Abstract
Dictyostelium DdINCENP is a chromosomal passenger protein associated with centromeres, the spindle midzone, and poles during mitosis and the cleavage furrow during cytokinesis. Disruption of the single DdINCENP gene revealed important roles for this protein in mitosis and cytokinesis. DdINCENP null cells lack a robust spindle midzone and are hypersensitive to microtubule-depolymerizing drugs, suggesting that their spindles may not be stable. Furthermore DdCP224, a protein homologous to the microtubule-stabilizing protein TOGp/XMAP215, was absent from the spindle midzone of DdINCENP null cells. Overexpression of DdCP224 rescued the weak spindle midzone defect of DdINCENP null cells. Although not required for the localization of the myosin II contractile ring and subsequent formation of a cleavage furrow, DdINCENP is important for the abscission of daughter cells at the end of cytokinesis. Finally, we show that the localization of DdINCENP at the cleavage furrow is modulated by myosin II but it occurs by a mechanism different from that controlling the formation of the contractile ring.
INTRODUCTION
Successful cell division requires tight coordination between the events of mitosis and those of cytokinesis. Late in mitosis, the contractile ring assembles at the equator of a dividing cell and is activated to constrict only when the daughter nuclei are safely separated. Simultaneously, membrane trafficking pathways deliver membranes and components necessary for furrow ingression and cell fission (O'Halloran, 2000). It is generally agreed that the spatial and temporal organization of the cytokinetic machinery is controlled by the microtubules of the mitotic apparatus. Depending on the cell type and cell geometry, either spindle microtubules or astral microtubules play key roles in controlling cytokinesis. However, it is not currently known whether the different components involved in cytokinesis are recruited to the cleavage furrow by a single mechanism or multiple converging mechanisms.
An interesting group of proteins found at the cleavage furrow are the chromosomal passenger proteins (Adams et al., 2001a). Chromosomal-passenger proteins typically localize in the nucleus during interphase and transfer to the chromosomes during prophase. By prometaphase, they begin to concentrate at the inner-centromeric regions of sister chromatids, where they reside throughout metaphase. At the onset of anaphase, chromosomal-passenger proteins transfer to the central spindle and remain through telophase. During cytokinesis, they are found at the midbody and the cortex region of the cleavage furrow. Several chromosomal-passenger proteins have been discovered, including INCENP, Aurora B, Survivin, TD-60, and Dasra/Borealin (Mollinari et al., 2003; Gassmann et al., 2004; Sampath et al., 2004). INCENP, Aurora B, and Survivin have been found to interact with each other forming a chromosomal passenger complex (Kang et al., 2001; Bolton et al., 2002; Cheeseman et al., 2002). This protein complex also interacts with Dasra/Borealin in both Xenopus and human cells (Gassmann et al., 2004; Sampath et al., 2004).
Considering their dynamic distribution during mitosis, it is not surprising that chromosomal-passenger proteins are emerging as critical regulators of mitosis. They have important functions in such diverse aspect of mitosis as chromosome condensation, metaphase chromosome congression, kinetochore bipolar spindle attachment, proper chromosome segregation and checkpoint control (Mackay et al., 1998; Kim et al., 1999; Giet and Glover, 2001; Cheeseman et al., 2002; Tanaka et al., 2002; Gassmann et al., 2004; Sampath et al., 2004). Additionally, chromosomal-passenger proteins also play a prominent role during cytokinesis (Adams et al., 2001a).
INCENP, the first identified chromosome passenger protein, has been found in a wide range of species from yeast to mammals. The most significant homology among these INCENP proteins is the C-terminal IN-box domain, which is involved in binding to Aurora B kinase (Kang et al., 2001; Bolton et al., 2002). INCENP has important functions during mitosis. It can stimulate the kinase activity of Aurora B and is also a substrate of Aurora B kinase (Kang et al., 2001; Honda et al., 2003). In both Drosophila and Caenorhabditis elegans, depletion of INCENP by RNAi causes failed chromosome congression at metaphase, mislocalization of Aurora B, and cytokinesis defects (Kaitna et al., 2000; Adams et al., 2001b). Also, in Xenopus mitotic egg extracts, immunodepletion of INCENP induced the formation of monopolar spindles (Sampath et al., 2004). Nonetheless, except as a possible regulator of Aurora B kinase activity, the mechanism of INCENP's function during mitosis is largely unknown (Kang et al., 2001; Bishop and Schumacher, 2002; Honda et al., 2003). INCENP is also known to play an essential role during cytokinesis. Two dominant-negative mutants of INCENP cause cytokinesis defects in mammalian cells (Eckley et al., 1997; Mackay et al., 1998). Additionally, in C. elegans embryos and Drosophila cultured cells, depletion of INCENP causes a cytokinesis defect (Kaitna et al., 2000; Giet and Glover, 2001). Some of the requirements for INCENP during cytokinesis may be mediated by its partner, Aurora B. Aurora B can phosphorylate MgcRacGAP, a RhoA GAP important for the completion of cytokinesis (Minoshima et al., 2003).
To explore the role of INCENP in mitosis and cytokinesis in more detail, we cloned and disrupted the corresponding gene in Dictyostelium, DdINCENP. We show here that DdINCENP plays important roles during both mitosis and cytokinesis. Importantly, we found that the localization of INCENP at the cleavage furrow is strongly influenced by myosin II but it occurs by a mechanism distinct from that controlling the formation of the actomyosin contractile ring.
MATERIALS AND METHODS
Cloning of DdINCENP cDNA and Construction of GFP-DdINCENP
A full-length cDNA of DdINCENP was constructed by the polymerase chain reaction (PCR) in two separate fragments as follows. A 0.7-kb N-terminal cDNA fragment of the gene was amplified from a Dictyostelium cDNA library using primers AO420 (5′-ATGGATTTTATTAAAAAAAATACAATTAATAGAATCAATGGGTTACC-3′) and AO441 (5′-GTGAAACAGCAATTGATTGAAACTCATGATAAAC-3′). A 3.2-kb C-terminal segment of the gene was generated from the AX2 genome DNA by using primers AO442 (5′-GTTTATCATGAGTTTCAATCAATTGCTGTTTCAC-3′) and AO387 (5′-CTCGAGTTATTTTTTATTAACAATGATTGGATTAGTAAAACCC-3′). These two segments were ligated to generate the full-length cDNA of DdINCENP. The GFP-DdINCENP construct was generated by cloning the DdINCENP cDNA downstream of the GFP sequence in the pTXGFP plasmid.
Cell Culture, Transformation, and Constructs
Three different parental cell lines were used in these experiments: AX2, NC4A2, and ORF+. The cells were cultured with HL-5 medium in Petri dishes at 19°C. When the cells were tested for sensitivity to the microtubule-depolymerizing drugs, they were grown in 50-ml flasks on a shaker at 200 rpm. The cultures all started at 2 × 105 cells/ml.
