved sequence was identified.
The gpr4 null mutant phenotypes are related to cAMP signaling and involve both capsule production and mating. But Gpr4 does not play a role in regulating melanin production, which is another important virulence factor controlled by the Gpa1-cAMP pathway. Epistasis analysis supports a model in which Gpr4 functions upstream of Gpa1, but Gpr4 may not be the only receptor coupled to Gpa1 (see model in Figure 10). cAMP assays after glucose induction revealed Gpr4 is not a major glucose sensor, which is functionally distinct from S. cerevisiae Gpr1, but similar to the Gpr1 receptor of C. albicans. That Gpr4 is not required for glucose sensing may further explain why gpr4 mutants produce wild-type levels of melanin because melanin induction is known to be triggered by low glucose levels. Other receptors may function to control melanization and act as glucose sensors because Gpa1 responds to glucose induction in a cAMP signaling-dependent manner. In addition to Gpr4, we also identified additional proteins having seven TMs, a feature of GPCRs. Gene deletion mutants were generated for seven of these putative GPCRs, but none appears to be involved in melanin or capsule production. Among them, Gpr5 has both sequence identity and structural similarity with Gpr4. However, gpr4 gpr5 double mutants produced phenotypes similar to that of gpr4 single mutations, indicating that Gpr4 and Gpr5 are not functionally redundant (unpublished data).
Figure 10.

Proposed model for the Gpr4, Gpa1-activated cAMP signaling pathway in C. neoformans. Two signal inputs to the cAMP signaling pathway are proposed in this model and involve nutrient transport and sensing. Fermentable carbon sources, such as glucose, are transported into the cell via hexose transporters and phosphorylated by hexose kinases to produce glucose-6-phosphate (Glu6P), which in turn regulates Cac1 or Gpa1 activation to engage cAMP signaling. Glucose may also be sensed by an unknown receptor to activate the cAMP pathway. Gpr4 may also play a role in low glucose concentration sensing based on the cAMP assay results. In this model, Gpr4 is an amino acid sensor and interacts with Gpa1 to regulate downstream elements of the cAMP pathway. This model does not exclude that other proteins such as methionine permease may participate in ligand sensing via direct or indirect interactions with Gpr4. Hxt, hexose transporters; Hxk, hexose kinases; Glu6P, glucose-6-phosphate.
Direct interactions between Gpr4 and Gpa1 provide further evidence that Gpr4 is a receptor upstream of Gpa1 and acts through cAMP signaling. The interactions between Gpr4 fragments indicate that Gpr4 may form dimeric or oligomeric structures, a property shared with other GPCR receptors. The importance of receptor oligomerization has only recently become apparent and is now a focus of study for GPCR activation (George et al., 2002; Bai, 2004; Ladds et al., 2005). One reason we did not detect an interaction between Gpr4 and Gpa1 using the conventional yeast two-hybrid assay could be that we did not use the proper fragment for interaction or those portions isolated from Gpr4 are not sufficient to mediate physical interactions with Gpa1. In contrast, the split-ubiquitin system is designed to monitor interactions between membrane proteins. With the split-ubiquitin system, we observed a physical interaction between Gpa1 and the Gpr4::Cub fusion construct, in which the Cub domain was fused to the C-terminus of Gpr4. This result supports our model that Gpr4 is a receptor of Gpa1-activated cAMP signaling. It also suggests that this interaction does not require the free carboxyl terminal tail, which is different to GCR1-Gpa1 interaction studies in Arabidopsis, which required the free C-tail of GCR1 (Pandey and Assmann, 2004). The failure to observe any interaction between Cub::Gpr4 or Cub::Gpr473-840 (N-terminal Cub fusions) and Gpa1 could indicate that the N-terminus is important for Gpr4 activation and other functions. Alternatively, the two halves of ubiquitin (Cub and NubG) might not be suitably oriented to activate the ubiquitin protease, which would result in a failure to release the artificial transcription factor (LexA-VP16).
