Abstract
In response to treatment with phorbol-12-myristate-13-acetate (PMA), the half-population of erythromyeloblast D2 cells, a cytokine-independent variant of TF-1 cells, displayed adhesion and differentiated into a monocyte/macrophage-like morphology, while the other half-population remained in suspension and underwent apoptosis. Expression of the cell cycle inhibitor p21Cip1/Waf1 was induced after PMA treatment in the adherent cells but not in the proapoptotic cells. We investigated the mechanism responsible for the impairment of p21Cip1/Waf1 induction in PMA-induced proapoptotic cells. We demonstrated that in PMA-induced adherent cells, upregulation of p21Cip1/Waf1 requires the activation and nuclear translocation of phosphorylated extracellular signal-regulated kinase (phospho-ERK). Although ERK was phosphorylated to comparable levels in PMA-induced proapoptotic and adherent cells, nuclear distribution of phospho-ERK was seen only in the adherent, not in the proapoptotic cells. We also found that only PMA-induced proapoptotic cells contained the phosphorylated form of myosin light chain, which is dependent on Rho-associated kinase (ROCK) activation, and that expression of a dominant-active form of ROCK suppressed activation of the p21Cip1/Waf1 promoter during PMA induction. Finally, we demonstrated that inhibition of ROCK restores nuclear distribution of phospho-ERK and activation of p21Cip1/Waf1 expression. Based on these findings, we propose that a ROCK-mediated signal is involved in interfering with the process of ERK-mediated p21Cip1/Waf1 induction in PMA-induced proapoptotic TF-1 and D2 cells.
Treatment of cells with phorbol-12-myristate-13-acetate (PMA), a phorbol ester that is known as a potent activator of protein kinase C (PKC), induces terminal differentiation or apoptosis, depending on the cell type. For instance, previous studies have reported that PMA induces apoptosis in LNCaP prostate cancer cells, MCF-7 breast cancer cells, U937 cells, promyeloleukemia HL-60 cells, and murine thymocytes (17, 18, 23, 28, 54, 61, 62). In contrast, many studies have shown that PMA induces differentiation of a number of myeloid leukemic cell lines and suppresses apoptosis (15, 16, 24, 38, 41, 46, 48, 53).
Since differentiation is often associated with growth arrest, expression of the cyclin-dependent kinase inhibitor p21Cip1/Waf1 is elevated in cells committed to differentiation (34, 70, 71). The p21Cip1/Waf1 gene was initially cloned independently by a number of research groups. It was identified by its association with cyclin-CDK complexes as an inhibitor (Cip-1) (27) and by its induction by p53 (Waf-1) (19). It was also designated melanoma differentiation-associated gene 6 (mda-6) and senescence-derived inhibitor-1 (sdi-1) by virtue of its enhanced expression in terminal differentiated melanoma cells (35) and in human senescent fibroblasts as a DNA synthesis inhibitor (52), respectively.
In addition to its function in antiproliferation, many lines of evidence have shown that p21Cip1/Waf1 can play an antiapoptotic role in response to various types of apoptotic stimulation (4, 22, 25, 65, 66, 67). Accordingly, it is expected that p21Cip1/Waf1 is usually not expressed in cells stimulated to undergo apoptosis. As stated earlier, PMA can induce differentiation or apoptosis in various types of cells. While the mechanism of PMA-induced transcriptional activation of p21Cip1/Waf1 has been well studied in a number of hematopoietic cells during terminal differentiation (7, 71), no information is available to explain the impaired expression of p21Cip1/Waf1 in PMA-induced apoptotic cells. The main purpose of this study is to understand why the PMA-activating signal elicits such a difference in p21CIP1/WAF1 gene expression in differentiating and apoptotic cells and to identify the signal present in the proapoptotic cells that can disrupt the mechanism of PMA-induced p21Cip1/Waf1 expression.
Here we used D2, a cytokine-independent variant derived from myeloid leukemia cell line TF-1, as a model system for this study, because 50% of D2 cells become differentiated and the rest are apoptotic when treated with PMA (13). In this study, we showed that treatment of D2 cells with PMA induces transcriptional activation of the p21CIP1/WAF1 gene in the differentiating cells but not in the proapoptotic fraction. Although studies have suggested that various responses to PMA treatment are partially due to differences in the expression of protein kinase C isoforms (17, 18, 49), our previous study found that there is no difference in the PMA-responsive translocation of protein kinase C isoforms α, β, γ, δ, ɛ, and θ between the differentiating and proapoptotic fractions of D2 cells (42).
It is known that activation of PKC can directly phosphorylate Raf-1 (10, 47), which in turn sequentially activates and phosphorylates the MEKs and extracellular signal-regulated kinase (ERK). Since the MEK/ERK pathway has been shown to be involved in transcriptional activation of p21Cip1/Waf1 in a variety of cell systems stimulated by different growth factors (6, 8, 29, 31, 45, 56), we asked whether there is a difference in activation of the MEK/ERK cascade in the proapoptotic and differentiating D2 cell populations, resulting in differential expression of p21Cip1/Waf1. Interestingly, we found that PMA-induced phosphorylation of ERK via the PKC/Raf-1/MEK cascade was similar in these two populations of cells; however, phospho-ERK was located in the nuclei of PMA-induced differentiating cells, whereas in PMA-induced proapoptotic cells it was retained in the cytosol. As our results showed that activation of MEK/ERK with subsequent nuclear translocation of phospho-ERK is a process necessary for PMA-induced activation of p21Cip1/Waf1 transcription, it is likely that cytosolic retention of phospho-ERK is responsible for the impairment of p21Cip1/Waf1 induction in proapoptotic cells.
Previously, we have shown that lysophosphatidic acid (LPA) and serum promote PMA-induced apoptosis in TF-1 and D2 cells via the Gα12/13/Rho-dependent pathway (42). In this study, we further investigated whether the RhoA-mediated signal transduction pathway is involved in prevention of ERK-mediated activation of p21Cip1/Waf1 induction in PMA-induced proapoptotic cells. RhoA is one member of the small G-protein family. When cells are stimulated with LPA or serum, RhoA is converted to a GTP-bound form, which binds to specific effectors and exerts its biological function (reviewed in reference 37).
Among the diverse effector pathways downstream of the Rho signal, Rho-associated kinase (ROCK) has been shown to increase the extent of myosin light chain (MLC) phosphorylation by directly phosphorylating MLC and inhibiting MLC phosphatase, thus activating myosin ATPase and contractility (37). Since the proapoptotic D2 cells in suspension always exhibit cellular contraction upon PMA stimulation, here we examined whether ROCK plays a role in the impairment of p21Cip1/Waf1 induction in the proapoptotic cells. Our experimental result showed that MLC is heavily phosphorylated in PMA-induced proapoptotic cells but not in the differentiating cells. Furthermore, we provide evidence that MLC phosphorylation in PMA-induced proapoptotic cells is indeed dependent on ROCK activation, which concomitantly downregulates PMA activation of p21Cip1/Waf1 induction. Most interestingly, inhibition of ROCK in the proapoptotic cells restored nuclear translocation of phospho-ERK and p21Cip1/Waf1 induction. Accordingly, we propose that upregulation of ROCK in PMA-induced proapoptotic cells, as indicated by MLC phosphorylation, provides the signal for cytosolic retention of phospho-ERK and impairs p21Cip1/Waf1 induction.
MATERIALS AND METHODS
Material.
PMA was purchased from Sigma Chemical Co. (St. Louis, Mo.) and dissolved in dimethyl sulfoxide. U0126 was from Promega (Madison, Wis.); Y-27632 [(+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl)cyclohexanecarboxamide dihydrochloride, monohydrate] was from Calbiochem-Novabiochem Corp. (San Diego, Calif.); LPA (1-oleoyl-2-sn-glycero-3-phosphate) was from Fluka (Basel, Switzerland) and dissolved in phosphate-buffered saline (PBS) containing 10 mg of fatty acid-free albumin per ml. Rabbit polyclonal anti-phospho-p44/p42 mitogen-activated protein (MAP) kinase (ERK1/2) was purchased from Cell Signaling Technology (Beverly, Mass.), and anti-Raf-1 was from Transduction Laboratories (Lexington, Ky.). Monoclonal anti-MLC antibody was from Sigma. Anti-phospho-MLC antibody (kindly supplied by J. M. Staddon, Eisai London Research Laboratories Ltd., London, United Kingdom) has been described previously (58). Human thymidine kinase (hTK) antibody was generated in our laboratory as described previously (12), and monoclonal antibodies against human p21Cip1/Waf1, Myc, and rabbit anti-ERK1/2 kinase were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.).
Constructs.
Plasmid pWWP-Luc, containing the promoter of human p21Cip1/Waf1 between −2300 and +8, was a gift from B. Vogelstein (Johns Hopkins University, Baltimore, Md.) (19). phTK-luc and pRhoAV14 constructed in the pCMV vector were as described previously (11, 42). The cDNA of ROCK(CAT) in the pEF-BOS-Myc vector was from K. Kaibuchi (Nara Institute of Science and Technology, Ikoma, Japan). The expression plasmids for MKP3 and MKP3(C/S) in the pSG5 vector were provided by J. Pouyssegur (Université de Nice, Nice, France).
Cell culture.