The GFP-DdCP224 construct is a generous gift from Dr. Graf (Graf et al., 2000). The fusion protein is driven by the constitutive actin 15 promoter. The GFP-α-tubulin construct is a gift from Dr. Gerisch (Neujahr et al., 1998). GFP-myosin construct was a gift from Dr. Spudich. All these three plasmids carry a G418-resistance cassette. The constructs were introduced into the cells with electroporation and the transformants were selected in the HL-5 medium with 10 μg/ml G418. Both thiabendazole and nocodazole are from Sigma Chemical Co. (St. Louis, MO).
Disruption of the DdINCENP Gene by Homologous Recombination
DdINCENP null cells were generated by homologous recombination between a DdINCENP knockout construct and the DdINCENP coding sequence on the genome. The knockout construct consisted of a blasticidin-resistance (BSR) cassette flanked by 5′ and 3′ fragments of the DdINCENP gene. The 1-kb 5′ fragment was amplified by AO386 (5′-GAGCTCGGTATTGCAAAGCCAACACCACTTAC-3′) and AO402 (5′-GGTACCGTTGCTGATGCTGTATTAGCAGC-3′), and the 1-kb 3′ fragment was amplified by AO403 (5′-AAGCTTGACGTTAATCAAAGTACAAAAGATAAATC-3′) and AO387 (5′-CTCGAGTTATTTTTTATTAACAATGATTGGATTAGTAAAACCC-3′). Both fragments were cloned into plasmid pSP72-Bsr. The construct was linearized by SacI and XhoI before its introduction into AX2, NC4A2, or ORF+ cells via electroporation. The transformants were then cultured on 96-well plates. They were screened by PCR and confirmed by Western blotting with rabbit anti-INCENP antibodies. A total of six DdINCENP null cell lines were identified. All the phenotypes of the DdINCENP null cells described in the article were verified by examining at least two of the knockout cell lines.
Western Blot Analysis
A GST-DdINCENP676-852 fusion protein was expressed in Escherichia coli and purified according to the previous published protocol (Kwak et al., 1999). The purified fusion protein was injected into rabbits to raise polyclonal anti-DdINCENP antibodies (Cocalico Biologicals, Reamstown, PA). For the Western blot analysis of DdINCENP, whole cell lysates of 1 × 106 cells were loaded in each lane and separated on a 6% SDS-PAGE gel (Koonce and McIntosh, 1990).
Microscopy of Live Cells
For live microscopy of mitotic cells, cells in log phase growth were plated on a small Petri dish with a coverslip on the bottom (MatTek, Ashland, MA). After the cells attached to the plate, the medium was removed and replaced with low-fluorescence (LF) medium for at least 30 min before observations (Bretschneider et al., 2002). The live imaging of the cells was conducted by using a Nikon Eclipse TE200 microscope (Nikon Instruments, Dallas, TX) equipped with a 100× 1.4 NA PlanFluor Objective, shuttered illumination, and a Quantix 57 camera (Roper Scientific, Tucson, AZ) controlled by Metamorph (Universal Imaging, West Chester, PA). The exposure time for the GFP fluorescence was 50 ms with the interval time being at least 10 s.
Immunostaining and Microscopy of Fixed Cells
The cells were allowed to attach to the acid-cleaned coverslips overnight in a 8.5-cm Petri dish before the fixation. Then they were fixed according to the protocol published by Dr. McIntosh (Koonce and McIntosh, 1990). Briefly, the cells were fixed at room temperature with 2.5% formaldehyde in a PIPES-EGTA buffer for 3 min followed by 1% formaldehyde in dehydrated methanol at -10°C for 5 min. The anti-alpha-tubulin antibody is a monoclonal antibody from Sigma Chemical Co. Anti-DdCP224 antibody is a gift from Dr. Graf. The second antibody is a Texas red-conjugated goat anti-mouse antibody (Molecular Probes, Eugene, OR). The protocol for DAPI staining was described previously (Gerald et al., 2001).
Coimmunoprecipitation of DdINCENP with DdAurora
We generated rabbit antibodies against the single Dictyostelium Aurora-like kinase gene (GenBank Accession XP_641803) and used them to immunoprecipitate DdAurora according to published protocols (Faix et al., 2001). The immunoprecipitates were probed by Western blot analysis with anti-DdINCENP antibodies.
RESULTS
Identification of the INCENP Homolog in Dictyostelium
Because the IN-box motif is the only sequence conserved among INCENP proteins from different species, we used this motif to search the Dictyostelium genome sequence for potential INCENP homologues. We found that Dictyostelium contains only one gene (entry DDB0189463 in http://dictybase.org; accession XP_647225) that encodes a protein similar to other INCENP proteins. This gene, encodes a predicted protein, named DdINCENP, of 1320 amino acids and 152 kDa. DdINCENP has the conserved C-terminal IN-box domain (Supplementary Figure 1), known to be involved in binding to Aurora B kinase (Kang et al., 2001). This domain includes two highly conserved serine residues that are phosphorylated by Aurora B (Bishop and Schumacher, 2002; Cheeseman et al., 2002; asterisks, Supplementary Figure 1). Within this region DdINCENP is 44% identical to fission yeast INCENP and 21% identical to vertebrate INCENP. DdINCENP also has a coil-coiled domain found in all other INCENP proteins. The function of this domain is not clear because a truncated chicken INCENP protein without the coil-coiled domain still localizes properly (Mackay et al., 1993). Besides these two conserved domains, no other sequence similarity can be discerned when comparing sequences of INCENP proteins from distant species. Although a microtubule-binding domain and a centromere-binding domain have been defined in vertebrate INCENP proteins, these sequences are not conserved in their invertebrate or single-celled organism counterparts (Ainsztein et al., 1998).
To confirm that DdINCENP is in fact an INCENP homolog, we determined whether it displayed the dynamic distribution characteristic of chromosomal passenger proteins during mitosis. For this purpose, DdINCENP tagged with GFP at the N-terminus was introduced into wild-type cells and the movement of GFP-DdINCENP during mitosis was studied by live fluorescence microscopy. To correlate the localization of GFP-DdINCENP with different stages of mitosis we also stained these cells with DAPI, because Dictyostelium cells have a very distinct morphology of condensed DNA during different stages of mitosis (Roos et al., 1984). After examining a large number of mitotic cells expressing GFP-DdINCENP, we concluded that DdINCENP has a pattern of localization during mitosis similar to that of other INCENP proteins (Video 1).
