Abstract
Hypoxia profoundly influences tumor development and response to therapy. While progress has been made in identifying individual gene products whose synthesis is altered under hypoxia, little is known about the mechanism by which hypoxia induces a global downregulation of protein synthesis. A critical step in the regulation of protein synthesis in response to stress is the phosphorylation of translation initiation factor eIF2α on Ser51, which leads to inhibition of new protein synthesis. Here we report that exposure of human diploid fibroblasts and transformed cells to hypoxia led to phosphorylation of eIF2α, a modification that was readily reversed upon reoxygenation. Expression of a transdominant, nonphosphorylatable mutant allele of eIF2α attenuated the repression of protein synthesis under hypoxia. The endoplasmic reticulum (ER)-resident eIF2α kinase PERK was hyperphosphorylated upon hypoxic stress, and overexpression of wild-type PERK increased the levels of hypoxia-induced phosphorylation of eIF2α. Cells stably expressing a dominant-negative PERK allele and mouse embryonic fibroblasts with a homozygous deletion of PERK exhibited attenuated phosphorylation of eIF2α and reduced inhibition of protein synthesis in response to hypoxia. PERK−/− mouse embryo fibroblasts failed to phosphorylate eIF2α and exhibited lower survival after prolonged exposure to hypoxia than did wild-type fibroblasts. These results indicate that adaptation of cells to hypoxic stress requires activation of PERK and phosphorylation of eIF2α and suggest that the mechanism of hypoxia-induced translational attenuation may be linked to ER stress and the unfolded-protein response.
Tumor hypoxia is a well-characterized feature of the solid-tumor microenvironment. The development of hypoxia has profound consequences on tumor growth characteristics and on tumor response to radiotherapy and chemotherapy. Hypoxic tumors are more metastatic, are more resistant to radiotherapy and chemotherapy, and have a poorer prognosis than better-oxygenated ones, irrespective of therapy (28, 29, 72). Delineating the mechanisms by which hypoxia affects tumor physiology at the cellular and molecular levels is crucial for a better understanding of the process of tumor development and metastasis and for the design of better antitumor modalities.
At the cellular level, exposure of cells to hypoxia has an immediate and reversible effect on proliferation. In Ehrlich ascites and HeLa cells, replicons stop firing within minutes of exposure to hypoxia, resulting in a G1/S-phase arrest. Upon reoxygenation, replicons begin extending, again within minutes (50-52). This hypoxia-induced G1/S arrest is independent of functional p53 tumor suppressor protein, since cells with mutant p53 and p53 knockout cells also arrest at the G1/S interface under hypoxic conditions (22). These studies point toward a unique mechanism of checkpoint control that differs from those induced by glucose deprivation or ionizing radiation, which require considerably more time for reinitiation of the cell cycle and, in the case of ionizing radiation, is p53 dependent (23).
Another well-characterized consequence of hypoxic stress is a pronounced repression in the rate of oxygen consumption and of energy turnover (36, 66). It has been estimated that, under severe hypoxia, the ATP demand for protein synthesis drops to about 7% of that of normoxic cells (27). This drop correlates with a substantial and rapid drop in the rate of protein synthesis, which occurs initially at the levels of translation and later extends to the level of transcription as well (47). Since protein synthesis is the second-costliest cellular process in terms of ATP demand, besides the Na+ pump (27), a decrease in the rate of translation may be crucial for cellular adaptation to the new environment of low oxygen and of energy deficiency.
Regulation of eukaryotic protein synthesis is controlled primarily at the level of translation initiation. This is a highly coordinated process, at the center of which lie at least nine eucaryotic initiation factors (eIFs) (15, 40, 48, 60). Translation of an mRNA begins when both the mRNA and the initiator methionyl (Met)-tRNA are bound to the 80S ribosome (40). This is achieved when initiation factor eIF2 binds to GTP and then to the initiator (Met)-tRNA, forming a ternary complex. The nucleotide hydrolysis and exchange by eIF-2 are critical steps in the formation of this ternary complex. The exchange is mediated by eIF2B, and this reaction is inhibited when one of the three subunits of eIF2, eIF2α, becomes phosphorylated at Ser51. Because eIF-2B exists in significantly smaller amounts than eIF2α (about 20 to 30% of eIF2α), a less than complete phosphorylation of eIF2α molecules is sufficient to inhibit the exchange activity of eIF-2B. The critical role of eIF2α phosphorylation for the maintenance of cellular homeostasis is further underscored by the finding that expression of a nonphosphorylatable point mutant form of eIF2 in NIH 3T3 cells causes malignant transformation, probably because of increased expression of oncogenes and the inability of the cell to regulate the rate of protein synthesis (11, 16). Phosphorylation of eIF2α and inhibition of protein synthesis have also been demonstrated to occur in response to ischemia-reperfusion injury in neuronal tissue (8, 21, 31, 45). However, in these cases, eIF2α phosphorylation was observed only upon reoxygenation. While seemingly similar to hypoxia, brain ischemia has distinct properties because of the more acute nature of the stress, the substantial vulnerability of the neurons to the reperfusion, and the fact that ischemia-reperfusion has a strong component of free-radical damage, which is not present in all modes of chronic hypoxia in tumor cells.
