Abstract
Rap1 is a small GTPase that regulates adherens junction maturation. It remains elusive how Rap1 is activated upon cell-cell contact. We demonstrate for the first time that Rap1 is activated upon homophilic engagement of vascular endothelial cadherin (VE-cadherin) at the cell-cell contacts in living cells and that MAGI-1 is required for VE-cadherin-dependent Rap1 activation. We found that MAGI-1 localized to cell-cell contacts presumably by associating with β-catenin and that MAGI-1 bound to a guanine nucleotide exchange factor for Rap1, PDZ-GEF1. Depletion of MAGI-1 suppressed the cell-cell contact-induced Rap1 activation and the VE-cadherin-mediated cell-cell adhesion after Ca2+ switch. In addition, relocation of vinculin from cell-extracellular matrix contacts to cell-cell contacts after the Ca2+ switch was inhibited in MAGI-1-depleted cells. Furthermore, inactivation of Rap1 by overexpression of Rap1GAPII impaired the VE-cadherin-dependent cell adhesion. Collectively, MAGI-1 is important for VE-cadherin-dependent Rap1 activation upon cell-cell contact. In addition, once activated, Rap1 upon cell-cell contacts positively regulate the adherens junction formation by relocating vinculin that supports VE-cadherin-based cell adhesion.
INTRODUCTION
Intercellular adhesion of vascular endothelial cells is essential for connecting neighboring endothelial cells to develop a vascular tree and to function as a barrier separating blood and tissues. Vascular endothelial cell adhesion is characterized by the overlapping of adherens junctions (AJs) and tight junctions (TJs). AJs are constituted by vascular endothelial cadherin (VE-cadherin) in close cooperation with platelet and endothelial adhesion molecule-1 (PECAM-1) and nectin. VE-cadherin-mediated cell adhesion depends on extracellular Ca2+, but not those mediated by PECAM-1 and nectin. TJs are made up of junctional adhesion molecule (JAM) family members, occuludin, claudin-5, and nectin (reviewed in Dejana, 2004).
VE-cadherin has an extracellular domain constituted by five cadherin domains, a transmembrane domain, and a cytoplasmic domain connected to p120 catenin and β-catenin (Iyer et al., 2004). Through β-catenin, VE-cadherin is linked to α-catenin that is associated with the actin cytoskeleton, which results in the maintenance of cell-cell adhesion in conjunction with cytoskeleton (Herren et al., 1998; Navarro et al., 1998; Kobielak and Fuchs, 2004). Tyrosine-phosphorylated VE-cadherin in its cytoplasmic domain provides docking sites for signal-transmitting molecules (Esser et al., 1998; Zanetti et al., 2002; Hudry-Clergeon et al., 2005). Conversely, cytoplasmic domain modified by phosphorylation or associated with signaling molecules triggers the inside-out signal that regulates the VE-cadherin-mediated cell adhesion (Nwariaku et al., 2004). β-catenin binds to other signaling molecules including PI3-K and MAGUK with inverted domain structure-1 (MAGI-1) as well as α-catenin (Kotelevets et al., 2005).
MAGI-1 consists of six PSD95/DiscLarge/ZO-1 (PDZ) domains, a guanylate kinase domain and two WW domains flanked by the first and second PDZ domain (Dobrosotskaya et al., 1997). Because PDZ domains are docking domains for PDZ-binding molecules, MAGI-1 associates with a variety molecules such as NMDA (N-methyl-d-aspartate) receptors, PTEN, BAI-1, δ-catenin, mNET1, and β-catenin (Hirao et al., 1998; Ide et al., 1999;Mino et al., 2000; Dobrosotskaya, 2001). These MAGI-1-associating molecules function at cell-cell contacts (Laura et al., 2002). MAGI-1, therefore, functions as a scaffold molecule by localizing to cell-cell contacts. Recently, MAGI-1 is reported to biochemically form a complex with E-cadherin and β-catenin (Kawajiri et al., 2000). However, the role of the E-cadherin/β-catenin-MAGI-1 complex in cell-cell junctional formation remains elusive.
Rap1 regulates cell-cell adhesion as well as cell-extracellular matrix (cell-ECM) adhesion (Bos, 2005). We have previously demonstrated that Epac-Rap1 signaling enhances VE-cadherin-dependent cell adhesion, thereby stabilizing vascular endothelial cell junctions (Fukuhara et al., 2005). On cell-cell contact, C3G, a guanine nucleotide exchange factor (GEF) for Rap1, is involved in the signaling mediated by E-cadherin and nectin in epithelial cells (Hogan et al., 2004; Fukuyama et al., 2005). Rap1 cycles between GDP-bound inactive form and GTP-bound active form; Rap1-specific GEFs and GTPase activating proteins (GAPs) activate and inactivate Rap1, respectively. Rap1 GEF family consists of C3G (RAPGEF1), PDZ-GEF1 (RAPGEF2), PDZ-GEF2, CalDAG-GEF1, Epac, and Epac2 (Bos et al., 2001).
We here investigate the involvement of MAGI-1-PDZ-GEF1 in the activation of Rap1 on vascular endothelial cell contact and demonstrate that MAGI-1 recruited to cell-cell junctions by associating β-catenin contributes to cell-cell contact-dependent activation of Rap1. In addition, the MAGI-1-mediated signal evoked upon cell-cell contact augments VE-cadherin-dependent endothelial cell adhesion. Thus, engagement of VE-cadherin activates Rap1 via MAGI-1, resulting in positive regulation of VE-cadherin-mediated cell adhesion.
MATERIALS AND METHODS
Plasmids and Adenovirus
pRaichu-Rap1, Rap1 activation monitoring-probe based on fluorescence resonance energy transfer (FRET), and Adeno-Raichu-Rap1, an adenovirus expressing Raichu-Rap1 were described previously (Mochizuki et al., 2001). Adenoviruses encoding Rap1GAPII and LacZ were obtained from S. Hattori (The Institute of Medical Science, University of Tokyo) and M. Matsuda (Research Institute for Microbial Disease, Osaka University, Osaka, Japan), respectively. Endothelial cells were infected with adenovirus at the appropriate multiplicity of infection for more than 24 h before imaging. The coding sequences of human MAGI-1b (hereafter MAGI-1) and PDZ-GEF1 were amplified by PCR using human heart cDNA library as a template and resultant DNAs were inserted into p3× FLAG-CMV-10 (Sigma, St. Louis, MO) and pEGFP-C1 (Clontech, Palo Alto, CA). cDNAs encoding truncated MAGI-1 as indicated in Figures 3B and 4A were similarly inserted into pEGFP-C1. pCALwL-FLAG-C3G, a FLAG-tagged mammalian expression vector, was obtained from M. Matsuda (Research Institute for Microbial Disease, Osaka University, Osaka, Japan; Ohba et al., 2001). pIRM21-PDZ5 expressed FLAG-tagged PDZ domain 5 of MAGI-1 and internal ribosomal entry site-driven dsFP593 (Nagashima et al., 2002). HcRed-p120 catenin-expressed HcRed-tagged p120 catenin was described previously (Kogata et al., 2003).
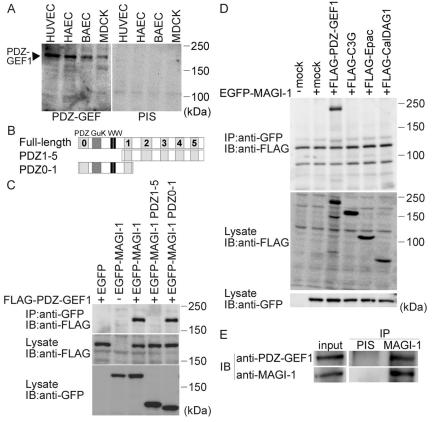
Figure 3.