During prometaphase, GFP-DdINCENP concentrated at a few dots adjacent to the condensed chromosomes (Figure 1, A and B). Occasionally, a dot of GFP-DdINCENP could be seen at one end of an isolated chromosome (Figure 1, A and B). Because Dictyostelium chromosomes are known to be telocentric, we interpret these images as representing the centromeric localization of GFP-DdINCENP. At metaphase, GFP-DdINCENP was concentrated at a single dot in the middle of the spindle and was surrounded by the chromosomes aligned on the metaphase plate (Figure 1, C and D). After the onset of anaphase, GFP-DdINCENP moved to the spindle midzone and the spindle poles (Figure 1E). GFP-DdINCENP remained at the spindle midzone and spindle poles through late telophase (Figure 1, F and G). When the cleavage furrow was initiated, GFP-DdINCENP began to concentrate increasingly at the furrow area, especially at the cortex region (Figure 2). As the furrow progressed, the cortical localization of GFP-INCENP at the furrow became even stronger (Figure 2). By the abscission stage of cytokinesis, GFP-INCENP was highly concentrated in the thin cytoplasmic bridge connecting the two daughter cells (Figure 2; also see Video 2). The dynamic localization of GFP-DdINCENP during mitosis and cytokinesis is typical of a chromosomal passenger protein.
Figure 1.
DdINCENP displays dynamic localization during mitosis. (A-G) Fluorescence images of AX2 cells expressing GFP-DdINCENP (green). DNA is shown in blue and tubulin is shown in red. (A and B) During prometaphase, GFP-DdINCENP concentrated at a few dots adjacent to the condensed chromosomes. These dots are consistent with a centromeric localization of DdINCENP. (C and D) During metaphase, GFP-DdINCENP concentrated on a single dot, which was surrounded by the chromosomes aligned on the metaphase plate. The dot may represent the conglomerate of the kinetochores at metaphase. The cell in C was imaged from one pole of the mitotic spindle. The cell in D was imaged from the side of the spindle. Notice that DdINCENP is not found at the spindle poles at this stage. (E-G) From anaphase to telophase, GFP-DdINCENP localized at the spindle poles and the spindle midzone. Also see Video 1. Bar, 5 μm.
Figure 2.
DdINCENP localizes at the cleavage furrow during cytokinesis. An AX2 cell expressing GFP-DdINCENP was followed by time-lapse video microcopy from early telophase. GFP-DdINCENP was especially concentrated on the cortex region of the furrow and the intracellular bridge during cytokinesis. The numbers in images indicate time in seconds. Bar, 5 μm.
Further evidence that DdINCENP is a bona fide INCENP protein also came from the study of the association of DdINCENP with Aurora kinase. The Dictyostelium genome contains a single Aurora-like kinase gene (GenBank Accession XP_641803). We generated antibodies against this predicted protein and used them to immunoprecipitate DdAurora from Dictyostelium extracts. We found that DdINCENP coimmunoprecipitated with DdAurora (Supplementary Figure 2), indicating that both form part of a chromosomal-passenger complex.
DdINCENP Is Important for Mitosis
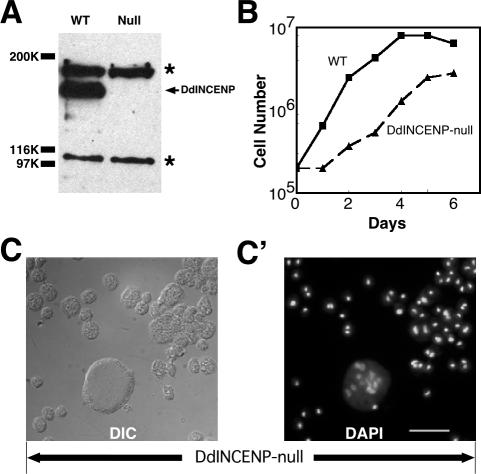
To determine the function of DdINCENP in Dictyostelium, we disrupted the DdINCENP gene by targeted gene knockout and characterized the phenotype of the DdINCENP null cells. The loss of DdINCENP in these cells was confirmed by PCR (unpublished data) and Western blot analysis (Figure 3A). DdINCENP null cells derived from different parental cell lines (AX2, NC4A2, and ORF+) shared a common phenotype: all DdINCENP null cells divided much more slowly than wild-type cells (Figure 3B), and a high percentage of mutant cells became multinucleate (39%, n = 395; Figure 3C). Furthermore, 33% (n = 395) of the mutant cells had very enlarged nuclei, a sign of failed mitosis. This phenotype suggested that the DdINCENP null cells harbored defects in both mitosis and cytokinesis. To further investigate this phenotype, we introduced a GFP-histone 2B construct into DdINCENP null cells to monitor their ability to segregate chromosomes. We found that some DdINCENP null cells had serious chromosome segregation defects; some of them were arrested at metaphase (Video 3), whereas some others had lagging chromosomes (Figure 4 and Video 4). The behavior of chromosomes in the DdINCENP null cells contrasted sharply with that of wild-type cells, which always displayed normal segregation of chromosomes (Graf et al., 2003; Figure 4; see Video 5). The mitotic and the cytokinetic defects displayed by the DdINCENP null cells were consistent with the observation in other organisms that INCENP plays important roles during mitosis (Kaitna et al., 2000; Adams et al., 2001b).
Figure 3.
DdINCENP null cells have mitosis and cytokinesis defects. (A) Immunoblot analysis of whole lysates of wild-type (WT) and DdINCENP null cells (Null) probed with anti-DdINCENP antibodies. The arrow points to the position of the DdINCENP protein (152 kDa) and the asterisk (*) indicates the positions of two unspecific bands recognized by the polyclonal antibodies. (B) Growth curve of the type cells (square solid line) and DdINCENP null cells (triangle dash line) in suspension cultures. DdINCENP null cells grew much more slowly than wild-type cells. (C-C′) A typical population of DdINCENP null cells. The cells were visualized by Differential interference contrast (DIC) microscopy (C) and stained with DAPI to visualize their nuclei (C′). Although some cells contained a single normal nucleus, many cells had multiple nuclei or enlarged nuclei. Bar, 15 μm.
Figure 4.
DdINCENP null cells have chromosome segregation defect. Time-lapse video microscopy of wild-type (top) and DdINCENP null cells (bottom) during mitosis, both of which expressed GFP-histone 2B. The micrographs were taken by fluorescence microscopy to show the movement of chromosomes (as indicated by GFP-histone 2B) from metaphase to anaphase in these two cells. The times are indicated in seconds. As shown, the wild-type cell segregated its chromosomes normally in a short time (86 s; see Video 5). However, the DdINCENP null cell displayed lagging chromosomes (arrows) during the segregation and stalled at anaphase (see Video 4). Other cells were arrested at metaphase for the entire observation period (see Video 3). Bar, 5 μm.
DdINCENP Regulates the Spindle Localization of Microtubule-stabilizing Protein DdCP224
To investigate the mitotic defect of DdINCENP null cells in more detail, we decided to compare the structure of the mitotic spindle of wild-type and mutant cells. Like fungi, the nuclear envelope of Dictyostelium cells does not dismantle during mitosis and the spindle is assembled inside the nucleus. Wild-type cells display robust spindles that have a defined midzone region of overlapping microtubules (Roos et al., 1984; McIntosh et al., 1985; Figure 5, A and B). In contrast, the spindle in most DdINCENP null cells was very thin and the midzone region was difficult to identify (Figure 5, C and D). Some DdINCENP null cells in anaphase or telophase had no perceptible central spindle (Figure 5E). This phenotype indicated that DdINCENP is critical for the assembly or maintenance of the spindle midzone during mitosis.
