Abstract
Cholera toxin (CT) is a potent adjuvant that activates dendritic cells (DC) by increasing intracellular cyclic AMP (cAMP) levels. In vivo and in vitro, very small amounts of CT induce potent adjuvant effects and activate DC. We hypothesized that DC intoxicated by CT may release factors that enhance their own maturation and induce the maturation of toxin-free bystander DC. Through the use of mixed cultures and transwell cultures, we found that human monocyte-derived DC (MDDC) pulsed with CT or other cAMP-elevating agonists induce the maturation of bystander DC. Many DC agonists including CT increase the production of prostaglandin E2 (PGE2) and nitric oxide (NO). For this reason, we determined whether the actions of PGE2 or NO are involved in the maturation of MDDC induced by CT or dibutyryl-cAMP (d-cAMP). We found that blocking the production of PGE2 or blocking prostaglandin receptors inhibited MDDC maturation induced by CT and d-cAMP. Likewise, sequestering NO or blocking the downstream actions of NO resulted in the inhibition of MDDC maturation induced by CT and d-cAMP. These results indicate that endogenously produced factors including PGE2 and NO contribute to the maturation of DC induced by CT and that these factors participate in bystander DC maturation. The results of this study may help explain why bacterial toxins that elevate cAMP are such potent adjuvants.
Understanding the intercellular and intracellular signaling processes that lead to dendritic cell (DC) maturation is important for determining how these cells initiate immune responses to foreign antigens. Monocyte-derived DC (MDDC), produced by culturing monocytes with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) for 6 to 7 days, are phenotypically equivalent to the immature DC that reside in peripheral tissues (45). However, it is becoming increasingly evident that the kinetics of in vivo DC differentiation from monocytes may not be reflected by the long-term in vitro culture conditions required to generate MDDC. A recent study showed that mature DC can be generated from human monocytes in as little as 48 h (14). We have studied the transition of monocytes to DC in vitro and have also generated a shortened protocol for the in vitro generation of DC. With our protocol, MDDC are matured after only 4 days of culture in the presence of GM-CSF and IL-4 (2-4). In our hands, MDDC at day 4 are more responsive to activation than MDDC at days 5 to 7.
Cholera toxin (CT) is an AB5 enterotoxin produced by Vibrio cholerae, the primary causative agent of the disease cholera. CT consists of a 27-kDa catalytic A domain anchored in a ring of five identical 11.7-kDa B subunits (40). The B pentamer of CT binds to GM1 gangliosides on cell membranes (28). CT has a Lys/Arg-Asp-Glu-Leu ([K/R]DEL) signal sequence at the C terminus of its A domain. This sequence is believed to target the toxin to the endoplasmic reticulum, where the A1 subunit (disguised as a misfolded host protein) is transported to the cytoplasm by the endoplasmic reticulum-associated degradation pathway (reviewed in reference 27). In the cytosol, the A1 subunit catalyzes the transfer of an ADP-ribose from NAD to stimulatory α-subunits of G proteins (Gsα). After ADP-ribosylation, Gsα binds to adenylate cyclase and constitutively activates it, leading to a sustained increase in the intracellular cyclic AMP (cAMP) concentration (12). The increased intracellular cAMP concentrations in intestinal epithelial cells are the primary cause of the massive fluid and electrolyte release associated with the disease cholera.
CT is also a powerful mucosal immunogen and adjuvant (reviewed in reference 35). When delivered mucosally, CT induces strong primary and secondary antibody responses and long-lasting immunologic memory to itself and to coadministered antigens (36, 48). In mice, antibody responses to CT and bystander antigens can last up to 2 years (36, 48). The activation/maturation of DC at sites of immunization may be responsible for the potent immunogenic and adjuvant effects of CT. In support of this, CT and the related toxins Escherichia coli heat-labile enterotoxin and pertussis toxin were found to induce the maturation of DC (2, 3, 21). With further study, the elevated cAMP levels induced by these toxins were found to be responsible for DC maturation (2, 3), and their enzymatic activity has been shown to play a dominant role in adjuvanticity (5, 6, 23, 34, 37).
Prostaglandins, most notably prostaglandin E2 (PGE2), have gained notoriety for their ability to synergize with other agonists to activate DC (42). Interestingly, CT has been shown to increase the production of PGE2 in many cell types, including DC, through the release of arachidonic acid (7, 8, 11, 18, 39). Nitric oxide (NO) and superoxide anions have also recently gained attention for their ability to directly activate DC or to synergize with other agonists that activate DC (29, 41). Like prostaglandins, NO can either work directly on the cells that produce it or exert effects on neighboring cells. One of the most well-known effects of NO is the activation of guanylate cyclase (15), which results in the conversion of GTP to cyclic GMP (cGMP) and the subsequent activation of cGMP-dependent protein kinase G.
In vitro and in vivo, low concentrations of CT activate large numbers of DC. For this reason, we hypothesized that DC activated by CT may release soluble factors that enhance their own maturation and induce the maturation of toxin-free bystander DC. In support of this hypothesis, we found that CT-stimulated MDDC produce PGE2 and NO and that these factors contribute to the maturation of the stimulated MDDC and most likely contribute to the maturation of bystander MDDC. The results of this study provide new insights into how immune responses are initiated at points of pathogen entry and may help explain the potent adjuvant effects of cholera-like enterotoxins.
MATERIALS AND METHODS
Reagents.
Indomethacin, aspirin, NS-398, bromoenol lactone, aristolochic acid, LNAC (N-acetyl-l-cysteine), PTIO (2-phenyl-4,4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide), carboxy-PTIO [2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazol-1-yloxy-3-oxide potassium salt), lipopolysaccharide (LPS), dibutyryl-cAMP (d-cAMP), forskolin, YC-1 (3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole), dibutyryl-cGMPs, and GM1 ganglioside were purchased from Sigma (St. Louis, MO). CT was purchased from List Biological Laboratories (Campbell, CA). DAF-2 (4,5-diaminofluorescein) diacetate was purchased from the Cayman Chemical Company (Ann Arbor, MI).
Cell culture medium.
Medium consisted of RPMI 1640 medium (Life Technologies, Carlsbad, CA) supplemented with 2 mM l-glutamine (Sigma), 1% stock nonessential amino acids (Life Technologies), 1% stock sodium pyruvate (Life Technologies), 50 μM 2-mercaptoethanol (Sigma), 50 μg/ml gentamicin (Life Technologies), and 10% fetal calf serum (Life Technologies).
Dendritic cell preparations.
All human specimens were obtained under informed consent as approved by the University of Maryland Baltimore Institutional Review Board. MDDC were generated as described previously, with minor modifications (45). Human peripheral blood mononuclear cells were enriched for CD14+ monocytes by negative selection (StemCell Technologies, Vancouver, Canada). The isolated monocytes were adhered to plastic and then washed with RPMI 1640 medium. The cells were cultured at 1 × 106 cells per/ml in DC culture medium supplemented with 50 ng/ml recombinant GM-CSF and 1,000 units/ml recombinant IL-4 (R&D Systems, Minneapolis, MN).
Mixed lymphocyte response.
MDDC from three different donors were prepared as described above and washed three times before addition to a single donor's T cells. Naïve CD4+ T cells were enriched from peripheral blood mononuclear cells by negative selection (StemCell Technologies). Naïve CD4+ T cells were cultured in triplicates at 1 × 105 cells/well with 3,000 allogeneic MDDC in 96-well U-bottom plates. The cells were pulsed with 1 μCi/well [3H]thymidine (Perkin-Elmer Life Sciences, Boston, MA) for the last 18 h of culture before measuring thymidine incorporation using a Packard Matrix 96 direct beta counter on the fifth day. Percent inhibition was calculated with the following formula: 100 − [(inhibited cpm − unstimulated cpm)/(uninhibited cpm − unstimulated cpm) × 100].
Flow cytometry.
