Abstract
To study the consequences of depleting the major membrane phospholipid phosphatidylcholine (PC), exponentially growing cells of a yeast cho2opi3 double deletion mutant were transferred from medium containing choline to choline-free medium. Cell growth did not cease until the PC level had dropped below 2% of total phospholipids after four to five generations. Increasing contents of phosphatidylethanolamine (PE) and phosphatidylinositol made up for the loss of PC. During PC depletion, the remaining PC was subject to acyl chain remodeling with monounsaturated species replacing diunsaturated species, as shown by mass spectrometry. The remodeling of PC did not require turnover by the SPO14-encoded phospholipase D. The changes in the PC species profile were found to reflect an overall shift in the cellular acyl chain composition that exhibited a 40% increase in the ratio of C16 over C18 acyl chains, and a 10% increase in the degree of saturation. The shift was stronger in the phospholipid than in the neutral lipid fraction and strongest in the species profile of PE. The shortening and increased saturation of the PE acyl chains were shown to decrease the nonbilayer propensity of PE. The results point to a regulatory mechanism in yeast that maintains intrinsic membrane curvature in an optimal range.
INTRODUCTION
Phosphatidylcholine (PC) is an abundant glycerophospholipid present in the membranes of eukaryotic cells. Apart from being a major structural component of all organellar membranes, it serves as a reservoir of signaling molecules (Exton, 1994; Kent and Carman, 1999), and it has been implicated in apoptosis (Cui and Houweling, 2002). In the model eukaryote Saccharomyces cerevisiae, mutations in the genes encoding PC biosynthetic enzymes lead to respiratory deficiency (Griac et al., 1996), indicating that PC is important for mitochondrial function. PC was found to interact with Gut2p, the mitochondrial glycerol-3-phosphate dehydrogenase, in a photolabeling study (Janssen et al., 2002). Furthermore, the biosynthesis of PC is involved in the regulation of intracellular vesicle trafficking in yeast (reviewed in Howe and McMaster, 2001).
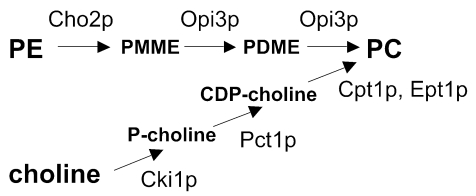
The triple methylation of phosphatidylethanolamine (PE), catalyzed by the methyltransferases Cho2p (Pem1p) and Opi3p (Pem2p), is the primary route for the synthesis of PC in yeast in the absence of exogenous choline (Carman and Henry, 1999). When choline is supplied in the growth medium, the CDP-choline pathway contributes to the net synthesis of PC (Figure 1). However, also in the absence of choline, the CDP-choline pathway contributes to PC synthesis using (phospho)choline derived from the turnover of PC (McMaster and Bell, 1994). Electrospray ionization tandem mass spectrometry (ESI-MS/MS) in combination with stable isotope labeling revealed that the two biosynthetic routes produce the PC molecular species, i.e., PC molecules with specific acyl chains, in different ratios (Boumann et al., 2003).
Figure 1.
The two biosynthetic pathways leading to PC in yeast with the enzymes indicated.
Whereas the biosynthesis of PC and its regulation have been extensively characterized (Carman and Henry, 1999), knowledge on the processes downstream of PC synthesis in yeast is fairly limited. The mechanism(s) responsible for distributing PC from its site of synthesis in the endoplasmic reticulum (ER) to the other organellar membranes is still obscure (e.g., de Kroon et al., 2003). With respect to the metabolic fates of PC, the turnover of PC by the SPO14 encoded phospholipase D yields phosphatidic acid and is essential for sporulation (Waksman et al., 1996; Rudge et al., 1998). PC was shown to be deacylated by Plb1p in vivo (Lee et al., 1994) and by Plb2p in vitro (Merkel et al., 1999). It can serve as acyl chain donor for the synthesis of triglycerides by Lro1p in vitro (Oelkers et al., 2000). In the presence of choline in the culture medium or upon raising the growth temperature to 37°C, PC synthesized by the CDP-choline route is converted to glycerophosphocholine by the enzyme Nte1p encoded by YML059c (Dowd et al., 2001; Zaccheo et al., 2004). Recently, the occurrence of remodeling by acyl chain exchange at the sn1 position of PC was demonstrated (Boumann et al., 2003).
According to the shape-structure concept of lipid polymorphism (Cullis and de Kruijff, 1979), PC has a cylindrical shape with similar cross-sectional areas for the headgroup and acyl chain parts of the molecule and prefers to organize as a bilayer, which makes it ideally suited to preserve membrane integrity. In contrast, PE and diacylglycerol (DAG), the lipid precursors of PC, are so-called type II lipids that have an overall conical molecular shape resulting from the comparatively small cross-sectional area of the polar head-group. The propensity of isolated type II lipids to organize as reversed nonlamellar structures upon hydration increases with increasing acyl chain length and unsaturation (Koynova and Caffrey, 1994). Type II lipids confer negative curvature stress to biological membranes (Gruner, 1985). Prokaryotes maintain intrinsic membrane curvature in an optimal window by adapting the membrane lipid composition in response to changes in growth conditions (reviewed by Dowhan, 1997).
We set out to investigate the consequences of lowering the cellular PC content in yeast as a novel approach to find clues regarding the specific cell biological roles of PC, the mechanism(s) of its intracellular transport, and the metabolism of PC downstream of its synthesis. Moreover, depletion of the bilayer-forming lipid PC affects the balance between bilayer- and nonbilayer-forming lipids in yeast, enabling us to study how a eukaryotic cell with its intracellular membrane trafficking processes responds to changes in membrane curvature stress. Deletion of the genes encoding Cho2p and Opi3p renders the yeast cell dependent on the supply of choline in the medium for the synthesis of PC (Summers et al., 1988; Kodaki and Yamashita, 1989). By transferring cho2opi3 cells in the exponential phase of growth to choline-free medium, net synthesis of PC was stopped, whereas growth continued for several generations. This strategy of choline deprivation was used to study the time-dependent effects of PC depletion on growth, cell viability, and lipid composition of the cho2opi3 cells.
It is reported here that yeast cells respond to depletion of the bilayer lipid PC by global changes in lipid acyl chain composition, that of the declining PC pool included. The shortening of the acyl chains and the increased saturation were most pronounced in PE. The results point to a regulatory mechanism in yeast that maintains the balance between bilayer and nonbilayer phospholipids by adjusting the acyl chain composition.
MATERIALS AND METHODS
Yeast Strains, Media, and Culture Conditions
The parental wild-type strain BY4742 (MATα hisΔ1 leu2Δ0 lys2Δ0 ura3Δ0) and the congenic cho2 strain (cho2::KanMX) were obtained from Invitrogen (Carlsbad, CA). The OPI3 gene of the cho2 strain was replaced by LEU2 using PCR-mediated one-step gene replacement to yield the cho2opi3 strain (cho2::KanMX opi3::LEU2) as described previously (Boumann et al., 2004). The SPO14 gene in the cho2opi3 strain (nucleotides -2 to 5052) was replaced with a PCR-generated construct containing the HIS5 gene of Schizosaccharamyces pombe flanked by coliphage loxP sites (kindly provided by J. Holthuis, Utrecht University, Utrecht, The Netherlands), to yield the cho2opi3spo14 strain (cho2::KanMX opi3::LEU2 spo14::HIS5). Correct integration of the HIS5 marker was verified by PCR. The cho2opi3 and cho2opi3spo14 strains were cultured under aerobic conditions at 30°C in complete vitamin-defined synthetic medium (Griac et al., 1996) containing 0.1% glucose and 2% lactate (SD-lactate) or 3% glucose (SD-glucose) as carbon source. SD-lactate medium was adjusted to pH 5.5. SD media contained 75 μM inositol and was supplemented with 1 mM choline as indicated.
Growth Phenotype
To analyze growth phenotypes, cells were cultured in YPD medium (1% yeast extract, 2% bactopeptone, and 2% glucose) to the mid-logarithmic phase of growth. Cells were collected by centrifugation, washed three times with sterile water, and adjusted to optical density (OD)600 = 1.0. Next, 10-μl aliquots of 1:10 serial dilutions were applied to solid SD-glucose medium containing 2% agar (Sigma-Aldrich, St. Louis, MO) with or without 75 μM inositol and the supplements indicated.
Assay for Inositol (Opi-) Phenotype
Overproduction of Opi- phenotypes was monitored as described previously (Greenberg et al., 1983). Briefly, yeast strains were patched onto synthetic inositol-free medium. After growth for 3 d at 30°C, the agar plates were sprayed with a suspension of the inositol auxotrophic indicator strain AID (MAT α/a ino1-13/ino1-13 ade1/ade1) and incubated for another 3 d at 30°C. Excretion of inositol was detected by a red halo of the AID strain.
