Abstract
Considering that little is known about the epidemiology of Neospora caninum infection in humans, particularly in populations with high Toxoplasma gondii infection rates, the present study aimed to investigate the presence of antibodies to N. caninum in T. gondii-seropositive and -seronegative individuals. A total of 256 serum samples divided into four groups (61 samples from human immunodeficiency virus [HIV]-positive patients, 50 samples from patients with neurological disorders, 91 samples from newborns, and 54 samples from healthy subjects) were assessed for N. caninum and T. gondii serologies by indirect fluorescent-antibody test, enzyme-linked immunosorbent assay, and immunoblotting (IB). Immunoglobulin G antibodies to N. caninum were predominantly detected in HIV-infected patients (38%) and patients with neurological disorders (18%), while newborns and healthy subjects showed lower seropositivity rates (5% and 6%, respectively). Seropositivity to N. caninum was significantly associated with seropositivity to T. gondii in both HIV-infected patients and patients with neurological disorders. Seroreactivity to N. caninum was confirmed by IB, with positive sera predominantly recognizing the 29-kDa antigen of N. caninum. The results of this study indicate the presence of N. caninum infection or exposure in humans, particularly in HIV-infected patients or patients with neurological disorders, who could have opportunistic and concurrent infections with T. gondii. These findings may bring a new concern for the unstable clinical health of HIV-infected patients and the actual role of N. caninum infection in immunocompromised patients.
Neospora caninum is a protozoan parasite closely related to Toxoplasma gondii, although they are genetically and antigenically distinct (10, 13). N. caninum infects a wide range of domestic and wild animals, among which cattle seem to be the most important, since the infection causes pregnancy failures, such as repeated abortions and stillbirths, producing enormous economic losses throughout the world (1, 9). Recently, dogs and coyotes have been established as definitive hosts of N. caninum (12, 17).
Many cases of animal neosporosis have been clinically and pathologically misdiagnosed as toxoplasmosis, since patients with both diseases may present with neuromuscular, gastrointestinal, and/or respiratory disorders (19). Although clinical signs are overlapping, N. caninum and T. gondii can be distinguished by serological and immunohistochemical methods when appropriate specific antibodies are used (11).
In nonhuman primates, N. caninum caused fetal infections when it was experimentally transferred to pregnant females, with the lesions closely resembling those caused by congenital toxoplasmosis (2). However, little is known about the epidemiology of N. caninum infection in humans, since only two seroepidemiological studies demonstrated seropositivity rates of about 6.7%, the first one in T. gondii-seropositive individuals (20) and the second one in blood donors (27). Another attempt to investigate the presence of human N. caninum infection was performed with a group of Danish women with repeated abortions of unknown cause, and no antibodies to the parasite were detected by enzyme-linked immunosorbent assay (ELISA), immunofluorescence assay, or Western blotting (21).
As the predominant effects of clinical canine neosporosis are progressive neurological signs, including paralysis, human patients with neurological disorders of unknown etiology should be investigated. Also, evaluation of immunocompromised individuals with suspected toxoplasmic encephalitis might reveal that a subpopulation of these patients may be infected with N. caninum. Thus, this study was designed to evaluate the presence of specific antibodies to N. caninum in human populations presenting with high rates of seropositivity to T. gondii, including patients who are infected by human immunodeficiency virus (HIV) or who have neurological disorders.
MATERIALS AND METHODS
Patients and serum samples.
A total of 256 serum samples were studied and divided into four groups: 61 samples from HIV-positive patients (group I), 50 samples from patients with neurological disorders (group II), 91 samples from newborns (group III), and 54 samples from healthy subjects (group IV). Patients from groups I and II had been referred to the Infectious Diseases Clinic, Clinical Hospital of the Federal University of Uberlāndia (HC-UFU), Minas Gerais, Brazil, from January 2000 to January 2002. All patients in group I were positive for HIV, as detected with an ELISA kit (AxSYM HIV 1/2 gO; Abbott Laboratórios do Brasil Ltda, São Paulo, Brazil), and the results were confirmed by an immunochromatographic test (Determine HIV 1/2; Abbott). Group II included sera from 20 patients who had confirmed neurocysticercosis by serology and neuroimaging techniques and 30 patients who presented with various neurological symptoms (headache, seizures, paresis, muscle weakness, dementia). The sera of group III were obtained from the cord blood of infants who were born at HC-UFU and private hospitals from Uberlāndia and who were sampled from January to August 2002. The serum samples of group IV were obtained from HC-UFU employees between August and September 2003. The present study was submitted to and approved by the Ethical Committee of HC-UFU.
Parasites and antigens.
