Abstract
Misfolded proteins are recognized in the endoplasmic reticulum (ER), transported back to the cytosol, and degraded by the proteasome. A number of proteins are processed and modified by a glycosylphosphatidylinositol (GPI) anchor in the ER, but the quality control mechanisms of GPI-anchored proteins remain unclear. Here, we report on the quality control mechanism of misfolded GPI-anchored proteins. We have constructed a mutant form of the β-1,3-glucanosyltransferase Gas1p (Gas1*p) as a model misfolded GPI-anchored protein. Gas1*p was modified with a GPI anchor but retained in the ER and was degraded rapidly via the proteasome. Disruption of BST1, which encodes GPI inositol deacylase, caused a delay in the degradation of Gas1*p. This delay was because of an effect on the deacylation activity of Bst1p. Disruption of genes involved in GPI-anchored protein concentration and N-glycan processing caused different effects on the degradation of Gas1*p and a soluble misfolded version of carboxypeptidase Y. Furthermore, Gas1*p associated with both Bst1p and BiP/Kar2p, a molecular chaperone, in vivo. Our data suggest that GPI inositol deacylation plays important roles in the quality control and ER-associated degradation of GPI-anchored proteins.
INTRODUCTION
Cells possess several quality control mechanisms for the maintenance of proper protein folding and function. On synthesis, membrane and secretory proteins are inserted into the lumen of the endoplasmic reticulum (ER), where they are folded and undergo oligomerization. There are a number of chaperones and enzymes in the ER required for proper protein folding (Ellgaard et al., 1999). Before exiting from the ER, proteins are monitored by a quality control system that ensures correct folding. Misfolded proteins that fail to pass the quality control checkpoint are transported back to the cytosol and degraded by an ER-associated degradation (ERAD) mechanism that involves the ubiquitin-proteasome pathway (Kopito, 1997; Kostova and Wolf, 2003). N-linked oligosaccharide, one of the major posttranslational modifications in the ER, is trimmed and processed by glucosidase I, glucosidase II, and mannosidase I (Jakob et al., 1998; Helenius and Aebi, 2001). The processing of N-linked oligosaccharides plays important roles in the quality control of glycoprotein folding in the ER (Helenius and Aebi, 2001, 2004).
A number of cell surface proteins are posttranslationally modified in the ER with glycosylphosphatidylinositol (GPI). Mechanisms for quality control and degradation of GPI-anchored proteins are important in folding diseases, including prion diseases and transmissible spongiform encephalopathies, which are caused by a conformational modification of prion, a GPI-anchored protein (Prusiner, 1998). There have been several biochemical studies on both the degradation of mutated prions and the quality control of proteins with mutated GPI attachment signals (Field et al., 1994; Oda et al., 1996; Wainwright and Field, 1997; Jin et al., 2000; Ito et al., 2002; Ishida et al., 2003). In contrast, the molecular mechanisms involved in the quality control and degradation of GPI-anchored proteins have not been elucidated.
The GPI anchor is synthesized in the ER by the stepwise addition of sugars and ethanolaminephosphate to phosphatidylinositol (Kinoshita and Inoue, 2000; Eisenhaber et al., 2003). At an early step in the biosynthesis, the GPI inositol is acylated by Gwt1p (Umemura et al., 2003) (Figure 1). The amounts of GPI-anchored proteins are greatly decreased in gwt1 mutant cells, indicating that this acylation is critical for the attachment of GPI to proteins (Umemura et al., 2003). Once the GPI anchor is attached to a protein, the inositol is usually deacylated in the ER (Figure 1) (Chen et al., 1998). Recently, mammalian PGAP1 and the yeast orthologue Bst1p were identified as GPI inositol deacylases (Tanaka et al., 2004). ER-to-Golgi transport of GPI-anchored proteins is defective in both PGAP1-deficient cells and bst1 mutant cells, which shows that the inositol deacylation of GPI is important for the efficient transport of GPI-anchored proteins from the ER to the Golgi (Vashist et al., 2001; Tanaka et al., 2004).
Figure 1.
Inositol acylation and deacylation of the GPI moiety during the biosynthesis of GPI in S. cerevisiae. GPI precursors are synthesized at the ER membrane. Gwt1p is required for the acylation of inositol at an early step in the biosynthesis of GPI. The complete GPI precursor is transferred to a nascent cleaved carboxy terminus of the GPI protein precursors. After attachment of GPI to proteins, the acyl group on the inositol is eliminated by Bst1p. PI, phosphatidylinositol; Acyl, acyl group; GlcN, glucosamine; Man, mannose; EtN-P, ethanolamine phosphate.
Here, we investigated the degradation of GPI-anchored proteins in yeast. We constructed a mutant Gas1p (Gas1*p) as a model misfolded GPI protein. Gas1*p was modified by GPI but retained in the ER. In addition, it was misfolded and rapidly degraded by the proteasome system. We found that the inositol deacylation of the misfolded GPI-anchored protein is required for its efficient degradation in the ER. Our results further suggest that the GPI inositol deacylase is a key enzyme in initiating the degradation of misfolded GPI-anchored proteins. This is the first report addressing the molecular mechanisms of the quality control of GPI-anchored proteins.
MATERIALS AND METHODS
Strains and Media
The yeast strains used in this study are listed in Table 1. The disruption of genes in yeast was performed using a one-step gene disruption method as described previously (Longtine et al., 1998). YPAD and synthetic complete (SC) media are described in Sherman (1991). SDCA contains 0.67% yeast nitrogen base without amino acids (Difco, Detroit, MI), 2% glucose, 0.5% casamino acids, and 0.004% adenine sulfate.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Origin |
|---|---|---|
| W303-1A | MATaade2-1 his3-11 leu2-3,-112 trp1-1 ura3-1 can1-100 | Sutton et al. (1991) |
| W303-1B | MATα ade2-1 his3-11 leu2-3,-112 trp1-1 ura3-1 can1-100 | Sutton et al. (1991) |
| KA31α | MATα trp1 ura3 leu2 his3 | Yashiroda et al. (1996) |
| YAT2-1C | MATα trp1 ura3 leu2 his3 rsp5-101 | Yashiroda et al. (1996) |
| YK109 | MATarpn12-1 leu2 his3 trp1 ura3 ade1 | Kominami et al. (1995) |
| YAT2579 | MATarpn3-1 W303 | Dr. Toh-e laboratory |
| YS63-1C | MATα sec18-1 trp1 ura3 leu2 his3 | Laboratory stock |
| MHY501 | MATα his3-200 leu2-3,-112 ura3-52 lys2-801 trp1-1 | Swanson et al. (2001) |
| MHY1703 | MATα hrd1Δ::LEU2 doa10Δ::HIS3 MHY501 | Swanson et al. (2001) |
| gwt1-20 | MATα gwt1Δ::his5+gwt1-20::TRP1 W303 | Umemura et al. (2003) |
| MFY11 | MATagpi7Δ::TRP1 W303 | Fujita et al. (2004) |
| MFY150 | MATabst1Δ::TRP1 W303 | This study |
| MFY156 | MATagas1Δ::his5+ W303 | This study |
| MFY157 | MATaemp24Δ::his5+ W303 | This study |
| MFY159 | MATabst1Δ::TRP1 emp24Δ::his5+ W303 | This study |
| MFY161 | MATagas1Δ::his5+GAS1::URA3 W303 | This study |
| MFY163 | MATagas1Δ::his5+gas1*::URA3 W303 | This study |
| MFY165 | MATamns1Δ::LEU2 W303 | This study |
| MFY166 | MATagas1Δ::his5+bst1Δ::TRP1 W303 | This study |
| MFY167 | MATaerv25Δ::LEU2 W303 | This study |
| MFY168 | MATabst1Δ::TRP1 mns1Δ::LEU2 W303 | This study |
| MFY176 | MATabst1Δ::TRP1 gas1Δ::his5+gas1*::URA3 W303 | This study |
| MFY182 | MATagas1Δ::his5+HA-GAS1::LEU2 W303 | This study |
| MFY183 | MATagas1Δ::his5+mRFP-GAS1::LEU2 W303 | This study |
| MFY188 | MATaHA-gas1*::URA3 W303 | This study |
| MFY189 | MATabst1Δ::TRP1 HA-gas1*::URA3 W303 | This study |
| MFY190 | MATagpi7Δ::TRP1 HA-gas1*::URA3 W303 | This study |
| MFY191 | MATagwt1Δ::his5+gwt1-20::TRP1 HA-gas1*::URA3 W303 | This study |
| MFY192 | MATaemp24Δ::his5+HA-gas1*::URA3 W303 | This study |
| MFY193 | MATaerv25Δ::LEU2 HA-gas1*::URA3 W303 | This study |
| MFY194 | MATamns1Δ::LEU2 HA-gas1*::URA3 W303 | This study |
| MFY195 | MATabst1Δ::TRP1 emp24Δ::his5+HA-gas1*::URA3 W303 | This study |
| MFY196 | MATabst1Δ::TRP1 mns1Δ::LEU2 HA-gas1*::URA3 W303 | This study |
| MFY197 | MATahtm1Δ::his5+ W303 | This study |
| MFY198 | MATabst1Δ::TRP1 htm1Δ::his5+ W303 | This study |
| MFY207 | MATaHA-GAS1::LEU2 W303 | This study |
| MFY208 | MATaHA-gas1*::LEU2 W303 | This study |
| MFY213 | MATabst1Δ::TRP1 mRFP-GAS1::LEU2 W303 | This study |
| MFY212 | MATabst1Δ::TRP1 mRFP-gas1*::LEU2 W303 | This study |
| MFY241 | MATahtm1Δ::his5+HA-gas1*::URA3 W303 | This study |
| MFY242 | MATabst1Δ::TRP1 htm1Δ::his5+HA-gas1*::URA3 W303 | This study |
| MFY215 | MATahrd1Δ::his5+ W303 | This study |
| MFY278 | MATadoa10Δ::his5+ W303 | This study |
| MFY255 | MATapep4Δ::his5+ W303 | This study |
| MFY327 | MATahrd1Δ::his5+HA-gas1*::URA3 W303 | This study |
| MFY328 | MATadoa10Δ::his5+HA-gas1*::URA3 W303 | This study |
| MFY329 | MATapep4Δ::his5+HA-gas1*::URA3 W303 | This study |
| MFY331 | MATα trp1 ura3 leu2 his3 HA-gas1*::URA3 | This study |
| MFY332 | MATα trp1 ura3 leu2 his3 rsp5-101 HA-gas1*::URA3 | This study |
| MFY336 | MATarpn12-1 HA-gas1*::URA3 leu2 his3 trp1 ura3 ade1 | This study |
| MFY337 | MATarpn3-1 HA-gas1*::URA3 W303 | This study |
| MFY324 | MATaSHG::LEU2 W303 | This study |
| MFY325 | MATaSHg*::URA3 W303 | This study |
| MFY326 | MATabst1Δ::TRP1 SHg*::URA3 W303 | This study |
| MFY341 | MATahrd1Δ::his5+doa10Δ::his5+ W303 | This study |
| MFY343 | MATahrd1Δ::his5+doa10Δ::his5+HA-gas1*::URA3 W303 | This study |
| MFY348 | MATα sec18-1 trp1 ura3 leu2 his3 HA-gas1*::URA3 | This study |
| MFY351 | MATα HA-gas1*::URA3 MHY501 | This study |
| MFY352 | MATα hrd1Δ::LEU2 doa10Δ::HIS3 HA-gas1*::URA3 MHY501 | This study |
Plasmids
The plasmids used in this study are listed in Table 2.
Table 2.