MAGI-1 interacts with PDZ-GEF1 in vascular endothelial cells. (A) Cell lysates from HUVECs, HAECs, BAECs, and MDCK cells were subjected to SDS-PAGE and immunoblotting with anti-PDZ-GEF1 antibody (left) or pre-immune serum (PIS, right). (B) Schematic illustration of MAGI-1 (full length) and its deletion mutants. MAGI-1 consists of six PDZ domains (PDZ0-5, indicated by gray boxes), a guanylate kinase domain (GuK), and two WW domains. Deletion mutants, PDZ1-5 and PDZ0-1, consist of PDZ1 to PDZ5 and the amino-terminus to PDZ1, respectively. (C) 293T cells were transfected with the plasmids together with (+) or without (-) FLAG-tagged PDZ-GEF1 expressing vector as indicated at the top. Cell lysates were subjected to immunoprecipitation (IP) with anti-GFP antibody followed by immunoblotting (IB) or directly to immunoblotting using the antibodies as indicated. Note that FLAG-tagged PDZ-GEF1 is coimmunoprecipitated with GFP-tagged PDZ0-1. (D) Cells transfected with a panel of FALG-tagged Rap1 GEF-expressing plasmids together with (+) or without (-) EGFP-tagged MAGI-1-expressing plasmid. Cell lysates were subjected to immunoprecipitation (IP) followed by immunoblotting (IB) similarly to C. Note that only FLAG-tagged PDZ-GEF-1 among several Rap1 GEFs is coimmunoprecipitated with MAGI-1. (E) The lysate of HUVECs was incubated with either pre-immune serum (PIS) or anti-MAGI-1 antibody, followed by immunoblotting with anti-PDZ-GEF1 antibody. Note that MAGI-1 is coimmunoprecipitated with PDZ-GEF1.
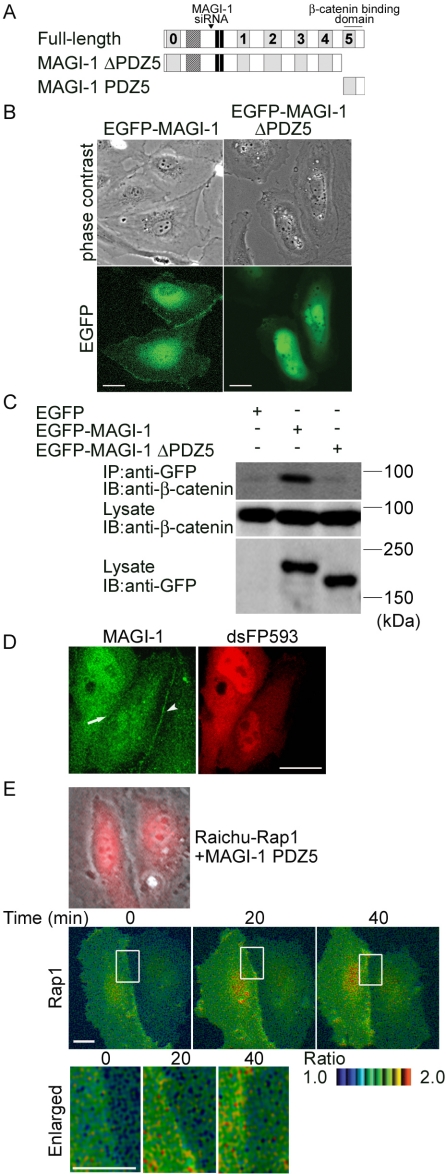
Figure 4.
MAGI-1 localizing to cell-cell contact via β-catenin is required for Rap1 activation. (A) Schematic illustration of MAGI-1 and its mutants. The corresponding region of siRNA for MAGI-1 used in Figure 5 is also indicated in this schema.(B) HUVECs were transfected with the plasmids indicated at the top and imaged on an Olympus IX-81 fluorescent microscope. Bars, 20 μm. Note the localization of EGFP-MAGI-1 at the cell-cell contact but not MAGI-1 lacking PDZ domain 5. (C) 293T cells were transfected with the plasmids as indicated at the top. Cell lysates were subjected to immunoprecipitation (IP) with anti-GFP followed by immunoblotting (IB) or directly to immunoblotting using the antibodies indicated at the left. Note that endogenous β-catenin is coimmunoprecipitated with EGFP-tagged MAGI-1, but not with that lacking PDZ5. (D) HUVECs expressing PDZ domain 5 of MAGI-1 was immunostained with anti-MAGI-1 antibody. No immunoreaction was detected at the cell-cell contact between PDZ domain 5-expressing cells (arrow), whereas immunoreaction was detected at the contact between PDZ domain 5-expressing cell and untransfected cell (arrow-head). (E) HUVECs transfected with Raichu-Rap1 and pIRM21-MAGI-1-PDZ5 were FRET-imaged (middle). Phase contrast image was overlaid onto the image for dsFP593 to distinguish HUVECs transfected with pIRM21-MAGI-1-PDZ5 from those transfected only with Raichu-Rap1 (top). Red and blue hues indicated by intensity modulated display reflect increased and decreased FRET, respectively. The boxed regions in the middle panels were enlarged (bottom). The upper and lower limits of the ratio range are shown at the bottom right. Bars, 20 μm.
Reagents and Antibodies
Purified human immunoglobulin (Ig) G Fc protein was purchased from ICN Biologicals (Cosa Mesa, CA). Glutathione Sepharose, protein A- and G-Sepharose were purchased from Amersham Biosciences (Piscataway, NJ). The rabbit polyclonal anti-MAGI-1b and anti-PDZ-GEF1 antibodies were developed in our laboratory by immunizing rabbits with recombinant glutathione S-transferase (GST)-tagged MAGI-1b (aa 1-140) or PDZ-GEF1 (aa 1-250) coupled with complete Freund's adjuvant, respectively. Anti-green fluorescent protein (GFP) antibody was generated in our laboratory. Other antibodies were purchased as follows: anti-Rap1 from Santa Cruz Biotechnology (Santa Cruz, CA); anti-FLAG (M2) and anti-vinculin from Sigma; anti-VE-cadherin, and anti-β-catenin from BD Bioscience (San Jose, CA); anti-ZO-1 from Zymed (South San Francisco, CA), Alexa 488- or Alexa 546-labeled secondary antibodies from Molecular Probes (Eugene, OR); horseradish peroxidase-coupled goat anti-mouse and anti-rabbit IgG from Amersham Biosciences.
Cell Culture and Transfection
Human umbilical vein endothelial cells (HUVECs) and human arterial endothelial cells (HAECs) were purchased from Kurabo (Kurashiki, Japan). The cells were maintained in HuMedia-EG2 with a growth additive set as described previously (Nagashima et al., 2002). Bovine aortic endothelial cells (BAECs), MDCK and 293T cells were maintained in DMEM (Nissui, Tokyo, Japan) supplemented with 10% fetal bovine serum and antibiotics (100 μg of streptomycin and 100 U of penicillin/ml). Endothelial cells and 293T cells were transfected by LipofectAMINE plus reagent (Invitrogen, Carlsbad, CA) and by the calcium phosphate method, respectively.
FRET Imaging and Fluorescence Imaging
HUVECs cultured on collagen-coated glass-base dishes were infected with Adeno-Raichu-Rap1 or transfected with pRaichu-Rap1. The structure of Raichu-Rap1 and the principle of FRET are illustrated as in Figure 1A. Cells were imaged on an Olympus IX-81 inverted fluorescence microscope (Lake Success, NY) as described previously (Nagashima et al., 2002). Dual images for cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) were obtained through an XF1071 excitation filter, an XF2034 dichroic filter, and an XF3075 emission filter for CFP and an XF 3079 for YFP (Omega Scientific, Tarzana, CA), respectively. The ratio image of YFP/CFP were created by MetaMorph 5.0 software (Universal Imaging, West Chester, PA) and displayed as an intensity-modulated display image as described previously (Nagashima et al., 2002).
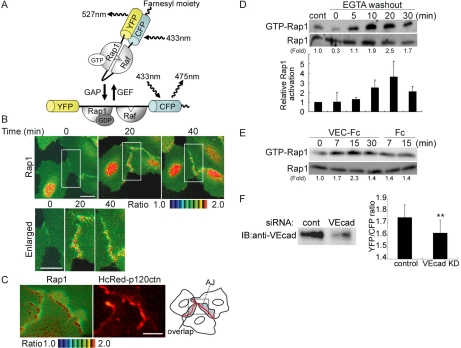
Figure 1.
