Abstract
Autoantibodies to the ribosomal phosphoproteins (Rib-P) are a serological feature of patients with systemic lupus erythematosus (SLE). The reported prevalence of anti-Rib-P antibodies in SLE ranges from 10 to 40%, being higher in Asian patients. The variation in the observed frequency may be related to a number of factors but is dependent in large part on the test system used to detect the autoantibodies. An association of anti-Rib-P with central nervous system involvement and neuropsychiatric manifestations of SLE has been controversial. In the present international multicenter study, we evaluated the clinical accuracy of a new sensitive Rib-P-specific enzyme-linked immunosorbent assay based on recombinant Rib-P polypeptides. The results showed that 21.3% of 947 SLE patients, but only 0.7% of 1,113 control patients, had a positive test result (P < 0.0001). The sensitivity, specificity, positive and negative predictive values, and diagnostic efficiency were determined to be 21.3%, 99.3%, 95.6%, 62.2%, and 65.3%, respectively. When evaluated in the context of participating centers, the prevalence of anti-Rib-P antibodies was found in descending frequency, as follows: China (35%) > Poland (34%) > Japan (28%) > United States (26%) > Germany (Freiburg; 23.3%) > Denmark (20.5%) > Germany (Berlin; 19%) > Mexico (15.7%) > Israel (11.7%) > Brazil (10%) > Canada (8%). The substantial data from this study indicate that the prevalence of anti-Rib-P antibodies may not be restricted to the genetic background of the patients or to the detection system but may depend on regional practice differences and patient selection. We confirm previously reported associations of antiribosomal antibodies with clinical symptoms and serological findings. Remarkably, we found a lower occurrence of serositis in Rib-P-positive lupus patients.
Autoantibodies to the ribosomal phosphoproteins (Rib-P) are a serological feature of patients with systemic lupus erythematosus (SLE) (4, 8, 9). The Rib-P autoantigen(s) consists of three protein components of the 60S ribosomal subunit, designated P0 (38 kDa), P1 (19 kDa), and P2 (17 kDa) (8, 12). A pentameric complex composed of one copy of P0 and two copies each of P1 and P2 interacts with the 28S rRNA molecule to form a GTPase domain, which is active during the elongation step of protein translation (8). The major immunoreactive epitope of this ribosomal autoantigen has been localized to the carboxy-terminal domain, which is highly conserved in all three proteins and contains two phosphorylated serine residues (e.g., Ser102 and Ser105 of human P2) (8, 16, 17). Several studies have shown that both the acidic and hydrophobic clusters, but not the phosphorylation of the P proteins, are critical for autoantibody binding (8, 16, 23). Furthermore, epitope mapping studies have shown that the major epitope domain is located within the last six C-terminal amino acids (GFGLFD) (8, 16, 23).
The reported prevalence of anti-Rib-P antibodies in SLE ranges from 10 to 40%, being higher in Asian patients and at a relatively lower prevalence in black and Caucasian patients (3, 12, 15, 18, 23, 30, 35). The variation in the observed frequency may be related to a number of factors but is dependent in large part on the test system used to detect the autoantibodies. In one study, an immunoblot technique was reported tobe the most sensitive (12). Several enzyme-linked immunosorbent assay (ELISA) systems designed for research studies as well as diagnostic applications have been evaluated. The antigenic analytes employed in these tests included purified native proteins, recombinant polypeptides, a synthetic peptide comprising the 22 C-terminal amino acids (C22), and a multiple antigen peptide construct (1, 12, 13, 21, 22, 23, 26, 30, 38). Recently, a Rib-P profile assay based on the three recombinant ribosomal P proteins and the C22 peptide in separate tests was developed and evaluated (22).
Anti-Rib-P antibodies were mainly detected in patients during the active phase of SLE and were believed to be correlated with lupus nephritis or hepatitis (4, 11, 12, 24, 28, 30, 36). Moreover, it was suggested that anti-Rib-P antibodies are more prevalent in juvenile-onset SLE than in adult-onset SLE (27). An association of anti-Rib-P with neuropsychiatric manifestations of SLE (NPSLE) has been more controversial (1, 4, 5, 11, 12, 15, 19, 25, 29, 31).
The current extended international multicenter study was designed to evaluate an ELISA for the detection of anti-Rib-P antibodies based on combinations of the three recombinant P polypeptides and to evaluate its clinical accuracy and utility. Another goal of the study was to elucidate the association of anti-Rib-P antibodies with clinical manifestations and with the demographic backgrounds of SLE patients in a large patient group, using a uniform detection system.
MATERIALS AND METHODS
Serum samples.
Sera from unselected SLE patients (n = 947) and various controls (n = 1,113) (Table 1) were collected in 11 centers and then retrospectively tested in the center where they were collected (Table 2) with the Rib-TriPlex assay (Sweden Diagnostics, Freiburg, Germany) developed for this investigation. Quality controls were included in each assay, and the validity of test results was ensured by the organizers of the study. The SLE patient cohort was classified according to the revised criteria for SLE (34). An index serum panel of 50 SLE and 100 control samples from a previous comparative study was used to evaluate different methods used to detect anti-Rib-P antibodies (22). Controls were classified at the participating centers based on the criteria for each disease, as done in a previous investigation (22). The collection and use of patient samples were done in accordance with local ethics committee regulations.