Plasmids used in this study
| Plasmid | Description | Origin |
|---|---|---|
| pRS305 | LEU2 | Sikorski and Hieter (1989) |
| pRS306 | URA3 | Sikorski and Hieter (1989) |
| pRS315 | CEN, ARS, LEU2 | Sikorski and Hieter (1989) |
| pRS316 | CEN, ARS, URA3 | Sikorski and Hieter (1989) |
| YEp351GAPII | 2μ, LEU2, TDH3 promoter and terminator | Abe et al. (2003) |
| YEp352GAPII | 2μ, URA3, TDH3 promoter and terminator | Abe et al. (2003) |
| pMO13 | GFP-HDEL, CEN, ARS, URA3 | Okamoto et al. (2006) |
| pMF600 | GAS1, pRS306 | This study |
| pMF605 | gas1*, pRS306 | This study |
| pMF607 | HA-GAS1, pRS305 | This study |
| pMF608 | mRFP-GAS1, pRS305 | This study |
| pMF615 | HA-gas1*, pRS305 | This study |
| pMF616 | HA-gas1*, pRS306 | This study |
| pMF617 | mRFP-gas1*, pRS305 | This study |
| pMF634 | BST1, YEp351GAPII | This study |
| pMF636 | BST1(S236A), YEp351GAPII | This study |
| pMF641 | BST1-FLAG, YEp351GAPII | This study |
| pMF642 | BST1(S236A)-FLAG, YEp351GAPII | This study |
| pMF643 | BST1-FLAG, YEp352GAPII | This study |
| pMF644 | BST1(S236A)-FLAG, YEp352GAPII | This study |
| pMF834 | FLAG-KAR2, YEp352GAPII | This study |
| pMF848 | CPY*HA, pRS316 | This study |
| pMF874 | SHG, pRS305 | This study |
| pMF876 | SHg*, pRS306 | This study |
| pMF880 | SEC18, pRS315 | This study |
Construction of GAS1. The promoter, coding, and terminator sequences of GAS1 were cloned by amplification of genomic DNA using PfuUltra high-fidelity DNA polymerase (Stratagene, La Jolla, CA) and the primers GAS1F (5′-AAAAGGATCCCGCCCATAATATTGTTACCA-3′) and GAS1R (5′-AAAAACTAGTCCTTCTAGTGATGCTATGGC-3′). The amplified 2875-base pair fragment was digested with BamHI and SpeI and then purified. The purified fragment was ligated into pRS306 (Sikorski and Hieter, 1989) digested with the same enzymes to generate pMF600 (GAS1, URA3).
Construction of HA-GAS1. Using pMF600 as a template, MluI and NdeI sites were introduced 69 base pairs downstream from the start codon of GAS1, and the fragment was subcloned into pRS305 (Sikorski and Hieter, 1989) to generate pMF606. Three copies of the HA epitope were amplified, inserted into the MluI-NdeI site of pMF606 to generate pMF607 (HA-GAS1, LEU2), and confirmed by sequencing. The DNA fragment containing monomeric red fluorescent protein (mRFP; kindly provided by Dr. Roger Tsien, University of California, San Diego, La Jolla, CA), was also amplified and inserted into the MluI-NdeI site of pMF606 to generate pMF608 (mRFP-GAS1, LEU2).
We substituted glycine 291 in Gas1p with arginine using a QuikChange site-directed mutagenesis kit (Stratagene) using pMF600, pMF607, or pMF608 as the template to generate pMF605 (gas1*, URA3), pMF615 (HA-gas1*, LEU2), and pMF617 (mRFP-gas1*, LEU2), respectively. Plasmid pMF615 was digested with BamHI and SpeI, and a fragment containing hemagglutinin (HA)-tagged gas1* was ligated into pRS306 to generate pMF616 (HA-gas1*, URA3).
Construction of SHG and SHg*. We substituted asparagine 528 in Gas1p with a stop codon using a QuikChange site-directed mutagenesis kit with pMF607 and pMF616 as the template to generate pMF874 (SHG, LEU2) and pMF876 (SHg*, URA3), respectively.
Construction of YEp-BST1. BST1 coding sequences were amplified from genomic DNA by PCR using primers BST1F (5′-AAAAGAGCTCGTTATGGGTATCAGGAGATTAG-3′) and BST1R (5′-AAAATCTAGAACTGGGTTGTAGTTCTAATGTAT-3′). The amplified 3107-base pair fragment was digested with SacI and XbaI and then purified. The purified fragment was ligated into YEp351GAPII (Abe et al., 2003) digested with the same enzymes to generate pMF634 (YEp351-BST1). We substituted serine 236 in Bst1p with alanine using the QuikChange site-directed mutagenesis kit with YEp351-BST1 as a template to generate pMF636 (YEp351-BST1S236A).
Construction of YEp351-BST1-FLAG, YEp351-BST1S236A-FLAG, YEp352-BST1-FLAG, and YEp352-BST1S236A-FLAG. Using YEp351-BST1 and YEp351-BST1S236A as templates, the DNA fragments containing the BST1 gene were amplified by PCR with primers BST1F and BST1-FLAG-R (5′-AAAATCTAGACTAGATATCATGATCCTTGTAATCACCGTCATGGTCTTTGTAGTCATGTATTGTTTCGAAAAATAG-3′). The amplified fragments were inserted into YEp351GAPII or YEp352GAPII (Abe et al., 2003) to generate pMF641 (YEp351-BST1-FLAG), pMF642 (YEp351-BST1S236A-FLAG), pMF643 (YEp352-BST1-FLAG), and pMF644 (YEp352-BST1S236A-FLAG), respectively.
Construction of GFP-HDEL. We constructed GFP-HDEL (pMO13; Okamoto et al., 2006) as an ER marker. Briefly, GFP-HDEL, which contains the Kar2p signal-peptide sequence (the first 135 nucleotides of the KAR2 gene) and the enhanced green fluorescent protein gene (BD Biosciences, San Jose, CA) modified to encode a C-terminal HDEL tetrapeptide, was amplified by PCR. The DNA fragments were cloned into the YCp50 (CEN, URA3) expression vector, which contains the TDH3 (glyceraldehyde-3-phosphate dehydrogenase) promoter and actin terminator to generate pMO13.
Construction of YEp352-FLAG-KAR2. A DNA fragment encoding the Kar2p signal peptide was amplified with primers KAR2s-F (5′-AAAAGAGCTCCATACCATGTTTTTCAAC-3′) and KAR2s-R (5′-AAAAGTCGACATCGATATCATCGGCACCTCTAAC-3′) and inserted into YEp352GAPII to generate pMF833. The Kar2p-coding sequence after the signal peptide was amplified from genomic DNA using primers FLAG-KAR2-F (5′-AAAAATCGATTACAAGGACGACGATGACAAGGTAGAAAACTACGGAACTGTTATCG-3′) and KAR2-R (5′-AAAAGTCGACCTACAATTCGTCGTGTTCG-3′). The amplified 3107-base pair fragment was digested with ClaI and SalI and then purified. The purified fragment was ligated into pMF833 digested with the same enzymes to generate pMF834 (YEp352-FLAG-KAR2).
Construction of Carboxypeptidase Y (CPY)*HA. An HA-tagged CPY* plasmid was constructed from YIp-CPY* (kindly provided by Dr. Tadashi Suzuki, Osaka University, Osaka, Japan), which contains the open reading frame of mutant prc1-1. We inserted a SpeI site just before the stop codon of PRC1 by QuikChange site-directed mutagenesis using YIp5-CPY* as a template to generate pMF845. Three copies of the HA epitope were amplified, inserted into the SpeI site of pMF845 to generate pMF846 (YIp5-CPY*HA), and confirmed by sequencing. Plasmid pMF846 was digested with EcoRI and HindIII, and the fragment containing HA-tagged prc1-1 was ligated into pRS316 to generate pMF848 (pRS316-CPY*HA).
Construction of pRS315-SEC18. The promoter, coding, and terminator sequences of SEC18 were amplified with primers SEC18F (5′-AAAAACTAGTAAAAGGTATGCTGGATGCTG-3′) and SEC18R (5′-AAAACTCGAGTCACCTGGCAAAGCTTCTC-3′). The amplified 3377-base pair fragment was digested with SpeI and XhoI and then purified. The purified fragment was ligated into pRS315 (Sikorski and Hieter, 1989) digested with the same enzymes to generate pMF880 (SEC18, LEU2).
Immunoblotting
Samples were denatured with SDS-sample buffer for 10 min at 37°C for membrane proteins and for 5 min at 95°C for soluble proteins and then separated by SDS-PAGE. For immunoblot analysis, 5 μl of sample was loaded in each lane. Gas1p was detected with anti-Gas1 peptide polyclonal antibody (1:2000; kindly provided by Dr. Katsura Hata, Eisai, Tokyo, Japan) and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:2000; Cell Signaling Technology, Beverly, MA). HA-Gas1p and CPY*HA were detected with anti-HA monoclonal antibody (mAb) 16B12 (1:10,000; BAbCO, Richmond, CA) and HRP-conjugated goat anti-mouse IgG (1:10,000; Cell Signaling Technology). Dpm1p was detected with anti-Dpm1p mAb (1:2000; Molecular Probes, Eugene, OR) and HRP-conjugated anti-mouse IgG (1:2000). Pgk1p was detected with anti-Pgk1p mAb (1:10,000; Molecular Probes) and HRP-conjugated anti-mouse IgG (1:10,000). Glutathione S-transferase (GST)-tagged proteins were detected with HRP-conjugated anti-GST antibody (1:10,000; Amersham Biosciences, Uppsala, Sweden). FLAG-Kar2p and Bst1p-FLAG were detected with anti-FLAG mAb M2 (1:5000; Sigma-Aldrich, St. Louis, MO) and HRP-conjugated goat anti-mouse IgG (1:5000). Immunoreactive bands were visualized by chemiluminescence with ECL Plus reagents (Amersham Biosciences).
Subcellular Fractionation and Glycosylation of Gas1*p
MFY163 cells were grown in YPAD medium to an optical density (OD)600 of 1.0-2.0, and 5 × 108 cells were washed twice with TNE buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail; Roche Diagnostics, Basel, Switzerland). One milliliter of TNE and the same volume of glass beads were added to the cells, and the cells were lysed with a FastPrep (Qbiogene, Morgan Irvine, CA). The lysate was centrifuged for 5 min at 300 × g to remove cell debris. The supernatant was further centrifuged for 15 min at 13,000 × g, yielding the P13 pellet and the S13 supernatant. The S13 supernatant was centrifuged for 1 h at 100,000 × g, yielding the P100 pellet and the S100 supernatant. The P13 and P100 fractions were washed twice with TNE and resuspended in 1 ml of TNE. To detect HA-Gas1p in culture medium, proteins were precipitated with 10% trichloroacetic acid and then washed with cold acetone. To study the glycosylation, cell lysates were solubilized in the same volume of 2× SDS-sample buffer and heated for 5 min at 95°C. Aliquots of the samples were treated with endoglycosidase H (Endo H)f (New England Biolabs, Beverly, MA) for 3 h at 37°C.
