Abstract
Interactions between transport receptors and phenylalanine-glycine (FG) repeats on nucleoporins drive the translocation of receptor-cargo complexes through nuclear pores. Tap, a transport receptor that mediates nuclear export of cellular mRNAs, contains a UBA-like and NTF2-like folds that can associate directly with FG repeats. In addition, two nuclear export sequences (NESs) within the NTF2-like region can also interact with nucleoporins. The Tap-RNA complex was shown to bind to three nucleoporins, Nup98, p62, and RanBP2, and these interactions were enhanced by Nxt1. Mutations in the Tap-UBA region abolished interactions with all three nucleoporins, whereas the effect of point mutations within the NTF2-like domain of Tap known to disrupt Nxt1 binding or nucleoporin binding were nucleoporin dependent. A mutation in any of these Tap domains was sufficient to reduce RNA export but was not sufficient to disrupt Tap interaction with the NPC in vivo or its nucleocytoplasmic shuttling. However, shuttling activity was reduced or abolished by combined mutations within the UBA and either the Nxt1-binding domain or NESs. These data suggest that Tap requires both the UBA- and NTF2-like domains to mediate the export of RNA cargo, but can move through the pores independently of these domains when free of RNA cargo.
INTRODUCTION
Nuclear transport plays an important role in cell function by selectively segregating macromolecules to the nuclear or cytoplasmic compartments. This biased distribution contributes to the regulation of many proteins including transcription factors, cell cycle regulators, and cell signaling components (Komeili and O'Shea, 2000; Macara, 2001; Carmo-Fonseca, 2002). Regulated nuclear export of mRNA serves as a quality control step to ensure that only properly spliced mRNA is exported to the cytoplasm for translation (Lei and Silver, 2002; Stutz and Izaurralde, 2003). All nucleocytoplasmic traffic must go through large, proteinaceous channels known as nuclear pore complexes (NPCs; Suntharalingam and Wente, 2003). Although small molecules (<40 kDa) can diffuse freely through the NPC, movement of most proteins and RNAs across nuclear pores requires binding to soluble import or export receptors (Macara, 2001; Weis, 2002; Bednenko et al., 2003; Pemberton and Paschal, 2005). Import and export receptors, in turn, mediate protein and RNA translocation through highly transient interactions with nucleoporins in the NPC.
The majority of nuclear transport receptors belong to the karyopherin/importin β family of proteins (Macara, 2001; Bednenko et al., 2003). Crm1, the best-characterized export receptor and a member of the importin β family, mediates the export of proteins, U snRNAs, and 5S RNAs (Fornerod et al., 1997; Mattaj and Englmeier, 1998; Cullen, 2003). The transport receptor believed to be responsible for the bulk of mRNA export in eukaryotes is Tap/NXF1, a factor that is unrelated to the importin β family (Katahira et al., 1999; Cullen, 2003). Tap also mediates nuclear export of certain retroviral mRNAs, such as the Mason-Pfizer monkey virus (MPMV; Grüter et al., 1998). A cis-acting element in the noncoding 3′ region of these viral transcripts, known as the constitutive transport element (CTE), forms a stem-loop structure recognized by Tap (Bray et al., 1994; Ernst et al., 1997a, 1997b; Grüter et al., 1998; Braun et al., 1999; Bachi et al., 2000; Liker et al., 2000). In contrast, the interaction between Tap and eukaryotic mRNAs appear to require a number of adaptor proteins that are recruited to messenger ribonucleoprotein (mRNP) complexes during transcription and processing (Izaurralde, 2002; Cullen, 2003; Stutz and Izaurralde, 2003; Vinciguerra and Stutz, 2004). RNA export mediated by Tap is also dependent on Nxt1/p15, a low-molecular-weight NTF2-like protein, that heterodimerizes with Tap (Black et al., 1999; Braun et al., 2001; Fribourg et al., 2001; Guzik et al., 2001). Nxt1 enhances Tap-dependent RNA export by stimulating Tap interactions with the NPC (Lévesque et al., 2001; Wiegand et al., 2002). Furthermore, the importance of Nxt1 for RNA export was extended by RNAi experiments in the Drosophila cell line, S2, in which suppression of Nxt1 expression caused a reduction in mRNA export, resulting in nuclear mRNA accumulation (Wiegand et al., 2002).
The NPC is assembled from ∼30 different proteins known as nucleoporins (Rout et al., 2000; Cronshaw et al., 2002). About a third of the nucleoporins play central roles in the transport of cargo-receptor complexes by providing binding sites for transport receptors. The receptor binding sites on nucleoporins are based on Phe-Gly (FG) repeat motifs occurring as FxFG, GLFG, or FG motifs flanked by polar residues, where “x” represents any amino acid (Bednenko et al., 2003; Suntharalingam et al., 2003). It is estimated that more than 3500 FG repeats are distributed throughout each NPC (Strawn et al., 2004). Recent evidence suggests that transport receptors interact with distinct subsets of FG repeats (Clarkson et al., 1997; Damelin and Silver, 2000; Strawn et al., 2001; Blevins et al., 2003; Strawn et al., 2004). This could provide part of the basis for coordinating bidirectional flow of traffic through the NPC, which is estimated to approach 1000 molecules per second (Ribbeck and Gorlich, 2001). Evidence for this type of organization was observed when examining the interaction of two transport receptors, Pse1p/Kap121p and Msn5p, with a number of nucleoporins using fluorescence resonance energy transfer (FRET) in yeast (Damelin and Silver, 2000). Although the two receptors converged on some of the same nucleoporins during translocation, each receptor also interacted with distinct nucleoporins. Additional evidence that transport receptors rely on distinct FG repeats and/or nucleoporins comes from yeast where mutations or over expression of individual nucleoporins can affect the translocation of particular transport receptors without affecting the movement of others (Bastos et al., 1996; Belgareh et al., 1998; Balasundaram et al., 1999). Mex67p, the Tap homologue in Saccharomyces cerevisiae, and Kap95p bind distinct repeats within Nup116 (Strawn et al., 2001). Moreover, the interaction between Tap and Nup98 is confined to a subset of the GLFG repeats in Nup98 (Blevins et al., 2003). Thus, the available data suggest that transport receptors rely on both common and distinct binding sites for movement through the NPC. Understanding how transport complexes interact with specific nucleoporins is required to define the mechanism(s) and regulation of translocation.
Structural analyses have thus far identified two regions of Tap that can interact with nucleoporins. The C-terminal domain of Tap (residues 551-619) was shown to contain a ubiquitin-associated (UBA) domain that has four α-helices folded against each other, forming a hydrophobic pocket capable of interacting with FG repeats (Grant et al., 2002, 2003). The UBA domain of Tap can bind the nucleoporin Nsp1p in vitro (Schmitt and Gerace, 2001; Grant et al., 2002). Tap mutants lacking the UBA domain are unable to interact with the nucleoporin p62 in vitro or to mediate RNA export in vivo (Guzik et al., 2001; Lévesque et al., 2001). A second hydrophobic pocket in Tap located in the NTF2-like domain (residues 372-550) was shown to interact with a small fragment of Can/Nup214 (residues 1805-1816) containing a single FG-repeat (Fribourg et al., 2001). Mutation of two residues (L383,386R) within this second nucleoporin-binding region (Tap-NBR2) decreases nuclear rim association in permeabilized cells and reduces RNA export activity in a transfected cell assay (Fribourg et al., 2001). In addition to the Tap-NBR2, functional analyses have identified two other regions, referred to as nuclear export sequences (NES) I (residues 473-505) and NES II (residues 505-546) within the NTF2-like domain of Tap that can interact directly with nucleoporins to mediate the export of tethered glucocorticoid receptor-GFP fusion protein in HeLa cells (Thakurta et al., 2004). Mutations of residues 495-497 (VNG→AAA; m9 mutant) within NES I and residues 529-530 (IV→AA; m6 mutant) within NES II abolished both the ability of these NESs to interact with nucleoporins and mediate Tap export function.
The goal of the present study was to assess the contribution of the nucleoporin-interacting domains of Tap, the UBA- and the NTF2-like regions (more specifically the Tap-NBR2 and both NESs), with regard to NPC binding, Nxt1 interaction and RNA export. We demonstrate that the UBA domain is required for association with all of the nucleoporins tested, whereas the requirement for the Tap-NBR2 and NESs are nucleoporin-specific. Interestingly we find that Nxt1 can still stimulate the binding of Tap to nucleoporins despite mutations of the Tap-NBR2, which suggest that this Nxt1 effect cannot simply be attributed to the stabilization of the NTF2-like Tap domain as previously suggested (Fribourg et al., 2001). Although efficient export of RNA was dependent on having both a functional UBA- and NTF2-like domain, Tap could still shuttle across the NPC despite point mutations in any of these regions. Shuttling was obliterated only when both the UBA domain together with the two NESs were mutated. Our data support the emerging view that transport receptors use multiple domains to contact nucleoporins in the NPC. Multidomain contact with nucleoporins is not required for Tap translocation through the NPC; multidomain contact is, however, required for Tap to mediate RNA export.
MATERIALS AND METHODS
Plasmids and Recombinant Proteins
FLAG-Tap1-619, FLAG-RevM10-Tap61-619, GFP-Tap61-619, glutathione-S-transferase (GST)-Tap WT, and pCMV-CTE plasmids have been described previously (Guzik et al., 2001; Lévesque et al., 2001). Point mutations were introduced into the FLAG-Tap, FLAG-RevM10-Tap, GFP-Tap, and GST-Tap vectors using the Quick-change site-directed mutagenesis protocol (Stratagene, La Jolla, CA). The GFP-NLS-Streptavidin construct has been described (Black et al., 2001). Dr. Larry Gerace kindly provided the pGEX vectors for expression of GST-p62 (Hu et al., 1996) and GST-RanBP2-4 (residues 996-1963; Yaseen and Blobel, 1999). The GST-Nup98 vector (GLFG domain residues 221-504) was a kind gift from Dr. Maureen Powers. All GST-tagged proteins were expressed in E. coli by induction with isopropyl fl-d-thiogalactoside and isolated on glutathione-Sepharose. Expression and purification of Nxt1 was described previously (Black et al., 1999). The plasmid encoding the GAC mutant (mutGAC) of the CTE was kindly provided by Dr. Bryan Cullen.