Cells were incubated for 30 min at 4°C with murine monoclonal antibodies specific for CD80, CD83, CD86, and HLA-DR (BD Pharmingen, San Diego, CA), washed, and then fixed with 2% paraformaldehyde for analysis using a FACScalibur flow cytometer (BD, San Jose, CA). Data analysis was carried out using FlowJo software (Tree Star Inc., San Carlos, CA).
Calculation of percent phenotypic activation and inhibition.
The fraction of MDDC that increased the expression of maturation markers on the cell surface (percent activation) was calculated with FlowJo software by overlaying the histograms of treated and untreated MDDC and Overton subtraction of the curves. Percent inhibition was calculated with the following formula: [(X − Y)/X] × 100, where X is the fraction of cells that increased the expression of a marker in the absence of the inhibitor and Y is the fraction of cells that increased the expression of a marker in the presence of the inhibitor.
Inhibitor concentrations.
The optimal concentration of each inhibitor used was determined as follows. Baseline concentrations were obtained from previously published reports. If the inhibitor did not have adverse effects on the cells (determined by trypan blue staining and changes in forward- or side-scatter profiles) after 20 h and if it displayed significant inhibition, this concentration was used for all other experiments. However, if the inhibitor displayed weak inhibition, the concentration was increased until adverse effects were apparent on the cells. The highest concentration of the inhibitor that did not induce adverse effects on the cells was then used for all further experiments.
Tumor necrosis factor alpha (TNF-α) ELISA.
Day 4 MDDC (2 × 106 cells/well in 12-well culture dishes in a total volume of 2 ml) were left untreated or were treated with 1 μg/ml of CT or 1 mM d-cAMP in the presence or absence of 1 μg/ml of LPS. Twelve hours later, supernatants were removed from the cells and analyzed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (R&D Systems).
PGE2 EIA.
Day 4 MDDC (2 × 106 cells/well in 12-well culture dishes in a total volume of 2 ml) were left untreated or were treated with 1 μg/ml of CT or LPS or 1 mM d-cAMP. Twelve hours later, supernatants were removed from the cells. The supernatants were diluted 1:10 with PGE2 enzyme immunoassay (EIA) buffer (Cayman). The diluted supernatants were analyzed by PGE2 EIA according to the manufacturer's instructions (Cayman).
DC migration assay.
DC migration was assessed using 24-well transwell plates with an 8-μm membrane (Costar, Corning, NY). MDDC (1 × 105 cells) were resuspended in 200 μl MDDC medium without GM-CSF or IL-4 and placed into the upper chamber of the transwell system. The lower chamber contained 500 μl of MDDC medium supplemented with 100 ng/ml each of macrophage inflammatory protein 3β (MIP-3β) and 6Ckine (R&D Systems). After 2 h of incubation at 37°C, the cells in the lower chamber were counted. Control cultures were kept in the absence of chemokines to assess baseline migration activity.
Statistics.
P values were determined using a Student's t test.
RESULTS
Bystander maturation of MDDC.
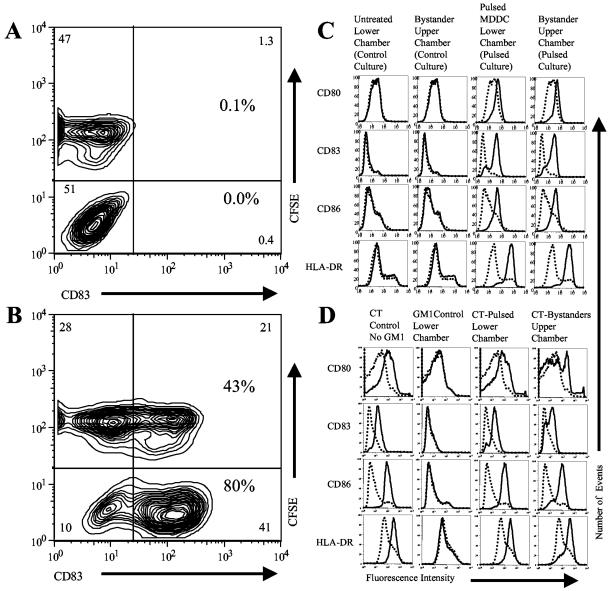
We hypothesized that DC stimulated with CT or other cAMP-elevating agonists (d-cAMP and forskolin) may release soluble factors that induce the maturation of bystander DC. Forskolin is a cell-permeable diterpenoid with adenylate cyclase-activating properties, and d-cAMP is a membrane-permeable analog of cAMP. As a first test of our hypothesis, MDDC were left unstimulated or were stimulated (incubated for 4 h with 1 mM d-cAMP and 100 μM forskolin and then washed three times to remove the agonist). These stimulated or unstimulated control MDDC (control MDDC were also washed three times) were replated at a 1:1 ratio with bystander MDDC (MDDC labeled with 1 μM carboxyfluorescein diacetate succinimidyl ester [CFSE]) in fresh culture medium. Twenty hours after replating, the cells were stained for flow cytometry with phycoerythrin (PE)-labeled anti-CD83.
Figure 1A shows that the unstimulated and bystander cells in the control cultures remained CD83 negative, indicating that these cells retained an immature phenotype. By contrast, as shown in Fig. 1B, the stimulated MDDC and the bystander MDDC in the stimulated cultures became CD83 positive, indicating that these cells had undergone maturation. It should be noted that the concentrations of residual d-cAMP and forskolin in the cultures of the stimulated cells, after three washes, were far less than the minimum concentrations necessary to induce MDDC maturation (data not shown). Therefore, the maturation of bystander MDDC is unlikely to be due to the effects of residual d-cAMP or forskolin in the cultures.
FIG. 1.
Bystander maturation of MDDC induced by cAMP-elevating agonists. (A and B) Bystander maturation of MDDC in mixed cultures. MDDC were pulsed (B) or not pulsed (A) with d-cAMP and forskolin (incubated for 4 h with 1 mM d-cAMP and 100 μM forskolin and then washed three times to remove the agonist). These pulsed or unpulsed control MDDC (control MDDC were washed three times) were then replated at a 1:1 ratio with unpulsed bystander MDDC (MDDC labeled with 1 μM CFSE) in fresh culture medium. Twenty hours after replating, the cells were harvested and stained for flow cytometry with PE-labeled anti-CD83. (A) Control cultures (unpulsed MDDC mixed with CFSE-labeled bystander MDDC). (B) Pulsed cultures (pulsed MDDC mixed with CFSE-labeled bystander MDDC). Data are representative of one experiment of three performed with similar results on MDDC derived from different donors. (C) Bystander maturation of MDDC by d-cAMP and forskolin-pulsed MDDC in transwell cultures. MDDC were pulsed or not pulsed with d-cAMP and forskolin (incubated for 4 h with 1 mM d-cAMP and 100 μM forskolin and then washed three times to remove the agonist). These pulsed or unpulsed control MDDC were then replated, and untreated bystander MDDC were plated in transwell inserts (upper chambers) and inserted into the culture wells containing the pulsed or unpulsed control MDDC (bottom chamber). Twenty hours later, the cells in the upper and lower chambers were harvested and stained for flow cytometry with PE-labeled anti-CD80, fluorescein isothiocyanate (FITC)-labeled anti-CD83, CyC-labeled anti-CD86, and PE-labeled anti-HLA-DR. Cells in transwell cultures (solid histograms) are compared to untreated MDDC from separate cultures (dotted histograms). Data are representative of one experiment of three performed with similar results on MDDC derived from different donors. (D) Bystander maturation of MDDC by CT-pulsed MDDC in transwell cultures. MDDC were pulsed with CT (incubated with 5 μg/ml of CT for 10 min before the addition of 10 μg/ml of GM1). In GM control cultures, GM1 was added 10 min before CT. In CT control cultures, no GM1 was added. Thirty minutes after the addition of CT, untreated bystander MDDC in transwell inserts (upper chambers) were inserted into the culture wells containing the CT-treated MDDC (lower chambers). Twenty hours later, the cells in the upper and lower chambers were harvested and stained for flow cytometry with PE-labeled anti-CD80, FITC-labeled anti-CD83, FITC-labeled anti-CD86, and PE-labeled anti-HLA-DR. Cells in transwell cultures (solid histograms) are compared to untreated MDDC from separate cultures (dotted histograms). Data are representative of one experiment of three performed with similar results on MDDC derived from different donors.