Rap1 is activated by VE-cadherin-mediated cell adhesion upon cell-cell contact. (A) Schematic illustration of Raichu-Rap1. FRET efficiency depends on the guanine nucleotide binding state of Rap1. GDP-bound Raichu-Rap1 emits 475-nm fluorescence when excited at 433 nm, whereas GTP-bound Raichu-Rap1 emits 527-nm fluorescence due to FRET. Raf; Ras/Rap1 binding domain of Raf. (B) Motile HUVECs infected with Adeno-Raichu-Rap1 were monitored by FRET time-lapse imaging every 20 s. A ratio image of YFP to CFP reflects FRET efficiency. Ratio images are shown by the intensity modulated display, in which the upper and lower limits of the ratio (the intensity of YFP divided by that of CFP) are indicated by the red and blue hues, respectively, and the average intensity of YFP and CFP is used. Time since starting FRET imaging is indicated on the top (min). The boxed regions in the top panels were enlarged and are shown in the bottom panels. Bars, 20 μm. (C) HUVECs expressing both Raichu-Rap1 and HcRed-tagged p120 catenin were FRET-imaged and red-fluorescence-imaged. The real images of boxed region of the schema are shown as FRET image (left panel) and red-fluorescence image (center). Areas indicated by the gray are regions where protruding and overlapping regions of the contacting cells. Note that Rap1 is activated at the adherens junctions where p120 catenin localizes. Bar, 20 μm. (D) Confluent HUVECs cultured in medium 199 containing 1% BSA without serum were treated with EGTA for 30 min to disrupt Ca2+-dependent cell adhesion. Subsequently, the cells were treated with Ca2+-containing medium. Cell lysates at the time points indicated at the top were subjected to pulldown assay for detecting GTP-bound Rap1 as described in Materials and Methods. A representative results from three independent experiments is shown (top). Fold activation indicates the ratio of the GTP-Rap1 intensity of total Rap1 intensity to the control GTP-Rap1 intensity of total Rap1 intensity. The result from three independent experiments were shown (bottom). Control (cont) was prepared from cells in medium 199 before calcium switch. Cells treated with EGTA for 30 min (time 0). (E) HUVECs sparsely cultured on the dish were stimulated with 10 μg/ml VEC-Fc or Fc for the time indicated at the top. Rap1 activity was examined as described for D. Fold activation is analyzed similarly to D. (F) The effect of VE-cadherin siRNA on VE-cadherin expression was examined by immunoblotting (left). FRET at the cell-cell contacts were quantitatively analyzed in control siRNA-treated HUVECs and VE-cadherin-depleted HUVECs (right), as explained in Supplementary Figure 2. Mean values with SDs obtained by 30 cell-cell contact sites are shown as a representative result of three independent experiments. Statistical significance was analyzed by Student's t test; ** p < 0.01.
Quantitative FRET analysis at the cell-cell contacts was performed by dividing the intensity of YFP by that of CFP in the area defined by randomly selected 30 cell-cell contact sites. The detail was explained in the figure legend of Supplementary Figure 2. Cells expressing either fluorescence-tagged proteins (GFP, dsFP593, and HcRed) were time-lapse imaged similar to FRET imaging on an IX-81 microscope using appropriate filter sets for GFP and dsFP593.
Calcium Switch
HUVECs serum-starved for 10 h in medium 199 (Invitrogen) containing 1% bovine serum albumin (BSA) were transiently exposed to 4 mM EGTA for 30 min to chelate extracellular calcium and disrupt Ca2+-dependent intercellular junctions (Volberg et al., 1986). After washing, the cells were allowed to recover in complete cell calcium-containing culture media for the time indicated in the figure (Figures 1D, 6, and 7).
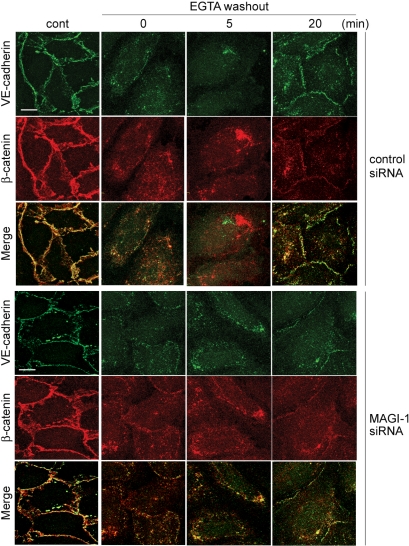
Figure 6.
Depletion of MAGI-1 impairs AJ formation. HUVECs transfected with control siRNAs (top three columns) or MAGI-1 siRNAs (bottom three columns) were cultured for 48 h. Cells were replated onto the glass-base dishes for another 24 h to constitute the cell-cell contacts. The cells were treated with EGTA for 30 min to disrupt VE-cadherin-dependent junctions and kept in the replaced medium containing Ca2+ for the time indicated at the top. The cells were immunostained with anti-VE-cadherin antibody (green) and anti-β-catenin antibody (red). The merged images are shown in the bottom panels (Merge). Bars, 20 μm. VE-cadherin remarkably accumulated 20 min after Ca2+ restoration in control siRNA-treated HUVECs, whereas slight accumulation was observed in MAGI-1 siRNA-treated HUVECs.
Figure 7.
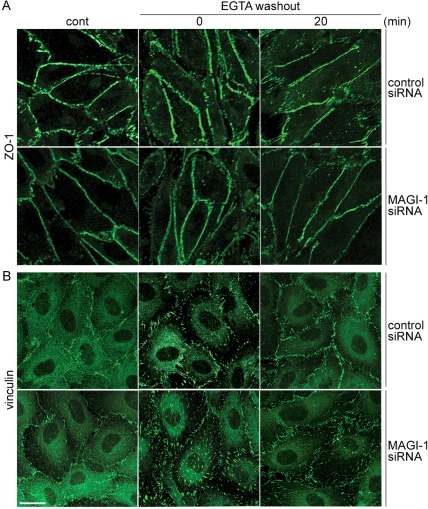
MAGI-1 depletion affects an AJ-supporting molecule, vinculin, but not a TJ-supporting molecule, ZO-1. (A) Similarly to Figure 6, HUVECs treated with control siRNAs (top panels) and MAGI-1 siRNAs (bottom panels) were immunostained with anti-ZO-1 after calcium switch. Bar, 20 μm. Note that ZO-1-positive cells were affected neither by calcium switch nor MAGI-1 depletion. (B) Similarly to A, control siRNA-treated cells and MAGI-1-depleted cells were immunostained with anti-vinculin before and after Ca2+ switch. Ca2+ depletion from the culture medium resulted in displacement of vinculin from cell-cell contacts to cell-ECM contacts and Ca2+-restoration induced relocalization from cell-ECM contacts to cell-cell contacts in control HUVECs. In clear contrast, vinculin remained at the cell-ECM contacts even 20 min after Ca2+ restoration in MAGI-1-depleted cells.
Detection of GTP-bound Rap1
GTP-bound active Rap1 was detected according to Bos's method (Franke et al., 1997). Briefly, cells starved in medium 199 containing 1% BSA for 10 h were subjected to a calcium switch or stimulated with 10 μg/ml VE-cadherin ectodomain-Fc (VEC-Fc) protein for the time indicated at the top of the figure (Figure 1E). The cells were lysed at 4°C in pulldown lysis buffer (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 1 mM EGTA, 1 mM dithiothreitol, 1 mM Na3VO4, 1× protease inhibitor cocktail). Precleared lysates were incubated with GST-Rap1 binding domain of RalGDS precoupled to glutathione-Sepharose beads. Proteins collected on the beads were subjected to SDS-PAGE followed by immunoblotting with anti-Rap1 antibody.
Immunocytochemistry and Confocal Imaging
Cells cultured on glass-bottom dishes were fixed with 2% formaldehyde in phosphate-buffered saline (PBS) for 30 min and permeabilized with 0.1% Triton X-100 for 10 min. Cells were blocked with 3% BSA for 30 min and incubated with anti-MAGI-1b, anti-VE-cadherin, anti-ZO-1, anti-vinculin, or anti-β-catenin antibody for 1 h at room temperature. Immunopositive reaction was visualized with Alexa 488- or Alexa 546-labeled secondary antibodies. Confocal images were obtained by an Olympus BX50WI microscope controlled by Fluoview.
Immunoprecipitation Assay
HUVECs were lysed with lysis buffer (100 mM NaCl, 50 mM Tris-HCl [pH 7.5], 1% Triton X-100, 2 mM Na3VO4, 1× protease inhibitor cocktail). Precleared cell lysates by centrifugation at 15,000 × g were incubated with antibodies. Immunoprecipitates collected on protein A- or G-Sepharose were subjected to SDS-PAGE and immunoblotting with antibodies as indicated in the figure (Figures 2D, 3, C, D, and E, and 4C).
Figure 2.