Depletion of Phosphatidylcholine in cho2opi3 Cells and Labeling with (D13)-Choline
The cho2opi3 strains were cultured to the mid-logarithmic phase of growth (OD600 of ∼0.5; Hitachi 150-20 spectrophotometer) in SD medium containing 1 mM choline. Cells were collected by filtration, washed thoroughly with choline-free SD medium (30°C), and used to inoculate fresh SD medium with (C+) or without 1 mM choline (C-) to an OD600 of 0.05 (t = 0), unless indicated otherwise. At the indicated times, OD600 values were measured, and samples were taken for further analysis. To determine the molecular species composition of PC newly synthesized after 16 h of growth in C- medium, the cells were pulsed for 10 min with 0.2 mM (D13)-choline (Cambridge Isotope Laboratories, Andover, MA), added to the culture medium. In the control experiment, cells cultured for 16 h in C+ medium were collected by centrifugation and transferred to C- medium containing 0.2 mM (D13)-choline. After 10 min of labeling with (D13)-choline, cells were inactivated by adding a mixture of KCN, NaF, and NaN3 to final concentrations of 15 mM each. The cells were pelleted, homogenized, and lipid extracts were prepared.
Phospholipid Analysis
The phospholipid class composition of the cho2opi3 cells was determined by labeling with [32P]orthophosphate. Briefly, cells were precultured as described above in the presence of 10 μCi/ml [32P]orthophosphate, collected by centrifugation, washed, and transferred at t = 0 to C+ or C- medium containing 10 μCi/ml [32P]orthophosphate, to an OD600 of 0.02. Cells corresponding to 2 OD600 units were harvested at the times indicated and treated with trichloroacetic acid. Lipids were extracted (Atkinson et al., 1980), and the phospholipid classes were resolved by two-dimensional paper chromatography (Steiner and Lester, 1972) and quantified using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Mass Spectrometry (MS) Analysis of Phospholipid Molecular Species
The molecular species compositions of PE, PC, and D13-labeled PC in total lipid extracts were analyzed by ESI-MS/MS, using neutral loss scanning for m/z 141 and parent ion scanning for m/z 184 and 197, respectively, on a Quattro Ultima triple quadrupole MS instrument (Micromass, Manchester, United Kingdom) in the positive ion mode. Total lipid extracts were dissolved at 0.5 mM phospholipid phosphorus in chloroform/methanol/water [2:15:3 (vol/vol/vol)], with 1% (vol/vol) formic acid added to reduce the content of [M + Na]+ adducts. The instrument settings and the collection and quantification of the ESI-MS/MS data, including the correction for the inverse relationship between mass and signal response, were as described previously (Boumann et al., 2004).
Fatty Acid Analysis
Total lipid extracts corresponding to 400 nmol of phospholipid phosphorus were applied to high-performance thin layer chromatography (HPTLC) silica 60 plates (Merck, Darmstadt, Germany) to separate phospholipids from neutral lipids by elution with chloroform/methanol [9:1 (vol/vol)]. The phospholipid and neutral lipid fractions were eluted from the silica with chloroform/methanol (1:1) and chloroform/methanol (9:1), respectively. The eluates and total lipid extracts were transesterified by heating at 70°C for 2 h in 2.5% (vol/vol) H2SO4 in dry methanol. The fatty acid methylesters were extracted in hexane and separated on a Chrompack CP-9001 gas chromatograph (Chrompack, Middelburg, The Netherlands) using a capillary column CP-WAX58 CB at 190°C. Fatty acid methylesters were identified, and signal intensities were calibrated using a fatty acid methylester standard (Nu-Chek-Prep, Elysian, MN).
Northern Blot Analysis
The isolation of total yeast RNA and quantitative Northern blot analysis, including the preparation of radiolabeled probes for the OLE1 mRNA and the internal control PGK1, were performed as described previously (Gonzalez and Martin, 1996).
Purification of the Phospholipid Fraction and PE
Total lipid extracts were prepared from yeast spheroplasts and subjected to silica gel column chromatography using chloroform as eluent to remove neutral lipids. Subsequently, the total phospholipid fraction was eluted with chloroform/methanol [1:1 (vol/vol)]. Purification of PE from the total phospholipid fraction involved repeated silica gel column chromatography using the following solvents: 1) chloroform/methanol/water/ammonia [30:70:2:2 (vol/vol/vol/vol)], 2) chloroform/methanol/water [65:25:4 (vol/vol/v)], and 3) chloroform/methanol/water [80:20:2 (vol/vol/v)]. The final product did not contain any impurities as judged by HPTLC.
31P-NMR
Samples were prepared by hydrating phospholipid films corresponding to at least 10 μmol of phospholipid phosphorus in 0.2 ml of 100 mM NaCl, 10 mM PIPES, pH 7.4, followed by 10 freeze-thaw cycles. 31P-NMR spectra were recorded on a Bruker Avance 500-MHz spectrometer (Bruker Biospin, Karlsruhe, Germany). 31P-NMR measurements were performed at 202.5 MHz, using a single-pulse experiment with a 12.0-μs pulse, 1.2-s relaxation delay time, and broadband proton decoupling. Typically, 4000 scans were acquired. An exponential multiplication, corresponding to a line broadening of 100 Hz, was applied to the free induction decay before Fourier transformation. The temperature was increased in steps of 5°C. Samples were allowed to equilibrate for 30 min at each temperature before data acquisition.
Other Methods
To determine cell viability, yeast samples were serially diluted, spread on YPD plates, and colonies were counted after 3 d of incubation at 30°C. For preparing total lipid extracts, cell homogenates were obtained by vortexing yeast cells (∼100 OD600 units) in the presence of glass beads (Boumann et al., 2003) and subjecting them to lipid extraction (Bligh and Dyer, 1959), unless indicated otherwise. Phospholipid-to-protein ratios were determined in yeast spheroplasts. Spheroplasts were prepared by zymolyase treatment (Daum et al., 1982), subjected to phospholipid extraction, and the phospholipid phosphate content of the organic phase was quantified (Fiske and Subbarow, 1925) after destruction with perchloric acid. The protein content of yeast samples solubilized with YPER (Pierce Chemical, Rockford, IL), according to the manufacturer's instructions, was determined using the bicinchoninic acid method (Pierce Chemical) with 0.1% (wt/vol) SDS added and BSA as a standard.
RESULTS
Phenotypic Characterization of the cho2opi3 Double Deletion Mutant
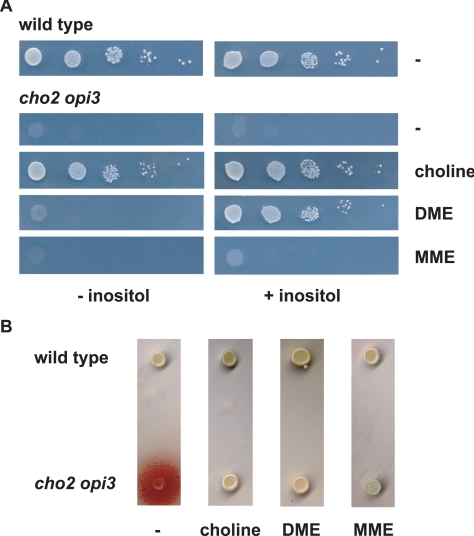
To characterize the cho2opi3 double deletion mutant constructed in the BY4742 genetic background, growth and overproduction of inositol (Opi- phenotype; Greenberg et al., 1983) were examined under several conditions (Figure 2). With choline present in the medium, cho2opi3 cells grew like wild type, irrespective of the presence of inositol. In the absence of inositol, the double mutant was strictly auxotrophic for choline, because neither 2-(methylamino)-ethanol (MME) nor N,N-dimethylamino-ethanol (DME) substrates for the synthesis of phosphatidylmonomethylethanolamine (PMME) and phosphatidyldimethylethanolamine (PDME) via the Kennedy pathways, respectively, supported growth. In the presence of inositol, DME could substitute for choline in supporting growth, whereas MME could not (Figure 2A). The Opi- phenotype of the cho2opi3 strain was suppressed by supplementing the medium with choline, DME, or MME (Figure 2B). The phenotype of the cho2opi3 double deletion mutant is comparable with that reported previously for cho2opi3 mutants (McGraw and Henry, 1989).
Figure 2.
Growth (A) and Opi- phenotypes (B) of the cho2opi3 mutant compared with wild type. (A) Cells cultured to mid-log phase in YPD medium were spotted as serial dilutions on SD-glucose plates containing 0 or 75 μM inositol and the supplements indicated at a concentration of 1 mM, and incubated at 30°C for 3 d. (B) Strains were patched on inositol-free SD-glucose agar plates containing the supplements indicated at 1 mM. Excretion of inositol results in growth of the inositol auxotrophic tester strain, as observed by a red halo around the patch.