Our study demonstrates that Gpr4 and Gpa1 play different roles in glucose sensing. Gpr4 is not essential for glucose sensing because a cAMP increase in response to glucose induction is still observed in the gpr4 mutant, even though its cAMP induction pattern is modestly different from that of H99. But Gpa1 is essential for glucose-mediated cAMP induction. There are several possibilities for the differing roles of Gpr4 and Gpa1 in glucose sensing. One is that other receptor(s) may exist to sense glucose and trigger cAMP signaling. There is no ScGpr1 homolog in C. neoformans, based on genomic sequence comparison. Although Gpr4 is structurally related to the S. cerevisiae Gpr1 receptor, no significant sequence identity was found between the two. Therefore, if there is a glucose receptor in C. neoformans, it may be very divergent from S. cerevisiae Gpr1, or multiple proteins might share glucose sensing functions. A second possibility is that C. neoformans and some other fungi may use a different mechanism to sense glucose, such as using glucose-6-phosphate to trigger Gpa1 activity instead of sensing extracellular glucose via receptors (see model in Figure 10). In S. cerevisiae, glucose-6-phosphate is produced in the cell by phosphorylation of glucose after its import by hexose transporters and is involved in promoting the cAMP pathway through adenylyl cyclase although the precise mechanism is as yet unclear (Rolland et al., 2002). Similar machinery may exist in C. neoformans because hexose transporters and hexose kinases homologues can also be found in the genome. To test this possibility in C. neoformans, generation of hexose transporter or hexose kinase mutants would be one approach. Caution will be required as functional redundancy may exist because both gene families have multiple members. A third model is that loss of Gpr4 may lead to partial constitutive activation of Gpa1. This may result in sufficient cAMP signaling to support melanin production but not mating or capsule production. In this model, the amplitude of pathway signaling (off, low, high) could enable at least three distinct biological read-outs from a single GPCR-Gα module rather than a simple binary switch with only off and on states.
The nature of the ligands for Gpr4 is a central question. In C. albicans, recent reports reveal that Cdc25 and Ras but not Gpr1 are responsible for glucose sensing, and instead Gpr1 may sense amino acids (Maidan et al., 2005a). In C. neoformans, Ras proteins are unlikely to play a role in glucose sensing because Ras1 has been reported to act independently from the cAMP signaling pathway (Alspaugh et al., 2000; Bahn et al., 2004). Amino acid rich media are known to induce morphological transitions in C. albicans. Van Dijck and colleagues further discovered that methionine is required for hyphal induction and both Gpr1 and Mup1 (a high-affinity methionine permease) are responsible for methionine induced filamentation in C. albicans. Deletion of GPR1 causes marked defects in true hypha formation and invasive growth, whereas the defect of the mup1 mutant strain in morphological transitions is in a methionine concentration-dependent manner. Their study revealed Gpr1 may sense methionine (Maidan et al., 2005a, 2005b). Methionine is also important for sexual development in the fission yeast S. pombe via a cAMP-dependent Ste11-signaling pathway (Schweingruber et al., 1998). In C. neoformans, we also observed that methionine can stimulate filamentation during mating filaments in a Gpr4, Gpa1-dependent manner, indicating that methionine is also important for sexual development in C. neoformans and could be one extracellular signal involved in Gpr4, Gpa1-activated cAMP signaling.
To test the possibility that Gpr4 may also function as a nutrient sensor, we tested cAMP production in response to amino acid induction and found that Gpr4 is important for sensing methionine. This conclusion is further supported by Gpr4 internalization assays, wherein Gpr4 is also rapidly internalized in response to high concentrations of methionine, indicating that amino acids can activate the Gpr4, Gpa1-activated cAMP pathway. Interestingly, we observed an additive increase in cAMP production in response to both glucose and methionine, indicating that cAMP signaling may be activated by multiple extracellular signals.
We also measured the expression of two glucose regulated genes, CAS8 (glucose inducible gene) and LAC1 (glucose reducible gene), using wild-type, gpr4, and gpa1 mutant strains in the presence and absence of either glucose and methionine. Expression of CAS8 was modestly induced by glucose both in the wild-type strain and the gpr4 mutant strain, consistent with a previous report (Pukkila-Worley et al., 2005). The wild type and the gpr4 mutant shared similar expression patterns indicating that Gpr4 is not involved in glucose sensing, and methionine is not important for CAS8 gene expression. No significant difference in expression was observed in the gpa1 mutant strain under these conditions. The expression of the LAC1 gene was significantly repressed by the glucose in wild-type and the gpr4 mutant strain, similar to the previous report (Pukkila-Worley et al., 2005). Surprisingly, we also observed a significant difference in LAC1 gene expression in the gpa1 mutant strain with or without glucose, indicating glucose signaling may in part bypass the Gpa1 protein to repress downstream target genes. These results also suggest that Gpr4 is not important for glucose sensing or glucose regulated gene expression, consistent with our findings in this study (see Supplementary Figure 2).