TF-1 cells were maintained in RPMI 1640 (Gibco-BRL, Gaithersburg, Md.) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah), 2 mM l-glutamine, 100 U of penicillin G per ml, 100 U of streptomycin per ml, and 1 ng of granulocyte-macrophage colony-stimulating factor (GM-CSF) per ml. Human GM-CSF was kindly provided by Schering-Plough Ltd., Taipei, Taiwan. D2 and K562 cells were maintained in the same medium without GM-CSF.
RNase protection analysis.
Plasmids containing cDNAs of the human p21Cip1/Waf1 and β-actin genes were linearized at the NciI and HindIII site, respectively, and added to a transcription reaction mixture containing Sp6 RNA polymerase and [α-32P]CTP for the synthesis of p21Cip1/Waf1 and β-actin riboprobes. Total RNA was isolated from fresh cell pellets as described previously (14) and hybridized to the riboprobes at 42°C overnight. The RNase protection analysis for detection of cellular p21CIP1/WAF1 RNA and β-actin RNA was performed as described previously (11). Autoradiography was performed on Kodak X-Omat film at −80°C.
Immunofluorescence.
D2 or TF-1 cells on coverslips were fixed for 30 min in PBS containing 3% paraformaldehyde (Merck) at room temperature and then treated with cold (−20°C) 100% methanol for 5 min. The fixed cells were washed with PBS, and nonspecific sites were blocked by incubation with TBST (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.1% Triton X-100) containing 5.5% normal goat serum. Cells were then incubated in a humidified atmosphere at 4°C with anti-phospho-ERK (1:250 dilution) in TBS (50 mM Tris-HCl [pH 7.4], 150 mM NaCl) containing 3% bovine serum albumin for 24 h. After being washed with TBST, cells were incubated with rhodamine-conjugated goat anti-rabbit immunoglobulin antibody (Sigma) at a 1:200 dilution in TBST-3% bovine serum albumin containing 4′,6′-diamidino-2-phenylindole (DAPI) for 1 h at room temperature. The cells were then washed with TBST three times and mounted for analysis with a Leica TCS SP2 confocal spectral microscope.
Transient-transfection and luciferase assays.
D2 cells were transiently transfected by the DEAE-dextran method as described previously (42). After transfection for 48 h, cells were washed and lysed in reporter lysis buffer (0.5 M HEPES [pH 7.8], 0.2% Triton X-100, 1 mM CaCl2, 1 mM MgCl2), and 50 μl of the cell lysates was mixed with 50 μl of luciferase assay buffer (Packard). The luciferase activity was measured with a luminescence counter (Packard). For K562 cells, we used electroporation for transfection; 5 × 106 cells were suspended in 0.4 ml of RPMI 1640 medium containing 30 μg of plasmid DNA and electroporated by Gene Pulser (Bio-Rad) at 300 mV. Cells were then resuspended in growth medium for 24 h for subsequent treatment.
Western blotting.
Samples containing equal amounts of proteins were separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to a polyvinylidene difluoride membrane (Millipore). The antibodies used and their dilutions were as follows: rabbit polyclonal anti-phospho-ERK1/2, 1:2,000; anti-ERK1/2, 1:1,000; human TK (hTK), 1:2,000; anti-MLC, 1:2,000; anti-phospho-MLC, 1: 250; anti-p21Cip1/Waf1, 1:1,000; and anti-Myc, 1:500. Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) or anti-mouse IgG antibody (Amersham) was used for detection of the primary antibodies. Enhanced chemiluminescence detection of the horseradish peroxidase reaction was performed according to the vendor's instructions.
RESULTS
Differential regulation of p21Cip1/Waf1 expression in PMA-induced differentiating and proapoptotic D2 cells.
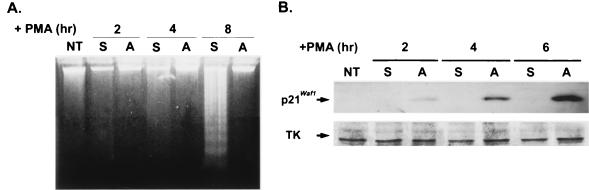
D2 is a cytokine-independent variant of a human hematopoietic progenitor cell line, TF-1. Addition of PMA to the culture medium induced 54% of D2 cells to an adherent phenotype in 2 h (13). The rest of the cells remained in suspension and became apoptotic in 8 h, as indicated by DNA laddering (Fig. 1A). When the suspended D2 cells collected after 30 min of PMA treatment were replated on the new culture dishes, all the cells still developed an apoptotic phenotype (data not shown). These observations suggested that PMA treatment triggers an early change in a subpopulation of D2 cells that leads to apoptosis. Since most of the attached cells survived and were induced to complete differentiation to monocyte-macrophage after 3 days, the attached cells obtained within 8 h of PMA induction are considered to be in the prodifferentiation state. As expected, the immunoblot analysis of these two populations of cells showed that the expression of p21Cip1/Waf1 was induced in the attached (i.e., prodifferentiating) cells but not in the suspended (i.e., the proapoptotic) fraction, compared to the nearly constant expression of TK in both fractions of cells (Fig. 1B).
FIG. 1.
Expression of p21Cip1/Waf1 in PMA-induced adherent but not in proapoptotic cells. D2 cells were treated with PMA (32 nM) in serum-containing medium for the indicated times. (A) After the PMA treatment, cells remaining in suspension (S) and attached (A) to the culture flask were harvested, and their genomic DNAs were analyzed by DNA fragmentation analysis. NT, nontreated. (B) Total cell lysates containing 50 μg of protein were separated by SDS-PAGE, followed by Western blot analysis with antibodies against p21Cip1/Waf1 and TK.
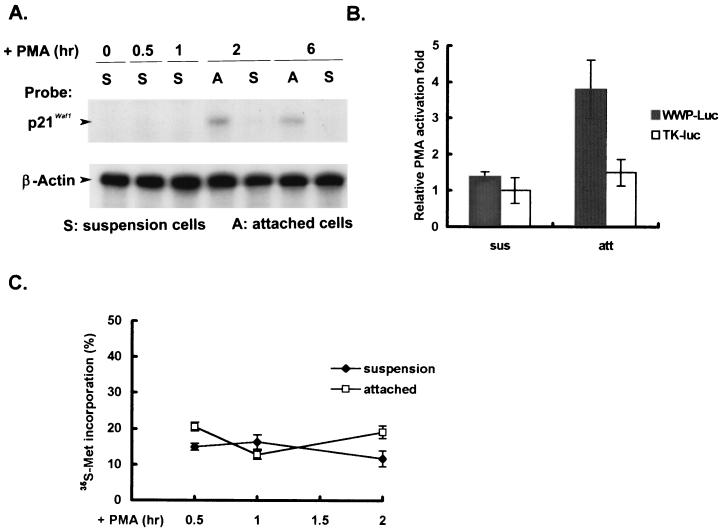
Using RNase protection analysis, we further demonstrated that increased expression of p21Cip1/Waf1 RNA was clearly associated with PMA-induced prodifferentiation but not with proapoptosis and that the induction was seen as early as 2 h after PMA treatment (Fig. 2A). To know whether transcription control is involved in the differential expression of p21Cip1/Waf1 RNA, we examined the transcriptional activity of the p21Cip1/Waf1 promoter in PMA-induced attached and suspended cells. We observed that the p21Cip1/Waf1 promoter was specifically activated to drive the expression of a luciferase reporter gene to a threefold increase after 2 h of PMA treatment in the attached but not in the suspended fraction (Fig. 2B). For comparison, the luciferase activities driven by the hTK promoter were similar in both fractions of cells regardless of PMA treatment. Note that here we treated cells with PMA for only 2 h in all assays related to transcriptional control to minimize possible side effects from the apoptotic process.
FIG. 2.
Preferential activation of p21Cip1/Waf1 transcription in PMA-induced attached cells. (A) Total cellular RNAs were isolated from cells that remained in suspension (S) and those attached to the flask (A) after PMA treatment for the times indicated. RNase protection assays were performed with RNA samples and specific riboprobes for detecting p21Cip1/Waf1 and β-actin RNA as described in Materials and Methods. (B) D2 cells were transfected with the p21Cip1/Waf1 promoter-luciferase, pWWP-Luc, or hTK-Luc plasmid. After transfection for 48 h, cells were treated or not with PMA for 2 h and harvested for luciferase assays. Activation of luciferase activity was expressed relative to the luciferase activities from the same set of transfected cells without PMA treatment. The values shown are the mean fold activation of duplicates ± standard deviation. The data shown are representative of three independent experiments. Note that here we treated cells with PMA for only 2 h to minimize possible side effects involving the apoptotic process in the suspended cells. sus, suspended; att, attached. (C) Following PMA treatment, the suspended and attached cells collected at the times indicated were washed separately with PBS three times,. These two fractions of cells obtained at same time point were pooled and incubated in methionine-free medium containing PMA in the presence of [35S]methionine (40 μCi/ml) for 10 min, after which the suspended and attached cells were collected separately and washed twice with PBS. The cells were then lysed in RIPA (radioimmunoprecipitation assay) lysis buffer. Radioactive methionine present in the cell lysates and incorporation into 10% trichloroacetic acid-precipitable material were individually determined with a liquid scintillation counter. Data are averages of two independent experiments and expressed as a percentage of radioactive methionine incorporation into acid-insoluble material.