Cycloheximide (CHX) Chase Analysis
CHX chase experiments were performed as described previously (Plemper et al., 1998; Jakob et al., 2001). Briefly, overnight cultures were inoculated into 5 ml of medium. Cells were grown to 2 × 107 cells/ml. After adding CHX (Nakalai Tesque, Kyoto, Japan) to a final concentration of 0.2 mg/ml, 1 × 107 cells were removed at specific time points, suspended in sodium azide solution (final concentration 10 mg/ml), and frozen at -80°C. The preparation of samples and immunoblotting were performed as described above. The effect of the proteasome inhibitor was analyzed as described previously (Suzuki et al., 2000). Ten minutes before CHX was added, MG-132 (Merck, Darmstadt, Germany) was added to a final concentration of 50 μM from a freshly made 5 mM solution in dimethyl sulfoxide (DMSO). For control cells, the same amount of DMSO was added. For GST-tagged α-toxin affinity precipitation, 3 × 107 cells were collected at specific time points after adding CHX. Cells were broken using glass beads (see Subcellular Fractionation and Glycosylation of Gas1*p), and the cell lysate was adjusted to 1% SDS, boiled, and mixed with 1 ml of TNET (100 mM Tris-HCl, pH 8, 100 mM NaCl, 5 mM EDTA, and 1% Triton X-100). The lysate was centrifuged at 13,000 × g for 15 min. The supernatant was incubated with 1 μg of GST-tagged α-toxin protein (kindly provided by Drs. Taroh Kinoshita and Yusuke Maeda, Osaka University) at 4°C for 30 min and then with 25 μl of glutathione-agarose beads (Sigma-Aldrich) at 4°C for 2 h. For SDS-PAGE, the beads were washed four times with TNET, resuspended in 30 μl of SDS-sample buffer, and boiled at 95°C for 5 min. Immunoblotting was performed as described above.
Inositol Labeling and immunoprecipitation of HA-Gas1p
Cells (5 × 107) were washed three times and resuspended in 1 ml of SC-inositol medium. Cells were incubated at 30°C for 30 min and then labeled with myo-[1,2-3H]inositol (PerkinElmer Life and Analytical Sciences, Boston, MA) for 3 h. The reaction was stopped by adding NaN3/NaF solution to a final concentration of 10 mM. The suspension was washed with 10 mM NaN3 and resuspended in 50 μl of TEPI (50 mM Tris-HCl, pH 7.5, 5 mM EDTA, and protease inhibitor cocktail). The cells were then broken with glass beads, and cell debris was removed (see Subcellular Fractionation and Glycosylation of Gas1*p). The cell lysate was adjusted to 1% SDS, boiled, and mixed with 1 ml of TNET. The lysate was centrifuged at 13,000 × g for 15 min. Then, the supernatant was incubated overnight at 4°C with 25 μl of anti-HA agarose beads (Roche Diagnostics). The agarose beads were washed four times with TNET and once with 20 mM Tris-HCl, pH 7.5, resuspended in 30 μl of SDS sample buffer, and boiled at 95°C for 5 min. Samples were separated by SDS-PAGE and analyzed using a Molecular Imager FX (Bio-Rad, Hercules, CA).
Fluorescence Microscopy
For the imaging of mRFP-Gas1 and mRFP-Gas1* proteins, cells grown to exponential phase were collected and washed with phosphate-buffered saline. Fluorescence images were observed using a BX50 fluorescence microscope (Olympus, Tokyo, Japan) and photographed with a MicroMax cooled charge-coupled device camera (Princeton Instruments, Trenton, NJ).
Immunoprecipitation
For detection of the Gas1p-Kar2p and Gas1p-Bst1p association, immunoprecipitation was performed as described previously with some modifications (Vallee and Riezman, 2005). Briefly, 100 OD600 of cells were washed twice with TNE buffer and disrupted with glass beads, after which cell debris and glass beads were removed by centrifugation (see Subcellular Fractionation and Glycosylation of Gas1*p). The supernatant was then centrifuged at 13,000 × g for 20 min at 4°C. The pellet was resuspended in TNE, and digitonin was added to a final concentration of 1%. The suspension was incubated for 1 h at 4°C with rotation, after which insoluble components were removed by centrifugation at 13,000 × g for 15 min at 4°C. For immunoprecipitation of HA-tagged proteins, anti-HA IgG-agarose was incubated with the sample at 4°C for 3 h. For immunoprecipitation of FLAG-tagged proteins, anti-FLAG beads (Sigma-Aldrich) were incubated with the sample at 4°C for 3 h. The immunoprecipitated beads were washed three times with TNE containing 1% digitonin and eluted with SDS-sample buffer. Immunoblotting was performed as described above.
RESULTS
Construction and Characterization of Misfolded Gas1 Proteins
To understand the process by which misfolded GPI-anchored proteins are degraded, we constructed a model misfolded GPI-anchored protein using the β-1,3-glucanosyltransferase Gas1p, one of the most abundant and well-characterized GPI-anchored proteins in Saccharomyces cerevisiae (Conzelmann et al., 1988; Popolo and Vai, 1999). Gas1p has also been used as a model to analyze the primary structural requirements for GPI anchoring (Nuoffer et al., 1991, 1993) and the transport of GPI-anchored proteins from the ER to the Golgi apparatus (Riezman et al., 1994; Muniz and Riezman, 2000; Watanabe et al., 2002). N-linked ER-type Man8 oligosaccharides, the O-linked Man1 residues, and a GPI anchor are transferred to the primary translation product of Gas1p (65 kDa) in the ER, yielding an immature polypeptide of 105 kDa. Further elaboration of the oligosaccharide chains takes place through the Golgi, resulting in a mature 125-kDa form.
First, we tried to obtain a mutant Gas1p that causes misfolding. In yeast, a mutated carboxypeptidase Y (CPY*) has been used as a model for misfolding of soluble luminal glycoproteins, and the process of CPY* degradation and its effects of N-glycan processing have been investigated in detail previously (Knop et al., 1996; Jakob et al., 1998). Mutant CPY carries an arginine instead of a glycine residue at position 255 of prepro-CPY (Finger et al., 1993). This mutated amino acid is located in a hydrophobic region in CPY. We speculated that the mutated CPY is misfolded because arginine, a hydrophilic and positive-charged amino acid, is placed in a hydrophobic region. Therefore, we adopted the same strategy to generate a misfolded form of Gas1p. We selected three hydrophobic regions for site-directed mutagenesis of Gas1p, excluding the secretion signal sequence, the GPI attachment signal, and the glycosylation sites. From a hydropathy plot of Gas1p, each amino acid—alanine 116, valine 258, and glycine 291—was replaced with arginine. One of the three mutant proteins, G291R (designated Gas1*p; Figure 2, A and B), could not rescue the slow growth phenotype of the gas1Δ strain (Figure 2C). Immunoblotting further revealed that, although the Golgi form of Gas1p (125 kDa) predominates in gas1Δ cells expressing wild-type GAS1, only a small amount of the ER form of Gas1p (105 kDa) was detected in gas1Δ cells expressing gas1* (Figure 2D).
Figure 2.
Construction and characterization of misfolded Gas1p. (A) Hydropathy plots of wild-type Gas1p (left) and mutant Gas1p (right; Gas1*p). Hydropathy plots were made using to the Kyte and Doolittle program. The arrows indicate the site of mutation in Gas1*p (G291R). (B) Schematic structure of Gas1p, including the sequence of the mutated form (Gas1*p). (C) The gas1* mutant gene does not rescue the slow growth phenotype of gas1Δ mutant cells. Isogenic W303-1A (WT), MFY156 (gas1Δ), MFY161 (gas1Δ URA3::GAS1), and MFY163 (gas1Δ URA3::gas1*) cells were spotted in serial dilutions on a YPAD plate and grown at 30°C for 2 d. (D) Gas1*p remains in the ER form, and its level is markedly decreased compared with wild-type Gas1p. MFY161 (GAS1) and MFY163 (gas1*) cells were grown overnight to mid-log phase. Equal cell numbers were disrupted, and total protein extracts were prepared and analyzed by immunoblotting using anti-Gas1p and anti-rabbit HRP-conjugated IgG or with anti-Dpm1p and anti-mouse HRP-conjugated IgG. (E) Gas1*p is correctly N-glycosylated. Cell lysates of MFY161 (GAS1) and MFY163 (gas1*) were digested with Endo H. The Endo H-treated or untreated protein extracts were separated by SDS-PAGE and analyzed by immunoblotting using anti-Gas1p or anti-Dpm1p. (F) Fractionation of Gas1*p. Exponentially growing MFY163 cells were broken, and the cell lysate was sedimented by sequential steps of centrifugation and ultracentrifugation. Proteins from the 13,000 × g pellet (P13), 100,000 × g pellet (P100), and 100,000 × g supernatant (S100) were processed for immunoblotting and detected with anti-Gas1p, anti-Dpm1p, or anti-Pgk1p.
To determine what causes the difference in molecular size between Gas1p and Gas1*p, we compared their sizes before and after digestion with Endo H. This treatment decreased the apparent molecular weight of Gas1*p, indicating the presence of N-linked sugar chains (Figure 2E). The difference in the mobility between Endo H-treated Gas1p and Gas1*p could be due to elongation of O-linked glycans of Gas1p but not Gas1*p in the Golgi. We further performed subcellular fractionation to investigate the subcellular localization of Gas1*p. The majority of Gas1*p was detected in the P13 fraction, which contains the ER membrane (Figure 2F). Gas1*p was detected not only in the membrane but also in the soluble fraction (S100) (Figure 2F). The molecular size of the major Gas1*p band was the same in the S100 and membrane fractions. This can be explained by the fact that the difference in the mobility of the non-GPI- and GPI-anchored forms of Gas1p is too small to be distinguished by SDS-PAGE (Nuoffer et al., 1991). We also detected a minor 65-kDa band in the S100 fraction corresponding to Gas1p form without any sugar chains. This might be a deglycosylated form of Gas1*p.
Additional gene disruption is difficult in gas1* cells because gas1Δ and gas1* cells grow slowly and because several genes involved in ER quality control (e.g., GLS1 and GLS2) and GPI-anchored protein transport (e.g., EMP24) are synthetic lethal in the gas1Δ background (Tong et al., 2004). Therefore, we constructed HA- and mRFP-tagged versions of Gas1p and Gas1*p to monitor their behaviors and localization. These proteins were generated by inserting the HA- or mRFP-tag in Gas1p after the cleavable N-terminal signal sequence (Figure 3A). Both HA-Gas1p and mRFP-Gas1p rescued the calcofluor white sensitivity of gas1Δ cells, indicating that the tagged proteins were functional (Figure 3B). In addition, immunoblotting revealed that HA-Gas1*p was in the ER form (Figure 3C). Also, mRFP-Gas1p was localized on the cell surface, whereas mRFP-Gas1*p was distributed in the intracellular compartment. Further analysis was difficult because of low fluorescence intensity in wild-type cells. These results indicate that the tagged Gas1*p behaves like Gas1*p.
Figure 3.
Construction of HA- or mRFP-tagged Gas1p and Gas1*p. (A) Schematic construction of HA-tagged and mRFP-tagged Gas1*p. A 3× HA epitope tag or mRFP sequence was inserted into Gas1p after the secretion signal sequence. (B) Tagged Gas1 proteins are functional and complement the calcofluor white (CFW) sensitivity of gas1Δ. Isogenic W303-1A (WT), MFY156 (gas1Δ), MFY182 (gas1Δ HA-GAS1), and MFY183 (gas1Δ mRFP-GAS1) cells were spotted on plates containing YPAD or YPAD with 5 μg/ml CFW and then incubated at 30°C for 2 d. (C) HA-Gas1p behaves like the native form of Gas1p, but the mutant of HA-Gas1p is unstable and remains as the ER form. MFY207 (HA-GAS1) and MFY208 (HA-gas1*) cells were grown overnight to mid-log phase. Equal cell numbers were disrupted, and total protein extracts were prepared and analyzed by immunoblotting using anti-HA and anti-mouse HRP-conjugated IgG, or anti-Dpm1p and anti-mouse HRP-conjugated IgG. ER and Golgi forms of HA-tagged Gas1p are shown by arrowheads. (D) The misfolded Gas1*p is modified by the GPI anchor. MFY207 (HA-GAS1) and MFY208 (HA-gas1*) cells were grown to a mid-log phase and labeled with myo-[1,2-3H]inositol for 3 h. Gas1p and Gas1*p in cell lysates were then immunoprecipitated with anti-HA-agarose and analyzed by SDS-PAGE, followed by image analysis with a Molecular Imager. The ER and Golgi forms are indicated by arrows.
