Abstract
The centrosome is the major microtubule-organizing center in animal cells. Although the cytoplasmic dynein regulator Nudel interacts with centrosomes, its role herein remains unclear. Here, we show that in Cos7 cells Nudel is a mother centriole protein with rapid turnover independent of dynein activity. During centriole duplication, Nudel targets to the new mother centriole later than ninein but earlier than dynactin. Its centrosome localization requires a C-terminal region that is essential for associations with dynein, dynactin, pericentriolar material (PCM)-1, pericentrin, and γ-tubulin. Overexpression of a mutant Nudel lacking this region, a treatment previously shown to inactivate dynein, dislocates centrosomal Lis1, dynactin, and PCM-1, with little influence on pericentrin and γ-tubulin in Cos7 and HeLa cells. Silencing Nudel in HeLa cells markedly decreases centrosomal targeting of all the aforementioned proteins. Silencing Nudel also represses centrosomal MT nucleation and anchoring. Furthermore, Nudel can interact with pericentrin independently of dynein. Our current results suggest that Nudel plays a role in both dynein-mediated centripetal transport of dynactin, Lis1, and PCM-1 as well as in dynein-independent centrosomal targeting of pericentrin and γ-tubulin. Moreover, Nudel seems to tether dynactin and dynein to the mother centriole for MT anchoring.
INTRODUCTION
In most animal cells, the centrosome serves as the microtubule-organizing center (MTOC). Each centrosome in G0 or G1 phase of the cell cycle contains a pair of barrel-shaped mother and daughter centrioles that usually lie closely and perpendicularly to each other. It also contains an amorphous cloud of the pericentriolar matrix (PCM). Microtubules (MTs) are nucleated from templates formed by the γ-tubulin ring complex in the PCM and are subsequently either released or stably anchored to the subdistal appendages on the mother centriole. The daughter centriole, however, lacks such accessories. Each centriole duplicates once during the cell cycle. The duplication occurs in S phase. Initially, the daughter centriole matures into a new mother centriole. From the side of each mother centriole then arises a daughter centriole de novo. After elongation of the new daughter centrioles, the PCM also expands. By the onset of mitosis, the two pairs of centrioles further separate and their associated PCM split to form two centrosomes that serve as spindle poles in M phase (Doxsey, 2001; Bornens, 2002; Job et al., 2003; Ou and Rattner, 2004).
In addition to γ-tubulin, a large number of proteins associate with the centrosome at certain stages of the cell cycle or remain associated throughout (Doxsey, 2001; Bornens, 2002; Ou and Rattner, 2004). For example, pericentrin tethers the γ-tubulin-containing complex to the spindle poles in M phase, whereas in interphase it may serve as a scaffold protein for organization of the centrosome (Dictenberg et al., 1998). PCM-1 is capable of oligomerization. In interphase, it may recruit certain centrosomal proteins into granules of 70-100 nm in diameter, or the “pericentriolar satellites” (Dammermann and Merdes, 2002; Kubo and Tsukita, 2003). Centrin-2 binds to the distal lumen of centrioles and is essential for centriole duplication (Paoletti et al., 1996; Salisbury et al., 2002). Ninein anchors the MTs to the subdistal appendages in nonpolarized cells (Mogensen et al., 2000). Dynactin, a multiprotein complex, is also implicated in MT anchoring to the centrosome (Quintyne et al., 1999).
The centrosome is a dynamic organelle. Its protein composition and stoichiometry vary considerably during progression of the cell cycle (Doxsey, 2001; Bornens, 2002; Blagden and Glover, 2003). Some proteins, such as PCM-1, pericentrin, and NuMA, are assembled to the centrosome through cytoplasmic dynein-mediated transport (Zimmerman and Doxsey, 2000). Dynein is an MT minus end-directed motor consisting of two dynein heavy chains (DHCs), three to four dynein intermediate chains (DICs), four light intermediate chains, and a variety of light chains. It is broadly involved in intracellular transport, mitosis, and other processes that require MT-based motility. On the other hand, dynein-independent protein assembly also exists but is poorly understood. Both pathways seem to be used by some proteins, such as γ-tubulin (Khodjakov and Rieder, 1999; Young et al., 2000). Therefore, they may be redundant and used preferentially in different cells or cell cycle stages (Doxsey, 2001; Blagden and Glover, 2003).
Mammalian Nudel and NudE are evolutionarily conserved dynein regulators with ∼53% amino acid sequence identity (Wynshaw-Boris and Gambello, 2001). Nudel seems to regulate the velocity of the dynein motor (Liang et al., 2004). This is distinct from dynactin, which stimulates the processivity of dynein movement and serves as an adaptor to many cargos and target sites (Karki and Holzbaur, 1999; King and Schroer, 2000; Dujardin and Vallee, 2002). Both Nudel and NudE are centrosomally localized. Through direct interaction with dynein, they function in neuronal migration during the development of the central nervous system, membrane trafficking, and mitosis (Feng et al., 2000; Sasaki et al., 2000; Yan et al., 2003; Yang et al., 2003; Feng and Walsh, 2004; Liang et al., 2004; Shu et al., 2004). Both proteins also bind directly to Lis1, another dynein regulator (Feng et al., 2000; Sasaki et al., 2000). At least in Nudel, its interactions with both dynein and Lis1 are critical for dynein activities (Yan et al., 2003; Liang et al., 2004). In addition, emerging evidence suggests their critical roles at the centrosome. First, murine NudE directly interacts with several centrosome proteins, including pericentrin in yeast two-hybrid assays (Feng et al., 2000). Second, depletion of Nudel in developing neocortex impairs neuronal positioning and the coupling of centrosome and nucleus (Shu et al., 2004). Third, both proteins are in a complex with γ-tubulin in coimmunoprecipitations (Feng et al., 2000; Liang et al., 2004). We thus investigated centrosome functions of Nudel in the current study.
MATERIALS AND METHODS
Plasmids
Plasmids for expressing FLAG-tagged or green fluorescent protein (GFP)-tagged human wild-type and mutant Nudel, NudE, and dynamitin were described previously (Yan et al., 2003; Liang et al., 2004). For vector-based RNA interference (RNAi), a pair of oligonucleotides containing the sequence 5′-GGATGAAGCAAGAGATTTA-3′ present in human Nudel cDNA was synthesized and cloned into pTER+ vector as described previously (van de Wetering et al., 2003). The resultant plasmid, pTER-Nudi, was capable of expressing a small interference RNA to target Nudel mRNA for degradation.
A partial murine cDNA coding for the C-terminal portion of pericentrin-2 from amino acid residues 988-2916 was kindly provided by Dr. H. Koga (GenBank accession no. AK122275) (Okazaki et al., 2003). In comparison with the full-length sequence under GenBank accession no NM_008787, it lacks coding sequences for amino acid residues 2279-2296, probably because of alternative splicing. It was cloned into pEGFP-C1 (Clontech, Mountain View, CA) to express GFP-PCNTMt1. A plasmid for expression of GFP-PCNTMt2 (containing residues 988-2335) was also constructed by truncating the cDNA fragment downstream of the unique KpnI site.