TABLE 1.
Summary of results for 947 SLE patients and 1,113 controls tested for anti-Rib-P antibodies by means of the Rib-TriPlex assay
| Disease or disorder | No. of patients | No. of positive results with Rib-TriPlex assay | % Positive results | Mean (RU) | SD | Maximum |
|---|---|---|---|---|---|---|
| SLE | 947 | 201 | 21.3 | 1.3 | 4 | 46.2 |
| All controls | 1,113 | 8 | 0.7 | 0.45 | 0.46 | 9.7 |
| Infectious diseases | 140 | 1 | 0.7 | 0.33 | 0.28 | 2.5 |
| Cytomegalovirus | 20 | 0 | 0.0 | 0.29 | 0.12 | 0.6 |
| Epstein-Barr virus | 20 | 0 | 0.0 | 0.5 | 0.29 | 1.3 |
| Human immunodeficiency virus | 20 | 0 | 0.0 | 0.34 | 0.13 | 0.7 |
| Hepatitis C virus | 68 | 1 | 1.5 | 0.3 | 0.35 | 2.3 |
| Unidentified infectious disease | 12 | 0 | 0.0 | 0.37 | 0.19 | 0.4 |
| Rheumatic disorders | 544 | 7 | 1.3 | 0.46 | 0.56 | 9.7 |
| RA | 284 | 3 | 1.1 | 0.44 | 0.27 | 8.4 |
| Systemic sclerosis | 131 | 2 | 1.5 | 0.43 | 0.44 | 3.7 |
| Mixed connective tissue disease | 36 | 1 | 2.8 | 0.38 | 0.21 | 1.9 |
| PM | 15 | 1 | 6.7 | 0.95 | 2.33 | 9.7 |
| Sjögren's syndrome | 41 | 0 | 0.0 | 0.5 | 0.22 | 1.11 |
| PM/dermatomyositis | 37 | 0 | 0.0 | 0.4 | 0.18 | 0.9 |
| Vasculitis/thrombosis | 32 | 0 | 0 | 0.4 | 0.22 | 1.0 |
| Wegener’s grandonatosis | 6 | 0 | 0.0 | 0.15 | 0.08 | 0.3 |
| Antiphospholipid syndrome | 26 | 0 | 0.0 | 0.45 | 0.2 | 1.0 |
| Organ-specific disorders | 23 | 0 | 0 | 0.18 | 0.8 | 0.4 |
| Hashimoto thyroiditis | 11 | 0 | 0.0 | 0.17 | 0.09 | 0.4 |
| Graves' disease | 12 | 0 | 0.0 | 0.19 | 0.6 | 0.3 |
| Others | 374 | 0 | 0 | 0.33 | 0.16 | 1.1 |
| Familial Mediterranean fever | 20 | 0 | 0.0 | 0.28 | 0.7 | 0.4 |
| Normal human serum | 208 | 0 | 0.0 | 0.32 | 0.15 | 0.8 |
| Crohn's disease | 25 | 0 | 0.0 | 0.41 | 0.23 | 1.1 |
| Diverse diseases | 121 | 0 | 0.0 | 0.36 | 0.17 | 0.6 |
TABLE 2.
Assay performance in the context of different centers
| Center no. | Location | No. of SLE patients/no. of controls | % Sensitivity/% specificity | AUC | PPV/NPV/ TE (%)a |
|---|---|---|---|---|---|
| 1 | Shanghai | 100/100 | 35.0/98.0 | 0.660 | 94.6/60.1/66.5 |
| 2 | Poland | 93/91 | 34.0/100 | 0.913 | 100/59.9/66.8 |
| 3 | Japan | 100/100 | 28.0/98.0 | 0.748 | 93.3/57.6/63 |
| 4 | United States | 100/70 | 26.0/98.7 | 0.739 | 96.3/50/57.1 |
| 5 | Freiburg | 51/259 | 23.3/100.0 | 0.788 | 100/71.7/74 |
| 6 | Denmark | 44/31 | 20.5/96.8 | 0.550 | 90/46.2/52 |
| 7 | Berlin | 100/87 | 19.0/100 | 0.853 | 100/51.8/56.7 |
| 8 | Mexico | 8295 | 15.7/99 | 0.520 | 92.9/57.6/60.3 |
| 9 | Israel | 77/97 | 11.7/100 | 0.679 | 100/58.8/60.9 |
| 10 | Brazil | 100/83 | 10.0/100.0 | 0.649 | 100/48/50.8 |
| 11 | Canada | 100/100 | 8.0/100.0 | 0.623 | 100/52.1/54 |
| All | 947/1,113 | 21.3/99.3 | 0.667 | 95.6/62.2/65.3 |
PPV, positive predictive value; NPV, negative predictive value; TE, test efficiency.
Preparation of recombinant proteins.