N. caninum (Nc-1 isolate) tachyzoites were cultured in bovine monocytes in RPMI 1640 supplemented with 3% calf fetal serum and harvested by scraping off the cell monolayer 2 to 3 days after infection (25). T. gondii (RH strain) tachyzoites were maintained in Swiss mice by serial passage for 48 to 72 h and were obtained from mouse peritoneal exudates, as described previously (18).
N. caninum and T. gondii soluble antigens were prepared as described by Scott et al. (22). Parasites were washed twice in phosphate-buffered saline (PBS; pH 7.2) and then submitted to freeze-thaw and sonication cycles. After centrifugation, supernatants were collected, the protein concentration was determined (16), and antigen aliquots were stored at −20°C until they were used as soluble antigen in ELISAs.
N. caninum and T. gondii whole antigens were prepared as described by Camargo (8). Parasites were washed in PBS, treated with 1% formaldehyde, fixed in microscopic slides, and stored at −20°C until they were used in indirect fluorescent-antibody tests (IFATs).
Laboratory assays. (i) ELISA.
Indirect ELISAs were carried out to detect immunoglobulin G (IgG) antibodies to N. caninum (ELISA-Nc) and T. gondii (ELISA-Tg), as described by Mineo et al. (18), with minor modifications. Optimization of the reaction was established in preliminary experiments through block titration of the reagents. Positive control sera were obtained from patients with chronic toxoplasmosis and from monkeys naturally infected by N. caninum (kindly provided by Rosangela Zacarias Machado, Laboratory of Immunoparasitology, FCAV, UNESP, Jaboticabal, SP, Brazil). Negative control sera were obtained from healthy patients with negative serologies for both parasites. Briefly, microtiter plates were separately coated with N. caninum (20 μg/ml) and T. gondii (10 μg/ml) soluble antigens and then incubated with samples diluted 1:50 (ELISA-Nc) and 1:64 (ELISA-Tg) in PBS containing 0.05% Tween 20 (PBS-T) and 5% skim milk. Subsequently, peroxidase-labeled anti-human IgG (Sigma Chemical Co., St. Louis, Mo.) diluted 1:1,000 (ELISA-Nc) and 1:2,000 (ELISA-Tg) was added, and the reaction was revealed by adding 0.03% H2O2 in chromogen buffer (o-phenylenediamine in 0.1 M citrate buffer, pH 5.5). The optical density (OD) was read at 492 nm by using a plate reader (Titertek Multiskan Plus spectrophotometer; Flow Laboratories). The cutoff of the reaction was determined as the mean OD of negative control sera plus 3 standard deviations. Antibody titers were arbitrarily expressed as ELISA indices (EIs), according to the formula ODsample/ODcutoff, as described by Silva et al. (24). Samples with EI values of >1.1 were considered positive.
(ii) IFAT.
Indirect fluorescent-antibody tests were carried out to detect IgG antibodies to N. caninum (IFAT-Nc) and T. gondii (IFAT-Tg), as described by Mineo et al. (19) and Camargo (8), respectively. Antigen slides of formolized tachyzoites were incubated with serum samples diluted 1:50 (IFAT-Nc) or 1:64 (IFAT-Tg) in PBS and then with fluorescein isothiocyanate-labeled anti-human IgG (Sigma Chemical Co.) diluted 1:80 in PBS plus 0.01% Evans blue. The slides were overlaid with carbonate-buffered glycerol (pH 8.5) and a coverslip and examined under a fluorescence microscopy. Only a bright, linear peripheral fluorescence of the tachyzoites was considered a positive result.
(iii) IB.
Immunoblotting (IB) was carried out in order to verify the reactivities to T. gondii (IB-Tg) and N. caninum (IB-Nc) immunodominant antigens in all serum samples with concordant or discordant results by ELISA and IFAT, as described by Mineo et al. (19). Briefly, T. gondii and N. caninum tachyzoites (approximately 108 organisms/ml) were lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and then submitted to electrophoresis in 12% SDS-PAGE under nonreducing conditions in parallel with molecular weight markers (Sigma Chemical Co.), as described by Laemmli (15). Proteins were electrophoretically transferred to nitrocellulose membranes (Sigma Chemical Co.), as described previously (26), and then blocked with PBS-T plus 5% skim milk for 2 h at room temperature. The strips were incubated overnight at 4°C with serum samples diluted 1:50 in PBS-T plus 1% skim milk and then with peroxidase-anti-human IgG diluted 1:500 (IB-Nc) or 1:1,000 (IB-Tg) for 2 h at room temperature. The reaction was developed by adding enzyme substrate (Fast 3,3′-diaminobenzidine tablet sets; Sigma Chemical Co.). Samples that showed reactivity to the T. gondii SAG1 (p30) antigen and at least one of two (29- and 35-kDa) immunodominant antigens of N. caninum were considered positive by IB.