Figure 5.
DdINCENP null cells have a defective spindle midzone. (A-E) Merged immunofluorescence images of wild-type (A and B) and DdINCENP null cells (C-E) during anaphase, showing DNA in blue and tubulin in red. The arrows point to the position of the spindle midzone in these cells. The mitotic wild-type cells had robust spindle midzone during anaphase. However, the DdINCENP null cells showed weak spindle midzone (D) and sometimes the spindle midzone was imperceptible (C and E). Bar, 5 μm.
Our results agree with previous studies showing a role for the chromosomal passenger protein complex in the stability of the mitotic spindle in yeast (Buvelot et al., 2003; Pereira and Schiebel, 2003). In vertebrate systems, the chromosomal passenger protein complex regulates the localization and activity of MCAK, a microtubule-depolymerizing protein crucial for spindle assembly (Lan et al., 2004; Sampath et al., 2004). The activity of MCAK is antagonized in vivo by XMAP215/TOGp, a microtubule-stabilizing protein (Cassimeris and Morabito, 2004; Holmfeldt et al., 2004). Thus, we considered the possibility that the spindle defect of our DdINCENP null cells may be due to misregulation of these microtubule regulatory proteins.
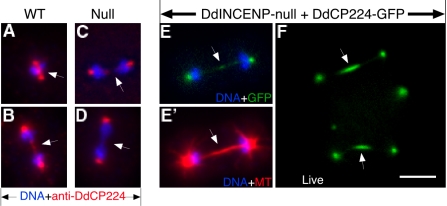
Although the protein MCAK has not been characterized in Dictyostelium, the Dictyostelium homolog of XMAP215, DdCP224, is a centrosome-localized protein known to be crucial for the stability of microtubules during interphase. During mitosis, DdCP224 localizes at both spindle pole bodies and the spindle midzone (Figure 6, A and B; Graf et al., 2000, 2003). We found that DdCP224 localized normally at the interphase centrosomes in the DdINCENP null cells. However, although DdCP224 still localized at the spindle pole bodies of these cells, it was absent from their spindle midzone (Figure 6, C and D). The absence of DdCP224 at the spindle midzone could underlie the spindle defect of DdINCENP null cells. To investigate this possibility, we tested whether enhanced expression of DdCP224 could stabilize the spindle midzone of DdINCENP null cells. We introduced into our mutant cells a vector for the overexpression of GFP-tagged DdCP224 (Graf et al., 2000) and found that this protein was localized on both the spindle pole bodies and the spindle midzone of dividing cells (Figure 6, E and F). Furthermore, the spindle midzone of these DdINCENP null cells was now as robust (9/10 cells) as those observed in wild-type cells (Figure 6E′). Therefore, DdINCENP is essential for the localization of DdCP224 at the spindle midzone and DdCP224 is a likely downstream target of the chromosome passenger protein complex for stabilizing the spindle midzone.
Figure 6.
DdINCENP is essential for the localization of DdCP224, an XMAP215/TOGp homologue, to the spindle midzone. (A-D) Merged immunofluorescence images of wild-type cells (A and B) and DdINCENP null cells (C and D) during anaphase. DdCP224 localized at the spindle poles and the spindle midzone during mitosis in wild-type cells. However, DdCP224 was absent from the spindle midzone in the DdINCENP null cells. DNA is shown in blue and DdCP224 is shown in red. Arrows point to the position of spindle midzone. (E and F) Overexpression of DdCP24 rescues the spindle midzone defect of DdINCENP null cells. (E and E′) The immunofluorescence pictures of a DdINCENP null cell overexpressing DdCP224-GFP (green), showing DNA in blue and tubulin in red. Arrows point to the position of spindle midzone. DdCP224-GFP was localized on the spindle midzone (E). As a result, the DdINCENP null cells had much more robust spindle midzone (E′) than those cells shown in Figure 5. (F) The fluorescence image of a live DdINCENP null cell expressing DdCP224-GFP. The cell was at anaphase. Overexpressed DdCP224-GFP was clearly localized on the spindle midzone. Bar, 5 μm.
DdINCENP Is Important for the Stability of the Mitotic Spindle
Although our data suggested that DdINCENP helps recruit DdCP224 to stabilize the midzone spindle, we suspected that this was not the sole function of DdINCENP during mitosis.
This was supported by the fact that DdINCENP mutant cells expressing DdCP224-GFP still divided much more slowly than wild-type cells (Supplementary Figure 3). To explore the mitosis defect of the DdINCENP null cells in more detail, we introduced GFP-tubulin into these cells to analyze their microtubule behavior during mitosis. Surprisingly, all the DdINCENP null cells expressing GFP-tubulin died shortly after transformation. Although GFP-tubulin has been widely used in Dictyostelium for live microscopy of microtubule structures (Neujahr et al., 1998), it has been shown that GFP-tubulin can slightly affect microtubule dynamics in vivo (Kimble et al., 2000). This raised the possibility that the microtubule organization in the DdINCENP mutant cells might be unstable relative to that in wild-type cells, thus making such cells hypersensitive to the destabilization of microtubules as a result of expressing GFP-tubulin. Therefore, we decided to examine the sensitivity of the mutant cells to microtubule-depolymerizing drugs.
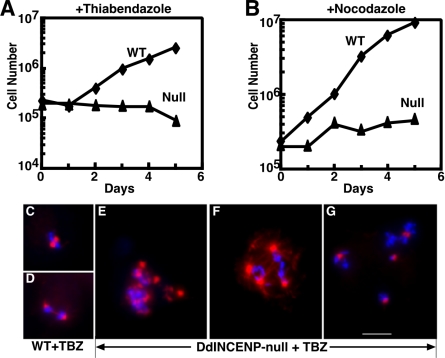
We found that DdINCENP null cells were highly sensitive to microtubule-depolymerizing drugs such as nocodazole and thiabendazole compared with wild-type cells (Figure 7, A and B). We considered two possible explanations for this hypersensitivity. First, DdINCENP null cells could have an unstable microtubule organization during interphase, which would indicate DdINCENP was essential for a stable microtubule network. However, this hypothesis was not supported by our data. We found that the depolymerization of interphase microtubules by nocodazole and thiabendazole was indistinguishable in wild-type and mutant cells (unpublished data). Similarly the recovery of interphase microtubules after drug washout was indistinguishable in both cell lines (unpublished data). A second possibility was that the depletion of DdINCENP impaired the ability of the mutant cells to establish a proper mitotic spindle structure. To test this, we examined whether wild-type and DdINCENP null cells showed differences in assembling the mitotic spindles in the presence of microtubule-depolymerizing drugs. We found that wild-type cells continued to establish normal bipolar spindles in the presence of 2 μg/ml thiabendazole, although their spindle midzones were not as robust as those formed in the absence of the drugs (Figure 7, C and D). In contrast, DdINCENP null cells exposed to 2 μg/ml thiabendazole could not form bipolar spindles. Instead 100% of the mitotic cells had aberrant spindle structures and were blocked at prometaphase, which was evident from the condensed but scattered chromosomes (Figure 7, E and G). As a result, these mutant cells died within 2 d of exposure to the drugs. In addition, the treated mutant cells displayed microtubule organizing centers of different sizes indicating a problem with spindle pole body duplication or separation (Figure 7, E and G). The formation of abnormal spindles occurred both in multinucleate and mononucleate DdINCENP null cells. Taken together, these data suggest that DdINCENP plays a vital role in the establishment and/or maintenance of a bipolar spindle.