Antibodies and Other Staining Reagents
Mouse antibodies directed against α-tubulin, γ-tubulin, FLAG, β-actin, DIC (70.1), and rabbit anti-γ-tubulin antibodies were from Sigma-Aldrich (St. Louis, MO). Monoclonal antibodies (mAbs) to p150glued were from BD Biosciences Transduction Laboratories (Lexington, KY). Rabbit polyclonal antibodies to GFP, Lis1, and DHC were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-PCM-1, anti-pericentrin, mouse anti-ninein, mouse anti-centrin-2, and rabbit anti-centrin-2 antibodies were kindly provided by A. Merdes (University of Edinburgh, Edinburgh, United Kingdom), S. J. Doxsey (University of Massachusetts Medical School, Worcester, MA), G. Chan (Alberta Cancer Board, Edmonton, Alberta, Canada), J. L. Salisbury (Tumor Biology Program, Mayo Clinic, Rochester, MN), and M. Bornens (Institute Curie, Paris, France), respectively. Anti-Nudel IgY was generated from chicken using Escherichia coli-expressed His-Nudel and affinity-purified using the antigen immobilized on Affigel (Bio-Rad, Hercules, CA). Secondary antibodies conjugated with Alexa 488, 546, 633, or 647 and LysoTracker Red were purchased from Invitrogen (Carlsbad, CA), whereas antibodies linked with peroxidase were from Promega (Madison, WI). Sypro Ruby was from Bio-Rad.
Cell Culture and Transfection
Human embryonic kidney (HEK)293T, HeLa, and Cos7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% (vol/vol) bovine serum (Sijiqing Company, Hangzhou, China) in an atmosphere containing 5% CO2. Cells were transfected using the conventional calcium phosphate method. When GFP-F was used as a transfection marker, the ratio of pEGFP-F (Clontech) to other plasmid(s) was kept at 1:10 to ensure its cotransfection. HEK293T cells were mainly used for biochemical assays because of their high transfection efficiency (∼80%). HeLa and Cos7 cells were used for cytological studies because of larger cell sizes. For overexpression experiments, cells were harvested or fixed at ∼48 h after transfection. For RNAi experiments, they were used ∼72 h after transfection unless otherwise stated.
Fluorescence Microscopy
Generally, cells grown on glass coverslips were fixed in cold methanol for 10 min before immunofluorescence staining. Proper antibody combinations were chosen for multicolor staining. Nuclear DNA was stained with 4,6-diamidino-2-phenylindole. To stain for endogenous Nudel, cells were permeabilized with extract buffer as described for 1 min, followed by fixation in cold methanol (Yan et al., 2003). Fluorescence images were captured with a cold charge-coupled device (SPOT II; Diagnostic Instruments, Sterling Heights, MI) on Olympus BX51 microscope or with a Leica TCS SP2 laser confocal microscope. For confocal microscopy with fixed cells, optical sections were scanned at 0.1- to 0.2-μm intervals when needed. Z-stack images were then formed by maximal projection. A Bio-Rad Radiance 2100 confocal system was used for live cell imaging. Grayscale images were converted to pseudocolor using Adobe Photoshop (Adobe Systems, Mountain View, CA). Statistical results were obtained in a blind manner and presented as mean ± SD. Image quantitations were performed as described previously (Howell et al., 2000).
Coimmunoprecipitation and Immunoblotting
Coimmunoprecipitation for FLAG-tagged proteins was performed as described previously (Yan et al., 2003). To deplete DIC, lysate from 2 × 107 HEK293T cells expressing FLAG-Nudel and GFP-PCNTMt2 was sequentially incubated at 4°C with 6 μg of mouse anti-DIC IgM (Sigma-Aldrich), 6 μg of anti-mouse IgM (Invitrogen), and protein G beads (Invitrogen), each for 1 h. Another aliquot of the lysate was treated only with protein G beads to serve as a control. The resulting supernatants were then used for immunoprecipitation with anti-FLAG mAb. For immunoblotting, proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes (Whatman Schleicher and Schuell, Dassel, Germany). Immunoblots were developed in Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life and Analytical Sciences, Boston, MA) and exposed to x-ray films (Eastman Kodak, Rochester, NY). When necessary, blots were stripped and reblotted with other antibodies.
Centrosome Fractionation
HEK293T cells were collected with a plastic scraper. Discontinuous sucrose gradient fractionations were performed to fractionate centrosomes as described previously (Yan et al., 2003). Eighteen fractions were collected from the bottom of the gradient, and those with even numbers were assayed by immunoblotting. For immunostaining, fraction 12 was directly blotted on a small piece of wet Immobilon-P membrane (Millipore, Billerica, MA) followed by methanol fixation and immunostaining performed as with cells on coverslips. Secondary antibodies labeled with Alexa 488 and 647 were used to avoid autofluorescence of the membrane.
Fluorescence Recovery after Photobleaching (FRAP)
FRAP experiments were performed at 25°C using a program in Bio-Rad Radiance 2100. To completely disassemble MTs, cells were treated with 5 μg/ml nocodazole for 3 h before photobleaching. Fluorescence intensities were measured as described previously (Howell et al., 2000). The relative intensity (FR) at a given time point “t” was calculated by the following equation: FRt = [Ft/Fpre] × [Fc-pre/Fc-t]. Fpre and Ft represent fluorescence of the same centrosomal region before and after photobleaching. Fc-pre and Fc-t stand for fluorescence of a corresponding control area outside the photobleached region, so that the ratio was used to compensate for quenching effects during image acquisition. The half-time of FRAP was measured as described previously (Howell et al., 2000). The percentage of FRAP was calculated as 100(FRt - FR0)/(1 - FR0), in which FR0 is the relative intensity immediately after bleaching (t = 0) (Howell et al., 2000).
RESULTS
Nudel Localizes Preferentially to the Mother Centriole
To further assess centrosome localization of human Nudel (Sasaki et al., 2000; Yan et al., 2003), we raised a new anti-Nudel antibody in chicken that showed little cross-reaction with NudE (Figure 1A). Association of endogenous Nudel with the centrosome marked by γ-tubulin was confirmed after fractionation of centrosomes from 293T cells by sucrose gradient centrifugation (Figure 1, B and C) (Yan et al., 2003). Centrosomal Nudel was also detected in Cos7 and HeLa cells, in which it only partially superimposed with centrin-2 (Figure 1D; our unpublished data), a protein located at the distal end of both centrioles (Paoletti et al., 1996). When Cos7 cells were treated with nocodazole at low concentrations (1 μM) to partly disassemble MTs (Mogensen et al., 2000), Nudel was found mainly to locate at the center of MTs stably anchored to the centrosome (Figure 1D).
Figure 1.