To determine if bystander maturation is the result of soluble factors, we designed experiments utilizing transwell culture dishes. MDDC were left unstimulated or were stimulated with d-cAMP and forskolin as described above. These stimulated or unstimulated control MDDC were replated in the lower chambers of transwell culture dishes. Bystander MDDC were placed in the upper transwell inserts. Twenty hours later, the cells in the upper and lower chambers were assayed by flow cytometry. Figure 1C shows that the bystander MDDC in the stimulated cultures (upper chamber) displayed phenotypic maturation. These results indicate that bystander maturation is due to the effects of soluble factors.
Initially, CT-stimulated MDDC were not tested for the ability to induce bystander maturation, as CT cannot be completely washed away from the stimulated cells. However, the effects of CT on MDDC can be completely blocked by the addition of soluble GM1 to the culture medium. In this regard, adding 10 μg/ml of GM1 to a culture of MDDC 10 min before the addition of 5 μg/ml of CT completely blocks the maturation of the MDDC induced by CT but not by LPS (GM1 control cells) (data not shown). By contrast, adding 10 μg/ml of GM1 to a culture of MDDC 10 min after the addition of 5 μg/ml of CT has no effect on MDDC maturation (CT-pulsed cells) (data not shown).
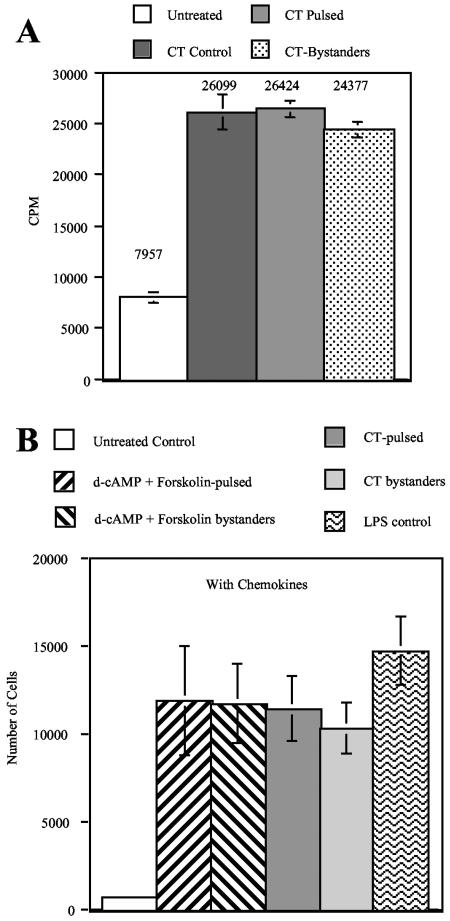
For these experiments, MDDC in the bottom chambers of transwell plates were pulsed with CT (incubated with 5 μg/ml of CT for 10 min before the addition of 10 μg/ml of GM1). Thirty minutes after the addition of CT, bystander MDDC were added to the upper chambers of the transwell cultures. In the GM1 control cultures, 10 μg/ml of GM1 was added 10 min before the addition of 5 μg/ml of CT. CT control cells were incubated with CT in the absence of GM1. Twenty hours later, the cells in the upper and lower chambers were harvested and assayed by flow cytometry and a mixed-lymphocyte reaction (MLR). Figure 1D shows that GM1 completely inhibited the ability of CT to induce the phenotypic maturation of MDDC in the GM1 control cultures. By contrast, in the pulsed cultures, the CT-pulsed MDDC (lower chamber) and the bystander MDDC (upper chamber) displayed phenotypic maturation. In addition, Fig. 2A shows that CT-pulsed MDDC and CT bystander MDDC from the pulsed cultures were equally potent antigen-presenting cells (APC) in an MLR, indicating that both populations were functionally mature. The increases in T-cell proliferation induced by the stimulated and bystander cells are statistically significant (P < 0.05).
FIG. 2.
Bystander MDDC are functionally mature. (A) Bystander MDDC induce robust T-cell proliferation. MDDC prepared in Fig. 1D were used in an MLR as described in Materials and Methods. Data shown are the means and standard errors from cells derived from three different donors. (B) Migration of MDDC in response to MIP-3β and 6Ckine. MDDC (1 × 105 cells) were placed into the upper chamber of the transwell system. The lower chamber contained medium supplemented with 100 ng/ml each of MIP-3β and 6Ckine. After 2 h of incubation at 37°C, the cells in the lower chamber were counted. Control cultures were kept in the absence of chemokines to assess random migration activity. Data shown are the means and standard errors of the means from cells derived from three different donors.
Cholera toxin-stimulated and bystander MDDC display similar migrations to CCR7-binding chemokines.
The maturation of DC has been reported to be associated with a switch in chemokine receptor expression from inflammatory to constitutive, such that CCR7 is up-regulated following maturation (16, 17, 19). CCR7 mediates DC migration by chemokines preferentially expressed in lymph nodes, such as 6Ckine and MIP-3β.
Transwell cultures were established using d-cAMP and forskolin-pulsed or CT-pulsed MDDC and bystanders, as described above. As an additional control, MDDC were matured in the presence of 100 ng/ml of LPS. Forty hours after the initiation of the cultures, the agonist-pulsed cells from the lower chambers and the bystander cells from the upper chambers were harvested, washed, and used in migration assays as follows. A total of 1 × 105 cells were placed into the upper chamber of a transwell system where the lower chamber contained medium supplemented with 100 ng/ml each of MIP-3β and 6Ckine. Control cultures were set up in the absence of chemokines to assess random migration activity. After 2 h of incubation at 37°C, the migrated MDDC in the lower chambers were harvested and counted.
Figure 2B shows that MDDC stimulated by d-cAMP and forskolin or CT showed similar migration activity to that of LPS-matured MDDC, a level far above that of the immature MDDC in the control cultures. Likewise, the bystander MDDC in the stimulated cultures showed similar migration activity to that of LPS-matured MDDC. The increases in migration of the stimulated and bystander cells are statistically significant (P < 0.05). For all of the tested MDDC, in the absence of chemokines, less than 1,000 cells migrated to the lower chambers (data not shown). These results, in addition to the results described above, indicate that MDDC stimulated to maturity by cAMP-elevating agonists release soluble factors that induce the full maturation of bystander MDDC not stimulated by the agonist.
MDDC incubated with CT or d-cAMP dominantly inhibit the production of TNF-α.
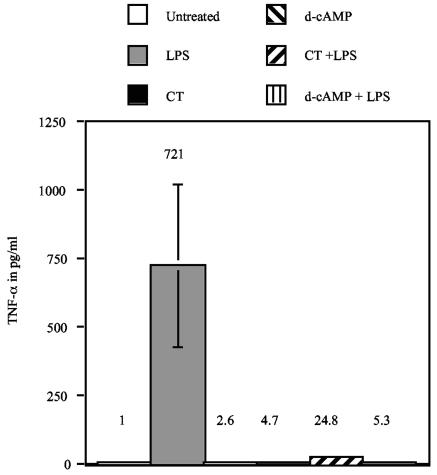
The most obvious candidate factors responsible for the bystander maturation described above are inflammatory cytokines. In this regard, TNF-α activates DC and is routinely used in cocktails for the maturation of MDDC used in clinical trials. TNF-α has also been shown to synergize with PGE2 for more potent activation of DC. However, it has been shown that cAMP-elevating agonists such as CT dominantly inhibit the production of inflammatory cytokines (2, 3, 21). For these reasons, we measured TNF-α concentrations in the supernatants of MDDC cultures stimulated with CT or d-cAMP. Day 4 MDDC were left untreated or were treated with 1 μg/ml CT or 1 mM d-cAMP in the presence or absence of 1 μg/ml LPS. After 12 h in culture, TNF-α concentrations in the supernatants were determined by ELISA.