Figure 7.
DdINCENP null cells are hypersensitive to microtubule-depolymerizing drugs. (A and B) Growth curve of wild-type cells (diamond) and DdINCENP null cells (triangle) in the presence of 2 μg/ml thiabendazole (A) or 2 μg/ml nocodazole (B). (C-G): Immunofluorescence images of mitotic wild-type cells (C and D) and DdINCENP null cells (E-G) in the presence of 2 μg/ml thiabendazole (TBZ). DNA is shown in blue and tubulin is shown in red. Wild-type cells displayed normal metaphase spindles (C) and anaphase spindles with a well-defined spindle midzone (D). In contrast, DdINCENP null cells were blocked at prometaphase by the drug treatment and contained either multipolar (E and F) or monopolar spindles (G, a single multinucleate mitotic cell is shown). Bar, 5 μm.
DdINCENP Plays an Important Role in the Completion of Cytokinesis But Not in the Formation of Normal or Ectopic Cleavage Furrows
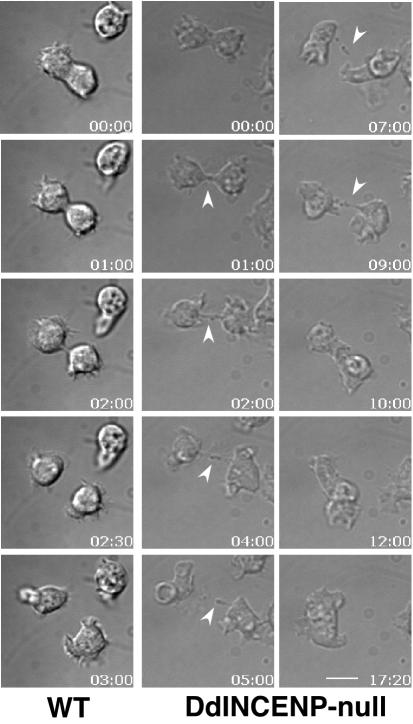
The localization of GFP-DdINCENP at the cleavage furrow throughout cytokinesis and the formation of multinucleate cells in the absence of DdINCENP indicate that DdINCENP plays a key role in this process. Because INCENP also localizes to ectopic, or Rappaport, furrows in vertebrate cells (Eckley et al., 1997), it has been suggested that INCENP may play a role in the formation and stabilization of cleavage furrows. To explore this role in more detail we imaged DdINCENP null cells undergoing cytokinesis (Figure 8). All DdINCENP null cells were able to form a cleavage furrow that constricted normally until the abscission stage, when the two daughter cells were only connected by a thin cytoplasmic bridge. In wild-type cells, the bridge was short and the abscission stage took no more than 2 min (Figure 8). In contrast, most DdINCENP null cells had a prolonged abscission stage that lasted as long as 13 min. About half of the cytokinesis events we observed failed (13 of 24) because the cleavage furrow eventually regressed and the daughter cells fused back with each other to become a multinucleate cell (Figure 8). The cytokinesis defect of these mutant cells was rescued by the reintroduction of GFP-DdINCENP.
Figure 8.
DdINCENP null cells have a late cytokinesis defect. Time-lapse video microscopy of a wild-type cell and a DdINCENP null cell undergoing cytokinesis. Cytokinesis of the wild-type cell was completed in ∼3 min. In contrast, DdINCENP null cell failed in cytokinesis because of the inability to break the thin cytoplasmic bridge connecting the daughter cells (arrowhead). The times are indicated in minutes:seconds. Bar, 5 μm.
We also determined the ability of DdINCENP null cells to form ectopic furrows. Dictyostelium cells, like animal cells, form Rappaport furrows when they are binucleate (Neujahr et al., 1998). When we analyzed binucleate INCENP null cells undergoing mitosis, we found that they could also form Rappaport furrows (Figure 9, A and A′). Thus, it seems that INCENP is not required for the formation and contraction of normal or ectopic cleavage furrows but that it is most important for the late stage of cytokinesis during abscission.
Figure 9.

DdINCENP is not essential for the formation of equatorial or ectopic cleavage furrows during cytokinesis. (A and A′) Microscopy images of a DdINCENP null cell forming Rappaport (ectopic) furrows during four-way cytokinesis. As shown, DdINCENP null cells are able to make normal and ectopic cleavage furrows. (B, B′, C, and C′) GFP-DdINCENP localized only to one pair of cleavage furrows in a four-way cell division. Arrows indicate the position of the presumptive equatorial cleavage furrows and arrowheads indicate the position of presumptive ectopic furrows. (B and B′) Live DIC and fluorescent images of an AX2 cell expressing GFP-DdINCENP ongoing a four-way division. (C and C′) Fluorescent image of a wild-type cells expressing GFP-DdINCENP. The cell was stained by DAPI to better illustrate the four-way cytokinesis. (D) Live fluorescent image of an AX2 cell expressing GFP-MHC (myosin II heavy chain) during a four-way cytokinesis. Myosin II localized to both normal and ectopic furrows (arrows). (E) Myosin II localizes normally at the cleavage furrow of DdINCENP null cells. Fluorescent microscopy image of a DdINCENP null cell expressing GFP-Myosin II heavy chain. The absence of DdINCENP does not affect the localization of myosin II at the cleavage furrow (arrows). Bar, 5 μm.
The Localization of DdINCENP at the Cleavage Furrow Is Influenced by Myosin II But It Occurs by a Different Mechanism
To date it is not known how chromosomal passenger proteins localize on the cleavage furrow. How do they transfer from the mitotic spindle to the cleavage furrow? Do they interact with cytoskeletal elements of the contractile ring or plasma membrane proteins at the furrow? Dictyostelium is a good system to explore these questions. Dictyostelium cells undergo closed mitosis forming an intranuclear spindle, yet can induce the formation of ectopic furrows between adjacent asters (Neujahr et al., 1997). We used these properties to determine what factors are important for INCENP localization at the furrow.
