Abstract
The only approved method of tuberculosis (TB) surveillance of reindeer within the United States is tuberculin skin testing; however, skin testing has an apparent lack of specificity, since numerous reindeer are classified as reactors, yet Mycobacterium bovis is not isolated from tissues upon necropsy. The objective of this study was to evaluate the ability of an in vitro assay (the Cervigam assay) to detect gamma interferon (IFN-γ) produced by blood leukocytes in response to mycobacterial antigens from M. bovis-infected reindeer. Thirteen male reindeer ∼9 months of age were inoculated with 105 CFU M. bovis in their tonsillar crypts. Stimulation of whole-blood cultures with a mitogen resulted in significant production of IFN-γ compared to that by nonstimulated samples. Responses by infected reindeer to M. bovis purified protein derivative (PPD) were as much as 3.5-fold higher than those by noninfected reindeer (n = 4). Despite differences in responses to PPD by the two groups, reindeer within the noninfected group had responses of >0.1 change in optical density (ΔOD) (a level generally considered positive) to PPD. Mean responses by infected reindeer to a rESAT-6-CFP-10 fusion protein (Mycobacterium tuberculosis complex specific) were as much as 20-fold higher than respective responses by noninfected reindeer at all time points. Additionally, responses by 3/4 noninfected reindeer were <0.1 ΔOD (considered negative) at each time point. To further evaluate the specificity of the assay, samples were collected from reindeer in a TB-free herd. All reindeer had responses to mitogen; however, only 1 of 38 had a response to PPD, and none of the reindeer responded to rESAT-6-CFP-10. Together, these findings indicate that IFN-γ-based tests may prove useful for TB surveillance of reindeer.
Mycobacterium bovis infection of reindeer (Rangifer tarandus) israre, especially in North America, where there are no published reports of tuberculosis occurring in reindeer. Despite the low incidence of disease, reindeer are subject to regulations in the USDA Uniform Methods and Rules for the Eradication of Bovine Tuberculosis (TB) (Animal and Plant Health Inspection Service [APHIS] circular 91-45-011) (25) requiring TB testing for interstate movement and herd accreditation. For reindeer within the United States, the single cervical test (SCT), a measure of delayed-type hypersensitivity, is the primary approved test for tuberculosis. The SCT relies on in vivo reactivity to M. bovis purified protein derivative (PPD) injected intradermally into the mid-cervical region. Reindeer classified as reactors or suspect with this test are often retested using the comparative cervical skin test (CCT), in which M. bovis PPD is injected at one site and Mycobacterium avium PPD at a separate site. In principle, the CCT provides an added ability to distinguish M. avium responders from M. bovis responders. Although exact numbers are difficult to ascertain, many reindeer have tested positive upon skin testing for TB surveillance. Neither Mycobacterium bovis nor any other species within the M. tuberculosis complex, however, has been isolated from these reindeer upon necropsy. Improved TB tests are urgently needed to avoid unnecessary slaughter of reindeer falsely identified as reactors.
An in vitro method of tuberculosis diagnosis has been developed (34) and approved for use in cattle in the United Sates as a complementary test (i.e., in conjunction with the skin test) (15). The in vitro assay detects gamma interferon (IFN-γ) produced by peripheral blood mononuclear cells exposed to no antigen (i.e., background response), M. avium PPD, M. bovis PPD, or a mitogen (e.g., pokeweed mitogen [PWM]) (33). The assay is easily adapted to the diagnostic laboratory setting, since whole-blood cultures are used, circumventing the need for cumbersome cell separation techniques. Recently, recombinant antigens specific for virulent tubercle bacilli (e.g., early secretory antigenic target 6 [ESAT-6], culture filtrate protein 10 [CFP-10], MPB-59, MPB-64, and MPB-70) have been evaluated for use in tests that discriminate between M. avium-exposed, M. bovis BCG-vaccinated, and tuberculous cattle (4, 5, 13, 27, 29). These antigens have demonstrated utility for both in vitro (i.e., IFN-γ test) and in vivo (i.e., skin test) use (7, 14, 20, 21, 26, 32). ESAT-6 and CFP-10 are particularly robust inducers of recall IFN-γ responses by infected cattle. A test similar to the Bovigam (Pfizer Animal Health, Kalamazoo, MI) assay yet designed to detect IFN-γ produced by red deer (Cervus elaphus) leukocytes (Cervigam; Pfizer Animal Health) also reacts with IFN-γ produced by white-tailed deer (Odocoileus virginianus) leukocytes, indicating that antibodies within the assay are cross-reactive with IFN-γ from at least two cervid species (17, 28). In vitro-based tests such as the Cervigam assay are particularly appealing for use on reindeer and other deer species because animals are handled only once for this test, thereby minimizing capture-associated injuries that are more likely with multiple handling events.
The primary objective of this study was to evaluate the ability of the Cervigam assay to detect IFN-γ produced by M. bovis-infected reindeer in response to in vitro stimulation with crude (i.e., PPDs) and specific (i.e., ESAT-6 and CFP-10) mycobacterial antigens. Secondary objectives were to evaluate the effects of skin testing and several whole-blood culture techniques on the IFN-γ response by reindeer leukocytes. These studies were designed to evaluate the potential for this assay to detect tuberculous reindeer. Further studies will be necessary to determine field applications for the test.
MATERIALS AND METHODS
Animals, M. bovis culture, and challenge procedures.
Castrated male reindeer (Rangifer tarandus; n = 17) were obtained from a tuberculosis-free herd in southern Michigan and were housed at the National Animal Disease Center [NADC], USDA, ARS, Ames, Iowa, according to institutional guidelines and approved animal care and use protocols. For M. bovis infection, the challenge inoculum (105 CFU in 0.2 ml of phosphate-buffered saline, 0.15 M, pH 7.2 [PBS]) was instilled directly into the tonsillar crypts of anesthetized reindeer (n = 13) as described previously for inoculation of white-tailed deer (19). The strain of M. bovis used for the challenge inoculum (95-1315; USDA APHIS designation) was originally isolated from a white-tailed deer in Michigan (24). The inoculum was prepared in a biosafety level-3 (BL-3) facility. The inoculum consisted of mid-log-phase M. bovis grown in Middlebrook 7H9 medium supplemented with 10% oleic acid-albumin-dextrose complex (Difco, Detroit, Mich.) plus 0.05% Tween 80 (Sigma Chemical Co., St. Louis, Mo.). At the time of inoculation, reindeer were moved from an outdoor pen into climate-controlled rooms (2 to 3 animals/room) within a BL-3 confinement facility. Negative airflow exited the building through high-efficiency particulate air (HEPA) filters; air from animal pens was pulled toward a central corridor and through HEPA filters before exiting the building. Airflow velocity was 10.4 air changes/h. All four noninfected reindeer were housed in a climate-controlled room in a separate building. At the initiation of the study, reindeer were approximately 9 months old.