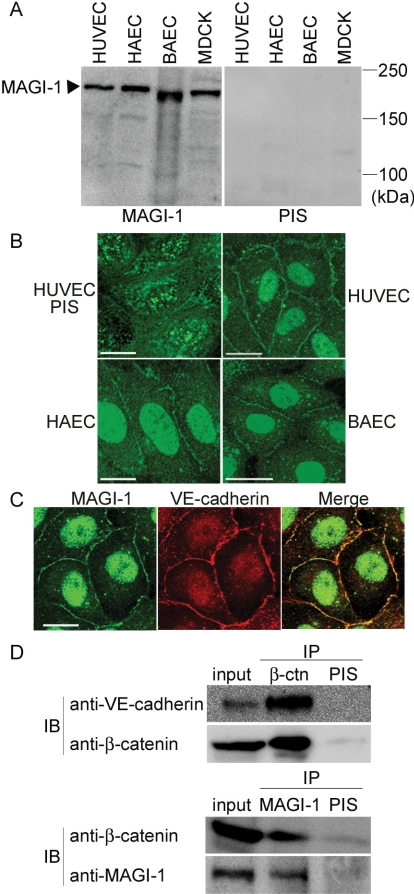
MAGI-1 localizes to cell-cell contacts and forms a complex with VE-cadherin and β-catenin. (A) Lysates from the cells indicated at the top were subjected to SDS-PAGE followed by immunoblotting with anti-MAGI-1 antibody (left) and with pre-immune serum (PIS, right). (B) Endothelial cells were immunostained with pre-immune serum (PIS, left top) and anti-MAGI-1 antibody. Immunoreaction was visualized by fluorescent microscopy. Bars, 20 μm. (C) HUVECs were immunostained with both anti-MAGI-1 antibody (green) and anti-VE-cadherin antibody (red). A merged image is shown in the right panel (Merge). Bar, 20 μm. (D) Cell lysates from HUVECs were subjected to either immunoprecipitation (IP) with antibodies as indicated at the top followed by immunoblotting (IB) with antibodies as indicated at the left. VE-cadherin was coimmunoprecipitated with β-catenin (top). β-catenin was coimmunoprecipitated with MAGI-1 (bottom)
siRNA-mediated Protein Knockdown
Small interfering RNAs (siRNAs) targeted to human MAGI-1; 5′-GGACCCUUCUCAGAAGUUCCCUCAA-3′ and 5′-UUGAGGGAACUUCUGAGAAGGGUCC-3, corresponding to nt 843-867 of coding sequence of MAGI-1 cDNA, and that for PDZ-GEF1; 5′-GGGAGUAAUCAAACAAAGAAGACUU3′ and 5′-AAGUCUUCUUUGUUUGAUUACUCCC3′, corresponding to nt 1980-2004 of PDZ-GEF1, were obtained from Invitrogen. VE-cadherin siRNAs were purchased from Santa Cruz Biotechnology. As a control, siRNA duplex with an irrelevant sequence was used. HUVECs were transfected with 20 nM siRNA duplexes using LipofectAMINE 2000 (Invitrogen) according to the manufacturer's instructions and incubated for 48 h after replacing fresh HuMedia-EG2.
Cell Adhesion Assay
Recombinant VEC-Fc chimeric protein was prepared as described previously (Fukuhara et al., 2005). Twenty-four-well tissue culture plates were coated with 10 μg VEC-Fc or Fc protein/ml in PBS-Ca2+/Mg2+ overnight at 4°C followed by blocking with 1% heat-inactivated BSA in PBS (inactivated at 85°C for 12 min) for 1 h at room temperature. HUVECs treated with control siRNAs or MAGI-1 siRNAs were cultured for 48 h and then suspended in 0.5% BSA-containing Medium 199. Resuspended cells, 2.0 × 105, were plated and adhered onto each VEC-Fc- or Fc-coated well at 37°C for the indicated time. To analyze cell adhesion to a collagen-covered surface, a collagen-coated 24-well plate (Asahi Technoglass, Chiba, Japan) was used instead of the VEC-Fc-coated plate. After washing with PBS-Ca2+/Mg2+ four times to remove nonadherent cells, adherent cells and input cells were quantified by measuring endogenous alkaline phosphatase (ALP) activity by using Atto-Phos AP fluorescent substrate system (Promega, Madison, WI).
RESULTS
Rap1 Is Activated on Homophilic VE-Cadherin Association at Cell-Cell Contacts
Rap1 is previously reported to localize to cell-cell contacts in vascular endothelial cells as well as epithelial cells to stabilize cell-cell contacts (Mandell et al., 2005; Wittchen et al., 2005). However, it remains elusive where Rap1 is activated on cell contacts. To monitor the spatiotemporal activation of Rap1 on vascular endothelial cell-cell contact, HUVECs expressing Raichu-Rap1 were time-lapse FRET-imaged. Raichu-Rap1 consists of YFP, Rap1, the Ras-binding domain of Raf, CFP, and a CAAX box of Ki-Ras. The intramolecular binding of GTP-Rap1 to Raf induces FRET from CFP to YFP (Figure 1A), whereas the dissociation of Rap1 from Raf reduces FRET. Increased FRET indicated by a red hue was observed at cell-cell contacts during spontaneous movement (Figure 1B and Supplementary Movie 1). Rap1 was constantly activated in the perinuclear region of the cells irrespective of cell-cell contact.
In vascular endothelial cells, the peripheral membrane of cells contacting each other was overlapped. Thus, AJs and TJs are intermingled (Dejana, 2004). To ascertain Rap1 activation at the adherens junctions, HUVECs expressing both Raichu-Rap1 and HcRed-tagged p120 catenin were imaged (Figure 1C). Most of Rap1 activation as indicated by red hue was observed at AJs where p120 catenin was localized. No remarkable Rap1 activation was detected within the protruding membrane overlapping region.
We further quantitatively examined whether the Rap1 is activated during cell adhesion after de-adhesion by chelating extracellular calcium and restoring calcium (hereafter, calcium switch). GTP-bound Rap1 was rapidly increased within 5 min and to a greater extent than the predisruption level by restoration of Ca2+ (Figure 1D, top panel). The quantitative results obtained from three independent experiments were shown (Figure 1D, bottom panel). These results suggest that the cell-cell contact triggers the Rap1 activation in a manner dependent on extracellular Ca2+. Although Rap1 is reported to be activated in a manner dependent on nectin, which is independent of extracellular Ca2+ (Fukuyama et al., 2005), we assumed that Ca2+-dependent cell-cell contact triggers Rap1 activation besides nectin-triggered Rap1 activation. We, therefore, examined the VE-cadherin engagement-dependent Rap1 activation. To mimic the VE-cadherin engagement in nascent cell-cell contacts, we used VEC-Fc chimeric protein, which consisted of the extracellular domain of VE-cadherin fused to the Fc portion of Ig. GTP-bound Rap1 was increased when cells were treated with VEC-Fc, but not with control Fc (Figure 1E).
To examine the requirement of VE-cadherin for Rap1 activation upon cell-cell contact, we imaged Rap1 activation in VE-cadherin-depleted HUVECs. Quantitative FRET imaging analysis upon cell-cell contacts demonstrated that Rap1 activation at the cell-cell contacts was less in VE-cadherin-depleted cells than those observed in control siRNA-treated cells (Figure 1F). Collectively, these data indicate that the engagement of VE-cadherin induces Rap1 activation.
