Abstract
Cryptosporidium species are ubiquitous in the environment and are frequently detected in the stools of children who live where sanitation conditions are poor. To better characterize the immune response to these parasites, we monitored immunoglobulin G (IgG) antibody levels in a cohort of children from Lima, Peru. Two new enzyme-linked immunosorbent assays based on the C. parvum (bovine, subtype IIa) Iowa strain 17-kDa and 27-kDa antigens were used to measure IgG antibody levels in longitudinal serum samples. Antibody responses were detected during infections with C. parvum, C. felis, and C. meleagridis and with four different subtypes of C. hominis. We also noted that the magnitude of the antibody response was related to the number of previous infections and that older children generally had higher levels of antibodies to the two C. parvum antigens. Antibody responses were not associated with infections with either Cyclospora sp. or Giardia sp. We believe the antibody assays will be important tools for monitoring the success of future public health interventions.
Cryptosporidium spp. are protozoan parasites that cause infection and diarrheal illness in a wide range of mammalian species. Several Cryptosporidium species, including C. parvum (formerly referred to as bovine or type 2 C. parvum), C. hominis (formerly referred to as human or type 1 C. parvum), C. felis, and C. meleagridis, have been identified as infectious to humans (23, 28, 30, 46). Transmission of the parasite is facilitated by a relatively low infectious dose and by the resistance of the parasite oocyst stage to commonly used disinfection techniques (9, 20). Cryptosporidium infections may be asymptomatic or may be associated with a broad range of enteric symptoms ranging from mild discomfort to severe diarrhea with dehydration (3, 5). Immunocompromised individuals, especially those with very low CD4-cell counts, often suffer from a chronic and severely debilitating form of the disease (15).
In the United States, outbreaks of cryptosporidiosis in both adults and children have been linked to improperly treated drinking water, exposure to contaminated recreational water, and contamination of uncooked food items (reviewed in reference 11). However, in the developing world, where sanitation conditions are poor and clean water supplies are limited, cryptosporidiosis is commonly an illness of childhood, and reported outbreaks are rare (3, 8, 13). Children, especially those living under lower socioeconomic conditions, may experience repeated Cryptosporidium infections from an early age (3, 25). Studies have suggested that early childhood infection may be associated with malnutrition and growth retardation and may have long-term consequences for the affected children (6, 7, 18, 19, 22, 40).
Many studies designed to characterize the epidemiology of Cryptosporidium infections have relied on microscopic examinations of stool to identify infection episodes. However, microscopy is relatively insensitive when small numbers of oocysts are present, especially in formed stools (45), and the sensitivity of stool detection may vary due to the intermittent nature of oocyst shedding (5). We have previously demonstrated that serologic assays based on the detection of immunoglobulin G (IgG) antibodies to the two immunodominant sporozoite surface antigens, the 27-kDa and 17-kDa antigens, can be used to identify infected individuals (31, 33). We have also shown that more infections are identified by serologic antibody assay than by traditional acid-fast stool microscopy (32). In the present study, we assayed serum samples from children who participated in a longitudinal study of diarrheal disease in Lima, Peru, in order to better characterize the immune response and to determine if the assays can detect antibody responses caused by different species of Cryptosporidium.
(Presented in part at the 49th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Houston, Tex., October 2000 [J. Kwon, C. Bern, P. Lammie, J. Roberts, W. Checkley, L. Cabrera, R. Gilman, and J. Priest, Am. J. Trop. Med. Hyg. 62:258-259, 2000].)
MATERIALS AND METHODS
Description of cohort study.
We conducted this work using serum samples from a birth cohort of children who participated in a longitudinal study of diarrheal disease. The study was conducted in Pampas de San Juan de Miraflores, a pueblo joven, or shantytown, of approximately 40,000 inhabitants on the outskirts of Lima, Peru. Previous reports have described the community and the design of the longitudinal study in detail (3, 8, 46) and have compared the sensitivity of the serologic IgG antibody assay to that of acid-fast microscopy (32).
Infants were enrolled at birth and monitored until December 1998. During the course of the surveillance, stool samples were collected weekly; additional samples were collected during episodes of diarrhea and when enteric protozoa were detected by microscopy. Stool specimens were processed by standard concentration techniques and examined by microscopy for Giardia sp., Cryptosporidium spp., and Cyclospora sp. in the pathology laboratory of the Universidad Peruana Cayetano Heredia (2, 10). In addition to the weekly stool collections, field workers visited households daily and maintained a daily log which recorded the number and consistency of the child's stools and the mother's assessment of whether the child had diarrhea that day. Serum samples were collected from the children at approximately 3- to 6-month intervals and more frequently when Cryptosporidium or Cyclospora oocysts were detected in stool.
The extraction of DNA from stored stool specimens and the PCR-restriction fragment length polymorphism (RFLP) technique used to identify which Cryptosporidium genotype or species was present in the stool specimens have been described in detail elsewhere (46). Only specimens that were positive for Cryptosporidium spp. by microscopy were analyzed. C. hominis and C. parvum isolates were further subtyped by PCR and sequence analysis of the GP60/Cp17 gene (1).
Written informed consent was obtained from the parent or guardian of all participating children. The original cohort study was approved by the institutional review boards of the Johns Hopkins University School of Public Health and Asociación Benefica PRISMA. The Cryptosporidium serology and genotype studies were approved by the Centers for Disease Control and Prevention Institutional Review Board.
Serum specimens.
As previously described, we selected 74 cohort children for serologic analysis based on availability of serum specimens and whether the children had cryptosporidiosis detected on stool examination (32). Serum samples were stored at −20°C until needed. Of the 676 samples that were originally collected from the identified children, 38 samples were either missing or did not contain sufficient volume for testing. The remaining 638 serum samples were diluted 1:50 with buffer containing 0.85% NaCl and 10 mM Na2HPO4 at pH 7.2 (phosphate-buffered saline) and 0.05% Tween 20 for the antibody assays.
ELISAs.
Serologic assays were performed to detect IgG antibodies to the 27-kDa and the 17-kDa Cryptosporidium sporozoite antigens using a recombinant form of the 27-kDa protein and a partially purified form of the native 17-kDa protein, respectively, from the C. parvum (bovine, subtype IIa) Iowa strain. Detailed methods for the expression and purification of the proteins and for the enzyme-linked immunosorbent assays (ELISAs) have been described previously (31, 33). Longitudinal samples from a single child were assayed on the same plate. Each plate included a standard curve consisting of a serial twofold dilution (1:50 to 1:12,800) of a strong positive control. Three positive control serum samples and four negative controls were also included on each plate. The negative controls were serum samples collected from North American adults who had no demonstrable antibody to crude Cryptosporidium oocyst/sporozoite antigens by Western blot assay. To detect bound antibodies, biotin-labeled anti-human IgG secondary antibody (1:1,000; clone HP6017; Zymed, South San Francisco, CA) and streptavidin alkaline phosphatase (1:500; Gibco BRL, Grand Island, NY) were added to each plate with washing steps in between. Color development was monitored following addition of p-nitrophenylphosphate substrate (Sigma, St. Louis, MO) dissolved in 3 mM MgCl2 and 10% diethanolamine at pH 9.8. Absorbance was read at 405 nm with a Molecular Devices (Sunnyvale, CA) UVmax microplate reader.