Fluorescence Microscopy
Cos 7 cells were transiently transfected with FLAG-Tap1-619 constructs (WT or mutants) using Fugene 6 (Roche, Indianapolis, IN). For standard immunofluorescence (IF), cells grown on glass coverslips were transfected and, 24-48 h later, fixed with 4% (wt/vol) formaldehyde in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4, pH 7.4) containing 2% (wt/vol) sucrose for 10 min at room temperature. Cells were then permeabilized with 0.2% (vol/vol) Triton X-100 in PBS for 20 min and incubated in blocking solution (2% [wt/vol] bovine serum albumin, 2% [vol/vol] new born calf serum, 0.2% [vol/vol] Tween 20, and 0.02% [wt/vol] NaN3) for 2 h before the addition of antibodies. FLAG-Tap was detected using M2 antibody (1:5000; Sigma, St. Louis, MO) and subsequent incubation with Cy3-conjugated anti-mouse antibody (1:800; Jackson ImmunoResearch Laboratories, West Grove, PA) diluted in blocking solution. After each antibody incubation, cells were washed with Tris-buffered saline (TBS) containing Tween 20 (TBS-T; 20 mM Tris, 154 mM NaCl, 0.1% [vol/vol] Tween-20, pH 7.4). Nuclei were stained with 4′,6-diamidino-2-phenyllindole (DAPI; 0.5 μg/ml) in the last washing step, and coverslips were mounted in Vectashield media (Vector Laboratories, Burlingame, CA). Digital images were captured by a charge-coupled device camera (Hamamatsu ORCA, Bridgewater, NJ) mounted on a Nikon Microphot-SA microscope (Melville, NY) using Openlab software 2.0.6 (Improvision I, Lexington, MA). Some images (Figures 8, GFP-STV-NLS, and 9A) were captured with Pictureframe software version 2.2 using a MicroFire digital camera (Optronics, Goleta, CA). Montages of digital images were assembled in Adobe Photoshop 7.0 (San Jose, CA).
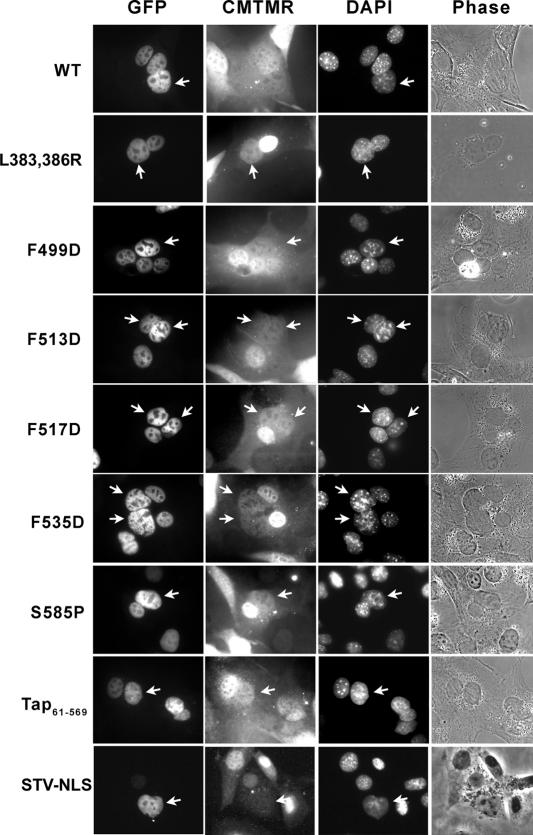
Figure 8.
Shuttling Assay of EGFP-Tap mutants. Donor Cos cells expressing GFP-Tap (WT or mutant) were fused to mouse NIH3T3 cells prelabeled with the cytoplasmic marker CMTMR. Cells were fixed in formaldehyde 4 h after fusion. Designation of a shuttling protein was made when GFP-Tap originating from the nuclei of donor cells (indicated by arrow) was detected in the nuclei of acceptor cells. The nonshuttling reporter GFP-STV-NLS was included as a negative control.
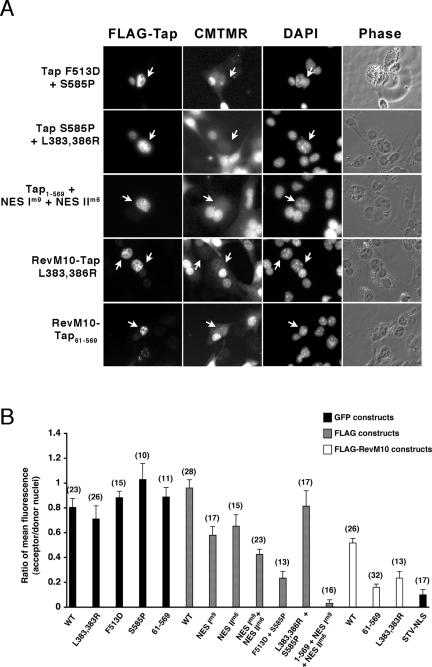
Figure 9.
Shuttling Assay of mutant FLAG-Tap. (A) Donor Cos cells expressing mutant FLAG-Tap or FLAG-RevM10-Tap were fused to mouse NIH3T3 cells prelabeled with the cytoplasmic marker CMTMR. Four hours after fusion, cells were fixed in formaldehyde and processed for IF. FLAG-fusion proteins were detected using α-FLAG mAb and anti-mouse Cy3. Nuclei were identified by DAPI stain. Designation of a shuttling protein was made when FLAG-Tap originating from the nuclei of donor cells (indicated by arrows) was detected in the nuclei of acceptor cells. (B) The shuttling ability of each GFP-(▪), FLAG-( ), and FLAG-RevM10-tagged (□) Tap construct from Figures 8 and 9A was assessed by comparing the ratio of mean nuclear pixel intensity (±SD) of donor from that of the acceptor nuclei. The number of heterokaryons used to assess shuttling of each construct is indicated (n = number of heterokaryon measured).
), and FLAG-RevM10-tagged (□) Tap construct from Figures 8 and 9A was assessed by comparing the ratio of mean nuclear pixel intensity (±SD) of donor from that of the acceptor nuclei. The number of heterokaryons used to assess shuttling of each construct is indicated (n = number of heterokaryon measured).
For IF-detection of Tap proteins on the cytoplasmic side of the NPC, cells were permeabilized with digitonin before fixation. Cos 7 cells were treated with 0.005% (wt/vol) digitonin in transport buffer (TB; 20 mM HEPES, 110 mM potassium acetate, 2 mM magnesium acetate, 1 mM EGTA, pH 7.4) for 6 min at 4°C. Soluble cytoplasmic factors were released by incubating the cells in TB for 20 min at room temperature, which was followed by fixation with 2% formaldehyde for 10 min. Cells were then processed for IF as described above except that Tween-20 and Triton X-100 were omitted throughout the remaining steps to maintain integrity of the nuclear envelope.
Heterokaryon Shuttling Assays
The ability of Tap mutants to move in and out of the nucleus was assessed using shuttling assays. The protocol for this assay was described in detail previously (Black et al., 2001). Briefly, donor Cos cells transfected with GFP-Tap (or FLAG-Tap) constructs and acceptor NIH3T3 cells labeled with Cell-Tracker dye, (5-(and-6)-(((4-chloromethyl) benzoyl) amino) tetramethylrhodamine (CMTMR; Molecular Probes, Eugene, OR), were fused in 50% (wt/vol) polyethylene glycol (Roche). Cycloheximide (100 μM) was added to the incubation media 1 h before fusion and was also present during the 4-h incubation that followed cytoplasmic fusion. In the experiments using GFP-Tap, cells were fixed in 4% (wt/vol) formaldehyde, permeabilized with 0.5% (vol/vol) Triton X-100 in PBS, incubated with DAPI, and mounted on slides as described above. FLAG-Tap and FLAG-RevM10-Tap (WT and mutants) were also tested and detected by standard IF as described above. Mean pixel intensity of donor and acceptor nuclei were measured using the NIH Image version 1.63. Background pixel intensity from an area adjacent to each cell and of the same size as the nucleus was subtracted from each nuclear pixel intensity. The ratio of pixel intensity of acceptor over that of donor nuclei was then calculated for each heterokaryon. At least 10 heterokaryons were analyzed for each Tap construct.
Solid Phase-binding Assays and Immunoprecipitation
The ability of Tap to interact with nucleoporins was assessed using a solid-phase binding assay as described (Lévesque et al., 2001). In this assay, 3 pmol of GST or GST-tagged nucleoporins were bound to 96-well plates. 35S-labeled FLAG-Tap (WT and mutants) were synthesized in vitro in rabbit reticulocyte lysate according to the manufacturer protocol (Promega, Madison, WI) and incubated with the immobilized nucleoporins for 24 h at 4°C in the presence or absence of recombinant (66 nM) Nxt1. For RNA binding experiments, 32P-labeled CTE RNA (WT or mutant) were synthesized in vitro using a pCMV template (Lévesque et al., 2001) and added to the binding reaction. After two wash steps to remove unbound proteins and RNAs, the specifically bound molecules were eluted with 5% SDS. The level of 35S-Tap and 32P-CTE RNA in the eluate was then determined by liquid scintillation counting.
Immunoprecipitation (IP) experiments combined 35S-labeled FLAG-Tap and 35S-labeled Nxt1 transcribed from a pSVK3 (GE Healthcare, Piscataway, NJ) vector (Lévesque et al., 2001). The 35S-labeled proteins were incubated overnight at 4°C with the anti-FLAG (M2) antibody pre-bound to protein-G beads. The IP-complexes were washed five times in PBS containing 0.1% Nonidet-P40. Samples were boiled in Laemmli sample buffer and separated by SDS-PAGE. Gels were treated with Autofluor (National Diagnostics, Atlanta, GA) for 30 min before drying. 35S-labeled proteins were then visualized by autoradiography.