Figure 3 shows that MDDC incubated with CT or d-cAMP do not produce TNF-α. In addition, these cAMP-elevating agents dominantly inhibit the production of this cytokine induced by LPS. The increase in TNF-α production by LPS-stimulated cells over that of untreated cells is statistically significant (P < 0.05). The differences in TNF-α production between CT- or d-cAMP-stimulated cells in the presence or absence of LPS and untreated cells are not statistically significant (P > 0.05). Similar results have been found with other cytokines such as IL-1, IL-6, and IL-12 (2, 3, 21). These results indicate that inflammatory cytokines such as TNF-α are unlikely to be responsible for the bystander maturation described above.
FIG. 3.
TNF-α is not responsible for bystander maturation. Day 4 MDDC were left untreated or were treated with 1 μg/ml CT or 1 mM d-cAMP in the presence or absence of 1 μg/ml LPS. After 12 h of culture, TNF-α concentrations in the supernatants were determined by ELISA. Data shown are the means and standard errors of the means from cells derived from at least three different donors.
Products of the COX enzymes contribute to the maturation of MDDC induced by CT and d-cAMP.
PGE2 has been shown to influence DC maturation. For this reason, we determined whether PGE2 or other products of the cycloxygenase (COX) enzymes contribute to the maturation of MDDC induced by elevated cAMP levels and/or contribute to bystander maturation. The effects of exogenous PGE2 on DC function have been extensively studied (for a review, see reference 38). The effects of endogenous PGE2 production on DC function are not as well studied but have been implicated in DC chemotaxis (46) and maturation (50).
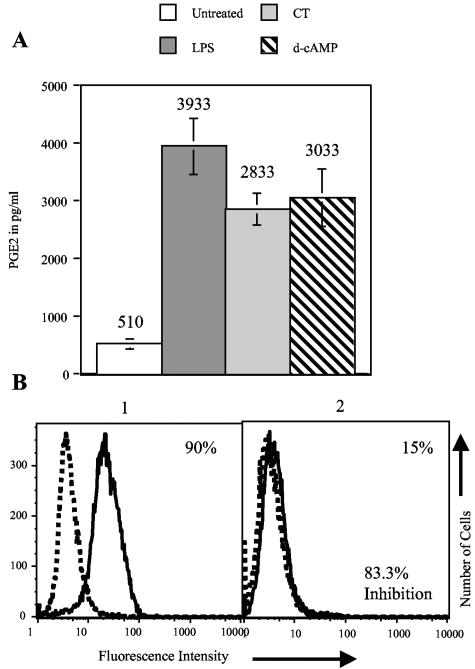
First, we tested PGE2 production by stimulated MDDC. Day 4 MDDC were treated with 1 μg/ml of CT, 1 mM d-cAMP, or 1 μg/ml LPS (as a control). Twelve hours later, supernatants were subjected to PGE2 EIA. Figure 4A shows that CT and d-cAMP as well as LPS increased PGE2 production by MDDC. The increases in PGE2 production by the stimulated cells are statistically significant (P < 0.05). Next, we determined whether prostaglandin production contributes to the maturation of MDDC induced by these agonists. To determine this, we incubated MDDC with these agonists in the presence of the COX-1-specific inhibitor aspirin (22) or the COX-2-specific inhibitor NS-398 (20). MDDC were incubated with 0.5 μg/ml CT, 500 μM d-cAMP, or 0.5 μg/ml LPS (as a control) with or without a prior 1-h incubation with 50 μg/ml of aspirin or 1 μg/ml of NS-398. Twenty hours later, the cells were assayed by flow cytometry. The percent inhibition was calculated as described in Materials and Methods and is illustrated in Fig. 4B.
FIG. 4.
PGE2 production by MDDC. (A) MDDC were treated with 1 μg/ml CT or LPS or 1 mM d-cAMP. Twelve hours later, supernatants were removed, and PGE2 concentrations in the supernatants were measured by EIA. Data were obtained from three experiments performed on MDDC derived from different donors. The means and standard errors of the means are shown. (B) Example calculation of percent inhibition. The formula used to calculate percent inhibition is [(X − Y)/X] × 100, where X is the fraction of cells that increased the expression of a marker in the absence of the inhibitor and Y is the fraction of cells that increased the expression of a marker in the presence of the inhibitor. Panel 1 shows CD83 expression in the absence of an inhibitor, and panel 2 shows CD83 expression in the presence of an inhibitor. X is 90, Y is 15, and the equation [(90 − 15) × 100]/90 equals 83.3% inhibition.
Table 1 shows that the phenotypic maturation of the MDDC induced by CT, d-cAMP, or LPS was weakly inhibited by aspirin or NS-398. For this reason, we determined whether inhibiting both isoforms of COX induces greater inhibition. MDDC were incubated with the agonists with or without a prior 1-h incubation with 10 μg/ml of the COX-1 and COX-2 inhibitor indomethacin (32). Twenty hours later, the cells were assayed for phenotypic maturation and for functional maturation in an MLR. Table 1 shows that indomethacin strongly inhibits the phenotypic maturation of MDDC while it moderately inhibits the functional maturation of the same cells induced by both agonists. It is not surprising that the inhibition of functional maturation is not as potent as the inhibition of phenotypic maturation. In this regard, only a small percentage of a population of MDDC needs to be phenotypically mature for that population to display near-maximal functional maturation in T-cell proliferation assays. It should also be noted that PGE2 is only one of many products produced by the COX enzymes. Therefore, it is possible that other products of the COX enzymes also contribute to MDDC maturation.
TABLE 1.
Prostaglandin production contributes to the maturation of MDDC induced by cAMP-elevating agonistsa
| Inhibitor | Concn | Marker | Avg % inhibition (SEM)
|
||
|---|---|---|---|---|---|
| LPS | CT | d-cAMP | |||
| Aspirin | 10 μg/ml | CD80 | 13.4 (5.1) | 11.7 (9.5) | 16.7 (9.1) |
| CD83 | 18.3 (7.6) | 15.0 (6.1) | 19.7 (3.3) | ||
| CD86 | 23.1 (6.7) | 17.3 (13.4) | 18.0 (8.7) | ||
| HLA-DR | 12.0 (3.4) | 16.7 (8.0) | 22.3 (4.8) | ||
| MLR | NT | NT | NT | ||
| NS-398 | 10 μM | CD80 | 0.7 (0.5) | 11.3 (6.3) | 18.3 (8.0) |
| CD83 | 5.3 (2.4) | 11.7 (6.2) | 17.0 (5.8) | ||
| CD86 | 3.7 (1.9) | 15.3 (9.1) | 9.0 (2.4) | ||
| HLA-DR | 3.3 (2.7) | 14.7 (7.0) | 15.3 (4.0) | ||
| MLR | NT | NT | NT | ||
| Indomethacin | 10 μg/ml | CD80 | 60.4 (8.8) | 83.1 (3.1) | 59.7 (6.7) |
| CD83 | 39.1 (4.6) | 43.5 (5.2) | 39.8 (3.2) | ||
| CD86 | 58.4 (6.7) | 54.8 (12.5) | 60.2 (6.3) | ||
| HLA-DR | 63.1 (3.7) | 71.2 (1.3) | 60.5 (3.5) | ||
| MLR | 26.5 (2.5) | 25.7 (4.0) | 23.5 (4.1) | ||
| Bromoenol lactone | 10 μM | CD80 | 67.4 (1.8) | 55.1 (10.0) | 55.8 (5.0) |
| CD83 | 70.5 (5.9) | 67.7 (5.2) | 52.9 (2.7) | ||
| CD86 | 67.3 (10.5) | 58.9 (1.9) | 54.9 (5.6) | ||
| HLA-DR | 52.7 (7.7) | 46.9 (7.2) | 46.7 (7.7) | ||
| MLR | 24.6 (2.6) | 26.2 (5.0) | 32.1 (2.7) | ||
| Aristolochic acid | 100 μM | CD80 | 37.1 (1.5) | 39.1 (9.0) | 51.7 (5.1) |
| CD83 | 37.6 (4.4) | 50.8 (3.4) | 32.8 (6.9) | ||
| CD86 | 35.9 (4.1) | 44.4 (8.7) | 47.4 (4.7) | ||
| HLA-DR | 5.3 (1.0) | 19.5 (7.7) | 23.2 (2.9) | ||
| MLR | NT | NT | NT | ||
| AH-6809 | 100 μM | CD80 | 75.9 (7.5) | 76.4 (6.0) | 75.3 (9.4) |
| CD83 | 78.4 (7.8) | 66.6 (14.8) | 62.5 (20.3) | ||
| CD86 | 80.7 (8.2) | 70.2 (6.3) | 50.7 (21.7) | ||
| HLA-DR | 73.5 (10.9) | 64.6 (12.1) | 57.1 (20.0) | ||
| MLR | 15.0 (1.9) | 22.7 (3.0) | 28.7 (6.1) | ||
MDDC were incubated with 0.5 μg/ml of LPS or CT or 500 μM d-cAMP with or without a prior 1-h incubation with the indicated concentrations of the indicated inhibitors for 20 h. The cells were then harvested and stained for four-color flow cytometry with PE-labeled anti-CD80, FITC-labeled anti-CD83, Cy-Chrome-labeled anti-CD86, and APC-labeled anti-HLA-DR. The MDDC were also tested in an MLR. The percent inhibition (averages are shown) was calculated as described in Materials and Methods. For reagents suspended in dimethyl sulfoxide (DMSO), control cultures were incubated with an equal volume of DMSO. DMSO at the concentrations used does not activate or inhibit the maturation of these cells (data not shown). The results are the means and standard errors of the means of at least three experiments performed on cells from different donors. NT, not tested.