Localization of Nudel on the mother centriole. (A) Antibody specificity. GFP-NudE (lane 1) or GFP-Nudel (lane 2) expressed in HEK293T cells was subjected to SDS-PAGE and immunoblotting (IB) with the indicated antibodies. (B and C) Endogenous Nudel in purified centrosomes. Centrosomes were fractionated from HEK293T cells by sucrose gradient ultracentrifugation. Fractions with even numbers were immunoblotted with antibodies to Nudel and γ-tubulin, respectively (B). Fraction 12 was then subjected to immunostaining (C) to reveal Nudel (1) and γ-tubulin (2). The merged image is shown in 3. Arrowheads point to representative centrosomes. Bar, 5 μm. (D) Correlation of centrosomal Nudel with MT anchoring. Cos7 cells treated with nocodazole (1 μM) for 20 min were immunostained with antibodies to α-tubulin, Nudel, and centrin-2. Bar, 5 μm. The 5× enlargements show merged images of MTs with Nudel or centrin-2 staining. (E-G) Relative distributions of centrosomal Nudel, centrin-2, γ-tubulin, Ninein, and p150glued in interphase. Cos7 cells were immunostained to visualize the indicated proteins. Arrowheads indicate centrosomes. Bar, 5 μm. Insets show 5× enlargements of Nudel staining alone or merged images with another protein.
For insight into functions of Nudel at the centrosome, its localization patterns were examined in detail. Initially, its distribution patterns were compared with those of centrin-2 and γ-tubulin in Cos7 cells. Centrin-2 served as a marker to indicate centriole numbers, thus also as a reference to cell cycle stages; γ-tubulin was a marker for the PCM (Bornens, 2002). Before centriole duplication, Nudel colocalized with only one of the two centrioles but in a more diffuse pattern than the centrin-2 staining (Figure 1E, 1 and 2). It only superimposed with a portion of centrosomal γ-tubulin (Figure 1E, 3). Nudel kept associated with one centriole until late stages of the centriole duplication: When centrin-2 staining exhibited four discrete foci, Nudel was seen at two centrioles that belonged to different pairs (Figure 1E, 4-9). In contrast, γ-tubulin associated with both pairs of the duplicating centrioles constitutively (Figure 1E, 6 and 9) (Piel et al., 2000).
Localization of Nudel at the center of MT focus (Figure 1D) suggested its association with the mother centriole (Mogensen et al., 2000; Piel et al., 2000). Indeed, Nudel and ninein, a known component of the subdistal appendages of the mother centriole (Mogensen et al., 2000), associated with the same centriole (Figure 1F, 1-9). Interestingly, during the centriole duplication, ninein targeted to the new mother centriole earlier than Nudel. In cells with two ninein-positive foci, 67.0% (n = 115) had only one Nudel spot (Figure 1F, 4-9). In contrast, p150glued, a subunit of dynactin that was also located on the maternal centriole (Quintyne and Schroer, 2002), coincided better with Nudel (Figure 1G, 1-9). Among cells with two Nudel-positive foci, only 20% (n = 90) contained one p150glued spot. Moreover, centrosomal Nudel superimposed better with p150glued than with ninein (Figure 1, F and G; our unpublished data), implying that Nudel and dynactin may not be located at the subdistal appendages.
Nudel Exhibits Fast Turnover at the Centrosome Independently of Dynein Activity
Cytoplasmic dynein/dynactin target to the centrosome in an MT-dependent manner (Quintyne et al., 1999). As a dynein regulator, Nudel was expected to exhibit similar properties. After nocodazole treatment for 3 h to completely depolymerize MTs (Figure 2A, 3), however, centrosomal Nudel still remained (1 and 2). Its average intensity was still 86.4% (n = 50) of that in the mock-treated control cells.
Figure 2.
Nudel binds to the centriole in a dynamic and dynein-independent manner. Arrowheads indicate centrosome positions. (A) Centrosomal localization of Nudel in the absence of MTs. Cos7 cells were treated with nocodazole (5 μg/ml) for 3 h to completely depolymerize MTs (3). Bar, 10 μm. (B and C) Dynamics of centrosomal Nudel in Cos7. Turnover rates of centrosomal GFP-Nudel were assayed by FRAP in the absence (B) or presence (C) of nocodazole. Centrosomes were photobleached at 0 min. Only one of the two centrosomal dots was bleached whenever possible. Bar, 2.5 μm. Average results from six cells are shown in each recovery curve in which centrosome intensities before photobleaching are defined as “1.” (D) Dynamitin overexpression fails to affect centrosomal Nudel in Cos7. FLAG-dynamitin was visualized with anti-FLAG mAb. Bar, 10 μm.
FRAP assays were then performed to examine the dynamics of centrosomal Nudel in the presence or absence of nocodazole. As shown in Figure 2B, GFP-Nudel exhibited clear centrosome localization as one or two closely spaced spots in Cos7 cells. After being bleached in live cells using a laser beam, the centrosomal GFP autofluorescence recovered rapidly over time, reaching 96% on average (n = 6) at 20 min postbleach, with t1/2 as 2.1 min (Figure 2B). Such a rate of FRAP reflected rapid turnover of GFP-Nudel at the centrosome (Khodjakov and Rieder, 1999). Nocodazole treatment for 3 h did not significantly alter the kinetics of the FRAP (92.1% at 20 min) (Figure 2C), indicating that Nudel is capable of MT-independent targeting to centrosomes.
To further corroborate these observations, we examined whether inactivating dynein influenced centrosomal Nudel. Overexpression of dynamitin/p50, a dynactin subunit, is known to inhibit dynein activities in both mitosis and retrograde transport of centrosomal proteins as well as membranous organelles in a variety of cell lines, including Cos7 and HeLa (Echeverri et al., 1996; Burkhardt et al., 1997; Quintyne et al., 1999; Kim et al., 2004). Nevertheless, overexpressing FLAG-dynamitin, which was previously shown to disrupt membrane traffic (Liang et al., 2004), failed to affect centrosomal Nudel (Figure 2D). Dynein activity is thus dispensable for centrosomal targeting of Nudel.
Nudel Uses Overlapping Domains to Bind Centrosome and Dynein and Overexpression of Mutant Nudel Missing a Common Region Abolishes Centrosomal Lis1, Dynactin, and PCM-1
To assess regions important for centrosome localization of Nudel, two mutants lacking residues 114-133 (NudelN20) or 256-291 (NudelC36) that are critical for binding Lis1 or dynein, respectively (Liang et al., 2004), were examined with γ-tubulin as a marker. GFP-NudelN20 was still localized at the centrosome (Figure 3A, 1 and 2). In contrast, GFP-NudelC36 was not seen at the centrosome (Figure 3A, 3 and 4). This was rather surprising, because our previous results (Figure 2) indicated dynein-independent centrosome targeting of Nudel. Despite this, its lack of dynein binding was an unlikely cause of the defect, because Nudel was able to bind to the centrosome in the absence of MTs or dynein activity (Figure 2). Rather, a logical explanation was that residues 256-291 were required for centrosome localization of Nudel through interaction with proteins other than dynein.
Figure 3.
Residues 256-291 are critical for centrosome localization of Nudel itself, and overexpression of a mutant Nudel missing this region dislocates Lis1, p150glued, and PCM-1. (A-F) Cos7 cells overexpressing GFP-tagged Nudel, NudelN20 (lacking residues 114-133) or NudelC36 (lacking residues 256-291) (1 or 3) were fixed and immunostained for the indicated proteins (2 and 4). To visualize centrosomal Lis1, cells were extracted for 10 s before fixation. Arrows and arrowheads point to transfectants and centrosome locations, respectively. Bar, 20 μm.