To address whether misfolded Gas1*p is modified with GPI, we labeled the cells with [1,2-3H]inositol and then specifically immunoprecipitated HA-Gas1*p with anti-HA-agarose. We detected HA-Gas1p at the predicted position (Golgi form), whereas labeled HA-Gas1*p was detected as the ER form (Figure 3D). These results show that Gas1*p is modified with GPI.
Figures 2D and 3C indicate that there is less Gas1*p than wild-type Gas1p. Therefore, we next examined whether the lowered steady-state level of Gas1*p is because of a rapid degradation of misfolded protein. A CHX chase experiment (Plemper et al., 1998; Jakob et al., 2001) showed that Gas1*p was present initially as the ER form but rapidly decreased to an undetectable level, whereas wild-type Gas1p and the control ER marker Dpm1p were stable even after protein synthesis was stopped by CHX (Figure 4A). In addition, in the CHX chase experiment, Gas1*p remained as the ER form but showed a gradually increased molecular weight. The reduced mobility of Gas1*p on SDS-PAGE was still apparent after Endo H treatment (Figure 4B), presumably because of the additional O-glycosylation (Nakatsukasa et al., 2004). We checked for the presence of Gas1*p in culture medium to confirm that the disappearance of Gas1*p in the chase analysis was not because of protein secretion. Neither Gas1p nor Gas1*p were detected in the culture medium (Figure 4C), indicating that Gas1*p is lost because of intracellular degradation and not secretion.
Figure 4.
Gas1*p is a substrate for ER-associated degradation via the proteasome. (A) Gas1*p is unstable and rapidly degraded. Isogenic MFY161 (GAS1) and MFY163 (gas1*) cells were grown to 2 × 107 cells/ml. Equal cell numbers were then incubated with 200 μg/ml CHX, and aliquots were removed at the indicated time points. Crude protein extract was separated by SDS-PAGE and analyzed by immunoblotting with anti-Gas1p. The immunoblot was subsequently probed with anti-Dpm1p as a control for loading. (B) Isogenic MFY163 cells were grown to 2 × 107 cells/ml, after which CHX chase analysis was performed. Aliquots of the sample were treated for 3 h with Endo H at 37°C and then analyzed by immunoblotting with anti-Gas1p. (C) Gas1*p is not secreted into the medium and is a target for intracellular degradation. Cells lysates were prepared from exponentially growing cells. Proteins secreted into the medium were precipitated with 10% trichloroacetic acid. Samples corresponding to 0.5 OD600 units of cells per lane were loaded and analyzed by immunoblotting using anti-HA antibodies. C, cell lysate; M, medium. (D) The degradation of Gas1*p was measured in MFY188 (WT) and MFY329 (pep4Δ) cells in which HA-gas1* is integrated at the URA3 locus, as described in A. HA-Gas1p was resolved by electrophoresis and analyzed by immunoblotting with anti-HA. The amounts of Gas1* protein were quantified with an image analyzer and plotted as means values ± SD from three independent experiments, with the quantity at the 0 time point (steady-state level) set at 100%. (E) The effect of MG-132, a specific proteasome inhibitor, on the degradation of Gas1*p. MG-132 in DMSO [MG-132 (+)] or DMSO only [MG-132 (-)] was added to cultures of MFY163 cells 10 min before starting CHX chase analysis. (F) The degradation of Gas1*p was measured in MFY188 (WT), MFY337 (rpn3-1), and MFY336 (rpn12-1) cells, in which HA-gas1* is integrated at the URA3 locus. Equal cell numbers (2 × 107 cells/ml) were incubated with 200 μg/ml CHX at 37°C. Cells were chased for the periods indicated. The amounts of Gas1* protein were quantified as described in D.
We next investigated what kind of cellular degradation system is involved in the degradation of Gas1*p. First, we investigated the role of proteolysis in the vacuole in the degradation of Gas1*p. We found that the degradation of HA-Gas1*p was not affected in pep4 mutant cells, which are defective in the vacuolar protease activity (Figure 4D), indicating that Gas1*p is not degraded in the vacuole. Next, we examined the involvement of the proteasome system using MG-132, a specific inhibitor of the proteasome (Suzuki et al., 2000). Gas1*p was dramatically stabilized by the addition of MG-132 (Figure 4E). In addition, the degradation of Gas1*p was significantly delayed in rpn3-1 and rpn12-1 proteasome mutant cells (Kominami et al., 1995, 1997) (Figure 4F), strongly suggesting that it is degraded by ERAD and subsequent proteasome-mediated degradation.
We further examined the role of ubiquitin ligases (E3) in the degradation of Gas1*p. We selected three kinds of E3 that are thought to be involved in ERAD pathway, including the gene products of HRD1, which is involved in ERAD-lumenal (ERAD-L) pathway (Ahner and Brodsky, 2004; Huyer et al., 2004; Vashist and Ng, 2004); DOA10, which is involved in ERAD-cytosolic (ERAD-C) pathway; and RSP5, which is assumed to be involved in ERAD of misfolded protein when overproduced (Haynes et al., 2002). Unexpectedly, the degradation of HA-Gas1*p was not stabilized in hrd1Δ and doa10Δ cells (Figure 5A). Furthermore, the degradation of Gas1*p without an HA-tag was unaffected in both the hrd1Δ and the doa10Δ cells (our unpublished data). Because it has reported that Hrd1p and Doa10p act redundantly (Swanson et al., 2001), we checked the effect of Gas1*p degradation in hrd1Δ doa10Δ double mutant cells. As shown in Figure 5A, the degradation rate of HA-Gas1*p was not delayed even in hrd1Δ doa10Δ double mutant cells. We also confirmed that HA-Gas1*p was not affected in the different background of hrd1Δ doa10Δ double mutant cells (our unpublished data). We used the rsp5-101 mutant cells to test the involvement of Rsp5p in the Gas1*p degradation (Yashiroda et al., 1996). HA-Gas1*p was not stabilized in this mutant (Figure 5B). Rsp5p is involved in several functions, including ERAD and ubiquitination for protein targeting to the multivesicular bodies. Although the degradation of Gas1*p might be affected by some other rsp5 alleles, no defects were observed in the degradation of Gas1*p at least in the rsp5-101 mutant cells. Together, our results suggest that misfolded GPI-anchored proteins are not degraded by a known pathway, such as ERAD-L or ERAD-C, but rather by an unknown pathway, whereas the final stages of degradation may be mediated by the proteasome system as reported for misfolded soluble proteins and membrane proteins (Kostova and Wolf, 2003).
Figure 5.
Effects of E3 ubiquitin ligases and ER-to-Golgi transport in the degradation of Gas1*p. (A) The degradation of Gas1*p was measured in MFY188 (WT), MFY327 (hrd1Δ), MFY328 (doa10Δ), and MFY343 (hrd1Δ doa10Δ) cells in which HA-gas1* is integrated at the URA3 locus, as described in Figure 4D. (B) The degradation of Gas1*p was measured in MFY331 (WT) and MFY332 (rsp5-101) cells, in which HA-gas1* is integrated at the URA3 locus, as described in Figure 4F. (C) The degradation of CPY* was measured in YS63-1C cells carrying pRS316-CPY*HA and pRS315-SEC18 (SEC18), and YS63-1C cells carrying pRS316-CPY*HA (sec18-1), as described in Figure 4F. (D) The degradation of Gas1*p was measured in MFY348 cells carrying pRS315-SEC18 (SEC18) and MFY348 cells (sec18-1), as described in Figure 4F.
It has been reported that ERAD-L substrates are transported from the ER to the Golgi and retrieved to the ER, whereas ERAD-C substrates are sorted for retention in the ER (Vashist et al., 2001; Ahner and Brodsky, 2004; Vashist and Ng, 2004). Gas1*p is synthesized and modified in the ER luminal side. We next investigated whether ER-to-Golgi transport is required for the degradation of Gas1*p, like ERAD-L substrates. We measured the HA-Gas1*p degradation in sec18-1 mutant cells. Sec18p is required for vesicular fusion to the Golgi (Eakle et al., 1988). We also used the HA-tagged misfolded CPY (CPY*HA), a model of misfolded glycoproteins in yeast, as a control (Ng et al., 2000; Vashist et al., 2001; Spear and Ng, 2003). The degradation of CPY*HA was delayed in sec18-1 cells, as reported previously (Figure 5C). In sec18-1 cells, the degradation of HA-Gas1*p was also delayed (Figure 5D), confirming that it uses the same pathway as the ERAD-L substrates. Interestingly, even at the restrictive temperature, a mobility shift of HA-Gas1*p was still observed in sec18-1 cells, suggesting that the shift is because of the reaction in the ER, but not in the Golgi. This mobility shift was not observed in other substrates (Vashist and Ng, 2004).
Degradation of Gas1* Protein Requires GPI Inositol Deacylation
The GPI moiety is transferred to proteins in the ER. The inositol in the complete GPI precursor is acylated. GWT1 is required for the acylation of the GPI inositol (Umemura et al., 2003). Once the GPI anchor is transferred to a protein, however, the acyl group is removed in the ER. Recently, it was shown that the deacylation of inositol in GPI is mediated by PGAP1 in mammalian cells and Bst1p in yeast (Tanaka et al., 2004). The maturation of GPI-anchored proteins is greatly delayed in both the PGAP1 mutant and bst1 mutant, suggesting that the inositol deacylation of GPI is important for the efficient transport of GPI-anchored proteins from the ER to the Golgi apparatus. BST1 was first identified as a mutant that rescued the lethality of the sec13 mutant (Elrod-Erickson and Kaiser, 1996). Interestingly, the bst1/per17-1 mutant was also identified, indicating a defect in the degradation of soluble misfolded proteins (Vashist et al., 2001). On the basis of these reports, we speculated that the inositol deacylation of GPI by Bst1p mediates ER quality control of GPI-anchored proteins.
To determine whether BST1 is involved in the degradation of GPI protein, we deleted the BST1 gene in wild-type cells harboring the HA-gas1* or the gas1* constructs. CHX chase analysis in bst1-deleted cells (bst1Δ) revealed a four-fold stabilization of both Gas1*p (Figure 6A) and HA-Gas1*p (Figure 6B). Notably, Gas1*p was modified with GPI (Figure 3D). To verify that GPI-anchored Gas1*p is efficiently degraded in wild-type cells but not in bst1Δ cells, we performed CHX chase analysis followed by affinity precipitation with α-toxin, which binds to GPI (Gordon et al., 1999; Hong et al., 2002). After chase analysis, Gas1*p was precipitated with anti-HA or GST-tagged α-toxin and detected with anti-HA antibodies. Because Dpm1p, which is not a GPI-anchored protein, was not detected by precipitation with α-toxin and because equal amounts of GST-tagged α-toxin were precipitated in each lane using glutathione-agarose beads, the results reflect a true association of GPI-anchored proteins with GST-tagged α-toxin (Figure 6C). Total Gas1*p immunoprecipitated with anti-HA behaved as in the standard CHX chase analysis (Figure 6, A-C). In wild-type cells, GPI-anchored Gas1*p was efficiently precipitated with α-toxin and gradually decreased during the chase time, indicating that Gas1*p is modified with GPI, after which it is degraded. In contrast, we observed a significant delay in Gas1*p degradation in bst1Δ cells (Figure 6C). The remaining GPI-anchored Gas1*p in bst1Δ cells seemed to reflect a delay in its degradation. These results suggest that the delay in degradation of Gas1*p in bst1Δ cells is because of the persistence of the inositol-acylated form of GPI-anchored Gas1*p caused by the defect in Bst1p function. Notably, in wild-type but not in bst1Δ cells, the amount of GPI-anchored Gas1*p transiently increased at 30 min and then gradually decreased during the chase period (Figure 6C). It is likely that the transient accumulation of GPI-anchored Gas1*p in wild-type cells is because of the modification of Gas1*p with GPI, followed by the gradual degradation of GPI-anchored Gas1*p after deacylation of the inositol ring by Bst1p.