To verify that products of the COX enzymes contribute to the maturation of MDDC induced by elevated cAMP levels, we incubated MDDC with CT, d-cAMP, or LPS in the presence or absence of the phospholipase A2 inhibitor bromoenol lactone (26) (10 μM) or aristolochic acid (44) (100 μM) or the prostaglandin d series (DP), prostaglandin E series 1 (EP1), and EP2 receptor antagonist AH-6809 (51) (100 μM). Phospholipase A2 is upstream of the COX enzymes in the prostaglandin production pathway, and blocking DP, EP1, and EP2 receptors blocks the activity of prostaglandins on target cells. Table 1 shows that all three inhibitors strongly inhibit the phenotypic maturation of MDDC, while bromoenol lactone and AH-6809 moderately inhibit the functional maturation of MDDC. These results further indicate that products of the COX enzymes, including PGE2, contribute to the maturation of MDDC induced by CT, d-cAMP, and LPS.
Nitric oxide contributes to the maturation of MDDC induced by CT and d-cAMP through the activation of guanylate cyclase.
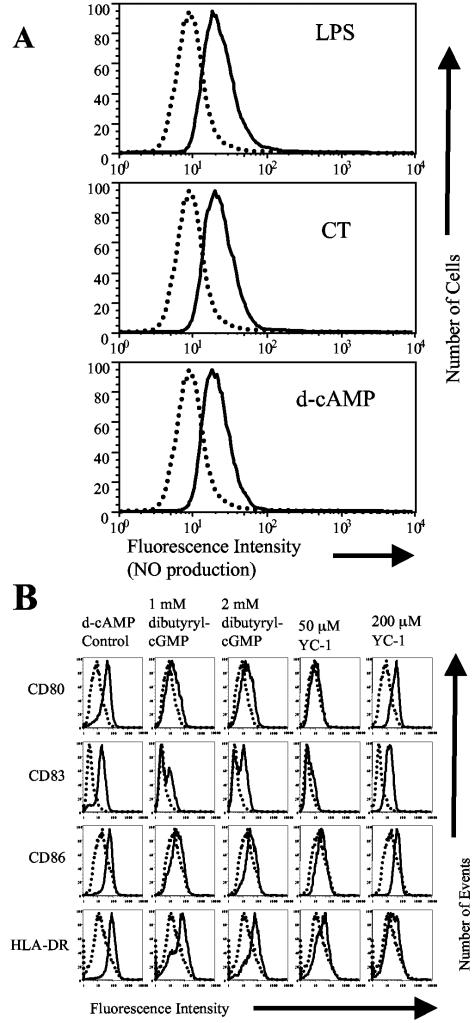
Elevated cAMP levels increase the expression of inducible NO synthase and increase the production of NO in many cell types (9, 13, 33). In addition, a possible role for NO in DC maturation is indicated by the ability of an NO-scavenging antioxidant to inhibit LPS-induced maturation of DC (49). For these reasons, we determined NO production by individual cells using DAF-2 diacetate, a sensitive and specific fluorescent indicator for the detection of NO (25, 31). MDDC were incubated for 20 h with 10 μM DAF-2 diacetate with or without 1 μg/ml CT, 1 mM d-cAMP, or 1 μg/ml LPS (as a control) and then analyzed by flow cytometry. Figure 5A shows that the mean channel fluorescence of MDDC incubated with DAF-2 increases when they are coincubated with CT, d-cAMP, or LPS. These results indicate that MDDC produce NO when stimulated with CT, d-cAMP, or LPS.
FIG. 5.
Nitric oxide contributes to the maturation of MDDC induced by cAMP-elevating agonists through the elevation of cGMP. (A) Nitric oxide production by MDDC. MDDC were incubated with 10 μM DAF-2 diacetate with or without 1 μg/ml LPS, 1 μg/ml CT, or 1 mM d-cAMP. Twenty hours later, the cells were assayed by flow cytometry. Stimulated MDDC (solid histograms) are compared to untreated MDDC (dotted histograms). Data are representative of one experiment of three performed with similar results on MDDC derived from different donors. (B) Dibutyryl-cGMP and YC-1 activate MDDC to mature. Cell surface expression of the indicated markers on untreated MDDC (dotted histograms) or stimulated MDDC (solid histograms) is shown. MDDC were incubated with 1 mM d-cAMP (as a control) or the indicated concentrations of dibutyryl-cGMP or YC-1 for 20 h. Cells were then harvested and stained for four-color flow cytometry with PE-labeled anti-CD80, FITC-labeled anti-CD83, Cy-Chrome-labeled anti-CD86, and APC-labeled anti-HLA-DR. Data are representative of one experiment of three performed with similar results on MDDC derived from different donors.
To test whether NO or other radicals are involved in MDDC maturation, we used radical scavengers to inhibit the effects of NO. LNAC is an agent that increases cellular pools of free radical scavengers, PTIO is a stable radical scavenger without the effect of NO synthase, and carboxy-PTIO is a scavenger that reacts with NO to form derivatives which inhibit NO synthase. For these experiments, MDDC were incubated with 0.5 μg/ml CT, 500 μM d-cAMP, or 0.5 μg/ml LPS (as a control) with or without a prior 1-h incubation with 10 μM LNAC (43), 500 μM PTIO, or 500 μM carboxy-PTIO (1). After 20 h, the cells were assayed by flow cytometry and an MLR. Table 2 shows that all three agents strongly inhibit the phenotypic maturation of MDDC while moderately inhibiting the functional maturation of MDDC, indicating that NO and/or other radicals contribute to the maturation of MDDC induced by these agonists.
TABLE 2.