RNA Export Assays and Northern Blots
293T/17 cells were maintained in Iscove's minimal essential medium supplemented with 10% bovine calf serum and transfected using a calcium phosphate protocol (Wigler et al., 1978). Supernatants from transfected cells were collected at 72 h posttransfection, centrifuged to remove residual cells and debris, and stored at -20°C until assayed. The p24 (HIV capsid protein) expression levels were determined by ELISA using a p24 antibody obtained from the AIDS Research and Reference Reagent Program. Secreted alkaline phosphatase (SEAP) activity in the supernatants was measured using the Tropix Phospha-Light Cheluminescent Reporter kit (cat. no. BP100, Tropix, Foster City, CA). The methods used for nuclear and cytoplasmic RNA extraction, poly(A) RNA selection, and Northern blot analyses were described previously (Hammarskjöld et al., 1986, 1994). 293T cells were harvested at 55 h posttransfection. A SacI-BglII (nucleotides 682-2093) fragment of the HIV-1 BH10 clone and BamHI fragment of the human SEAP cDNA (nucleotides 213-1698) were labeled with a 32P-dCTP by using the Rediprime II Kit (GE Healthcare). Northern blots were quantitated with a Molecular Dynamics PhosphorImager (Sunnyvale, CA) and ImageQuant analysis software (GE Healthcare).
RESULTS
Formation of Tap/CTE RNA Complexes on Nucleoporins
Translocation of export complexes across the NPC relies on interactions between nuclear transport receptors and nucleoporins (Suntharalingam and Wente, 2003). Tap can bind a number of nucleoporins in vitro including p62, Can/Nup214, Nup98, RanBP2, and CG1 (Katahira et al., 1999; Bachi et al., 2000; Lévesque et al., 2001; Katahira et al., 2002; Grant et al., 2003; Forler et al., 2004). Using a solid-phase binding assay, we have observed that Nxt1 could stimulate binding of both cargo-free Tap and the Tap/CTE RNA complex to p62 (Lévesque et al., 2001). In addition, Nxt1-stimulates the RNA export activity of Tap in vivo (Braun et al., 2001; Guzik et al., 2001; Lévesque et al., 2001; Wiegand et al., 2002). These observations suggest that the interaction between Tap/Nxt1 and the nucleoporin p62, which is located in the central channel of the NPC, could be rate limiting for RNA export. It is also possible that Nxt1 enhances RNA export by facilitating the recruitment of Tap to other nucleoporin binding sites. In the present study, one of the questions addressed is whether Nxt1 facilitates the interaction between Tap and nucleoporins located on nucleoplasmic and cytoplasmic sides of the NPC that are distal to the central channel.
We examined the interaction of the Tap/CTE RNA complex with the nucleoporins Nup98 (Figure 1, A and B) and RanBP2 (Figures 1, C and D). Nup98 is localized to both the nuclear and cytoplasmic sides of the NPC, whereas RanBP2 is localized to the cytoplasmic filaments (Griffis et al., 2003; Suntharalingam and Wente, 2003). Binding of Tap to these two nucleoporins was investigated by incubating in vitro-translated 35S-FLAG-Tap in microtiter wells containing either immobilized GST or GST fusion recombinant nucleoporins. Recombinant Nxt1 and 32P-CTE RNA (WT or mutGAC, deficient for Tap binding) were also added to the incubation reaction where indicated. Binding of 35S-Tap and/or 32P-CTE-RNA to immobilized nucleoporins was measured by liquid scintillation counting.
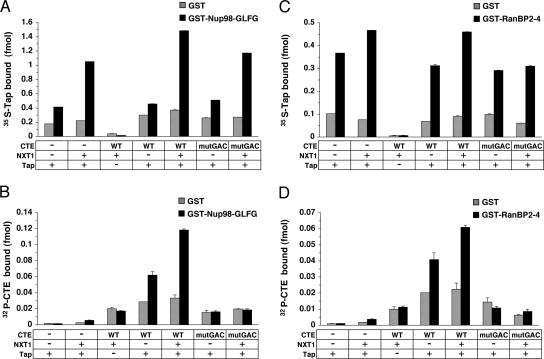
Figure 1.
Tap recruits CTE RNA to Nup98 and RanBP2. Solid-phase binding assay of WT Tap (A and C) and CTE RNA (B and D) to Nup98-GLFG (A and B) or RanBP2-4 (C and D). GST-fusion nucleoporins and GST alone were immobilized to microtiter plates. Binding reactions containing various combinations of 35S-FLAG-Tap, recombinant Nxt1 (66 nM) and 32P-CTE RNA (WT or mutGAC), as indicated, as well as unlabeled tRNA (14 μg/ml) and ribonuclease inhibitor (14.3 U/ml) were incubated with the immobilized nucleoporins for 24 h. Levels of bound proteins and RNAs were measured by liquid scintillation counting. Each data point represents the mean of three replicates (±SD).
In the absence of recombinant Nxt1, 35S-Tap bound to immobilized Nup98 (Figure 1A) and RanBP2 (Figure 1C); the addition of Nxt1 stimulated the binding of Tap to Nup98 by 2.5-fold and to RanBP2 by 1.3-fold. The level of Tap bound to Nup98 was also enhanced 1.4-fold by the presence of WT CTE RNA in the binding reaction (Figure 1A). Tap recruited CTE RNA to these same nucleoporins in vitro (Figure 1, B and D). No significant binding of CTE RNA to Nup98 or RanBP2 was obtained when Tap was omitted from the binding reaction or when mutGAC CTE RNA was used. Recruitment of the Tap/CTE RNA complex to Nup98 was enhanced twofold and binding to RanBP2 increased 1.5-fold by the addition of Nxt1. Because RanBP2 is located on the cytoplasmic fibrils and Nup98 maps primarily to the cytoplasmic face and nuclear basket, Nxt1 can modulate Tap interactions with spatially distinct sites within the NPC.
Tap Residues Involved in Nxt1 Binding
Crystallographic and biochemical analyses have characterized three main functional domains on Tap (Liker et al., 2000; Fribourg et al., 2001; Grant et al., 2002, 2003; Figure 2A): a N-terminal leucine-rich region of the protein that interacts with RNA, the Tap-NTF2-like domain that also binds nucleoporins and can heterodimerize with Nxt1 (Fribourg et al., 2001) and a C-terminal Tap-UBA domain that can associate also with nucleoporins (Katahira et al., 1999; Lévesque et al., 2001; Schmitt and Gerace, 2001). To better differentiate between the Nxt1 and NPC binding properties of the Tap-NTF2-like region, we set out to identify mutations that could disrupt the Tap interaction with Nxt1 without affecting NPC binding. These mutants were generated before the crystal structure of the Tap-Nxt1 interaction interface became available and were restricted to residues 507-540 of Tap because we found previously that deletion of this region abolished Tap binding to Nxt1 (Guzik et al., 2001). A report demonstrating that the F499D point mutant of Tap was deficient for Nxt1 binding (Suyama et al., 2000) led to the hypothesis that phenylalanine residues within the 507-540 region were involved in the Tap-Nxt1 interaction. To test this hypothesis, each of the three phenylalanines within the 507-540 region (F513, F517, and F535) were mutated to aspartates. In addition, we generated mutations within the newly identified NES I (m9; V495A and G497A) and NES II (m6; I529A and V530A) located within the Nxt1 heterodimerization region of Tap. Similar mutations were shown to disrupt the ability of these NESs to bind nucleoporins and disrupted their ability to exit the nucleus but the effect of these mutations on Nxt1 binding was not examined (Thakurta et al., 2004). Tap constructs (L383,386R, S585P, and Tap1-569) with mutations in one of the two nucleoporin-binding domains were also tested.
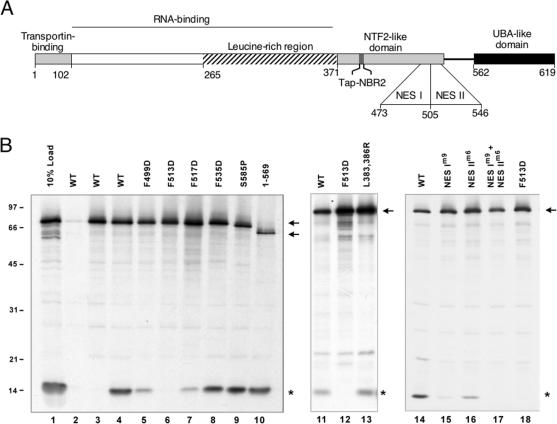
Figure 2.
Interaction between Tap mutants and Nxt1. (A) Schematic representation of Tap domains. The N-terminal of Tap contains the Transportin-binding domain and a RNA-binding domain that includes a leucine-rich region (LRRs). The NTF2-like domain heterodimerizes with Nxt1 and binds nucleoporin repeats. The nucleoporin binding function within the NTF2-like domain is mediated by a hydrophobic fold (Tap-NBR2) and two NESs. Residues L383 and L386 are part of the Tap-NBR2 that binds FG repeats directly. An additional nucleoporin-binding region is present in the UBA-like domain of Tap (Tap-UBA). (B) Lysate containing in vitro synthesized 35S-labeled FLAG-Tap (arrows), WT (lanes 2-4, 11, and 14) or mutants (lanes 5-10, 12-13, and 15-18), and 35S-labeled Nxt1 (*) were coimmunoprecipitated using anti-FLAG antibody. No protein was IP by protein G alone (lane 2). The addition of recombinant Nxt1 in the reaction competed for the 35S-labeled Nxt1 binding to Tap (lane 3).
Mutations at F499 (Figure 2B, lane 5) and F517 (Figure 2B, lane 7) greatly diminished Tap binding to Nxt1 when compared with WT Tap (Figure 2B, lane 4). Tap/Nxt1 interaction was completely abolished by the F513D mutation (Figure 2B, lanes 6, 12, and 18). In contrast, the F535D mutations had no detectable effect on Nxt1-binding (Figure 2B, lane 8). Hence, mutations within a relatively small region of Tap (residues 499-517) were sufficient to disrupt the Tap/Nxt1 interaction. As we reported previously (Guzik et al., 2001; Lévesque et al., 2001), mutations in the C-terminal domain of Tap did not affect binding to Nxt1 (S585P and 570-619 deletion; Figure 2B, lanes 9 and 10, respectively). The L383,386R double mutation of the Tap-NBR2 also had no effect on Nxt1 binding (Figure 2B, lane 13). Mutation of either NES diminished binding to Nxt1 (lanes 15 and 16), and combining mutations in both NESs abolished all Nxt1 binding (lane 17).