To further evaluate the specificity of the Cervigam assay, blood samples were obtained from an additional 38 reindeer in a TB-free herd in Milltown, WI. These samples were delivered overnight to NADC, Ames, IA, for evaluation of IFN-γ production in response to mycobacterial antigens and mitogen.
Whole-blood cultures and analysis of IFN-γ production.
Blood was collected at 45 and 7 days prior to inoculation and at approximately monthly intervals after inoculation. Additional blood samples were collected immediately prior to and following skin testing procedures. Heparinized blood samples were dispensed in 1.5-ml aliquots into individual wells of a 24-well plate (Falcon 353047; Becton Dickinson and Company, Franklin Lakes, N.J.). Treatments included no stimulation (PBS), M. avium PPD (Pfizer Animal Health; 40 μg/ml), M. bovis PPD (Pfizer Animal Health; 40 μg/ml), recombinant ESAT-6 (rESAT-6; 10 μg/ml), rCFP-10 (10 μg/ml), rESAT-6-CFP-10 (10 μg/ml), or PWM (Sigma; 20 μg/ml). Optimal concentrations of stimulants were determined by evaluation of responses to 1, 10, 20, 40, and 80 μg/ml of each stimulant. Cloning, expression, and purification of recombinant proteins were performed as described elsewhere (29). Blood cultures were incubated for 48 h, and plasma was harvested and stored at −80°C. IFN-γ concentrations in nonstimulated and stimulated plasma were determined using an enzyme-linked immunosorbent assay (ELISA)-based kit (Pfizer Animal Health). Absorbencies of standards and test samples were read at 450 nm using an ELISA plate reader (Molecular Devices, Menlo Park, CA). Individual treatments were analyzed in duplicate and the assay repeated if duplicate responses were not consistent. Data are presented as optical density (OD) readings or changes in optical density readings upon stimulation compared to no stimulation (ΔOD, calculated as the OD of the antigen- or mitogen-stimulated sample minus the OD of the nonstimulated sample).
Skin testing procedures.
Three months after M. bovis inoculation, reindeer were tested for in vivo responsiveness to mycobacterial antigens by a modified CCT technique enabling collection of biopsy specimens showing dermal reactions to PPDs at 24, 48, and 72 h postinjection (16, 30). Briefly, the cervical region was clipped, and animals were injected intradermally at three separate locations with M. bovis PPD and at a single location with M. avium PPD (PPDs for skin tests were obtained from the National Veterinary Services Laboratory, Ames, Iowa). Biopsy procedures are provided elsewhere (16). A standard CCT (i.e., a single intradermal injection each of M. avium PPD and M. bovis PPD) was performed 8 months after M. bovis inoculation (18, 25). For each of the skin tests, 100 μg of M. bovis PPD and 40 μg of M. avium PPD were administered at each respective location according to guidelines described in USDA APHIS circular 91-45-011. Skin thickness was measured immediately prior to administration of PPD and at 24, 48, and 72 h after injection by using calipers.
Necropsy procedures.
Thirteen months after inoculation, all reindeer were euthanized by intravenous administration of sodium pentobarbital. A thorough postmortem examination was done. Specimens collected for bacteriologic culture and microscopic examination from inoculated and naïve reindeer included tonsil, brain, lung, liver, spleen, and kidney; as well as mandibular, parotid, medial retropharyngeal, tracheobronchial, mediastinal, mesenteric, hepatic, ileocecal, superficial cervical, and prefemoral lymph nodes. Specimens for bacteriologic culture were placed individually in sterile bags and stored at −80°C until processing. Specimens were processed as previously described (19). Mycobacterial isolates were identified using standard growth and biochemical characteristics. Isolates were confirmed to belong to the M. tuberculosis complex by genetic probe analysis (AccuProbe; Gen-Probe Inc., San Diego, CA). Results were considered positive if M. bovis was isolated. Isolates of mycobacteria that were not members of the M. tuberculosis complex were further identified using 16S rRNA gene sequencing. Sequences were then identified through the use of a mycobacterial species sequence database.
Samples for microscopic examination were fixed in neutral buffered 10% formalin and processed by routine paraffin embedment techniques. Sections were cut 3 μm thick, stained with hematoxylin and eosin, and examined by light microscopy. Adjacent 3-μm-thick sections were cut from specimens with lesions suggestive of tuberculosis (caseonecrotic granulomata) and stained by the Ziehl-Neelsen technique for visualization of acid-fast bacteria. Microscopic findings were considered positive when lesions consistent with tuberculosis contained acid-fast bacilli.
Statistics.
Data were analyzed by either Student's t test or one-way analysis of variance followed by a Tukey-Kramer multiple-comparison test using commercially available software (InStat 2.00; GraphPAD Software, San Diego, CA). Differences between groups were considered significant if probability values (P) of <0.05 were obtained.
RESULTS
Inoculation of M. bovis into tonsillar crypts of reindeer resulted in gross and microscopic lesions in all animals inoculated (16). Tuberculous lesions were most prominent in medial retropharyngeal lymph nodes and were also detected in tonsils, mesenteric lymph nodes, lungs, and lung-associated lymph nodes. Infected reindeer did not have clinical signs of TB or disseminated disease after 1 year of colonization, a finding suggestive of low-grade chronic infection. M. bovis was not isolated from control reindeer, and bacteriologic results for infected reindeer are presented elsewhere (16). While control reindeer were not infected with M. bovis, lesions were detected within the tracheobronchial lymph nodes of 2/4 noninoculated reindeer. Histologically, the lesions in these two lymph nodes were characterized by pyogranulomatous inflammation with no acid-fast bacteria. No mycobacteria were isolated upon culture, and no other etiologic agents were detected.