Counts of CD4 and CD8 T cells.
CD4- and CD8-positive T cells were counted on a FACSort flow cytometer by using the Immunocytometry Systems (Becton-Dickinson, São José, Calif.), and the results were expressed as the number of cells/μl.
HIV load evaluation.
HIV loads were quantitatively assessed by a nucleic acid sequence-based amplification assay (Nasba; Organon Technika, Inc., Boxtel, The Netherlands). This assay had a detection limit of 80 copies/ml.
Statistical analysis.
The seropositivity percentages found in the different groups were compared by the χ2 test (Statistic for Windows 4.5, 1993; Statesoft Inc.). Associations between serology for N. caninum and laboratory parameters for HIV-infected patients were determined by the Fisher exact test. P values of <0.05 were considered statistically significant.
RESULTS
IgG antibodies to N. caninum were predominantly detected in HIV-infected patients (38%) and patients with neurological disorders (18%), whereas newborns and healthy subjects showed lower seropositivity rates (5% and 6%, respectively) (Table 1). The rate of seropositivity among HIV-infected patients was significantly higher than that among patients with neurological disorders (P = 0.0227), and both groups showed positivity rates significantly higher than those found in newborns (P = 0.0001 and P = 0.0134, respectively) and healthy individuals (P = 0.0001 and P = 0.0560, respectively).
TABLE 1.
Occurrence of anti-Neospora caninum IgG antibodies by ELISA, IFAT, and IB in different groups of patients
| Patient group | No. of serum samples | No. (%) of samples seropositive for N. caninum
|
|||
|---|---|---|---|---|---|
| Concordant resultsa | Discordant resultsb | IBc | Totald | ||
| HIV positive | 61 | 18 (30) | 9 (15) | 5 (8) | 23 (38)* |
| Neurological disorders | 50 | 1 (2) | 11 (22) | 8 (16) | 9 (18)** |
| Newborns | 91 | 1 (1) | 6 (7) | 4 (4) | 5 (5)† |
| Control | 54 | 1 (2) | 5 (9) | 2 (4) | 3 (6)† |
Concordant results by ELISA and IFAT.
Discordant results by ELISA and IFAT.
Positive results by IB of sera with discordant results by ELISA and IFAT.
The total number of positive serum samples includes the number of samples with concordant results plus the number of serum samples with positive results by IB. Different symbols indicate statistically significant differences between the groups (P < 0.05).
Seroreactivity to N. caninum was confirmed by IB-Nc by testing all sera from different groups of patients with positive concordant results or discordant results for IgG anti-N. caninum antibodies by ELISA and IFAT (Table 2). All sera with positive concordant results (ELISA positive and IFAT positive) recognized at least one of two (29- and 35-kDa) immunodominant antigens of N. caninum, with the great majority (>83%) showing strong recognition of the 29-kDa antigen and weak staining of the 35- and/or 46-kDa antigen, regardless of the group of patients analyzed. In addition, a strongly stained double antigenic band (62 and 66 kDa) was recognized by more than 78% of the serum samples, particularly those from the groups of HIV-infected patients and patients with neurological disorders. Sera with discordant results also showed similar patterns of reactivity; that is, the 29-kDa antigen was consistently detected. Among 11 serum samples with ELISA-negative and IFAT-positive results, 9 (82%) were confirmed to be seropositive by IB-Nc by recognizing predominantly the 29-kDa antigen; and among 20 serum samples with ELISA-positive and IFAT-negative results, 10 (50%) were confirmed to be positive by IB-Nc by recognizing the 29-kDa antigen and other antigens.
TABLE 2.
Reactivity to Neospora caninum antigens by IB of sera from different groups of patients with concordant or discordant results by ELISA and IFAT for IgG antibodies to N. caninum
| Patient group | Resultsa | No. of serum samples | No. of serum samples positive by IB | No. (%) of serum samples reactive to the following N. caninum antigens:
|
|||
|---|---|---|---|---|---|---|---|
| 29 kDa | 35 kDa | 46 kDa | 62 and 66 kDa | ||||
| HIV positive | ELISA+/IFAT+ | 18 | 18 | 15 (83) | 11 (61) | 11 (61) | 14 (78) |
| ELISA+/IFAT− | 6 | 2 | 2 (100) | 1 (50) | |||
| ELISA−/IFAT+ | 3 | 3 | 3 (100) | ||||
| Neurological disorders | ELISA+/IFAT+ | 1 | 1 | 1 (100) | 1 (100) | 1 (100) | |
| ELISA+/IFAT− | 5 | 3 | 3 (100) | 1 (50) | 1 (50) | ||
| ELISA−/IFAT+ | 6 | 5 | 5 (100) | ||||
| Newborns | ELISA+/IFAT+ | 1 | 1 | 1 (100) | |||
| ELISA+/IFAT− | 5 | 4 | 1 (25) | 3 (75) | 1 (25) | 1 (25) | |
| ELISA−/IFAT+ | 1 | 0 | |||||
| Control | ELISA+/IFAT+ | 1 | 1 | 1 (100) | 1 (100) | ||
| ELISA+/IFAT− | 4 | 1 | 1 (100) | ||||
| ELISA−/IFAT+ | 1 | 1 | 1 (100) | ||||
+, positive result by the indicated assay; −, negative result by the indicated assay.