Recombinant P proteins were produced in SF9 insect cells by using a baculovirus expression system (23). The purity of the different P proteins was determined to be >95% by Western blot analysis. Protein concentrations were determined using bicinchoninic acid reagents (Sigma-Aldrich Corporation, St. Louis, MO).
Commercial ELISA systems for detection of anti-Rib-P antibodies.
Fifty sera from SLE patients were tested with ELISA kits that were based on three different ribosomal antigens (native ribosomal P antigen, recombinant ribosomal P0, and the synthetic peptide C22) which were compared in a previous study (22). The cutoff values were used as suggested by the manufacturer.
Rib-TriPlex ELISA.
To achieve a highly sensitive screening assay for anti-Rib-P autoantibodies, equimolar mixtures of the recombinant Rib-P proteins in various concentrations were allowed to adsorb to ELISA plates (Maxisorb; Nunc, Roskilde, Denmark). Furthermore, the recombinant proteins were combined at the molar ratio of the native, pentameric hetero-Rib-P complex [P0(P1/P2)2] (referred to Rib-TriPlex in this publication) in phosphate-buffered saline (pH 7.6)byovernight incubation at 15°C followed by blocking with 0.5% bovine serum albumin for 30 min at room temperature. A calibrator was developed using a human index anti-Rib-P serum, and the optical density was adjusted to 1.0. The index serum panel (n = 150) was used to evaluate different absorption conditions and for comparison with other methods used to detect anti-Rib-P antibodies (22). A conversion factor was calculated for semiquantitative expression of results, and ratios of >1.0 (optical density of sample × conversion factor/optical density of calibrator) were finally defined as above the cutoff. All results of the Rib-TriPlex are given in relative units (RU).
Statistical evaluations.
Statistical evaluations, including receiver operating characteristic analysis and calculations of positive predictive and negative predictive values and test efficiency, were carried out using Analyze-it software (version 1.62; Analyze-it Software, Ltd., Leeds, United Kingdom). Differences between anti-Rib-P antibodies and clinical features were calculated by chi-square analysis and by Fisher's exact test as 99.5% confidence intervals (CI) around the odds ratio (OR), using Woolf's approximation. Correlations with P values of ≤0.05 were considered significant. Agreement between anti-Rib-P antibody assays was analyzed using the kappa method.
RESULTS
Development of Rib-P screening ELISA.
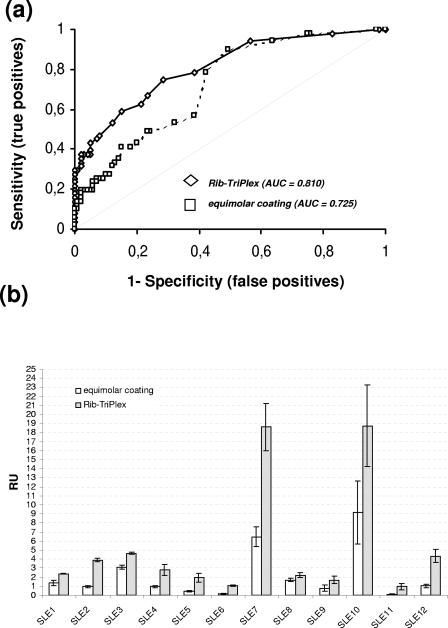
ELISA plates coated with different concentrations and molar ratios of the three ribosomal polypeptides and tested with the index serum panel showed no significant increase in sensitivity compared to ELISA plates coated with the three Rib-P proteins in the same molar ratio regardless of the overall coating concentration (22). In contrast, ELISA plates coated with the three polypeptides in the molar ratio as they occur in their native pentameric complex (Rib-TriPlex assay) displayed a significantly improved differentiation of SLE patients and controls, as revealed by the area under the curve (AUC) (0.810 [P < 0.0001] versus 0.725 [P < 0.0001]) as well as the increased sensitivity of 30% (Fig. 1a). A more detailed analysis revealed that 12/50 SLE samples, but no control sera, showed statistically significant enhanced reactivity in the Rib-TriPlex assay compared to the coating variants with equal molar ratios of the three polypeptides (Fig. 1b). The Rib-TriPlex assay demonstrated a higher sensitivity than three commercial anti-Rib-P ELISA systems based on different antigens (Fig. 2a). Therefore, this sensitive assay was selected for an international multicenter evaluation.
FIG. 1.
Comparison of two ELISA coating variants. ELISA plates coated with the three recombinant ribosomal P proteins at equal molar ratios and at the molar ratio of the native, pentameric complex [P0(P1/P2)2] were assayed with an index patient group of 50 SLE patients and 100 controls taken from a previous study (22), and the results were compared. (a) ELISA plates coated with the three polypeptides in the molar ratio of the heterocomplex P0(P1/P2)2 displayed a significantly improved differentiation of SLE patients and controls, as revealed by the AUC (0.810 versus 0.725). (b) A more detailed analysis revealed that 12/50 SLE samples, but no control sera, showed statistically significantly enhanced reactivities in the Rib-TriPlex assay compared to the coating variant with equal molar ratios of the three polypeptides (error bars show 2 standard deviations). Six of those sera were negative in the assay based on equimolar coating of Rib-P proteins but were positive in the Rib-TriPlex assay. Fisher's exact test revealed a P value of 0.0000007041 for the increased reactivities, and a paired-sample t test yielded a two-tailed P value of 0.0160 comparing the two assays.