The 1:50 dilution of the standard curve was arbitrarily assigned a value of 6,400 units. Antibody levels of unknown serum samples were determined by comparison to the standard curve serum dilution series with a four-parameter curve fit. Antibody values of >160 and >57 arbitrary units for the 27-kDa and 17-kDa ELISAs, respectively, were considered to be positive (21). These cutoffs were based on the means plus three standard deviations of the values of the four negative control sera.
Definitions.
A stool-confirmed episode of Cryptosporidium, Cyclospora, or Giardia infection began when oocysts or cysts were detected in one or more stool specimens and ended when the last day of detection was followed by an interval of at least 4 weeks with no further detections (minimum of three stool samples tested) (3). An episode of diarrhea began when a child had three or more liquid or semiliquid stools in a 24-hour time period and the mother or other primary caregiver reported that the child had diarrhea. An episode of diarrhea was considered to have ended when the child had at least 3 days that did not satisfy this definition. A day of diarrhea was considered to be associated with a stool-confirmed Cryptosporidium episode if it occurred during or within 1 week of the beginning or the end of the infection episode.
Consecutive serum antibody responses were compared in pairwise fashion in order to identify intervals during which infection with Cryptosporidium was likely to have occurred. Using our previously described definition (32), a serologic episode of Cryptosporidium infection was identified when the following conditions were met: (i) the interval between the two consecutive serum samples was ≤180 days; (ii) antibody levels in the second serum were above the identified cutoff for both the 27-kDa and 17-kDa antigens; (iii) antibody levels for both the 27-kDa and 17-kDa antigens were elevated in the second serum sample relative to the first sample; and (iv) the antibody response to at least one of the two antigens demonstrated an increase of ≥50% during the interval. Consecutive serologic episodes were considered to represent separate events if the interval between them was >90 days.
A serologic episode of Cryptosporidium infection was considered to be associated with a stool microscopy-defined cryptosporidiosis episode if the second serum sample of the interval was collected within a window of 7 days before the first oocyst detection to 90 days after last oocyst detection. We excluded a serologic episode from analysis if both of the sera in the interval were collected >14 days after the last oocyst detection.
Data analysis.
All proportions and means not involving multiple measures per child were compared using the chi-square test and pooled t test, respectively. The associations between magnitude of antibody response, previous infection experience, and/or age at time of infection were evaluated by multiple linear regression. Poisson regression analysis was used to investigate the effect of pre- and postinfection antibody levels on the risk of symptom development during an episode of cryptosporidiosis. The association between number of infections diagnosed by serology and repeat infection was tested using the Cochran-Armitage test. All regressions were run using SAS Proc Genmod implementing the generalized estimating equations procedure, when applicable, to adjust for correlation among multiple responses and/or episodes from the same child. Geometric means were calculated by exponentiating of the log mean of the antibody values. One was added to each antibody value in order to derive logs of zero values. Analyses were performed using SAS version 9.0, Sudaan version 8.0.2, SigmaStat version 2.03.0 (SPSS, Inc.), or StatXact5 (Cytel Software Corp.). Statistical significance was set at an alpha level of 0.05.
RESULTS
PCR confirmation of infection status.
Of the 74 children in our study, 57 (77%) had one or more episodes of microscopy-confirmed cryptosporidiosis as follows: 29 (39%) children experienced one episode, 22 (30%) had two episodes, 5 (7%) had three episodes, and 1 (1%) child had four episodes. Stool samples were available for PCR and RFLP analysis from 73 ofthe 92 microscopically defined cryptosporidiosis episodes (Table 1). Genotyping was successful for 55 (75%) of the episodes with available samples: 39 C. hominis (or human type 1 Cryptosporidium) infections, 6 C. parvum (or bovine type 2 Cryptosporidium) infections, 5 C. meleagridis infections, 2 C. felis infections, 1 mixed C. meleagridis and C. canis or “dog” genotype infection, and 2 mixed C. hominis and C. parvum infections were identified. PCR analysis was unable to confirm the presence of Cryptosporidium oocysts for 18 (25%) of the infection episodes for which stool samples were available. Because some degradation may have occurred in these stool samples as a result of repeated freeze-thaw cycles during 3 to 5 years of storage, no conclusions could be drawn regarding the specificity of the acid-fast microscopy assay.
TABLE 1.
ELISA detection of antibody responses caused by various species of Cryptosporidium
| Speciesa | n | Subtypeb | Serum antibody response
|
||
|---|---|---|---|---|---|
| Positive | Negative | Unknownc | |||
| C. hominis | 39 | Ia | 7 | 1 | 0 |
| Ib | 5 | 1 | 3 | ||
| Id | 3 | 2 | 2 | ||
| Ie | 4 | 0 | 0 | ||
| Id + Ie | 1 | 0 | 0 | ||
| Unidentifiedd | 3 | 5 | 2 | ||
| C. parvum | 6 | Ic | 1 | 0 | 2 |
| Unidentifiedd | 2 | 0 | 1 | ||
| C. meleagridis | 5 | NAe | 3 | 1 | 1 |
| C. felis | 2 | NAe | 2 | 0 | 0 |
| C. hominis + C. parvum | 2 | Ic | 2 | 0 | 0 |
| C. meleagridis + C. canis (dog) | 1 | NAe | 1 | 0 | 0 |
| Unidentifiedf | 18 | NAe | 6 | 9 | 3 |
| Not testedg | 19 | NAe | 8 | 6 | 5 |
Species determined by PCR and RFLP of small subunit rRNA.
Subtype determined by PCR amplification and sequencing of the GP60/ Cp17 antigen gene.
Serum samples not available in appropriate window for testing.
GP60/ Cp17 antigen gene PCR unsuccessful.
NA, not applicable.
Stool samples were available, but PCR failed.
No stool samples were available for PCR analysis.
The GP60/Cp17 antigen gene sequence was successfully used to subtype 29 of the 39 C. hominis infections: one mixed subtype Id and subtype Ie infection, eight subtype Ia infections, nine subtype Ib infections, seven subtype Id infections, and four subtype Ie infections were identified (1, 29, 34, 38). In addition, three of the C. parvum infections were identified as subtype Ic organisms.
Cryptosporidium infection can be diagnosed by serologic antibody analysis.