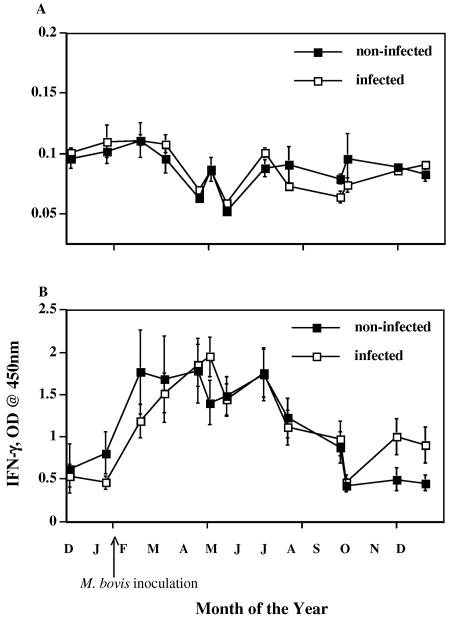
At all time points, polyclonal stimulation of whole-blood cultures with PWM resulted in significant (P < 0.05) production of IFN-γ compared to that by nonstimulated samples (Fig. 1). Responses to PWM were greatest from February to June and did not differ (P > 0.05) between infected and noninfected reindeer. Mean responses to PWM stimulation over the course of the study (ΔOD, 1.03 ± 0.06 for infected [n = 201] and 0.99± 0.08 for noninfected [n = 68] reindeer) were similar to those reported for white-tailed deer (17). Mean responses to no stimulation (i.e., PBS) over the course of the study (ΔOD, 0.085 ± 0.002 for infected [n = 201] and 0.086 ± 0.003 for noninfected [n = 68] reindeer) were also similar to those reported for white-tailed deer (17). Neither infection status nor time of year affected responses to no stimulation.
FIG. 1.
IFN-γ responses to no stimulation (A) and PWM stimulation (B) by M. bovis-infected (n = 13) (open squares) and noninfected (n = 4) (solid squares) reindeer. Heparinized blood samples were obtained at various time points (months, starting with December, indicated on x axis) and dispensed immediately in 1.5-ml aliquots into individual wells of a 24-well plate. Treatment was no stimulation (PBS) or PWM (20 μg/ml). Blood cultures were incubated for 48 h, plasma was harvested, and IFN-γ concentrations were determined using an ELISA-based kit (Cervigam; Pfizer Animal Health). Absorbencies of standards and test samples were read at 450 nm using an ELISA plate reader. Duplicate samples for individual treatments were analyzed. Data are presented as mean OD readings ± standard errors of the means. Responses to either no stimulation (A) or PWM stimulation (B) did not differ (P > 0.05) between infected and noninfected reindeer. Due to disparate responses to no stimulation versus PWM, the scales for the two graphs are different.
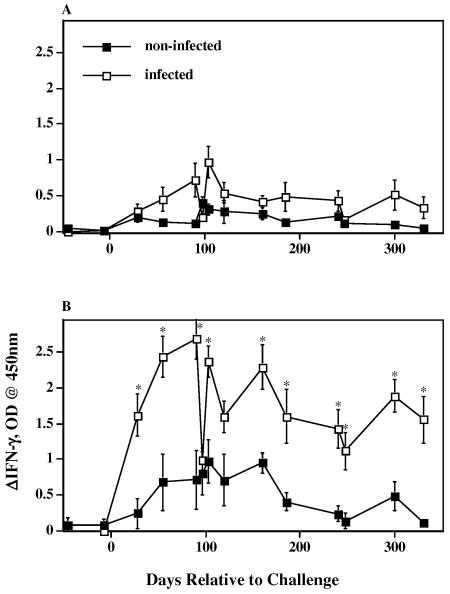
As early as 30 days after inoculation and at most time points thereafter, responses by infected reindeer to M. bovis PPD exceeded (P < 0.05) those by noninfected reindeer (Fig. 2). Responses to M. avium PPD did not differ between groups. Despite differences in responses to M. bovis PPD by the two groups, reindeer within the noninfected group had responses that were considered positive (>0.1 ΔOD). Each of the four noninfected reindeer had positive responses to M. bovis PPD, although not at each time point. Nonspecific responses to M. bovis PPD by the two control animals that had nonmycobacterial lesions within the tracheobronchial lymph nodes did not exceed those responses by the two control animals that did not have lesions. Mean responses (i.e., averages of individual responses over the course of the study [n = 64]) by noninfected reindeer to M. bovis PPD (0.47 ± 0.06 ΔOD) exceeded (P < 0.0001) concurrent responses to M. avium PPD (0.16 ± 0.02 ΔOD).
FIG. 2.
IFN-γ responses to M. avium PPD stimulation (A) and M. bovis PPD stimulation (B) by M. bovis-infected (n = 13) (open squares) and noninfected (n = 4) (solid squares) reindeer. Heparinized blood samples were obtained at the indicated time points and dispensed immediately in 1.5-ml aliquots into individual wells of a 24-well plate. Treatment was no stimulation (PBS), M. avium PPD (40 μg/ml), or M. bovis PPD (40 μg/ml). Blood cultures were incubated for 48 h, plasma was harvested, and IFN-γ concentrations were determined using an ELISA-based kit (Cervigam; Pfizer Animal Health). Absorbencies of standards and test samples were read at 450 nm using an ELISA plate reader. Duplicate samples for individual treatments were analyzed. Data are presented as the response to PPD stimulation minus the response to no stimulation (ΔIFN-γ, OD) and are means ± standard errors of the means. Asterisks indicate responses by M. bovis-infected reindeer that differ (P < 0.05) from the respective responses by noninfected reindeer.