The Effect of Choline Deprivation on Growth and Viability of cho2opi3 Cells
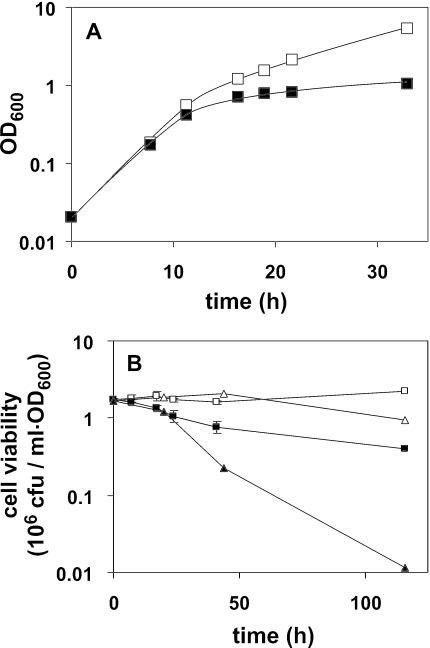
The effects of removing choline from the culture medium of cho2opi3 cells were studied in liquid media containing 75 μM inositol, to preclude any limitation on the ability of the cells to synthesize phosphatidylinositol (PI). Mid-log cho2opi3 cells were transferred within 2 min from C+ to fresh C+ or C- liquid medium by filtration and incubated at 30°C. Exponential growth continued for ∼12 h corresponding to ∼4.5 generations, irrespective of the presence of choline, and independent of lactate (Figure 3A) or glucose (our unpublished data) serving as carbon source. After 12 h, the growth in C- medium ceased compared with growth in C+ medium (Figure 3A), where the cells reached densities comparable with wild-type cells (our unpublished data).
Figure 3.
Effect of choline deprivation on the growth and viability of cho2opi3 cells cultured in liquid medium at 30°C. (A) Growth of cho2opi3 upon transfer of mid-log cells from synthetic complete lactate medium containing 1 mM choline to synthetic complete lactate medium with (□) or without 1 mM choline (▪. Growth rates were monitored by OD600. (B) Viability of cho2opi3 cells after transfer of mid-log cells from lactate- (□) or glucose-based (▵) synthetic defined medium containing 1 mM choline to the corresponding medium with (open) or without 1 mM choline (solid). Averaged values from two independent experiments are shown with the error bars representing the variation. For experimental details, see Materials and Methods.
During the first 20 h of choline deprivation, >60% of the cells maintained viability (Figure 3B) in agreement with published data (Summers et al., 1988). After 5 d, cell survival in choline-free lactate medium decreased to 25%, whereas <1% of the cells survived in choline-free glucose medium. Because of the more favorable cell survival, all following experiments were carried out using lactate-based culture media.
Effect of Choline Deprivation on the Phospholipid Class Composition of cho2opi3 Cells
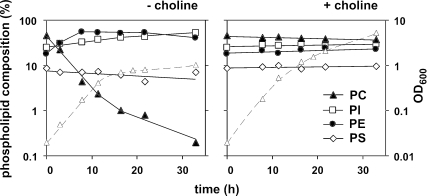
The effect of depriving cho2opi3 cells from choline on the phospholipid composition was examined by steady-state labeling with 32Pi. The results from lactate-grown cells have been plotted on a logarithmic scale for the major phospholipid classes in Figure 4. In C+ medium, the phospholipid composition of cho2opi3 cells remained essentially constant during growth, with ∼42% PC, 27% PI, 20% PE, and 9% phosphatidylserine (PS) (Figure 4, right). After removing choline from the medium, the PC content decreased with an apparent rate constant that was similar to but of opposite sign as the rate of cell proliferation (Figure 4, left). Because the overall phospholipid-to-protein ratio of the cells was not affected by choline deprivation for up to 20 h (our unpublished data), this indicates that the PC content of the cells was halved with each generation. Consistent with the above-mentioned result, growth ceased when the PC level dropped below 2%. The loss of PC was compensated for by a rapid increase in PE content from 20 to 55% during the first 8 h of choline starvation and a gradual increase in PI from 27 to 50% in 30 h (Figure 4, left).
Figure 4.
The PC content of cho2opi3 cells in choline-deficient medium is halved with every doubling of the cells, until growth ceases at a PC content below 2% of total phospholipids. Mid-log cho2opi3 cells (OD600 = 0.4) cultured in synthetic complete lactate medium containing 1 mM choline were harvested, washed, and used to inoculate fresh synthetic complete lactate medium with (right) or without 1 mM choline (left) to an OD600 of 0.02 at time 0. At the indicated times, samples were analyzed for phospholipid composition by steady-state labeling with [32P]orthophosphate as detailed in the experimental section. The contents of the four most abundant phospholipid classes are shown on a logarithmic scale (left y-axis) as percentages of the total label incorporated into glycerophospholipids and together account for >95% of the incorporated label. Growth rates (▵, dashed lines) were monitored in parallel by the OD600 (right y-axis).
During PC Depletion, the PC Pool Remaining Is Subject to Extensive Acyl Chain Remodeling
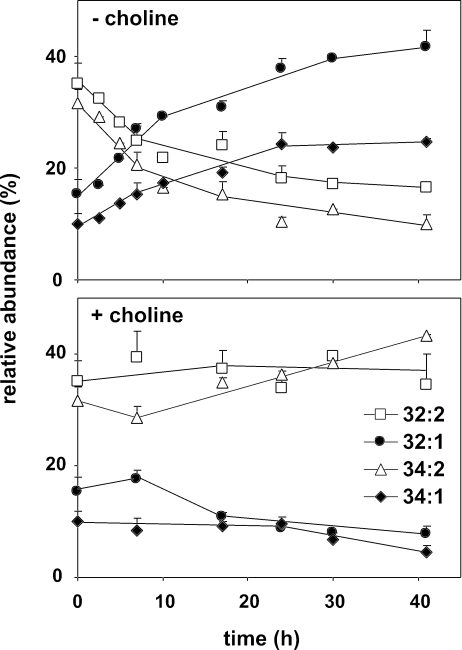
To investigate whether the existing PC pool was metabolically silent during choline starvation of cho2opi3 cells, the molecular species composition of PC was monitored by ESI-MS/MS, using parent ion scanning for m/z 184, corresponding to the phosphocholine headgroup. The most abundant PC species in yeast are 32:2, 32:1, 34:2, and 34:1 that predominantly have combinations of C16:0, C16:1, C18:0, and C18:1 acyl chains esterified at the sn1 and sn2 positions of the glycerol backbone (Wagner and Paltauf, 1994; Schneiter et al., 1999). The cho2opi3 strain cultured in C+ lactate medium exhibited a PC species profile similar to that of the parental wild-type strain (Boumann et al., 2003), with a slight increase in 34:2 at the expense of 32:1 as the cells progressed into stationary phase (Figure 5, bottom). On transfer to choline-free medium, the PC species profile changed dramatically with strong increases in the proportions of 32:1 and 34:1 at the expense of diunsaturated 32:2 and 34:2 (Figure 5, top). This result shows that during PC depletion, in the absence of any net biosynthesis of PC, the preexisting PC pool is metabolized to attain a more saturated species profile.
Figure 5.
During PC depletion in cho2opi3 cells, the acyl chain profile of the PC remaining is rearranged. Mid-log cho2opi3 cells were transferred from choline-containing medium to medium with or without 1 mM choline as indicated. At the indicated times, samples were subjected to lipid extraction and analyzed for PC species composition by ESI-MS/MS in the parent ion scan mode (m/z 184). The time-dependent changes in the PC acyl chain profile are shown for the four most abundant PC species as percentages of the total PC pool. Under the conditions tested, 32:2, 32:1, 34:2, and 34:1 account for at least 90% of total PC. Data are averaged from three independent experiments; error bars represent the SD (n ≥ 3).
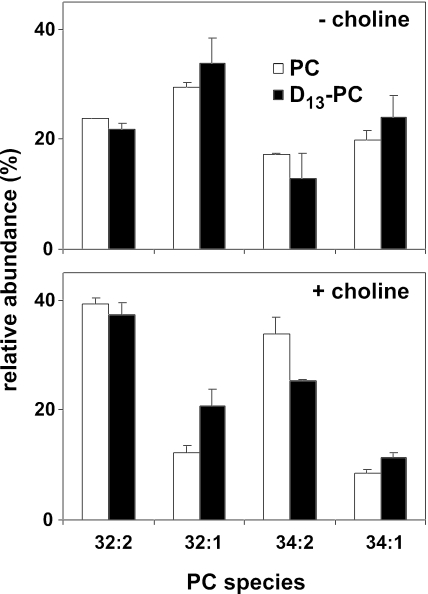
The change in PC species composition upon PC depletion could be because of PC turnover and recycling of the released (phospho)choline via the CDP-choline route, or alternatively, it could be because of exchange of the acyl chains via deacylation-reacylation, or transacylation reactions. To address the first possibility, the species composition of PC newly synthesized after 16 h of choline deprivation was determined by pulsing the cells for 10 min with (D13)-choline. Parent ion scanning at m/z 197 revealed that the species profile of the newly synthesized (D13)-PC closely resembled that of the PC pool remaining after 16 h of choline deprivation (Figure 6, top). This result is consistent with the changes in the PC species profile resulting from turnover and rein-corporation of the released (phospho)choline moiety into PC. Comparison of the profiles of newly synthesized PC between cells grown in the presence and absence of choline (black columns in Figure 6, bottom and top, respectively) implies that the species composition of the DAG pool serving as substrate for the CDP-choline pathway has been enriched in monounsaturated species in response to PC depletion.