Because the induction difference in luciferase activity might be due to a difference in translational efficiency between the attached and suspended cells during 2 h of PMA induction, we then examined the amounts of [35S]methionine incorporated into acid-insoluble materials in these two fractions of cells and found that the levels of general protein newly synthesized were quite similar in the two populations of cells during the 2-h induction period (Fig. 2C). Together with the results of the RNase protection analysis, it is reasonable to conclude that transcription activation of p21Cip1/Waf1 is preferentially enhanced in prodifferentiating but not in proapoptotic D2 cells.
Upregulation of p21Cip1/Waf1 expression by PMA requires activation of the MEK pathway, which is not defective in proapoptotic cells.
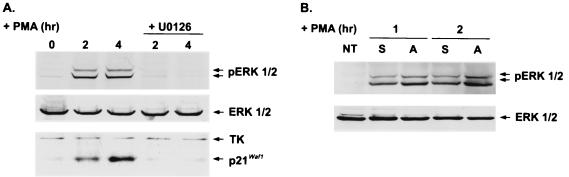
Although it has been reported that Sp1- and Ap2-mediated transcriptional activation is involved in PMA-induced activation of the p21Cip1/Waf1 promoter (7, 41, 71), we did not find any difference in Sp1 and Ap2 binding activities in nuclear extracts of PMA-treated suspended and adherent D2 cells (data not shown), indicating that the transcriptional factors that are involved in activation of the p21Cip1/Waf1 promoter are not defective in the proapoptotic cells. Using the MEK inhibitor U0126 (20), we showed that PMA-induced expression of the p21Cip1/Waf1 protein and phosphorylation of ERK1/2 were completely abolished by blocking MEK activation, suggesting that MEK activation is necessary for p21Cip1/Waf1 induction (Fig. 3A).
FIG. 3.
Activation of MEK pathway is required for PMA-induced p21Cip1/Waf1 expression and is not impaired in proapoptotic cells. (A) D2 cells were treated with PMA (32 nM) in the presence or absence of U0126 (50 μM). Total cells treated as indicated were harvested for Western blotting with antibodies against phospho-ERK (pp42, pp44), ERK, TK, and p21Cip1/Waf1. (B) D2 cells were treated with PMA (32 nM) for the times indicated. The attached (A) and suspended (S) cells were harvested for Western blot analysis using antibodies against phospho-ERK (pp42, pp44), and ERK. NT, not treated.
We then sought to determine whether there is a disruption in the Raf-1/MEK/ERK cascade triggered by PKC activation in the PMA-induced proapoptotic cells. Because activation of ERK is indicated by its phosphorylation status, we next examined whether ERKs are activated differently in PMA-induced attached and proapoptotic cells by directly measuring the amount of phospho-ERK. We found that the extent of PMA-induced ERK phosphorylation was comparable in both attached and proapoptotic suspended cells (Fig. 3B). These data suggest that the PKC/Raf1/MEK/ERK pathway is still intact in PMA-induced apoptotic cells.
Translocation of phospho-ERK from cytoplasm to nucleus occurs in adherent cells but not in proapoptotic cells.
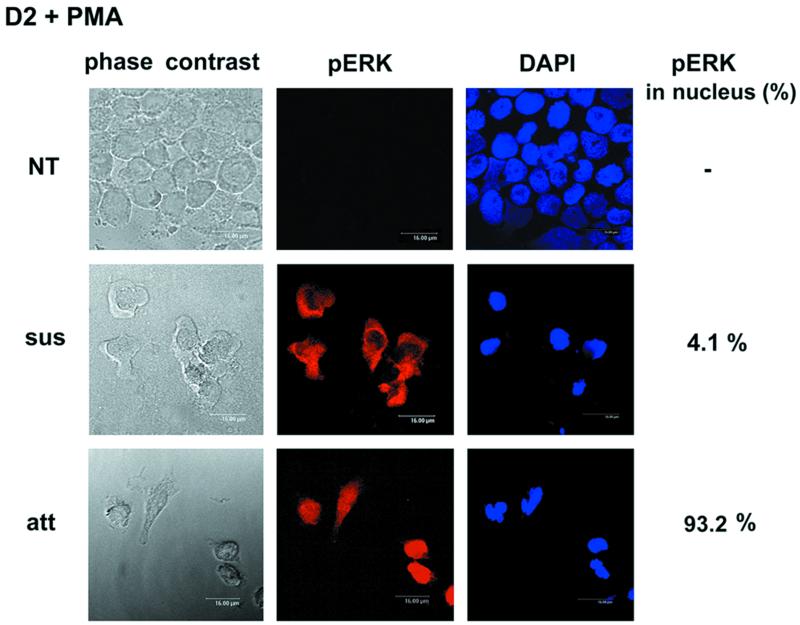
Given that MEK activation is necessary for PMA-induced activation of p21Cip1/Waf1 induction, the above findings prompted us to further examine whether a signal downstream of ERK phosphorylation is disrupted in the proapoptotic cells. In view of the facts that many ERK substrates are transcription factors and ERK nuclear translocation is thought to be crucial for its role in modulating gene expression (3, 9, 39, 44), we used immunostaining processed by confocal microscopy to observe the cellular distribution of phospho-ERK. As shown in Fig. 4, phospho-ERK could be found in the nuclei of PMA-induced attached cells, whereas in proapoptotic suspended cells it remained in the cytosol. This observation pointed out the possibility that the difference in subcellular localization of phospho-ERK is responsible for the differential expression of p21Cip1/Waf1 in these two populations of cells.
FIG. 4.
Distribution of phospho-ERK in D2 cells after PMA treatment. Suspended and attached D2 cells treated or not with PMA (32 nM) for 3 h were fixed and incubated with phospho-ERK antibody, followed by visualization with rhodamine-conjugated secondary antibody. Corresponding nuclear staining was performed with DAPI. The number of cells containing nuclear phospho-ERK was counted in randomly chosen fields and expressed relative to total phospho-ERK-positive cell number (>200 cells). NT, not treated.
PMA-induced activation of the p21Cip1/Waf1 promoter requires ERK nuclear translocation.
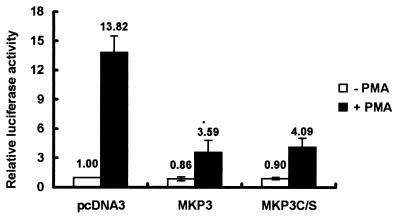
Because the substrates of ERK are widely distributed in various subcellular locations, including membrane, cytoplasm, and nuclei, it is uncertain whether nuclear translocation of phospho-ERK is a critical event needed for PMA-induced activation of p21Cip1/Waf1 gene expression. To determine whether activation of the p21Cip1/Waf1 promoter requires phospho-ERK nuclear translocation, we expressed the wild-type MKP3 [MKP3(wt)] or mutant MKP3(C/S) in D2 cells to examine their effects on PMA-induced activation of the p21Cip1/Waf1 promoter. MKP3, a cytosolic phosphatase of dual specificity (26, 50), has been shown to specifically dephosphorylate phospho-ERK, and its inactive mutant MKP3(C/S) can retain phospho-ERK in the cytosol and abolish Elk-mediated transcriptional activation (9). In addition, it has been shown that overexpression of MKP3(C/S), which carries a mutation causing loss of phosphatase activity, specifically prevents phospho-ERK from entering the nucleus without dephosphorylating and inactivating phospho-ERK (9).
In this experiment, a 4-h PMA induction was conducted in serum-free medium for cells transfected with the p21Cip1/Waf1 promoter-luciferase plasmid and the indicated expression vector. Under these serum-free conditions, more than 95% of D2 cells were attached and expressed about 14-fold more p21Cip1/Waf1 promoter activity in response to PMA treatment. In the presence of MKP3(wt) or MKP3(C/S) expression, PMA-induced activation of the p21Cip1/Waf1 promoter was significantly reduced (Fig. 5). These results indicated that not only ERK activation but also its nuclear translocation is required for PMA-induced activation of the p21Cip1/Waf1 promoter and suggested that the lack of phospho-ERK in the nuclei of PMA-induced proapoptotic cells contributes to the impairment in p21Cip1/Waf1expression in these cells.
FIG. 5.
Expression of active and inactive forms of MKP3 diminishes activation of the p21Cip1/Waf1 promoter by PMA. D2 cells were transfected with pWWP-Luc plasmid (1 μg) and pCMV-β-galactosidase (1 μg) in combination with 3 μg of pcDNA3, pMKP3, or pMKP3(C/S) expression plasmid. After transfection for 2 days, cells were washed and incubated in serum-free medium in the presence or absence of PMA for 4 h. Cells were then harvested for luciferase assays. Luciferase activity in the cells without PMA treatment was normalized by β-galactosidase activity, and the activity of cells cotransfected with the control vector, pcDNA3, was set arbitrarily to 1. Because PMA treatment can rapidly activate the cytomegalovirus promoter in hematopoietic cells, the relative luciferase activities of the basal and PMA-driven p21Cip1/Waf1 promoter were calculated directly from the same set of transfected cells. The data represent averages for three independent experiments.
Involvement of LPA signaling in PMA-induced activation of p21Cip1/Waf1 transcription and nuclear translocation of phospho-ERK.