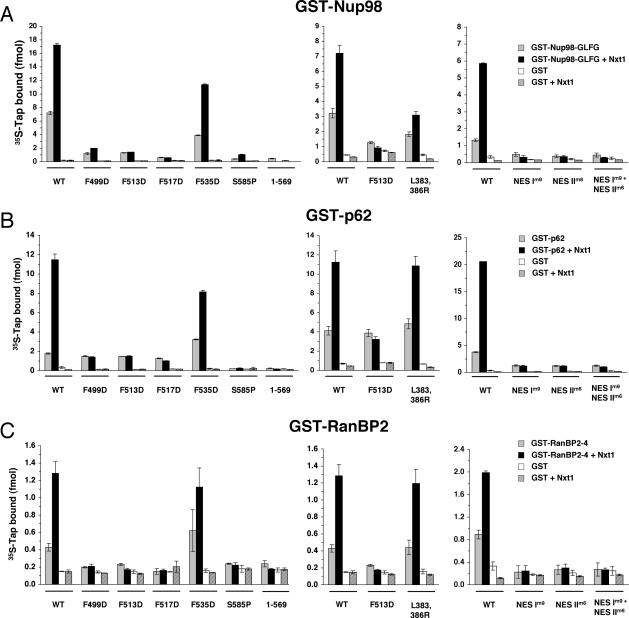
Tap Mutations That Affect In Vitro Binding to Nucleoporins
Two hydrophobic surfaces on Tap have been suggested to mediate binding to nucleoporin FG repeats (Fribourg et al., 2001; Grant et al., 2002, 2003). We reported previously that deletion of one of these hydrophobic surfaces, the Tap-UBA domain, obliterated binding to p62 in vitro (Lévesque et al., 2001). A single point mutation within Tap-UBA (S585P) has also been shown to disrupt the association of Tap with the nuclear envelope (Bear et al., 1999) and was thus incorporated into the present study. The second NPC-binding hydrophobic interface, Tap-NBR2, resides within the NTF2-like domain. Mutation of two leucine residues within Tap-NBR2 to arginine (L383,386R) reduces the association of Tap with the nuclear rim and decreases the efficiency of RNA export in vivo (Fribourg et al., 2001). This double-point mutant was included in the present study to elucidate the role of the NTF2-like hydrophobic fold in the interaction of Tap with nucleoporins. We also tested the effects of two additional mutations within the NTF2-like region, NES Im9 and NES IIm6, which have also been shown to disrupt nucleoporin binding (Thakurta et al., 2004). Maximal binding of Tap to the nucleoporins only occurs when its Nxt1-binding domain is intact and Nxt1 is included in the binding reaction (Figure 3, present article; Lévesque, 2001). Whether the stimulation in FG binding produced by Nxt1 involves modulation of Tap-UBA, Tap-NBR2, Tap-NESs, or all three is not known. The goal was to try and discriminate between these possibilities by selectively mutating each of these NPC-binding domains.
Figure 3.
Solid-phase binding assay of Tap mutants with nucleoporins. (A) In vitro-translated 35S-Tap, WT or mutant, was incubated in the presence or absence of recombinant Nxt1 and added to immobilized GST-Nup98 fragment or GST. WT or mutant Tap was also added to immobilized GST-p62 (B) and GST-RanBP2 (C). Levels of bound proteins were measured by liquid scintillation counting. Each data point represents the mean of three replicates (±SD).
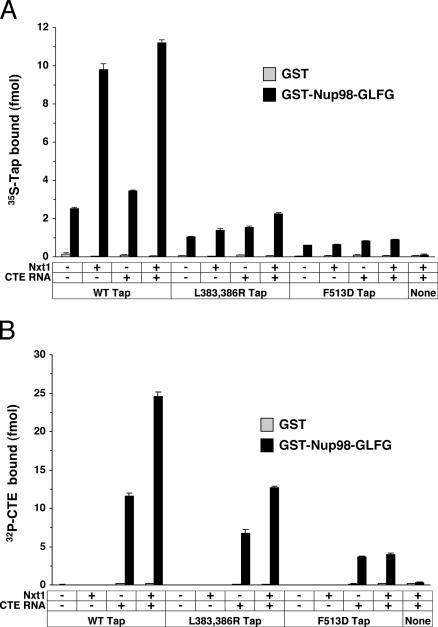
Solid-phase binding assays examining the interaction of Tap with Nup98 (Figure 3A), p62 (Figure 3B), or RanBP2 (Figure 3C) were carried out in the presence or absence of recombinant Nxt1. As shown previously for p62 (Lévesque et al., 2001), the association of Tap with Nup98 and RanBP2 requires an intact UBA domain. This was evident by the loss of binding of the S585P Tap and Tap1-569 mutants (Figure 3). The addition of Nxt1 to the binding reactions did not restore binding for those mutants. The Tap L383,386R mutant bound to p62 and RanBP2 to the same extent as WT Tap, both in the absence and presence of Nxt1 (Figure 3, B and C, respectively). Interaction of the L383,386R mutant with Nup98, though lower than with WT Tap, was still enhanced by Nxt1 (Figure 3A). Characterization of the interaction of the Tap NTF2-like domain with nucleoporins was deduced from a cocrystal containing a 12-amino acid peptide with a single FG motif (GQSPGFGQGGSV; Fribourg et al., 2001). The Nup98 (amino acids 43-498) construct used in the present study contains four such GFG motifs. There are no such GFG within the RanBP2-4 or the p62 construct. Although it has been suggested that the structure of the Tap-NBR2 is also compatible for binding FXFG or GLFG motifs, this has not been tested (Fribourg et al., 2001). Therefore, it is possible that the Tap-NBR2 preferentially binds GFG motifs and that this association is important for Tap binding to Nup98. Whether Nxt1 is required for the binding of this domain to Nup98 cannot be determined unequivocally from our experiments. Mutations in the Nxt1-binding domain of Tap (F499D, F513D, and F517D) decreased the binding of Tap to Nup98 (Figure 3A) and RanBP2 (Figure 3C), but did not affect binding to p62 (Figure 3B), compared with WT Tap. These same mutations also abolished the Nxt1-induced stimulation of Tap binding to all three nucleoporins (Figure 3, A-C). Binding of Tap to p62 was greatly reduced in the NES Im9 and NES IIm6 mutants (Figure 3B). Nxt1 failed to stimulate their recruitment to p62 as expected because they are also defective for Nxt1 binding. These same mutants failed to bind to RanBP2 or Nup98 above background levels (Figures 3, A and B). Because of the reduced binding observed with both the Tap F513D and L383,386R mutants to Nup98, we wanted to test whether these mutants could still recruit CTE RNA to Nup98 in the solid-phase binding assay (Figure 4B). We observed that both the L383,386R and F513D mutants could still do so but to a lower level than WT Tap. Nxt1 stimulated CTE RNA recruitment by the L383,386R mutant but had no effect on the level of RNA recruitment by the F513D mutant. No CTE RNA bound to GST-Nup98 in the absence of Tap.
Figure 4.
Recruitment of CTE RNA to Nup98 by Tap mutants. Solid-phase binding assay of (A) Tap (WT or mutant) and (B) CTE RNA to Nup98-GLFG. GST-Nup98 and GST alone were immobilized to microtiter plates. Binding reactions containing various combinations of 35S-FLAG Tap, recombinant Nxt1, and 32P-CTE RNA, as indicated, as well as unlabeled tRNA (7 μg/ml) and ribonuclease inhibitor (14.3 U/ml) were incubated with the immobilized nucleoporins for 24 h. Levels of bound proteins and RNAs were measured by liquid scintillation counting. Each data point represents the mean of three replicates (±SD).
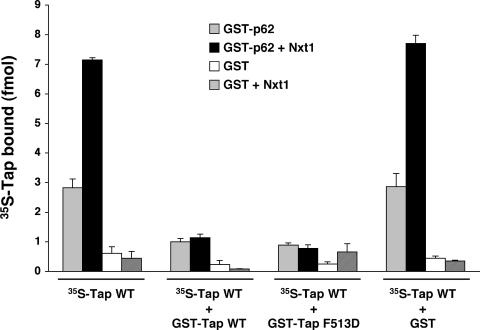
It has been hypothesized that the function of Nxt1 is to stabilize the NTF2-like domain and thus facilitate its binding to nucleoporins (Fribourg et al., 2001). Although our experiments did not specifically test this hypothesis, they do demonstrate that such a stabilization effect cannot account for the increase in Tap binding to nucleoporins observed in our solid-phase binding assays after the addition of Nxt1. Nxt1 was able to stimulate the recruitment of a Tap mutant lacking a functional NBR2 (L383,386R) to two nucleoporins, p62 and RanBP2, to the same level as that obtained with WT Tap (Figure 3, B and C, respectively). Therefore, our data do not support a role for the Tap-NBR2 in the Nxt1-induced stimulation of nucleoporin-binding by Tap but instead suggest that Nxt1 promotes binding via another region of Tap. This Nxt1 effect may be due to an increase in Tap affinity for individual FG repeats, the recruitment of Nxt1-bound Tap to additional FG repeats, corresponding to an increased in avidity, or a combination of the two. To differentiate between these possibilities, we used a Tap mutant deficient for binding Nxt1 (F513D) to compete with the binding of WT Tap to p62 in a solid-phase assay (Figure 5). If Nxt1 increases the avidity of Tap for nucleoporins by engendering additional Tap-FG repeat contact sites, then the F513D mutant should not be able to compete for these Nxt1-dependent binding regions. However, Figure 5 shows that the recombinant GST-Tap F513D prevented WT 35S-Tap from binding p62 as well as GST-Tap WT even in the presence of Nxt1, consistent with a modulation of Tap affinity by Nxt1 rather than avidity.
Figure 5.
Competition of WT Tap binding to p62 by the Tap F513D mutant. Solid-phase binding assay of WT Tap to p62. GST-p62 (100 ng) or GST alone (150 ng) was immobilized to microtiter plates. Binding reactions containing various combinations of 35S-FLAG-Tap, recombinant Nxt1 (66 nM), and recombinant GST-Tap WT (2 μM) or F513D mutant (1.5 μM), as indicated, were incubated with the immobilized nucleoporins for 24 h. Levels of bound proteins were measured by liquid scintillation counting. Each data point represents the mean of three replicates (±SD).