Figure 6.
After depriving cho2opi3 cells of choline for 16 h, the acyl chain profile of newly synthesized PC (black columns) strongly resembles that of the remaining PC pool (white columns). D13-choline was added to a concentration of 0.2 mM, to cho2opi3 cells cultured without choline for 16 h. After 10 min, cells were harvested, and total lipid extracts were prepared. The PC species profiles were analyzed by ESI-MS/MS in the parent ion scan mode at m/z 197 for newly synthesized D13-PC and at m/z 184 for unlabeled PC. For comparison, cho2opi3 cells grown in the presence of 1 mM choline for 16 h were pulse labeled for 10 min with 0.2 mM D13-choline after transfer to choline-free medium and analyzed for PC species profiles (bottom). The relative abundances of the four major PC species are shown as percentages of total PC, averaged from two independent experiments with the error bars representing the variation.
Remodeling of the Remaining PC Pool Does Not Require Turnover Mediated by Spo14p
The phospholipase D encoded by the SPO14 gene presented a potential candidate responsible for the turnover of PC (Patton-Vogt et al., 1997; Sreenivas et al., 1998). To test the involvement of Spo14p, a triple cho2opi3spo14 mutant was constructed. It was verified that deletion of the SPO14 gene abolished the Ca2+-independent conversion of fluorescent 7-nitro-2-1,3-benzoxadiazol-4-yl (NBD)-labeled PC to NBD-PA (our unpublished data; Waksman et al., 1996). The effect of 24 h of choline deprivation on the PC species profile of the cho2opi3spo14 mutant was indistinguishable from that of the cho2opi3 mutant (compare Figures 7 and 5), demonstrating that SPO14 is not required for the remodeling of PC.
Figure 7.
PC depletion in cho2opi3spo14 cells reveals that PC turnover mediated by Spo14p (Pld1p) is not required for the changes in the PC species profile occurring during PC depletion. The distribution of the four major PC species of cho2opi3spo14 cells after 24 h of culturing in the absence or presence of choline is shown. Experimental conditions were as described in the legend of Figure 5.
Lipid Class-dependent Changes in Acyl Chain Composition in Response to PC Depletion
The PC depletion-induced changes in the species profiles of PC and its lipid precursor DAG may reflect a global change in the cellular fatty acid composition of cho2opi3 cells. To address this possibility, cho2opi3 cells were cultured in C+ or C- medium and examined for their fatty acid content by gas chromatography of the fatty acid methyl esters (Table 1). The acyl chain composition of cho2opi3 cells cultured in C+ medium was comparable with that of wild-type yeast (Daum et al., 1999). After 20 h of choline deprivation, the content of unsaturated acyl chains has decreased by some 10%, whereas that of C16 acyl chains has increased by 7% at the expense of C18 chains in total lipid extracts, compared with cells cultured in C+ medium. Examination of the acyl chain compositions of the separate phospholipid and neutral lipid fractions revealed that the former is more strongly affected by PC depletion, with the contents of C16:0 and C18:0 more than doubling at the expense of C18:1 and, to a lesser extent, C16:1 (Table 1). In both phospholipid and neutral lipid fractions similar increases in the content of C16 chains were apparent. The fatty acid compositions (Table 1) are in agreement with neutral lipids and phospholipids contributing more or less equally to the total cellular acyl chain content in the late log phase of growth (Tung et al., 1991; Sandager et al., 2002).
Table 1.
Acyl chain composition (mol%) of a total lipid extract (TLE) and of the phospholipid and neutral lipid fractions of cho2opi3 cells after 20 h of growth in the presence or absence of 1 mM choline
| TLE
|
Phospholipids
|
Neutral lipids
|
||||
|---|---|---|---|---|---|---|
| Fatty acid | + Choline | − Choline | + Choline | − Choline | + Choline | − Choline |
| C12:0 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.9 ± 0.4 | 1.0 ± 0.1 | 2.6 ± 0.4 | 1.5 ± 0.3 |
| C14:0 | 1.1 ± 0.1 | 1.7 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.3 | 2.1 ± 0.6 | 2.3 ± 0.5 |
| C16:0 | 10.6 ± 0.3 | 18.3 ± 0.4 | 9.6 ± 0.3 | 24.3 ± 0.6 | 12.8 ± 0.7 | 16.1 ± 0.4 |
| C16:1 | 52.5 ± 0.4 | 51.8 ± 0.5 | 51.2 ± 1.1 | 45.5 ± 1.3 | 47.4 ± 0.5 | 52.7 ± 0.8 |
| C18:0 | 4.0 ± 0.1 | 5.7 ± 0.2 | 3.0 ± 0.2 | 6.4 ± 0.5 | 5.9 ± 0.3 | 6.0 ± 0.2 |
| C18:1 | 30.5 ± 0.3 | 21.5 ± 0.4 | 33.2 ± 1.4 | 21.4 ± 0.2 | 29.3 ± 1.3 | 21.4 ± 0.6 |
| % Unsaturated | 83 | 73 | 84 | 67 | 77 | 74 |
| % C16 | 63 | 70 | 61 | 70 | 60 | 69 |
Values represent means ± SD of three independent experiments. For experimental details, see Materials and Methods.
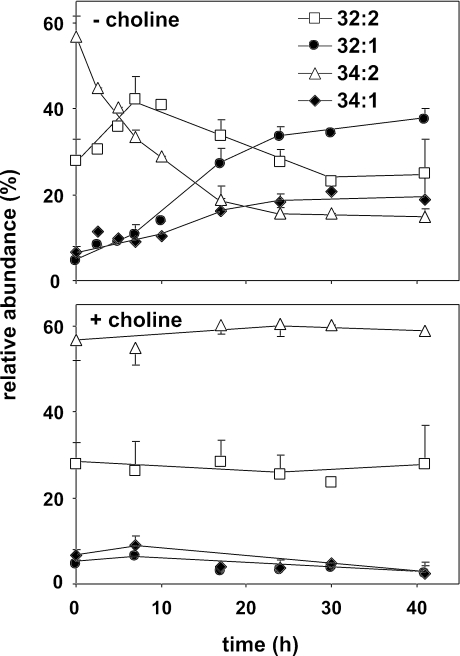
To further investigate the changes in the phospholipid species, the species profile of PE was monitored by ESI-MS/MS using neutral loss scanning for m/z 141. PE rapidly became the most abundant phospholipid class in cho2opi3 cells during choline deprivation, accounting for >50% of the total phospholipids (Figure 4). The PE species composition of cho2opi3 cells cultured in the presence of choline is similar to that of the parental wild type, with 34:2 the most prominent PE species, followed by 32:2, and with relatively low levels of monounsaturated 32:1 and 34:1 (Boumann et al., 2003). In C+ medium the PE profile is hardly affected by the growth phase (Figure 8, bottom). In response to choline deprivation, during the first 7 h, the contents of 32:2 and the monounsaturated species increased at the expense of 34:2 (Figure 8, top). Subsequently, the contents of both diunsaturated PE species decreased, whereas the contents of 32:1 and 34:1 PE strongly increased reaching levels 7 and 2.5 times higher than under C+ conditions, respectively (Figure 8).
Figure 8.
PC depletion in cho2opi3 cells is accompanied by shortening and increasing saturation of the acyl chains of PE. Experimental conditions were as described in the legend of Figure 5. The PE species composition was analyzed by ESI-MS/MS in the neutral loss mode (m/z 141). The time-dependent changes in the PE acyl chain profile are shown for the four most abundant PE species as percentages of the total PE pool. Under the conditions tested, 32:2, 32:1, 34:2, and 34:1 account for at least 94% of total PE. Data are averaged from three independent experiments; the error bars represent the SD (n ≥ 3).
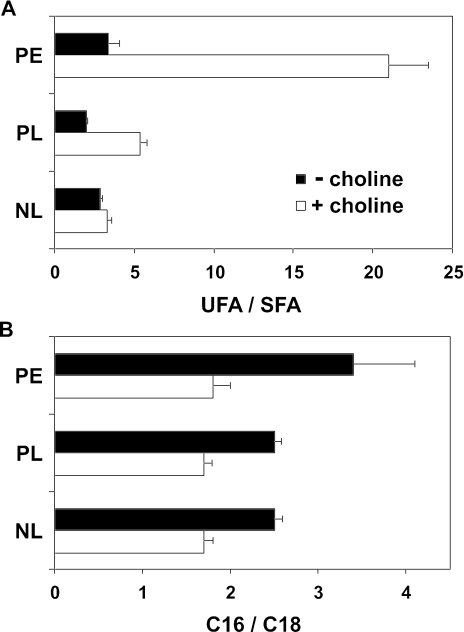
As summarized in Figure 9, after 20 h of PC depletion the shift toward shorter (C16) acyl chains was stronger in PE than in the total phospholipid or neutral lipid fractions. Furthermore, the increase in saturation degree was largest in PE and larger in the phospholipid than in the neutral lipid fraction. The combined results indicate lipid class-specific adaptations in acyl chain composition in response to PC depletion.
Figure 9.