MAGI-1 Localizes to Cell-Cell Contacts and Binds to β-Catenin
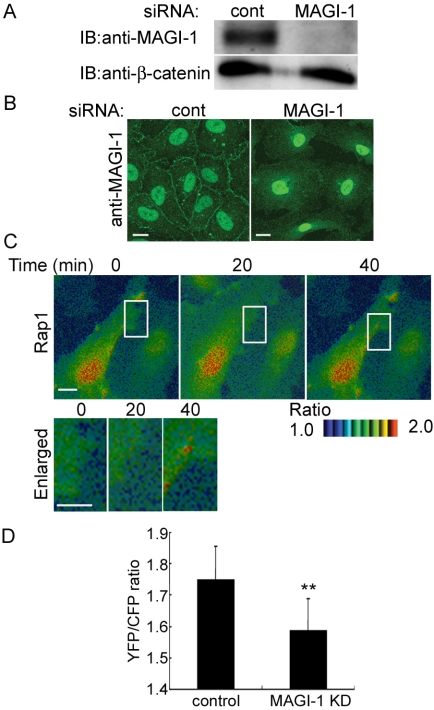
MAGI-1 constitutes a complex with E-cadherin/β-catenin and associates with a GEF for Rap1, PDZ-GEF1 (Kawajiri et al., 2000), implying that MAGI-1 may link the cadherin-mediated signal to PDZ-GEF1 for the activation of Rap1. To investigate the involvement of MAGI-1 in Rap1 activation on VE-cadherin-mediated cell-cell contact, we first developed an anti-MAGI-1 antibody and examined the expression of MAGI-1 in vascular endothelial cells. MAGI-1 was expressed in all cultured vascular endothelial cells we tested, because it was found in MDCK epithelial cells used as a positive control (Figure 2A). Next, we examined the localization of MAGI-1 in vascular endothelial cells by immunostaining. MAGI-1 was localized to the cell-cell contacts (Figure 2B) and colocalized with VE-cadherin (Figure 2C). The immunopositive reaction in the nucleus appeared to be non-specific, because it was detected in the nucleus by immunostaining using preabsorbed anti-MAGI-1 (unpublished data) and after knockdown of MAGI-1 (see Figure 5B).
Figure 5.
Depletion of MAGI-1 inhibits Rap1 activation upon cell-cell contact. (A) HUVECs transfected with control siRNAs or MAGI-1 siRNAs were cultured for 48 h. The cells were lysed, subjected to SDS-PAGE, and immunoblotted with anti-MAGI-1 and anti-β-catenin. (B) HUVECs transfected with control siRNAs or MAGI-1 siRNAs were cultured for 48 h and immunostained with anti-MAGI-1. Bars, 20 μm. (C) MAGI-1-depleted HUVECs were infected with Adeno-Raichu-Rap1 and FRET-imaged. The ratio-images indicate Rap1 activation by red hue and Rap1 inactivation by blue hue (top). The boxed region between two neighboring cells is enlarged (bottom). Bars, 20 μm. (D) Quantitative FRET analysis at the cell-cell contacts were performed in cells treated with control siRNA-treated HUVECs (control) and with MAGI-1-depleted cells (MAGI-1 KD). Quantitative FRET analysis is explained in Supplementary Figure 2. Mean values with standard deviations obtained by 30 cell-cell contact sites are shown as a representative result of three independent experiments. Statistical significance was analyzed by Student's t test and is indicated as ** p < 0.01.
To investigate how MAGI-1 localizes to cell-cell contacts, we tested the link between VE-cadherin and MAGI-1 by β-catenin. We examined this link by immunoprecipitation assay (Figure 2D). β-catenin bound to both VE-cadherin (Figure 2D, top panel) and MAGI-1 (Figure 2D, bottom panel). These results indicate that MAGI-1 appears to localize to VE-cadherin-based cell adhesion through β-catenin in vascular endothelial cells.
MAGI-1 Interacts with PDZ-GEF1 in Vascular Endothelial Cells
It has been shown that MAGI-1 binds to PDZ-GEF1 localized to cell-cell contacts in epithelial cells (Dobrosotskaya and James, 2000; Kawajiri et al., 2000). We hypothesized that PDZ-GEF1 is associated with MAGI-1 in vascular endothelial cells and that it is involved in the activation of Rap1 on VE-cadherin-mediated cell-cell contact. PDZ-GEF1 was expressed in vascular endothelial cells similarly to MAGI-1 (Figure 3A). The interaction between MAGI-1 and PDZ-GEF1 was examined by the immunoprecipitation using the full-length and the truncated mutants of MAGI-1 (Figure 3, B and C). The PDZ-GEF1 bound to the N-terminus of MAGI-1 (Figure 3C). EGFP-tagged MAGI-1 coimmunoprecipitated PDZ-GEF1, but not other GEFs for Rap1, C3G, Epac1, and CalDAG-GEF-I (Figure 3D). We further examined the interaction between endogenous MAGI-1 and PDZ-GEF1 in HUVECs. Both MAGI-1 and PDZ-GEF1 were coimmunoprecipitated from the lysate of HUVECs (Figure 3E), indicating that PDZ-GEF1 associates with MAGI-1 in vascular endothelial cells.
Localization of MAGI-1 to Cell-Cell Contact is Important for Rap1 Activation on Cell Contact
To understand the role of MAGI-1 in activating Rap1 when forming cell-cell contacts, we proceeded to investigate the localization of MAGI-1 using EGFP-tagged MAGI-1 in motile endothelial cells. EGFP-MAGI-1 was accumulated at cell-cell contacts (Figure 4B, left panels, and Supplementary Movie 2). Removal of the carboxy terminal PDZ domain (delta PDZ5) resulted in the dissociation of MAGI-1 from cell-cell contacts (Figure 4B, right panels, and Supplementary Movie 3). Because it was reported that MAGI-1 binds to β-catenin through PDZ5 (Dobrosotskaya and James, 2000), we tested the requirement of PDZ5 for the association of MAGI1- with β-catenin. β-catenin was coimmunoprecipitated with EGFP-tagged full-length MAGI-1 but not with MAGI-1 lacking PDZ5 (Figure 4C). These results suggest that MAGI-1 localizes to vascular endothelial cell-cell contacts in a manner dependent on β-catenin. We further revealed that MAGI-1 was dislocated from the cell-cell contact of the PDZ5-expressing cells as marked by red fluorescence but not from that of wild-type cells (Figure 4D), indicating that PDZ5 is important for the localization of MAGI-1 to cell-cell contacts.
To examine the requirement of the association of MAGI-1 with β-catenin for the cell-cell contact-induced Rap1 activation, we checked the effect of disconnection of MAGI-1 to β-catenin by overexpressing MAGI-1 PDZ domain 5 on Rap1 activation. In HUVECs expressing MAGI-1 PDZ domain 5, as marked by dsFP593 (Figure 4E and Supplementary Movie 4), Rap1 activation upon cell-cell contacts was not observed in FRET imaging. These results indicate that the dislocation of MAGI-1 from the cell-cell contact inhibits Rap1 activation at the cell-cell contacts in motile vascular endothelial cells.
To confirm the requirement of MAGI-1 in Rap1 activation upon vascular endothelial cell-cell contact, we knocked down MAGI-1 in HUVECs using RNA interference. MAGI-1 was almost completely reduced, as examined by Western blotting (Figure 5A). MAGI-1 at the cell-cell junction was not found in the cells treated with siRNA, as examined by immunostaining (Figure 5B). In the same setting, siRNA-introduced HUVECs expressing Raichu-Rap1 were subjected to FRET imaging. Rap1 activation upon cell-cell contact was significantly suppressed in MAGI-1-depleted cells (Figure 5C and Supplementary Movie 5). Quantitative FRET imaging analysis was performed to quantitatively analyze the activation of Rap1 at the cell-cell contacts in MAGI-1-depleted cells (Figure 5D and Supplementary Figure 2). We notice that Rap1 activation was detected at the free ruffled membrane without cell-cell contacts, similarly to control cells (Supplementary Movies 1 and 5), even in the MAGI-1-depleted cells. Collectively, these data suggest that the localization of MAGI-1 to cell-cell contacts through binding to β-catenin is involved in Rap1 activation.
Depletion of MAGI-1 Results in Impairment of VE-Cadherin-based Cell Adhesion
To elucidate the role of activated Rap1 downstream of MAGI-1 upon cell-cell contact, we examined the effect of depletion of MAGI-1 on VE-cadherin/β-catenin-based cell-cell contact after calcium switch. The localization of VE-cadherin and β-catenin at cell-cell contacts in confluent monolayer-cultured HUVECs was unchanged by MAGI-1 siRNA treatment. Because calcium switch induces cadherin-mediated cell junction after its disruption, we looked at the localization of VE-cadherin and β-catenin during calcium switch by immunostaining for VE-cadherin and β-catenin. VE-cadherin was reaccumulated at cell-cell junctions together with β-catenin within 20 min in control HUVECs. In clear contrast, there was a significant impairment of the formation of VE-cadherin/β-catenin-based cell junction in MAGI-1-depleted HUVECs (Figure 6).
TJ formation was not affected by MAGI-1 depletion and calcium switch (Figure 7A), whereas the recovery of VE-cadherin-based cell adhesion was substantially impaired in MAGI-1-depleted cells. These results indicate that MAGI-1-mediated signal is important for VE-cadherin/β-catenin-based cell adhesion.