Gpr4 is not essential for virulence and H99 and the gpr4 mutant strain were indistinguishable in our virulence study. Virulence is controlled by several attributes, including melanin production, capsule formation, and growth at body temperature. The gpr4 mutant strain produces reduced capsule size but has normal melanin production compared with H99, and no growth defect at 37°C. The virulence test results indicate that reduction of capsule size may not be sufficient to affect virulence if enough capsule is present to provide protection against the host immune response and secrete normal amounts of glucuronoxylomannan (GXM). As an important virulence factor, the presence of a capsule is required for optimal pathogenicity in C. neoformans, but its size is not necessarily essential to produce clinical disease. In nonisogenic strains there is no direct correlation between the size of the capsule and the virulence of the strain (Dykstra et al., 1977), so the relative size of the capsule does not ensure pathogenicity.
Overall, our study identified a novel GPCR, which is a receptor for the cAMP pathway that can sense amino acids such as methionine. Our study also indicates that C. neoformans may have a more complicated signal sensing system than S. cerevisiae and could involve multiple sensors for the cAMP signal pathway. To fully understand the extracellular signals and their sensors that activate the cAMP pathway, additional receptor proteins may need to be identified, especially those involved in glucose sensing and that may potentially affect the production of melanin.
Supplementary Material
Acknowledgments
We thank Julie Hicks, James Fraser, and Toshiaki Harashima for plasmids and strains; Jenny Lodge for providing signature-tagged markers; Charles Hall for assistance with phylogenetic tree generation; Andy Alspaugh, Kirsten Nielsen, Julie Hicks, and Hiroaki Matsunami for critical reading and comments on the manuscript; and Cristl Arndt for technical assistance. This study was supported in part by National Institute of Allergy and Infectious Diseases R01 grants AI50113 and AI39115 to J.H. and P01 program project grant AI44975 to the Duke University Mycology Unit. G.M.C. was a Burroughs Wellcome New Investigator and J.H. was a Burroughs Wellcome Scholar in Molecular Pathogenic Mycology and an Investigator of the Howard Hughes Medical Institute.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-07-0699) on November 16, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Alspaugh, J. A., Cavallo, L. M., Perfect, J. R., and Heitman, J. (2000). RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36, 352-365. [DOI] [PubMed] [Google Scholar]
- Alspaugh, J. A., Perfect, J. R., and Heitman, J. (1997). Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 11, 3206-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh, J. A., Pukkila-Worley, R., Harashima, T., Cavallo, L. M., Funnell, D., Cox, G. M., Perfect, J. R., Kronstad, J. W., and Heitman, J. (2002). Adenylyl cyclase functions downstream of the Galpha protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 1, 75-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn, Y. S., Hicks, J. K., Giles, S. S., Cox, G. M., and Heitman, J. (2004). Adenylyl cyclase-associated protein Aca1 regulates virulence and differentiation of Cryptococcus neoformans via the cyclic AMP-protein kinase A cascade. Eukaryot. Cell 3, 1476-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn, Y. S., Kojima, K., Cox, G. M., and Heitman, J. (2005). Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol. Biol. Cell 16, 2285-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, M. (2004). Dimerization of G-protein-coupled receptors: roles in signal transduction. Cell Signal. 16, 175-186. [DOI] [PubMed] [Google Scholar]
- Bolker, M., Urban, M., and Kahmann, R. (1992). The a mating type locus of U. maydis specifies cell signaling components. Cell 68, 441-450. [DOI] [PubMed] [Google Scholar]
- Casadevall, A., and Perfect, J. R. (1998). Cryptococcus neoformans, Washington, DC: ASM Press.