The increase in lipid acyl chain saturation and the shortening of the acyl chains accompanying PC depletion in cho2opi3 cells are most pronounced in PE. The ratios of unsaturated to saturated acyl chains (unsaturated fatty acid/saturated fatty acid, UFA/SFA) (A) and the ratios of 16-carbon atom chain length to 18-carbon atom chain length (C16/C18) were calculated for the neutral lipid (NL) and the phospholipid fractions (PL), and for PE, isolated from cho2opi3 cells after 20 h (NL, PL; data taken from Table 1) and 17 h (PE; data from Figure 8) of growth in the presence or absence of 1 mM choline. Ratios are averaged from three independent experiments with the error bars representing the SD.
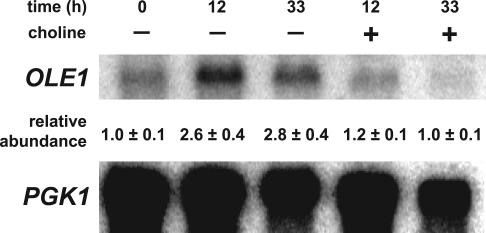
PC Depletion Is Accompanied by a Rise in the Level of OLE1 mRNA
The increase in saturation of total fatty acids during PC depletion (Table 1) suggests that the activity of the only fatty acid Δ-9 desaturase in yeast encoded by the essential OLE1 gene (Stukey et al., 1990) is reduced. Regulation of OLE1 gene expression by nutrient fatty acids has been extensively investigated and occurs via transcriptional and mRNA stability controls (McDonough et al., 1992; Gonzalez and Martin, 1996; Hoppe et al., 2000; Kandasamy et al., 2004). To find a first clue with regard to the apparent change in Ole1p activity in choline-deprived cho2opi3 cells, the OLE1 mRNA levels were examined in Northern blots. Whereas in C+ medium the OLE1 mRNA level remained constant during growth, after 12 h of choline deprivation the OLE1 mRNA level had increased 2.6-fold, and this level was maintained for at least another 20 h (Figure 10). We conclude from these data that OLE1 gene expression is regulated under these conditions by one or more mechanisms that act to compensate for the effects produced by PC depletion.
Figure 10.
Northern blot analysis reveals an increased level of OLE1 mRNA under conditions of PC depletion. A Northern blot of total RNA isolated from the cho2opi3 strain cultured in the presence or absence of 1 mM choline for the times indicated was probed with OLE1- and PGK1-specific probes. Phosphorimaging data were normalized using the PGK1 transcript as an internal standard, yielding values of the relative abundance of the OLE1 transcript that were averaged from two independent experiments with the errors representing the variation.
The Rise in PE Level Is Compensated for by an Increase in the Bilayer-to-Nonbilayer Transition Temperature of PE
The increased PE content resulting from PC depletion is expected to enhance the intrinsic membrane negative curvature stress. The accompanying shortening and increased saturation of the acyl chains of PE (Figures 8 and 9) could serve as a compensatory mechanism reducing membrane negative curvature. The tendency of PE to adopt nonlamellar phase is a measure for its contribution to membrane negative curvature and can be measured by 31P-NMR, the method of choice for studying aggregate structures of phospholipids in aqueous dispersion. Therefore, the phase behavior of PE purified from cho2opi3 cells cultured for 16 h in the absence or presence of choline was compared by 31P-NMR.
As shown in Figure 11, at 10°C the 31P-NMR signals of both PE samples showed line shapes with a high-field peak and a low-field shoulder and a chemical shift anisotropy around -40 ppm, characteristic for PE in the lamellar liquid crystalline phase (Cullis and de Kruijff, 1978). The spectrum of the PE sample from the cells cultured with choline also revealed a minor superimposed isotropic component. At 20°C, the isotropic component slightly increased and an additional component occurred in the spectrum, indicating the onset of a phase transition. At 30°C the shift to a line shape with a reversed asymmetry and reduced line width, characteristic for the nonlamellar hexagonal HII phase, was complete. The superimposed signal intensity at the isotropic position originates from motional averaging of a minor fraction of the lipids due to the formation of small vesicles or cubic-like phase, which is often observed in systems undergoing a bilayer to hexagonal HII phase transition. The PE sample from the PC-depleted cells maintained lamellar phase up to 45°C (our unpublished data). Here, the phase transition to nonlamellar phase was first visible at 50°C (Figure 11), and it was completed at 55°C (our unpublished data). The combined results show that the lamellar to nonlamellar phase transition temperature of PE increased by ∼30°C in response to PC depletion.
Figure 11.
PE extracted from choline-deprived cho2opi3 cells exhibits a higher bilayer to hexagonal HII phase transition temperature than PE from cho2opi3 cells cultured in the presence of choline.31P-NMR spectra of dispersions in buffer of PE purified from cho2opi3 cells cultured for 16 h in the presence or absence of choline were recorded at increasing temperatures as indicated and as detailed in Materials and Methods.
The phase behavior of the total phospholipid extracts of cho2opi3 cells cultured in the absence or presence of choline for 16 h was also investigated by 31P-NMR. The dispersions of both phospholipid extracts maintained lamellar liquid crystalline phase up to a temperature of 50°C (our unpublished data). The data indicate that yeast counterbalances the rising level of the type II lipid PE by increasing the lamellar-to-nonlamellar phase transition temperature of PE.
DISCUSSION
The effects of depleting the major membrane phospholipid PC on cell growth, viability, and lipid composition have been studied in yeast. After cessation of de novo synthesis of PC upon transferring cho2opi3 cells to choline-free medium, cell growth remained unaffected for four to five generations, providing a window for examining the effects in exponentially growing cells. The main findings were that the PC remaining during choline deprivation is subject to extensive acyl chain remodeling and that PC depletion induces an overall shift to shorter and more saturated acyl chains that is most pronounced in PE. The implications of the results for PC function, PC turnover, and the regulation of membrane lipid composition in yeast will be discussed.
In the absence of choline or a substrate replacing choline, growth of cho2opi3 cells ceased four to five generations after deprivation of choline, as was observed previously (Kodaki and Yamashita, 1989; Howe et al., 2002). Subsequently, a gradual, time-dependent loss of cell viability was observed, which was more severe in medium containing the fermentable carbon source glucose than in medium containing nonfermentable lactate. Possibly, the low PC levels interfere with the diauxic shift, leading to increased cell death. Previously, a virtually complete loss of cell viability within 23 h was reported upon inhibition of PC synthesis in yeast cells cultured on glucose at 37°C, whereas cells cultured at 25°C stayed viable (Howe et al., 2002). The increased cell death was attributed to the enhanced turnover of PC at 37°C via deacylation (Dowd et al., 2001). Even in the presence of choline, cho2opi3 cells grow very slowly at 37°C compared with wild-type cells (our unpublished data; McGraw and Henry, 1989), most likely because of increased PC turnover.
In the absence of choline, the PC content of the cho2opi3 cells was halved with each division cycle, whereas the total phospholipid content of the cells remained constant. Under these conditions, the remaining PC pool was turned over with an apparent t1/2 in the order of 10 h to yield PC with a more saturated acyl chain composition (Figure 5). Compared with the half-life values of PC in yeast at 30°C determined in radiolabeling experiments (Patton-Vogt et al., 1997; Dowd et al., 2001), the rate of PC turnover in the cho2opi3 cells may seem fast. However, it should be realized that the occurrence of acyl chain exchange could not be detected in these studies, because the isotope labels were present in the glycerophosphocholine moiety of PC.
The acyl chain remodeling of PC could be because of several metabolic conversions. The resemblance of the species profiles of newly synthesized and existing PC pools after PC depletion (Figure 6) suggests recycling of (phospho)choline derived from PC degradation to PC via the CDP-choline pathway. In this scenario, the yeast SPO14 (PLD1) gene product that is known to hydrolyze PC (Waksman et al., 1996) was shown not to be required (Figure 7). Involvement of the only other, Ca2+-dependent phospholipase D activity in yeast that so far escaped identification at the gene level, is unlikely because this enzyme has a strong substrate preference for PE over PC (Mayr et al., 1996; Waksman et al., 1997). In the absence of any known PC-degrading, phospholipase C activity in yeast, this leaves the possibility of PC deacylation by B type phospholipases, followed by degradation of glycerophosphocholine to choline mediated by Gde1p (Fernandez-Murray and McMaster, 2005; Fisher et al., 2005). Candidate phospholipase Bs capable of degrading PC include Plb1p (Lee et al., 1994), Plb2p (Merkel et al., 1999), and Nte1p (Zaccheo et al., 2004). In the alternative scenario, PC could be remodeled independently of the CDP-choline pathway by acyl chain exchange via de- and reacylation, or transacylation reactions. The occurrence of PC remodeling by acyl chain exchange was recently demonstrated in live yeast cells (Boumann et al., 2003).
The change in the PC species profile was found to reflect a global shift in the composition of the fatty acid content of the cells. To our knowledge, this is the first report of an isothermal shift in acyl chain composition in yeast with an intact fatty acid synthesis machinery, and cultured aerobically in the absence of any fatty acid supplements. As the PC content of the cells decreased, the ratios of C16 over C18 acyl chains and that of saturated over unsaturated acyl chains increased. The changes were larger in the phospholipid than in the neutral lipid fraction and were most pronounced in PE that becomes the most abundant phospholipid during PC depletion.