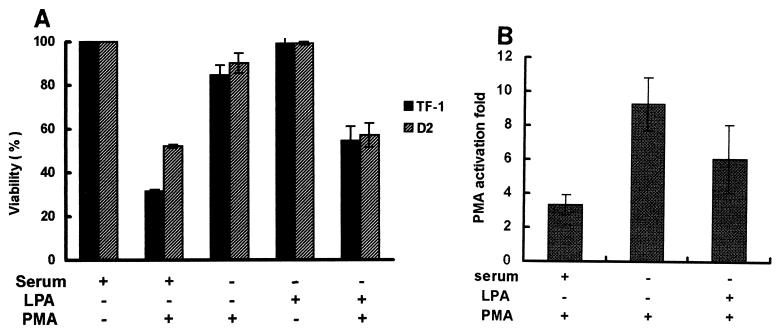
As mentioned above, unlike in the serum-containing medium, most (90 to 95%) D2 and TF-1 cells in serum-free medium became attached to the culture plate and survived after PMA treatment, instead of turning apoptotic (Fig. 6A). After addition of serum or LPA to the serum-free medium, 40 to 55% and 30 to 35% of the cells, respectively, underwent PMA-induced apoptosis. The presence of serum or LPA in the serum-free medium in the absence of PMA did not affect cell viability. Presumably, the LPA- or serum-mediated signaling occurs through the Rho-dependent pathway to interfere with adhesion (42). The activity of the p21Cip1/Waf1 promoter was significantly increased about ninefold by PMA treatment for 3 h in serum-free medium, compared to a three- and fivefold increase in serum- and LPA-containing medium, respectively (Fig. 6B).
FIG. 6.
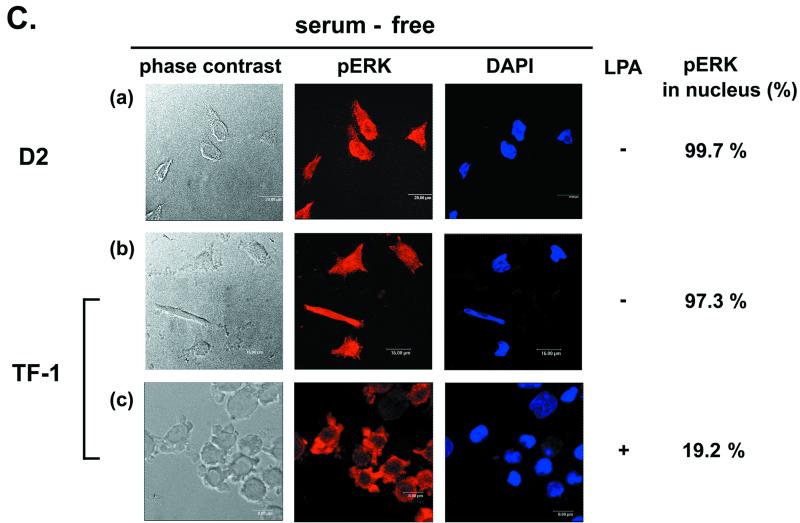
Serum and LPA can affect activation of the p21Cip1/Waf1 promoter and phospho-ERK nuclear translocation during PMA treatment. (A) TF-1 and D2 cells (2 × 105/ml) were suspended in serum-free medium. After incubation of cells without or with 10% heat-inactivated fetal bovine serum or LPA (10 μM), as indicated, for 10 min, PMA (32 nM) was added to the medium. After 12 h of PMA treatment, viable cells adhering to the plates were counted. (B) D2 cells were transfected with the pWWP-Luc plasmid. After transfection for 2 days, cells were washed and suspended in serum-containing, LPA-containing, or serum-free medium, followed by treatment with PMA (32 nM) or no treatment for 3 h for luciferase assays. PMA-induced activation was calculated as described in the legend to Fig. 2B. For the cells treated with PMA in serum-containing medium, the suspended and attached cells were pooled for the luciferase activity assay. The data represent averages for three independent experiments. (C) D2 and TF-1 cells treated with PMA in serum-free medium for 3 h were fixed for immunofluorescent detection of phospho-ERK and nuclear DAPI staining (a and b). TF-1 cells incubated with LPA (10 μM) in serum-free medium were treated with PMA for 3 h for immunofluorescent detection as described above (c). The percentage of nuclear phospho-ERK-containing cells in the total number of phospho-ERK-positive cells was calculated as described in the legend to Fig. 4.
As expected, phospho-ERK was well distributed in the nuclei of D2 and TF-1 cells cultured in serum-free medium with PMA treatment (Fig. 6C). These results indicated that the machinery for nuclear translocation of phospho-ERK and activation of the p21Cip1/Waf1 promoter is functional in most cells under the serum-free conditions. In another experiment, we showed that addition of LPA to the serum-free medium caused TF-1 cells to retain phospho-ERK in the cytosol after PMA treatment (Fig. 6C). Together, it seems that the LPA signaling pathway in the proapoptotic fraction of cells can influence ERK-mediated p21Cip1/Waf1 induction.
ROCK is involved in preferential phosphorylation of MLC and impairment of p21Cip1/Waf1 induction in PMA-induced proapoptotic cells.
Next, we turned to investigating whether the downstream event activated by LPA/RhoA signaling is specifically upregulated in PMA-induced proapoptotic cells. It is well established that the serine/threonine kinase Rho-associated kinase, also known as ROCK, is one of the downstream targets of RhoA (1). ROCK activated by Rho signaling has been shown to phosphorylate MLC and also to inhibit MLC phosphatase activity by phosphorylating its myosin-binding subunit (2, 40). Therefore, the MLC phosphorylation status may serve as an indicator of whether ROCK-mediated signaling is active in the cells.
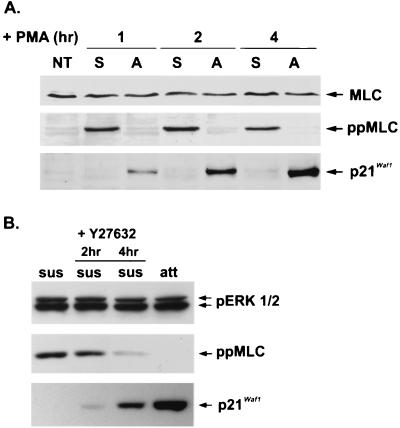
Using the ppMLC antibody, specifically recognizing MLC phosphorylated on Thr18 and Ser19 (58), we found that MLC phosphorylation was readily detected in PMA-induced proapoptotic cells but not in adherent cells. An inverse relationship between MLC phosphorylation and p21Cip1/Waf1 expression in response to PMA was observed in these two populations of cells (Fig. 7A). We then used a ROCK-specific inhibitor, Y27632 (51, 63), to determine whether the active ROCK was involved in both MLC phosphorylation and impairment of p21Cip1/Waf1 expression in the proapoptotic cells. Following PMA treatment for 30 min, suspended cells, which were in the early proapoptotic stage, were collected and replated in a new culture dish in the presence or absence of Y27632 for another 2 or 4 h of incubation in PMA-containing medium. MLC phosphorylation was decreased and p21Cip1/Waf1 expression was increased in the suspended cells treated with Y27632, while the level of phospho-ERK was not affected (Fig. 7B). In contrast, the same set of cells incubated in the absence of Y27632 contained phosphorylated MLC and did not express p21Cip1/Waf1. These findings not only suggested that ROCK-mediated signal is necessary for MLC phosphorylation in the suspended cells, but also implied that regulation of ROCK can affect p21Cip1/Waf1 expression in the proapoptotic fraction of cells.
FIG. 7.
Reverse relationship between MLC phosphorylation and p21Cip1/Waf1 induction in D2 cells in response to PMA treatment. (A) D2 cells were treated with PMA (32 nM) in serum-containing medium for the indicated times. Cells remaining in the suspension (S) and those attached (A) to the culture dish were harvested. Equal amounts of cell extracts were analyzed by Western blotting with antibodies against p21Cip1/Waf1, phospho-MLC, and MLC. NT, not treated. (B) After treatment with PMA for 30 min, cells remaining in suspension (sus) were transferred to a fresh dish for incubation for another 2 and 4 h in the presence or absence of Y27632 (20 μM), as indicated. The remaining attached cells (att) that were continuously incubated in the PMA-containing medium and the suspended cells treated or not with Y27632 as described above were collected at the same time for Western blot analysis with antibodies against p21Cip1/Waf1, phospho-ERK (pp42, pp44), and phospho-MLC.
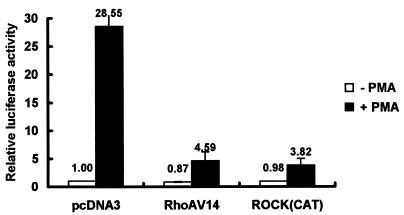
To further substantiate these findings, we cotransfected a dominant active form of RhoAV14 or ROCK(CAT) with a p21Cip1/Waf1 promoter-luciferase plasmid into D2 cells and examined PMA-induced activation of the p21Cip1/Waf1 promoter under serum-free conditions, which minimized serum- or LPA-mediated signaling as described for Fig. 6A. RhoAV14 is a constitutively active form of RhoA that carries a mutation at its GAP (GTPase-activating protein) interaction site (37). ROCK(CAT), which contains only an amino-terminal kinase domain, is also a constitutively active form of ROCK (33). Clearly, both RhoAV14 and ROCK(CAT) had a repressive effect on activation of the p21Cip1/Waf1 promoter induced by PMA (Fig. 8), implying that activation of ROCK signaling in proapoptotic D2 cells can simultaneously suppress PMA-induced transcriptional activation of p21Cip1/Waf1 expression.