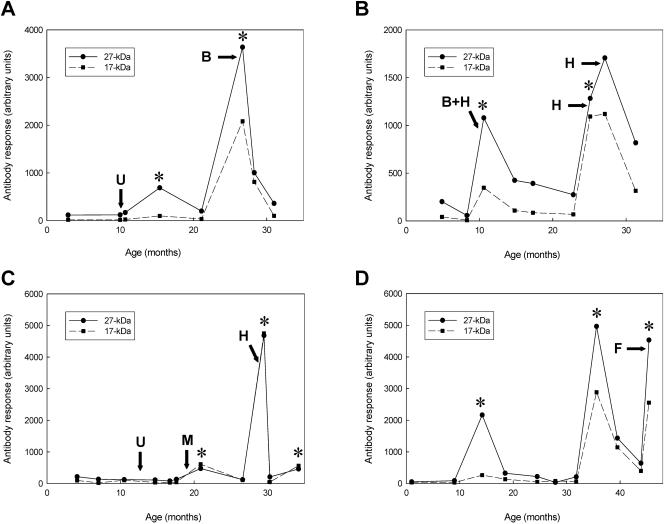
As we previously reported, 108 serologic antibody episodes were identified in our 74 study children; 10 of these episodes were identified in 6 children who were consistently negative for Cryptosporidium infection by stool microscopy (32). Figure 1 shows representative antibody, microscopic stool assay, and PCR confirmation results from 4 of the 74 children. In the first example (Fig. 1A), the child had two separate oocyst-confirmed Cryptosporidium infections, one at 10.6 months that lasted 6 days and that could not be genotyped (no stool samples were available) and a second at 26.4 months that lasted only 1 day and that was shown to be C. parvum. Neither infection was associated with diarrhea according to our case definition. Peaks of antibody reactivity were identified at 15.3 and 26.7 months of age by serologic assay. Because the oocyst detection and serum collection dates were separated by more than 90 days, the first antibody peak and the oocyst detection at 10.6 months were treated as unrelated events. We cannot, however, exclude the possibility that both may have been the result of a single Cryptosporidium infection. The levels of antibodies to both the 27-kDa and the 17-kDa antigens were markedly higher during the second serologic response.
FIG. 1.
Representative profiles showing the diagnosis of Cryptosporidium episodes by stool assay and by serologic antibody assay. Longitudinal serum samples from four children (A to D) were analyzed by ELISA for antibodies to the 17-kDa (dashed line with closed square) and 27-kDa (solid line with closed circle) antigens as described in Materials and Methods. Responses are presented in arbitrary units based upon a standard curve serum with a 6,400 maximum value. An asterisk indicates a serum sample that meets the definition for a positive antibody response as described in Materials and Methods. Regularly collected stool samples from the children were screened for the presence of Cryptosporidium oocysts by acid-fast microscopy, and the first day of each identified infection episode is indicated by an arrow. Microscopy-positive stool samples were subjected to PCR and RFLP analysis (46) in order to identify the genotype of Cryptosporidium parasite: C. parvum of the bovine genotype (B), C. felis (F), C. hominis or human genotype (H), and C. meleagridis (M) were detected. Stool samples that were unavailable for analysis are indicated by a U.
Three separate stool-confirmed Cryptosporidium infections were diagnosed in the child represented in Fig. 1B: a mixed C. parvum and C. hominis infection at 10.4 months (12 day duration with 2 days of diarrhea; subtype Ic) and two C. hominis infections at 24.7 (26 day duration with 12 days of diarrhea; subtypes Id and Ie) and 26.6 months of age (1 day duration with 2 days of diarrhea). The mixed infection and the first C. hominis infection were associated with serum IgG responses at 10.6 and 25.1 months of age, respectively. The level of antibody to the 17-kDa antigen, but not that to the 27-kDa antigen, was higher during the second infection. Although an increase in antibody was also observed at 27.1 months of age, 11 days after the second C. hominis infection, the increase did not reach the 50% threshold required by our definition.
Figure 1C shows the assay profile from a child who had three separate stool-confirmed infections at 12.9 (unknown genotype; no stool samples available), 19.2 (C. meleagridis), and 29.3 months of age (C. hominis; subtype Ia) with durations of 1, 6, and 15 days, respectively. Only the C. meleagridis infection at 19.2 months was associated with diarrheal symptoms (11 days duration). Although no peak of antibody reactivity was detected after the first stool-confirmed infection, antibody peaks at 20.9 and 29.5 months of age were associated with the C. meleagridis and C. hominis infections, respectively. As observed in Fig. 1A, the second antibody response was elevated relative to the first response for both antigens. An additional antibody peak was detected at 34.2 months of age without an associated oocyst excretion event. The third antibody response was not elevated relative to the previous two responses but, because this response was detected during the last interval of the study, we cannot be certain that it had reached its maximum value.
The assay profile of the child shown in Fig. 1D shows one stool-confirmed C. felis infection at 44.9 months of age that lasted for 8 days. The episode was not associated with diarrheal symptoms according to our case definition. Serologic responses were detected at 14.2 and 35.6 months of age in the absence of oocyst detection and at 45.4 months of age in association with the C. felis infection. A memory response was apparent for the second serologic response. The third antibody response detected in the final serum sample in association with the C. felis infection was approximately equal in magnitude to the preceding response, but it may not have reached its maximum value.
As suggested by the individual profiles described above, most of the Cryptosporidium infections diagnosed by stool assay were not associated with an episode of diarrhea according to our case definition: 68 (74%) of the 92 stool assay-positive infections were considered asymptomatic. An analysis of the relationship between antibody level and diarrheal illness among the 73 stool-confirmed infections with serum coverage showed that asymptomatic infections (n = 54) were associated with significantly higher postinfection antibody levels to the 27-kDa antigen than symptomatic infections (n = 19) (geometric mean level, 863 versus 441, respectively; P = 0.039). The differences between the levels of antibody to the 17-kDa antigen were not significant (asymptomatic geometric mean level, 296 versus 166 for symptomatic; P = 0.26). No significant differences in preinfection antibody levels were observed for either antigen. However, these results may be impacted by the fact that the intervals between infection and serum collection and between repeat infections were not uniform (32).
Effect of species and subtype on antibody response.
As demonstrated by the profiles in Fig. 1, serologic assays based upon the binding of antibodies to C. parvum (bovine) subtype IIa antigens were able to detect infections caused by various species and subtypes of parasite. When appropriately spaced serum samples were available for analysis, we were able to detect an antibody response for 23 of 32 C. hominis infections (72% with a response), 3 of 4 C. meleagridis infections (75% with a response), and 2 C. felis infections (100% with a response) as well as 3 infections caused by the homologous C. parvum parasite (100% with a response) (Table 1). Three additional stool-positive episodes that were associated with an antibody response were a mixed infection of C. canis or “dog” genotype and C. meleagridis and two mixed infections of C. parvum and C. hominis. Of the 23 antibody-positive C. hominis episodes (Table 1), 7 were subtype Ia infections, 5 were subtype Ib infections, 3 were subtype Id infections, 4 were subtype Ie infections, 1 was a mixed subtype Id and Ie infection, and 3 infections could not be subtyped by GP60/Cp17 antigen gene sequence (29, 38). These results would suggest that there is significant overlap of 17- and 27-kDa antigen epitopes between these different species and subtypes of parasites and that our assays are sensitive to the responses caused by a variety of Cryptosporidium organisms.