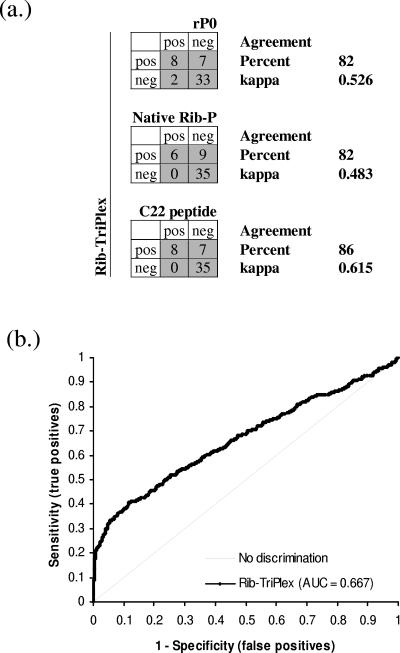
FIG. 2.
Evaluation of the Rib-TriPlex assay. (a) Comparison of the Rib-TriPlex assay with three commercial assays, based on recombinant ribosomal P0 (rP0), native ribosomal antigen, and the C22 peptide. The agreement between the methods was moderate for Rib-TriPlex versus the rP0 and native Rib-P assays and good for Rib-TriPlex versus the C22 assay. (b) Samples (947) from SLE patients and 1,113 controls were assayed for anti-Rib-P antibodies, and the results were used to perform receiver operating characteristic analysis. The results show a clear discrimination between SLE patients and the various controls.
International multicenter evaluation of Rib-TriPlex assay.
When sera from SLE patients (n = 947) and various controls (n = 1113) were assayed for anti-ribosomal P protein antibodies, a clear discrimination between SLE patients and various controls was found (Table 1; Fig. 2b). In total, 21.3% of SLE patients and only 0.7% of control patients had a positive test using the Rib-TriPlex ELISA (Table 1). Statistical evaluation by chi-square analysis and Fisher's exact test showed a statistically relevant difference between SLE patients and various controls (P < 0.0001; χ2 = 233.76; OR = 37.216; CI = 13.411 to 103.279).
The eight control patients that tested positive included three males and five females from six centers. Three patients had rheumatoid arthritis (RA) (8.4, 1.5, and 2.1 RU), one had polymyositis (PM) (RU 9.7), two had systemic sclerosis (1.7 and 3.7 RU), one had mixed connective tissue disease (1.9 RU), and one had hepatitis C virus infection (2.3 RU).
When evaluated in the context of participating centers, the prevalence of anti-Rib-P antibodies was found in descending order as follows: China > Poland > Japan > United States > Germany (Freiburg) > Denmark > Germany (Berlin) > Mexico >Israel > Brazil > Canada. Based on these results, sensitivities, specificities, positive and negative predictive values, and efficiencies were calculated and are summarized in Table 2.
Correlation of anti-Rib-P reactivity with the demographic background of SLE patients and with clinical parameters.
The mean age of the SLE patients was 40.2 ± 14.4 years (range, 9 to 86 years), and there was no difference in the gender ratios between Rib-P-positive and Rib-P-negative patients. Among the anti-Rib-P antibody-positive SLE patients, the mean age was 36.3 ± 13.6 years (12 to 73 years), whereas the mean age for the Rib-P-negative group was 41.1 ± 14.6 years. To evaluate the effect of the racial background of the patient cohort under investigation, we evaluated two homogeneous Caucasian (Berlin, Germany, and Poland) and two homogeneous Asian (Japan and Shanghai, China) SLE patient cohorts. We did not find a statistically higher prevalence in either of these racial groups (for the Asian group versus the Caucasian group, χ2 = 0.98, P = 0.3213, OR = 1.275, and CI = 0.686 to 2.372). When autoantibody specificities were correlated with clinical features of SLE patients, there was a statistically significant association of anti-Rib-P autoantibodies with malar rash (χ2 = 9.72, P = 0.0018, OR = 2.107, CI = 1.095 to 4.057), renal disorders (χ2 = 7.95, P = 0.0048, OR = 1.825, CI = 1.020 to 3.266), and to a lesser extent, neurological complications (χ2 = 4.09, P = 0.0432, OR = 1.771, CI = 0.839 to 3.736) but not with other clinical features. In addition, there was a negative association of anti-Rib-P reactivity with serositis (χ2 = 6.10, P = 0.0135, OR = 0.452, CI = 0.189 to 1.082). When evaluated in the context of participating centers, the association of anti-Rib-P antibodies and clinical features showed significant differences. A statistically significant association could only be reported for anti-Rib-P and malar rash in the patient groups from Berlin (χ2 = 17.36, P < 0.0001, OR = 36.913, CI = 1.589 to 858.194) and Shanghai (χ2 = 4.32, P = 0.0376, OR = 2.708, CI = 0.785 to 9.350), for lupus nephritis in the patient group from Berlin (χ2 = 4.12, P = 0.0424, OR = 3.245, CI = 0.733 to 14.360), and for NPSLE in the patient groups from Japan (χ2 = 11.58, P = 0.0007, OR = 7.118, CI = 1.414 to 35.833) and Berlin (χ2 = 6.26, P = 0.0124, OR = 4.970, CI = 0.929 to 26.588).