Changes in acyl chain composition may serve to adjust membrane fluidity, membrane thickness, or intrinsic membrane curvature. The combination of shortening and increased saturation of acyl chains is not compatible with unidirectional changes in membrane fluidity or thickness. However, it is consistent with regulation of intrinsic membrane curvature maintaining the balance between bilayer and nonbilayer lipids. Because bilayer lipids have a different intrinsic radius of curvature compared with nonbilayer lipids, changes in their relative proportions affect membrane properties, in particular the membrane's intrinsic curvature (Gruner, 1985). Whereas PC is considered a bilayer-forming lipid, PE can form nonbilayer structures under physiological conditions (Cullis and de Kruijff, 1979). The changes in the acyl chain composition of PE were found to increase the bilayer to hexagonal HII phase transition temperature of this type II phospholipid (Figure 11), reflecting a reduction of the contribution of the PE molecules to negative membrane curvature. The increase in transition temperature is in agreement with physicochemical studies showing that the transition temperature from liquid crystalline to hexagonal HII phase increases as the acyl chains of PE become shorter, and more drastically, as the acyl chains become saturated (Koynova and Caffrey, 1994). The results provide evidence for a novel regulatory mechanism enabling yeast to counteract the increased negative membrane curvature stress conferred by increasing PE levels by reducing the propensity of the PE molecules to adopt the HII phase.
Polymorphic regulation of membrane lipid composition in response to changes in growth conditions is well established in prokaryotes (reviewed in Dowhan, 1997). Comparably to the response of yeast to rising PE levels, Escherichia coli with a PE content of 70% of total glycerophospholipids adapts to rising growth temperatures by a shortening and increased saturation of its acyl chains (Morein et al., 1996). The purpose of polymorphic regulation of membrane lipid composition is to maintain the intrinsic membrane curvature within certain limits to provide an optimum environment for the functioning of membrane proteins and to facilitate dynamic membrane processes such as fusion and fission (van den Brink-van der Laan et al., 2004). In this context, it should be noted that reduction of the PE content in yeast is deleterious for growth, particularly on nonfermentable carbon sources (Birner et al., 2001).
Interestingly, the results from previous studies in yeast, in which phospholipid class compositions, the PE/PC ratios in particular, were found to change in response to fatty acid supplements in the culture medium, support the concept of yeast regulating the relative proportions of bilayer and nonbilayer lipids within a certain window. A strong increase in PE content at the expense of PC was observed when wild-type cells were cultured in the presence of short chain C14:1 (Schneiter et al., 2000), in line with the HII phase propensity of PE declining with decreasing acyl chain length. A yeast fatty acid auxotroph cultured in the presence of trans C16:1 was found to have an increased PE/PC ratio compared with the same strain cultured with cis C16:1 (Tung et al., 1991), consistent with the bilayer to HII phase transition temperatures for PEs with trans unsaturated acyl chains being higher than for PEs with cis unsaturated acyl chains. Similarly, polymorphic regulation of membrane lipid composition may explain why yeast cells cultured in the presence of C18:2 have an almost threefold lower PE/PC ratio than cells cultured in the presence of C16:0 (Mizoguchi and Hara, 1997).
The model eukaryote S. cerevisiae is very tolerant with regard to its membrane lipid composition. Of the major membrane phospholipids, PI (Nikawa et al., 1987) and PE (Birner et al., 2001; Storey et al., 2001) are essential in yeast, whereas PS (Atkinson et al., 1980) and cardiolipin (Jiang et al., 1997) are not. Contrary to the situation in mammals (Waite and Vance, 2004), PC is not essential in yeast either. In cho2opi3 cells, PC could be replaced by PDME, its precursor in the PE methylation pathway, provided that the culture medium contained inositol, substrate for the synthesis of PI (Figure 2; McGraw and Henry, 1989). In contrast PMME failed to compensate for the loss of PC irrespective of the presence of inositol. These findings lend further support to the importance of balancing bilayer and nonbilayer lipids in yeast membranes, given that the propensities of PMME and PDME to adopt nonbilayer structures are intermediate between those of PE and PC (Gagné et al., 1985) and that PI is a bilayer-forming phospholipid (Nayar et al., 1982). PDME in combination with an increased PI level may compensate for the increased membrane curvature stress resulting from the loss of PC, whereas PDME by itself, or PMME with or without extra PI, do not. A recent study demonstrated that the nonnatural, nonbilayer forming phospholipid phosphatidylpropanolamine could substitute for the methylated phospholipids in a cho2opi3 mutant altogether (Choi et al., 2004). In line with the proposed polymorphic regulation, the lipid composition of cells cultured in the presence of propanolamine revealed a strongly increased PI level, and a shift to shorter acyl chains in PE compared with the choline-grown cells; however, the degree of PE saturation was not affected.
How does yeast accomplish the adaptations in acyl chain composition in response to PC depletion? Shortening of the acyl chains could result from an earlier release of newly synthesized fatty acids from the fatty acid synthase and/or by reduced activity of the elongases Elo1p and/or Elo2p that reportedly have the ability to elongate C16-acyl-CoA to C18-acyl-CoA (Schneiter et al., 2000; Rossler et al., 2003). The decreased unsaturation must be because of a reduction of the activity of Ole1p, the only fatty acid desaturase in yeast. Interestingly, the reduction in Ole1p activity is accompanied by an increase in the level of OLE1 mRNA (Figure 10). This reduced Ole1p enzyme activity could be a consequence of the changing lipid composition and may reflect a mechanism by which the OLE1 transcript level is up-regulated to compensate for the lower desaturase activity. OLE1 has been previously shown to be regulated by a complex set of physiological and nutrient factors (including molecular oxygen, nutrient fatty acids, cobalt, and iron chelators) at the levels of transcription and mRNA stability (Stukey et al., 1990; Hoppe et al., 2000; Chellappa et al., 2001; Vasconcelles et al., 2001; Kandasamy et al., 2004). Although we speculate that the OLE1 transcript levels are up-regulated in phosphatidylcholine-deprived cells to compensate for the lower desaturase activity, it is not clear whether the increased mRNA levels result from an increase in the relative rate of OLE1 transcription or through a combination of transcriptional and mRNA stability controls.
Superimposed on the changes in fatty acid synthesis, regulatory mechanisms must be in place to afford the lipid class specific adaptations in acyl chain composition. Somehow the changing membrane lipid composition must be conveyed to the fatty acid and lipid-synthesizing machinery. The membrane-associated enzymes involved may directly sense and respond to changes in membrane curvature stress or in the membrane lateral pressure profile (Attard et al., 2000; van den Brink-van der Laan et al., 2004). Alternatively, sensor proteins in the membrane may transmit signals affecting lipid synthesis, as has been proposed for Spt23p (Hoppe et al., 2000; Chellappa et al., 2001). Whether Spt23p or its homologue Mga2p, known activators of Ole1 transcription (Hoppe et al., 2000), is involved in sensing and transmitting changes in membrane curvature stress will be subject of future research. It is of interest to consider the effect of varying the growth temperature on the acyl chain composition of yeast. As the growth temperature is decreased from 37 to 10°C, the average acyl chain length decreases, whereas the degree of unsaturation is unaffected (Suutari et al., 1990). In response to heat shock, yeast slightly decreases the degree of acyl chain unsaturation (Swan and Watson, 1997). The sensing mechanism(s) and regulatory network(s) underlying these adaptations may in part overlap with those responding to PC depletion.
In conclusion, depletion of PC in yeast unveiled changes in acyl chain composition that are fully consistent with regulation of intrinsic membrane curvature, which so far has only been documented in prokaryotes. Because PC depletion is unlikely to occur in yeast in its natural habitat, we speculate that the polymorphic regulation of membrane lipid composition is a reflection of a versatile mechanism to maintain optimal membrane function in changing environments.
Choline deprivation of cho2opi3 cells provides a promising experimental system for identifying the factors involved in regulating and accomplishing the adaptations in acyl chain composition.
Acknowledgments
We thank Ineke Rood, Rutger Staffhorst, and Drs. Manuel Villa-Garcia, Thomas Nyholm, and Antoinette Killian for experimental support and fruitful discussions. This work was supported by The Netherlands Division of Chemical Sciences with financial aid from the Netherlands Organization for Scientific Research, and by the National Institutes of Health Grant GM-19629 (to S.A.H.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-04-0344) on December 7, 2005.
Abbreviations used: DAG, diacylglycerol; DME, N,N-dimethylamino-ethanol; ESI-MS/MS, electrospray ionization tandem mass spectrometry; MME, 2-(methylamino)-ethanol; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine.