- Chang, Y. C., Miller, G. F., and Kwon-Chung, K. J. (2003). Importance of a developmentally regulated pheromone receptor of Cryptococcus neoformans for virulence. Infect. Immun. 71, 4953-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, S., Karos, M., Chang, Y. C., Lukszo, J., Wickes, B. L., and Kwon-Chung, K. J. (2002). Molecular analysis of CPRalpha, a MATalpha-specific pheromone receptor gene of Cryptococcus neoformans. Eukaryot. Cell 1, 432-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, G. M., Mukherjee, J., Cole, G. T., Casadevall, A., and Perfect, J. R. (2000). Urease as a virulence factor in experimental cryptococcosis. Infect. Immun. 68, 443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, R. C., Blankenship, J. R., Kraus, P. R., de Jesus Berrios, M., Hull, C. M., D'Souza, C., Wang, P., and Heitman, J. (2002). A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148, 2607-2615. [DOI] [PubMed] [Google Scholar]
- Davidson, R. C., Cruz, M. C., Sia, R. A., Allen, B., Alspaugh, J. A., and Heitman, J. (2000). Gene disruption by biolistic transformation in serotype D strains of Cryptococcus neoformans. Fungal Genet. Biol. 29, 38-48. [DOI] [PubMed] [Google Scholar]
- Davidson, R. C., Nichols, C. B., Cox, G. M., Perfect, J. R., and Heitman, J. (2003). A MAP kinase cascade composed of cell type specific and non-specific elements controls mating and differentiation of the fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 49, 469-485. [DOI] [PubMed] [Google Scholar]
- Dohlman, H. G. (2002). G proteins and pheromone signaling. Annu. Rev. Physiol. 64, 129-152. [DOI] [PubMed] [Google Scholar]
- D'Souza, C. A., Alspaugh, J. A., Yue, C., Harashima, T., Cox, G. M., Perfect, J. R., and Heitman, J. (2001). Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol. 21, 3179-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra, M. A., Friedman, L., and Murphy, J. W. (1977). Capsule size of Cryptococcus neoformans: control and relationship to virulence. Infect. Immun. 16, 129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, J. A., Subaran, R. L., Nichols, C. B., and Heitman, J. (2003). Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans var. gattii: implications for an outbreak on Vancouver Island, Canada. Eukaryot. Cell 2, 1036-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galagan, J. E. et al. (2003). The genome sequence of the filamentous fungus Neurospora crassa. Nature 422, 859-868. [DOI] [PubMed] [Google Scholar]
- George, S. R., O'Dowd, B. F., and Lee, S. P. (2002). G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat. Rev. Drug Discov. 1, 808-820. [DOI] [PubMed] [Google Scholar]
- Granger, D. L., Perfect, J. R., and Durack, D. T. (1985). Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J. Clin. Invest. 76, 508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, K. H., Seo, J. A., and Yu, J. H. (2004). A putative G protein-coupled receptor negatively controls sexual development in Aspergillus nidulans. Mol. Microbiol. 51, 1333-1345. [DOI] [PubMed] [Google Scholar]
- Harashima, T., and Heitman, J. (2002). The Galpha protein Gpa2 controls yeast differentiation by interacting with kelch repeat proteins that mimic Gbeta subunits. Mol. Cell 10, 163-173. [DOI] [PubMed] [Google Scholar]
- Idnurm, A., and Heitman, J. (2005). Light controls growth and development via a conserved pathway in the fungal kingdom. PLoS Biol. 3, e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, M., Arai, M., Lao, D. M., and Shimizu, T. (2002). Transmembrane topology prediction methods: a re-assessment and improvement by a consensus method using a dataset of experimentally-characterized transmembrane topologies. In Silico Biol. 2, 19-33. [PubMed] [Google Scholar]
- Iyer, K., Burkle, L., Auerbach, D., Thaminy, S., Dinkel, M., Engels, K., and Stagljar, I. (2005). Utilizing the split-ubiquitin membrane yeast two-hybrid system to identify protein-protein interactions of integral membrane proteins. Sci. STKE 2005, pl3. [DOI] [PubMed] [Google Scholar]