Antibody levels increased with age and infection experience in the study children.
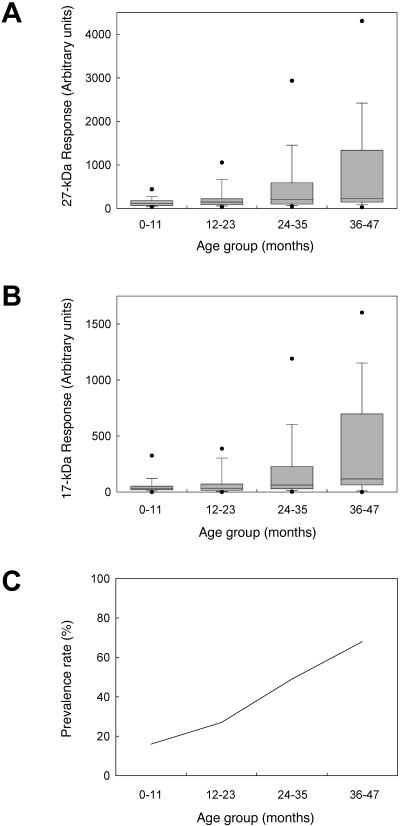
To examine the longitudinal trends in the serologic assay values for our study group as a whole, we identified the first serum from each child that was collected during each year of life and plotted these values versus age. We observed a significant (P < 0.0001 for both antigens) upward trend in the antibody levels to the 27-kDa antigen (Fig. 2A) and the 17-kDa antigen (Fig. 2B) with the age of the child. Median antibody levels increased threefold for the 27-kDa antigen and fourfold for the 17-kDa antigen over the 4-year study period. Sixty-eight percent of children ≥3 years of age were positive for antibodies to both the 17-kDa antigen (>57 arbitrary units) and 27-kDa antigen (>160 arbitrary units) compared to 16% for children under 1 year of age (Fig. 2C). The prevalence curve determined for the children in our study is very similar to those reported from an earlier study in Peru, from a study in rural China, and from a study in Guatemala (37, 43, 47). However, the results observed for the 74 children in our study must be interpreted with some caution, as they may not be representative of the entire birth cohort.
FIG. 2.
The magnitude of the antibody response is correlated with age. The first available sample collected from each child (n = 61, 71, 63, and 28) in each age group (<12 months, 12 to 23 months, 24 to 35 months, and 36 to 47 months, respectively) was analyzed for antibodies to the 27-kDa (A) and 17-kDa (B) antigens by ELISA as previously described. Boxes enclose the 25th to 75th percentiles, whiskers indicate the 10th to 90th percentiles, and closed circles indicate the 5th and 95th percentiles for each age group. The medians (indicated by bars) are 120, 162, 250, and 359 arbitrary units for the 27-kDa antigen assay and 35, 35, 63, and 116 arbitrary units for the 17-kDa antigen assay, respectively. The first available sample collected from each child in each age group was also used to calculate a prevalence rate (C). A child was considered to be positive at a given time point for antibodies to Cryptosporidium if the antibody levels to both the 27-kDa and 17-kDa antigens were above the cutoff values (>160 and >57 arbitrary units, respectively).
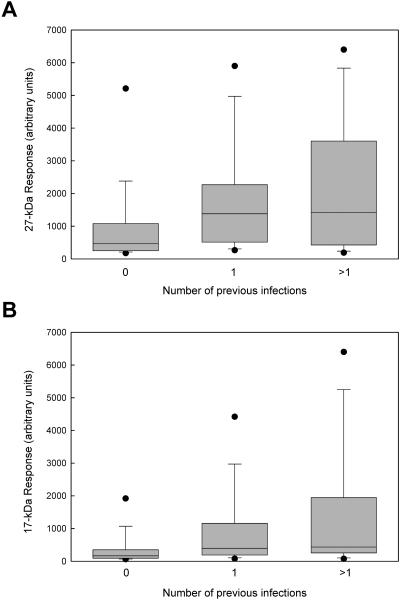
As suggested by the individual antibody response profiles shown in Fig. 1, a history of previous infection does affect the magnitude of the antibody response. Figure 3 shows the distributions of the 108 antibody responses identified in our study categorized according to the total number of previous infections (0, 1, or >1 previous infections). Infections diagnosed only by microscopy were included in the count of previous infections (n = 44). Geometric mean antibody levels were significantly higher for repeat infections (n = 69) than for primary infections (n = 39) for both the 27-kDa antigen (Fig. 3A) (1,258 versus 534, respectively; P = 0.0044) and the 17-kDa antigen (Fig. 3B) (584 versus 204, respectively; P = 0.0006). However, when adjusted for the number of previous infections, age was not significantly associated with the magnitude of the antibody response (P = 0.11 for the 27-kDa antibody level and P = 0.29 for the 17-kDa antibody level). These results would suggest that the older children in our study group have higher antibody levels than the younger children because they are more likely to have been repeatedly infected with Cryptosporidium during a longer lifetime of exposure.
FIG. 3.
The magnitude of the antibody response increases with experience of infection. A total of 108 Cryptosporidium infection episodes were diagnosed by serologic assay using the definitions described in Materials and Methods. Antibody levels to the 27-kDa (A) and 17-kDa (B) antigens were grouped according to the number of previous infections (0, n = 39; 1, n = 36; >1, n = 33). Boxes enclose the 25th to 75th percentiles, whiskers indicate the 10th to 90th percentiles, and closed circles indicate the 5th and 95th percentiles for each age group. The medians (indicated by bars) are 464, 1,380, and 1,414 arbitrary units, respectively, for the 27-kDa assay and 166, 397, and 435 arbitrary units, respectively, for the 17-kDa assay.
Increasing experience of infection also had an impact on the sensitivity of the serologic assay. When we compared the number of infections that were detected by microscopy to the total number of infections detected by either method, we found that microscopy diagnosed comparable proportions of first (63%), second (63%), and third infections (62%) (Table 2). There were too few subsequent infections to assess the significance of the decrease in stool positivity after three infections (35%). The serologic assay diagnosed the same proportion of primary infections as stool microscopy (62% versus 63%), but the proportion of infections associated with an antibody response increased with the number of previous infections (P = 0.012). We conclude that, although primary infection episodes can be detected by serologic assay as efficiently as by microscopy, repeat infections are diagnosed by serologic assay with increased efficiency. This increase in diagnosis efficiency probably reflects the increase in the magnitude of the antibody response observed upon repeated infection.