References
- Atkinson, K., Fogel, S., and Henry, S. A. (1980). Yeast mutant defective in phosphatidylserine synthesis. J. Biol. Chem. 255, 6653-6661. [PubMed] [Google Scholar]
- Attard, G. S., Templer, R. H., Smith, W. S., Hunt, A. N., and Jackowski, S. (2000). Modulation of CTP:phosphocholine cytidylyltransferase by membrane curvature elastic stress. Proc. Natl. Acad. Sci. USA 97, 9032-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birner, R., Burgermeister, M., Schneiter, R., and Daum, G. (2001). Roles of phosphatidylethanolamine and of its several biosynthetic pathways in Saccharomyces cerevisiae. Mol. Biol. Cell 12, 997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh, E. G., and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911-917. [DOI] [PubMed] [Google Scholar]
- Boumann, H. A., Chin, P. T., Heck, A. J., De Kruijff, B., and De Kroon, A. I. (2004). The yeast phospholipid N-methyltransferases catalyzing the synthesis of phosphatidylcholine preferentially convert di-C 16, 1 substrates both in vivo and in vitro. J. Biol. Chem. 279, 40314-40319. [DOI] [PubMed] [Google Scholar]
- Boumann, H. A., Damen, M. J., Versluis, C., Heck, A. J., de Kruijff, B., and de Kroon, A. I. (2003). The two biosynthetic routes leading to phosphatidylcholine in yeast produce different sets of molecular species. Evidence for lipid remodeling. Biochemistry 42, 3054-3059. [DOI] [PubMed] [Google Scholar]
- Carman, G. M., and Henry, S. A. (1999). Phospholipid biosynthesis in the yeast Saccharomyces cerevisiae and interrelationship with other metabolic processes. Prog. Lipid Res. 38, 361-399. [DOI] [PubMed] [Google Scholar]
- Chellappa, R., Kandasamy, P., Oh, C. S., Jiang, Y., Vemula, M., and Martin, C. E. (2001). The membrane proteins, Spt23p and Mga2p, play distinct roles in the activation of Saccharomyces cerevisiae OLE1 gene expression. Fatty acid-mediated regulation of Mga2p activity is independent of its proteolytic processing into a soluble transcription activator. J. Biol. Chem. 276, 43548-43556. [DOI] [PubMed] [Google Scholar]
- Choi, J. Y., Martin, W. E., Murphy, R. C., and Voelker, D. R. (2004). Phosphatidylcholine and N-methylated phospholipids are non-essential in Saccharomyces cerevisiae. J. Biol. Chem. 279, 42321-42330. [DOI] [PubMed] [Google Scholar]
- Cui, Z., and Houweling, M. (2002). Phosphatidylcholine and cell death. Biochim. Biophys. Acta 1585, 87-96. [DOI] [PubMed] [Google Scholar]
- Cullis, P. R., and de Kruijff, B. (1978). The polymorphic phase behaviour of phosphatidylethanolamines of natural and synthetic origin. Biochim. Biophys. Acta 513, 31-42. [DOI] [PubMed] [Google Scholar]
- Cullis, P. R., and de Kruijff, B. (1979). Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta 559, 399-420. [DOI] [PubMed] [Google Scholar]
- Daum, G., Bohni, P. C., and Schatz, G. (1982). Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J. Biol. Chem. 257, 13028-13033. [PubMed] [Google Scholar]
- Daum, G., et al. (1999). Systematic analysis of yeast strains with possible defects in lipid metabolism. Yeast 15, 601-614. [DOI] [PubMed] [Google Scholar]
- de Kroon, A. I., Koorengevel, M. C., Vromans, T. A., and de Kruijff, B. (2003). Continuous equilibration of phosphatidylcholine and its precursors between endoplasmic reticulum and mitochondria in yeast. Mol. Biol. Cell 14, 2142-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd, S. R., Bier, M. E., and Patton-Vogt, J. L. (2001). Turnover of Phosphatidylcholine in Saccharomyces cerevisiae. The role of the CDP-choline pathway. J. Biol. Chem. 276, 3756-3763. [DOI] [PubMed] [Google Scholar]
- Dowhan, W. (1997). Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu. Rev. Biochem. 66, 199-232. [DOI] [PubMed] [Google Scholar]
- Exton, J. H. (1994). Phosphatidylcholine breakdown and signal transduction. Biochim. Biophys. Acta 1212, 26-42. [DOI] [PubMed] [Google Scholar]
- Fernandez-Murray, J. P., and McMaster, C. R. (2005). Glycerophosphocholine catabolism as a new route for choline formation for phosphatidylcholine synthesis by the Kennedy pathway. J. Biol. Chem. 280, 38290-38296. [DOI] [PubMed] [Google Scholar]
- Fisher, E., Almaguer, C., Holic, R., Griac, P., and Patton-Vogt, J. (2005). Glycerophosphocholine-dependent growth requires Gde1p (YPL110c) and Git1p in Saccharomyces cerevisiae. J. Biol. Chem. 280, 36110-36117. [DOI] [PubMed] [Google Scholar]
- Fiske, L. M., and Subbarow, Y. (1925). The colorimetric determination of phosphorus. J. Biol. Chem. 66, 375-389. [Google Scholar]
- Gagné, J., Stamatatos, L., Diacovo, T., Hui, S. W., Yeagle, P. L., and Silvius, J. R. (1985). Physical properties and surface interactions of bilayer membranes containing N-methylated phosphatidylethanolamines. Biochemistry 24, 4400-4408. [DOI] [PubMed] [Google Scholar]
- Greenberg, M. L., Klig, L. S., Letts, V. A., Loewy, B. S., and Henry, S. A. (1983). Yeast mutant defective in phosphatidylcholine synthesis. J. Bacteriol. 153, 791-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griac, P., Swede, M. J., and Henry, S. A. (1996). The role of phosphatidylcholine biosynthesis in the regulation of the INO1 gene of yeast. J. Biol. Chem. 271, 25692-25698. [DOI] [PubMed] [Google Scholar]
- Gonzalez, C. I., and Martin, C. E. (1996). Fatty acid-responsive control of mRNA stability. Unsaturated fatty acid-induced degradation of the Saccharomyces cerevisiae OLE1 transcript. J. Biol. Chem. 271, 25801-25809. [DOI] [PubMed] [Google Scholar]
- Gruner, S. M. (1985). Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc. Natl. Acad. Sci. USA 82, 3665-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe, T., Matuschewski, K., Rape, M., Schlenker, S., Ulrich, H. D., and Jentsch, S. (2000). Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell 102, 577-586. [DOI] [PubMed] [Google Scholar]
- Howe, A. G., and McMaster, C. R. (2001). Regulation of vesicle trafficking, transcription, and meiosis: lessons learned from yeast regarding the disparate biologies of phosphatidylcholine. Biochim. Biophys. Acta 1534, 65-77. [DOI] [PubMed] [Google Scholar]
- Howe, A. G., Zaremberg, V., and McMaster, C. R. (2002). Cessation of growth to prevent cell death due to inhibition of phosphatidylcholine synthesis is impaired at 37 degrees C in Saccharomyces cerevisiae. J. Biol. Chem. 277, 44100-44107. [DOI] [PubMed] [Google Scholar]
- Janssen, M. J., van Voorst, F., Ploeger, G. E., Larsen, P. M., Larsen, M. R., de Kroon, A. I., and de Kruijff, B. (2002). Photolabeling identifies an interaction between phosphatidylcholine and glycerol-3-phosphate dehydrogenase (Gut2p) in yeast mitochondria. Biochemistry 41, 5702-5711. [DOI] [PubMed] [Google Scholar]
- Jiang, F., Rizavi, H. S., and Greenberg, M. L. (1997). Cardiolipin is not essential for the growth of Saccharomyces cerevisiae on fermentable or non-fermentable carbon sources. Mol. Microbiol. 26, 481-491. [DOI] [PubMed] [Google Scholar]
- Kandasamy, P., Vemula, M., Oh, C.-S., Chellappa, R., and Martin, C. E. (2004). Regulation of unsaturated fatty acid biosynthesis in Saccharomyces. The endoplasmic reticulum membrane protein, Mga2p, a transcription activator of the OLE1 gene, regulates the stability of the OLE1 mRNA through exosome-mediated mechanisms. J. Biol. Chem. 279, 36586-36592. [DOI] [PubMed] [Google Scholar]
- Kent, C., and Carman, G. M. (1999). Interactions among pathways for phosphatidylcholine metabolism, CTP synthesis and secretion through the Golgi apparatus. Trends Biochem. Sci. 24, 146-150. [DOI] [PubMed] [Google Scholar]
- Kodaki, T., and Yamashita, S. (1989). Characterization of the methyltransferases in the yeast phosphatidylethanolamine methylation pathway by selective gene disruption. Eur. J. Biochem. 185, 243-251. [DOI] [PubMed] [Google Scholar]
- Koynova, R., and Caffrey, M. (1994). Phases and phase transitions of the hydrated phosphatidylethanolamines. Chem. Phys. Lipids 69, 1-34. [DOI] [PubMed] [Google Scholar]