- Johnsson, N., and Varshavsky, A. (1994). Split ubiquitin as a sensor of protein interactions in vivo. Proc. Natl. Acad. Sci. USA 91, 10340-10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H., and Borkovich, K. A. (2004). A pheromone receptor gene, pre-1, is essential for mating type-specific directional growth and fusion of trichogynes and female fertility in Neurospora crassa. Mol. Microbiol. 52, 1781-1798. [DOI] [PubMed] [Google Scholar]
- Kostenis, E. (2004). A glance at G-protein-coupled receptors for lipid mediators: a growing receptor family with remarkably diverse ligands. Pharmacol. Ther. 102, 243-257. [DOI] [PubMed] [Google Scholar]
- Kraakman, L., Lemaire, K., Ma, P., Teunissen, A. W., Donaton, M. C., Van Dijck, P., Winderickx, J., de Winde, J. H., and Thevelein, J. M. (1999). A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol. Microbiol. 32, 1002-1012. [DOI] [PubMed] [Google Scholar]
- Krogh, A., Larsson, B., von Heijne, G., and Sonnhammer, E. L. (2001). Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567-580. [DOI] [PubMed] [Google Scholar]
- Ladds, G., Davis, K., Das, A., and Davey, J. (2005). A constitutively active GPCR retains its G protein specificity and the ability to form dimers. Mol. Microbiol. 55, 482-497. [DOI] [PubMed] [Google Scholar]
- Leberer, E., Harcus, D., Dignard, D., Johnson, L., Ushinsky, S., Thomas, D. Y., and Schroppel, K. (2001). Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol. Microbiol. 42, 673-687. [DOI] [PubMed] [Google Scholar]
- Lemaire, K., Van de Velde, S., Van Dijck, P., and Thevelein, J. M. (2004). Glucose and sucrose act as agonist and mannose as antagonist ligands of the G protein-coupled receptor Gpr1 in the yeast Saccharomyces cerevisiae. Mol. Cell 16, 293-299. [DOI] [PubMed] [Google Scholar]
- Lengeler, K. B., Davidson, R. C., D'Souza, C., Harashima, T., Shen, W. C., Wang, P., Pan, X., Waugh, M., and Heitman, J. (2000). Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64, 746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus, B. J. et al. (2005). The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307, 1321-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, M. C., Pan, X., Harashima, T., Cardenas, M. E., Xue, Y., Hirsch, J. P., and Heitman, J. (2000). The G protein-coupled receptor Gpr1 is a nutrient sensor that regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Genetics 154, 609-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidan, M. M., De Rop, L., Serneels, J., Exler, S., Rupp, S., Tournu, H., Thevelein, J. M., and Van Dijck, P. (2005a). The G protein-coupled receptor Gpr1 and the G{alpha} protein Gpa2 act through the cAMP-Protein Kinase A pathway to induce morphogenesis in Candida albicans. Mol. Biol. Cell 16, 1971-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidan, M. M., Thevelein, J. M., and Van Dijck, P. (2005b). Carbon source induced yeast-to-hypha transition in Candida albicans is dependent on the presence of amino acids and on the G-protein-coupled receptor Gpr1. Biochem. Soc. Trans. 33, 291-293. [DOI] [PubMed] [Google Scholar]
- Maller, J. L. (2003). Signal transduction. Fishing at the cell surface. Science 300, 594-595. [DOI] [PubMed] [Google Scholar]
- Miwa, T., Takagi, Y., Shinozaki, M., Yun, C. W., Schell, W. A., Perfect, J. R., Kumagai, H., and Tamaki, H. (2004). Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans. Eukaryot. Cell 3, 919-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman, A. (2004). Yeast two-hybrid assay for studying protein-protein interactions. Methods Mol. Biol. 270, 403-422. [DOI] [PubMed] [Google Scholar]
- Overton, M. C., Chinault, S. L., and Blumer, K. J. (2003). Oligomerization, biogenesis, and signaling is promoted by a glycophorin A-like dimerization motif in transmembrane domain 1 of a yeast G protein-coupled receptor. J. Biol. Chem. 278, 49369-49377. [DOI] [PubMed] [Google Scholar]