TABLE 2.
Efficiency of diagnosis of Cryptosporidium infection by assay technique
| No. of previous infections | na | No. (%) diagnosed by:
|
|
|---|---|---|---|
| Stool microscopy | Serologyb | ||
| 0 | 63 | 40 (63) | 39 (62) |
| 1 | 51 | 32 (63) | 36 (71) |
| 2 | 24 | 15 (62) | 21 (87) |
| >2 | 14 | 5 (35) | 12 (86) |
Number of Cryptosporidium infections diagnosed by any method. Total n = 152; 44 infections were diagnosed by microscopy only, 60 infections were diagnosed by serologic assay only, and 48 infections were diagnosed by both methods.
Significant trend detected by Cochran-Armitage test (P = 0.012).
Specificity of serologic assay for Cryptosporidium.
The extensive serologic and stool surveillance of this study provided opportunities to determine whether peaks of antibody reactivity to the 27- and 17-kDa Cryptosporidium antigens coincided with detection of Cyclospora or Giardia parasites by stool assay. Using the same definition of a stool-confirmed infection episode as that used for Cryptosporidium, 32 of the children from our study had 47 Cyclospora infections (range, one to four infections/child). Twelve (26%) of these Cyclospora episodes were likely to have been mixed infections with Cryptosporidium, because oocysts from both species were found either on the same day (n = 8) or within 7 days of each other (n = 4). Twenty-five stool-confirmed Cyclospora episodes (53%) were not temporally associated with an antibody response to Cryptosporidium antigens, 13 (28%) were temporally associated with an antibody response, and 9 (19%) did not have sera collected in an appropriate window of time for analysis. Of the 13 serologic antibody responses, 5 were found in intervals containing both Cyclospora- and Cryptosporidium-positive stool samples. Thus, only 8 (21%) of 38 Cyclospora infections with appropriate serum collection were uniquely associated with an antibody response to the Cryptosporidium 17- and 27-kDa antigens. By comparison, 48 (66%) of 73 stool-confirmed Cryptosporidium infections with appropriate serum collection were associated with an antibody response (32), and 43 (59%) of these had no concurrent Cyclospora detections.
When the 48 antibody responses associated with stool detection of Cryptosporidium oocysts were deleted from the analysis, antibody levels to the 27-kDa and 17-kDa antigens were not significantly higher (P = 0.11 and P = 0.15, respectively) in sera collected in a window from 7 days before Cyclospora oocyst excretion began to 90 days after oocyst excretion ended (n = 65; geometric mean for 27-kDa level, 244; geometric mean for 17-kDa level, 55) than in sera collected outside this window (n = 525; geometric mean for 27-kDa level, 185; geometric mean for 17-kDa level, 47). Although not conclusive, these results suggest that there was no relationship between Cyclospora infection and the development of an antibody response to the two Cryptosporidium antigens.
In contrast to the sporadic nature of Cyclospora and Cryptosporidium infections, Giardia was found as a cause of frequent, sometimes persistent, infections in 68 (92%) of the children of our study. For instance, the child represented in Fig. 1A had episodes of Giardia infection at 1, 7, and 31 months of age, and the child represented in Fig. 1B had episodes at 8, 17, 26, 28, 30, and 36 months of age. The child represented in Fig. 1C had nine episodes beginning at 2 months of age. Similarly, the child represented in Fig. 1D became infected at 13 months of age and had detectable Giardia cysts for 27 of the following 34 months of study. We saw no evidence of a temporal association between Giardia infection and the development of an antibody response to the two Cryptosporidium antigens: 15 of the 18 children who had no detectable antibody response to the Cryptosporidium antigens had at least one Giardia infection by stool microscopy.
DISCUSSION
In a previous study of the entire birth cohort of Peruvian children, Xiao et al. (46) demonstrated that most (79%) of the Cryptosporidium infections that could be confirmed by PCR were caused by C. hominis rather than by zoonotic species/genotypes; however, infections with C. parvum, C. felis, C. meleagridis, and C. canis were also diagnosed. Because the serologic assays used in our study were based on Triton X-114-extracted C. parvum (bovine, subtype IIa) 17-kDa antigens and on a recombinant 27-kDa antigen from the same organism, the high proportion of non-C. parvum infections in the cohort was of some concern. Sestak et al. (36) recently demonstrated that convalescent-phase sera from a C. hominis-inoculated gnotobiotic pig did not efficiently react by Western blot assay with the 17-kDa antigen from C. parvum parasites. We were, however, able to detect antibody responses in 90% of the infection episodes with Cryptosporidium spp. other than C. hominis and in 73% of the C. hominis infections when appropriate serum samples were available. As described by others, no evidence of cross-reactivity with Giardia was noted (17, 44), nor did we see convincing evidence of cross-reactivity caused by Cyclospora.
Our results provide strong evidence that infections caused by the various subtypes and species of Cryptosporidium elicit antibody responses to common antigen epitopes that can then be detected by ELISA. In fact, a comparison of the C. parvum (bovine, subtype IIa) 17-kDa antigen sequence (4, 34, 38), those of various C. hominis human isolates (38), and partial sequences from two human C. meleagridis isolates from Peru (14) suggests that some regions of the antigen are highly conserved. Similarly, the C. parvum and C. hominis 27-kDa antigen genes were recently shown to be 97% identical at the amino acid level (39). It remains to be seen whether the lower sensitivity of our assays towards C. hominis infections is indicative of more extensive variation in the antigen gene sequences within this species. We are currently mapping the epitopes of the antigens to identify the regions that are critical to the human immune response.