We have shown that NudelC36 overexpression inactivates dynein in membrane traffic (Liang et al., 2004). To test whether Nudel also regulated dynein-mediated centrosomal protein transport and assembly, effects of NudelC36 on a variety of centrosomal proteins were examined. The centrosomal proteins Lis1, p150glued, and PCM-1, although unaffected by GFP-Nudel, were disrupted in GFP-NudelC36 expressors (Figure 3, B-D). The PCM-1-containing pericentriolar satellites, which are usually enriched in the vicinity of the centrosome (Figure 3D, 1 and 2) (Kubo and Tsukita, 2003), also became randomly dispersed (Figure 3D, 3 and 4). In contrast, centrosomal γ-tubulin, ninein, and pericentrin were not affected (Figure 3A, E and F). Because dynactin and the PCM-1-containing pericentriolar satellites depend on dynein-mediated transport for centrosomal targeting (Kubo et al., 1999; Dammermann and Merdes, 2002; Quintyne and Schroer, 2002), current results are consistent with involvement of Nudel in centrosomal protein transport mediated by dynein.
Nudel Complexes with Centrosomal Proteins in Both Dynein-dependent and -independent Ways
As dynein regulators, Nudel and NudE are expected to complex, through dynein, with proteins whose assembly to the centrosome requires dynein-mediated transport. Indeed, they are able to immunoprecipitate endogenous dynactin and γ-tubulin in addition to dynein (Feng et al., 2000; Sasaki et al., 2000; Liang et al., 2004). Moreover, we found that FLAG-tagged NudE or Nudel also precipitated pericentrin and PCM-1 but not centrin-2 (Figure 4A, lanes 6 and 7). In contrast, NudelC36 associated with none of these proteins (Figure 4A, lane 8), although it still bound Lis1 (lane 8) (Liang et al., 2004). Consistently, direct interaction between pericentrin and dynein light intermediate chain has been documented (Purohit et al., 1999).
Figure 4.
Associations of Nudel and NudE with other centrosomal proteins. (A) Identification of Nudel or NudE-associated centrosomal proteins. HEK293T cells (2 × 107) transfected with vector or plasmids to express FLAG-tagged NudE, Nudel, and NudelC36 were processed for immunoprecipitation (IP) using anti-FLAG mAb. Immunoblotting was then performed with antibodies to the indicated proteins. Lanes 1-4, proteins in total cell lysates. Lanes 5-8, proteins in immunoprecipitates. To avoid overloading, FLAG-fusion proteins were detected from 1/13 of the samples for other proteins. The top bands, which are more evident in samples from cells overexpressing NudelC36 because of a mitotic block (our unpublished data), represent phosphorylated isoforms of Nudel in M phase (Yan et al., 2003). (B) Pericentrin is able to associate with Nudel in much higher stoichiometry than dynein. Cells (6 × 107) coexpressing FLAG-Nudel and GFP (lane 2) or a GFP-tagged pericentrin mutant PCNTMt2 (lane 1) were subjected to immunoprecipitation using anti-FLAG mAb. Immunoprecipitates were resolved by 5-15% gradient SDS-PAGE followed by either IB to visualize the indicated proteins (lanes 3 and 4) or by in-gel staining with Sypro Ruby to reveal total proteins (lanes 5 and 6). The same blot for lanes 3 and 4 was restripped to allow immunoblotting for DHC (top) and FLAG-Nudel (bottom) serially. Positions of protein size markers in kilodaltons are indicated on the right side. (C) Depleting dynein has little effect on pericentrin-Nudel interaction. Lysates from cells coexpressing FLAG-Nudel and GFP-PCNTMt2 were either treated with anti-DIC antibody to immunodeplete dynein (lane 3) or mock-treated (lane 2). The resulting supernatants (lanes 2 and 3) and the cell lysate containing FLAG-Nudel and GFP (lane 1) were then subjected to immunoprecipitation using anti-FLAG mAb. Immunoblotting (lanes 4-6) was then performed as described in B.
Nevertheless, recent findings that murine NudE can interact with several centrosome proteins, including pericentrin in yeast two-hybrid assays (Feng et al., 2000), challenged the above-mentioned assumption, suggesting that NudE may be able to bind certain centrosome proteins directly. Coimmunoprecipitation of endogenous pericentrin with FLAG-NudE (Figure 4A, lane 6) actually confirmed their interaction in vivo. Therefore, a portion of pericentrin in the immunoprecipitates (Figure 4A, lane 6) seemed to bind FLAG-NudE directly. Nudel shares many properties with NudE (Feng et al., 2000; Sasaki et al., 2000; Yan et al., 2003; Liang et al., 2004) and thus might be able to associate directly with pericentrin as well.
To verify existence of dynein-independent association between Nudel and pericentrin, we investigated whether dynein was dispensable for their association. Initially, we tested whether the stoichiometry of pericentrin in the immunoprecipitates could exceed that of dynein by overexpressing exogenous pericentrin before immunuprecipitation. Because of alternative splicing of the same gene, pericentrin has several isoforms (Flory and Davis, 2003), one of which is pericentrin-2 (GenBank accession no. NM_008787). There is no information available on which region of pericentrin interacts with NudE (Feng et al., 2000). We therefore used a partial murine cDNA encoding a C-terminal fragment (residues 988-2916) of pericentrin-2 (GenBank accession no. AK122275) to express a mutant named PCNTMt1. This mutant contains additional 995 residues after the extreme C terminus of the original pericentrin (Doxsey et al., 1994). GFP-PCNTMt1 immunoprecipitated with FLAG-Nudel but was expressed at very low levels (our unpublished data). Truncating residues 2336-2916 from PCNTMt1 to form GFP-PCNTMt2 dramatically increased the expression levels while maintaining the Nudel-association activity (Figure 4B, lanes 1-4). Moreover, staining with Sypro Ruby, a fluorescent dye for in-gel protein detection, revealed GFP-PCNTMt2 as a major protein in the immunoprecipitates in addition to several unidentified protein bands (Figure 4B, lanes 5 and 6). Although DHC was detected through immunoblotting (top, lanes 3 and 4), it was not visualized in the gel stained by Sypro Ruby (Figure 4B, lanes 5 and 6). This indicates a much lower stoichiometry than GFP-PCNTMt2 because immunoblotting is much more sensitive than Sypro Ruby staining. Thus, the majority of GFP-PCNTMt2 was unlikely precipitated via interaction with dynein.
To further corroborate the above-mentioned results, DIC was immunodepleted from the cell lysate using anti-DIC IgM before immunoprecipitation with anti-FLAG mAb (see Materials and Methods). Such a process eliminated 98% of DHC, thus dynein, from the lysate (Figure 4C, lane 3), whereas the mock depletion with only protein G resin had little effect (Figure 4C, lane 2). After the subsequent immunoprecipitation with anti-FLAG antibody, however, a similar amount of GFP-PCNTMt2 was found in the immunoprecipitates regardless of the dramatically different dynein levels (Figure 4C, lanes 5 and 6). These results further confirmed that Nudel was able to associate with pericentrin in a dynein-independent way.