Here we report that exposure of untransformed human diploid fibroblasts and transformed human and rodent cells to anoxia and moderate hypoxia induces a rapid upregulation of the phosphorylation levels of eIF2α that is readily reversible upon reoxygenation. This hyperphosphorylation correlates with a decrease in the rates of protein synthesis and is independent of the function of hypoxia-inducible factor 1α (HIF-1α). The endoplasmic reticulum (ER)-resident kinase PERK, which was previously shown to phosphorylate eIF2α in response to ER stress, becomes hyperphosphorylated under hypoxia. Phosphorylation of eIF2α and the inhibition of protein synthesis are significantly attenuated in PERK knockout mouse embryonic fibroblasts (MEFs) or in cells expressing a dominant-negative PERK construct, and PERK−/− cells exhibit lower levels of clonogenic survival than PERK+/+ cells after prolonged exposure to hypoxia. Taken together, these results indicate that eIF2α phosphorylation plays a critical role in the regulation of protein synthesis rates in the hypoxic cell and suggest that an ER-generated signal may be important for initiating this adaptive cellular response to hypoxia.
MATERIALS AND METHODS
Cell lines.
A549, AG1522, and HeLa cells were obtained from the American Type Culture Collection (ATCC, Manassas, Va.). The NIH 3T3 cell line was obtained from the tissue culture core facility of the Wake Forest University School of Medicine. A549 cells were maintained in Hanks F-12 medium, AG1522 cells were maintained in alpha-modified Eagle’s medium, and HeLa and 3T3 cells were maintained in Dulbecco modified Eagle medium. All media were supplemented with antibiotics (penicillin, streptomycin, and amphotericin) and 10% fetal calf serum. PKR+/+ and PKR−/− cells (1), PERK+/+ and PERK−/− MEFs (24), and wild-type and HIF-1α−/− MEFs (56) have all been previously described. The PERK MEFs used in the experiments described in this report were transformed with the simian virus 40 (SV40) large-T antigen.
Hypoxia treatments.
All experiments were performed with exponentially growing cells. For all experiments except labeling with [35S]methionine, cells were plated in 100-mm-diameter culture dishes at a density of ∼2 × 106 cells/plate. After approximately 18 h, the culture dishes were placed in a hypoxic culture chamber (either a Bactron 1 Anaerobe Chamber [Cornelius] or a MACS VA500 microaerophilic workstation [Don Whitley Scientific, Shipley, United Kingdom]). The oxygen concentration in the chamber was maintained as desired and monitored with a polarographic, membrane-covered oxygen sensor (Animas Corporation, Malvern, Pa.). The sensor has a Pt cathode, an Ag anode, and a ceramic body and is connected to an amplifier and a chart recorder. The design of this chamber allows easy entry and exit and sample manipulations (e.g., radiolabeling) without compromising the hypoxic environment. For measurements at anoxia, cells were plated on glass dishes to minimize leakage of oxygen from the plastic into the culture medium.
[35S]methionine incorporation assays.
For in vivo labeling experiments, cells were plated in 60-mm-diameter plates at a density of 5 × 105/plate. Ten hours before labeling began, the culture medium of the cells was changed to one without methionine to ensure optimal incorporation of [35S]methionine. Thirty minutes before the end of treatments (drugs or hypoxia), cells were labeled with [35S]methionine (50 μCi ml−1). Cells were washed three times with phosphate-buffered saline (PBS), scraped off the dishes in PBS, and centrifuged at 5,000 × g. The cell pellet was resuspended in PBS containing 2 mM EDTA and lysed by three rounds of freeze-thawing. Trichloroacetic acid (TCA) precipitation of macromolecules and scintillation counting were performed as follows: Briefly, 10 μl of sample was added to a mixture of 250 μl of water, 50 μl of bovine serum albumin (1 mg/ml), and 100 μl of TCA (50%, wt/vol). The samples were kept on ice for <20 min and then filtered trough glass filters (GC filters; VWR Scientific) under vacuum. The filter was washed three times with ice-cold TCA (5%, wt/vol) and with ethanol (95%, vol/vol) and then immersed into 5 ml of scintillation liquid (Ecolume; ICN). Disintegrations per minute were calculated as the percentage of [35S]methionine incorporation relative to that incorporated by untreated cells. Results were normalized as the percent increase or decrease of [35S]methionine incorporation compared to that of untreated control cells.