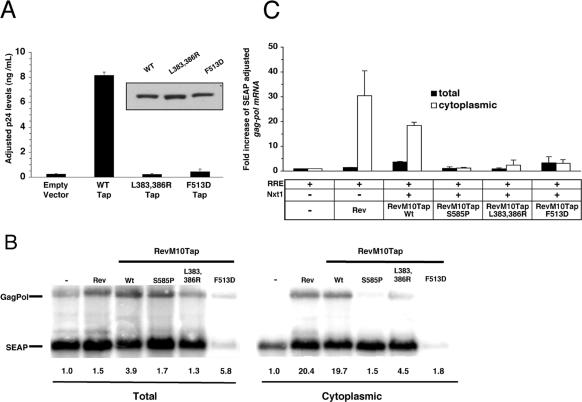
We previously demonstrated that the nucleoporin-binding defect observed with the Tap-UBA mutant (Tap1-569) is correlated with a failure to mediate RNA export in vivo (Guzik et al., 2001; Lévesque, 2001). Using the same export assay, we found that a single point mutation in the Nxt1 binding region (F513D) or mutation of Tap-NBR2 (L383,386R) significantly reduced RNA export (Figure 6A). In this assay, efficient export of a RNA transcript containing the coding region for gag-pol was correlated to the levels of p24 translated. Because the end point of our export assay could be affected by alterations in protein expression and because both Tap and Nxt1 have been shown to affect translation (Jin et al., 2003), we performed Northern blot analyses to verify that the low p24 levels observed with these Tap mutants were due to a defect in mRNA export to the cytoplasm, as opposed to disruption of p24 translation. Indeed, Figure 6, B and C, confirms that the defect in export was directly correlated to the decrease in the translocation of the gag-pol transcript to the cytoplasm in both Tap mutants. The defect in RNA export obtained with the Tap L383,386R mutant was unexpected because this same mutant was still able to bind and recruit CTE RNA to Nup98 in vitro (Figure 4B). The discrepancy may be due to the different RNAs used between the two assays. Tap binds CTE RNA directly in a sequence-specific manner that involves both the RNP and LRR region of Tap (Liker et al., 2000). Formation of a Tap/CTE RNA complex may change Tap conformation and allow alternate sites on Tap to bind nucleoporins, thus making the Tap/CTE RNA less susceptible to mutations in the Tap-NBR2. Indeed, addition of CTE RNA to our assays resulted in a slight increase in WT Tap bound to p62 (Lévesque et al., 2001) and Nup98 (this article, Figures 1 and 4), consistent with the recruitment of additional Tap binding sites. In contrast, the export assay does not rely on direct binding of Tap to the RNA reporter. Instead, the RRE-gag-pol transcript associates directly with the RevM10 protein tethered to the amino terminus of Tap. Export of the RRE construct by RevM10-Tap may be more dependent on the NBR2 region than CTE RNA for translocation across the NPC. The requirement for the NTF2-like domain for RNA export was also shown to depend on the type of cargo used. In a Xenopus oocyte export assay, the NTF2-like domain was shown to be necessary for the export of a CTE-intron lariat but irrelevant for the export of a CTE-U6 construct (Bachi et al., 2000). Unlike the direct interaction occurring between Tap and CTE, the association of Tap with cellular mRNA is not sequence-specific and depends on the recruitment of additional proteins, such as SR proteins and REF, which bind both mRNA and the amino terminus of Tap (Liker et al., 2000; Stutz et al., 2000; Huang et al., 2003). Therefore the export of our RRE-gag-pol transcript by the RevM10-Tap fusion protein may mimic the Tap/mRNA export mechanism better than CTE RNA export.
Figure 6.
Tap mutants fail to promote RNA export. (A) 293T/17 cells (1 × 107 in 15-cm culture dish) were transfected with 15 μg of pCMVGagPol-RRE and 5 μg of pCMVSEAP in the absence or presence of 3 μg of pCMVRev. For transfections involving FLAG-M10-Tap and its derived mutants, cells were cotransfected with same amount of pCMVGagPol-RRE and pCMVSEAP vectors with either 6 μg of FLAG-RevM10-Tap, 6 μg of FLAG-RevM10-TapS585P, 10 μg of FLAG-RevM10-TapL383, 386R, or 10 μg of FLAG-RevM10-TapF513D plasmids and 3 μg of pCMVFLAG-NXT1. Levels of RNA export for each condition were measured as a function of p24 levels in the media. Levels of p24 were normalized to SEAP and expressed as fold increase over levels of p24 in cells transfected with RRE alone. Each value represents the average of two data points obtained from independent transfection experiments. The error bars represent differences between duplicates. Inset, Western blot for RevM10-Tap protein expression of transfection cells used in the export experiment. Each lane corresponds to the combined extract from each set of duplicates. Proteins were detected using anti-Rev antibody. (B) Northern blot analyses of total and cytoplasmic mRNA from 293T/17 cells transfected as described in (A). Fifty-five hours posttransfection, poly (A)+ mRNA was isolated from the transfected cells as described in Materials and Methods. The blot contains 5 μg of poly (A)+ mRNA per lane and was hybridized with 32P-labeled gag-pol and SEAP probes. Values shown under each figure represent the fold difference in the levels of the gag-pol RNA bands between the RRE-containing vector with or without cotransfected plasmids. All values have been normalized using the SEAP band. (C) Levels of total and cytoplasmic gag-pol mRNA for each transfected conditions were normalized to SEAP and expressed as fold increase over levels of mRNAs in cells transfected with RRE alone. Each value represents the average of two Northern blots obtained from independent transfection experiments. Error bars represent the range.
Distribution of the Tap Mutants In Vivo
We next performed a series of experiments to determine if mutations that reduce RNA export activity have an effect on the steady state distribution of Tap. Cos 7 cells were transiently transfected with FLAG-Tap (WT and mutant) constructs and detected by immunofluorescence microscopy. The distribution of all Tap mutants was nuclear and indistinguishable from that of WT Tap (Figure 7, Total). Transfected cells were also analyzed after permeabilization of the plasma membrane with a low level of digitonin (0.005%), a procedure that leaves the nuclear envelope intact. Under this condition, only the cytoplasmic pool of FLAG-Tap is detected by antibodies and the distribution of WT Tap appeared as punctate and restricted to the nuclear rim (Lévesque et al., 2001). Figure 7 shows that all of the mutant proteins were detected on the cytoplasmic face of the nuclear membrane at a level comparable to WT Tap (Cytoplasmic), suggesting that the reduced level of RNA export observed with these mutants is not due to a defect in Tap release from the cytoplasmic side of the NPC. An independent study had previously shown that mutations in Tap-UBA or Tap-NBR2 significantly reduced the association of Tap with the nuclear rim (Fribourg et al., 2001). The discrepancy between our two studies may be explained by differences in the way cells were treated before fixation. Fribourg et al. (2001) treated their cells with 0.5% Triton X-100 before fixation, whereas the current study used 0.005% (wt/vol) digitonin. Beside the obvious difference in detergent concentration, digitonin selectively solubilizes cholesterol, whereas Triton X-100 affects a wide range of molecules (Le Maire et al., 1983; Ray et al., 1983; Adam et al., 1990). Therefore treatment with Triton X-100 is more likely to disrupt weak protein-protein interactions such as may be the case between cytoplasmic nucleoporins and mutant Tap and possibly accounts for the discrepancy between our two studies.
Figure 7.
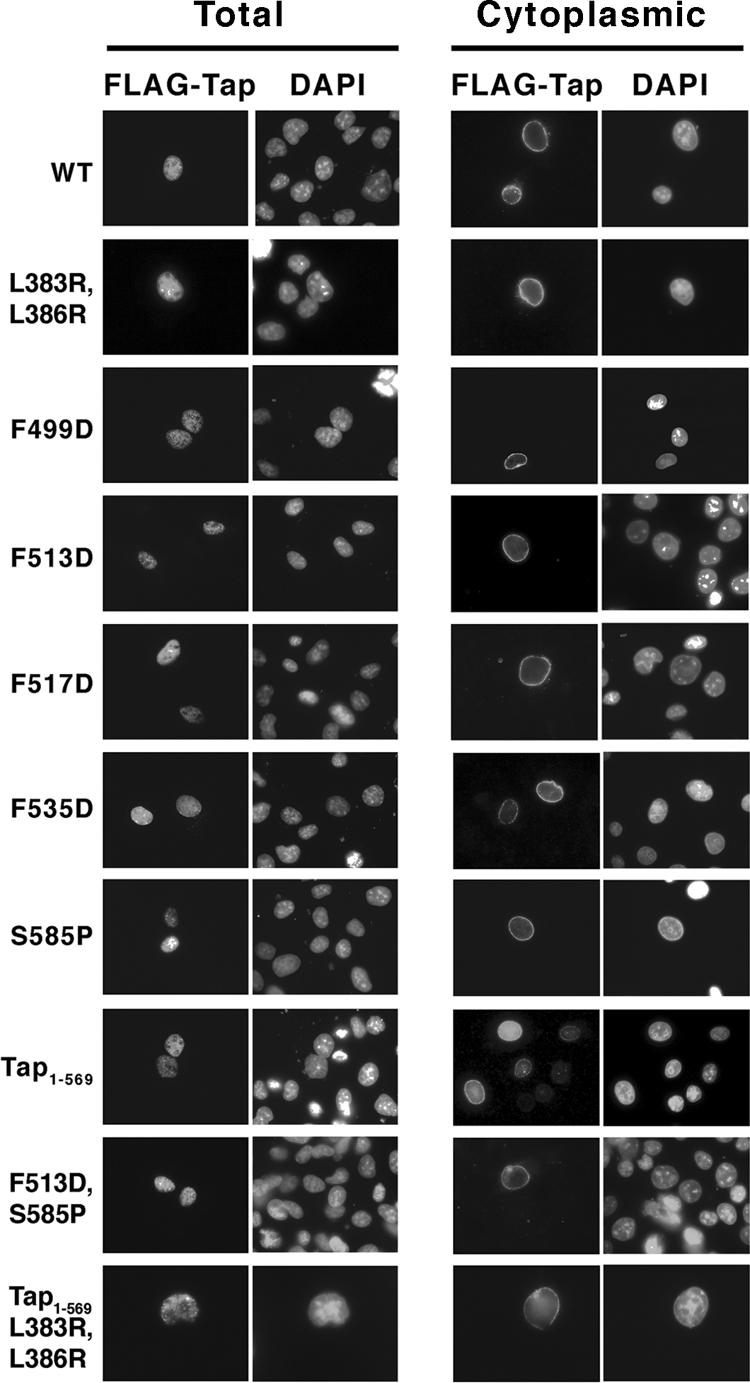
Cellular Distribution of FLAG-Tap mutants. Cos cells were transfected with pcDNA FLAG-Tap (WT or mutant) and processed for IF 24-36 h later. Cells were fixed in formaldehyde and permeabilized Triton X-100 before antibody detection (Total) or cells were treated with 0.005% digitonin before fixation and detergents were omitted from the IF protocol (Cytoplasmic). FLAG-Tap was detected using α-FLAG monoclonal antibody (mAb) and anti-mouse Cy3. Nuclei were identified by DAPI stain.