We and others have previously reported that Rap1 activation enhances cell adhesions (Fukuhara et al., 2005; Kooistra et al., 2005). Cortical actin formation is enhanced by Rap1 activation and strengthens VE-cadherin-based cell-cell adhesion. Vinculin supports the cortical actin by linking α-catenin to α-actinin and by directly functioning as an actin-bundling molecule (Kobielak and Fuchs, 2004). Thus we investigated the vinculin localization after calcium switch by immunostaining. Vinculin was observed at the cell-ECM contacts presumably by translocating from cell-cell contacts after calcium depletion. Calcium restoration induced the relocation of vinculin from cell-ECM to cell-cell contact in control siRNA-treated cells. In clear contrast, vinculin remained at the focal adhesions in MAGI-1-depleted cells after calcium switch (Figure 7B). These data suggest that MAGI-1-dependent Rap1 activation at cell-cell contact may affect the vinculin localization, thereby regulating VE-cadherin-based cell adhesion.
MAGI-1 Is Required for VE-Cadherin-mediated Cell Adhesion
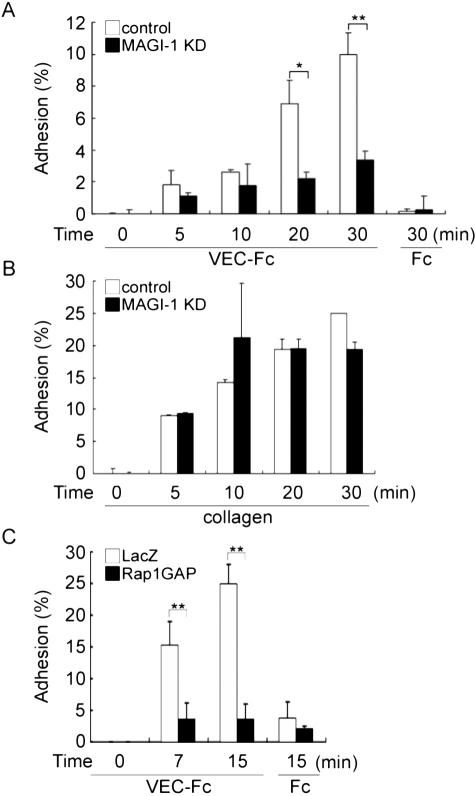
Vascular endothelial cell adhesion entails VE-cadherin-based adhesion and other cell adhesion molecules-based cell adhesion. To directly assess the involvement of MAGI-1 in VE-cadherin-mediated cell adhesion, we examined the adhesion of control siRNA-treated HUVECs and MAGI-1-depleted HUVECs onto VEC-Fc-coated dishes. The adhesion was quantified by the ALP activity of cells attaching to the dish after washing. Control HUVECs adhered to the VEC-Fc-coated dish in a time-dependent manner, whereas MAGI-1-depleted HUVECs exhibited significantly impaired adhesion to the VEC-Fc-coated dish (Figure 8A). No cells attached to the Fc-coated dish. MAGI-1-depleted HUVECs adhered to the collagen-coated dish comparably to control HUVECs (Figure 8B). We proceeded to examine the effect of inactivation of Rap1 on VE-cadherin-dependent adhesion. Control adenovirus-infected HUVECs adhered to VEC-Fc-coated dish, whereas Rap1GAPII-expressing adenovirus-infected HUVECs did not (Figure 8C). These results indicate that MAGI-1 and Rap1 activation is required for VE-cadherin-dependent cell adhesion.
Figure 8.
Depletion of MAGI-1 and inactivation of Rap1 inhibits VE-cadherin-mediated cell adhesion. (A) HUVECs transfected with control siRNAs (white column) or MAGI-1 siRNAs (black column) were cultured for 48 h, suspended in 0.5% BSA-containing medium 199, and incubated for 30 min at 37°C. Cells, 2.0 × 105, were plated onto either a VEC-Fc- or Fc-coated well for the time indicated at the bottom. Cell adhesion was quantified as described in Materials and Methods. The averages of triplicate (plus SDs) are presented. A representative result of three independent experiments is shown. Statistical significance was analyzed by Student's t test; * p < 0.05 and ** p < 0.01. Note that adhesion of HUVECs treated with MAGI-1 siRNAs to the VEC-Fc-coated dish was significantly reduced compared with mock-treated HUVECs. (B) Adhesion of MAGI-1-depleted cells to a collagen-coated dish was comparable to mock-treated HUVECs, as analyzed by the same method described in the legend for A. (C) HUVECs infected with either LacZ-expressing adenovirus (LacZ) or Rap1GAPII-expressing virus (Rap1GAP) were analyzed for adhesion to a VEC-Fc-coated dish similarly to A.
DISCUSSION
FRET imaging enabled us for the first time to show the activation of Rap1 at the endothelial cell-cell junction, although previously Rap1 was suggested to be activated upon cell adhesion. In epithelial cells, C3G associating with E-cadherin is responsible for Rap1 activation upon cell contacts (Hogan et al., 2004). Rap1, vice versa, regulates E-cadherin-mediated cell adhesion (Price et al., 2004). In addition to E-cadherin, homophilic dimerization of nectin at the AJs triggers Rap1 activation downstream of Src-Crk (Fukuyama et al., 2005). During the calcium switch experiment, which requires extracellular Ca2+, we found that Rap1 was activated (Figure 1C), indicating extracellular Ca2+-dependent signal, namely cadherin- and nectin-independent signal, appears to be involved in Rap1 activation upon cell-cell contact. In the present study, we propose the involvement of the MAGI-1/PDZ-GEF1 complex in Rap1 activation, besides nectin-mediated Rap1 activating signal and the subsequent positive feedback regulation of VE-cadherin-mediated cell adhesion.
Rap1 is responsible for maintenance and maturation of AJs. The establishment of cadherin-dependent cell-cell contacts is attributable to Rap1 in Drosophila melanogaster and mammalian cells (Knox and Brown, 2002; Price et al., 2004). Consistently, we show here that VE-cadherin-dependent cell adhesion triggers a signal implicating MAGI-1 in Rap1 activation, presumably the MAGI-1-PDZ-GEF1-Rap1 pathway. VE-cadherin engagement-induced Rap1 activation may contribute to AJ formation in addition to homophilic engagement of nectin-dependent Rap1 activation (Fukuyama et al., 2005).
Here we demonstrate VE-cadherin-dependent Rap1 activation besides nectin-dependent Rap1 activation. Calcium switch does not alter localization of nectin at the cell-cell contacts (Yamada et al., 2005). We found that Rap1 was activated after calcium restoration in the calcium switch experiment that mimics nascent cell-cell contact formation (Figure 1), indicating that Ca2+-dependent cell-cell contact is involved in Rap1 activation. We first assumed that VE-cadherin is responsible for Rap1 activation. Indeed, VE-cadherin depletion inhibited cell-cell contact-mediated Rap1 activation (Figure 1F).
These two AJ molecules, VE-cadherin and nectin, are linked by their cytoplasmic domain-associating proteins (Tachibana et al., 2000). L-afadin, a nectin cytoplasmic domain-binding molecule, binds to α-catenin and subsequently locates cadherin to AJs without the transinteraction of cadherin (Tanaka et al., 2003). L-afadin, s-afadin (AF-6), and Canoe (Drosophila orthologue of AF-6) contain a Rap1-binding domain (Boettner et al., 2000, 2003). Thus, afadin regulated by activated Rap1 at cell-cell contacts may enhance AJ formation constituted by both cadherin and nectin.
We explored the requirement of MAGI-1 for Rap1 activation at cell adhesion. The association of MAGI-1 with β-catenin via the PDZ domain 5 is critical for its localization to VE-cadherin-based cell-cell contact. MAGI-1 also interacts with endothelial cell-selective adhesion molecule (ESAM) and JAM-4 at TJs (Hirabayashi et al., 2003). Other JAM family members (JAM-A, B, and C) do not bind to MAGI-1 (our unpublished data). The carboxy-terminal sequence of ESAM and JAM-4 provides the class I PDZ-binding motif, whereas that of JAM family members contains the class II PDZ-binding motif (Hung and Sheng, 2002). Thus, ESAM-mediated MAGI-1 recruitment may contribute to Rap1-regulated cell adhesion at TJs as β-catenin recruits MAGI-1 at AJs. It will be interesting to explore the TJ-dependent Rap1 activation.