As with the microscopic stool assay method, the serologic antibody assay method did not capture all of the Cryptosporidium episodes. Some microscopy-positive episodes lacked appropriate serum samples for antibody assay confirmation, while others did not appear to elicit a detectable antibody response. The failure to detect an antibody response in association with 25 of the stool-confirmed episodes may have several possible explanations. First, our definition of a serologic response may have been too narrow. We observed 13 intervals where both antibody responses were above the positive cutoff and where antibody responses to one antigen increased ≥50% while responses to the other antigen remained the same or decreased. Two of these intervals included detection of oocysts by microscopy. For 3 more of the 25 stool-confirmed episodes, antibody levels were quite high (27-kDa responses of 1,707, 903, and 439; 17-kDa responses of 1,120, 460, and 422, respectively) and increases in antibody levels to one or both antigens were detected (as in Fig. 1B), but the increases did not reach the 50% threshold. Similarly, an antibody response was sometimes detected following an oocyst positive stool sample (Fig. 1A) but, because of the time constraints imposed by our definition, these were considered separate events. Second, some of the 25 oocyst detections that lacked an antibody response may not have been newly acquired infections: single-day detections of oocysts sometimes occurred during the decay phase of a previous antibody responses. It is difficult to be certain whether these detections were oocysts from a new infection episode or were intermittently shed oocysts from the previous infection episode. Third, it is possible that some of the organisms detected by acid-fast microscopy in these stool specimens may not have been viable or may have been species that were noninfectious for humans. While we were able to confirm the presence of Cryptosporidium by PCR in 55 of the microscopy-positive infection episodes, we were unable to draw definitive conclusions on the specificity of the microscopy assay because of a number of missing or improperly treated samples. Fourth, the timing of serum collection may not have been optimal for the detection of an antibody response, especially of a weak response resulting from a mild infection of short duration. We observed that the interval between oocyst detection and the collection of a serum sample was significantly longer for the 25 episodes that lacked a response than for the 48 episodes with a response (32). Finally, it is possible that, even with optimal timing, a serum antibody response would not have been detected in all of the infected individuals. Using a more sensitive Western blot technique and regularly spaced serum samples, Moss et al. were able to detect IgG antibody responses in only 12 of 18 (67%) adult human volunteers who were shown to be infected with C. parvum by stool assay. The sensitivity of our serologic assay compared to the stool assay was nearly identical (66%) to that reported by Moss et al. when serum samples were available in the appropriate timeframe (24).
Reports in the literature on the usefulness of the IgG response for serologic diagnosis of Cryptosporidium infection are inconsistent. Okhuysen et al. reported that IgA and IgM were the predominant serum antibody responses observed during primary infection of adults with C. parvum and that a serum IgG antibody response tended to develop only upon reinfection (26). However, when a more sensitive Western blot technique was used, IgG and IgM responses were both detected in primary challenge serum samples and were more frequent than IgA responses (24). Another study, conducted in southern Israel by Robin et al., found that 100% of the children had IgM responses by 6 months of age and 89% of the children had IgA responses by 1 year of age, but only 42% of the children were positive for IgG antibodies by 2 years of age (35). Although serum samples were collected at regular intervals, their analysis of IgG prevalence was complicated by high levels of maternal antibodies in the baseline cord blood samples. Based on our results, we have no reason to believe that repeated infection is a requirement for the development of an IgG response: we saw 10 primary infection IgG antibody responses in children under 12 months of age (earliest infection at 4.6 months) who were not likely to have had a previous infection, and we saw no significant difference in the age at primary infection between those children who were positive by stool assay only and those children who were positive by serologic assay (data not shown). A more likely explanation of the discrepant results is that the crude, whole-oocyst antigen ELISA used in previous studies is less sensitive than our new assays and could only detect IgG antibodies during a repeat infection when an anamnestic response was present. We and others have previously demonstrated that the crude, whole-oocyst antigen ELISA does not correlate well with the Western blot assay (24, 33, 41).
Our study had several limitations that may restrict our ability to generalize these results to study populations in other settings. First, we do not know whether children and adults are equally likely to mount a detectable antibody response to infection, nor do we know how the antibody half-life compares between adults and children. Finding a naive adult human population for comparison to the study children is likely to be problematic given that a large proportion (perhaps more than 75%) of the adult population in the United States and Canada are antibody positive (12, 16, 27, 42; J. W. Priest, unpublished data). In addition, many of the assumptions built into our definition of an antibody response are based upon antibody half-life calculations in adults (31). Attempts to calculate a half-life from the antibody responses observed in our study were confounded by uneven serum collection intervals and by the high frequency of repeat infections. Second, our selection of children for this study was not random in that we oversampled children with repeated stool-positive episodes of infection and we may, therefore, have unknowingly introduced some bias into results. Finally, while we did observe a statistically significant difference in levels of antibody to the 27-kDa antigen between symptomatic and asymptomatic infections, the relatively small proportion of cryptosporidiosis episodes associated with diarrhea made it difficult to accurately assess the impact of preexisting antibody on the development or severity of disease symptoms. With these limitations in mind, we have attempted to be conservative in our interpretation of the results.
In conclusion, ELISA analysis of longitudinal serum samples is a sensitive tool for the identification of Cryptosporidium infection in children: the assays detect antibody responses caused by at least four of the major human-infective species of parasite and capture more infections than stool-based assays alone. The ELISAs also appear to be specific, since no cross-reactivities were noted with Giardia or Cyclospora infections. We would propose that, with the collection of evenly spaced serum samples, a serologic-based antibody assay would be a simple and reliable method for following the Cryptosporidium infection status of a study population. New public health interventions that target chlorine-resistant pathogens such as Cryptosporidium are clearly needed in the poorer, underdeveloped regions of the world, and the serologic assays described here may provide a useful tool for monitoring the success of novel interventions in trial studies.
Acknowledgments
We thank Carmen Taquiri and Manuela Verastegui for laboratory diagnostics and specimen handling, Marco Varela for data management, Paula Maguiña, Ana Rosa Contreras, and Paola Maurtua for administrative support, and J. B. Phu and D. Sara for their technical assistance.
The pediatric cohort study was supported by an ICTDR grant from the National Institutes of Allergy and Infectious Diseases awarded to the Johns Hopkins Bloomberg School of Public Health (UA01-AI035894), by a grant awarded to Universidad Peruana Cayetano Heredia (1PO1-AI51976), and by the charitable RG-ER fund, which is concerned with childhood health in developing countries.
Use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Services.
REFERENCES
- 1.Alves, M., L. Xiao, I. Sulaiman, A. A. Lal, O. Matos, and F. Antunes. 2003. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J. Clin. Microbiol. 41:2744-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrowood, M. J. 1997. Diagnosis, p. 65-92. In R. Fayer (ed.), Cryptosporidium and cryptosporidiosis. CRC Press, Boca Raton, Fla.