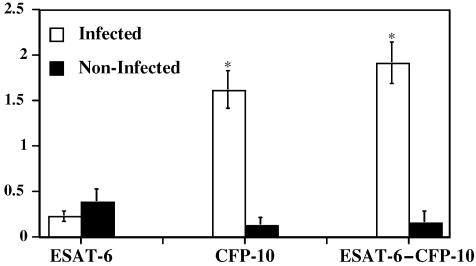
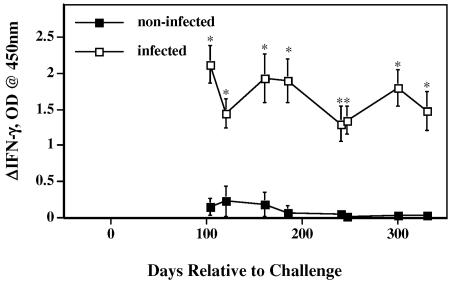
Antigens produced predominantly by tuberculous mycobacteria and not by many environmental nontuberculous mycobacteria were evaluated as an approach to increase the specificity of the test. Mean responses (averaged over various time points) by infected reindeer to rCFP-10 and to rESAT-6-CFP-10, but not to rESAT-6, exceeded (P < 0.01) the respective responses by noninfected reindeer (Fig. 3). Responses by infected reindeer to rCFP-10 and to rESAT-6-CFP-10 exceeded (P < 0.01) responses to rESAT-6 at all time points tested (data not shown). Responses by infected reindeer to rCFP-10 (data not shown) and to rESAT-6-CFP-10 (Fig. 4) exceeded (P < 0.01) the respective responses by noninfected reindeer at all time points. No correlations between responses to TB antigens and bacillary loads were detected (data not shown). In contrast to responses to M. bovis PPD, responses to rCFP-10 and to rESAT-6-CFP-10 by 3/4 noninfected reindeer were considered negative (<0.1 ΔOD) at each time point. One noninfected reindeer, however, had positive responses to rCFP-10 and to rESAT-6-CFP-10 at each time point tested except one. While 2/4 noninfected reindeer had granulomatous lesions within the tracheobronchial lymph nodes with no acid-fast bacteria present, the 1 reindeer responding to rCFP-10 and to rESAT-6-CFP-10 had no detectable mycobacterial lesions upon necropsy.
FIG. 3.
IFN-γ responses to recombinant ESAT-6, CFP-10, and ESAT-6-CFP-10. Heparinized blood samples were obtained from reindeer and dispensed immediately in 1.5-ml aliquots into individual wells of a 24-well plate. Treatment was either no stimulation (PBS), rESAT-6 (10 μg/ml), rCFP-10 (10 μg/ml), or rESAT-6-CFP-10 (10 μg/ml). Whole-blood cultures were incubated for 48 h, plasma was harvested, and IFN-γ concentrations were determined using an ELISA-based kit (Cervigam; Pfizer Animal Health). Absorbencies of standards and test samples were read at 450 nm using an ELISA plate reader. Duplicate samples for individual treatments were analyzed. Data are presented as the response to antigen stimulation minus the response to no stimulation (ΔIFN-γ, OD) and are means ± standard errors of the means. Responses at 90, 103, 120, and 160 days after challenge were averaged (n = 4). Open bars, responses by M. bovis-infected reindeer (n = 10); solid bars, responses by noninfected reindeer (n = 4). Asterisks indicate responses by M. bovis-infected reindeer that differ (P < 0.05) from the respective responses by noninfected reindeer.
FIG. 4.
Longitudinal IFN-γ responses by M. bovis-infected (n = 10) and noninfected (n = 4) reindeer to rESAT-6-CFP-10. Heparinized blood samples were obtained and dispensed immediately in 1.5-ml aliquots into individual wells of a 24-well plate. Treatments included no stimulation (PBS) and rESAT-6-CFP-10 (10 μg/ml) stimulation. Whole-blood cultures were incubated for 48 h, plasma was harvested, and IFN-γ concentrations were determined using an ELISA-based kit (Cervigam; Pfizer Animal Health). Absorbencies of standards and test samples were read at 450 nm using an ELISA plate reader. Duplicate samples for individual treatments were analyzed. Data are presented as the response to antigen stimulation minus the response to no stimulation (ΔIFN-γ, OD) and are means ± standard errors of the means. Asterisks indicate responses by M. bovis-infected reindeer that differ (P< 0.05) from the respective responses by noninfected reindeer.
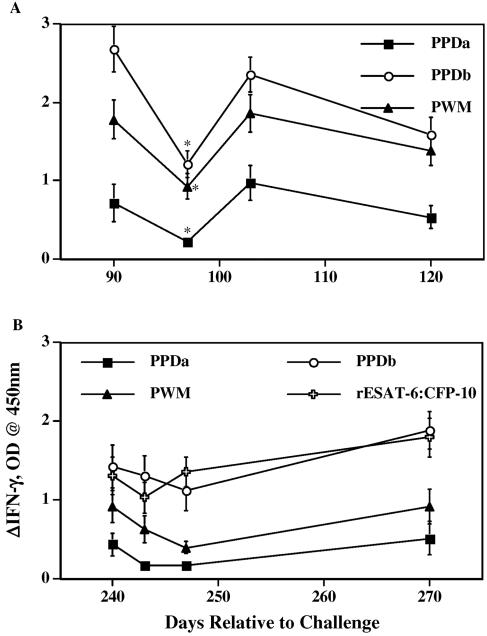
Because skin testing is the only approved test for tuberculosis surveillance of reindeer within the United States, it is likely that field evaluation of candidate tests will initially be performed in conjunction with skin testing. At 90 and 240 days after inoculation, experimentally infected reindeer were injected with PPDs for determination of in vivo responsiveness to mycobacterial antigens (16). With the initial test, animals received three injections of M. bovis PPD (300 μg total, as a means to collect three daily skin biopsy samples [16]) and one injection of M. avium PPD (40μg). Quantities of PPDs injected at each injection site were in accordance with USDA APHIS circular 91-45-011. IFN-γ responses to M. avium PPD, M. bovis PPD, and PWM were reduced (P < 0.05) 1 week after PPD administration compared to the respective responses at the time of injection (Fig. 5A). IFN-γ responses were only transiently depressed; responses returned to near-preinjection levels by 2 weeks after PPD administration. For the second skin test at 8 months after inoculation, 40 μg of M. avium and 100 μg of M. bovis PPD were injected according to the standard CCT technique as outlined by USDA APHIS circular 91-45-011. While the difference was not statistically significant, IFN-γ responses after this test were generally decreased for the 7-day period following PPD injection (Fig. 5B).
FIG. 5.