MAGI-1 together with MAGI-2 (S-SCAM), and MAGI-3 constitute the MAGI family (Hirao et al., 2000; Franklin et al., 2005). It has been shown that MAGI-2 binds to β-catenin and that MAGI-3 colocalized to β-catenin in astrocytes expressing E-cadherin (Adamsky et al., 2003; Subauste et al., 2005). Although MAGI-2 is exclusively expressed in the brain, MAGI-3 is ubiquitously expressed. Although we cannot exclude the involvement of MAGI-3 in the activation of Rap1 in vascular endothelial cells, the disconnection of MAGI family members from β-catenin by overexpressing the PDZ domain 5 of MAGI-1 perturbed the Rap1 activation upon cell-cell contact (Figure 4, D and E). Furthermore, depletion of MAGI-1 by siRNA hampered the Rap1 activation (Figure 5), suggesting that MAGI-1 is indispensable for Rap1 activation based on the linkage between VE-cadherin-β-catenin complex and MAGI-PDZ-GEF1 complex in vascular endothelial cells.
To delineate the VE-cadherin engagement-triggered Rap1 activation signal, we tried to test the requirement of PDZ-GEF1 for Rap1 activation because we found that MAGI-1 associated with PDZ-GEF1 in vascular endothelial cells (Figure 3), as this association reported previously (Dobrosotskaya and James, 2000; Kawajiri et al., 2000). PDZ-GEF1-depleted cells seemed to be detached from the collagen-coated dishes and the adhesive activity to collagen-coated dish was significantly inhibited. Thus we assumed that there might be other signaling besides MAGI-1-PDZ-GEF1-mediated signal for cell adhesion and thus concluded that PDZ-GEF1-depleted cells were not appropriate for further evaluation to delineate the signaling. At least, VE-cadherin engagement-triggered Rap1 activation requires MAGI-1.
Activated Rap1 upon cell-cell contact further strengthens the VE-cadherin-dependent cell-cell adhesion. Rap1 activation is required for VE-cadherin-mediated cell adhesion (Figure 8B). We previously reported that the inside-out signal regulated by cAMP-Epac-Rap1 signal enhances the VE-cadherin-mediated cell-cell contacts by regulating cortical actin (Fukuhara et al., 2005). Although we did not observe significant cortical actin distribution after calcium switch experiment (unpublished data), we noticed that the relocation of vinculin from cell-ECM to cell-cell contacts was inhibited in MAGI-1-depleted cells. Because vinculin supports the cadherin-based cell contacts by linking actin to cytoplasmic domain of cadherin through α-catenin, inhibition of Rap1 activation by MAGI-1 depletion might affect AJ formation. These results imply a positive feedback loop in cell-cell contact regulated by Rap1; namely, cell-cell contact promotes transdimerization of cell surface adhesion molecules, inducing Rap1 activation followed by further tightening of VE-cadherin-mediated cell adhesion.
In conclusion, we revealed that MAGI-1 is required for Rap1 activation upon cell-cell contacts and in turn for AJ formation. The translocation of MAGI-1 to cell-cell contacts is ascribed to its association with β-catenin. The MAGI-1-associating molecule, PDZ-GEF1, may account for Rap1 activation.
Supplementary Material
Acknowledgments
We are grateful to M. Matsuda for his advice, plasmids, and virus; K. Kaibuchi for anti-PDZ-GEF1 antibody; J. T. Pearson for his critical reading of this manuscript; and M. Sone, Y. Mizushima, and Y. Matsuura for their technical assistance. This work was supported by grants from the Ministry of Health, Labor, and Welfare Foundation of Japan; from the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation; from the Ministry of Education, Science, Sports and Culture of Japan; from the Mochida Memorial Foundation for Medical and Pharmaceutical Research; and from Astellas Foundation for Research on Metabolic Disorders.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-07-0647) on December 7, 2005.
Abbreviations used: AJ, adherens junction; CFP, cyan fluorescent protein; ECM, extracellular matrix; EGFP, enhanced green fluorescent protein; FRET, fluorescence resonance energy transfer; GEF, guanine nucleotide exchange factor; GAP, GTPase activating protein; HAEC, human aortic endothelial cell; HUVEC, human umbilical vascular endothelial cell; JAM, junctional adhesion molecule; MAGI-1, MAGUK with inverted domain structure-1; GFP, green fluorescent protein; PBS, phosphate-buffered saline; PDZ, PSD95/DiscLarge/ZO-1; PECAM-1, platelet and endothelial cell adhesion molecule-1; siRNAs, small interfering RNAs; TJ, tight junction; VE-cadherin, vascular endothelial cadherin; VEC-Fc, recombinant VE-cadherin ectodomain-Fc chimera; YFP, yellow fluorescent protein.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Adamsky, K., Arnold, K., Sabanay, H., and Peles, E. (2003). Junctional protein MAGI-3 interacts with receptor tyrosine phosphatase beta (RPTP beta) and tyrosine-phosphorylated proteins. J. Cell Sci. 116, 1279-1289. [DOI] [PubMed] [Google Scholar]
- Boettner, B., Govek, E. E., Cross, J., and Van Aelst, L. (2000). The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc. Natl. Acad. Sci. USA 97, 9064-9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner, B., Harjes, P., Ishimaru, S., Heke, M., Fan, H. Q., Qin, Y., Van Aelst, L., and Gaul, U. (2003). The AF-6 homolog canoe acts as a Rap1 effector during dorsal closure of the Drosophila embryo. Genetics 165, 159-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos, J. L. (2005). Linking Rap to cell adhesion. Curr. Opin. Cell Biol. 17, 123-128. [DOI] [PubMed] [Google Scholar]
- Bos, J. L., de Rooij, J., and Reedquist, K. A. (2001). Rap1 signalling: adhering to new models. Nat. Rev. Mol. Cell Biol. 2, 369-377. [DOI] [PubMed] [Google Scholar]
- Dejana, E. (2004). Endothelial cell-cell junctions: happy together. Nat. Rev. Mol. Cell Biol. 5, 261-270. [DOI] [PubMed] [Google Scholar]
- Dobrosotskaya, I., Guy, R. K., and James, G. L. (1997). MAGI-1, a membrane-associated guanylate kinase with a unique arrangement of protein-protein interaction domains. J. Biol. Chem. 272, 31589-31597. [DOI] [PubMed] [Google Scholar]
- Dobrosotskaya, I. Y. (2001). Identification of mNET1 as a candidate ligand for the first PDZ domain of MAGI-1. Biochem. Biophys. Res. Commun. 283, 969-975. [DOI] [PubMed] [Google Scholar]
- Dobrosotskaya, I. Y., and James, G. L. (2000). MAGI-1 interacts with beta-catenin and is associated with cell-cell adhesion structures. Biochem. Biophys. Res. Commun. 270, 903-909. [DOI] [PubMed] [Google Scholar]
- Esser, S., Lampugnani, M. G., Corada, M., Dejana, E., and Risau, W. (1998). Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J. Cell Sci. 111(Pt 13,) 1853-1865. [DOI] [PubMed] [Google Scholar]
- Franke, B., Akkerman, J. W., and Bos, J. L. (1997). Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J. 16, 252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, J. L., Yoshiura, K., Dempsey, P. J., Bogatcheva, G., Jeyakumar, L., Meise, K. S., Pearsall, R. S., Threadgill, D., and Coffey, R. J. (2005). Identification of MAGI-3 as a transforming growth factor-alpha tail binding protein. Exp. Cell Res. 303, 457-470. [DOI] [PubMed] [Google Scholar]
- Fukuhara, S., Sakurai, A., Sano, H., Yamagishi, A., Somekawa, S., Takakura, N., Saito, Y., Kangawa, K., Mochizuki, N. (2005). Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol. Cell Biol. 25, 136-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama, T., Ogita, H., Kawakatsu, T., Fukuhara, T., Yamada, T., Sato, T., Shimizu, K., Nakamura, T., Matsuda, M., and Takai, Y. (2005). Involvement of the c-Src-Crk-C3G-Rap1 signaling in the nectin-induced activation of Cdc42 and formation of adherens junctions. J. Biol. Chem. 280, 815-825. [DOI] [PubMed] [Google Scholar]