Nitric oxide production contributes to the maturation of MDDC induced by cAMP-elevating agonistsa
| Inhibitor | Concn (μM) | Marker | Avg % inhibition (SEM)
|
||
|---|---|---|---|---|---|
| LPS | CT | d-cAMP | |||
| LNAC | 10 | CD80 | 57.6 (14.1) | 59.2 (12.5) | 83.7 (3.6) |
| CD83 | 58.0 (11.8) | 60.9 (16.5) | 87.5 (0.4) | ||
| CD86 | 57.7 (18.9) | 53.2 (9.7) | 86.5 (1.6) | ||
| HLA-DR | 59.2 (21.4) | 52.1 (15.3) | 83.2 (6.3) | ||
| MLR | 15.2 (1.5) | 14.6 (2.3) | 19.8 (3.9) | ||
| PTIO | 500 | CD80 | 53.4 (11.4) | 65.0 (7.8) | 58.7 (9.2) |
| CD83 | 45.9 (8.3) | 65.2 (7.9) | 59.1 (10.9) | ||
| CD86 | 64.1 (14.0) | 56.2 (7.2) | 44.4 (11.1) | ||
| HLA-DR | 41.3 (17.7) | 49.6 (8.5) | 36.5 (8.0) | ||
| MLR | 21.1 (3.5) | 20.1 (1.9) | 17.6 (1.8) | ||
| Carboxy-PTIO | 500 | CD80 | 83.3 (9.1) | 98.7 (1.1) | 98.5 (1.2) |
| CD83 | 82.4 (7.3) | 94.0 (2.8) | 96.8 (1.6) | ||
| CD86 | 82.7 (6.2) | 87.9 (4.5) | 92.8 (2.8) | ||
| HLA-DR | 81.3 (8.7) | 76.7 (5.8) | 89.1 (4.9) | ||
| MLR | NT | NT | NT | ||
| ODQ | 100 | CD80 | 54.7 (5.8) | 98.4 (0.5) | 86.7 (5.5) |
| CD83 | 78.0 (7.7) | 94.4 (1.4) | 84.1 (0.6) | ||
| CD86 | 54.3 (6.6) | 81.6 (1.0) | 70.2 (10.9) | ||
| HLA-DR | 25.4 (6.1) | 58.9 (2.1) | 49.4 (8.7) | ||
| MLR | 20.5 (4.8) | 19.7 (6.2) | 17.5 (3.9) | ||
| KT5823 | 8 | CD80 | 78.7 (2.7) | 80.6 (8.0) | 93.2 (2.7) |
| CD83 | 72.5 (5.4) | 73.7 (10.4) | 92.9 (3.6) | ||
| CD86 | 76.7 (5.5) | 48.2 (8.6) | 66.9 (11.8) | ||
| HLA-DR | 72.1 (4.8) | 64.5 (5.9) | 53.9 (15.3) | ||
| MLR | 18.9 (6.6) | 21.8 (4.2) | 27.3 (3.6) | ||
MDDC were incubated with 0.5 μg/ml LPS or CT or 500 μM d-cAMP with or without a prior 1-h incubation with the indicated concentrations of the indicated inhibitors for 20 h. The cells were then harvested and stained for four-color flow cytometry with PE-labeled anti-CD80, FITC-labeled anti-CD83, Cy-Chrome-labeled anti-CD86, and APC-labeled anti-HLA-DR. The MDDC were also tested in an MLR. The percent inhibition (averages are shown) was calculated as described in Materials and Methods. For reagents suspended in DMSO, control cultures were incubated with an equal volume of DMSO. DMSO at the concentrations used does not activate or inhibit the maturation of these cells (data not shown). The results are the means and standard errors of the means of at least three experiments performed on cells from different donors. NT, not tested.
To determine if NO directly activates DC, we treated MDDC with the NO donors 3-[(±)-(E)-ethyl-2′-[(E)-hydroxyimino]-5-nitro-3-hexene carbanoyl]-pyridine (NOR-4) and 3-morpholino-sydnonimine (SIN-1). We found that neither 1 mM NOR-4 nor 1 mM SIN-1 alone or together increased the expression of any maturation markers tested (data not shown). As we could not determine whether the failure of the NO donors to activate MDDC was due to an inability of NO to activate the cells or a failure to mimic the type of NO production (time of production and amount of production) by the stimulated MDDC, we determined if a downstream signaling pathway activated by NO is involved in MDDC maturation.
One of the key downstream signaling pathways activated by NO is the guanylate cyclase/cGMP pathway. Therefore, we determined whether increased intracellular levels of cGMP activate MDDC. MDDC were incubated for 20 h with increasing concentrations of the membrane-permeable analog of cGMP, dibutyryl-cGMP, or the guanylate cyclase activator YC-1 (30). Twenty hours later, the cells were assayed by flow cytometry. Figure 5B shows that dibutyryl-cGMP and YC-1 induce phenotypic MDDC maturation in a dose-dependent manner. These results demonstrate that signaling through guanylate cyclase, a pathway known to be activated by NO, induces the phenotypic maturation of MDDC.
To test whether cGMP production by guanylate cyclase contributes to the maturation of MDDC induced by CT, d-cAMP, or LPS, we incubated MDDC for 20 h with these agonists in the presence or absence of the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a1]quinoxalin-1-one (ODQ) (10) (100 μM) or the protein kinase G inhibitor KT5823 (24) (8 μM). Table 2 shows that ODQ and KT5823 strongly inhibit the phenotypic maturation and moderately inhibit the functional maturation of MDDC induced by these agonists. Together, these results indicate that induction of guanylate cyclase, possibly by NO, contributes to the maturation of MDDC induced by elevated cAMP levels and LPS.
PGE2 and dibutyryl-cGMP synergize for the maturation of MDDC.
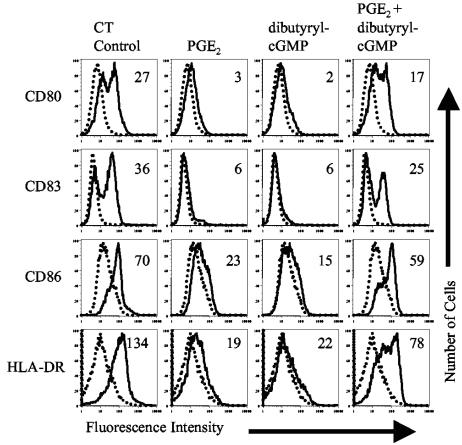
The above-described results indicate that products of the COX enzymes and NO contribute to the maturation of MDDC and most likely contribute to the maturation of bystander MDDC. However, the potency of either factor alone is not able to fully explain the observed bystander maturation. For this reason, we determined whether PGE2 and elevated cGMP levels (dibutyryl-cGMP) synergize for the activation of MDDC. MDDC were incubated with 1 μg/ml of CT (as a control) or suboptimal doses of PGE2 (1 μM) and dibutyryl-cGMP (250 μM), alone or together. Twenty hours later, the cells were analyzed by flow cytometry. Figure 6 shows that PGE2 and dibutyryl-cGMP synergize for the phenotypic maturation of MDDC.
FIG. 6.
Synergy between PGE2 and dibutyryl-cGMP. Cell surface expression of the indicated markers on untreated MDDC (dotted histograms) or MDDC treated with the indicated agonist (solid histograms) is shown. MDDC were incubated with 1 μg/ml of CT (as a control) or 1 μM PGE2 or 250 μM dibutyryl-cGMP alone or together for 20 h. The cells were then harvested and stained for four-color flow cytometry with PE-labeled anti-CD80, FITC-labeled anti-CD83, Cy-Chrome-labeled anti-CD86, and APC-labeled anti-HLA-DR. Numbers in histograms indicate the increase in mean fluorescence of treated MDDC over untreated MDDC. Data are representative of one experiment of three performed with similar results on MDDC derived from different donors.
DISCUSSION
CT and d-cAMP activate cAMP-responsive intracellular pathways while LPS activates other intracellular pathways. Surprisingly, our data show that the maturation of MDDC induced by all of these agonists is blocked by inhibiting the production of prostaglandins or NO or by inhibiting their effects on target cells. Therefore, our data indicate that the intracellular signaling pathways activated by these agonists are not sufficient to induce the maturation of the MDDC. There are two possible explanations for these results. First, the maturation of MDDC induced by these agonists may be the result of synergy between signal transduction pathways directly activated by the agonists and pathways activated by the soluble factors produced by the stimulated MDDC. Alternatively, the maturation induced by these agonists may entirely be the result of the actions of the soluble factors. In this regard, the only role in MDDC maturation played by the signaling pathways activated directly by cAMP may be to induce the production of the soluble factors. Indeed, the ability of these soluble factors to induce the maturation of bystander MDDC demonstrates that these factors can induce MDDC maturation without synergy from the cAMP-elevating agonists. Unfortunately, the data generated in this study are not sufficient to determine which of the above-described possibilities is correct.