DISCUSSION
Although anti-ribosomal P protein autoantibodies have been known for approximately 25 years, they have not achieved the attention and clinical impact that anti-Sm or anti-double-stranded DNA (anti-dsDNA) antibodies have (34, 39). This might be explained by the limited reliability of indirect immunofluorescence (IIF) assays for the detection of anti-Rib-P antibodies or by the absence of an international reference serum. It is noteworthy that a reference standard human Rib-P antibody with a reactivity of 8.3 RU in the Rib-TriPlex assay, but only weak cytoplasmic IIF staining, has recently become available (Centers for Disease Control and Prevention, Atlanta, GA), and this should be an important step in standardizing assays from different sources. Furthermore, the relatively late discovery of anti-Rib-P antibodies in 1979 compared to those of anti-dsDNA (in 1968) and anti-Sm (in 1966) has also likely contributed to the marginal impact of anti-ribosomal antibodies on clinical practice and the development of classification criteria for SLE (20, 39).
Various techniques have been proposed for the detection of anti-Rib-P antibodies (1, 12, 13, 14, 21, 23, 26, 37, 38). A more recent study has confirmed the high efficiency of the immunoblot technique compared to peptide ELISAs (13). An obvious advantage of immunoblotting with native antigen is that the detection of antibodies to the individual Rib-P proteins is possible.
In a recent study, this feature was incorporated into a semiquantitative Rib-P profile ELISA, which in addition to the recombinant antigens contains the C22 peptide, with each of the four ribosomal antigens used to coat separate wells (22). The clinical sensitivity when this approach was used was significantly improved. Thus, the best method for detecting antiribosomal antibodies appears to be the combination of different Rib-P antigens. These findings are in keeping with the N-terminal epitopes reported by Fabien and colleagues, who argued against the use of the C22 peptide for the detection of antiribosomal antibodies (10). Based on this background, an anti-Rib-P screening ELISA using the three recombinant proteins in the molar ratio of the native heterocomplex, P0(P1/P2)2, was developed for this study. The feature of the ribosomal P polypeptide assay that apparently conferred heightened sensitivity was the heterocomplex coating, i.e., P0(P1/P2)2. This is likely due to the formation of a conformational epitope, as this assay gave positive results for 15/50 (30%) SLE patients, in contrast to three other commercial Rib-P kits, which detected 12%, 16%, and 20% of SLE patients, respectively (22). Similar findings were observed by Lin and colleagues, who showed that a combination of all three recombinant ribosomal P proteins yielded a fivefold increased sensitivity compared to that with the C22 peptide used in ELISA (21). Since the recombinant antigens used in the ELISAs of both studies were at least partially denatured during purification, it is unclear if they refold and form the native heterocomplex P0(P1/P2)2. Further studies should be done to address this question.
Despite substantial investigations, the relationship between antiribosomal antibodies and organic central nervous system involvement is still controversial (4, 5, 11, 12, 15, 19, 23, 25, 29, 31). Anti-Rib-P antibodies have also been reported to be correlated with lupus nephritis (24, 28). Reasons for the discrepancy between correlation studies may be the difference in the criteria used to define and detect various disease features or the demographics and/or makeup of the patient cohorts being investigated. Therefore, this study was organized to study antibodies to Rib-P in a broad genetic and geographic background. The genetic and demographic background showed a lower significance for the prevalence of anti-Rib-P, since the results for a Caucasian patient cohort were comparable to the data for an Asian patient group. Since we used a unique anti-Rib antibody assay for all centers, we could rule out that the selection of an anti-Rib-P antibody test has the most pronounced effect on the differences in frequency. Variations in the prevalence of certain autoantibodies (anti-dsDNA, anti-Sm, and anti-U1-RNP) between the different centers suggested a strong influence of the makeup of the SLE patient group, although all participating centers included unselected SLE patients in the study. This is in keeping with two independent studies which have shown the critical importance of accuracy in both the performance of serological assays and the selection of SLE subpopulations (19, 37). When clinical data were analyzed in the context of all participating centers, we found an association of anti-Rib-P with malar rash, renal disorders, anemia, and to a lesser extent, neurological complications. In the context of the individual centers, significant associations could only be confirmed for malar rash (Berlin and Shanghai), lupus nephritis (Berlin), and NPSLE (Canada). Whether the lack of associations in the individual centers is due to the limited number of samples, and thus to limited statistical reliability, remains unclear. Although our study is, to the best of our knowledge, the largest published analysis of anti-Rib-P antibodies and the first study that includes a broad range of control patients, it may be limited in accessing an association between anti-Rib-P and clinical manifestations because it was a retrospective evaluation and the diagnosis of NPSLE did not include uniform clinical evaluation and assessment of disease status such as the SLE Disease Activity Index (2). Other variables likely included the use of the same diagnostic tools and approaches such as sensitive tests that can detect minor neurological and behavioral deficits. Furthermore, out of necessity, the patients were examined and diagnosed by different physicians and in different centers. It is likely that the patient groups from the different centers were not homogeneous and that patients with milder disease were more common in one center while the majority of patients from other centers had more severe disease. Moreover, it remains unclear whether there was a difference in the mean interval between diagnosis and antibody testing and treatment. It is possible that patients in some centers (i.e., in Western countries) were treated earlier and more aggressively.