Figure 6.
Deletion of BST1 stabilizes the degradation of misfolded Gas1p. (A) The degradation of Gas1*p in MFY163 (gas1Δ URA3::gas1*) and MFY176 (bst1Δ gas1Δ URA3::gas1*) mutant cells. Equal cell numbers (2 × 107 cells/ml) were incubated with 200 μg/ml CHX. Cells were chased for the indicated periods. Gas1p was resolved by electrophoresis and analyzed by immunoblotting with anti-Gas1p. The blot was subsequently probed with anti-Dpm1p as a control for loading. The results were quantified with an image analyzer and are plotted as mean values ± SD from three independent experiments, with the quantity at the 0 time point (steady-state level) set at 100%. (B) The degradation of HA-tagged Gas1*p in MFY188 (WT URA3::HA-gas1*) and MFY189 (bst1Δ URA3::HA-gas1*). The turnover of HA-Gas1*p in MFY188 (WT) cells and MFY189 (bst1Δ) cells was examined as described in A. The results were quantified with an image analyzer and plotted as mean values ± SD from five independent experiments, with the quantity at the 0 time point (steady-state level) set at 100%. (C) Gas1*p receives a GPI anchor before its degradation. MFY188 (WT) and MFY189 (bst1Δ) cells were analyzed by a CHX experiment, followed by immunoprecipitation (IP) with anti-HA-agarose or affinity precipitation (AP) with α-toxin-GST and glutathione-agarose beads. IP fractions were separated by SDS-PAGE and analyzed by immunoblotting with anti-HA, anti-Dpm1p, and anti-GST.
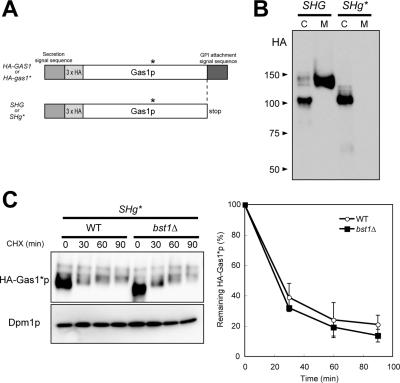
To determine whether Gas1*p stabilization in bst1Δ cells is specific to GPI-anchored proteins, we further constructed a soluble form of Gas1*p (SHg*) by adding a stop codon just before the signal sequence for GPI anchoring (Figure 7A). A soluble form of wild-type Gas1p (SHG) is efficiently secreted into the culture medium. In contrast, SHg* was not present in the medium but rather was inside the cell as an ER form (Figure 7B). These results strongly suggest that soluble Gas1*p is also misfolded. We also compared the rate of degradation of mutant Gas1*p (SHg*) between wild-type and bst1Δ cells. As shown in Figure 7C, we did not observe any differences in the degradation of SHg* between wild-type and bst1Δ cells, further supporting the idea that the degradation of misfolded non-GPI protein is independent of Bst1p function.
Figure 7.
Degradation of a soluble form of Gas1*p is independent of Bst1p function. (A) Construction of soluble forms of Gas1p (SHG) and Gas1*p (SHg*). SHG and SHg* carry a stop codon instead of an asparagine (N528) just before the GPI anchoring signal of Gas1p. (B) SHG but not SHg* is secreted into the medium. Cell lysates were prepared from exponentially growing cells. Proteins secreted into the medium were precipitated with 10% trichloroacetic acid. Samples corresponding to 0.5 OD600 units of cells per lane were analyzed by immunoblotting. C, cell lysate; M, medium. (C) The degradation of SHg* was measured in MFY325 (WT) and MFY326 (bst1Δ) cells, in which SHg* is integrated at the URA3 locus as described in Figure 6. The results were quantified with an image analyzer, and the mean values ± SDs of three independent experiments are plotted, with the quantity at the 0 time point (steady-state level) set at 100%.
Both mammalian PGAP1 and yeast Bst1p possess a consensus motif for lipases. This motif contains a serine residue that is thought to be part of the active site of deacylases. In fact, substitution of this putative catalytic serine residue of PGAP1 with alanine causes a loss of PGAP1 activity (Tanaka et al., 2004). To confirm that the stabilization of Gas1*p is because of the deacylation activity of Bst1p rather than a defect in binding, we substituted the catalytic serine 236 with alanine (S236A). The amounts of FLAG-tagged Bst1(S236A)p and FLAG-tagged Bst1p were similar, suggesting that Bst1(S236A)p is as stable as the wild-type Bst1p (Figure 8A). HA-Gas1*p was stabilized in bst1Δ cells containing a control vector, whereas BST1 restored the degradation of Gas1*p in bst1Δ cells (Figure 8B). We also found that bst1-S236A did not restore the degradation of Gas1*p in bst1Δ cells (Figure 8B). These results indicate that the GPI inositol deacylase activity of Bst1p is important for the degradation of Gas1*p.
Figure 8.
GPI inositol deacylase activity is important for the efficient degradation of misfolded Gas1p. (A) The expression of an S236A mutant of Bst1p. MFY150 (bst1Δ) cells were transformed with the plasmid containing the wild-type (YEp-BST1-FLAG) or S236A mutant (YEp-BST1SA-FLAG) of FLAG-tagged Bst1p. Equal cell numbers were disrupted, and total protein extracts were analyzed by immunoblotting with anti-FLAG antibody and anti-mouse HRP-conjugated IgG or with anti-Dpm1p antibody and anti-mouse HRP-conjugated IgG. (B) Degradation of HA-Gas1*p was measured in MFY189 cells carrying YEp351 (bst1Δ -), YEp351-BST1 (bst1Δ BST1), or YEp351-BST1S236A (bst1Δ S236A). The rate of degradation of HA-Gas1*p was determined as described in Figure 4.
We also examined the localization of mRFP-tagged Gas1 and Gas1* proteins in bst1Δ cells. We found that mRFP-Gas1p is mainly localized in the plasma membrane, which is the native location of Gas1p (Figure 9, top). In contrast, mRFP-Gas1*p was present not in the plasma membrane but rather in an intracellular compartment, probably in the ER membrane (Figure 9, bottom). We next transformed the bst1Δ cells expressing mRFP-Gas1*p with GFP-HDEL as a marker of the ER (Monnat et al., 2000). We found that mRFP-Gas1*p colocalized with HDEL-GFP in the ER (Figure 9, bottom). As we found in our initial experiments, the fluorescence of mRFP-Gas1*p in wild-type cells was too weak to detect. These results also indicate that the Gas1* protein, which was degraded through the ERAD pathway, accumulates in the ER in bst1Δ cells and that the degradation of Gas1*p is dependent on inositol deacylation of the GPI.
Figure 9.
Localization of mRFP-Gas1p and mRFP-Gas1*p in bst1Δ cells. (A) MFY213 (bst1Δ mRFP-GAS1) cells or (B) MFY212 (bst1Δ mRFP-gas1*) cells expressing GFP-HDEL were visualized by fluorescence microscopy. DIC, differential interference contrast microscopy. Bar, 10 μm.
Degradation of Gas1* Protein in Mutants of GPI Biosynthesis, Concentration, and N-Glycosylation
Our results show that inositol deacylation of GPI is required for the ER quality control of misfolded GPI-anchored proteins (Figures 6 and 8). To identify other factors required for quality control in the ER, we performed CHX chase experiments of Gas1*p in mutants defective in the biosynthesis of the GPI anchor, the concentration of GPI-anchored proteins, and the folding of N-glycosylated proteins. We also performed the same experiments in the mutants carrying CPY*HA to compare with the degradation of HA-Gas1*p.
First, we examined the effects of mutants involved in the biosynthesis of GPI on the degradation of Gas1*p. GWT1 is required for inositol acylation early in the GPI biosynthetic pathway, which is the opposite reaction of Bst1p (Umemura et al., 2003). GPI7 is involved in the addition of ethanolamine phosphate to the second mannose of GPI (Benachour et al., 1999; Fujita et al., 2004). We used a gwt1-20 mutant that has a partial defect in inositol acylation activity at 30°C, and a gpi7Δ mutant that shows a temperature-sensitive growth phenotype. HA-Gas1*p was not stabilized in these mutants (Figures 10, A and B). Also, the degradation of CPY*HA was not delayed in any of the mutants (Figure 10, E and F). The results are consistent with our finding that the degradation of SHg* is independent of Bst1p (Figure 7C). It is not clear why the degradation of CPY* and KHN proteins are delayed in the per17-1/bst1 mutant (Vashist et al., 2001), but, based on our finding that Bst1p deacylates inositol in GPI anchors, it is most likely that it is an indirect effect. The steady-state levels of CPY*HA seemed to be slightly higher in GPI biosynthetic mutants than in wild-type cells. Therefore, it is possible that the degradation of other proteins such as CPY* is affected because pre-GPI proteins or immature GPI-anchored proteins are accumulated and degraded in the ER because of general defects in GPI anchor biosynthesis.
Figure 10.
(facing page). Effects of mutations in GPI biosynthesis, cargo receptors, and N-glycan processing on the degradation of Gas1*p and CPY*. (A) The degradation of Gas1*p was measured in MFY188 (WT), MFY189 (bst1Δ), MFY191 (gwt1), MFY190 (gpi7Δ), MFY192 (emp24Δ), MFY193 (erv25Δ), MFY195 (bst1Δ emp24Δ), MFY194 (mns1Δ), MFY196 (bst1Δ mns1Δ), MFY241 (htm1Δ), and MFY242 (bst1Δ htm1Δ) cells, in which HA-gas1* is integrated at the URA3 locus. The rate of degradation of HA-Gas1*p was determined as described in Figure 4. (B-D) The amount of Gas1* protein was quantified and is plotted as the means ± SD of at least two independent experiments, with the quantity at the 0 time point (steady-state level) set at 100%. (E) The degradation of CPY* was measured in W303-1A (WT), MFY150 (bst1Δ), gwt1-20 (gwt1), MFY11 (gpi7Δ), MFY157 (emp24Δ), MFY167 (erv25Δ), MFY159 (bst1Δ emp24Δ), MFY165 (mns1Δ), MFY168 (bst1Δ mns1Δ), MFY197 (htm1Δ), and MFY198 (bst1Δ htm1Δ) cells, all of which carried pRS316-CPY*HA. The rate of degradation of HA-Gas1*p was determined as described in Figure 4. (F-H) The amount of CPY* protein was quantified and plotted as described above.
We next addressed whether mutations in the process of vesicular protein concentration of GPI-anchored proteins affect the degradation of GPI-anchored proteins. Members of the p24 family, such as EMP24 and ERV25, have been shown to be involved in the concentration of GPI-anchored proteins during vesicular transport (Muniz et al., 2000; Watanabe and Riezman, 2004). Deletion of p24 family genes activates the unfolded protein response (Belden and Barlowe, 2001). However, as reported previously (Caldwell et al., 2001), the degradation of CPY*HA was not affected when these genes are deleted (emp24Δ and erv25Δ; Figure 10, E and G). In contrast, HA-Gas1*p was stabilized in emp24Δ, erv25Δ, and double bst1Δ emp24Δ mutants (Figure 10, A and C). These results indicate that the processes of concentrating GPI-anchored proteins and degrading misfolded GPI-anchored proteins are closely related. Thus, the activation of the unfolded protein response in emp24Δ cells and erv25Δ cells (Belden and Barlowe, 2001) may be because of a defect in the degradation of GPI-anchored proteins.