- 3.Bern, C., Y. Ortega, W. Checkley, J. M. Roberts, A. G. Lescano, L. Cabrera, M. Verastegui, R. E. Black, C. Sterling, and R. H. Gilman. 2002. Epidemiologic differences between cyclosporiasis and cryptosporidiosis in Peruvian children. Emerg. Infect. Dis. 8:581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cevallos, A. M., X. Zhang, M. K. Waldor, S. Jaison, X. Zhou, S. Tzipori, M. R. Neutra, and H. D. Ward. 2000. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect. Immun. 68:4108-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chappell, C. L., P. C. Okhuysen, C. R. Sterling, and H. L. DuPont. 1996. Cryptosporidium parvum: intensity of infection and oocyst excretion patterns in healthy volunteers. J. Infect. Dis. 173:232-236. [DOI] [PubMed] [Google Scholar]
- 6.Checkley, W., L. D. Epstein, R. H. Gilman, R. E. Black, L. Cabrera, and C. R. Sterling. 1998. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am. J. Epidemiol. 148:497-506. [DOI] [PubMed] [Google Scholar]
- 7.Checkley, W., R. H. Gilman, L. D. Epstein, M. Suarez, J. F. Diaz, L. Cabrera, R. E. Black, and C. R. Sterling. 1997. Asymptomatic and symptomatic cryptosporidiosis: their acute effect on weight gain in Peruvian children. Am. J. Epidemiol. 145:156-163. [DOI] [PubMed] [Google Scholar]
- 8.Checkley, W., R. H. Gilman, R. E. Black, L. D. Epstein, L. Cabrera, C. R. Sterling, and L. H. Moulton. 2004. Effect of water and sanitation on childhood health in a poor Peruvian peri-urban community. Lancet 363:112-118. [DOI] [PubMed] [Google Scholar]
- 9.Dupont, H. L., C. L. Chappell, C. R. Sterling, P. C. Okhuysen, J. B. Rose, and W. Jakubowski. 1995. The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 332:855-859. [DOI] [PubMed] [Google Scholar]
- 10.Eberhard, M. L., N. J. Pieniazek, and M. J. Arrowood. 1997. Laboratory diagnosis of Cyclospora infections. Arch. Pathol. Lab. Med. 121:792-797. [PubMed] [Google Scholar]
- 11.Fayer, R., U. Morgan, and S. J. Upton. 2000. Epidemiology of Cryptosporidium: transmission, detection, and identification. Int. J. Parasitol. 30:1305-1322. [DOI] [PubMed] [Google Scholar]
- 12.Frost, F. J., T. Muller, G. F. Craun, R. L. Calderon, and P. A. Roefer. 2001. Paired city Cryptosporidium serosurvey in the southwest USA Epidemiol. Infect. 126:301-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilman, R. H., G. S. Marquis, G. Ventura, M. Campos, W. Spira, and F. Diaz. 1993. Water cost and availability: key determinants of family hygiene in a Peruvian shantytown. Am. J. Public Health 83:1554-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glaberman, S., I. M. Sulaiman, C. Bern, J. Limor, M. M. Peng, U. Morgan, R. Gilman, A. A. Lal, and L. Xiao. 2001. A multilocus genotypic analysis of Cryptosporidium meleagridis. J. Eukaryot. Microbiol. 2001(Suppl.):19S-22S. [DOI] [PubMed] [Google Scholar]
- 15.Goodgame, R. W., R. M. Genta, A. C. White, and C. L. Chappell. 1993. Intensity of infection in AIDS-associated cryptosporidiosis. J. Infect. Dis. 167:704-709. [DOI] [PubMed] [Google Scholar]
- 16.Isaac-Renton, J., J. Blatherwick, W. R. Bowie, M. Fyfe, M. Khan, A. Li, A. King, M. McLean, L. Medd, W. Moorehead, C. S. Ong, and W. Robertson. 1999. Epidemic and endemic seroprevalence of antibodies to Cryptosporidium and Giardia in residents of three communities with different drinking water supplies. Am. J. Trop. Med. Hyg. 60:578-583. [DOI] [PubMed] [Google Scholar]
- 17.Isaac-Renton, J. L., C. S. L. Ong, W. R. Bowie, P. J. Lammie, and J. W. Priest. 2003. Cryptosporidium serology in human populations. AWWA Research Foundation, Denver, Colo.
- 18.Janoff, E. N., P. S. Mead, J. R. Mead, P. Echeverria, L. Bodhidatta, M. Bhaibulaya, C. R. Sterling, and D. H. Taylor. 1990. Endemic Cryptosporidium and Giardia lamblia infections in a Thai orphanage. Am. J. Trop. Med. Hyg. 43:248-256. [DOI] [PubMed] [Google Scholar]
- 19.Kirkpatrick, B. D., M. M. Daniels, S. S. Jean, J. W. Pape, C. Karp, B. Littenberg, D. W. Fitzgerald, H. M. Lederman, J. P. Nataro, and C. L. Sears. 2002. Cryptosporidiosis stimulates an inflammatory intestinal response in malnourished Haitian children. J. Infect. Dis. 186:94-101. [DOI] [PubMed] [Google Scholar]
- 20.Korich, D. G., J. R. Mead, M. S. Madore, N. A. Sinclair, and C. R. Sterling. 1990. Effects of ozone, chlorine dioxide, chlorine, and monochoramin on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 56:1423-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald, A. C., W. R. Mac Kenzie, D. G. Addiss, M. S. Gradus, G. Linke, E. Zembrowski, M. R. Hurd, M. J. Arrowood, P. J. Lammie, and J. W. Priest. 2001. Cryptosporidium parvum-specific antibody responses among children residing in Milwaukee during the 1993 waterborne outbreak. J. Infect. Dis. 183:1373-1379. [DOI] [PubMed] [Google Scholar]
- 22.Molbak, K., M. Anderson, P. Aaby, N. Hojlyng, M. Jakobsen, M. Sodemann, and A. P. J. da Silva. 1997. Cryptosporidium infection in infancy as a cause of malnutrition: a community study from Guinea-Bissau, West Africa. Am. J. Clin. Nutr. 65:149-152. [DOI] [PubMed] [Google Scholar]
- 23.Morgan-Ryan, U. M., A. Fall, L. A. Ward, N. Hijawi, I. Sulaiman, R. Fayer, R. C. A. Thompson, M. Olson, A. Lal, and L. Xiao. 2001. Cryptosporidium hominis n. sp. (Apicomplexa: Cryptosporidiidae) from Homo sapiens. J. Eukaryot. Microbiol. 49:433-440. [DOI] [PubMed] [Google Scholar]
- 24.Moss, D. M., C. L. Chappell, P. C. Okhuysen, H. L. DuPont, M. J. Arrowood, A. W. Hightower, and P. J. Lammie. 1998. The antibody response to 27-, 17-, and 15-kDa Cryptosporidium antigens following experimental infection in humans. J. Infect. Dis. 178:827-833. [DOI] [PubMed] [Google Scholar]
- 25.Newman, R. D., C. L. Sears, S. R. Moore, J. P. Narato, T. Wuhib, D. A. Agnew, R. L. Guerrant, and A. A. M. Lima. 1999. Longitudinal study of Cryptosporidium infection in children in northeastern Brazil. J. Infect. Dis. 180:167-175. [DOI] [PubMed] [Google Scholar]