FIG. 8.
Expression of dominant active form of RhoA or ROCK suppressed PMA-induced activation of the p21Cip1/Waf1 promoter. (A) D2 cells were transfected with 1 μg each of plasmids pWWP-Luc and pCMV-β-galactosidase in combination with 3 μg of pcDNA3, pCMV-RhoAV14, or pEF-ROCK(CAT) as indicated. After transfection for 2 days, cells were washed and incubated in serum-free medium in the presence or absence of PMA (32 nM) for 5 h. Cells were harvested for luciferase and β-galactosidase assays. PMA-induced activation was calculated as described in the legend to Fig. 5. The data represent averages for three independent experiments.
PMA-induced p21Cip1/Waf1 expression is suppressed by overexpressing ROCK(CAT) in K562 cells.
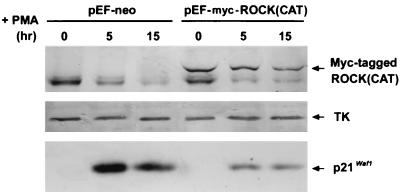
We further used K562 cells, which underwent only the megakaryocytic differentiation program accompanied by p21Cip1/Waf1 induction after PMA treatment, to test whether activated ROCK can also suppress PMA-induced expression of p21Cip1/Waf1 in other suspended cells. The induction of p21Cip1/Waf1 by PMA was examined in K562 cells that were transfected with an expression vector of the dominant active form of ROCK(CAT) or a control vector. Although the transfection efficiency was about 40%, as judged by a parallel transfection with an expression vector of green fluorescent protein (pEGFP), we could still observe that the expressed level of p21Cip1/Waf1 was reduced in K562 cells expressing Myc-tagged ROCK(CAT) during PMA treatment (Fig. 9). Thus, activated ROCK is also able to generate a signal to suppress PMA-induced expression of p21Cip1/Waf1 in another hematopoietic suspended cell line.
FIG. 9.
Overexpression of ROCK(CAT) suppresses p21Cip1/Waf1 induction in PMA-treated K562 cell. K562 cells were transfected with pEF-neo or pEF-Myc-ROCK(CAT) by electroporation as described in Materials and Methods. After 24 h, cells were treated with PMA (50 nM) for the times indicated and harvested for Western blot analysis with antibodies against p21Cip1/Waf1, Myc, and TK.
Inhibition of ROCK switches on p21Cip1/Waf1 induction and phospho-ERK nuclear translocation in TF-1 cells with PMA stimulation.
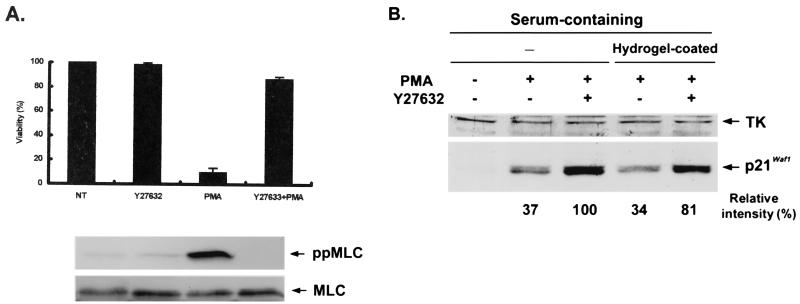
Reciprocally, we tested whether inhibition of ROCK in TF-1 cells can lead to a different outcome in response to PMA, because it has been shown previously that about 80% of TF-1 cells remain in suspension and become proapoptotic when treated with PMA in serum-containing medium (13, 42). As shown in Fig. 10A, preincubation of TF-1 cells with Y27632 for 30 min in serum-containing medium significantly reduced PMA-induced apoptosis, and more than 90% of the TF-1 cells became attached to the plate and viable. PMA-induced MLC phosphorylation was detected in TF-1 cells and was abolished by pretreatment of the cells with Y276323. In accordance with the results from D2 cells, a concomitant increase in p21Cip1/Waf1 induction (Fig. 10B) and nuclear translocation of phospho-ERK (Fig. 10C, a versus b) were also observed in PMA-treated TF-1 cells pretreated with Y27632.
FIG. 10.
Inhibition of ROCK affects PMA-induced apoptosis, p21Cip1/Waf1 induction, and phospho-ERK distribution in TF-1 cells. (A) TF-1 cells in serum-containing medium were incubated with or without Y27632 (20 μM) for 30 min prior to treatment with PMA (32 nM). After 12 h, viability was determined for each sample by the trypan blue dye exclusion method and expressed relative to that of cells incubated in the control medium (NT). Cells from a parallel set were harvested at 3 h after PMA treatment for Western analysis of phospho-MLC and total MLC. (B) TF-1 cells in serum-containing medium were treated or not with Y27632 and plated in the culture dish without (−) or with Hydrogel coating. The Hydrogel coating prevents the attachment of cells to the plastic. After treatment with PMA for 5 h, cells were harvested for Western blot analysis with antibodies against TK and p21Cip1/Waf1. For the cells plated in the regular dishes (−) after PMA treatment, the suspended and attached cells were pooled for cell lysate preparation. By densitometric scanning, the intensity of p21Cip1/Waf1 in the cells treated with PMA and Y27632 was set to 100%, and the relative intensity of p21Cip1/Waf1 expression in each lane is shown. (C) TF-1 cells treated as described above were collected for immunofluorescent detection of phospho-ERK and nuclear staining. The percentage of nuclear phospho-ERK-containing cells among all phospho-ERK-positive cells was calculated as described in the legend to Fig. 4.
Because adhesion is always associated with the extent of p21Cip1/Waf1 induction, we further examined whether restoration of p21Cip1/Waf1 induction and phospho-ERK translocation by ROCK inhibition are dependent on its effect on cell adhesion. TF-1 cells were treated with Y27632 before PMA stimulation and plated on Hydrogel-coated dishes to prevent cell adhesion. TF-1 cells that were treated with Y27632 and plated on the Hydrogel-coated dishes following PMA treatment still displayed an increase in p21Cip1/Waf1 induction and phospho-ERK nuclear translocation (Fig. 10B and C, c) and became rounded again and viable after longer treatment. Although the extents of phospho-ERK in nuclei and p21Cip1/Waf1 induction in these cells plated in Hydrogel-coated dishes were not as great as that seen in the adherent cells, i.e., about 20% less, as determined by densitometric scanning of the expressed levels of p21Cip1/Waf1, our results, to some extent, suggested that nuclear translocation of phospho-ERK and PMA-activated p21Cip1/Waf1 induction can occur in nonadherent TF-1 cells when the ROCK-mediated signal is inhibited.
DISCUSSION
In this study, we explored the mechanism by which the PMA-activated signal interferes in the process leading to transcriptional activation of p21Cip1/Waf1 in PMA-induced proapoptotic cells. Several observations were made. First, the transcriptional activation of p21Cip1/Waf1 in PMA-induced differentiating cells requires activation of MEK/ERK and nuclear translocation of phospho-ERK. Phospho-ERK was found to be retained in the cytosol of PMA-induced proapoptotic cells regardless of whether the PKC/Raf1/MEK/ERK pathway is intact. Second, ROCK-dependent MLC phosphorylation is detected only in PMA-induced proapoptotic cells, not in the differentiating cells. Third, activation of ROCK impairs PMA-induced activation of the p21Cip1/Waf1 promoter in D2 cells. Last, inhibition of the ROCK pathway restores phospho-ERK nuclear translocation and p21Cip1/Waf1 induction in PMA-treated cells. From these findings, we propose that the ROCK-mediated signaling pathway may contribute, in part, to a cellular context that prohibits nuclear translocation of phospho-ERK and impairs subsequent p21Cip1/Waf1 induction in the PMA-induced proapoptotic cells.
The most striking result of this study was that ROCK-mediated MLC phosphorylation was detected only in the apoptotic cells, not in the surviving D2 cells, after PMA treatment. Similar results were also obtained upon PMA treatment of TF-1 cells. We found that MLC kinase and ERK are not involved in MLC phosphorylation in the proapoptotic cells, because pretreatment of D2 and TF-1 cells with ML-7 or ML-9, two specific inhibitors of MLC kinase, or U0126 did not cause inhibition of MLC phosphorylation after PMA stimulation (data not shown). Since MLC phosphorylation is a PMA-induced event, it is possible that another molecular event is also involved in MLC phosphorylation in PMA-induced proapoptotic cells. In this regard, the CPI protein, an inhibitor of MLC phosphatase, has been reported to be phosphorylated by protein kinase C and to cooperate with ROCK to account for MLC phosphorylation in human platelet secretion (68). Whether CPI is also involved in MLC phosphorylation in PMA-induced proapoptotic cells remains to be studied.