We confirmed the reported association between anti-Rib-P and anti-dsDNA, anti-SS-A/Ro, and anti-cardiolipin when analyzing the entire patient cohort. In contrast, previously published associations between anti-Rib-P and anti-dsDNA (4, 11, 14) were found in only two centers (Berlin and Canada). This phenomenon is most likely due to the heterogeneity of the other autoantibody assays used in the individual laboratories. The relationship of anti-Rib-P and anti-dsDNA antibodies observed in this study supports the reported coexistence and cross-reactivity of anti-dsDNA and anti-Rib-P antibodies (6, 32, 33). Associations between anti-Rib-P and anti-Ro (SS-A) or anti-cardiolipin have previously been described, and these are in general agreement with the observations of this study (4, 11, 14). Whether these associations are also due to cross-reactivity remains speculative and requires further research. Although not all patients in our study had been tested for anti-dsDNA and anti-Sm, the evidence strongly suggested that anti-Rib-P autoantibodies frequently occur without other lupus-specific antibodies. Remarkably, 52/143 (36.4%), 108/131 (82.4%), and 34/124 (27.4%) Rib-P-positive samples were negative for anti-dsDNA, anti-Sm, or both antibodies, respectively (data not shown). With regard to the sensitivity of >20% for SLE patients of the Rib-TriPlex assay, we conclude that about 8% of SLE patients are anti-Rib-P positive, anti-dsDNA negative, and anti-Sm negative, and may therefore fail to fulfill the diagnostic criteria for SLE since anti-Rib-P reactivity is difficult to detect by IIF.
Due to the high positive predictive value of anti-Rib-P antibodies, the Rib-P-positive control patients should be carefully monitored and clinically examined. For example, follow-up of one anti-Rib-P-positive RA patient from Mexico revealed that the patient subsequently developed renal disease and sufficient criteria to be classified as having SLE. Based on our study and other related studies, we propose that, akin to anti-Sm and anti-dsDNA, anti-Rib-P antibodies detected by one agreed method may be considered for inclusion as a criterion for the classification of SLE.
Acknowledgments
We thank Yun Zhang (University of Calgary, Canada) and Lucyna Korman (Wroclaw University of Medicine) for technical assistance, Heike Berthold (Sweden Diagnostics) for providing recombinant antigens, and Jacek Szechinski (Rheumatology Department, Wroclaw University of Medicine) for valuable suggestions and delivery of clinically defined sera.
REFERENCES
- 1.ACR Ad Hoc Committee on Neuropsychiatric Lupus Syndromes. 1999. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 42:599-608. [DOI] [PubMed] [Google Scholar]
- 2.American College of Rheumatology. Systemic lupus erythematosus disease activity index (SLEDAI). [Online.] http://www.rheumatology.org/sections/pediatric/sledai.asp?aud=mem.