Nudel Depletion Markedly Reduces Centrosome Targeting of Its Associated Proteins
To further pinpoint roles of Nudel at the centrosome, vector-based RNA interference (van de Wetering et al., 2003) was performed to repress its expression. Transfection of pTER-Nudi, a plasmid designed to express a small interference RNA against human Nudel mRNA, efficiently inhibited exogenous Nudel expression but not NudE in HEK293T cells (Figure 5A, lanes 2 and 4), whereas the vector pTER had no effect on either protein (Figure 5A, lanes 1 and 3). pTER-Nudi also repressed endogenous Nudel by 64.0 ± 3.0 or 92.8 ± 1.2% after transfection for 48 or 72 h, respectively, compared with the levels in vector-transfected samples (Figure 5B). In contrast, the levels of PCM-1, pericentrin, p150glued, DIC, and γ-tubulin were not affected.
Figure 5.
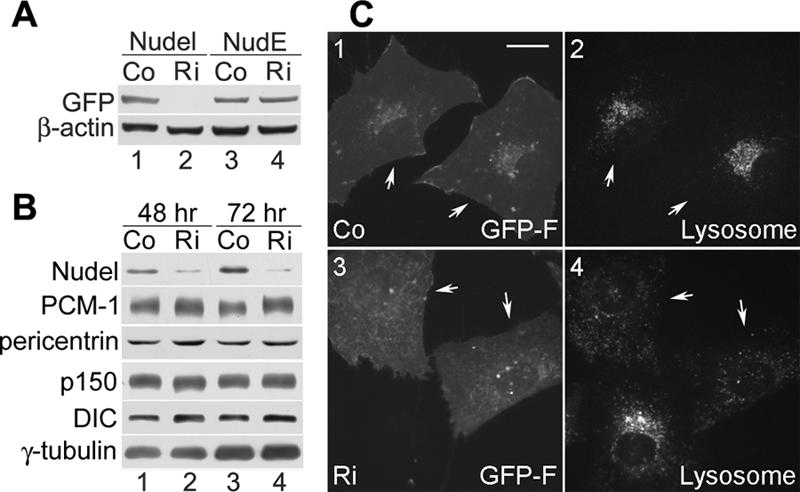
Silencing Nudel by plasmid-based RNAi. (A) Specificity of RNAi. Plasmid for expression of either GFP-Nudel (lanes 1 and 2) or GFP-NudE (lanes 3 and 4) was cotransfected with pTER (Co) or pTER-Nudi (Ri) at a ratio of 1:10 into HEK293T cells. Cell lysates were prepared 48 h after transfection and subjected to immunoblotting with anti-GFP and anti-β-actin antibodies. (B) Efficiency of RNAi on endogenous Nudel. HEK293T cells transfected with pTER or pTER-Nudi for 48 or 72 h were subjected to 3-12% gradient SDS-PAGE followed by immunoblotting to visualize the indicated proteins. (C) Silencing Nudel causes dispersed lysosome distribution. pEGFP-F was cotransfected with pTER or pTER-Nudi at a ratio of 1:10 into HeLa cells to express GFP-F as a transfection marker. Lysosomes were labeled with LysoTracker Red 72 h after transfection. Arrows indicate transfectants. Bar, 20 μm.
We then examined lysosome distributions to assess dynein activity in Nudel-depleted HeLa cells (Liang et al., 2004). For easy identification of transfectants, GFP-F, a membrane-attached isoform of GFP (Jiang and Hunter, 1998), was used as the transfection marker. When pEGFP-F was cotransfected with pTER-Nudi at a ratio of 1:10, lysosomes were indeed dispersed in GFP-F expressors 72 h posttransfection (Figure 5C, panels 3 and 4). In contrast, GFP-F-positive cells cotransfected with pTER still exhibited normal perinuclear lysosome distributions (Figure 5C, 1 and 2). Therefore, silencing Nudel expression alone in HeLa cells was sufficient to impair dynein activity in membrane traffic.
Next, we examined whether Nudel repression affected distributions of centrosomal proteins. The control transfection had little effect on tested proteins (Figure 6, A and C). In contrast, in populations cotransfected with pTER-Nudi and pEGFP-F, 97 ± 1% of GFP-F positive HeLa cells (n = 400, two experiments) exhibited little or only weak centrosomal Nudel staining (our unpublished data), suggestive of Nudel depletion. Moreover, centrosomal Lis1, pericentrin, and p150glued were disrupted (Figure 6, B and C). The centriolar satellites labeled with PCM-1 also became dispersed in the cytoplasm (Figure 6, B and C), although a small fraction of PCM-1 still localized at the centrosome (Figure 6B, 7-9, arrowhead in the GFP-F-positive cell). γ-Tubulin was also affected. Its centrosomal staining was not seen in 20 ± 3% of pTER-Nudi transfectants (n = 200), compared with 5 ± 3% in control cells (Figure 6C). Another 56 ± 1% of transfectants exhibited significantly diminished centrosomal γ-tubulin (Figure 6B, 9, arrowhead in the GFP-F-positive cell). Quantitation indicated a reduction by 70 ± 11% on average (n = 30) compared with the average centrosome intensity of the remainder of 24 ± 1% transfectants whose γ-tubulin staining showed little difference to that of the surrounding untransfected cells. In control transfectants, however, only 14 ± 1% showed significantly reduced centrosomal γ-tubulin.
Figure 6.
Nudel depletion attenuates centrosomal pericentrin, p150glued, PCM-1, Lis1, and γ-tubulin. (A and B) Effects of Nudel depletion on other centrosomal proteins. pEGFP-F was cotransfected with pTER (A) or pTER-Nudi (B) at a ratio of 1:10 into HeLa cells. After 72 h, cells were processed to visualize the indicated proteins. Arrows indicate transfected cells, whereas arrowheads point to centrosomes. Bar, 20 μm. (C) Statistics for centrosomal localization of the indicated proteins. n = 200, two experiments. (D) Dynamitin overexpression does not affect γ-tubulin and pericentrin. HeLa cells were transfected for 48 h to express GFP-p50 before fixation. Bar, 20 μm.
The centriolar satellites and dynactin are targeted to centrosomes mainly through dynein-mediated transport (Zimmerman and Doxsey, 2000; Quintyne and Schroer, 2002). Reports differ, however, regarding whether pericentrin and γ-tubulin rely on dynein (Khodjakov and Rieder, 1999; Quintyne et al., 1999; Young et al., 2000; Dammermann and Merdes, 2002; Kim et al., 2004). In our hands, overexpressing GFP-dynamitin in HeLa cells affected neither centrosomal γ-tubulin nor pericentrin (Figure 6D), although it caused lysosome dispersions indicative of dynein inactivation in 86 ± 3% (n = 200) of expressors (Burkhardt et al., 1997). Together, pericentrin and a major portion of γ-tubulin required Nudel but not dynein for their centrosomal localizations in interphase HeLa cells.