It has been shown previously that Rho-mediated signaling suppresses p21Cip1/Waf1 expression, thus enabling Ras to stimulate cell cycle progression in 3T3 fibroblasts (55), and this mechanism is important for the role of Rho in oncogenic Ras-induced transformation (5, 57). Although there are diverse pathways downstream of the RhoA signal (5), the mechanism responsible for RhoA-mediated suppression of p21Cip1/Waf1 has remained unclear. This study provides the first evidence that activation of ROCK, one of the downstream effectors, downregulates the expression of p21Cip1/Waf1 in TF-1 and D2 cells undergoing PMA-induced apoptosis. Our results showed that activation of ROCK generates a signal that interferes with the transcriptional activation of the p21Cip1/Waf1 promoter induced by PMA.
A recent report, in contrast, has demonstrated that treatment of Ras-transformed 3T3 cells with Y27632 cannot restore the expression of p21Cip1/Waf1, whereas the RhoA inhibitor C3 can activate p21Cip1/Waf1 induction (60). These data suggest that another downstream effector of RhoA, which is not present in ROCK, is responsible for RhoA-mediated inhibition of p21Cip1/Waf1 activation in Ras-transformed 3T3 cells, where ROCK 1 and 2 are found to be present only in the Triton X-100-insoluble fraction and are probably inactivated. It has also been shown that Rat1 cells expressing a dominant active form of RasV12 are defective in ROCK-mediated stress fiber formation (33). These studies suggest that sustained MEK/ERK activation resulted from oncogenic Ras signaling downregulates ROCK. In our case, however, ROCK-dependent phosphorylation of MLC was clearly demonstrated in PMA-induced proapoptotic D2 and TF-1 cells, indicating that the MEK/ERK pathway in these cells does not lead to the inactivation of ROCK, which is therefore susceptible to Y27632-mediated inhibition.
Here, we speculate that the discrepancy in the effect of Y27632 on ERK-mediated p21Cip1/Waf1 induction is probably due to the differential regulation of ROCK activation by the MEK/ERK pathway in these cells, which in turn contributes to the differences in expression of p21Cip1/Waf1 in response to the PMA signal. Our results that overexpression of the dominant active form of ROCK markedly decreased ERK-mediated transcriptional activation of the p21Cip1/Waf1 promoter in D2 cells and the expression of p21Cip1/Waf1 protein in PMA-treated K562 cells support the notion that activation of ROCK confers a mechanism leading to the suppression of p21Cip1/Waf1 induction in the proapoptotic cells.
In addition to MEK/ERK, c-Jun N-terminal protein kinase (JNK) and p38 MAP kinase are two other members of separate MAP kinase modules, which are generally linked to apoptosis (21, 30, 32, 36, 43, 64, 69). Therefore, it is also possible that JNK or p38 MAP kinase is preferentially activated in the proapoptotic cells, resulting in upregulation of ROCK. However, we did not find a difference in JNK activity in extracts prepared from PMA-induced suspended and attached cells (data not shown), nor did the inhibitor of p38 MAP kinase, SB 203580, have an effect on the PMA-induced outcome in terms of p21Cip1/Waf1 induction or cell survival.
To explore whether the status of individual cell cycle phases is relevant to the differential regulation of ROCK in these two populations of cells, flow cytometric analysis of PMA-treated cells was performed. Although the suspended fraction consisted of more cells in the S and G2/M phases, whereas the attached population contained a larger proportion of cells in the G0/G1 phase (data not shown), it appeared that both the suspended and attached cell populations contained a significant proportion of cells distributed among the G0/G1, S, and G2/M phases. Therefore, it is very unlikely that a particular cell cycle phase determines whether cells stay in suspension or become adherent upon PMA stimulation.
Numerous studies have shown that disruption of p21Cip1/Waf1 induction using the antisense expression approach can result in a switch from a differentiation to an apoptotic program in U937 and HL-60 leukemia cells during treatment with phorbol ester or 1-β-d-arabinofuranosylcytosine (22, 65, 66, 67). It is believed that p21Cip1/Waf1 expression confers a survival advantage during differentiation induction. Accordingly, it therefore seems possible that the expression of p21Cip1/Waf1 plays a role in modulating the Rho/ROCK pathway. However, we found that ectopic overexpression of p21Cip1/Waf1 did not prevent the PMA-induced suspended cells from undergoing apoptosis (data not shown), indicating that the lack of p21Cip1/Waf1 expression in this fraction of cells is not responsible for the occurrence of apoptosis. Rather, it is clearly the ROCK-mediated signal that is necessary for the apoptotic stimulation induced at the early stage, since pretreatment of D2 and TF-1 cells with Y27632 can cause more than 95% of cells to become attached and survive during PMA induction (Fig. 10A). In this case, restoration of p21Cip1/Waf1 induction by ROCK inhibition may represent one of the early events associated with survival during PMA induction.
In addition, we found that pretreatment of cells with cycloheximide had no effect on the occurrence of apoptosis and adhesion following PMA treatment for 8 h (data not shown). Therefore, the decision for apoptosis during the early stage examined in this study is independent of protein synthesis and does not require the presence of p21Cip1/Waf induction. In view of the fact that p21Cip1/Waf is required for the engagement of a differentiation program, we believe that its role in providing a survival signal is probably engaged at the later stage, i.e., following PMA treatment for 24 h, as seen in another report (65). Further detailed experiments, such as blocking p21Cip1/Waf1 in the attached cells, is certainly required to address the question of whether disruption of p21Cip1/Waf1 in the attached cells can affect the regulation of ROCK at the later stage and then trigger the apoptotic stimulation.
Our previous study has shown that the activated RhoA signal promotes PMA-induced apoptosis by interfering with PMA-induced adhesion (42). Therefore, it is possible that the effect of ROCK inhibition occurs through promotion of cell adhesion, enhancing p21Cip1/Waf1 induction and phospho-ERK nuclear translocation. However, our results showed that nuclear distribution of phospho-ERK and p21Cip1/Waf1 induction still occurred in those cells plated on a Hydrogel-coated dish when ROCK was inhibited by Y27632, suggesting that adhesion is not a prerequisite step for phospho-ERK nuclear translocation in TF-1 cells when ROCK activity is blocked. It has been shown that phospho-ERK fails to accumulate in the nuclei of suspended 3T3 fibroblasts (3). On the other hand, another report has demonstrated that Rho activity remains elevated in suspended 3T3 fibroblasts compared to its downregulation during cell adhesion (59). Linking these observations with the results obtained in this study, it will be interesting to further examine whether there is a regulatory relationship between the Rho/ROCK pathway and nuclear translocation of phospho-ERK in the suspended cells.
Finally, it is worth noting that inhibition of ROCK by Y27632 in the suspended cells did not restore expression of p21Cip1/Waf1 to a level similar to that in the PMA-induced attached cells during the 4 h of treatment (Fig. 7B). Therefore, it is possible that a change at the level of posttranscriptional control may also contribute to the difference in p21Cip1/Waf1 induction in PMA-induced proapoptotic and prodifferentiating cells. In brief, our overall data presented here identify the ROCK-mediated signal and cytosolic retention of phospho-ERK as a part of the cellular context in the proapoptotic cells that contributes to the impairment of p21Cip1/Waf1 induction during PMA stimulation.
Acknowledgments
We are grateful to H.-F. Yang-Yen (Academia Sinica, Taipei, Taiwan) for providing D2 and TF-1 cells, T.-S. Jou (National Taiwan University, Taipei, Taiwan) for expression plasmids RhoAV14, and ROCK(CAT), which were originally obtained from K. Kaibuchi (Nara Institute of Science and Technology, Ikoma, Japan), B. Vogelstein (Johns Hopkins University School of Medicine, Baltimore, Md.) for pWWP-Luc, J. Pouyssegur (Université de Nice, Nice, France) for expression vectors MKP3(wt) and MKP3(C/S), and J. M. Staddon (University College London, London, United Kingdom) for antibody against ppMLC. We also thank C.-L. Chien (National Taiwan University, Taipei, Taiwan) for guidance on confocal microscopy, which was supported by grant 89-B-FA01-1-4, J.-Y. Chen and W.-N. Wen for critical reading of the manuscript, and A. Hall for the suggestion on MLC phosphorylation.
This research is supported by grant NSC90-2320-B-002-171 from the National Science Council, Taiwan, Republic of China.