To investigate possible concomitant infections with N. caninum and T. gondii and to confirm the specificity for N. caninum, all sera were tested in parallel for T. gondii serology by ELISA-Tg and IFAT-Tg (Table 3). Also at this time, the results for sera with discordant results were confirmed by IB-Tg by considering the reactivity to the SAG1 (p30) immunodominant antigen of T. gondii as a marker for positivity. Among 61 HIV-infected patients, 18 (30%) were seropositive for T. gondii only, 3 (5%) were seropositive for N. caninum only, and 20 (33%) were seropositive for both parasites. As a result, among the 23 N. caninum-seropositive samples, 20 (87%) were also seropositive for T. gondii. In group II, all 9 N. caninum-seropositive samples (18%) were also T. gondii seropositive, with no sample showing seroreactivity to N. caninum only. Thus, seropositivity for N. caninum was strongly and significantly associated with seropositivity for T. gondii in these two groups (P = 0.0001 and P = 0.0443, respectively) but not in newborns or healthy individuals (P > 0.05).
TABLE 3.
Seropositivity to Toxoplasma gondii and Neospora caninum by ELISA, IFAT, and IB in different groups of patients
| Patient group | No. of serum samples | No. (%) of serum samples seropositive for:
|
||
|---|---|---|---|---|
| T. gondii only | N. caninum only | Both parasites | ||
| HIV positive | 61 | 18 (30) | 3 (5) | 20 (33)a |
| Neurological disorders | 50 | 27 (54) | 0 (0) | 9 (18)b |
| Newborns | 91 | 37 (41) | 3 (3) | 2 (2) |
| Control | 54 | 24 (44) | 1 (2) | 2 (4) |
P < 0.0001 by comparison of seropositivity for both parasites and for N.caninum only.
P < 0.05 by comparison of seropositivity for both parasites and for N.caninum only.
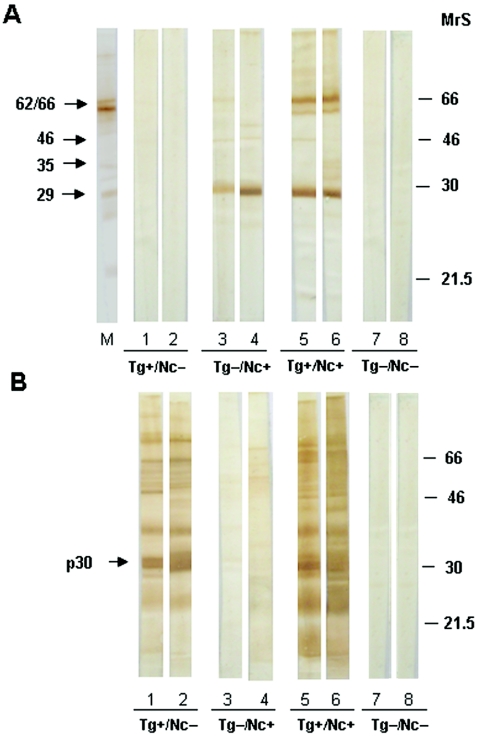
Figure 1 illustrates representative IB assay results for the reactivities of human sera against N. caninum (Fig. 1A) and T. gondii (Fig. 1B) antigens and shows the different serological results (T. gondii positive and N. caninum negative, T. gondii negative and N. caninum positive, T. gondii positive and N. caninum positive, and T. gondii negative and N. caninum negative) obtained as demonstrated in Table 3. A blot proved with monkey anti-N. caninum serum is also depicted (Fig. 1A), showing that the antigenic bands recognized by human serum samples comigrated with those recognized by the monkey immune serum. When the reactivities of T. gondii-seropositive samples against N. caninum were analyzed, no reactivity was found for samples that were N. caninum seronegative (T. gondii positive and N. caninum negative). On the other hand, samples that were T. gondii negative and N. caninum positive showed reactivity only to N. caninum antigens by IB. All T. gondii-positive and N. caninum-positive samples were strongly reactive to major immunodominant antigens of T. gondii (p30) and N. caninum (29 kDa), and none of the T. gondii-negative and N. caninum-negative samples showed any reactivity against antigens from both parasites.