- Lee, K. S., Patton, J. L., Fido, M., Hines, L. K., Kohlwein, S. D., Paltauf, F., Henry, S. A., and Levin, D. E. (1994). The Saccharomyces cerevisiae PLB1 gene encodes a protein required for lysophospholipase and phospholipase B activity. J. Biol. Chem. 269, 19725-19730. [PubMed] [Google Scholar]
- Mayr, J. A., Kohlwein, S. D., and Paltauf, F. (1996). Identification of a novel, Ca2+-dependent phospholipase D with preference for phosphatidylserine and phosphatidylethanolamine in Saccharomyces cerevisiae. FEBS Lett. 393, 236-240. [DOI] [PubMed] [Google Scholar]
- McDonough, V. Stukey, J. E., and Martin, C. E. (1992). Specificity of unsaturated fatty acid-regulated expression of the Saccharomyces cerevisiae OLE1 gene. J. Biol. Chem. 267, 5931-5936. [PubMed] [Google Scholar]
- McGraw, P., and Henry, S. A. (1989). Mutations in the Saccharomyces cerevisiae opi3 gene: effects on phospholipid methylation, growth and cross-pathway regulation of inositol synthesis. Genetics 122, 317-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster, C. R., and Bell, R. M. (1994). Phosphatidylcholine biosynthesis in Saccharomyces cerevisiae. Regulatory insights from studies employing null and chimeric sn-1,2-diacylglycerol choline- and ethanolaminephosphotransferases. J. Biol. Chem. 269, 28010-28016. [PubMed] [Google Scholar]
- Merkel, O., Fido, M., Mayr, J. A., Pruger, H., Raab, F., Zandonella, G., Kohlwein, S. D., and Paltauf, F. (1999). Characterization and function in vivo of two novel phospholipases B/lysophospholipases from Saccharomyces cerevisiae. J. Biol. Chem. 274, 28121-28127. [DOI] [PubMed] [Google Scholar]
- Mizoguchi, H., and Hara, S. (1997). Ethanol-induced alterations in lipid composition of Saccharomyces cerevisiae in the presence of exogenous fatty acid. J. Ferment. Bioeng. 83, 12-16. [Google Scholar]
- Morein, S., Andersson, A., Rilfors, L., and Lindblom, G. (1996). Wild-type Escherichia coli cells regulate the membrane lipid composition in a “window” between gel and non-lamellar structures. J. Biol. Chem. 271, 6801-6809. [DOI] [PubMed] [Google Scholar]
- Nayar, R., Schmid, S. L., Hope, M. J., and Cullis, P. R. (1982). Structural preferences of phosphatidylinositol and phosphatidylinositol-phosphatidylethanolamine model membranes. Influence of Ca2+ and Mg2+. Biochim. Biophys. Acta 688, 169-176. [DOI] [PubMed] [Google Scholar]
- Nikawa, J., Kodaki, T., and Yamashita, S. (1987). Primary structure and disruption of the phosphatidylinositol synthase gene of Saccharomyces cerevisiae. J. Biol. Chem. 262, 4876-4881. [PubMed] [Google Scholar]
- Oelkers, P., Tinkelenberg, A., Erdeniz, N., Cromley, D., Billheimer, J. T., and Sturley, S. L. (2000). A lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast. J. Biol. Chem. 275, 15609-15612. [DOI] [PubMed] [Google Scholar]
- Patton-Vogt, J. L., Griac, P., Sreenivas, A., Bruno, V., Dowd, S., Swede, M. J., and Henry, S. A. (1997). Role of the yeast phosphatidylinositol/phosphatidylcholine transfer protein (Sec14p) in phosphatidylcholine turnover and INO1 regulation. J. Biol. Chem. 272, 20873-20883. [DOI] [PubMed] [Google Scholar]
- Rossler, H., Rieck, C., Delong, T., Hoja, U., and Schweizer, E. (2003). Functional differentiation and selective inactivation of multiple Saccharomyces cerevisiae genes involved in very-long-chain fatty acid synthesis. Mol. Genet. Genomics 269, 290-298. [DOI] [PubMed] [Google Scholar]
- Rudge, S. A., Morris, A. J., and Engebrecht, J. (1998). Relocalization of phospholipase D activity mediates membrane formation during meiosis. J. Cell Biol. 140, 81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandager, L., Gustavsson, M. H., Stahl, U., Dahlqvist, A., Wiberg, E., Banas, A., Lenman, M., Ronne, H., and Stymne, S. (2002). Storage lipid synthesis is non-essential in yeast. J. Biol. Chem. 277, 6478-6482. [DOI] [PubMed] [Google Scholar]
- Schneiter, R., et al. (1999). Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J. Cell Biol. 146, 741-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiter, R., Tatzer, V., Gogg, G., Leitner, E., and Kohlwein, S. D. (2000). Elo1p-dependent carboxy-terminal elongation of C 14, 1Delta(9) to C 16, 1Delta(11) fatty acids in Saccharomyces cerevisiae. J. Bacteriol. 182, 3655-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivas, A., Patton-Vogt, J. L., Bruno, V., Griac, P., and Henry, S. A. (1998). A role for phospholipase D (Pld1p) in growth, secretion, and regulation of membrane lipid synthesis in yeast. J. Biol. Chem. 273, 16635-16638. [DOI] [PubMed] [Google Scholar]
- Steiner, S., and Lester, R. L. (1972). Studies on the diversity of inositol-containing yeast phospholipids: incorporation of 2-deoxyglucose into lipid. J. Bacteriol. 109, 81-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey, M. K., Clay, K. L., Kutateladze, T., Murphy, R. C., Overduin, M., and Voelker, D. R. (2001). Phosphatidylethanolamine has an essential role in Saccharomyces cerevisiae that is independent of its ability to form hexagonal phase structures. J. Biol. Chem. 276, 48539-48548. [DOI] [PubMed] [Google Scholar]
- Stukey, J. E., McDonough, V. M., and Martin, C. E. (1990). The OLE1 gene of Saccharomyces cerevisiae encodes the Δ-9 fatty acid desaturase and can be functionally replaced by the rat stearoyl CoA reductase gene. J. Biol. Chem. 265, 20144-20149. [PubMed] [Google Scholar]
- Summers, E. F., Letts, V. A., McGraw, P., and Henry, S. A. (1988). Saccharomyces cerevisiae cho2 mutants are deficient in phospholipid methylation and cross-pathway regulation of inositol synthesis. Genetics 120, 909-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suutari, M., Liukkonen, K., and Laakso, S. (1990). Temperature adaptation in yeasts: the role of fatty acids. J. Gen. Microbiol. 136, 1469-1474. [DOI] [PubMed] [Google Scholar]
- Swan, T. M., and Watson, K. (1997). Membrane fatty acid composition and membrane fluidity as parameters of stress tolerance in yeast. Can. J. Microbiol. 43, 70-77. [DOI] [PubMed] [Google Scholar]
- Tung, B. S., Unger, E. R., Levin, B., Brasitus, T. A., and Getz, G. S. (1991). Use of an unsaturated fatty acid auxotroph of Saccharomyces cerevisiae to modify the lipid composition and function of mitochondrial membranes. J. Lipid Res. 32, 1025-1038. [PubMed] [Google Scholar]
- van den Brink-van der Laan, E., Killian, J. A., and de Kruijff, B. (2004). Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim. Biophys. Acta 1666, 275-288. [DOI] [PubMed] [Google Scholar]
- Vasconcelles, M. J., Jiang, Y., McDaid, K., Gilooly, L., Wretzel, S., Porter, D., Martin, C. E., and Goldberg, M. A. (2001). Identification and Characterization of a low oxygen response element involved in the hypoxic induction of a family of Saccharomyces cerevisiae genes. J. Biol. Chem. 276, 14374-14384. [DOI] [PubMed] [Google Scholar]
- Wagner, S., and Paltauf, F. (1994). Generation of glycerophospholipid molecular species in the yeast Saccharomyces cerevisiae. Fatty acid pattern of phospholipid classes and selective acyl turnover at sn-1 and sn-2 positions. Yeast 10, 1429-1437. [DOI] [PubMed] [Google Scholar]
- Waite, K. A., and Vance, D. E. (2004). Dimethylethanolamine does not prevent liver failure in phosphatidylethanolamine N-methyltransferase-deficient mice fed a choline-deficient diet. Biochim. Biophys. Acta 1636, 175-182. [DOI] [PubMed] [Google Scholar]
- Waksman, M., Eli, Y., Liscovitch, M., and Gerst, J. E. (1996). Identification and characterization of a gene encoding phospholipase D activity in yeast. J. Biol. Chem. 271, 2361-2364. [DOI] [PubMed] [Google Scholar]
- Waksman, M., Tang, X., Eli, Y., Gerst, J. E., and Liscovitch, M. (1997). Identification of a novel Ca2+-dependent, phosphatidylethanolamine-hydrolyzing phospholipase D in yeast bearing a disruption in PLD1. J. Biol. Chem. 272, 36-39. [DOI] [PubMed] [Google Scholar]
- Zaccheo, O., Dinsdale, D., Meacock, P. A., and Glynn, P. (2004). Neuropathy target esterase and its yeast homologue degrade phosphatidylcholine to glycerophosphocholine in living cells. J. Biol. Chem. 279, 24024-24033. [DOI] [PubMed] [Google Scholar]