We first determined whether DdINCENP could localize on the cleavage furrow in the absence of a contractile ring. Dictyostelium myosin II null cells are still able to form cleavage furrows by an attachment-mediated process, cytokinesis B, that is not dependent on a contractile ring (Zang et al., 1997; Uyeda et al., 2000). We expressed GFP-DdINCENP in myosin II null cells and examined its localization in dividing cells attached to a substrate. The localization of GFP-DdINCENP to the spindle midzone and spindle poles was completely normal in the myosin II null cells (Supplementary Figure 4). However, when these cells entered into cytokinesis B, GFP-DdINCENP localized to a very narrow band at the equator of the cells (Figure 10). This distribution is remarkably different from the broad equatorial distribution of GFP-DdINCENP in wild-type cells (Figure 2). Optical sectioning of multiple cells revealed that GFP-DdINCENP is found as a hemicircle at the top surface of the cell (see Video 6). This hemicircle can be seen to narrow concomitantly with the constriction of the cleavage furrow (Figure 10; see Video 7).
Figure 10.
The distribution of DdINCENP in the cleavage furrow area is different in the absence of myosin II. Time-lapse video microscopy was performed on a myosin heavy chain null cell expressing GFP-DdINCENP. The live images show a mutant cell attached to a substrate undergoing myosin II-independent cytokinesis B (Zang et al., 1997). In contrast to the cortical distribution of GFP-DdINCENP in wild-type cells (see Figure 2), GFP-DdINCENP was found as a narrow band at the equatorial plane of the dividing cell. This band of GFP-DdINCENP decreased in size as the cleavage furrow ingressed. The times are indicated in minutes:seconds. Bar, 5 μm.
We next determined whether the localization of myosin II to the cleavage furrow was dependent on DdINCENP. We expressed GFP-myosin II heavy chain in DdINCENP null cells and found that this protein localized normally to the cleavage furrow (Figure 9E). Thus, although myosin and INCENP do not depend on each other to localize to the cleavage furrow, myosin II strongly influences the organization of INCENP at the furrow.
Finally, we determined the localization of DdINCENP in binucleate cells. Myosin II is known to localize to ectopic as well as equatorial furrows, suggesting that its localization is controlled by the position of astral microtubules (Figure 9D). Surprisingly, we found that DdINCENP localized only on two of the four cleavage furrows (Figure 9, B and C). These results suggest that the localization of INCENP at the furrow must occur by a mechanism distinct from that driving myosin II localization.
DISCUSSION
We have identified the protein DdINCENP as a new member of the INCENP protein family in Dictyostelium. In addition to conserved domains, DdINCENP also displays the dynamic behavior of other chromosomal passenger proteins but with some additional unique characteristics. DdINCENP localizes at the centromeres from prophase to metaphase and then transfers to the spindle midzone and spindle poles at the onset of anaphase. This is different from metazoan cells where INCENP translocates from the centromeres to the spindle midzone but is not found at the spindle poles.
DdINCENP associates with the cleavage furrow during cytokinesis and concentrates in the intercellular bridge connecting daughter cells. In metazoans INCENP is also known to localize to the midbody. However, in Dictyostelium the central spindle breaks well before the abscission stage of cytokinesis and a microtubule-containing midbody is not formed in this organism (Roos et al., 1984; McIntosh et al., 1985; Neujahr et al., 1998). This suggests that the accumulation of INCENPs at the intercellular cytoplasmic bridge is not dependent on a midbody.
DdINCENP and Spindle Assembly
Considering the localization of INCENP in mitosis, it is not surprising that it plays important roles in several mitotic events (Mackay et al., 1998; Giet and Glover, 2001; Tanaka et al., 2002; Sampath et al., 2004). Analysis of our DdINCENP null cell lines confirmed a role for this protein in spindle assembly and chromosome segregation. Our results further suggest a role for DdINCENP in spindle stability and localization of proteins at the spindle midzone.
The hypersensitivity of DdINCENP null cells to microtubule-depolymerizing drugs revealed the importance of DdINCENP for the stability of the bipolar spindle. In the presence of these drugs mitotic DdINCENP null cells failed to establish a normal bipolar spindle structure. It seems likely that DdINCENP exerts this role by controlling the activity of proteins that modulate microtubule stability. For example, the localization of metazoan MKLP1, a kinesin essential for central spindle formation, is dependent on INCENP function (Zhu et al., 2005). Similarly, the activity of vertebrate MCAK, a microtubule-depolymerizing protein, is regulated through its phosphorylation by Aurora B kinase (Andrews et al., 2004; Lan et al., 2004). Although a Dictyostelium homolog of MCAK has yet to be identified, the homolog of MCAK's antagonist, XMAP215/TOGp, has been characterized as the Dictyostelium DdCP224 protein. In contrast to MCAK, XMAP215/TOGp is known to stabilize mitotic spindles (Holmfeldt et al., 2004). We found that DdCP224 is absent from spindles of DdINCENP null cells and that DdCP224 overexpression enhances the ability of DdINCENP null cells to build spindles. Although these results do not demonstrate a direct interaction between DdINCENP and DdCP224, they suggest that DdINCENP may regulate the localization and/or activity of DdCP224. One possible model is that DdCP224, like MCAK, is directly phosphorylated by the INCENP-Aurora B protein complex during mitosis. Interestingly, it has been reported that the homolog of DdCP224, XMAP215, is hyperphosphorylated during mitosis (Vasquez et al., 1999).
DdINCENP and Cytokinesis
DdINCENP begins to localize at the cortex region of the cleavage furrow during late telophase. When cells reach late cytokinesis, DdINCENP is highly concentrated at the cortical region of the furrow area, suggesting an important role for DdINCENP during cytokinesis. Indeed, DdINCENP null cells have a severe cytokinesis defect: at the end of cytokinesis the mutant cells are impaired in severing the thin cytoplasmic bridge connecting two daughter cells. Similarly, the mammalian cells expressing a dominant-negative INCENP mutant also had a late cytokinesis defect (Mackay et al., 1998). However, because Dictyostelium does not have a spindle midzone or midbody structure during late cytokinesis (Roos et al., 1984; McIntosh et al., 1985), the cytokinesis defect of DdINCENP null cells cannot be attributed solely to their defective spindle midzone. Additionally, it is also unlikely that their cytokinesis defect is due to a malfunction of the actin-myosin ring, because myosin II localizes normally at the furrow area in the mutant cells. Instead, we postulate that the cytokinesis defect of DdINCENP null cells may be due to a failure in the fusion of the membranes of the cytoplasmic bridge. Several membrane-trafficking proteins have been shown to be required late in cytokinesis including dynamin, clathrin, LvsA, syntaxin, rabs, and others (O'Halloran, 2000). Interestingly, the depletion of dynamin in Dictyostelium cause a very similar late cytokinesis defect because the daughter cells were often connected by a thin cytoplasmic bridge for prolonged period (Wienke et al., 1999).