FIG. 1.
Reactivities of human sera by IB of lysates of N. caninum (A) and T. gondii (B). A monkey anti-N. caninum serum (lane M) and eight human sera (lanes 1 to 8, respectively) are shown as representative immunoblots for each result obtained from T. gondii and N. caninum serological analysis: T. gondii positive and N. caninum negative (Tg+/Nc−; seropositive for T. gondii only), T. gondii negative and N. caninum positive (Tg−/Nc+; seropositive for N. caninum only), T. gondii positive and N. caninum positive (Tg+/Nc+; seropositive for both parasites), and T. gondii negative and N. caninum negative (Tg−/Nc−; seronegative for both parasites). Molecular size markers (MrS) are indicated in kDa on the right. The immunodominant antigens of N. caninum (29, 35, 46, and 62 and 66 kDa) and T. gondii (p30) are shown on the left.
To verify probable associations between laboratory parameters for HIV-positive patients and serology for N. caninum, the CD4 T-cell counts and the viral loads of HIV-seropositive patients were analyzed (Table 4). No significant association was found between the counts of the CD4-positive T cells or HIV viral load and the serological results obtained by ELISA-Nc (P > 0.05).
TABLE 4.
Associations between CD4-positive T-cell counts and HIV viral load of HIV-positive patients and serology for N. caninum
| HIV-positive patientsa | No. (%) of patients with the following characteristics:
|
|||||
|---|---|---|---|---|---|---|
| CD4-positive T cells (no./μl)
|
HIV viral load (log/ml)
|
|||||
| <200 | 200-400 | >400 | <3.0 | 3.0-5.0 | >5.0 | |
| N. caninum seropositive (n = 21) | 7 (33) | 5 (24) | 9 (43) | 4 (19) | 12 (57) | 5 (24) |
| N. caninum seronegative (n = 27) | 9 (33) | 7 (26) | 11 (41) | 4 (15) | 16 (59) | 7 (26) |
Data for CD4 T-cell counts and HIV viral loads were available for 48 HIV-positive patients.
DISCUSSION
Due to the biologic similarities between N. caninum and T. gondii, in addition to the important role of dogs as companion animals for humans and as definitive hosts of N. caninum, the potential for human exposure to N. caninum cannot be ruled out. In the present study, the presence of antibodies to N. caninum in different groups of human populations with high T. gondii infection rates was demonstrated by using different but complementary serological assays.
Serology for N. caninum has been performed with different animal species through ELISA with immunostimulating complex (ISCOM) or crude antigens and IFAT, which has been considered the reference test for N. caninum antibodies (4, 5). As no reference N. caninum-negative or -positive human sera were available, we arbitrarily established cutoff values for ELISA-Nc (EI > 1.1) and IFAT-Nc titer (>1:50) based on the findings from serological assays used in previous studies (19, 20, 27). Sera were further analyzed by IB-Nc, with the presence of at least one of two (p29 and p35) immunodominant antigens of N. caninum considered a positive serological result. Interestingly, all sera with concordant positive results (ELISA and IFAT positive) consistently recognized the 29-kDa antigen, regardless of the group of patients analyzed. In addition, more than 80% of sera with ELISA-negative and IFAT-positive results recognized the 29-kDa antigen, thus confirming the specificity of IFAT.
The 29-kDa surface protein of N. caninum, designated Ncp29, is considered one of the two major antigens recognized by antisera from N. caninum-infected mice, dogs, and cattle (14). Considering that the N. caninum-seropositive samples showed uniform peripheral fluorescence localized to the tachyzoite surface, it can be hypothesized that the 29-kDa antigen may be Ncp29. Although many host species may develop their own patterns of profiles of antibodies to N. caninum, the reactive bands in human sera comigrated with those recognized by monkey anti-N. caninum control serum. In this context, Bjerkas et al. (3) have demonstrated similar profiles of reactive bands in several host species, including cows, dogs, sheep, goats, and pigs, with predominant recognition of the 17-, 29- to 30-, 37-, and 46-kDa antigenic bands by sera of all species analyzed. Thus, our results indicate that the 29-kDa protein can be considered an immunodominant antigen of N. caninum for humans. In a previous study, a 35-kDa protein was found to be the immunodominant antigen for human N. caninum infection (27). This small difference in kDa between these antigens may be due to variations in the techniques used for gel electrophoresis, specimen preparation, antigen source, and antibody detection. As these immunoreactive dominant antigens were not cloned, it is likely that we are describing the same antigen that Tranas et al. (27) described.