Quality control of glycoproteins is well-characterized and involves the specific oligosaccharide structures Glc1Man9GlcNAc2 and Man8GlcNAc2 (Helenius and Aebi, 2004). The Gas1 protein contains 10 potential sites of N-glycosylation and is highly O-mannosylated (Popolo and Vai, 1999). We used mns1Δ and htm1Δ mutants to determine whether the quality control mechanisms of glycoproteins are involved in the degradation of misfolded GPI-anchored proteins. MNS1 is required for the mannose trimming of an oligosaccharide (Camirand et al., 1991), and HTM1 is involved in the recognition of the oligosaccharide portion of misfolded glycoproteins (Jakob et al., 2001). The degradation of misfolded glycoproteins was stabilized in these mutants. We also confirmed that the degradation of CPY* is delayed in mns1Δ, htm1Δ, and double bst1Δ mns1Δ mutants (Figure 10, E and H). The degradation of Gas1*p was not stabilized in mns1Δ, whereas the deletion of HTM1 slightly delayed the degradation of Gas1*p, although the stabilization was less efficient in these cells than in bst1Δ cells (Figure 10, A and D). Gas1*p was stabilized in double bst1Δ mns1Δ and bst1Δ htm1Δ mutants, but the efficiency of stabilization in these double mutants was similar to that in bst1Δ single-mutant cells (Figure 10, A and D).
Misfolded Gas1 Proteins Associate with Both the Chaperone BiP/Kar2p and GPI Inositol Deacylase In Vivo
If Gas1*p is misfolded, several chaperones should be associated with Gas1*p in the ER. A previous study showed that a precursor of GPI-anchored protein associates with the ER chaperone BiP but not with calnexin in mammalian cells (Oda et al., 1996). BiP also binds to mutant prion proteins (Jin et al., 2000). First, we examined whether Gas1*p could associate with yeast BiP (Kar2p) in vivo. For this purpose, we fused the FLAG epitope to Kar2p after the signal peptide in Kar2p and expressed the construct in yeast cells containing HA-Gas1p or HA-Gas1*p. Immunoprecipitation in 1% digitonin-solubilized extracts revealed that FLAG-Kar2p coprecipitated with a fraction of HA-Gas1*p but not with HA-Gas1p (Figure 11A, left). We examined the ability of anti-HA-agarose to coimmunoprecipitate FLAG-Kar2p with HA-Gas1p. We found that FLAG-Kar2p coprecipitated with HA-Gas1*p but not with HA-Gas1p (Figure 11A, right), suggesting that Gas1*p is misfolded and that Kar2p is involved in the folding of Gas1*p. Because FLAG-Kar2p was not detected by anti-HA immunoprecipitation in WT cells carrying FLAG-KAR2 (Figure 11A, right), the results reflect a true association of HA-Gas1*p with FLAG-Kar2p.
Figure 11.
BiP/Kar2p and Bst1p associate with Gas1*p in vivo. (A) Gas1*p associates with Kar2p. W303-1A (WT), MFY207 (HA-GAS1), and MFY208 (HA-gas1*) cells, all of which carried YEp352-FLAG-KAR2 (FLAG-KAR2), were analyzed by immunoprecipitation (IP) with anti-FLAG-agarose or anti-HA-agarose, followed by immunoblotting with anti-FLAG (top) or anti-HA (bottom). (B) Gas1*p associates with Bst1p. W303-1A (WT), MFY207 (HA-GAS1), and MFY208 (HA-gas1*) cells, all of which carried YEp352-BST1-FLAG (BST1-FLAG), were analyzed by IP with anti-FLAG-agarose or anti-HA-agarose, followed by immunoblotting with anti-FLAG (top) or anti-HA (bottom). (C) Lipase-inactive Bst1p associates with Gas1*p. W303-1A (WT) cells carrying YEp352-BST1-FLAG (BST1-FLAG), WT cells carrying YEp352-BST1S236A-FLAG (S236A-FLAG), MFY208 (HA-gas1*) cells carrying YEp352-BST1-FLAG, and MFY208 cells carrying YEp352-BST1S236A-FLAG were analyzed by IP with anti-FLAG-agarose or anti-HA-agarose, followed immunoblotting with anti-FLAG (top) or anti-HA (bottom).
In these studies, we demonstrated that inositol deacylation of GPI is required for the degradation of Gas1*p (Figures 6 and 8). To determine whether Bst1p interacts with Gas1*p in vivo, we coexpressed Bst1p-FLAG with HA-Gas1p or HA-Gas1*p in yeast cells. We immunoprecipitated Bst1p-FLAG from the microsomal fraction and examined for the presence of HA-Gas1p or HA-Gas1*p by immunoblot analysis. Figure 11B shows that wild-type Gas1p associates weakly with Bst1p, whereas there is a strong association of Gas1*p with Bst1p (Figure 11B, left). We also used anti-HA-agarose, to immunoprecipitate HA-Gas1p or HA-Gas1*p and then checked for associated Bst1p. We found that a small amount of Bst1p-FLAG coimmunoprecipitated with wild-type HA-Gas1p, whereas there was a substantial amount of Bst1p-FLAG that coprecipitated with HA-Gas1*p (Figure 11B, right), supporting the idea that Gas1*p associates with Bst1p. Because Bst1p has GPI inositol deacylase activity, it is possible that it associates with GPI-anchored proteins by binding to the GPI moiety. Finally, we examined whether Bst1(S236A)p, the lipase-inactive form of Bst1p, associates with Gas1*p. We found that Gas1*p associates with both wild-type Bst1p and Bst1(S236A)p in vivo (Figure 11C), supporting the idea that the stabilization of Gas1*p in S236A cells (Figure 8B) is because of a loss in the GPI inositol deacylase activity rather than reduced binding of Gas1*p. These results suggest that Bst1p associates with misfolded GPI-anchored proteins and that deacylation activity is required for their degradation.
DISCUSSION
Posttranslational modification of proteins, including N-linked and O-linked glycosylation, that occur in the lumen of the ER, is involved in folding and ER quality control (Helenius and Aebi, 2004; Nakatsukasa et al., 2004). Our current report is the first on the molecular mechanisms of the quality control and degradation of GPI-anchored proteins, and we showed that the GPI anchor itself is involved in the quality control process. The first key finding was that a misfolded Gas1p, a model of misfolded GPI-anchored proteins, is GPI-anchored but retained in the ER and degraded via the proteasome pathway. We also found that deacylation of the inositol on GPI plays a crucial role in the degradation of GPI-anchored proteins.
In the CHX chase experiment, Gas1*p remained as the ER form, but its molecular weight gradually increased. Immunoblot analysis showed that the steady-state forms of Gas1*p are broad. The increases in the molecular weight of Gas1*p could not be because of reactions in the Golgi apparatus, such as outer chain elongation of N-glycans, because GPI-anchored Gas1*p was only present as the ER form and because the mobility of Gas1*p increased during the chase experiment, even after Endo H treatment, and the mobility shift of HA-Gas1*p was still observed in sec18-1 mutant cells at the restrictive temperature. Rather, the increases in molecular weight seem to be because of modification in the ER. We suspect that the mobility shift of Gas1*p during the chase period is caused by O-linked mannosylation because several aberrant proteins receive additional O-mannosyl residues in the ER (Nakatsukasa et al., 2004). Gas1p has serine/threonine-rich regions that are potential sites of O-mannosylation, and misfolded Gas1p might also receive additional O-mannosyl residues in the ER.
To investigate the involvement of N-glycans in the degradation of GPI-anchored proteins, we measured the degradation of Gas1*p in mns1Δ and htm1Δ cells. The deletion of HTM1 slightly stabilized Gas1*p, whereas the deletion of MNS1 had little effect. The effect of the htm1 deletion on the delay of the Gas1*p degradation was weaker than that of the bst1 deletion, suggesting that the degradation of Gas1*p is mainly affected by the GPI inositol deacylase activity. In yeast, GPI-anchored proteins are major components of the mannan layer in the cell wall. Almost all cell wall proteins and GPI-anchored proteins at the plasma membrane are highly N- and O-mannosylated (Orlean, 1997). Gas1p contains several modifications, including 10 potential N-glycosylation sites and a number of potential O-glycosylation sites. It might be difficult for Mns1p-Htm1p quality control systems to access highly glycosylated misfolded GPI-anchored proteins. Further analysis using a simpler system would be helpful, for example, a study of folding and degradation using a non-N-glycosylated GPI-anchored protein.
Recently, it was reported that a membrane protein and a soluble luminal protein in the ER were degraded by distinct cellular mechanisms (Taxis et al., 2003; Huyer et al., 2004; Vashist and Ng, 2004). In addition, two ER surveillance mechanisms have been proposed: ERAD-L, which monitors the folded state of luminal domains; and ERAD-C, which monitors that of cytosolic domains (Ahner and Brodsky, 2004; Vashist and Ng, 2004). Several specific factors have been identified for the ERAD-L and ERAD-C pathways. The proposed models could explain how misfolded proteins are degraded through the ERAD pathway, but there still are several exceptions to this pathway (Hampton, 2002; Schmitz and Herzog, 2004; Meusser et al., 2005). In this study, we could not identify the E3 ubiquitin ligases that are involved in Gas1*p degradation. Although we investigated the effect of three kinds of E3 on Gas1*p degradation, neither ERAD-L nor ERAD-C had an apparent effect on the degradation of Gas1*p.
We suspect that the ERAD-L and ERAD-C pathways are not involved because of the unique properties of GPI-anchored proteins. First, GPI-anchored proteins are bound to the ER membrane by a lipid, which is different from membrane or soluble proteins. Second, GPI-anchored proteins are a component of lipid rafts, which are sphingolipid- and sterol-rich membrane microdomains, and Gas1p has been found to associate with a raft-like microdomain in the ER (Bagnat et al., 2000). Third, GPI-anchored proteins are transported from the ER to the Golgi apparatus in distinct vesicles from those containing non-GPI proteins (Mayor and Riezman, 2004; Watanabe and Riezman, 2004). Apparently, specific components are required for sorting GPI-anchored proteins from other secretory proteins upon exit from the ER (Muniz et al., 2000; Muniz and Riezman, 2000). These properties also suggest the presence of specific components that are required for the degradation of GPI-anchored proteins, wherein GPI anchoring might change the accessibility to chaperones and unique ubiquitin ligases might act on the GPI-anchored proteins. In fact, our results suggest that the inositol deacylation of GPI by Bst1p plays an important role in this degradation pathway.
It remains unclear when the folding of GPI-anchored proteins occurs. We demonstrated that Gas1*p is modified by the GPI anchor, suggesting that the unfolded protein precursor is transferred to the GPI anchor. GPI-protein precursors that fail to be processed and retain the C-terminal GPI signal peptide are accumulated and degraded through the ERAD pathway in mammalian cells (Wainwright and Field, 1997; Wilbourn et al., 1998; Ali et al., 2000). A precursor Gas1p that could not be modified by the GPI anchor is also retained in the ER (Nuoffer et al., 1993; Doering and Schekman, 1996). In Trypanosome brucei GPI8-knockout (TbGPI8KO) cells, which lack a member of the GPI transamidase complex, GPI transamidase cannot process the trans-sialidase precursors by removing their GPI signals. TbGPI8KO cells have low trans-sialidase activity even in the cell lysate, and trans-sialidase is probably degraded in the ER (Nagamune et al., 2004), suggesting that GPI signal cleavage of the precursor protein is important for the folding of GPI-proteins. These reports indicate that unanchored precursor GPI-proteins are not sufficient for complete folding of the protein, which seems to be carried out after the cleavage of GPI signals.