Silencing Nudel Attenuates MT Nucleation and Anchoring
Centrosomal γ-tubulin and pericentrin are essential for MT nucleation from the centrosome, whereas centrosomal localization of dynactin is crucial for subsequent anchoring of MTs to this organelle (Quintyne et al., 1999; Job et al., 2003). We therefore examined the MT organizations in Nudel-depleted cells. Sixty-nine hours posttransfection, cells were treated with nocodazole for 3 h to completely disassemble MTs (our unpublished data). At 3 min after removal of the drug, 88 ± 3% of control transfectants (n = 200) exhibited bright asters with short nascent MTs (Figure 7A). At 8 min, a similar amount of transfectants (83 ± 4%) showed clear asters with longer radiant MTs over a background of randomly polymerized MTs (Figure 7A). At 60 min, MT arrays were fully established, and 73 ± 4% of transfectants showed radial arrays apparently focusing at the MTOC (Figure 7A). Untransfected cells showed similar kinetics (Figure 7, A and B). In contrast, only ∼60% pTER-Nudi transfectants showed asters at 3 or 8 min (n = 200). In the remaining 40%, asters were not obvious (Figure 7B). At 20 min, a radial MT array was seen in most untransfected cells and in 80 ± 6% control transfectants (our unpublished data). In pTER-Nudi transfectants, however, the value was 50 ± 6% (our unpublished data). At 60 min, only 27 ± 3% pTER-Nudi transfectants displayed radial MT arrays. MTs failed to focus at the MTOC in the majority of cells (Figure 7B). Therefore, pTER-Nudi transfectants exhibited weaker MT nucleation activity correlated with reduced centrosomal γ-tubulin, and the nucleated MTs tended to detach from the centrosome over time (Figures 6 and 7C).
Figure 7.
Nudel depletion attenuates MT nucleation and anchoring. (A and B) MT regrowth assays. pEGFP-F was cotransfected with pTER (A) or pTER-Nudi (B) at a ratio of 1:10 into HeLa for 72 h. Cells were then treated with nocodazole (5 μg/ml) for additional 3 h to disassemble MTs. After removal of the drug, cells were incubated at 37°C again and fixed at the indicated time. Arrows indicate transfected cells, whereas arrowheads point to MTOC. Bar, 20 μm. (C) Graph showing percentage of cells with radial MT organization at different time points after release from nocodazole treatment. n = 200, two experiments.
DISCUSSION
Nudel Contributes to MT Anchoring as a Dynamic Mother Centriole Protein Upstream of Dynein/Dynactin
We find that centrosomal Nudel is associated with the mother centriole. In G0 or G1 phase, the mother centriole is distinguished from the daughter at the ultrastructural level mainly by its appendages (Doxsey, 2001; Bornens, 2002). So far, several proteins, including ninein, dynactin, cenexin, ε-tubulin, and centriolin, have been found to localize to the mother centriole (Lange and Gull, 1995; Piel et al., 2000; Quintyne and Schroer, 2002; Gromley et al., 2003). Association of Nudel with the mother centriole was determined through comparison with centrosomal ninein, dynactin, and centrin-2, a centriole marker, and was confirmed by its colocalization with the centriole that anchors MTs (Figure 1) (Piel et al., 2000). Moreover, although ninein seems to associate constitutively with the mother centriole (Piel et al., 2000), Nudel and dynactin showed slightly different behaviors: During the centriole duplication, Nudel is recruited to the new mother centriole earlier than dynactin but later than ninein (Figure 1).
Nudel mainly targets to the mother centriole independently of MTs and dynactin/dynein. Although it can be transported to centrosomes by dynein (Yan et al., 2003), inactivating dynein by dynamitin overexpression or disassembling MTs with nocodazole only showed little or weak influence on its centrosome levels in both Cos7 and HeLa cells (Figure 2; our unpublished data). Centrosomal localization of NudelN20, whose overexpression also inactivates dynein (Yan et al., 2003; Liang et al., 2004), again suggests that dynein activity is dispensable (Figure 3A). The rapid turnover of centrosomal Nudel even in the presence of nocodazole (Figure 2) further strengthens this notion.
Nudel is involved in MT anchoring at the centrosome. We found that repressing Nudel in HeLa cells resulted in loss of MT focus (Figure 7). This was correlated with loss of centrosomal dynactin (Figure 6), which is also critical for MT anchoring (Quintyne et al., 1999). Ninein distributions, however, were not disturbed (Figure 6). Thus, Nudel and dynactin, perhaps dynein and Lis1 also (Smith et al., 2000; Malikov et al., 2004), are likely to function in the same pathway of MT anchoring. Nudel represents the most upstream factor. In addition, centrosomal Nudel overlapped with dynactin more closely than ninein (Figures 1, F and G), suggesting that the dynein pathway proteins may locate on the mother centriole differently than ninein.
Together, we propose that Nudel may associate with the wall of the mother centriole and then recruits Lis1, dynactin, and dynein in an MT-dependent manner (Figure 8A). The complex may play a role in transport of crescent MTs to be anchored to the subdistal appendages (Figure 8A) (Bornens, 2002). However, details on the mechanism are unknown. In G0/G1 stages, centrosomal dynein is undetectable (Quintyne and Schroer, 2002), and dynactin may associate with Nudel through Lis1, which binds to the dynamitin subunit (Tai et al., 2002). The p150glued subunit then anchors MTs (Waterman-Storer et al., 1995). In S/G2 phases, dynein also remains at the centrosome through dynactin and may serve as an MT anchor as well (Quintyne and Schroer, 2002; Malikov et al., 2004).
Figure 8.
Models delineating roles of Nudel at the centrosome. (A) Nudel recruits Lis1, dynactin, and dynein to the mother centriole and may transport MTs to be anchored to the subdistal appendages. See text for detailed descriptions. (B) Nudel functions in both dynein-dependent and -independent centrosome protein assembly. Nudel activates dynein for centripetal transport of Lis1, dynactin, and PCM-1 (a). It also exhibits rapid turnover between cytosol and PCM (b) and facilitates centrosome targeting of pericentrin through direct interaction (c). Centrosomal targeting of γ-tubulin is promoted possibly through association with percentrin (d). It should be noted that pericentrin and γ-tubulin can also be transported by dynein (Young et al., 2000). See text for detailed discussions.
Nudel Functions in Both Dynein-dependent and -independent Centrosome Protein Assembly
Nudel is critical for dynein-mediated centrosome protein assembly (Figure 8B). Although dynactin binds centrosomes in G0/G1 in the absence of centrosomal dynein, its centrosomal assembly relies on dynein-mediated transport along MTs (Quintyne and Schroer, 2002). The pericentriolar particles are also transported by dynein (Kubo et al., 1999), presumably through the Bardet-Biedl syndrome protein BBS4 that binds both PCM-1 and p150glued (Kim et al., 2004). Therefore, associations of Nudel with dynactin and PCM-1 (Figure 4A) are likely mediated by dynein (Figure 8B) (Sasaki et al., 2000; Liang et al., 2004). In the presence of NudelC36 or absence of Nudel, dynein is inactivated (Figure 5) (Liang et al., 2004). Loss or attenuation of centrosomal dynactin and PCM-1 (Figures 3 and 6) was thus mainly attributed to lack of dynein-mediated transport. The centrosomal localization of Lis1 is sensitive to MT assembly (Tanaka et al., 2004). Therefore, such a localization is probably dynein dependent, although Lis1 binds Nudel directly in addition to dynein (Sasaki et al., 2000). Disruption of the localization upon Nudel depletion or NudelC36 overexpression (Figures 3B and 6) should be attributed to inactivation of dynein as well.