Immunoblotting.
Following treatments, cells were washed three times with ice-cold PBS and resuspended in PBS with 1% NP-40 containing 2 μg of leupeptin per ml, 2 μg of pepstatin per ml, 2 μg of aprotinin per ml, 2 μg of antipain per ml, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaVO5, 1 mM NaF, 1 μM microcystin L, and 2 mM EDTA. Cells were lysed on ice for 15 min and centrifuged for 10 min to separate cell debris. Protein concentrations of each sample were determined by the modified Bradford assay as recommended by the manufacturer (Bio-Rad, Hercules, Calif.). A 40- to 50-μg sample of whole-cell protein extract was mixed with an equal volume of 2× Laemmli sample buffer (62 mM Tris [pH 6.8], 10% glycerol, 2% sodium dodecyl sulfate, 5% β-mercaptoethanol, 0.003% bromophenol blue) and heated at 95°C for 5 min. Proteins were resolved on a sodium dodecyl sulfate-10 to 12% polyacrylamide gel and transferred onto Hybond ECL nitrocellulose membrane (Amersham, Arlington Heights, Ill.) with a wet transfer system (Bio-Rad). Membranes were stained with 0.15% Ponceau red (Sigma) to ensure equal loading and transfer and then blocked with 5% (wt/vol) dried nonfat milk in TBS buffer (20 mM Tris base, 137 mM NaCl, pH 7.6). Immunoblotting for eIF2α was performed with either a rabbit polyclonal antibody raised against the phosphorylated form of eIF2α (anti-Pser51) (Cell Signaling Technologies, Beverly, Mass., or Research Genetics, Inchinnan, Scotland) or a rabbit polyclonal antibody recognizing both phosphorylated and unphosphorylated eIF2α (Cell Signaling Technologies) in Tris-buffered saline with 0.1% Tween 20. Immunoblotting for PERK was performed with a goat polyclonal antibody, and immunoblotting for PKR was performed with a rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.). Following incubation with the primary antibody, the membranes were washed in Tris-buffered saline with 0.1% Tween 20 and incubated with the secondary antibody and immunoreactive bands were visualized with an enhanced-chemiluminescence (ECL-Plus) reagent kit in accordance with the manufacturer's (Amersham, Arlington Heights, Ill.) recommendations. Films were exposed to the membranes for various periods of time and scanned with a personal scanner (Microtec, San Jose, Calif.). Optical densities of the immunoreactive bands were measured with the ScionImage Analysis software (commercial version of the NIH Image analysis software).
Plasmids and cell transfections and infections.
The wt-PERK and K618A-PERK plasmids have been previously described (26). The nonphosphorylatable eIF2α plasmid (S51A) has also been described previously (16). A549 cells were transfected with 10 μg of plasmid pCMV-neo or pCMV-eIF2α-S51A. Forty-eight hours later, cells were trypsinized, counted, and plated in 100-mm-diameter dishes at three different cell densities (10,000, 1,000, and 100 cells/plate). Twenty-four hours later, neomycin (G418; Geneticin; Gibco BRL), at 600 μg/ml, was added to the medium and the cells were incubated in the presence of the drug (replaced every 4 days) for a total of 2 weeks. Individual clones (<200 cells/clone) were isolated with Pyrex cloning rings, trypsinized, and added to 24-well plates. The isolated clones were expanded, and aliquots were frozen at −80°C. All subsequent culture of the clones was performed in the presence of neomycin (100 μg/ml) to minimize the expansion of revertant cells. Viral infection of 3T3 cells with retrovirus expressing the PERKΔC construct or a control retrovirus carrying only the puromycin resistance gene was performed as described elsewhere (3), with 10 μg of Polybrene per ml. Cells were further selected in 1.5 μg of puromycin per ml for 7 days.
Clonogenic-survival assays.