Effects of injection of PPDs for skin test on IFN-γ responses to mycobacterial antigens and mitogen. Reindeer were tested for delayed-type hypersensitive responses to mycobacterial antigens after administration of CCT at 90 (A) and 240 (B) days after challenge with M. bovis. Heparinized blood samples were obtained from reindeer at the indicated time points and dispensed immediately in 1.5-ml aliquots into individual wells of a 24-well plate. Treatments included no stimulation (PBS), M. avium PPD (PPDa; 40 μg/ml), M. bovis PPD (PPDb; 40 μg/ml), rESAT-6-CFP-10 (10 μg/ml), and PWM (20 μg/ml). Whole-blood cultures were incubated for 48 h, plasma was harvested, and IFN-γ concentrations were determined using an ELISA-based kit (Cervigam; Pfizer Animal Health). Absorbencies of standards and test samples were read at 450 nm using an ELISA plate reader. Duplicate samples for individual treatments were analyzed. Data are presented as the response to antigen stimulation minus the response to no stimulation (ΔIFN-γ, OD) and are means ± standard errors of the means. Asterisks indicate responses that differ (P < 0.05) from preinjection responses (responses at 90 days after challenge).
In field situations, blood samples for IFN-γ-based assays are routinely delivered via overnight courier to reference laboratories. To evaluate effects of delayed setup, whole-blood cultures were processed either immediately or after a 24-h hold at room temperature (RT). The 24-h delay in processing did not alter (P > 0.05) the response (Table 1). To evaluate a potential strategy to enhance the response, antigens or a mitogen was added and samples held at RT for 24 h (to mimic overnight shipment with stimulation). Following the 24-h hold with stimulation, samples were incubated at 37°C for 48 h. Addition of antigen for 24 h prior to the 48-h incubation did not alter the response (Table 1). Addition of PWM for 24 h prior to incubation did decrease (P < 0.05) the response compared to a 24-h hold without stimulation prior to incubation with PWM. Samples prestimulated for 24 h prior to incubation were also evaluated after 24 and 48 h of incubation at 37°C (Table 2). Responses of samples stimulated with M. avium PPD, M. bovis PPD, or PWM for 48 h exceeded (P < 0.05) the respective responses after 24 h of stimulation. Responses to rESAT-6-CFP-10, however, were not different (P > 0.05) after the two incubation periods.
TABLE 1.
Effects of culture techniques on IFN-γ responses by infected reindeera
| Stimulant | Mean ΔOD ± SEM for:
|
||
|---|---|---|---|
| Immediate setup | 24-h delayed setup
|
||
| Without stimulation | With stimulation | ||
| M. avium PPD | 0.303 ± 0.049 (n = 56) | 0.300 ± 0.040 (n = 56) | 0.183 ± 0.029 (n = 30) |
| M. bovis PPD | 1.285 ± 0.105 (n = 56) | 1.191 ± 0.089 (n = 56) | 1.054 ± 0.112 (n = 30) |
| rESAT-6-CFP-10 | 1.291 ± 0.103 (n = 43) | 1.268 ± 0.104 (n = 43) | 1.072 ± 0.144 (n = 20) |
| PWM | 0.839 ± 0.084 (n = 56) | 0.758 ± 0.075 (n = 56) | 0.355 ± 0.050b (n = 30) |
Heparinized blood samples were obtained from M. bovis-infected reindeer at various time points after challenge. Blood was dispensed in 1.5-ml aliquots either immediately or after a 24-h delay at RT into individual wells of a 24-well plate. Treatments included no stimulation (PBS), M. avium PPD (40 μg/ml), M. bovis PPD (40 μg/ml), rESAT-6-CFP-10 (10 μg/ml), and PWM (20 μg/ml). Blood cultures were incubated for 48 h (with either an immediate or a delayed setup), plasma was harvested, and IFN-γ concentrations were determined using an ELISA-based kit. Absorbencies of standards and test samples were read at 450 nm using an ELISA plate reader. Duplicate samples for individual treatments were analyzed.
Differs from PWM responses obtained with an immediate setup and with a delayed setup without stimulation (P < 0.01).
TABLE 2.
Effects of culture duration on IFN-γ responses by infected reindeera
| Stimulant | Mean ΔOD ± SEMb with:
|
|
|---|---|---|
| 24-h stimulation | 48-h stimulation | |
| M. avium PPD | 0.118 ± 0.034 | 0.314 ± 0.056* |
| M. bovis PPD | 0.686 ± 0.111 | 1.417 ± 0.211* |
| rESAT-6-CFP-10 | 1.004 ± 0.223 | 1.244 ± 0.193 |
| PWM | 0.110 ± 0.024‡ | 0.392 ± 0.092*‡ |
Heparinized blood samples were obtained from M. bovis-infected reindeer 8 months after challenge. Blood was dispensed in 1.5-ml aliquots immediately into individual wells of a 24-well plate. Treatments included no stimulation (PBS), M. avium PPD (40 μg/ml), M. bovis PPD (40 μg/ml), rESAT-6-CFP-10 (10 μg/ml), and PWM (20 μg/ml). Blood cultures were incubated for 24 or 48 h, plasma was harvested, and IFN-γ concentrations were determined using an ELISA-based kit (Cervigam; Pfizer Animal Health). Absorbencies of standards and test samples were read at 450 nm using an ELISA plate reader. Duplicate samples for individual treatments were analyzed. Data are means of results for 10 samples.
*, response differs from responses to respective antigens/mitogen after a 24-h stimulation (P < 0.01). ‡, effects of culture duration on PWM responses by noninfected reindeer (ΔOD, 0.132 ± 0.037 with a 24-h stimulation versus 0.446 ± 0.159 with a 48-h stimulation [P = 0.07]) were similar to those of infected reindeer.
To further evaluate the potential for nonspecific responses to M. bovis antigens by noninfected reindeer, samples were collected from reindeer in a TB-free herd and evaluated for production of IFN-γ in response to mycobacterial antigens and mitogen (Table 3). All reindeer had responses to PWM; however, only 1 of 38 (97% specificity) had a response to M. bovis PPD, and none of the reindeer responded to rESAT-6-CFP-10.
TABLE 3.