Except in human erythrocytes, almost all GPI-anchored proteins are deacylated before their exit from the ER. In mammalian cells, a mammalian model GPI-anchored protein, DAF, was 56% deacylated in the ER within as little as 5 min (Chen et al., 1998). Inositol deacylation of GPI is important for the efficient transport of GPI-anchored proteins from the ER to the Golgi (Tanaka et al., 2004). We further demonstrated that the degradation of Gas1*p was significantly delayed in bst1Δ cells. The stabilization of Gas1*p in bst1Δ cells was because of the loss of GPI inositol deacylation activity. In addition, we found that Gas1*p associates with BiP and Bst1p in vivo. Our results suggest that GPI inositol deacylation is further required for the efficient degradation of misfolded GPI-anchored proteins.
There are several numbers of ERAD substrates that exit from the ER and their efficient degradation requires ER-to-Golgi transport (Vashist et al., 2001; Ahner and Brodsky, 2004; Vashist and Ng, 2004). In this study, we showed Gas1*p is capable of exiting from the ER to a certain extent, and this ER-to-Golgi transport is also important for the efficient degradation of Gas1*p. The degradation rate of SHg*, a misfolded non-GPI protein, was not changed between WT and bst1Δ cells, indicating that Bst1p affected only GPI-anchored proteins in exiting from the ER. One possibility for the degradation delay of Gas1*p in bst1Δ cells might be because of the delay of Gas1*p transport from the ER. The degradation of Gas1*p was also delayed in emp24Δ and erv25Δ cells. Emp24p and Erv25p are involved in the concentration of GPI-anchored proteins for the ER exit (Muniz et al., 2000; Watanabe and Riezman, 2004). These finding support the idea that a delay of Gas1*p transport from the ER affects its degradation in these mutant cells. Our results also indicates that misfolded GPI-anchored proteins, as folded GPI-anchored proteins, would exit from the ER in certain vesicles that are distinct from those that contain many other secretory proteins (Mayor and Riezman, 2004; Watanabe and Riezman, 2004), because the degradation of CPY* was not affected in emp24Δ and erv25Δ cells.
It is an important issue whether Bst1p is indeed involved in the ER quality control of GPI-anchored proteins. As shown in Figure 11B, Gas1*p was stably associated with Bst1p, whereas Gas1p was not so much. These results imply that Bst1p might wait for the correct folding of the GPI-anchored protein and deacylate the correctly folded proteins positively but deacylate less efficiently the misfolded GPI-anchored proteins. Once GPI-anchored proteins are deacylated, they are rapidly sorted to exit from the ER (Tanaka et al., 2004). Therefore, inositol deacylation activity should be strictly regulated. Bst1p could receive information on the status of protein folding after the protein is anchored by GPI. Stable association between Gas1*p and Bst1p suggests that there are unknown molecules that participate in the sensing of the protein folding status of GPI-anchored proteins and then transmit this information to Bst1p before it deacylates GPI-anchored proteins. When the misfolded GPI-anchored proteins are expressed, chaperones such as BiP try to assist in their folding. If proteins could not form a proper folding within an appropriate period of time, Bst1p may function to transport them to the Golgi preventing the accumulation of aberrant proteins in the ER. The Bst1p function positions at the border between protein folding in the ER and ER exit for the GPI-anchored proteins. We suspect that the inositol deacylation of GPI-anchored proteins by Bst1p is involved in the ER quality control and acts as a gatekeeper that leads GPI-anchored proteins to the ER exit after receiving some information on their folding status.
The quality control and degradation pathway for GPI-anchored proteins remains unknown, although we uncovered one aspect of the process in this study. We developed Gas1*p, which enables the study of the degradation of GPI-anchored proteins and therefore provides a unique and useful addition to the existing models of misfolded soluble glycoproteins and membrane proteins. The degradation of Gas1*p undergoes the same pathway with ERAD-L substrates in some respects (e.g., ER-to-Golgi transport), but it passes different route with ERAD-L and ERAD-C substrates in other points (e.g., Bst1p dependency and effect of ubiquitin ligases). However, several questions remain. For example, which components are involved in the degradation of misfolded GPI-anchored proteins? Are common factors or specific factors used in the degradation of GPI-anchored proteins, soluble glycoproteins, and membrane proteins? What factor distinguishes between folded and misfolded GPI-anchored proteins? When does Bst1p deacylate the misfolded GPI-anchored proteins? Although these questions remain to be answered, the analysis of molecules that interact with Gas1*p may reveal how the ER quality control machinery for GPI-anchored proteins is linked to proteasome-mediated degradation.
Acknowledgments
We are grateful to Drs. Taroh Kinoshita and Xiao-Dong Gao (AIST) for critical reading of this manuscript and for helpful comments. We also thank Dr. Yusuke Maeda for providing GST-tagged α-toxin protein; Dr. Tadashi Suzuki for providing the YIp-CPY* plasmid; Dr. Roger Tsien for providing the mRFP plasmid; Dr. Katsura Hata for providing the anti-Gas1 peptide polyclonal antibody; and Drs. Akio Toh-e (University of Tokyo, Tokyo, Japan), Yoshiko Kikuchi (University of Tokyo), and Mark Hochstrasser (Yale University, New Haven, CT) for providing the yeast strains. Finally, we thank Dr. Yasunori Chiba (AIST), Dr. Ken-ichi Nakayama (AIST), Dr. Yoh-ichi Shimma (AIST), Dr. Toru Sumita (AIST), Hiroto Hirayama (University of Tsukuba, Tsukuba, Japan), Mariko Umemura (AIST), and Michiyo Okamoto (AIST) for helpful discussions.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-05-0443) on November 30, 2005.
Abbreviations used: CHX, cycloheximide; CPY, carboxypeptidase Y; ER, endoplasmic reticulum; ERAD, endoplasmic reticulum-associated degradation; GPI, glycosylphosphatidylinositol; HRP, horseradish peroxidase; mRFP, monomeric red fluorescent protein.
References
- Abe, H., Shimma, Y., and Jigami, Y. (2003). In vitro oligosaccharide synthesis using intact yeast cells that display glycosyltransferases at the cell surface through cell wall-anchored protein Pir. Glycobiology 13, 87-95. [DOI] [PubMed] [Google Scholar]
- Ahner, A., and Brodsky, J. L. (2004). Checkpoints in ER-associated degradation: excuse me, which way to the proteasome? Trends Cell Biol. 14, 474-478. [DOI] [PubMed] [Google Scholar]
- Ali, B. R., Claxton, S., and Field, M. C. (2000). Export of a misprocessed GPI-anchored protein from the endoplasmic reticulum in vitro in an ATP- and cytosol-dependent manner. FEBS Lett. 483, 32-36. [DOI] [PubMed] [Google Scholar]
- Bagnat, M., Keranen, S., Shevchenko, A., Shevchenko, A., and Simons, K. (2000). Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 97, 3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden, W. J., and Barlowe, C. (2001). Deletion of yeast p24 genes activates the unfolded protein response. Mol. Biol. Cell 12, 957-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benachour, A., Sipos, G., Flury, I., Reggiori, F., Canivenc-Gansel, E., Vionnet, C., Conzelmann, A., and Benghezal, M. (1999). Deletion of GPI7, a yeast gene required for addition of a side chain to the glycosylphosphatidylinositol (GPI) core structure, affects GPI protein transport, remodeling, and cell wall integrity. J. Biol. Chem. 274, 15251-15261. [DOI] [PubMed] [Google Scholar]
- Caldwell, S. R., Hill, K. J., and Cooper, A. A. (2001). Degradation of endoplasmic reticulum (ER) quality control substrates requires transport between the ER and Golgi. J. Biol. Chem. 276, 23296-23303. [DOI] [PubMed] [Google Scholar]
- Camirand, A., Heysen, A., Grondin, B., and Herscovics, A. (1991). Glycoprotein biosynthesis in Saccharomyces cerevisiae. Isolation and characterization of the gene encoding a specific processing alpha-mannosidase. J. Biol. Chem. 266, 15120-15127. [PubMed] [Google Scholar]
- Chen, R., Walter, E. I., Parker, G., Lapurga, J. P., Millan, J. L., Ikehara, Y., Udenfriend, S., and Medof, M. E. (1998). Mammalian glycophosphatidylinositol anchor transfer to proteins and posttransfer deacylation. Proc. Natl. Acad. Sci. USA 95, 9512-9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann, A., Riezman, H., Desponds, C., and Bron, C. (1988). A major 125-kd membrane glycoprotein of Saccharomyces cerevisiae is attached to the lipid bilayer through an inositol-containing phospholipid. EMBO J. 7, 2233-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering, T. L., and Schekman, R. (1996). GPI anchor attachment is required for Gas1p transport from the endoplasmic reticulum in COP II vesicles. EMBO J. 15, 182-191. [PMC free article] [PubMed] [Google Scholar]
- Eakle, K. A., Bernstein, M., and Emr, S. D. (1988). Characterization of a component of the yeast secretion machinery: identification of the SEC18 gene product. Mol. Cell. Biol. 8, 4098-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhaber, B., Maurer-Stroh, S., Novatchkova, M., Schneider, G., and Eisenhaber, F. (2003). Enzymes and auxiliary factors for GPI lipid anchor biosynthesis and post-translational transfer to proteins. Bioessays 25, 367-385. [DOI] [PubMed] [Google Scholar]
- Ellgaard, L., Molinari, M., and Helenius, A. (1999). Setting the standards: quality control in the secretory pathway. Science 286, 1882-1888. [DOI] [PubMed] [Google Scholar]
- Elrod-Erickson, M. J., and Kaiser, C. A. (1996). Genes that control the fidelity of endoplasmic reticulum to Golgi transport identified as suppressors of vesicle budding mutations. Mol. Biol. Cell 7, 1043-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, M. C., Moran, P., Li, W., Keller, G. A., and Caras, I. W. (1994). Retention and degradation of proteins containing an uncleaved glycosylphosphatidylinositol signal. J. Biol. Chem. 269, 10830-10837. [PubMed] [Google Scholar]
- Finger, A., Knop, M., and Wolf, D. H. (1993). Analysis of two mutated vacuolar proteins reveals a degradation pathway in the endoplasmic reticulum or a related compartment of yeast. Eur. J. Biochem. 218, 565-574. [DOI] [PubMed] [Google Scholar]
- Fujita, M., Yoko-o, T., Okamoto, M., and Jigami, Y. (2004). GPI7 involved in glycosylphosphatidylinositol biosynthesis is essential for yeast cell separation. J. Biol. Chem. 279, 51869-51879. [DOI] [PubMed] [Google Scholar]
- Gordon, V. M., Nelson, K. L., Buckley, J. T., Stevens, V. L., Tweten, R. K., Elwood, P. C., and Leppla, S. H. (1999). Clostridium septicum alpha toxin uses glycosylphosphatidylinositol-anchored protein receptors. J. Biol. Chem. 274, 27274-27280. [DOI] [PubMed] [Google Scholar]
- Hampton, R. Y. (2002). ER-associated degradation in protein quality control and cellular regulation. Curr. Opin. Cell Biol. 14, 476-482. [DOI] [PubMed] [Google Scholar]
- Haynes, C. M., Caldwell, S., and Cooper, A. A. (2002). An HRD/DER-independent ER quality control mechanism involves Rsp5p-dependent ubiquitination and ER-Golgi transport. J. Cell Biol. 158, 91-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius, A., and Aebi, M. (2001). Intracellular functions of N-linked glycans. Science 291, 2364-2369. [DOI] [PubMed] [Google Scholar]