To determine whether the RNA export defect results from a reduced level of nucleocytoplasmic shuttling, we analyzed each of the mutants in heterokaryon shuttling assays (Figures 8 and 9). These assays involve the formation of heterokaryons between Cos cells expressing GFP-Tap or FLAG-Tap (donor) and NIH3T3 cells labeled with CMTMR (acceptor) using polyethylene glycol. After fusion, cells are incubated for 4 h in the presence of cycloheximide to inhibit new protein synthesis. Proteins are scored positive for shuttling if, within the fusion, similar levels of GFP-Tap (or FLAG-Tap) are detected in both the donor cell nuclei and the acceptor cell nuclei. Dense chromatin foci can be detected in nuclei derived from NIH3T3 cells when stained with DAPI and are thus readily distinguishable morphologically from Cos cell nuclei. Fusion of cells is evidenced by CMTMR staining throughout the cytoplasm of heterokaryons (Figures 8 and 9A). CMTMR is a cell permeant fluorescent dye in living cells until it interacts with GST inside the cells and becomes cell-impermeant (Molecular Probes technical information). A large pool of membrane bound GST is found at the surface of the nuclear envelope and endoplasmic reticulum, which explains the seemingly higher level of CMTMR fluorescence detected in the nucleus of our acceptor cells (Surapureddi et al., 2000). Ratios of the level of Tap within the acceptor nuclei over that within the donor nuclei were calculated for at least 10 heterokaryons from each construct and shown in Figure 9B. The GFP-STV-NLS construct, which can enter the nucleus via its NLS but is unable to be exported out of the nucleus, was used as a negative control in these assays.
Unexpectedly, GFP-Tap constructs with mutations within the UBA (S585P and 61-569) or Tap-NBR2 (L383,386R) regions and a Tap mutant deficient for Nxt1-binding (F513D) displayed shuttling that was comparable to WT Tap (Figure 8). To determine if there were subtle differences in the rate of shuttling between mutant and WT proteins, we also examined the heterokaryons at a time shortly after fusion (1 h). Again, no significant differences were observed with these mutants (unpublished data). We also tested the shuttling ability of FLAG-RevM10-Tap (WT and mutant) constructs used in our RNA export assay to rule out any effect of the RevM10 fusion on Tap shuttling. As shown in Figure 9B, shuttling was decreased for the WT FLAG-RevM10-Tap construct compared with WT GFP-Tap or WT FLAG-Tap. The disruption of shuttling was even more pronounced for FLAG-RevM10-Tap constructs bearing mutations in one of the two NPC-binding domains (L383,386R or 61-569), whereas these same mutations did not disrupt shuttling of GFP-Tap (Figure 9B). However, the decrease in Tap shuttling caused by the RevM10 tethering is not sufficient to account for the greatly reduced ability of the RevM10-Tap mutants to export RRE RNA compared with WT RevM10-Tap.
Shuttling was also intact in FLAG-Tap with mutations in both hydrophobic pockets (Figure 9A; FLAG-Tap L383,386R+S585P) suggesting that Tap shuttling can be mediated by alternate domain(s). The alternate domain(s) must rely on Nxt1-binding because FLAG-Tap F513D + S585P, deficient for Nxt1-binding, had reduced shuttling activity compared with the S585P mutant alone. Two novel nucleoporin-binding sites, NES I and NES II, located within residues 473-546 of Tap would satisfy the requirements for such alternate NPC-interacting regions. Each of these NESs can drive the nuclear export of fusion proteins (Thakurta et al., 2004). Mutations in the NES I (m9 mutation:V495A and G496A) and NES II (m6 mutation: I529A and V530A), previously shown to disrupt the interaction of these domains with nucleoporins (Thakurta et al., 2004), significantly reduced shuttling (Figure 9). These same mutations also disrupted Nxt1 binding (Figure 2B). Incorporation of both NES mutations within our FLAG-Tap1-569 construct completely prevented shuttling (Figure 9).
DISCUSSION
The Two NPC-binding Domains of Tap Interact with Nucleoporins Differently
Direct interactions between nuclear transport receptors and FG repeats in nucleoporins is thought to provide the physical basis for translocation through the NPC (Suntharalingam and Wente, 2003). Defining how transport receptors bind FG repeats and the spatial arrangement of FG repeats within the NPC is, therefore, critical to understanding the mechanisms underlying transport. Here we present evidence that the mode of interaction between Tap and nucleoporins can be nucleoporin specific and demonstrate that distinct domains on Tap contribute to these preferences. Using a solid-phase binding assay, we found that a Tap/CTE RNA complex could bind to FG repeat regions on three different nucleoporins and that binding was enhanced by the presence of Nxt1 (Figure 1, present article; Lévesque et al., 2001). We also engineered point mutations in each of the major structural domains of Tap to assess their contribution in mediating the interaction with FG repeats. We concluded that the Tap-NBR2 is critical for Tap binding to Nup98, but not for Tap binding to p62 or RanBP2. Significant binding of Tap to Nup98, p62, or RanBP2, is only detected when the Tap-UBA domain remains intact. Mutations within the NESs I+II regions of the Tap-NTF2-like domain greatly reduced binding to p62 and eliminated binding to Nup98 and RanBP2 (Figure 3). These results suggest that Tap contains both specific and general binding sites for nucleoporins.
The Tap-UBA domain has been shown to be critical for nucleoporin binding and RNA export function (Katahira et al., 1999; Bachi et al., 2000; Lévesque et al., 2001; Schmitt and Gerace, 2001; Grant et al., 2002, 2003). The crystal structure of the Tap-UBA domain bound to an FxFG peptide shows both Phe side-chains of the FG repeat inserted into the hydrophobic pocket of the Tap-UBA. Tap-UBA was also shown to bind a GLFG repeat containing peptide, though with half the affinity of FxFG peptides (Grant et al., 2003). This structural arrangement is similar to what had been described previously for importin β/FxFG interaction (Bayliss et al., 2000; Grant et al., 2002, 2003).
Mapping of the second NPC-binding domain of Tap (Tap-NBR2) was done using a Tap/Nxt1 heterodimer and a peptide containing a single GFG motif (Fribourg et al., 2001). Residues L383 and L386 within the hydrophobic region of Tap-NBR2 were shown to surround the aromatic ring of Phe on the GFG peptide and were then proposed to be important for the interaction between Tap-NBR2 and FG repeats. Indeed, the Tap L383,386R mutant has decreased efficiency for RNA export in a transfected cell assay (Fribourg et al., 2001 and current study). It was therefore unexpected to find that the L383,386R Tap mutant bound to p62 and RanBP2 with levels comparable to that of WT Tap. These results indicate that the Tap-NBR2 does not contribute to the binding of Tap to these two nucleoporins in vitro (Figure 3, B and C). For that reason we proposed that the hydrophobic domain within the NTF2-like region of Tap may preferentially bind GFG motifs because the L383,386R mutation disrupted binding of Tap to our Nup98-GLFG construct, which has three GFGs, but had no effect on the interaction of Tap with RanBP2 or p62, which do not contain GFG motifs. Nup98 may thus rely more heavily on the Tap-NBR2 than other nucleoporins for its interaction with Tap. It is not known whether the Tap-NBR2 region is capable of binding GLFG or FxFG motifs.
A Tap470-619 fragment, lacking a significant part of Tap-NBR2 hydrophobic pocket, bound to five nucleoporins (Can/Nup214, Nup98, Nup153, p62, and hCG1) at levels comparable to full-length Tap in a GST pulldown assay (Bachi et al., 2000). In addition, a Tap-UBA construct (GST-Tap540-619) bound to Nup214, Nup153, Nup98, Nup62, and Nup58, despite its lack of a Tap-NBR2 domain (Schmitt and Gerace, 2001). In contrast, Tap61-610, which is missing the last nine residues of Tap-UBA, failed to bind p62 or Nup153 but showed no defect in Nup98 binding (Bachi et al., 2000). These results support the hypothesis that the Tap-UBA domain is important for binding most nucleoporins, whereas the Tap-NBR2 domain is important for binding Nup98. Interestingly, the Tap-UBA domain was shown to have half the affinity for GLFG repeats, also found in Nup98, compared with FxFG repeats (Grant et al., 2003). Thus Tap may rely more on NPC binding domains other than the Tap-UBA for its interaction with GLFG-containing nucleoporins such as Nup98. Such specificity of transport receptors for repeat motifs has been demonstrated for NTF2, which binds FxFG with micromolar affinity but cannot bind GLFG motifs (Clarkson et al., 1997). A similar case has been described for importin β in a study comparing the affinity of its two NPC-binding sites (Bednenko et al., 2003). A N-terminal region of importin β was shown to bind to three nucleoporins, Nup153, Nup358, and p62. Although the NPC-binding site at the C-terminal of importin β could also bind Nup153, it did so with much lower affinity than the N-terminal region and was found defective for Nup358 or p62 binding. As is the case for Tap, both NPC-domains of importin β were reported to be necessary for cargo transport. The Tap L383,386R mutant is defective for mediating RNA export (Figure 5A), which could be interpreted as evidence that Tap interaction with Nup98 is an early critical, rate-limiting step in nuclear export of RNA. One possible mechanism for the role of the Tap-NBR2 domain in this process would be to facilitate the initial docking of the Tap-CTE complex to the NPC.