IFN-γ responses by reindeer from a TB-free herd in Wisconsina
| Stimulant | IFN-γ, OD (mean ± SEM) | No. of responses of >0.1 ΔOD |
|---|---|---|
| None | 0.092 ± 0.010 | Not applicable |
| M. avium PPD | 0.086 ± 0.003 | 0 |
| M. bovis PPD | 0.091 ± 0.006 | 1 |
| rESAT-6-CFP-10 | 0.098 ± 0.005 | 0 |
| PWM | 1.526 ± 0.128 | 38 |
Heparinized blood samples were obtained from 38 reindeer in a TB-free herd in Milltown, WI, and shipped to NADC, Ames, IA, overnight (24-h delay prior to setup). Blood was dispensed in 1.5-ml aliquots into individual wells of a 24-well plate. Treatments included no stimulation (PBS), M. avium PPD (40 μg/ml), M. bovis PPD (40 μg/ml), rESAT-6-CFP-10 (10 μg/ml), or PWM (20 μg/ml). Blood cultures were incubated for 48 h, plasma was harvested, and IFN-γ concentrations were determined using an ELISA-based kit (Cervigam; Pfizer Animal Health). Absorbencies of standards and test samples were read at 450 nm using an ELISA plate reader. Duplicate samples for individual treatments were analyzed. ΔOD is calculated as response to antigen or mitogen stimulation minus response to no stimulation.
DISCUSSION
The present findings indicate that the Cervigam assay detects IFN-γ produced by blood leukocytes isolated from reindeer. Experimental infection elicited IFN-γ responses to M. bovis PPD that exceeded the respective responses by noninfected reindeer. While cutoff values have yet to be determined, responses greater than 0.1 ΔOD are generally considered positive. All noninfected reindeer had responses to M. bovis PPD greater than 0.1 ΔOD at certain time points during the study, indicating a false-positive reaction to this antigen. Exposure to M. avium or environmental nontuberculous Mycobacterium spp. may induce responses cross-reactive with M. bovis antigens. With both IFN-γ-based assays and skin tests, these responses are generally differentiated from those elicited by tuberculous mycobacteria by the degree of the response to the various PPDs. Responses by noninfected reindeer to M. bovis PPD, however, exceeded concurrent responses to M. avium PPD, indicating that M. avium exposure did not elicit the response. Exposure to other Mycobacterium spp. (e.g., Mycobacterium fortuitum) may also result in cross-reactive responses to M. bovis PPD (11), yet M. fortuitum PPD was not evaluated in the present study, and the effects of M. fortuitum on the differential response to M. bovis and M. avium PPD are not clear. Another “environmental” mycobacterial species, Mycobacterium kansasii, has been shown to induce disseminated infection of reindeer (12). Host responses, however, were not evaluated with this case study. It would be of interest to determine the effects of M. kansasii or other mycobacterial infections/sensitization on the IFN-γ response to M. avium and M. bovis PPDs. Regardless, the present findings indicate that PPDs may not provide the specificity required for TB surveillance of reindeer, at least using IFN-γ-based tests and as previously demonstrated with field use of skin tests within the United States.
IFN-γ responses to PWM increased from February to June. The relevance of these findings is unclear; however, there is anecdotal evidence that nonspecific immune reactivity to various antigens, including M. bovis PPD, occurs during these months (Tom Scheib, Reindeer Owners and Breeders Association, personal communication). Both nonspecific responses by noninfected reindeer and specific responses by M. bovis-infected reindeer coincided with increased responses to PWM. While PPD-specific responses by infected reindeer persisted throughout the remainder of the study, subtle decreases in the response may have resulted from effects of seasonal changes, decreased antigen load resulting from control of infection, or alterations in the immune response associated with the kinetics of the response. The natural breeding season of reindeer generally lasts from mid-September to late October, with calving from April through May. All reindeer used in the experimental challenge study were castrated males; thus, the increased IFN-γ response to polyclonal and/or antigenic stimulation was not likely linked to breeding cycle hormonal changes. Additionally, the reindeer in the experimental challenge study were housed in a biocontainment facility with a controlled temperature, humidity, and light cycle (12 h of light, 12 h of darkness). It has been observed that feed consumption by both captive and free-ranging reindeer is diminished during the winter months. In general, feed consumption remained consistent throughout the present study. Further studies are necessary to determine potential mechanisms associated with the seasonal increases in responsiveness to polyclonal and/or antigenic stimulation observed in the present study.
Proteins encoded within the ESAT-6 gene cluster (including ESAT-6 and CFP-10) of tuberculous mycobacteria evoke potent T-cell responses that have been utilized in TB diagnostic tests (2, 3, 4, 20, 21, 27). In particular, use of these antigens can enhance the specificity of IFN-γ-based tests over that achieved when standard PPDs are used as the eliciting agent. However, humans infected with Mycobacterium leprae, Mycobacterium marinum, or M. kansasii exhibit recall IFN-γ responses to ESAT-6 and/or CFP-10, indicating that T-cell responses to these two proteins are not invariably specific for infection with M. tuberculosis complex mycobacteria (1, 8, 9, 10). In the present study, CFP-10 and a fusion protein of ESAT-6 and CFP-10 elicited robust recall IFN-γ responses by blood leukocytes from M. bovis-infected reindeer. More importantly, responses to these proteins were not detected for 3/4 noninfected reindeer, each of which responded to M. bovis PPD. The one reindeer that did respond to these two proteins did so on multiple occasions, and the eliciting agent or reason for reactivity was not detected. However, the present findings indicate that use of rESAT-6-CFP-10 will enhance the specificity of the Cervigam assay compared to use of standard PPDs.
In contrast to responses to CFP-10 and rESAT-6-CFP-10, responses by M. bovis-infected reindeer to rESAT-6 did not exceed those of noninfected reindeer. ESAT-6 and CFP-10 are cosecreted proteins that naturally form a tight 1:1 complex upon export (22). The recombinant form of ESAT-6 used for the present study is poorly soluble in PBS. Diluents other than PBS were not tested and may provide enhanced solubility without loss of antigenicity and cell viability. Addition of CFP-10 as a fusion protein (rESAT-6-CFP-10) resulted in a more stable compound, as occurs naturally, with improved solubility. Poor responses elicited by rESAT-6 were likely due to physical shortcomings of the recombinant protein, although it is possible that the genetic background of these reindeer rendered them unresponsive to ESAT-6 and not to CFP-10. Further studies with rESAT-6 homodimers, soluble peptides, additional stabilizing proteins, and improved diluents for rESAT-6 are necessary to determine the reasons for the lack of reactivity of samples from infected reindeer to rESAT-6.