When seropositivity to N. caninum was analyzed, newborns and healthy individuals showed low seropositivity rates (5 to 6%), suggesting an environmental exposure without infection. In contrast, HIV-infected patients demonstrated the highest rate of seropositivity (38%) among all other groups analyzed, indicating a N. caninum exposure with a possible productive infection. Evidence in the literature suggests that concomitant infections with some immunosuppressive viruses in cattle, such as bovine viral diarrhea virus, may increase the pathogenesis of N. caninum (6).
The gastrointestinal tract is one of the major target organs for secondary infections and malignancies in HIV-infected patients, indicating disturbed local immune defense mechanisms. Zeitz et al. (29) have demonstrated a pronounced loss of CD4+ T cells and the presence of more activated CD8+ T cells in the intestinal mucosa compared to their numbers in the peripheral blood of humans infected with HIV. CD4+ T-cell loss in the intestinal mucosa of monkeys may occur as early as 2 weeks after infection with simian immunodeficiency virus (SIV) (28). Depletion and functional impairment of mucosal CD4+ T lymphocytes, followed by altered cytokine secretion in HIV or SIV infection, may explain the breakdown of the mucosal immune barrier that leads to secondary opportunistic or nonopportunistic infections. In addition, due to the interrelation between the mucosal immune system and the epithelium, these changes might be responsible for the partial small intestinal mucosal atrophy and maturation defects in enterocytes observed in HIV-infected patients. Altogether, these findings could be a reasonable explanation for the high prevalence of N. caninum antibodies in HIV-infected patients, given that a major route of N. caninum transmission is from the oral cavity to the gastrointestinal tract. Due to the impairment of mucosal protection in HIV-infected patients, N. caninum may be an efficient opportunistic pathogen for this group of patients.
The second highest rate of seropositivity for N. caninum (18%) was observed in the group of patients with neurological disorders. Among nine N. caninum-seropositive patients from this group, five had confirmed neurocysticercosis and the four remaining patients had neurological disorders of unknown etiology. Although the results for this patient group are suggestive of a productive N. caninum infection, a cause-and-effect relationship to human disease has not yet been shown.
The low seropositivity rates (5 to 6%) found for newborns and healthy individuals were consistent with the findings for immunocompetent individuals presented in previous reports (20, 27). Due to the transplacental passage of maternal IgG antibodies, the presence of IgG antibodies to N. caninum in the cord blood of infants reflects the seropositivity of the mothers based on serological screening of the newborns. Thus, immunocompetent pregnant women with no history of reproductive disorders do not seem to present significant serological evidence of N. caninum. In this context, no evidence of N. caninum infection was detected in women with repeated abortions (21).
It is noteworthy that the IFAT antibody titers (1:50 to 1:100) and the ELISA indices (EI = 1.1 to 3.4) for N. caninum obtained in human serum samples were extremely low compared to those for T. gondii (titers of 1:64 to 1:4,096 for IFAT and EI values of 2.0 to 14.5 for ELISA), suggesting a lack of replication of Neospora and exposure without infection. Also, these data seem to indicate important differences in the immunogenicities of both parasites, since an apparent reduction in antigen complexity in N. caninum has been demonstrated previously, thus reflecting its decreased pathogenicity and narrower host range relative to those of T. gondii (14). In addition, Tranas et al. (27) suggest that the low titers of antibodies to N. caninum can attest to past infections that have been overcome or that are inactive or to the ingestion of nonviable parasites, or perhaps the systemic humoral response remained low due to the restriction of the infection to the gastrointestinal mucosa.
A significant association between the rates of seropositivity for N. caninum and T. gondii was observed for HIV-positive patients and those with neurological disorders. These findings are in disagreement with those of previous reports that showed that the reactivities for N. caninum are not associated with the presence of T. gondii antibodies (21, 27). However, this divergence can be attributed to the fact that the population examined in both previous studies consisted of immunocompetent individuals, whereas in the present work the immunocompromised patients studied may be more susceptible to infection with opportunistic pathogens, such as T. gondii and N. caninum. Although N. caninum and T. gondii tachyzoites share a few cross-reacting antigens, the immunodominant antigens of both parasites are not recognized by heterologous sera (3). In the present study, the immunoblot banding patterns were unique for samples positive for a single organism; that is, T. gondii-negative and N. caninum-positive samples did not bind to immunodominant T. gondii antigens, and likewise, T. gondii-positive and N. caninum-negative samples did not bind to immunodominant N. caninum antigens. On the other hand, antigens of higher molecular mass, particularly the strongly stained double antigenic band of 62 and 66 kDa, were predominantly recognized by samples positive for both parasites, suggesting that these could be cross-reactive antigens of N. caninum and T. gondii for humans. Indeed, a previous study based on the N. caninum proteome map also pointed out the occurrence of a high degree of homology among components from 60 to 70 kDa from both parasites (23).