Nxt1 Stimulates the Recruitment of Tap/CTE RNA Complex to Nucleoporins
Nxt1 functions on a number of proteins and RNAs export pathways (Ossareh-Nazari et al., 2000; Braun et al., 2001; Guzik et al., 2001; Lévesque et al., 2001; Wiegand et al., 2002). We demonstrated that Nxt1 plays an important role in Tap-mediated RNA transport in vivo by enhancing the export efficiency by as much as 10-fold (Guzik et al., 2001) and that its function is to enhance Tap interaction with the NPC (Lévesque et al., 2001). Although others have reported that complex formation of Tap-CTE RNA precludes Nxt1 binding to Tap (Bachi et al., 2000), the results presented here show that Nxt1 promotes the association of the Tap/CTE-RNA complex with two different nucleoporins. The discrepancy between these two studies may perhaps arise from the fact that Bachi et al. (2000) used GST-Nxt1, which is three times the size of the untagged recombinant Nxt1 utilized in our studies, and this GST moiety may have interfered with the formation of the CTE RNA/Tap/Nxt1 complex. It has been suggested that the predominant role of Nxt1 during RNA export is to mediate the folding of the Tap-NBR2 and allow it to interact with nucleoporins (Izaurralde, 2002). Although our present study cannot rule out the possibility that Nxt1 stimulates the binding of the Tap-NBR2 to nucleoporins, we conclude that such an interaction would have little impact on Tap/RNA translocation across the pores. This conclusion is supported by our evidence that the binding of Tap to all three nucleoporins tested in vitro could still be stimulated by Nxt1 despite mutations in the NBR2 of Tap. Therefore we suggest that Nxt1 binding to Tap modulates the conformation of Tap domains, thus allowing it to bind more efficiently to nucleoporins. This increased binding may reflect increased Tap affinity or avidity for FG repeats.
Binding of Tap to RNA Cargo Alters the Requirements for Its Translocation through the NPC
Export of a RNA reporter by Tap was disrupted by mutations in either one of the two NPC-binding domains or in the Nxt1-binding domain, suggesting that all three of these regions are required for Tap translocation across the NPC in vivo. However, our shuttling experiments challenge this idea by showing that none of these mutations prevented nuclear import or nuclear export of Tap.
On the basis of our data, we propose a model in which the interaction between Tap and the NPC involves two distinct modes of transport. The first, or cargo-bound, mode of Tap-mediated RNA export requires multiple NPC- and Nxt1-binding domains of Tap. The second, or cargo-free, mode of NPC interaction is sufficient to allow cargo-free Tap to move through the NPC despite mutations in the known functional domains of Tap. Cargo-free Tap can, therefore, associate with the NPC using sites other than the Tap-UBA or Tap-NBR2 domains. These additional sites could still interact with the same three nucleoporins tested in this study but with affinities too low to be detected by our in vitro solid-phase assay. These lower affinity regions may be sufficient to drive the translocation of cargo-free Tap but not of cargo-bound Tap. Our findings also raise the possibility that cargo-free Tap may be able to utilize novel NPC-binding sites on Tap that can associate with nucleoporins other than those tested in our assays.
The existence of such alternate NPC-binding regions was recently reported by Thakurta et al. (2004) and referred to as NES I (residues 473-505) and NES II (residues 505-546). These authors determined that GST-Tap473-546, containing both of these NESs but missing the Tap-UBA and part of the Tap-NBR2 domains could still interact directly with spNup159, a homologue of vertebrate Nup214/Can, and spNup98p. This same Tap fragment however failed to associate with human p62 by pulldown assays. Point mutations within the NES I and NES II regions of GST-Tap473-546, NES Im9, and NES II m6, abolished the binding to spNup159. Mutation of both these sites within Tap1-569 was sufficient to disrupt Tap shuttling (Figure 8), suggesting that these sites are required to mediate the movement of Tap across the NPC, at least in the absence of a functional Tap-UBA domain. We previously demonstrated that the NES II mutant (Δ 507-540), missing most of the NES II, was deficient for RNA export (Guzik et al., 2001). However, because that same mutant was also deficient for Nxt1 binding, we cannot conclude that the requirement for this domain for RNA export is solely because of its lack of nucleoporin-binding ability (Guzik et al., 2001).
Further evidence for the existence of alternative NPC-binding sites of Tap comes from a study in which GFP-β-Gal-Tap61-120, missing Tap-UBA,Tap-NBR2, and the two NESs was shown to shuttle (Bear et al., 1999). In addition, a separate study found that the truncated Tap540-619 mutant, missing the Tap-NBR2 and Nxt1-binding domain, and the two NESs, could also shuttle through the NPC (Schmitt and Gerace, 2001). Neither of these deletion mutants, Tap61-120 and Tap540-619, included the RNA-binding domain and therefore were not tested for RNA export. Whether these additional sites mediate a novel direct interaction of Tap with nucleoporins and whether they are required for RNA export remains to be determined.
The suggestion that the binding of a cargo to its transport receptor can influence the interaction between the receptor and nucleoporins was proposed previously for two karyopherin receptors (Lyman et al., 2002). That study demonstrated that the size of a cargo influenced the requirement of importin β and Transportin for RanGTP during import. The efficient import of larger cargos by both receptors requires the presence of hydrolysable RanGTP. However, the presence of a nonhydrolysable Ran was sufficient to support import of a small cargo by importin β, whereas the import of small cargos by Transportin did not require any Ran. In fact, large cargos were shown to associate with Nup153 only in the presence of RanGTP, whereas small cargo could bind Nup153 independently of Ran. Conceptually, these findings support our hypothesis that cargo-free and cargo-bound Tap have different requirements for translocation.
Acknowledgments
We thank Dr. Craig Mizzen for his valuable comments and discussions and Nima Ashar for reading the manuscript. We also thank Elizabeth King for her help with some of the immunofluorescence data. L.L. was supported by the American Heart Foundation fellowship 0225421U and Grant 0335362Z. B.M.P. is supported by National Institutes of Health Grant GM58639. Funding for M.L.H. was provided by NIH (National Institute of Allergy and Infectious Diseases) R01 AI054335 and NIH (NIAID) Grant RO1 AI34721.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-07-0634) on November 28, 2005.
References
- Adam, S. A., Sterne Marr, R., and Gerace, L. (1990). Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J. Cell Biol. 111, 807-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachi, A. et al. (2000). The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA 6, 136-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasundaram, D., Benedik, M. J., Morphew, M., Dang, V. D., and Levin, H. L. (1999). Nup124p is a nuclear pore factor of Schizosaccharomyces pombe that is important for nuclear import and activity of retrotransposon Tf1. Mol. Cell. Biol. 19, 5768-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos, R., Lin, A., Enarson, M., and Burke, B. (1996). Targeting and function in mRNA export of nuclear pore complex protein Nup153. J. Cell Biol. 134, 1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss, R., Littlewood, T., and Stewart, M. (2000). Structural basis for the interaction between FxFG nucleoporin repeats and importin-beta in nuclear trafficking. Cell 102, 99-108. [DOI] [PubMed] [Google Scholar]
- Bear, J., Tan, W., Zolotukhin, A. S., Tabernero, C., Hudson, E. A., and Felber, B. K. (1999). Identification of novel import and export signals of human TAP, the protein that binds to the constitutive transport element of the type D retrovirus mRNAs. Mol. Cell. Biol. 19, 6306-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednenko, J., Cingolani, G., and Gerace, L. (2003). Nucleocytoplasmic transport: navigating the channel. Traffic 4, 127-135. [DOI] [PubMed] [Google Scholar]
- Belgareh, N., Snay-Hodge, C., Pasteau, F., Dagher, S., Cole, C. N., and Doye, V. (1998). Functional characterization of a Nup159p-containing nuclear pore subcomplex. Mol. Biol. Cell 9, 3475-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, B. E., Holaska, J. M., Lévesque, L., Ossareh-Nazari, B., Gwizdek, C., Dargemont, C., and Paschal, B. M. (2001). NXT1 is necessary for the terminal step of Crm1-mediated nuclear export. J. Cell Biol. 152, 141-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, B. E., Lévesque, L., Holaska, J. M., Wood, T. C., and Paschal, B. M. (1999). Identification of an NTF2-related factor that binds Ran-GTP and regulates nuclear protein export. Mol. Cell. Biol. 19, 8616-8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins, M. B., Smith, A. M., Phillips, E. M., and Powers, M. A. (2003). Complex formation among the RNA export proteins Nup98, Rae1/Gle2, and TAP. J. Biol. Chem. 278, 20979-20988. [DOI] [PubMed] [Google Scholar]
- Braun, I. C., Herold, A., Rode, M., Conti, E., and Izaurralde, E. (2001). Overexpression of TAP/p15 heterodimers bypasses nuclear retention and stimulates nuclear mRNA export. J. Biol. Chem. 276, 20536-20543. [DOI] [PubMed] [Google Scholar]
- Braun, I. C., Rohrbach, E., Schmitt, C., and Izaurralde, E. (1999). TAP binds to the constitutive transport element (CTE) through a novel RNA-binding motif that is sufficient to promote CTE-dependent RNA export from the nucleus. EMBO J. 18, 1953-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray, M., Prasad, S., Dubay, J. W., Hunter, E., Jeang, K. T., Rekosh, D., and Hammarskjold, M. L. (1994). A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc. Natl. Acad. Sci. USA 91, 1256-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca, M. (2002). The contribution of nuclear compartmentalization to gene regulation. Cell 108, 513-521. [DOI] [PubMed] [Google Scholar]
- Clarkson, W. D., Corbett, A. H., Paschal, B. M., Kent, H. M., McCoy, A. J., Gerace, L., Silver, P. A., and Stewart, M. (1997). Nuclear protein import is decreased by engineered mutants of nuclear transport factor 2 (NTF2) that do not bind GDP-Ran. J. Mol. Biol. 272, 716-730. [DOI] [PubMed] [Google Scholar]