REFERENCES
- 1.Amano, M., Y. Fukata, and K. Kaibuchi. 2000. Regulation and functions of Rho-associated kinase. Exp. Cell Res. 261:44-51. [DOI] [PubMed] [Google Scholar]
- 2.Amano, M., M. Ito, K. Kimura, Y. Fukata, K. Chihara, T. Nakano, Y. Matsuura, and K. Kaibuchi. 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271:20246-20249. [DOI] [PubMed] [Google Scholar]
- 3.Aplin, A. E., S. A. Stewart, R. K. Assoian, and R. L. Juliano. 2001. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J. Cell Biol. 153:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asada, M., T. Yamada, H. Ichijo, D. Delia, K. Miyazono, K. Fukumuro, and S. Mizutani. 1999. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 18:1223-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-Sagi, D., and A. Hall. 2000. Ras and Rho GTPases: a family reunion. Cell 103:227-238. [DOI] [PubMed] [Google Scholar]
- 6.Beier, F., A. C. Taylor, and P. LuValle. 1999. The Raf-1/MEK/ERK pathway regulates the expression of the p21Cip1/Waf1 gene in chondrocytes. J. Biol. Chem. 274:30273-30279. [DOI] [PubMed] [Google Scholar]
- 7.Biggs, J. R., J. E. Kudlow, and A. S. Kraft. 1996. The role of the transcription factor Sp1 in regulating the expression of the WAF1/CIP1 gene in U937 leukemic cells. J. Biol. Chem. 271:901-906. [DOI] [PubMed] [Google Scholar]
- 8.Bottazzi, M. E., X. Zhu, R. M. Bohmer, and R. K. Assoian. 1999. Regulation of p21Cip1 expression by growth factors and the extracellular matrix reveals a role for transient ERK activity in G1 phase. J. Cell Biol. 146:1255-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunet, A., D. Roux, P. Lenormand, S. Dowd, S. Keyse, and J. Pouyssegur. 1999. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 18:664-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll, M. P., and W. S. May. 1994. Protein kinase C-mediated serine phosphorylation directly activates Raf-1 in murine hematopoietic cells. J. Biol. Chem. 269:1249-1256. [PubMed] [Google Scholar]
- 11.Chang, Z. F., D. Y. Huang, and T. C. Lai. 1995. Different regulation of human thymidine kinase promoter in normal human diploid IMR-90 fibroblasts and HeLa cells. J. Biol. Chem. 270:27374-27379. [DOI] [PubMed] [Google Scholar]
- 12.Chang, Z. F., D. Y. Huang, and N. C. Hsue. 1994. Differential phosphorylation of human thymidine kinase in proliferating and M phase-arrested human cells. J. Biol. Chem. 269:21249-21254. [PubMed] [Google Scholar]
- 13.Chao, J. R., C. S. Chen, T. F. Wang, L. H. Tseng, J. J. Tsai, M. L. Kuo, J. J. Yen, and H. F. Yang-Yen. 1997. Characterization of factor-independent variants derived from TF-1 hematopoietic progenitor cells: the role of the Raf/MAP kinase pathway in the anti-apoptotic effect of GM-CSF. Oncogene 14:721-728. [DOI] [PubMed] [Google Scholar]
- 14.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 16:156-159. [DOI] [PubMed] [Google Scholar]
- 15.Collins, S. J. 1987. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood 70:1233-1244. [PubMed] [Google Scholar]
- 16.Davis, T. A., A. A. Saini, P. J. Blair, B. L. Levine, N. Craighead, D. M. Harlan, C. H. June, and K. P. Lee. 1998. A phorbol esters induce differentiation of human CD34+ hemopoietic progenitors to dendritic cells: evidence for protein kinase C-mediated signaling. J. Immunol. 160:3689-3697. [PubMed] [Google Scholar]
- 17.de Vente, J. E., C. A. Kukoly, W. O. Bryant, K. J. Posekany, J. Chen, D. J. Fletcher, P. J. Parker, G. J. Pettit, G. Lozano, P. P. Cook, and D. K. Ways. 1995. Phorbol esters induce death in MCF-7 breast cancer cells with altered expression of protein kinase C isoforms. Role for p53-independent induction of gadd-45 in initiating death. J. Clin. Investig. 96:1874-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vente, J., S. Kiley, T. Garris, W. Bryant, J. Hooker, K. Posekany, P. Parker, P. Cook, D. Fletcher, and D. K. Ways. 1995. Phorbol ester treatment of U937 cells with altered protein kinase C content and distribution induces cell death rather than differentiation. Cell Growth Differ. 6:371-382. [PubMed] [Google Scholar]
- 19.El-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, W. E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817-825. [DOI] [PubMed] [Google Scholar]
- 20.Favata, M. F., K. Y. Horiuchi, E. J. Manos, A. J. Daulerio, D. A. Stradley, W. S. Feeser, D. E. Van Dyk, W. J. Pitts, R. A. Earl, F. Hobbs, R. A. Copeland, R. L. Magolda, P. A. Scherle, and J. M. Trzaskos. 1998. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273:18623-18632. [DOI] [PubMed] [Google Scholar]
- 21.Frasch, S. C., J. A. Nick, V. A. Fadok, D. L. Bratton, G. S. Worthen, and P. M. Henson. 1998. p38 mitogen-activated protein kinase-dependent and independent pathways leading to apoptosis in human neutrophils. J. Biol. Chem. 273:8389-8397. [DOI] [PubMed] [Google Scholar]
- 22.Freemerman, A. J., J. A. Vrana, R. M. Tombes, H. Jiang, S. P. Chellappan, P. B. Fisher, and S. Grant. 1997. Effects of antisense p21WAF1/CIP1/MDA6 expression on the induction of differentiation and drug-mediated apoptosis in human myeloid leukemia cells (HL-60). Leukemia 11:504-513. [DOI] [PubMed] [Google Scholar]
- 23.Garzotto, M., M. White-Jones, Y. Jiang, D. Ehleiter, W. C. Liao, A. Haimovitz-Friedman, Z. Fuks, and R. Kolesnick. 1998. 12-O-tetradecanoylphorbol-13-acetate-induced apoptosis in LNCaP cells is mediated through ceramide synthase. Cancer Res. 58:2260-2264. [PubMed] [Google Scholar]
- 24.Girard, P. R., V. L. Stevens, P. J. Blackshear, A. H. Merrill, Jr., J. G. Wood, and J. F. Kuo. 1987. Immunocytochemical evidence for phorbol ester-induced directional translocations of protein kinase C in HL60, K562, CHO, and E7SKS cells: possible role in differentiation. Cancer Res. 47:2892-2898. [PubMed] [Google Scholar]
- 25.Gorospe, M., W. Wang, K. Z. Guyton, and N. J. Holbrook. 1996. Protective role of p21Waf1/Cip1 against prostaglandin A2-mediated apoptosis of human colorectal carcinoma cells. Mol. Cell. Biol. 16:6654-6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groom, L. A., A. A. Sneddon, D. R. Alessi, S. Dowd, and S. M. Keyse. 1996. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 15:3621-3632. [PMC free article] [PubMed] [Google Scholar]
- 27.Harper, J. W., G. R. Adami, N. Wei, K. Keyomarsi, and S. J. Elledge. 1993. The p21 cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75:805-816. [DOI] [PubMed] [Google Scholar]
- 28.Harutoshi, K., T. Takushi, O. Cachexia, M. Jun, and Y. Ishimura. 1989. Activation of a suicide process of thymocytes through DNA fragmentation by calcium ionophores and phorbol esters. J. Immunol. 143:1790-1794. [PubMed] [Google Scholar]
- 29.He, H., X. Wang, M. Gorospe, N. J. Holbrook, and M. A. Trush. 1999. Phorbol ester-induced mononuclear cell differentiation is blocked by the mitogen-activated protein kinase kinase (MEK) inhibitor PD98059. Cell Growth Differ. 10:307-315. [PubMed] [Google Scholar]
- 30.Herr, I., D. Wilhelm, E. Meyer, I. Jeremais, P. Angel, and K. M. Debatin. 1999. JNK/SAPK activity contributes to TRAIL-induced apoptosis. Cell Death Differ. 6:130-135. [DOI] [PubMed] [Google Scholar]
- 31.Hu, P. P., X. Shen, D. Huang, Y. Liu, C. Counter, and X. F. Wang. 1999. The MEK pathway is required for stimulation of p21WAF1/CIP1 by transforming growth factor-beta. J. Biol. Chem. 274:35381-35387. [DOI] [PubMed] [Google Scholar]
- 32.Ichijo, H., E. Nishida, Irie, K., P. ten Dijke, M. Saitoh, T. Moriguchi, M. Takagi, K. Matsumoto, K. Miyazono, and Y. Gotoh. 1997. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275:90-94., [DOI] [PubMed] [Google Scholar]
- 33.Izawa, I., M. Amano, K. Chihara, T. Yamamoto, and K. Kaibuchi. 1998. Possible involvement of the inactivation of the Rho-Rho-kinase pathway in oncogenic Ras-induced transformation. Oncogene 17:2863-2871. [DOI] [PubMed] [Google Scholar]
- 34.Jiang, H., J. Lin, Z. Z. Su, F. R. Collart, E. Huberman, and P. B. Fisher. 1994. Induction of differentiation in human promyelocytic HL-60 leukemia cells activates p21WAF1/CIP1 expression in the absence of p53. Oncogene 9:3397-3406. [PubMed] [Google Scholar]
- 35.Jiang, H., J. Lin, Z. Z. Su, M. Herlyn, R. S. Kerbel, B. E. Weissman, D. R. Welch, and P. B. Fisher. 1995. The melanoma differentiation-associated gene mda-6, which encodes the cyclin-dependent kinase inhibitor p21, is differentially expressed during growth, differentiation and progression in human melanoma cells. Oncogene 10:1855-1864. [PubMed] [Google Scholar]