The formation of Rappaport or ectopic furrows in sand dollar eggs has been a classical example of the ability of the cytokinesis machinery to assemble between two mitotic asters in the absence of a spindle (Rappaport, 1961). The formation of ectopic furrows in mammalian cells has also been used to determine the requirements for furrow formation (Eckley et al., 1997; Savoian et al., 1999). These studies revealed that, although not required for the establishment of a furrow, INCENP is required for the complete constriction of the furrow and formation of a midbody. Similarly, we have shown that in Dictyostelium INCENP is not required for the formation of either equatorial or ectopic furrows. Therefore, it is clear that from Dictyostelium to mammals, the chromosomal passenger proteins do not determine the spatial organization of the cytokinetic machinery. Indeed, myosin II localized normally in our INCENP null cells. Myosin II and other components of the contractile ring must assemble at the equator of a cell by a mechanism independent of INCENP and other chromosomal passenger proteins.
The Mechanism Involved in the Localization of DdINCENP at the Cleavage Furrow Is Different from that Controlling the Formation of the Contractile Ring
Although some aspects of regulation of the contractile ring are known, nothing is yet known about the recruitment of chromosomal passengers to the cleavage furrow. We found that the localization of DdINCENP at the cleavage furrow was strongly influenced by myosin II. Although not required for the furrow localization of DdINCENP, myosin II is involved in broadening the distribution of DdINCENP at the furrow. These results highlight for the first time a potential interaction between INCENP and the actomyosin contractile ring. The nature of this interaction and its significance for INCENP function remain to be elucidated.
A general assumption in the field of cytokinesis has been that a common mechanism must be involved in the determination of the plane of division and the recruitment of the different components of the cleavage furrow. Our results clearly indicate that the mechanism involved in the equatorial localization of DdINCENP is different from that regulating the distribution of myosin II at the cleavage furrow. This distinction cannot be made in normal mononucleate cells undergoing mitosis because there is a single plane of division. However, the formation of ectopic, or Rappaport furrows in binucleate cells provides a unique system to clearly distinguish these two localization mechanisms. The myosin II contractile ring is always formed at both equatorial furrows and ectopic furrows in Dictyostelium and other systems. These results strongly suggest that the contractile ring must be positioned wherever microtubules of opposite polarities meet the cortical cytoskeleton. In contrast, DdINCENP localized only at two of the four cleavage furrows, presumably at the equator. This equatorial localization of DdINCENP was found in both wild-type and myosin II mutant cells. Thus, the distribution of DdINCENP is not controlled by the encounter of antiparallel microtubules, but must be regulated by the position of the spindle midzone. Because the Dictyostelium spindle midzone is no longer surrounded by the nuclear envelope in late anaphase and telophase it is possible that DdINCENP transfers directly from the spindle midzone, where it is highly concentrated during mitosis, to the cortex of the cell. Future experiments will dissect the requirements of DdINCENP localization at the cleavage furrow.
Supplementary Material
Acknowledgments
We thank all members of the De Lozanne and O'Halloran laboratories for their comments and encouragement throughout this work. We also thank Dr. Gunther Gerisch for providing us with expression vectors for GFP-Tubulin and Dr. Graf for anti-DdCP224 antibodies and GFP-DdCP224 expression vectors. We shall also thank Dr. Clarence Chan for reading the manuscript and providing very valuable advice. This work was supported by National Institutes of Health Grant GM48745.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-08-0704) on December 7, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Adams, R. R., Carmena, M., and Earnshaw, W. C. (2001a). Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 11, 49-54. [DOI] [PubMed] [Google Scholar]
- Adams, R. R., Maiato, H., Earnshaw, W. C., and Carmena, M. (2001b). Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 153, 865-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsztein, A. M., Kandels-Lewis, S. E., Mackay, A. M., and Earnshaw, W. C. (1998). INCENP centromere and spindle targeting: identification of essential conserved motifs and involvement of heterochromatin protein HP1. J. Cell Biol. 143, 1763-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews, P. D., Ovechkina, Y., Morrice, N., Wagenbach, M., Duncan, K., Wordeman, L., and Swedlow, J. R. (2004). Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6, 253-268. [DOI] [PubMed] [Google Scholar]
- Bishop, J. D., and Schumacher, J. M. (2002). Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J. Biol. Chem. 277, 27577-27580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton, M. A., Lan, W., Powers, S. E., McCleland, M. L., Kuang, J., and Stukenberg, P. T. (2002). Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol. Biol. Cell 13, 3064-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretschneider, T., Jonkman, J., Kohler, J., Medalia, O., Barisic, K., Weber, I., Stelzer, E. H., Baumeister, W., and Gerisch, G. (2002). Dynamic organization of the actin system in the motile cells of Dictyostelium. J. Muscle Res. Cell Motil. 23, 639-649. [DOI] [PubMed] [Google Scholar]
- Buvelot, S., Tatsutani, S. Y., Vermaak, D., and Biggins, S. (2003). The budding yeast Ipl1/Aurora protein kinase regulates mitotic spindle disassembly. J. Cell Biol. 160, 329-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris, L., and Morabito, J. (2004). TOGp, the human homolog of XMAP215/Dis1, is required for centrosome integrity, spindle pole organization, and bipolar spindle assembly. Mol. Biol. Cell 15, 1580-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman, I. M., Anderson, S., Jwa, M., Green, E. M., Kang, J., Yates, J. R., 3rd, Chan, C. S., Drubin, D. G., and Barnes, G. (2002). Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 111, 163-172. [DOI] [PubMed] [Google Scholar]
- Eckley, D. M., Ainsztein, A. M., Mackay, A. M., Goldberg, I. G., and Earnshaw, W. C. (1997). Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and inner centromere protein positioning in mitotic heterokaryons and mid-anaphase cells. J. Cell Biol. 136, 1169-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faix, J., Weber, I., Mintert, U., Kohler, J., Lottspeich, F., and Marriott, G. (2001). Recruitment of cortexillin into the cleavage furrow is controlled by Rac1 and IQGAP-related proteins. EMBO J. 20, 3705-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann, R., Carvalho, A., Henzing, A. J., Ruchaud, S., Hudson, D. F., Honda, R., Nigg, E. A., Gerloff, D. L., and Earnshaw, W. C. (2004). Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 166, 179-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerald, N. J., Damer, C. K., O'Halloran, T. J., and De Lozanne, A. (2001). Cytokinesis failure in clathrin-minus cells is caused by cleavage furrow instability. Cell Motil. Cytoskelet. 48, 213-223. [DOI] [PubMed] [Google Scholar]