Nudel is also important for dynein-independent protein assembly. Centrosomal assembly of pericentrin and γ-tubulin are usually considered dynein dependent (Purohit et al., 1999; Young et al., 2000; Zimmerman and Doxsey, 2000). Nevertheless, their centrosomal localizations are not as sensitive to dynein inactivation as dynactin and PCM-1 (Quintyne et al., 1999; Dammermann and Merdes, 2002; Kim et al., 2004), suggesting existence of dynein-free mechanisms for them. A solid evidence is that centrosomal γ-tubulin in the presence or absence of nocodazole exhibits similar rapid turnover in PtK1 cells (Khodjakov and Rieder, 1999). Consistently, in our hands, centrosomal pericentrin and γ-tubulin in Cos7 or HeLa cells were rarely affected by inactivation of dynein upon overexpression of either dynamitin or NudelC36 (Figures 3A and F, and 6D). Therefore, their significantly reduced centrosomal signals upon Nudel depletion (Figure 6) could only be attributed to dynein-independent activities of Nudel for their centrosomal assembly. Two lines of evidence for dynein-insensitive association of pericentrin and Nudel further strengthened such a conclusion. First, an overexpressed pericentrin mutant was able to associate with Nudel in much higher stoichiometry than dynein (Figure 4B). Second, immunodepletion of dynein had little effect on pericentrin-Nudel interaction (Figure 4C). Because NudE, with which Nudel shares many properties (Figure 4A) (Yan et al., 2003; Liang et al., 2004), physically binds pericentrin in vivo (Feng et al., 2000) (Figure 4A), Nudel may modulate the latter's centrosomal localization through direct interaction as well (Figure 8B). γ-tubulin, on the other hand, might be linked to dynein-free Nudel by pericentrin (Dictenberg et al., 1998; Zimmerman et al., 2004) or other unidentified proteins (Figure 4B). In NudelC36 expressors, pericentrin and γ-tubulin are recruited to the centrosome, probably through endogenous Nudel.
We found that Nudel used overlapping C-terminal domains to directly or indirectly associate with many centrosomal proteins (Figure 4A) (Liang et al., 2004). Because NudelC36 was not centrosomal (Figure 3), proteins that tethered Nudel to the mother centriole were also expected to bind such domains independently of dynein. The identity of these proteins, however, still remains unknown. γ-tubulin and pericentrin exhibited a broader distribution pattern at the centrosome than Nudel (Figure 1) (Bornens, 2002). They are thus unlikely centrosomal anchors for Nudel, and vice versa. Instead, Nudel might function to facilitate their assembly by shuttling protein complexes dynamically between the cytosol and PCM (Figures 2 and 8B).
Acknowledgments
We thank Qiongping Huang, Wei Yu, Yan Li, Chong Ding, and Wei Bian for technical assistance and acknowledge Dr. Friedhelm Hildebrandt (University of Michigan, Ann Arbor, MI), Dr. Naihe Jing (Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai, China), and the anonymous reviewers for helpful comments. We are grateful to Drs. Stephen J. Doxsey, Gordon Chan, and Andreas Merdes for kindly providing antibodies to pericentrin, ninein, and PCM-1, respectively. We are also in debt to Drs. Jeffrey L. Salisbury and M. Bornens for gifts of mouse and rabbit antibodies to centrin-2 and to Dr. Hisashi Koga (Kazusa DNA Research Institute, Chiba, Japan) for pericentrin-2 cDNA. This work was supported by Grants 30330330, 30421005, and 30270665 from the National Science Foundation of China; Grants 2002CB713802 and 2005CB522701 from the Ministry of Science and Technology of China; and Grant S048014317 from the Council of Shanghai Municipal for Science and Technology.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-04-0360) on November 16, 2005.
Abbreviations used: DHC, dynein heavy chain; DIC, dynein intermediate chain; FRAP, fluorescence recovery after photobleaching; MT, microtubule; MTOC, microtubule-organizing center; PCM, pericentriolar material; RNAi, RNA interference.
References
- Blagden, S. P., and Glover, D. M. (2003). Polar expeditions-provisioning the centrosome for mitosis. Nat. Cell Biol. 5, 505-511. [DOI] [PubMed] [Google Scholar]
- Bornens, M. (2002). Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14, 25-34. [DOI] [PubMed] [Google Scholar]
- Burkhardt, J. K., Echeverri, C. J., Nilsson, T., and Vallee, R. B. (1997). Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139, 469-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann, A., and Merdes, A. (2002). Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 159, 255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictenberg, J. B., Zimmerman, W., Sparks, C. A., Young, A., Vidair, C., Zheng, Y., Carrington, W., Fay, F. S., and Doxsey, S. J. (1998). Pericentrin and gamma-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 141, 163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey, S. (2001). Re-evaluating centrosome function. Nat. Rev. Mol. Cell. Biol. 2, 688-698. [DOI] [PubMed] [Google Scholar]
- Doxsey, S. J., Stein, P., Evans, L., Calarco, P. D., and Kirschner, M. (1994). Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell 76, 639-650. [DOI] [PubMed] [Google Scholar]
- Dujardin, D. L., and Vallee, R. B. (2002). Dynein at the cortex. Curr. Opin. Cell Biol. 14, 44-49. [DOI] [PubMed] [Google Scholar]
- Echeverri, C. J., Paschal, B. M., Vaughan, K. T., and Vallee, R. B. (1996). Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132, 617-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y., Olson, E. C., Stukenberg, P. T., Flanagan, L. A., Kirschner, M. W., and Walsh, C. A. (2000). LIS1 regulates CNS lamination by interacting with mNudE, a central component of the centrosome. Neuron 28, 665-679. [DOI] [PubMed] [Google Scholar]
- Feng, Y., and Walsh, C. A. (2004). Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron 44, 279-293. [DOI] [PubMed] [Google Scholar]
- Flory, M. R., and Davis, T. N. (2003). The centrosomal proteins pericentrin and kendrin are encoded by alternatively spliced products of one gene. Genomics 82, 401-405. [DOI] [PubMed] [Google Scholar]
- Gromley, A., Jurczyk, A., Sillibourne, J., Halilovic, E., Mogensen, M., Groisman, I., Blomberg, M., and Doxsey, S. (2003). A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J. Cell Biol. 161, 535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, B. J., Hoffman, D. B., Fang, G., Murray, A. W., and Salmon, E. D. (2000). Visualization of Mad2 dynamics at kinetochores, along spindle fibers, and at spindle poles in living cells. J. Cell Biol. 150, 1233-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, W., and Hunter, T. (1998). Analysis of cell-cycle profiles in transfected cells using a membrane-targeted GFP. Biotechniques 24, 349-350, 352, 354 [PubMed] [Google Scholar]