- 3.Arnett, F. C., J. D. Reveille, H. M. Moutsopoulos, L. Georgescu, and K. B. Elkon. 1996. Ribosomal P autoantibodies in systemic lupus erythematosus. Frequencies in different ethnic groups and clinical and immunogenetic associations. Arthritis Rheum. 39:1833-1839. [DOI] [PubMed] [Google Scholar]
- 4.Bonfa, E., and E. K. Elkon. 1986. Clinical and serologic associations of the antiribosomal P protein antibody. Arthritis Rheum. 29:981-985. [DOI] [PubMed] [Google Scholar]
- 5.Bonfa, E., S. J. Golombek, L. D. Kaufman, S. Skelly, H. Weissbach, N. Brot, and K. B. Elkon. 1987. Association between lupus psychosis and anti-ribosomal P protein antibodies. N. Engl. J. Med. 317:265-271. [DOI] [PubMed] [Google Scholar]
- 6.Caponi, L., D. Chimenti, F. Pratesi, and P. Migliorini. 2002. Anti-ribosomal antibodies from lupus patients bind DNA. Clin. Exp. Immunol. 130:541-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caponi, L., S. Pegoraro, V. Di Bartolo, P. Rovero, R. Revoltella, and S. Bombardieri. 1995. Autoantibodies directed against ribosomal P proteins: use of a multiple antigen peptide as the coating agent in ELISA. J. Immunol. Methods 179:193-202. [DOI] [PubMed] [Google Scholar]
- 8.Elkon, K. 1994. Ribosomal RNP, p. 1-11. In W. J. Van Venrooij and R. N. Maini (ed.), Manual of biological markers of disease. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 9.Elkon, K. B., A. P. Parnassa, and C. L. Foster. 1985. Lupus autoantibodies target ribosomal P proteins. J. Exp. Med. 162:459-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabien, N., A. Moreira, J. P. Lavergne, A. Desbos, P. Surgey, C. Alves de Olivera, P. Gonzalo, A. Venot, J. Bienvenu, H. Perrier, J. P. Reboud, and J. C. Monier. 1999. Autoantibodies directed against the ribosomal P proteins are not only directed against a common epitope of the P0, P1 and P2 proteins. J. Autoimmun. 13:103-110. [DOI] [PubMed] [Google Scholar]
- 11.Gerli, R., L. Caponi, A. Tincani, R. Scorza, M. G. Sabbadini, M. G. Danieli, V. De Angelis, M. Cesarotti, M. Piccirilli, R. Quartesan, P. Moretti, C. Cantoni, F. Franceschini, I. Cavazzana, L. Origgi, M. Vanoli, E. Bozzolo, L. Ferrario, A. Padovani, O. Gambini, L. Vanzulli, D. Croce, and S. Bombardieri. 2002. Clinical and serological associations of ribosomal P autoantibodies in systemic lupus erythematosus: prospective evaluation in a large cohort of Italian patients. Rheumatology 41:1357-1366. [DOI] [PubMed] [Google Scholar]
- 12.Gerli, R., and L. Caponi. 2005. Anti-ribosomal P protein antibodies. Autoimmunity 38:85-92. [DOI] [PubMed] [Google Scholar]
- 13.Ghirardello, A., L. Caponi, F. Franceschini, S. Zampieri, M. Quinzanini, R. Bendo, S. Bombardieri, P. F. Gambari, and A. Doria. 2002. Diagnostic tests for antiribosomal P protein antibodies: a comparative evaluation of immunoblotting and ELISA assays. J. Autoimmun. 19:71-77. [DOI] [PubMed] [Google Scholar]
- 14.Ghirardello, A., A. Doria, S. Zampieri, R. Gerli, E. Rapizzi, and P. F. Gambari. 2000. Anti-ribosomal P protein antibodies detected by immunoblotting in patients with connective tissue diseases: their specificity for SLE and association with IgG anticardiolipin antibodies. Ann. Rheum. Dis. 59:975-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenwood, D. L., V. M. Gitlits, F. Alderuccio, J. W. Sentry, and B. H. Toh. 2002. Autoantibodies in neuropsychiatric lupus. Autoimmunity 35:79-86. [DOI] [PubMed] [Google Scholar]
- 16.Hasler, P., N. Brot, H. Weissbach, W. Danho, Y. Blount, J. L. Zhou, and K. B. Elkon. 1994. The effect of phosphorylation and site-specific mutations in the immunodominant epitope of the human ribosomal P proteins. Clin. Immunol. Immunopathol. 72:273-279. [DOI] [PubMed] [Google Scholar]
- 17.Hasler, P., N. Brot, H. Weissbach, A. P. Parnassa, and K. B. Elkon. 1991. Ribosomal proteins P0, P1, and P2 are phosphorylated by casein kinase II at their conserved carboxyl termini. J. Biol. Chem. 266:13815-13820. [PubMed] [Google Scholar]
- 18.Isenberg, D. A., M. Garton, M. W. Reichlin, and M. Reichlin. 1997. Long-term follow-up of autoantibody profiles in black female lupus patients and clinical comparison with Caucasian and Asian patients. Br. J. Rheumatol. 136:229-233. [DOI] [PubMed] [Google Scholar]
- 19.Isshii, K., and S. Hirohata. 1998. Differential roles of the anti-ribosomal P antibody and antineuronal antibody in the pathogenesis of central nervous system involvement in systemic lupus erythematosus. Arthritis Rheum. 41:1819-1827. [DOI] [PubMed] [Google Scholar]
- 20.Koffler, D., T. E. Miller, and R. G. Lahita. 1979. Studies on the specificity and clinical correlation of antiribosomal antibodies in systemic lupus erythematosus sera. Arthritis Rheum. 22:463-470. [DOI] [PubMed] [Google Scholar]