IFN-γ responses to M. bovis antigens and PWM by infected reindeer were decreased immediately after skin testing (Fig. 2B, day 96). In contrast, cattle sensitized to M. bovis antigens exhibit increased IFN-γ responses for 3 to 28 days after PPD administration for CCT (31). In other studies, using M. bovis-infected cattle, results of IFN-γ tests were not affected by PPD administration for skin testing (6, 23). For the first skin test, reindeer received three separate injections of M. bovis PPD to enable daily skin biopsy collection. The increased dose of in vivo PPD administered may have resulted in lymphocyte anergy or redistribution of reactive lymphocytes. For the second skin test, reindeer received standard amounts of PPDs for CCT. Again, IFN-γ responses were diminished after skin testing, but only slightly. Further studies are necessary to clearly define the effects of skin testing on IFN-γ responses and, potentially, to evaluate the mechanisms of these effects. Considering that false-positive tests using M. bovis PPD for skin testing and IFN-γ-based assays for reindeer appear to be common, it is intriguing that injection of PPDs for CCT appears to inhibit blood IFN-γ responses to M. bovis PPD.
For field usage, IFN-γ-based assays require shipment of whole-blood samples to diagnostic laboratories capable of running the assay. As with cattle (23), it was determined that a 24-h delay at RT prior to incubation with stimulating agents did not interfere with the performance of the test. Strategies to enhance the response after delays associated with overnight delivery are currently being planned for use with TB surveillance samples. With this strategy, antigen is added to tubes prior to blood collection. Blood is then collected into the tube with antigen, and the tube is delivered to the laboratory, where the sample is then incubated without the need for reallocation to tubes or wells in plates. Additionally, incubation containers for delivery, which prevent delays in incubation, are being tested. In the present study, however, addition of antigen to whole-blood samples during the 24-h delay did not increase the response. As determined with white-tailed deer (17), responses were increased with a 48-h incubation period compared to a 24-h incubation period. The increased flexibility of overnight delivery should prove useful for field application of the IFN-γ assay with samples from reindeer.
Together, these findings indicate that the Cervigam assay may prove useful for TB surveillance of reindeer. However, specific antigens such as rESAT-6-CFP-10 may be needed to achieve adequate specificity of the test. As with cattle, samples may be shipped overnight and responses will not be compromised. In contrast to a 24-h incubation for samples from cattle, samples from reindeer that are incubated for 48 h provide better responses than those elicited by a 24-h incubation, as with samples from white-tailed deer. Additional studies with samples obtained from reindeer across the United States will be useful for determining the specificity of the test, especially in comparison to skin testing.
Acknowledgments
USDA APHIS provided partial funding for these studies.
We are especially grateful to Tom Scheib, Kyle Wilson, and other members of the Reindeer Owners and Breeders Association for helpful comments on reindeer handling/care and specific concerns with current TB testing procedures. We thank Ryan Cook, Peter Lasley, Rebecca Lyon, Jody Mentele, Jessica Pollock, and Shelly Zimmerman for excellent technical support. We also thank Richard Auwerda, Doug Ewing, Andrew Gibson, Don Hackbarth, Todd Holtz, Nate Horman, Terry Krausman, David Panthen, Brian Pottebaum, Don Robinson, Jay Steffen, Johann Thiel, Wayne Varland, and Larry Wright for excellent animal care.
REFERENCES
- 1.Arend, S. M., K. E. van Meijgaarden, K. de Boer, E. C. de Palou, D. van Soolingen, T. H. Ottenhoff, and J. T. van Dissel. 2002. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J. Infect. Dis. 186:1797-1807. [DOI] [PubMed] [Google Scholar]
- 2.Arend, S. M., T. H. Ottenhoff, P. Andersen, and J. T. van Dissel. 2001. Uncommon presentations of tuberculosis: the potential value of a novel diagnostic assay based on the Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung. Dis. 5:680-686. [PubMed] [Google Scholar]
- 3.Buddle, B. M., A. R. McCarthy, T. J. Ryan, J. M. Pollock, H. M. Vordermeier, R. G. Hewinson, P. Andersen, and G. W. de Lisle. 2003. Use of mycobacterial peptides and recombinant proteins for the diagnosis of bovine tuberculosis in skin test-positive cattle. Vet. Rec. 153:615-620. [DOI] [PubMed] [Google Scholar]
- 4.Buddle, B. M., T. J. Ryan, J. M. Pollock, P. Andersen, and G. W. de Lisle. 2001. Use of ESAT-6 in the interferon-gamma test for diagnosis of bovine tuberculosis following skin testing. Vet. Microbiol. 80:37-46. [DOI] [PubMed] [Google Scholar]
- 5.Buddle, B. M., N. A. Parlane, D. L. Keen, F. E. Aldwell, J. M. Pollock, K. Lightbody, and P. Andersen. 1999. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin. Diagn. Lab. Immunol. 6:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doherty, M. L., M. L. Monaghan, H. F. Bassett, and P. F. Quinn. 1995. Effect of a recent injection of purified protein derivative on diagnostic tests for tuberculosis in cattle infected with Mycobacterium bovis. Res. Vet. Sci. 58:217-221. [DOI] [PubMed] [Google Scholar]
- 7.Elhay, M. J., T. Oettinger, and P. Andersen. 1998. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect. Immun. 66:3454-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geluk, A., K. E. Van Meijgaarden, K. L. Franken, B. Wieles, S. M. Arend, W. R. Faber, B. Naafs, and T. H. Ottenhoff. 2004. Immunological crossreactivity of the Mycobacterium leprae CFP-10 with its homologue in Mycobacterium tuberculosis. Scand. J. Immunol. 59:66-70. [DOI] [PubMed] [Google Scholar]
- 9.Geluk, A., K. E. van Meijgaarden, K. L. Franken, Y. W. Subronto, B. Wieles, S. M. Arend, E. P. Sampaio, T. de Boer, W. R. Faber, B. Naafs, and T. H. Ottenhoff. 2002. Identification and characterization of the ESAT-6 homologue of Mycobacterium leprae and T-cell cross-reactivity with Mycobacterium tuberculosis. Infect. Immun. 70:2544-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gey van Pittius, N. C., R. M. Warren, and P. D. van Helden. 2002. ESAT-6 and CFP-10: what is the diagnosis? Infect. Immun. 70:6509-6510; author reply 6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grobler, D. G., A. L. Michel, L. M. De Klerk, and R. G. Bengis. 2002. The gamma-interferon test: its usefulness in a bovine tuberculosis survey in African buffaloes (Syncerus caffer) in the Kruger National Park. Onderstepoort J. Vet. Res. 69:221-227. [PubMed] [Google Scholar]
- 12.Kiupel, M., H. Simmons, D. Berry, et al. 2003. Mycobacterium kansasii in reindeer (Rangifer tarandus), p. 120. In Proceedings of the 46th Annual Conference of the American Association of Veterinary Laboratory Diagnosticians. Pat Campbell & Associates, Richmond, Va.