- Helenius, A., and Aebi, M. (2004). Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73, 1019-1049. [DOI] [PubMed] [Google Scholar]
- Hong, Y., Ohishi, K., Inoue, N., Kang, J. Y., Shime, H., Horiguchi, Y., van der Goot, F. G., Sugimoto, N., and Kinoshita, T. (2002). Requirement of N-glycan on GPI-anchored proteins for efficient binding of aerolysin but not Clostridium septicum alpha-toxin. EMBO J. 21, 5047-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyer, G., Piluek, W. F., Fansler, Z., Kreft, S. G., Hochstrasser, M., Brodsky, J. L., and Michaelis, S. (2004). Distinct machinery is required in Saccharomyces cerevisiae for the endoplasmic reticulum-associated degradation of a multi-spanning membrane protein and a soluble luminal protein. J. Biol. Chem. 279, 38369-38378. [DOI] [PubMed] [Google Scholar]
- Ishida, Y., Komaru, K., Ito, M., Amaya, Y., Kohno, S., and Oda, K. (2003). Tissue-nonspecific alkaline phosphatase (ALP) with an Asp(289)→Val mutation fails to reach the cell surface and undergoes proteasome-mediated degradation. J. Biochem. (Tokyo) 134, 63-70. [DOI] [PubMed] [Google Scholar]
- Ito, M., Amizuka, N., Ozawa, H., and Oda, K. (2002). Retention at the cis-Golgi and delayed degradation of tissue-non-specific ALP with an Asn153→Asp substitution, a cause of perinatal hypophosphatasia. Biochem. J. 361, 473-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob, C. A., Bodmer, D., Spirig, U., Battig, P., Marcil, A., Dignard, D., Bergeron, J. J., Thomas, D. Y., and Aebi, M. (2001). Htm1p, a mannosidase-like protein, is involved in glycoprotein degradation in yeast. EMBO Rep. 2, 423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob, C. A., Burda, P., Roth, J., and Aebi, M. (1998). Degradation of misfolded endoplasmic reticulum glycoproteins in Saccharomyces cerevisiae is determined by a specific oligosaccharide structure. J. Cell Biol. 142, 1223-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, T., Gu, Y., Zanusso, G., Sy, M., Kumar, A., Cohen, M., Gambetti, P., and Singh, N. (2000). The chaperone protein BiP binds to a mutant prion protein and mediates its degradation by the proteasome. J. Biol. Chem. 275, 38699-38704. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T., and Inoue, N. (2000). Dissecting and manipulating the pathway for glycosylphos-phatidylinositol-anchor biosynthesis. Curr. Opin. Chem. Biol. 4, 632-638. [DOI] [PubMed] [Google Scholar]
- Knop, M., Hauser, N., and Wolf, D. H. (1996). N-Glycosylation affects endoplasmic reticulum degradation of a mutated derivative of carboxypeptidase yscY in yeast. Yeast 12, 1229-1238. [DOI] [PubMed] [Google Scholar]
- Kominami, K., et al. (1995). Nin1p, a regulatory subunit of the 26S proteasome, is necessary for activation of Cdc28p kinase of Saccharomyces cerevisiae. EMBO J. 14, 3105-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominami, K., et al. (1997). Yeast counterparts of subunits S5a and p58 (S3) of the human 26S proteasome are encoded by two multicopy suppressors of nin1-1. Mol. Biol. Cell 8, 171-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito, R. R. (1997). ER quality control: the cytoplasmic connection. Cell 88, 427-430. [DOI] [PubMed] [Google Scholar]
- Kostova, Z., and Wolf, D. H. (2003). For whom the bell tolls: protein quality control of the endoplasmic reticulum and the ubiquitin-proteasome connection. EMBO J. 22, 2309-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M. S., McKenzie, A., 3rd, Demarini, D. J., Shah, N. G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J. R. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953-961. [DOI] [PubMed] [Google Scholar]
- Mayor, S., and Riezman, H. (2004). Sorting GPI-anchored proteins. Nat. Rev. Mol. Cell. Biol. 5, 110-120. [DOI] [PubMed] [Google Scholar]
- Meusser, B., Hirsch, C., Jarosch, E., and Sommer, T. (2005). ERAD: the long road to destruction. Nat. Cell Biol. 7, 766-772. [DOI] [PubMed] [Google Scholar]
- Monnat, J., Neuhaus, E. M., Pop, M. S., Ferrari, D. M., Kramer, B., and Soldati, T. (2000). Identification of a novel saturable endoplasmic reticulum localization mechanism mediated by the C-terminus of a Dictyostelium protein disulfide isomerase. Mol. Biol. Cell 11, 3469-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz, M., Nuoffer, C., Hauri, H. P., and Riezman, H. (2000). The Emp24 complex recruits a specific cargo molecule into endoplasmic reticulum-derived vesicles. J. Cell Biol. 148, 925-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz, M., and Riezman, H. (2000). Intracellular transport of GPI-anchored proteins. EMBO J. 19, 10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamune, K., Acosta-Serrano, A., Uemura, H., Brun, R., Kunz-Renggli, C., Maeda, Y., Ferguson, M. A., and Kinoshita, T. (2004). Surface sialic acids taken from the host allow trypanosome survival in tsetse fly vectors. J. Exp. Med. 199, 1445-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsukasa, K., Okada, S., Umebayashi, K., Fukuda, R., Nishikawa, S., and Endo, T. (2004). Roles of O-mannosylation of aberrant proteins in reduction of the load for endoplasmic reticulum chaperones in yeast. J. Biol. Chem. 279, 49762-49772. [DOI] [PubMed] [Google Scholar]
- Ng, D. T., Spear, E. D., and Walter, P. (2000). The unfolded protein response regulates multiple aspects of secretory and membrane protein biogenesis and endoplasmic reticulum quality control. J. Cell Biol. 150, 77-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuoffer, C., Horvath, A., and Riezman, H. (1993). Analysis of the sequence requirements for glycosylphosphatidylinositol anchoring of Saccharomyces cerevisiae Gas1 protein. J. Biol. Chem. 268, 10558-10563. [PubMed] [Google Scholar]
- Nuoffer, C., Jeno, P., Conzelmann, A., and Riezman, H. (1991). Determinants for glycophospholipid anchoring of the Saccharomyces cerevisiae GAS1 protein to the plasma membrane. Mol. Cell. Biol. 11, 27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda, K., Wada, I., Takami, N., Fujiwara, T., Misumi, Y., and Ikehara, Y. (1996). Bip/GRP78 but not calnexin associates with a precursor of glycosylphosphatidylinositol-anchored protein. Biochem. J. 316 (Pt 2), 623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, M., Yoko-o, T., Umemura, M., Nakayama, K., and Jigami, Y. (2006). Glycosylphosphatidyl-inositol-anchored proteins are required for the transport of detergent-resistant microdomain-associated membrane proteins, Tat2p and Fur4p. J. Biol. Chem. (in press). [DOI] [PubMed]
- Orlean, P. (1997). Biogenesis of yeast wall and surface components. In: The Molecular and Cellular Biology of the Yeast Saccharomyces. Cell Cycle and Biology, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 229-362.
- Plemper, R. K., Egner, R., Kuchler, K., and Wolf, D. H. (1998). Endoplasmic reticulum degradation of a mutated ATP-binding cassette transporter Pdr5 proceeds in a concerted action of Sec61 and the proteasome. J. Biol. Chem. 273, 32848-32856. [DOI] [PubMed] [Google Scholar]
- Popolo, L., and Vai, M. (1999). The Gas1 glycoprotein, a putative wall polymer cross-linker. Biochim. Biophys. Acta 1426, 385-400. [DOI] [PubMed] [Google Scholar]
- Prusiner, S. B. (1998). Prions. Proc. Natl. Acad. Sci. USA 95, 13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman, H., Horvath, A., Manning-Krieg, U., and Movva, R. (1994). Intracellular transport of GPI-anchored proteins by yeast. Braz. J. Med. Biol. Res. 27, 323-326. [PubMed] [Google Scholar]
- Schmitz, A., and Herzog, V. (2004). Endoplasmic reticulum-associated degradation: exceptions to the rule. Eur. J. Cell Biol. 83, 501-509. [DOI] [PubMed] [Google Scholar]
- Sherman, F. (1991). Getting started with yeast. Methods Enzymol. 194, 3-21. [DOI] [PubMed] [Google Scholar]
- Sikorski, R. S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear, E. D., and Ng, D. T. (2003). Stress tolerance of misfolded carboxypeptidase Y requires maintenance of protein trafficking and degradative pathways. Mol. Biol. Cell 14, 2756-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, A., Immanuel, D., and Arndt, K. T. (1991). The SIT4 protein phosphatase functions in late G1 for progression into S phase. Mol. Cell. Biol. 11, 2133-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T., Park, H., Hollingsworth, N. M., Sternglanz, R., and Lennarz, W. J. (2000). PNG1, a yeast gene encoding a highly conserved peptide:N-glycanase. J. Cell Biol. 149, 1039-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, R., Locher, M., and Hochstrasser, M. (2001). A conserved ubiquitin ligase of the nuclear envelope/endoplasmic reticulum that functions in both ER-associated and Matalpha2 repressor degradation. Genes Dev. 15, 2660-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, S., Maeda, Y., Tashima, Y., and Kinoshita, T. (2004). Inositol deacylation of glycosylphosphatidylinositol-anchored proteins is mediated by mammalian PGAP1 and yeast Bst1p. J. Biol. Chem. 279, 14256-14263. [DOI] [PubMed] [Google Scholar]
- Taxis, C., Hitt, R., Park, S. H., Deak, P. M., Kostova, Z., and Wolf, D. H. (2003). Use of modular substrates demonstrates mechanistic diversity and reveals differences in chaperone requirement of ERAD. J. Biol. Chem. 278, 35903-35913. [DOI] [PubMed] [Google Scholar]
- Tong, A. H., et al. (2004). Global mapping of the yeast genetic interaction network. Science 303, 808-813. [DOI] [PubMed] [Google Scholar]
- Umemura, M., Okamoto, M., Nakayama, K., Sagane, K., Tsukahara, K., Hata, K., and Jigami, Y. (2003). GWT1 gene is required for inositol acylation of glycosylphosphatidylinositol anchors in yeast. J. Biol. Chem. 278, 23639-23647. [DOI] [PubMed] [Google Scholar]
- Vallee, B., and Riezman, H. (2005). Lip1p: a novel subunit of acyl-CoA ceramide synthase. EMBO J. 24, 730-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist, S., Kim, W., Belden, W. J., Spear, E. D., Barlowe, C., and Ng, D. T. (2001). Distinct retrieval and retention mechanisms are required for the quality control of endoplasmic reticulum protein folding. J. Cell Biol. 155, 355-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist, S., and Ng, D. T. (2004). Misfolded proteins are sorted by a sequential checkpoint mechanism of ER quality control. J. Cell Biol. 165, 41-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright, L. J., and Field, M. C. (1997). Quality control of glycosylphosphatidylinositol anchor attachment in mammalian cells: a biochemical study. Biochem. J. 321, 655-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, R., Funato, K., Venkataraman, K., Futerman, A. H., and Riezman, H. (2002). Sphingolipids are required for the stable membrane association of glycosylphosphatidylinositol-anchored proteins in yeast. J. Biol. Chem. 277, 49538-49544. [DOI] [PubMed] [Google Scholar]
- Watanabe, R., and Riezman, H. (2004). Differential ER exit in yeast and mammalian cells. Curr. Opin. Cell Biol. 16, 350-355. [DOI] [PubMed] [Google Scholar]
- Wilbourn, B., Nesbeth, D. N., Wainwright, L. J., and Field, M. C. (1998). Proteasomeandthiolinvolvementinqualitycontrolofglycosylphosphatidylinositol anchor addition. Biochem. J. 332, 111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiroda, H., Oguchi, T., Yasuda, Y., Toh-e, A., and Kikuchi, Y. (1996). Bul1, a new protein that binds to the Rsp5 ubiquitin ligase in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 3255-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]