- 21.Lin, J. L., V. Dubljevic, M. J. Fritzler, and B. H. Toh. 2005. Major immunoreactive domains of human ribosomal P proteins lie N-terminal to a homologous C-22 sequence: application to a novel ELISA for systemic lupus erythematosus. Clin. Exp. Immunol. 141:155-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahler, M., K. Kessenbrock, J. Raats, and M. J. Fritzler. 2004. Technical and clinical evaluation of anti-ribosomal P protein immunoassays. J. Clin. Lab. Anal. 18:215-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahler, M., K. Kessenbrock, J. Raats, R. Williams, M. J. Fritzler, and M. Blüthner. 2003. Characterization of the human autoimmune response to the major C-terminal epitope of the ribosomal P proteins. J. Mol. Med. 81:194-204. [DOI] [PubMed] [Google Scholar]
- 24.Martin, A. L., and M. Reichlin. 1996. Fluctuations of antibody to ribosomal P proteins correlate with appearance and remission of nephritis in SLE. Lupus 5:22-29. [DOI] [PubMed] [Google Scholar]
- 25.Nojima, Y., S. Minota, A. Yamada, F. Takaku, S. Aotsuka, and R. Yokohari. 1992. Correlation of antibodies to ribosomal P protein with psychosis in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 51:1053-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rayno, K., and M. Reichlin. 2000. Evaluation of assays for the detection of autoantibodies to the ribosomal P proteins. Clin. Immunol. 95:99-103. [DOI] [PubMed] [Google Scholar]
- 27.Reichlin, M., T. F. Broyles, O. Hubscher, J. James, T. A. Lehman, R. Palermo, H. A. Stafford, E. Taylor-Albert, and M. Wolfson-Reichlin. 1999. Prevalence of autoantibodies to ribosomal P proteins in juvenile-onset systemic lupus erythematosus compared with the adult disease. Arthritis Rheum. 42:69-75. [DOI] [PubMed] [Google Scholar]
- 28.Reichlin, M., and M. Wolfson-Reichlin. 1999. Evidence for the participation of anti-ribosomal P antibodies in lupus nephritis. Arthritis Rheum. 42:2728-2729. [DOI] [PubMed] [Google Scholar]
- 29.Reichlin, M. 2003. Ribosomal P antibodies and CNS lupus. Lupus 12:916-918. [DOI] [PubMed] [Google Scholar]
- 30.Sato, T., T. Uchiumi, T. Ozawa, M. Kikuchi, M. Nakano, R. Kominami, and M. Arakawa. 1991. Autoantibodies against ribosomal proteins found with high frequency in patients with systemic lupus erythematosus with active disease. J. Rheumatol. 18:1681-1684. [PubMed] [Google Scholar]
- 31.Schneebaum, A. B., J. D. Singleton, S. G. West, J. K. Blodgett, L. G. Allen, J. C. Cheronis, and B. L. Kotzin. 1991. Association of psychiatric manifestations with antibodies to ribosomal P proteins in systemic lupus erythematosus. Am. J. Med. 90:54-62. [DOI] [PubMed] [Google Scholar]
- 32.Sun, K. H., C. C. Hong, S. J. Tang, G. H. Sun, W. T. Liu, S. H. Han, and C. L. Yu. 1999. Anti-dsDNA autoantibody cross-reacts with the C-terminal hydrophobic cluster region containing phenylalanines in the acidic ribosomal phosphoprotein P1 to exert a cytostatic effect on the cells. Biochem. Biophys. Res. Commun. 263:334-339. [DOI] [PubMed] [Google Scholar]
- 33.Takeda, I., K. Rayno, M. Wolfson-Reichlin, and M. Reichlin. 1999. Heterogeneity of anti-dsDNA antibodies in their cross-reaction with ribosomal P protein. J. Autoimmun. 13:423-428. [DOI] [PubMed] [Google Scholar]
- 34.Tan, E. M., A. S. Cohen, J. F. Fries, A. T. Masi, D. J. McShane, N. F. Rothfield, J. G. Schaller, N. Talal, and R. J. Winchester. 1982. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25:1271-1277. [DOI] [PubMed] [Google Scholar]
- 35.Teh, L. S., M. K. Lee, F. Wang, M. Manivasagar, P. J. Charles, G. D. Nicholson, E. M. Hay, D. A. Isenberg, N. Amos, and B. D. Williams. 1993. Antiribosomal P protein antibodies in different populations of patients with systemic lupus erythematosus. Br. J. Rheumatol. 32:663-665. [DOI] [PubMed] [Google Scholar]
- 36.Torre, J. C., L. Mozo, A. Suarez, E. Ramos, and C. Gutierrez. 1996. Antibodies to ribosomal P proteins and hepatic damage in undifferentiated CTD. Ann. Rheum. Dis. 55:562-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzioufas, A. G., N. G. Tzortzakis, E. Panou-Pomonis, K. A. Boki, M. Sakarellos-Daitsiotis, C. Sakarellos, and M. H. Moutsopoulos. 2000. The clinical relevance of antibodies to ribosomal-P common epitope in two targeted systemic lupus erythematosus populations: a large cohort of consecutive patients and patients with active central nervous system disease. Ann. Rheum. Dis. 59:99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshio, T., J. Masuyama, M. Ikeda, K. Tamai, T. Hachiya, T. Emori, A. Mimori, A. Takeda, S. Minota, and S. Kano. 1995. Quantification of antiribosomal P0 protein antibodies by ELISA with recombinant P0 fusion protein and their association with central nervous system disease in systemic lupus erythematosus. J. Rheumatol. 22:1681-1687. [PubMed] [Google Scholar]
- 39.von Mühlen, C. A., and E. M. Tan. 1995. Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin. Arthritis Rheum. 24:323-358. [DOI] [PubMed] [Google Scholar]