- Cronshaw, J. M., Krutchinsky, A. N., Zhang, W., Chait, B. T., and Matunis, M. J. (2002). Proteomic analysis of the mammalian nuclear pore complex. J. Cell Biol. 158, 915-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, B. R. (2003). Nuclear RNA export. J. Cell Sci. 116, 587-597. [DOI] [PubMed] [Google Scholar]
- Damelin, M., and Silver, P. A. (2000). Mapping interactions between nuclear transport factors in living cells reveals pathways through the nuclear pore complex. Mol. Cell 5, 133-140. [DOI] [PubMed] [Google Scholar]
- Ernst, R. K., Bray, M., Rekosh, D., and Hammarskjold, M. L. (1997a). Secondary structure and mutational analysis of the Mason-Pfizer monkey virus RNA constitutive transport element. RNA 3, 210-222. [PMC free article] [PubMed] [Google Scholar]
- Ernst, R. K., Bray, M., Rekosh, D., and Hammarskjold, M. L. (1997b). A structured retroviral RNA element that mediates nucleocytoplasmic export of intron-containing RNA. Mol. Cell. Biol. 17, 135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forler, D., Rabut, G., Ciccarelli, F. D., Herold, A., Kocher, T., Niggeweg, R., Bork, P., Ellenberg, J., and Izaurralde, E. (2004). RanBP2/Nup358 provides a major binding site for NXF1-p15 dimers at the nuclear pore complex and functions in nuclear mRNA export. Mol. Cell. Biol. 24, 1155-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornerod, M., Ohno, M., Yoshida, M., and Mattaj, I. W. (1997). CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90, 1051-1060. [DOI] [PubMed] [Google Scholar]
- Fribourg, S., Braun, I. C., Izaurralde, E., and Conti, E. (2001). Structural basis for the recognition of a nucleoporin FG repeat by the NTF2-like domain of the TAP/p15 mRNA nuclear export factor. Mol. Cell 8, 645-656. [DOI] [PubMed] [Google Scholar]
- Grant, R. P., Hurt, E., Neuhaus, D., and Stewart, M. (2002). Structure of the C-terminal FG-nucleoporin binding domain of Tap/NXF1. Nat. Struct. Biol. 9, 247-251. [DOI] [PubMed] [Google Scholar]
- Grant, R. P., Neuhaus, D., and Stewart, M. (2003). Structural basis for the interaction between the Tap/NXF1 UBA domain and FG nucleoporins at 1A resolution. J. Mol. Biol. 326, 849-858. [DOI] [PubMed] [Google Scholar]
- Griffis, E. R., Xu, S., and Powers, M. A. (2003). Nup98 localizes to both nuclear and cytoplasmic sides of the nuclear pore and binds to two distinct nucleoporin subcomplexes. Mol. Biol. Cell 14(2): 600-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüter, P., Tabernero, C., von Kobbe, C., Schmitt, C., Saavedra, C., Bachi, A., Wilm, M., Felber, B. K., and Izaurralde, E. (1998). TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol. Cell 1, 649-659. [DOI] [PubMed] [Google Scholar]
- Guzik, B. W., Lévesque, L., Prasad, S., Bor, Y. C., Black, B. E., Paschal, B. M., Rekosh, D., and Hammarskjöld, M. L. (2001). NXT1 (p15) is a crucial cellular cofactor in TAP-dependent export of intron-containing RNA in mammalian cells. Mol. Cell. Biol. 21, 2545-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarskjöld, M. L., Li, H., Rekosh, D., and Prasad, S. (1994). Human immunodeficiency virus env expression becomes Rev-independent if the env region is not defined as an intron. J. Virol. 68, 951-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarskjöld, M. L., Wang, S. C., and Klein, G. (1986). High-level expression of the Epstein-Barr virus EBNA1 protein in CV1 cells and human lymphoid cells using a SV40 late replacement vector. Gene 43(1-2), 41-50. [DOI] [PubMed] [Google Scholar]
- Hu, T., Guan, T., and Gerace, L. (1996). Molecular and functional characterization of the p62 complex, an assembly of nuclear pore complex glycoproteins. J. Cell Biol. 134, 589-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y., Gattoni, R., Stevenin, J., and Steitz, J. A. (2003). SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol. Cell 11, 837-843. [DOI] [PubMed] [Google Scholar]
- Izaurralde, E. (2002). A novel family of nuclear transport receptors mediates the export of messenger RNA to the cytoplasm. Eur J. Cell Biol. 81, 577-584. [DOI] [PubMed] [Google Scholar]
- Jin, L., Guzik, B. W., Bor, Y. C., Rekosh, D., and Hammarskjöld, M. L. (2003). Tap and NXT promote translation of unspliced mRNA. Genes Dev. 17, 3075-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira, J., Sträβer, K., Saiwaki, T., Yoneda, Y., and Hurt, E. (2002). Complex formation between Tap and p15 affects binding to FG-repeat nucleoporins and nucleocytoplasmic shuttling. J. Biol. Chem. 277, 9242-9246. [DOI] [PubMed] [Google Scholar]
- Katahira, J., Sträβer, K., Podtelejnikov, A., Mann, M., Jung, J. U., and Hurt, E. (1999). The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J. 18, 2593-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeili, A., and O'Shea, E. K. (2000). Nuclear transport and transcription. Curr. Opin. Cell Biol. 12, 355-360. [DOI] [PubMed] [Google Scholar]
- Lei, E. P., and Silver, P. A. (2002). Protein and RNA export from the nucleus. Dev. Cell 2, 261-272. [DOI] [PubMed] [Google Scholar]
- Le Maire, M., Kwee, S., Andersen, J. P., and Moller, J. V. (1983). Mode of interaction of polyoxyethyleneglycol detergents with membrane proteins. Eur. J. Biochem. 129(3): 525-532. [DOI] [PubMed] [Google Scholar]
- Lévesque, L., Guzik, B., Guan, T., Coyle, J., Black, B. E., Rekosh, D., Hammarskjöld, M. L., and Paschal, B. M. (2001). RNA export mediated by tap involves NXT1-dependent interactions with the nuclear pore complex. J. Biol. Chem. 276, 44953-44962. [DOI] [PubMed] [Google Scholar]
- Liker, E., Fernandez, E., Izaurralde, E., and Conti, E. (2000). The structure of the mRNA export factor TAP reveals a cis arrangement of a non-canonical RNP domain and an LRR domain. EMBO J. 19, 5587-5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman, S. K., Guan, T., Bednenko, J., Wodrich, H., and Gerace, L. (2002). Influence of cargo size on Ran and energy requirements for nuclear protein import. J. Cell Biol. 159, 55-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara, I. G. (2001). Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 65, 570-594, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj, I. W., and Englmeier, L. (1998). Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem. 67, 265-306. [DOI] [PubMed] [Google Scholar]
- Ossareh-Nazari, B., Maison, C., Black, B. E., Lévesque, L., Paschal, B. M., and Dargemont, C. (2000). RanGTP-binding protein NXT1 facilitates nuclear export of different classes of RNA in vitro. Mol. Cell. Biol. 20, 4562-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton, L. F., and Paschal, B. M. (2005). Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic 6, 187-198. [DOI] [PubMed] [Google Scholar]
- Ray, T. K., Nandi, J., Dannemann, A., and Gordon, G. B. (1983). Role of cholesterol in the structure and function of gastric microsomal vesicles. J. Cell. Biochem. 21(2): 141-150. [DOI] [PubMed] [Google Scholar]
- Ribbeck, K., and Gorlich, D. (2001). Kinetic analysis of translocation through nuclear pore complexes. EMBO J. 20, 1320-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout, M. P., Aitchison, J. D., Suprapto, A., Hjertaas, K., Zhao, Y., and Chait, B. T. (2000). The yeast nuclear pore complex: composition, architecture, and transport mechanism. J. Cell Biol. 148, 635-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt, I., and Gerace, L. (2001). In vitro analysis of nuclear transport mediated by the C-terminal shuttle domain of Tap. J. Biol. Chem. 276, 42355-42363. [DOI] [PubMed] [Google Scholar]
- Strawn, L. A., Shen, T., Shulga, N., Goldfarb, D. S., and Wente, S. R. (2004). Minimal nuclear pore complexes define FG repeat domains essential for transport. Nat. Cell Biol. 6, 197-206. [DOI] [PubMed] [Google Scholar]
- Strawn, L. A., Shen, T., and Wente, S. R. (2001). The GLFG regions of Nup116p and Nup100p serve as binding sites for both Kap95p and Mex67p at the nuclear pore complex. J. Biol. Chem. 276, 6445-6452. [DOI] [PubMed] [Google Scholar]
- Stutz, F., Bachi, A., Doerks, T., Braun, I. C., Seraphin, B., Wilm, M., Bork, P., and Izaurralde, E. (2000). REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA 6, 638-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz, F., and Izaurralde, E. (2003). The interplay of nuclear mRNP assembly, mRNA surveillance and export. Trends Cell Biol. 13, 319-327. [DOI] [PubMed] [Google Scholar]
- Suntharalingam, M., and Wente, S. R. (2003). Peering through the pore: nuclear pore complex structure, assembly, and function. Dev. Cell 4, 775-789. [DOI] [PubMed] [Google Scholar]
- Surapureddi, A., Svartz, J., Magnusson, K.-E., Hammarström, S., and Söderström, M. (2000). Colocalization of leukotriene C synthase and microsomal glutathione S-transferase elucidated by indirect immunofluorescence analysis. FEBS Lett. 480(2): 239-243. [DOI] [PubMed] [Google Scholar]
- Suyama, M., Doerks, T., Braun, I. C., Sattler, M., Izaurralde, E., and Bork, P. (2000). Prediction of structural domains of TAP reveals details of its interaction with p15 and nucleoporins. EMBO Rep. 1, 53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakurta, A. G., Gopal, G., Yoon, J. H., Saha, T., and Dhar, R. (2004). Conserved nuclear export sequences in Schizosaccharomyces pombe Mex67p and human Tap function in mRNA export by direct nuclear pore interactions. J. Biol. Chem. 279, 17434-17442. [DOI] [PubMed] [Google Scholar]
- Vinciguerra, P., and Stutz, F. (2004). mRNA export: an assembly line from genes to nuclear pores. Curr. Opin. Cell Biol. 16, 285-292. [DOI] [PubMed] [Google Scholar]
- Weis, K. (2002). Nucleocytoplasmic transport: cargo trafficking across the border. Curr. Opin. Cell Biol. 14, 328-335. [DOI] [PubMed] [Google Scholar]
- Wiegand, H. L., Coburn, G. A., Zeng, Y., Kang, Y., Bogerd, H. P., and Cullen, B. R. (2002). Formation of Tap/NXT1 heterodimers activates Tap-dependent nuclear mRNA export by enhancing recruitment to nuclear pore complexes. Mol. Cell. Biol. 22, 245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler, M., Pellicer, A., Silverstein, S., and Axel, R. (1978). Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell 14, 725-731. [DOI] [PubMed] [Google Scholar]
- Yaseen, N. R., and Blobel, G. (1999). Two distinct classes of Ran-binding sites on the nucleoporin Nup-358. Proc. Natl. Acad. Sci. USA 96, 5516-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]