The data presented in this study indicate that human DC stimulated to mature by cAMP-elevating agonists release soluble factors that induce the maturation of human bystander DC not stimulated by the agonist. A separate study showed a similar phenomenon in the mouse (47). In this regard, wild-type mouse bone marrow-derived DC activated by LPS can induce the maturation of bystander TLR4−/− DC (47). In addition, it was shown using bone marrow chimeras that bystander maturation occurs in vivo using Toll-like receptor (TLR) agonists (47). That study found that although the bystander DC displayed all of the characteristics of fully mature DC, they failed to produce IL-12 and expanded T cells that did not display a Th1 or Th2 phenotype. Those authors hypothesized that the bystander DC may induce T cells with a regulatory phenotype that help control the immune response (47).
We have a slightly different interpretation of the data (47). Although it has been published that MDDC matured by CT expand T cells with a Th2 phenotype (21), in our hands, CT-matured DC consistently generate T cells that fail to produce Th1 or Th2 effector cytokines. Therefore, in our hands, DC matured with CT strongly resemble bystander DC matured by factors released from DC stimulated with cAMP-elevating agonists or TLR agonists. In contrast to generating tolerance, CT is known to be a potent adjuvant. Therefore, we believe that bystander DC do not exclusively expand T cells with a regulatory phenotype but rather expand effector T cells with an undetermined phenotype.
Although the other study (47) convincingly showed bystander maturation using TLR agonists in the mouse, no experiments were undertaken to determine the nature of the soluble factors involved (47). Those authors hypothesized that inflammatory mediators including inflammatory cytokines such as alpha/beta interferon are responsible for the observed bystander maturation (47). This is supported by the fact that cytokines such as TNF-α induce the maturation of DC and are routinely used in maturation cocktails for the maturation of MDDC used in clinical trials. However, DC matured by inflammatory cytokines generally produce IL-12 and expand T cells with a Th1 phenotype. Therefore, it is difficult to reconcile how bystander DC matured by inflammatory cytokines would fail to produce IL-12 and expand T cells with a regulatory phenotype.
Our data indicate that the bystander maturation may be the result of factors that are not proteins. In this regard, unlike TLR agonists, it has been shown that cAMP-elevating agonists dominantly inhibit the production of inflammatory cytokines (2, 3, 21). Therefore, the soluble mediators that are responsible for bystander maturation are more likely factors such as prostaglandins and NO that activate DC without inducing cytokine production. The full elucidation of the factors involved in this bystander phenomenon should provide valuable insights into how immune responses are initiated at points of pathogen entry.
Acknowledgments
This work is supported by NIH grants AI38192 and AI43046 to G.K.L.
REFERENCES
- 1.Akaike, T., M. Yoshida, Y. Miyamoto, K. Sato, M. Kohno, K. Sasamoto, K. Miyazaki, S. Ueda, and H. Maeda. 1993. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction. Biochemistry 32:827-832. [DOI] [PubMed] [Google Scholar]
- 2.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, T. R. Fouts, and G. K. Lewis. 2002. Cholera toxin and heat-labile enterotoxin activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cyclic AMP-dependent pathway. Infect. Immun. 70:5533-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, T. R. Fouts, and G. K. Lewis. 2002. Pertussis toxin and the adenylate cyclase toxin from Bordetella pertussis activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cAMP-dependent pathway. J. Leukoc. Biol. 72:962-969. [PubMed] [Google Scholar]
- 4.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, and G. K. Lewis. 2004. Calcium signaling through phospholipase C activates dendritic cells to mature and is necessary for the activation and maturation of dendritic cells induced by diverse agonists. Clin. Diagn. Lab. Immunol. 11:77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, and G. K. Lewis. 2003. An enzymatically active A domain is required for cholera-like enterotoxins to induce a long-lived blockade on the induction of oral tolerance: new method for screening mucosal adjuvants. Infect. Immun. 71:6850-6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagley, K. C., M. T. Shata, D. Y. Onyabe, A. L. DeVico, T. R. Fouts, G. K. Lewis, and D. M. Hone. 2003. Immunogenicity of DNA vaccines that direct the coincident expression of the 120 kDa glycoprotein of human immunodeficiency virus and the catalytic domain of cholera toxin. Vaccine 21:3335-3341. [DOI] [PubMed] [Google Scholar]
- 7.Beubler, E., G. Kollar, A. Saria, K. Bukhave, and J. Rask-Madsen. 1989. Involvement of 5-hydroxytryptamine, prostaglandin E2, and cyclic adenosine monophosphate in cholera toxin-induced fluid secretion in the small intestine of the rat in vivo. Gastroenterology 96:368-376. [DOI] [PubMed] [Google Scholar]
- 8.Beubler, E., R. Schuligoi, A. K. Chopra, D. A. Ribardo, and B. A. Peskar. 2001. Cholera toxin induces prostaglandin synthesis via post-transcriptional activation of cyclooxygenase-2 in the rat jejunum. J. Pharmacol. Exp. Ther. 297:940-945. [PubMed] [Google Scholar]
- 9.Billiar, T. R., R. D. Curran, D. J. Stuehr, M. A. West, B. G. Bentz, and R. L. Simmons. 1989. An L-arginine-dependent mechanism mediates Kupffer cell inhibition of hepatocyte protein synthesis in vitro. J. Exp. Med. 169:1467-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunner, F., H. Stessel, and W. R. Kukovetz. 1995. Novel guanylyl cyclase inhibitor, ODQ reveals role of nitric oxide, but not of cyclic GMP in endothelin-1 secretion. FEBS Lett. 376:262-266. [DOI] [PubMed] [Google Scholar]
- 11.Burch, R. M., C. Jelsema, and J. Axelrod. 1988. Cholera toxin and pertussis toxin stimulate prostaglandin E2 synthesis in a murine macrophage cell line. J. Pharmacol. Exp. Ther. 244:765-773. [PubMed] [Google Scholar]
- 12.Cassel, D., and Z. Selinger. 1977. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc. Natl. Acad. Sci. USA 74:3307-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, C. C., K. T. Chiu, Y. T. Sun, and W. C. Chen. 1999. Role of the cyclic AMP-protein kinase A pathway in lipopolysaccharide-induced nitric oxide synthase expression in RAW 264.7 macrophages. Involvement of cyclooxygenase-2. J. Biol. Chem. 274:31559-31564. [DOI] [PubMed] [Google Scholar]
- 14.Dauer, M., B. Obermaier, J. Herten, C. Haerle, K. Pohl, S. Rothenfusser, M. Schnurr, S. Endres, and A. Eigler. 2003. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J. Immunol. 170:4069-4076. [DOI] [PubMed] [Google Scholar]
- 15.Denninger, J. W., and M. A. Marletta. 1999. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim. Biophys. Acta 1411:334-350. [DOI] [PubMed] [Google Scholar]
- 16.Dieu, M. C., B. Vanbervliet, A. Vicari, J. M. Bridon, E. Oldham, S. Ait-Yahia, F. Briere, A. Zlotnik, S. Lebecque, and C. Caux. 1998. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188:373-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dieu-Nosjean, M. C., A. Vicari, S. Lebecque, and C. Caux. 1999. Regulation of dendritic cell trafficking: a process that involves the participation of selective chemokines. J. Leukoc. Biol. 66:252-262. [DOI] [PubMed] [Google Scholar]