- Herren, B., Levkau, B., Raines, E. W., and Ross, R. (1998). Cleavage of beta-catenin and plakoglobin and shedding of VE-cadherin during endothelial apoptosis: evidence for a role for caspases and metalloproteinases. Mol. Biol. Cell 9, 1589-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi, S., Tajima, M., Yao, I., Nishimura, W., Mori, H., and Hata, Y. (2003). JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1. Mol. Cell Biol. 23, 4267-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao, K., Hata, Y., Ide, N., Takeuchi, M., Irie, M., Yao, I., Deguchi, M., Toyoda, A., Sudhof, T. C., and Takai, Y. (1998). A novel multiple PDZ domain-containing molecule interacting with N-methyl-D-aspartate receptors and neuronal cell adhesion proteins. J. Biol. Chem. 273, 21105-21110. [DOI] [PubMed] [Google Scholar]
- Hirao, K., Hata, Y., Yao, I., Deguchi, M., Kawabe, H., Mizoguchi, A., and Takai, Y. (2000). Three isoforms of synaptic scaffolding molecule and their characterization. Multimerization between the isoforms and their interaction with N-methyl-D-aspartate receptors and SAP90/PSD-95-associated protein. J. Biol. Chem. 275, 2966-2972. [DOI] [PubMed] [Google Scholar]
- Hogan, C., Serpente, N., Cogram, P., Hosking, C. R., Bialucha, C. U., Feller, S. M., Braga, V. M., Birchmeier, W., and Fujita, Y. (2004). Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol. Cell Biol. 24, 6690-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudry-Clergeon, H., Stengel, D., Ninio, E., and Vilgrain, I. (2005). Platelet-activating factor increases VE-cadherin tyrosine phosphorylation in mouse endothelial cells and its association with the PtdIns3′-kinase. FASEB J. 19, 512-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung, A. Y., and Sheng, M. (2002). PDZ domains: structural modules for protein complex assembly. J. Biol. Chem. 277, 5699-5702. [DOI] [PubMed] [Google Scholar]
- Ide, N., Hata, Y., Deguchi, M., Hirao, K., Yao, I., and Takai, Y. (1999). Interaction of S-SCAM with neural plakophilin-related Armadillo-repeat protein/delta-catenin. Biochem. Biophys. Res. Commun. 256, 456-461. [DOI] [PubMed] [Google Scholar]
- Iyer, S., Ferreri, D. M., DeCocco, N. C., Minnear, F. L., and Vincent, P. A. (2004). VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am. J. Physiol Lung Cell Mol. Physiol. 286, L1143-L1153. [DOI] [PubMed] [Google Scholar]
- Kawajiri, A., Itoh, N., Fukata, M., Nakagawa, M., Yamaga, M., Iwamatsu, A., and Kaibuchi, K. (2000). Identification of a novel beta-catenin-interacting protein. Biochem. Biophys. Res. Commun. 273, 712-717. [DOI] [PubMed] [Google Scholar]
- Knox, A. L., and Brown, N. H. (2002). Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science 295, 1285-1288. [DOI] [PubMed] [Google Scholar]
- Kobielak, A., and Fuchs, E. (2004). Alpha-catenin: at the junction of intercellular adhesion and actin dynamics. Nat. Rev. Mol. Cell Biol. 5, 614-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogata, N., Masuda, M., Kamioka, Y., Yamagishi, A., Endo, A., Okada, M., and Mochizuki, N. (2003). Identification of Fer tyrosine kinase localized on microtubules as a platelet endothelial cell adhesion molecule-1 phosphorylating kinase in vascular endothelial cells. Mol. Biol. Cell 14, 3553-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra, M. R., Corada, M., Dejana, E., and Bos, J. L. (2005). Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 579, 4966-4972. [DOI] [PubMed] [Google Scholar]
- Kotelevets, L., van Hengel, J., Bruyneel, E., Mareel, M., van Roy, F., and Chastre, E. (2005). Implication of the MAGI-1b/PTEN signalosome in stabilization of adherens junctions and suppression of invasiveness. FASEB J. 19, 115-117. [DOI] [PubMed] [Google Scholar]
- Laura, R. P., Ross, S., Koeppen, H., and Lasky, L. A. (2002). MAGI-1, a widely expressed, alternatively spliced tight junction protein. Exp. Cell Res. 275, 155-170. [DOI] [PubMed] [Google Scholar]
- Mandell, K. J., Babbin, B. A., Nusrat, A., and Parkos, C. A. (2005). Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on beta1 integrins and Rap1 activity. J. Biol. Chem. 280, 11665-11674. [DOI] [PubMed] [Google Scholar]
- Mino, A., Ohtsuka, T., Inoue, E., and Takai, Y. (2000). Membrane-associated guanylate kinase with inverted orientation (MAGI)-1/brain angiogenesis inhibitor 1-associated protein (BAP1) as a scaffolding molecule for Rap small G protein GDP/GTP exchange protein at tight junctions. Genes Cells 5, 1009-1016. [DOI] [PubMed] [Google Scholar]
- Mochizuki, N., Yamashita, S., Kurokawa, K., Ohba, Y., Nagai, T., Miyawaki, A., and Matsuda, M. (2001). Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature 411, 1065-1068. [DOI] [PubMed] [Google Scholar]
- Nagashima, K., Endo, A., Ogita, H., Kawana, A., Yamagishi, A., Kitabatake, A., Matsuda, M., Mochizuki, N. (2002). Adaptor protein Crk is required for Ephrin-B1-induced membrane ruffling and focal complex assembly of human aortic endothelial cells. Mol. Biol. Cell 13, 4231-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, P., Ruco, L., and Dejana, E. (1998). Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J. Cell Biol. 140, 1475-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwariaku, F. E., Liu, Z., Zhu, X., Nahari, D., Ingle, C., Wu, R. F., Gu, Y., Sarosi, G., and Terada, L. S. (2004). NADPH oxidase mediates vascular endothelial cadherin phosphorylation and endothelial dysfunction. Blood 104, 3214-3220. [DOI] [PubMed] [Google Scholar]
- Ohba, Y. et al. (2001). Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis. EMBO J. 20, 3333-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, L. S., Hajdo-Milasinovic, A., Zhao, J., Zwartkruis, F. J., Collard, J. G., and Bos, J. L. (2004). Rap1 regulates E-cadherin-mediated cell-cell adhesion. J. Biol. Chem. 279, 35127-35132. [DOI] [PubMed] [Google Scholar]
- Subauste, M. C., Nalbant, P., Adamson, E. D., and Hahn, K. M. (2005). Vinculin controls PTEN protein level by maintaining the interaction of the adherens junction protein beta-catenin with the scaffolding protein MAGI-2. J. Biol. Chem. 280, 5676-5681. [DOI] [PubMed] [Google Scholar]
- Tachibana, K., Nakanishi, H., Mandai, K., Ozaki, K., Ikeda, W., Yamamoto, Y., Nagafuchi, A., Tsukita, S., and Takai, Y. (2000). Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J. Cell Biol. 150, 1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, Y., Nakanishi, H., Kakunaga, S., Okabe, N., Kawakatsu, T., Shimizu, K., and Takai, Y. (2003). Role of nectin in formation of E-cadherin-based adherens junctions in keratinocytes: analysis with the N-cadherin dominant negative mutant. Mol. Biol. Cell 14, 1597-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg, T., Geiger, B., Kartenbeck, J., and Franke, W. W. (1986). Changes in membrane-microfilament interaction in intercellular adherens junctions upon removal of extracellular Ca2+ ions. J. Cell Biol. 102, 1832-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen, E. S., Worthylake, R. A., Kelly, P., Casey, P. J., Quilliam, L. A., and Burridge, K. (2005). Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J. Biol. Chem. 280, 11675-11682. [DOI] [PubMed] [Google Scholar]
- Yamada, A., Irie, K., Hirota, T., Ooshio, T., Fukuhara, A., and Takai, Y. (2005). Involvement of the annexin II-S100A10 complex in the formation of E-cadherin-based adherens junctions in Madin-Darby canine kidney cells. J. Biol. Chem. 280, 6016-6027. [DOI] [PubMed] [Google Scholar]
- Zanetti, A., Lampugnani, M. G., Balconi, G., Breviario, F., Corada, M., Lanfrancone, L., and Dejana, E. (2002). Vascular endothelial growth factor induces SHC association with vascular endothelial cadherin: a potential feedback mechanism to control vascular endothelial growth factor receptor-2 signaling. Arterioscler. Thromb. Vasc. Biol. 22, 617-622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.