- 26.Okhuysen, P. C., C. L. Chappell, C. R. Sterling, W. Jakubowski, and H. L. DuPont. 1998. Susceptibility and serologic response of healthy adults to reinfection with Cryptosporidium parvum. Infect. Immun. 66:441-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong, C. S., A. S. Li, J. W. Priest, R. Copes, M. Khan, M. W. Fyfe, S. A. Marion, J. M. Roberts, P. J. Lammie, and J. L. Isaac-Renton. 2005. Enzyme immunoassay of Cryptosporidium-specific immunoglobulin G antibodies to assess longitudinal infection trends in six communities in British Columbia, Canada. Am. J. Trop. Med. Hyg. 73:288-295. [PubMed] [Google Scholar]
- 28.Peng, M. M., L. Xiao, A. R. Freeman, M. J. Arrowood, A. A. Escalante, A. C. Weltman, C. S. L. Ong, W. R. Mac Kenzie, A. A. Lal, and C. B. Beard. 1997. Genetic polymorphism among Cryptosporidium parvum isolates: evidence of two distinct human transmission cycles. Emerg. Infect. Dis. 3:567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng, M. M., O. Matos, W. Gatei, P. Das, M. Stantic-Pavlinic, C. Bern, I. M. Sulaiman, S. Glaberman, A. A. Lal, and L. Xiao. 2001. A comparison of Cryptosporidium subgenotypes from several geographic regions. J. Eukaryot. Microbiol. 2001(Suppl.):28S-31S. [DOI] [PubMed] [Google Scholar]
- 30.Pieniazek, N. J., F. J. Bornay-Llinares, S. B. Slemenda, A. J. da Silva, I. N. S. Moura, M. J. Arrowood, O. Ditrich, and D. G. Addiss. 1999. New Cryptosporidium genotypes in HIV-infected persons. Emerg. Infect. Dis. 5:444-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priest, J. W., A. Li, M. Khan, M. J. Arrowood, P. J. Lammie, C. S. Ong, J. M. Roberts, and J. Isaac-Renton. 2001. Enzyme immunoassay detection of antigen-specific immunoglobulin G antibodies in longitudinal serum samples from patients with cryptosporidiosis. Clin. Diag. Lab. Immunol. 8:415-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Priest, J. W., C. Bern, J. M. Roberts, J. P. Kwon, A. G. Lescano, W. Checkley, L. Cabrera, D. M. Moss, M. J. Arrowood, C. R. Sterling, R. H. Gilman, and P. J. Lammie. 2005. Changes in serum immunoglobulin G levels as a marker for Cryptosporidium infection in Peruvian children. J. Clin. Microbiol. 43:5298-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Priest, J. W., J. P. Kwon, D. M. Moss, J. M. Roberts, M. J. Arrowood, M. S. Dworkin, D. D. Juranek, and P. J. Lammie. 1999. Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigens. J. Clin. Microbiol. 37:1385-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Priest, J. W., J. P. Kwon, M. J. Arrowood, and P. J. Lammie. 2000. Cloning of the immunodominant 17-kDa antigen from Cryptosporidium parvum. Mol. Biochem. Parasitol. 106:261-271. [DOI] [PubMed] [Google Scholar]
- 35.Robin, G., D. Fraser, N. Orr, T. Sela, R. Slepon, R. Ambar, R. Dagan, S. Le Blancq, R. J. Deckelbaum, and D. Cohen. 2001. Cryptosporidium infection in Bedouin infants assessed by prospective evaluation of anticryptosporidial antibodies and stool examination. Am. J. Epidemiol. 153:194-201. [DOI] [PubMed] [Google Scholar]
- 36.Sestak, K., L. A. Ward, A. Sheoran, X. Feng, D. E. Akiyoshi, H. D. Ward, and S. Tzipori. 2002. Variability among Cryptosporidium parvum genotype 1 and 2 immunodominant surface glycoproteins. Parasite Immunol. 24:213-219. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg, E. B., C. E. Mendoza, R. Glass, B. Arana, M. B. Lopez, M. Mejia, B. D. Gold, J. W. Priest, W. Bibb, S. S. Monroe, C. Bern, B. P. Bell, R. M. Hoekstra, R. Klein, E. D. Mintz, and S. Luby. 2004. Prevalence of infection with waterborne pathogens: a seroepidemiologic study in children 6-36 months old in San Juan Sacatepequez, Guatemala. Am. J. Trop. Med. Hyg. 70:83-88. [PubMed] [Google Scholar]
- 38.Strong, W. B., J. Gut, and R. G. Nelson. 2000. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect. Immun. 68:4117-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sturbaum, G. D., B. H. Jost, and C. R. Sterling. 2003. Nucleotide changes within three Cryptosporidium parvum surface protein encoding genes differentiate genotype I from genotype II isolates. Mol. Biochem. Parasitol. 128:87-90. [DOI] [PubMed] [Google Scholar]
- 40.Tumwine, J. K., A. Kekitiinwa, N. Nabukeera, D. E. Akiyoshi, S. M. Rich, G. Widmer, X. Feng, and S. Tzipori. 2003. Cryptosporidium parvum in children with diarrhea in Mulago Hospital, Kampala, Uganda. Am. J. Trop. Med. Hyg. 68:710-715. [PubMed] [Google Scholar]
- 41.Ungar, B. L., and T. E. Nash. 1986. Quantification of specific antibody response to Cryptosporidium antigens by laser densitometry. Infect. Immun. 53:124-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ungar, B. L. P., M. Mulligan, T. B. Nutman. 1989. Serologic evidence of Cryptosporidium infection in US volunteers before and during Peace Corps service in Africa. Arch. Intern. Med. 149:894-897. [PubMed] [Google Scholar]
- 43.Ungar, B. L. P., R. H. Gilman, C. F. Lanata, and I. Perez-Schael. 1988. Seroepidemiology of Cryptosporidium infection in two Latin American populations. J. Infect. Dis. 157:551-556. [DOI] [PubMed] [Google Scholar]
- 44.Ungar, B. L. P., R. Soave, R. Fayer, and T. E. Nash. 1986. Enzyme immunoassay detection of immunoglobulin M and G antibodies to Cryptosporidium in immunocompetent and immunocompromised persons. J. Infect. Dis. 153:570-578. [DOI] [PubMed] [Google Scholar]
- 45.Weber, R., R. T. Bryan, H. S. Bishop, S. P. Wahlquist, J. J. Sullivan, and D. D. Juranek. 1991. Threshold of detection of Cryptosporidium oocysts in human stool specimens: evidence for low sensitivity of current diagnostic methods. J. Clin. Microbiol. 29:1323-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao, L., C. Bern, J. Limor, I. Sulaiman, J. Roberts, W. Checkley, L. Cabrera, R. H. Gilman, and A. A. Lal. 2001. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J. Infect. Dis. 183:492-497. [DOI] [PubMed] [Google Scholar]
- 47.Zu, S.-X., J.-F. Li, L. J. Barrett, R. Fayer, S.-Y. Shu, J. F. McAuliffe, J. K. Roche, and R. L. Guerrant. 1994. Seroepidemiologic study of Cryptosporidium infection in children from rural communities of Anhui, China and Fortaleza, Brazil. Am. J. Trop. Med. Hyg. 51:1-10. [DOI] [PubMed] [Google Scholar]