- 18.Fogel-Petrovic, M., J. A. Long, D. A. Knight, P. J. Thompson, and J. W. Upham. 2004. Activated human dendritic cells express inducible cyclo-oxygenase and synthesize prostaglandin E2 but not prostaglandin D2. Immunol. Cell Biol. 82:47-54. [DOI] [PubMed] [Google Scholar]
- 19.Forster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Muller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99:23-33. [DOI] [PubMed] [Google Scholar]
- 20.Futaki, N., S. Takahashi, M. Yokoyama, I. Arai, S. Higuchi, and S. Otomo. 1994. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins 47:55-59. [DOI] [PubMed] [Google Scholar]
- 21.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur. J. Immunol. 30:2394-2403. [DOI] [PubMed] [Google Scholar]
- 22.Gilroy, D. W., A. Tomlinson, and D. A. Willoughby. 1998. Differential effects of inhibition of isoforms of cyclooxygenase (COX-1, COX-2) in chronic inflammation. Inflamm. Res. 47:79-85. [DOI] [PubMed] [Google Scholar]
- 23.Giuliani, M. M., G. Del Giudice, V. Giannelli, G. Dougan, G. Douce, R. Rappuoli, and M. Pizza. 1998. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J. Exp. Med. 187:1123-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grider, J. R. 1993. Interplay of VIP and nitric oxide in regulation of the descending relaxation phase of peristalsis. Am. J. Physiol. 264:G334-G340. [DOI] [PubMed] [Google Scholar]
- 25.Havenga, M. J., B. van Dam, B. S. Groot, J. M. Grimbergen, D. Valerio, A. Bout, and P. H. Quax. 2001. Simultaneous detection of NOS-3 protein expression and nitric oxide production using a flow cytometer. Anal. Biochem. 290:283-291. [DOI] [PubMed] [Google Scholar]
- 26.Hazen, S. L., L. A. Zupan, R. H. Weiss, D. P. Getman, and R. W. Gross. 1991. Suicide inhibition of canine myocardial cytosolic calcium-independent phospholipase A2. Mechanism-based discrimination between calcium-dependent and -independent phospholipases A2. J. Biol. Chem. 266:7227-7232. [PubMed] [Google Scholar]
- 27.Hazes, B., and R. J. Read. 1997. Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry 36:11051-11054. [DOI] [PubMed] [Google Scholar]
- 28.Heyningen, S. V. 1974. Cholera toxin: interaction of subunits with ganglioside GM1. Science 183:656-657. [DOI] [PubMed] [Google Scholar]
- 29.Kantengwa, S., L. Jornot, C. Devenoges, and L. P. Nicod. 2003. Superoxide anions induce the maturation of human dendritic cells. Am. J. Respir. Crit. Care Med. 167:431-437. [DOI] [PubMed] [Google Scholar]
- 30.Ko, F. N., C. C. Wu, S. C. Kuo, F. Y. Lee, and C. M. Teng. 1994. YC-1, a novel activator of platelet guanylate cyclase. Blood 84:4226-4233. [PubMed] [Google Scholar]
- 31.Kojima, H., K. Sakurai, K. Kikuchi, S. Kawahara, Y. Kirino, H. Nagoshi, Y. Hirata, and T. Nagano. 1998. Development of a fluorescent indicator for nitric oxide based on the fluorescein chromophore. Chem. Pharm. Bull. (Tokyo) 46:373-375. [DOI] [PubMed] [Google Scholar]
- 32.Laneuville, O., D. K. Breuer, D. L. Dewitt, T. Hla, C. D. Funk, and W. L. Smith. 1994. Differential inhibition of human prostaglandin endoperoxide H synthases-1 and -2 by nonsteroidal anti-inflammatory drugs. J. Pharmacol. Exp. Ther. 271:927-934. [PubMed] [Google Scholar]
- 33.Lowenstein, C. J., C. S. Glatt, D. S. Bredt, and S. H. Snyder. 1992. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc. Natl. Acad. Sci. USA 89:6711-6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lycke, N. 2004. From toxin to adjuvant: the rational design of a vaccine adjuvant vector, CTA1-DD/ISCOM. Cell. Microbiol. 6:23-32. [DOI] [PubMed] [Google Scholar]
- 35.Lycke, N. 1997. The mechanism of cholera toxin adjuvanticity. Res. Immunol. 148:504-520. [DOI] [PubMed] [Google Scholar]
- 36.Lycke, N., and J. Holmgren. 1987. Long-term cholera antitoxin memory in the gut can be triggered to antibody formation associated with protection within hours of an oral challenge immunization. Scand. J. Immunol. 25:407-412. [DOI] [PubMed] [Google Scholar]
- 37.Lycke, N., T. Tsuji, and J. Holmgren. 1992. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur. J. Immunol. 22:2277-2281. [DOI] [PubMed] [Google Scholar]
- 38.Morelli, A. E., and A. W. Thomson. 2003. Dendritic cells under the spell of prostaglandins. Trends Immunol. 24:108-111. [DOI] [PubMed] [Google Scholar]
- 39.Norgauer, J., Y. Ibig, D. Gmeiner, Y. Herouy, and B. L. Fiebich. 2003. Prostaglandin E2 synthesis in human monocyte-derived dendritic cells. Int. J. Mol. Med. 12:83-86. [PubMed] [Google Scholar]
- 40.Ohtomo, N., T. Muraoka, A. Tashiro, Y. Zinnaka, and K. Amako. 1976. Size and structure of the cholera toxin molecule and its subunits. J. Infect. Dis. 133(Suppl.):31-40. [DOI] [PubMed] [Google Scholar]
- 41.Paolucci, C., S. E. Burastero, P. Rovere-Querini, C. De Palma, S. Falcone, C. Perrotta, A. Capobianco, A. A. Manfredi, and E. Clementi. 2003. Synergism of nitric oxide and maturation signals on human dendritic cells occurs through a cyclic GMP-dependent pathway. J. Leukoc. Biol. 73:253-262. [DOI] [PubMed] [Google Scholar]
- 42.Rieser, C., G. Bock, H. Klocker, G. Bartsch, and M. Thurnher. 1997. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J. Exp. Med. 186:1603-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roederer, M., F. J. Staal, P. A. Raju, S. W. Ela, and L. A. Herzenberg. 1990. Cytokine-stimulated human immunodeficiency virus replication is inhibited by N-acetyl-L-cysteine. Proc. Natl. Acad. Sci. USA 87:4884-4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenthal, M. D., B. S. Vishwanath, and R. C. Franson. 1989. Effects of aristolochic acid on phospholipase A2 activity and arachidonate metabolism of human neutrophils. Biochim. Biophys. Acta 1001:1-8. [DOI] [PubMed] [Google Scholar]
- 45.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scandella, E., Y. Men, D. F. Legler, S. Gillessen, L. Prikler, B. Ludewig, and M. Groettrup. 2004. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood 103:1595-1601. [DOI] [PubMed] [Google Scholar]
- 47.Sporri, R., and C. Reis e Sousa. 2005. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat. Immunol. 6:163-170. [DOI] [PubMed] [Google Scholar]
- 48.Vajdy, M., and N. Y. Lycke. 1992. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology 75:488-492. [PMC free article] [PubMed] [Google Scholar]
- 49.Verhasselt, V., W. Vanden Berghe, N. Vanderheyde, F. Willems, G. Haegeman, and M. Goldman. 1999. N-acetyl-L-cysteine inhibits primary human T cell responses at the dendritic cell level: association with NF-kappaB inhibition. J. Immunol. 162:2569-2574. [PubMed] [Google Scholar]
- 50.Whittaker, D. S., K. S. Bahjat, L. L. Moldawer, and M. J. Clare-Salzler. 2000. Autoregulation of human monocyte-derived dendritic cell maturation and IL-12 production by cyclooxygenase-2-mediated prostanoid production. J. Immunol. 165:4298-4304. [DOI] [PubMed] [Google Scholar]
- 51.Woodward, D. F., D. J. Pepperl, T. H. Burkey, and J. W. Regan. 1995. 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH 6809), a human EP2 receptor antagonist. Biochem. Pharmacol. 50:1731-1733. [DOI] [PubMed] [Google Scholar]