Cells were plated into 100-mm-diameter dishes until they reach approximately 80% confluency. Cells were exposed to hypoxia (0.02% O2) for 24 h or left untreated (Normoxia). Following hypoxia, treated cells were reoxygenated and allowed to recover for 2 h. Cells were then trypsinized, counted with a hemocytometer, and plated into 60-mm-diameter dishes at densities of 300 cells/plate. Platings were performed in triplicate, and each experiment was performed three times. After incubation for 12 days, cells were fixed with methanol-acetic acid and stained with crystal violet. Colonies containing more than 50 cells were counted. The plating efficiencies were determined for each treatment and normalized to the control. The average normalized surviving fraction from three independent experiments and the standard deviation were reported.
RESULTS
Exposure of cells to hypoxia reduces the rate of protein synthesis and induces phosphorylation of eIF2α.
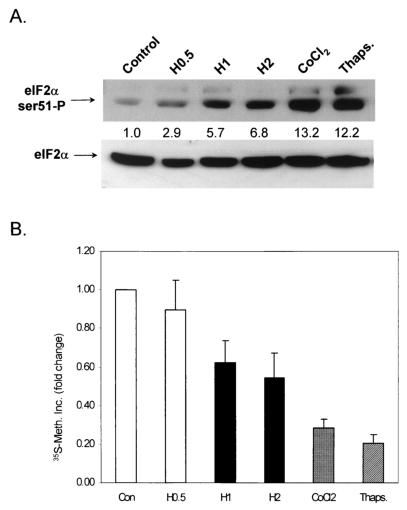
We first sought to determine the effect of exposure to hypoxia on protein synthesis in lung carcinoma A549 cells. Exponentially growing A549 cells were exposed to stringent hypoxia (0.02% O2) for 30 min, 1 h, and 2 h or treated with the heavy metal CoCl2 (a hypoxia-mimicking agent). Cells treated with thapsigargin served as positive controls, since this agent has been shown to induce a robust increase in the phosphorylation of eIF2α (24). Inhibition of protein synthesis correlated with the levels of eIF2α phosphorylation shown in Fig. 1. The top part in Fig. 1A depicts phosphorylation of eIF2α by immunoblotting with an antibody that reacts with eIF2α only when it is phosphorylated on Ser51 (anti-ser51-P antibody) (see also Fig. 3A for a demonstration of the specificity of this antibody). The bottom part depicts an immunoblot of the same membrane that was stripped and reblotted with an anti-eIF2α antibody that recognizes both phosphorylated and unphosphorylated (total) eIF2α. The levels of total eIF2α remain unchanged by all treatments, indicating that hypoxia alters the phosphorylation status and not the levels of this protein. As shown in Fig. 1B, exposure of cells to increasing durations of hypoxia resulted in progressively decreased protein synthesis, as measured by [35S]methionine incorporation. The 30-min exposure did not cause a significant change in the rate of [35S]methionine incorporation, while the 1- and 2-h exposures reduced the rate of protein synthesis by about 40 to 50%. CoCl2 reduced protein synthesis even further, while a 2-h treatment with thapsigargin induced the largest inhibition (approximately 80%). The reduction of the rate of protein synthesis in response to hypoxic stress was not due to a reduction in the rate of mRNA synthesis, as hypoxia treatments of up to 4 h did not decrease the incorporation of [3H]uridine into poly(A)+ RNA in hypoxic cells (results not shown).
FIG. 1.
(A) Hypoxia, CoCl2, and thapsigargin (Thaps.) induce phosphorylation of eIF2α on Ser51. Cells were exposed to 0.02% O2 for the indicated times or treated with 100 μM CoCl2 for 2 h or 1 μM thapsigargin for 2 h. Shown at the top is an immunoblot with a rabbit polyclonal antibody raised against eIF2α phosphorylated on Ser51. The membrane was stripped and reprobed with a rabbit polyclonal antibody that recognizes both phosphorylated and unphosphorylated (total) eIF2α (bottom). The values between the two parts represent the fold induction of phosphorylated eIF2α levels compared to control levels after normalization to the total eIF2α levels, as determined by densitometric analysis of the film and use of the ScionImage densitometric analysis program (a commercial version of the NIH Image shareware program). (B) Hypoxia, CoCl2, and thapsigargin reduce the rates of protein synthesis. Cells were treated as described above and labeled with [35S]methionine (50 μCi/ml) during the last 20 min of treatment. TCA-precipitable counts were measured as described in Materials and Methods. Concentrations of O2, CoCl2, and thapsigargin were the same ones used in the experiment shown in Fig. 1A. Experiments were performed in triplicate, and averages are reported along with errors. Con, control; 35S-Meth. Inc., [35S]methionine incorporation.