- 36.Jun, C. D., H. O. Pae, H. J. Kwak, J. C. Yoo, B. M. Choi, C. D. Oh, J. S. Chun, S. G. Paik, Y. H. Park, and H. T. Chung. 1999. Modulation of nitrix oxide-induced apoptotic death of HL-60 cells by protein kinase C and protein kinase A through mitogen-activated protein kinases and CPP32-like protease pathways. Cell. Immunol. 194:36-46. [DOI] [PubMed] [Google Scholar]
- 37.Kaibuchi, K., S. Kuroda, and M. Amano. 1999. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPase in mammalian cells. Annu. Rev. Biochem. 68:459-486. [DOI] [PubMed] [Google Scholar]
- 38.Kanto, T., P. Kalinski, O. C. Hunter, M. T. Lotze, and A. A. Amoscato. 2001. Ceramide mediates tumor-induced dendritic cell apoptosis. J. Immunol. 167:3773-3784. [DOI] [PubMed] [Google Scholar]
- 39.Khokhlatchev, A. V., B. Canagarajah, J. Wilsbacher, M. Robinson, M. Atkinson, E. Goldsmith, and M. H. Cobb. 1998. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 93:605-615. [DOI] [PubMed] [Google Scholar]
- 40.Kimura, K., M. Ito, M. Amano, K. Chihara, Y. Fukata, M. Nakafuku, B. Yamamori, J. Feng, T. Nakano, K. Okawa, A. Iwamatsu, and K. Kaibuchi. 1996. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273:245-248. [DOI] [PubMed] [Google Scholar]
- 41.Kivinen, L., M. Tsubari, T. Haapajarvi, M. B. Datto, X. F. Wang, and M. Laiho. 1999. Ras induces p21Cip1/Waf1 cyclin kinase inhibitor transcriptionally through Sp1-binding sites. Oncogene 18:6252-6261. [DOI] [PubMed] [Google Scholar]
- 42.Lai, J. M., C. Y. Lu, H. F. Yang-Yen, and Z. F. Chang. 2001. Lysophosphatidic acid promotes phorbol-ester-induced apoptosis in TF-1 cells by interfering with adhesion. Biochem. J. 359:227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le-Niculescu, H., E. Bonfoco, Y. Kasuva, F. X. Claret, D. R. Green, and M. Karin. 1999. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol. Cell. Biol. 19:751-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lenormand, P., C. Sardet, G. Pagès, G. L'Allemain, A. Brunet, and J. Pouysségur. 1993. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J. Cell Biol. 122:1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu, Y., J. L. Martindale, M. Gorospe, and N. J. Holbrook. 1996. Regulation of p21WAF1/CIP1 expression through mitogen-activated protein kinase signaling pathway. Cancer Res. 56:31-35. [PubMed] [Google Scholar]
- 46.Lotem, J., E. J. Jr. Cragoe, and L. Sachs. 1991. Rescue from programmed cell death in leukemic and normal myeloid cells. Blood 78:953-960. [PubMed] [Google Scholar]
- 47.Marquardt, B., D. Frith, and S. Stabel. 1994. Signalling from TPA to MAP kinase requires protein kinase C, Raf and MEK: reconstitution of the signalling pathway in vitro. Oncogene 9:3213-3218. [PubMed] [Google Scholar]
- 48.Mayumi, T., K. Nagasawa, T. Horiuchi, and T. Kusaba. 1988. Phorbol ester receptors and the induction of differentiation in the human T lymphoblastic cell line MOLT-3. Exp. Cell Biol. 56:12-19. [DOI] [PubMed] [Google Scholar]
- 49.Mizuno, K., K. Noda, T. Araki, T. Imaoka, Y. Kobayashi, Y. Akita, M. Shimonaka, S. Kishi, and S. Ohno. 1997. The proteolytic cleavage of protein kinase C isotypes, which generates kinase and regulatory fragments, correlates with Fas-mediated and 12-O-tetradecanoyl-phorbol-13-acetate-induced apoptosis. Eur. J. Biochem. 250:7-18. [DOI] [PubMed] [Google Scholar]
- 50.Muda, M., A. Theodosiou, N. Rodrigues, U. Boschert, M. Camps, C. Gillieron, K. Davies, A. Ashworth, and S. Arkinstall. 1996. The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J. Biol. Chem. 271:27205-27208. [DOI] [PubMed] [Google Scholar]
- 51.Narumiya, S., T. Ishizaki, and M. Uehata. 2000. Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol. 325:273-284. [DOI] [PubMed] [Google Scholar]
- 52.Noda, A., Y. Ning, S. F. Venable, O. M. Pereira-Smith, and J. R. Smith. 1994. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp. Cell Res. 211:90-98. [DOI] [PubMed] [Google Scholar]
- 53.Obeid, L., C. M. Linardic, L. A. Karolak, and Y. A. Hannun. 1993. Programmed cell death induced by ceramide. Science 259:1769-1771. [DOI] [PubMed] [Google Scholar]
- 54.Ohta, H., E. A. Sweeney, A. Masamune, Y. Yatomi, S. Hakomori, and Y. Igarashi. 1995. Induction of apoptosis by sphingosine in human leukemic HL-60 cells: a possible endogenous modulator of apoptotic DNA fragmentation occurring during phorbol ester-induced differentiation. Cancer Res. 55:691-697. [PubMed] [Google Scholar]
- 55.Olson, M. F., H. F. Paterson, and C. J. Marshall. 1998. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature 394:295-299. [DOI] [PubMed] [Google Scholar]
- 56.Pumiglia, K. M., and S. J. Decker. 1997. Cell cycle arrest mediated by the MEK/mitogen-activated protein kinase pathway. Proc. Natl. Acad. Sci. USA 94:448-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu, R. G., J. Chen, F. McCormick, and M. Symons. 1995. A role for Rho in Ras transformation. Proc. Natl. Acad. Sci. USA 92:11781-11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ratcliffe, M. J., C. Smales, and J. M. Staddon. 1999. Dephosphorylation of the catenins p120 and p100 in endothelial cells in response to inflammatory stimuli. Biochem. J. 338:471-478. [PMC free article] [PubMed] [Google Scholar]
- 59.Ren, X.-D., W. B. Kiosses, and M. A. Schwartz. 1999. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18:578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sahai, E., M. F. Olson, and C. J. Marshall. 2001. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 20:755-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solary, E., R. Bertrand, and Y. Pommier. 1994. Apoptosis of human leukemic HL-60 cells induced to differentiate by phorbol ester treatment. Leukemia 8:792-797. [PubMed] [Google Scholar]
- 62.Takada, Y., M. Hachiya, Y. Osawa, Y. Hasegawa, K. Ando, Y. Kobayashi, and M. Akashi. 1999. 12-O-Tetradecanoylphorbol-13-acetate-induced apoptosis is mediated by tumor necrosis factor alpha in human monocytic U937 cells. J. Biol. Chem. 274:28286-28292. [DOI] [PubMed] [Google Scholar]
- 63.Uehata, M., T. Ishizaki, H. Satoh, T. Ono, T. Kawahara, T. Morishita, H. Tamakawa, K. Yamagami, J. Inui, M. Maekawa, and S. Narumiya. 1997. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389:990-994. [DOI] [PubMed] [Google Scholar]
- 64.Verheij, M., R. Bose, X. H. Lin, B. Yao, W. D. Jarvis, S. Grant, M. J. Birrer, E. Szabo, L. I. Zon, J. M. Kyriakis, A. Haimovitz-Friedman, Z. Fuks, and R. N. Kolesnic. 1996. Requirement for ceramide initiated JNK/SAPK signalling in stress-induced apoptosis. Nature 380:75-79. [DOI] [PubMed] [Google Scholar]
- 65.Wang, Z., Z. Z. Su, P. B. Fisher, S. Wang, G. VanTuyle, and S. Grant. 1998. Evidence of a functional role for the cyclin-dependent kinase inhibitor p21WAF1/CIP1/MDA6 in the reciprocal regulation of protein kinase C activator-induced apoptosis and differentiation in human myelomonocytic leukemia cells. Exp Cell Res. 244:105-116. [DOI] [PubMed] [Google Scholar]
- 66.Wang, Z., G. Van Tuyle, D. Conrad, P. B. Fisher, P. Dent, and S. Grant. 1999. Dysregulation of the cyclin-dependent kinase inhibitor p21WAF1/CIP1/MDA6 increases the susceptibility of human leukemia cells (U937) to 1-β-d-arabinofuranosylcytosine-mediated mitochondrial dysfunction and apoptosis. Cancer Res. 59:1259-1267. [PubMed] [Google Scholar]
- 67.Wang, Z., S. Wang, P. B. Fisher, P. Dent, and S. Grant. 2000. Evidence of a functional role for the cyclin-dependent kinase inhibitor p21CIP1 in leukemic cell (U937) differentiation induced by low concentrations of 1-β-d-arabinofuranosylcytosine. Differentiation 66:1-13. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe, Y., M. Ito, Y. Kataoka, H. Wada, M. Koyama, J. Feng, H. Shiku, and M. Nishikawa. 2001. Protein kinase C-catalyzed phosphorylation of an inhibitory phosphoprotein of myosin phosphatase is involved in human platelet secretion. Blood 975:3798-3805. [DOI] [PubMed] [Google Scholar]
- 69.Yang, X., R. Khosravi-Far, H. Y. Chang, and D. Baltimore. 1997. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 89:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng, Y. X., and W. S. El-Deiry. 1996. Regulation of p21WAF1/CIP1 expression by p53-independent pathways. Oncogene 12:1557-1564. [PubMed] [Google Scholar]
- 71.Zeng, Y. X., K. Somasundaram, and W. S. El-Deiry. 1997. AP2 inhibits cancer cell growth and activates p21WAF1/CIP1 expression. Nat. Genet. 15:78-82. [DOI] [PubMed] [Google Scholar]