- Job, D., Valiron, O., and Oakley, B. (2003). Microtubule nucleation. Curr. Opin. Cell Biol. 15, 111-117. [DOI] [PubMed] [Google Scholar]
- Karki, S., and Holzbaur, E. L. (1999). Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr. Opin. Cell Biol. 11, 45-53. [DOI] [PubMed] [Google Scholar]
- Khodjakov, A., and Rieder, C. L. (1999). The sudden recruitment of gamma-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 146, 585-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. C., et al. (2004). The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat. Genet. 36, 462-470. [DOI] [PubMed] [Google Scholar]
- King, S. J., and Schroer, T. A. (2000). Dynactin increases the processivity of the cytoplasmic dynein motor. Nat. Cell Biol. 2, 20-24. [DOI] [PubMed] [Google Scholar]
- Kubo, A., Sasaki, H., Yuba-Kubo, A., Tsukita, S., and Shiina, N. (1999). Centriolar satellites: molecular characterization, ATP-dependent movement toward centrioles and possible involvement in ciliogenesis. J. Cell Biol. 147, 969-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo, A., and Tsukita, S. (2003). Non-membranous granular organelle consisting of PCM-1, subcellular distribution and cell-cycle-dependent assembly/disassembly. J. Cell Sci. 116, 919-928. [DOI] [PubMed] [Google Scholar]
- Lange, B. M., and Gull, K. (1995). A molecular marker for centriole maturation in the mammalian cell cycle. J. Cell Biol. 130, 919-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Y., Yu, W., Li, Y., Yang, Z., Yan, X., Huang, Q., and Zhu, X. (2004). Nudel functions in membrane traffic mainly through association with Lis1 and cytoplasmic dynein. J. Cell Biol. 164, 557-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malikov, V., Kashina, A., and Rodionov, V. (2004). Cytoplasmic dynein nucleates microtubules to organize them into radial arrays in vivo. Mol. Biol. Cell 15, 2742-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen, M. M., Malik, A., Piel, M., Bouckson-Castaing, V., and Bornens, M. (2000). Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J. Cell Sci. 113, 3013-3023. [DOI] [PubMed] [Google Scholar]
- Okazaki, N., Kikuno, R., Ohara, R., Inamoto, S., Aizawa, H., Yuasa, S., Nakajima, D., Nagase, T., Ohara, O., and Koga, H. (2003). Prediction of the coding sequences of mouse homologues of KIAA gene: II. The complete nucleotide sequences of 400 mouse KIAA-homologous cDNAs identified by screening of terminal sequences of cDNA clones randomly sampled from size-fractionated libraries. DNA Res. 10, 35-48. [DOI] [PubMed] [Google Scholar]
- Ou, Y., and Rattner, J. B. (2004). The centrosome in higher organisms: structure, composition, and duplication. Int. Rev. Cytol. 238, 119-182. [DOI] [PubMed] [Google Scholar]
- Paoletti, A., Moudjou, M., Paintrand, M., Salisbury, J. L., and Bornens, M. (1996). Most of centrin in animal cells is not centrosome-associated and centrosomal centrin is confined to the distal lumen of centrioles. J. Cell Sci. 109, 3089-3102. [DOI] [PubMed] [Google Scholar]
- Piel, M., Meyer, P., Khodjakov, A., Rieder, C. L., and Bornens, M. (2000). The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 149, 317-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit, A., Tynan, S. H., Vallee, R., and Doxsey, S. J. (1999). Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 147, 481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne, N. J., Gill, S. R., Eckley, D. M., Crego, C. L., Compton, D. A., and Schroer, T. A. (1999). Dynactin is required for microtubule anchoring at centrosomes. J. Cell Biol. 147, 321-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne, N. J., and Schroer, T. A. (2002). Distinct cell cycle-dependent roles for dynactin and dynein at centrosomes. J. Cell Biol. 159, 245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury, J. L., Suino, K. M., Busby, R., and Springett, M. (2002). Centrin-2 is required for centriole duplication in mammalian cells. Curr. Biol. 12, 1287-1292. [DOI] [PubMed] [Google Scholar]
- Sasaki, S., Shionoya, A., Ishida, M., Gambello, M. J., Yingling, J., Wynshaw-Boris, A., and Hirotsune, S. (2000). A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron 28, 681-696. [DOI] [PubMed] [Google Scholar]
- Shu, T., Ayala, R., Nguyen, M. D., Xie, Z., Gleeson, J. G., and Tsai, L. H. (2004). Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron 44, 263-277. [DOI] [PubMed] [Google Scholar]
- Smith, D. S., Niethammer, M., Ayala, R., Zhou, Y., Gambello, M. J., Wynshaw-Boris, A., and Tsai, L. H. (2000). Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat. Cell Biol. 2, 767-775. [DOI] [PubMed] [Google Scholar]
- Tai, C. Y., Dujardin, D. L., Faulkner, N. E., and Vallee, R. B. (2002). Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J. Cell Biol. 156, 959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, T., Serneo, F. F., Higgins, C., Gambello, M. J., Wynshaw-Boris, A., and Gleeson, J. G. (2004). Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J. Cell Biol. 165, 709-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering, M., Oving, I., Muncan, V., Pon Fong, M. T., Brantjes, H., van Leenen, D., Holstege, F. C., Brummelkamp, T. R., Agami, R., and Clevers, H. (2003). Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep. 4, 609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer, C. M., Karki, S., and Holzbaur, E. L. (1995). The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1). Proc. Natl. Acad. Sci. USA 92, 1634-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynshaw-Boris, A., and Gambello, M. J. (2001). LIS1 and dynein motor function in neuronal migration and development. Genes Dev. 15, 639-651. [DOI] [PubMed] [Google Scholar]
- Yan, X., Li, F., Liang, Y., Shen, Y., Zhao, X., Huang, Q., and Zhu, X. (2003). Human Nudel and NudE as regulators of cytoplasmic dynein in poleward protein transport along the mitotic spindle. Mol. Cell. Biol. 23, 1239-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. Y., Guo, J., Li, N., Qian, M., Wang, S. N., and Zhu, X. L. (2003). Mitosin/CENP-F is a conserved kinetochore protein subjected to cytoplasmic dynein-mediated poleward transport. Cell Res. 13, 275-283. [DOI] [PubMed] [Google Scholar]
- Young, A., Dictenberg, J. B., Purohit, A., Tuft, R., and Doxsey, S. J. (2000). Cytoplasmic dynein-mediated assembly of pericentrin and gamma tubulin onto centrosomes. Mol. Biol. Cell 11, 2047-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, W., and Doxsey, S. J. (2000). Construction of centrosomes and spindle poles by molecular motor-driven assembly of protein particles. Traffic 1, 927-934. [PubMed] [Google Scholar]
- Zimmerman, W. C., Sillibourne, J., Rosa, J., and Doxsey, S. J. (2004). Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell 15, 3642-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]