- 13.Lyashchenko, K., A. O. Whelan, R. Greenwald, J. M. Pollock, P. Andersen, R. G. Hewinson, and H. M. Vordermeier. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 72:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyashchenko, K., C. Manca, R. Colangeli, A. Heijbel, A. Williams, and M. L. Gennaro. 1998. Use of Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect. Immun. 66:3606-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massengill, C. E. 2002. Report of the committee on tuberculosis, p. 590-611. In Proceedings of the United States Animal Health Association Annual Meeting. Pat Campbell & Associates, Richmond, Va.
- 16.Palmer, M. V., W. R. Waters, T. C. Thacker, and W. C. Stoffregen. 2006. Experimental infection of reindeer (Rangifer tarandus) with Mycobacterium bovis: pathological and immunological findings. J. Vet. Diagn. Investig., 18:51-59. [DOI] [PubMed]
- 17.Palmer, M. V., W. R. Waters, D. L. Whipple, R. E. Slaughter, and S. L. Jones. 2004. Analysis of interferon-γ production by Mycobacterium bovis infected white-tailed deer (Odocoileus virginianus) using an in-vitro based assay. J. Vet. Diagn. Investig. 16:16-21. [DOI] [PubMed] [Google Scholar]
- 18.Palmer, M. V., D. L. Whipple, and W. R. Waters. 2001., Tuberculin skin testing in white-tailed deer (Odocoileus virginianus). J. Vet. Diagn. Investig. 13:530-533. [DOI] [PubMed] [Google Scholar]
- 19.Palmer, M. V., D. L. Whipple, and S. C. Olsen. 1999. Development of a model of natural infection with Mycobacterium bovis in white-tailed deer. J. Wildl. Dis. 35:450-457. [DOI] [PubMed] [Google Scholar]
- 20.Pollock, J. M., R. M. Girvin, K. A. Lightbody, R. A. Clements, S. D. Neill, B. M. Buddle, and P. Andersen. 2000. Assessment of defined antigens for the diagnosis of bovine tuberculosis in skin test-reactor cattle. Vet. Rec. 146:659-665. [DOI] [PubMed] [Google Scholar]
- 21.Pollock, J. M., and P. Andersen. 1997. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J. Infect. Dis. 175:1251-1254. [DOI] [PubMed] [Google Scholar]
- 22.Renshaw, P. S., P. Panagiotidou, A. Whelan, S. V. Gordon, R. G. Hewinson, R. A. Williamson, and M. D. Carr. 2002. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6-CFP-10 complex. Implications for pathogenesis and virulence. J. Biol. Chem. 277:21598-21603. [DOI] [PubMed] [Google Scholar]
- 23.Ryan, T. J., B. M. Buddle, and G. W. De Lisle. 2000. An evaluation of the gamma interferon test for detecting bovine tuberculosis in cattle 8 to 28 days after tuberculin skin testing. Res. Vet. Sci. 69:57-61. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt, S. M., S. D. Fitzgerald, T. M. Cooley, C. S. Bruning-Fann, L. Sullivan, D. Berry, T. Carlson, R. B. Minnis, J. B. Payeur, and J. Sikarskie. 1997. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. J. Wildl. Dis. 33:749-758. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Department of Agriculture. 1999. Uniform methods and rules for the eradication of bovine tuberculosis. APHIS circular 91-45-011. U.S. Government Printing Office, Washington, D.C.
- 26.van Pinxteren, L. A., P. Ravn, E. M. Agger, J. Pollock, and P. Andersen. 2000. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 7:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waters, W. R., M. V. Palmer, D. L. Whipple, R. E. Slaughter, and S. L. Jones. 2004. Immune responses of white-tailed deer (Odocoileus virginianus) to Mycobacterium bovis BCG vaccination. J. Wildl. Dis. 40:66-78. [DOI] [PubMed] [Google Scholar]
- 29.Waters, W. R., B. J. Nonnecke, M. V. Palmer, S. Robbe-Austermann, J. P. Bannantine, J. R. Stabel, D. L. Whipple, J. B. Payeur, D. M. Estes, J. E. Pitzer, and F. C. Minion. 2004. Use of recombinant ESAT-6:CFP-10 fusion protein for differentiation of infections of cattle by Mycobacterium bovis and by M. avium subsp. avium and M. avium subsp. paratuberculosis. Clin. Diagn. Lab Immunol. 11:729-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waters, W. R., M. V. Palmer, S. C. Olsen, R. E. Sacco, and D. L. Whipple. 2003. Immune responses of elk to Mycobacterium bovis bacille Calmette Guerin vaccination. Vaccine 21:1518-1526. [DOI] [PubMed] [Google Scholar]
- 31.Whipple, D. L., M. V. Palmer, R. E. Slaughter, and S. L. Jones. 2001. Comparison of purified protein derivatives and effect of skin testing on results of a commercial gamma interferon assay for diagnosis of tuberculosis in cattle. J. Vet. Diagn. Investig. 13:117-122. [DOI] [PubMed] [Google Scholar]
- 32.Wilcke, J. T., B. N. Jensen, P. Ravn, A. B. Andersen, and K. Haslov. 1996. Clinical evaluation of MPT-64 and MPT-59, two proteins secreted from Mycobacterium tuberculosis, for skin test reagents. Tuber. Lung Dis. 77:250-256. [DOI] [PubMed] [Google Scholar]
- 33.Wood, P. R., L. A. Corner, J. S. Rothel, C. Baldock, S. L. Jones, D. B. Cousins, B. S. McCormick, B. R. Francis, J. Creeper, and N. E. Tweddle. 1991. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust. Vet. J. 68:286-290. [DOI] [PubMed] [Google Scholar]
- 34.Wood, P. R., and J. S. Rothel. 1994. In vitro immunodiagnostic assays for bovine tuberculosis. Vet. Microbiol. 40:125-135. [DOI] [PubMed] [Google Scholar]