In an attempt to verify if CD4 T-cell counts or the viral load in HIV-positive patients could be associated with serology for N. caninum, these laboratory parameters were stratified and analyzed for the groups of N. caninum-seropositive and N. caninum-seronegative patients. Even in the presence of a relatively preserved immune system (>200 CD4-positive T cells), no evident association was found with the serological findings for N. caninum. Similar findings were reported in analyses of Leishmania DNA in HIV-infected patients, in which no correlation between clinical or laboratory parameters and Leishmania infection were found (7).
We provide evidence that N. caninum infects humans. Although disease has not been described, differences between T. gondii and N. caninum must be considered, especially due to their distinct epidemiological characteristics. In this context, while this issue is not fully clarified, the same procedures adopted for cats and toxoplasmosis should be carried out for dogs and neosporosis, particularly when these pets are in contact with HIV-infected patients. Also, it should be considered that the epidemiological chain of N. caninum has not been entirely uncovered, and therefore, other transmission routes should be evaluated.
In conclusion, the results of this study indicate the presence of N. caninum exposure or infection in humans, particularly in HIV-infected patients and patients with neurological disorders, who could have opportunistic and concurrent infections with T. gondii. In addition, we emphasize the importance of complementary serological methods or multidisciplinary diagnostic procedures, such as PCR and immunohistochemical assays with material collected by biopsy or at autopsy, for a better characterization of human N. caninum infection. These findings may bring a new concern to the unstable clinical health of HIV-infected patients and the actual role of N. caninum infection in immunocompromised patients.
Acknowledgments
This work was supported by Brazilian research agencies (CNPq and FAPEMIG).
REFERENCES
- 1.Barling, K. S., J. W. Mcneill, J. A. Thompson, J. C. Paschal, F. T. McCollum III, T. M. Craig, and L. G. Adams. 2000. Association of serologic status for Neospora caninum with post weaning weight gain and carcass measurements in beef calves. J. Am. Vet. Med. Assoc. 217:1356-1360. [DOI] [PubMed] [Google Scholar]
- 2.Barr, B. C., P. A. Conrad, K. W. Sverlow, A. F. Tarantal, and A. G. Hendrickx. 1994. Experimental fetal and transplacental Neospora infection in the nonhuman primate. Lab. Investig. 71:236-242. [PubMed] [Google Scholar]
- 3.Bjerkas, I., M. C. Jenkins, and J. P. Dubey. 1994. Identification and characterization of Neospora caninum tachyzoite antigens useful for diagnosis of neosporosis. Clin. Diagn. Lab. Immunol. 1:214-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorkman, C., A. Lunden, J. Holmdahl, J. Barber, A. J. Trees, and A. Uggla. 1994. Neospora caninum in dogs: detection of antibodies by ELISA using an iscom antigen. Parasite Immunol. 16:643-648. [DOI] [PubMed] [Google Scholar]
- 5.Bjorkman, C., and A. Uggla. 1999. Serological diagnosis of Neospora caninum infection. Int. J. Parasitol. 29:1497-1507. [DOI] [PubMed] [Google Scholar]
- 6.Bjorkman, C., S. Alenius, U. Manuelsson, and A. Uggla. 2000. Neospora caninum and bovine virus diarrhea virus infections in Swedish dairy cows in relation to abortion. Vet. J. 159:201-206. [DOI] [PubMed] [Google Scholar]
- 7.Bossolasco, S., G. Gaiera, D. Olchini, M. Gulletta, L. Martello, A. Bestetti, L. Bossi, L. Germagnoli, A. Lazzarin, C. Uberti-Foppa, and P. Cinque. 2003. Real-time PCR assay for clinical management of human immunodeficiency virus-infected patients with visceral leishmaniasis. J. Clin. Microbiol. 41:5080-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camargo, M. E. 1964. Improved technique of indirect immunofluorescence for serological diagnosis of toxoplasmosis. Rev. Inst. Med. Trop. 6:117-118. [PubMed] [Google Scholar]