- Pandey, S., and Assmann, S. M. (2004). The Arabidopsis putative G protein-coupled receptor GCR1 interacts with the G protein alpha subunit GPA1 and regulates abscisic acid signaling. Plant Cell 16, 1616-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, P. S., Filipek, S., Wells, J. W., and Palczewski, K. (2004). Oligomerization of G protein-coupled receptors: past, present, and future. Biochemistry 43, 15643-15656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukkila-Worley, R., Gerrald, Q. D., Kraus, P. R., Boily, M. J., Davis, M. J., Giles, S. S., Cox, G. M., Heitman, J., and Alspaugh, J. A. (2005). Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot. Cell 4, 190-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupillo, M., Insall, R., Pitt, G. S., and Devreotes, P. N. (1992). Multiple cyclic AMP receptors are linked to adenylyl cyclase in Dictyostelium. Mol. Biol. Cell 3, 1229-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisley, B., Zhang, M., Hereld, D., and Hadwiger, J. A. (2004). A cAMP receptor-like G protein-coupled receptor with roles in growth regulation and development. Dev. Biol. 265, 433-445. [DOI] [PubMed] [Google Scholar]
- Rolland, F., Winderickx, J., and Thevelein, J. M. (2002). Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res. 2, 183-201. [DOI] [PubMed] [Google Scholar]
- Schweingruber, A. M., Hilti, N., Edenharter, E., and Schweingruber, M. E. (1998). Methionine induces sexual development in the fission yeast Schizosaccharomyces pombe via an ste11-dependent signalling pathway. J. Bacteriol. 180, 6338-6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, J. A., Han, K. H., and Yu, J. H. (2004). The gprA and gprB genes encode putative G protein-coupled receptors required for self-fertilization in Aspergillus nidulans. Mol. Microbiol. 53, 1611-1623. [DOI] [PubMed] [Google Scholar]
- Sprang, S. R. (1997). G protein mechanisms: insights from structural analysis. Annu. Rev. Biochem. 66, 639-678. [DOI] [PubMed] [Google Scholar]
- Stagljar, I., Korostensky, C., Johnsson, N., and te Heesen, S. (1998). A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc. Natl. Acad. Sci. USA 95, 5187-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, K., Davey, J., Imai, Y., and Yamamoto, M. (1993). Schizosaccharomyces pombe map3+ encodes the putative M-factor receptor. Mol. Cell. Biol. 13, 80-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., and Higgins, D. G. (1997). The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusnady, G. E., and Simon, I. (2001). The HMMTOP transmembrane topology prediction server. Bioinformatics 17, 849-850. [DOI] [PubMed] [Google Scholar]
- Versele, M., Lemaire, K., and Thevelein, J. M. (2001). Sex and sugar in yeast: two distinct GPCR systems. EMBO Rep. 2, 574-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P., and Heitman, J. (1999). Signal transduction cascades regulating mating, filamentation, and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol. 2, 358-362. [DOI] [PubMed] [Google Scholar]
- Wang, P., Nichols, C. B., Lengeler, K. B., Cardenas, M. E., Cox, G. M., Perfect, J. R., and Heitman, J. (2002). Mating-type-specific and nonspecific PAK kinases play shared and divergent roles in Cryptococcus neoformans. Eukaryot. Cell 1, 257-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P., Perfect, J. R., and Heitman, J. (2000). The G-protein beta subunit GPB1 is required for mating and haploid fruiting in Cryptococcus neoformans. Mol. Cell. Biol. 20, 352-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton, R. M., and Hoffman, C. S. (2000). Glucose monitoring in fission yeast via the gpa2 Galpha, the git5 Gbeta and the git3 putative glucose receptor. Genetics 156, 513-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, Y., Batlle, M., and Hirsch, J. P. (1998). GPR1 encodes a putative G protein-coupled receptor that associates with the Gpa2p Galpha subunit and functions in a Ras-independent pathway. EMBO J. 17, 1996-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, C. W., Tamaki, H., Nakayama, R., Yamamoto, K., and Kumagai, H. (1997). G-protein coupled receptor from yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 240, 287-292. [DOI] [PubMed] [Google Scholar]
- Yun, C. W., Tamaki, H., Nakayama, R., Yamamoto, K., and Kumagai, H. (1998). Gpr1p, a putative G-protein coupled receptor, regulates glucose-dependent cellular cAMP level in yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 252, 29-33. [DOI] [PubMed] [Google Scholar]
- Zaragoza, O., Fries, B. C., and Casadevall, A. (2003). Induction of capsule growth in Cryptococcus neoformans by mammalian serum and CO(2). Infect. Immun. 71, 6155-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.