- Giet, R., and Glover, D. M. (2001). Drosophila Aurora B Kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152, 669-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf, R., Daunderer, C., and Schliwa, M. (2000). Dictyostelium DdCP224 is a microtubule-associated protein and a permanent centrosomal resident involved in centrosome duplication. J. Cell Sci. 113(Pt 10), 1747-1758. [DOI] [PubMed] [Google Scholar]
- Graf, R., Euteneuer, U., Ho, T. H., and Rehberg, M. (2003). Regulated expression of the centrosomal protein DdCP224 affects microtubule dynamics and reveals mechanisms for the control of supernumerary centrosome number. Mol. Biol. Cell 14, 4067-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmfeldt, P., Stenmark, S., and Gullberg, M. (2004). Differential functional interplay of TOGp/XMAP215 and the KinI kinesin MCAK during interphase and mitosis. EMBO J. 23, 627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, R., Korner, R., and Nigg, E. A. (2003). Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol. Biol. Cell 14, 3325-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitna, S., Mendoza, M., Jantsch-Plunger, V., and Glotzer, M. (2000). Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr. Biol. 10, 1172-1181. [DOI] [PubMed] [Google Scholar]
- Kang, J., Cheeseman, I. M., Kallstrom, G., Velmurugan, S., Barnes, G., and Chan, C. S. (2001). Functional cooperation of Dam1, Ipl1, and the inner centromere protein (INCENP)-related protein Sli15 during chromosome segregation. J. Cell Biol. 155, 763-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. H., Kang, J. S., and Chan, C. S. (1999). Sli15 associates with the ipl1 protein kinase to promote proper chromosome segregation in Saccharomyces cerevisiae. J. Cell Biol. 145, 1381-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble, M., Kuzmiak, C., McGovern, K. N., and de Hostos, E. L. (2000). Microtubule organization and the effects of GFP-tubulin expression in Dictyostelium discoideum. Cell Motil. Cytoskelet. 47, 48-62. [DOI] [PubMed] [Google Scholar]
- Koonce, M. P., and McIntosh, J. R. (1990). Identification and immunolocalization of cytoplasmic dynein in Dictyostelium. Cell Motil. Cytoskelet. 15, 51-62. [DOI] [PubMed] [Google Scholar]
- Kwak, E., Gerald, N., Larochelle, D. A., Vithalani, K. K., Niswonger, M. L., Maready, M., and De Lozanne, A. (1999). LvsA, a protein related to the mouse beige protein, is required for cytokinesis in Dictyostelium. Mol. Biol. Cell 10, 4429-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, W., Zhang, X., Kline-Smith, S. L., Rosasco, S. E., Barrett-Wilt, G. A., Shabanowitz, J., Hunt, D. F., Walczak, C. E., and Stukenberg, P. T. (2004). Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 14, 273-286. [DOI] [PubMed] [Google Scholar]
- Mackay, A. M., Ainsztein, A. M., Eckley, D. M., and Earnshaw, W. C. (1998). A dominant mutant of inner centromere protein (INCENP), a chromosomal protein, disrupts prometaphase congression and cytokinesis. J. Cell Biol. 140, 991-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay, A. M., Eckley, D. M., Chue, C., and Earnshaw, W. C. (1993). Molecular analysis of the INCENPs (inner centromere proteins): separate domains are required for association with microtubules during interphase and with the central spindle during anaphase. J. Cell Biol. 123, 373-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh, J. R., Roos, U. P., Neighbors, B., and McDonald, K. L. (1985). Architecture of the microtubule component of mitotic spindles from Dictyostelium discoideum. J. Cell Sci. 75, 93-129. [DOI] [PubMed] [Google Scholar]
- Minoshima, Y. et al. (2003). Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev. Cell 4, 549-560. [DOI] [PubMed] [Google Scholar]
- Mollinari, C. et al. (2003). The mammalian passenger protein TD-60 is an RCC1 family member with an essential role in prometaphase to metaphase progression. Dev. Cell 5, 295-307. [DOI] [PubMed] [Google Scholar]
- Neujahr, R., Albrecht, R., Kohler, J., Matzner, M., Schwartz, J. M., Westphal, M., and Gerisch, G. (1998). Microtubule-mediated centrosome motility and the positioning of cleavage furrows in multinucleate myosin II-null cells. J. Cell Sci. 111(Pt 9), 1227-1240. [DOI] [PubMed] [Google Scholar]
- Neujahr, R., Heizer, C., and Gerisch, G. (1997). Myosin II-independent processes in mitotic cells of Dictyostelium discoideum: redistribution of the nuclei, re-arrangement of the actin system and formation of the cleavage furrow. J. Cell Sci. 110(Pt 2), 123-137. [DOI] [PubMed] [Google Scholar]
- O'Halloran, T. J. (2000). Membrane traffic and cytokinesis. Traffic 1, 921-926. [PubMed] [Google Scholar]
- Pereira, G., and Schiebel, E. (2003). Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science 302, 2120-2124. [DOI] [PubMed] [Google Scholar]
- Rappaport, R. (1961). Experiments concerning the cleavage stimulus in sand dollar eggs. J. Exp. Zool. 148, 81-89. [DOI] [PubMed] [Google Scholar]
- Roos, U. P., De Brabander, M., and De Mey, J. (1984). Indirect immunofluorescence of microtubules in Dictyostelium discoideum. A study with polyclonal and monoclonal antibodies to tubulins. Exp. Cell Res. 151, 183-193. [DOI] [PubMed] [Google Scholar]
- Sampath, S. C., Ohi, R., Leismann, O., Salic, A., Pozniakovski, A., and Funabiki, H. (2004). The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell 118, 187-202. [DOI] [PubMed] [Google Scholar]
- Savoian, M. S., Earnshaw, W. C., Khodjakov, A., and Rieder, C. L. (1999). Cleavage furrows formed between centrosomes lacking an intervening spindle and chromosomes contain microtubule bundles, INCENP, and CHO1 but not CENP-E. Mol. Biol. Cell 10, 297-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, T. U., Rachidi, N., Janke, C., Pereira, G., Galova, M., Schiebel, E., Stark, M. J., and Nasmyth, K. (2002). Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell 108, 317-329. [DOI] [PubMed] [Google Scholar]
- Uyeda, T. Q., Kitayama, C., and Yumura, S. (2000). Myosin II-independent cytokinesis in Dictyostelium: its mechanism and implications. Cell Struct. Funct. 25, 1-10. [DOI] [PubMed] [Google Scholar]
- Vasquez, R. J., Gard, D. L., and Cassimeris, L. (1999). Phosphorylation by CDK1 regulates XMAP215 function in vitro. Cell Motil. Cytoskelet. 43, 310-321. [DOI] [PubMed] [Google Scholar]
- Wienke, D. C., Knetsch, M. L., Neuhaus, E. M., Reedy, M. C., and Manstein, D. J. (1999). Disruption of a dynamin homologue affects endocytosis, organelle morphology, and cytokinesis in Dictyostelium discoideum. Mol. Biol. Cell 10, 225-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang, J. H., Cavet, G., Sabry, J. H., Wagner, P., Moores, S. L., and Spudich, J. A. (1997). On the role of myosin-II in cytokinesis: division of Dictyostelium cells under adhesive and nonadhesive conditions. Mol. Biol. Cell 8, 2617-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, C., Bossy-Wetzel, E., and Jiang, W. (2005). Recruitment of MKLP1 to the spindle midzone/midbody by INCENP is essential for midbody formation and completion of cytokinesis in human cells. Biochem. J. 389, 373-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.