- 9.Chi, J., J. A. Vanleeuwen, A. Weersink, and G. P. Keefe. 2002. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leucosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum. Prev. Vet. Med. 55:137-153. [DOI] [PubMed] [Google Scholar]
- 10.Dubey, J. P., J. L. Carpenter, C. A. Speer, M. J. Topper, and A. Uggla. 1988. Newly recognized fatal protozoan disease of dogs. J. Am. Vet. Med. Assoc. 192:1269-1285. [PubMed] [Google Scholar]
- 11.Dubey, J. P., and D. S. Lindsay. 1996. A review of Neospora caninum and neosporosis. Vet. Parasitol. 67:1-59. [DOI] [PubMed] [Google Scholar]
- 12.Gondim, L. F., M. M. McAllister, W. C. Pitt, and D. E. Zemlicka. 2004. Coyotes (Canis latrans) are definitive hosts of Neospora caninum. Int. J. Parasitol. 34:159-161. [DOI] [PubMed] [Google Scholar]
- 13.Holmdahl, O. J., J. G. Mattsson, A. Uggla, and K. E. Johansson. 1994. The phylogeny of Neospora caninum and Toxoplasma gondii based on ribosomal RNA sequences. FEMS Microbiol. Lett. 119:187-192. [DOI] [PubMed] [Google Scholar]
- 14.Howe, D. K., A. C. Crawford, D. Lindsay, and L. D. Sibley. 1998. The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii. Infect. Immun. 66:5322-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 17.McAllister, M. M., J. P. Dubey, D. S. Lindsay, W. R. Jolley, R. A. Wills, and A. M. McGuire. 1998. Dogs are definitive hosts of Neospora caninum. Int. J. Parasitol. 28:1473-1478. [PubMed] [Google Scholar]
- 18.Mineo, J. R., M. E. Camargo, and A. W. Ferreira. 1980. Enzyme-linked immunosorbent assay for antibodies to Toxoplasma gondii polysaccharides in human toxoplasmosis. Infect. Immun. 27:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mineo, T. W. P., D. A. O. Silva, G. H. N. Costa, A. C. B. von Ancken, L. H. Kasper, M. A. Souza, D. D. Cabral, A. J. Costa, and J. R. Mineo. 2001. Detection of IgG antibodies to Neospora caninum and Toxoplasma gondii in dogs examined in a veterinary hospital from Brazil. Vet. Parasitol. 98:239-245. [DOI] [PubMed] [Google Scholar]
- 20.Nam, H., S. Kang, and W. Choi. 1998. Antibody reaction of human anti-Toxoplasma gondii positive and negative sera with Neospora caninum antigens. Korean J. Parasitol. 36:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen, E., M. Lebech, L. Jensen, P. Lind, M. Rask, P. Bagger, C. Bjorkman,and A. Uggla. 1999. Neospora caninum infection and repeated abortions in humans. Emerg. Infect. Dis. 5:278-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott, P., E. Pearce, P. Natovitz, and A. Sher. 1987. Vaccination against cutaneous leishmaniasis in murine model. Induction of protective immunity with a soluble extract of promastigotes. J. Immunol. 139:221-227. [PubMed] [Google Scholar]
- 23.Shin, Y. S., E. G. Lee, G. W. Shin, Y. R. Kim, E. Y. Lee, J. H. Kim, H. Jang, L. J. Gershwin, D. Y. Kim, Y. H. Kim, G. S. Kim, M. D. Suh, and T. S. Jung. 2004. Identification of antigenic proteins from Neospora caninum recognized by bovine immunoglobulins M, E, A and G using immunoproteomics. Proteomics 4:3600-3609. [DOI] [PubMed] [Google Scholar]
- 24.Silva, D. A. O., N. M. Silva, T. W. P. Mineo, A. A. Pajuaba Neto, E. A. Ferro, and J. R. Mineo. 2002. Heterologous antibodies to evaluate the kinetics of the humoral immune response in dogs experimentally infected with Toxoplasma gondii RH strain. Vet. Parasitol. 107:181-195. [DOI] [PubMed] [Google Scholar]
- 25.Speer, C. A., D. W. Reduker, D. E. Burgess, W. M. Whitmire, and G. A. Splitter. 1985. Lymphokine-induced inhibition of growth of Eimeria bovis and Eimeria papillata (Apicomplexa) in cultures of bovine monocytes. Infect. Immun. 50:566-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tranas, J., R. A. Heinzen, L. M. Weiss, and M. M. McAllister. 1999. Serological evidence of human infection with the protozoan Neospora caninum. Clin. Diagn. Lab. Immunol. 6:765-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 29.Zeitz, M., R. Ullrich, T. Schneider, S. Kewenig, and E. Riecken. 1998. Mucosal immunodeficiency in HIV/SIV infection. Pathobiology 66:151-157. [DOI] [PubMed] [Google Scholar]