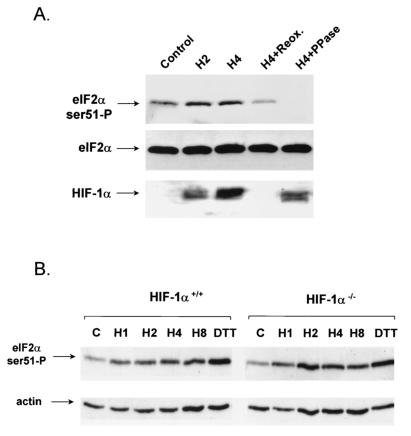
FIG. 3.
Hypoxia-induced phosphorylation of eIF2α correlates with, but is independent of, HIF-1α accumulation. (A) A549 cells were treated with 2 or 4 h of hypoxia or with 4 h of hypoxia, followed by 30 min of reoxygenation (Reox.). Fifty micrograms of the protein extract from the 4-h hypoxia treatment was pretreated with 10 U of calf intestinal phosphatase (PPase) prior to gel electrophoresis. Membranes were immunoblotted with the anti-phospho-specific eIF2α antibody (top), anti-eIF2α antibody (middle), or a mouse monoclonal antibody raised against human HIF-1α (bottom). (B) HIF-1α+/+ and HIF-1α−/− cells were exposed to hypoxia for 1, 2, 4, and 8 h. Cell lysates were probed by Western analysis for eIF2α phosphorylation as described in the legend to Fig. 1. DTT, dithiothreitol.
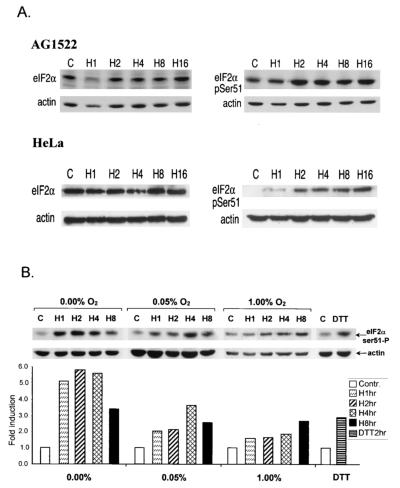
To examine whether the hypoxia-induced phosphorylation of eIF2α is a general property of cells (untransformed and transformed), we exposed normal human fibroblasts (AG1522) and HeLa cervical carcinoma cells to different durations of hypoxia. Hypoxia induced an increase in the phosphorylation levels of eIF2α in both untransformed (AG1522) and transformed (HeLa) cells (Fig. 2A). The magnitude of the increase in phosphorylation in AG1522 fibroblasts was smaller than that observed in A549 or HeLa cells. This difference may be attributed to the higher basal phosphorylation levels of eIF2α in AG1522 cells and is consistent with the slower rate of growth of these cells.
FIG. 2.
Kinetics and oxygen dependency of eIF2α phosphorylation in normal human fibroblasts and HeLa cells. (A) AG1522 and HeLa cells were exposed to hypoxia for the times indicated. Immunoblotting was performed with the anti-total eIF2α antibody (left) or the anti-Ser51-specific antibody (right). An anti-actin monoclonal antibody was used as a control (C or Contr.). (B) HeLa cells were exposed to different oxygen concentrations for the times indicated. The levels of phosphorylation were analyzed by densitometry and are presented as fold induction compared to that of untreated control cells (bottom graph). Cells were also treated with 1 mM dithiothreitol (DTT) as a positive control.
The experiments described above were performed under relatively stringent hypoxic conditions (0.02% O2). Although extremely hypoxic areas can be found in human tumors and mouse xenografts, the oxygen concentration in tumors is a continuum of 5% to <0.01% (68, 71). This oxygen concentration gradient also exists in cells grown as spheroids an in vitro model of tumor growth, and in this model, the oxygen gradient closely correlates with a decrease in the rate of cell proliferation (66, 67). Therefore, we sought to determine the oxygen dependency of this phosphorylation at three different O2 concentrations: at 1% (which we will refer to as mild hypoxia), 0.05% (moderate hypoxia), and ≤0.02% (denoted as 0.0%, extreme hypoxia). As shown in Fig. 2B, induction of eIF2α phosphorylation in HeLa cells was found to be dependent on O2 concentration, with the highest levels under extreme hypoxia-anoxia and the lowest induction under mild hypoxia. In this cell line, under conditions of extreme hypoxia, the induced phosphorylation of eIF2α was transient, peaking at ∼2 h of exposure and declining somewhat at 4 and 8 h of exposure. Under mild and moderate hypoxia, eIF2α phosphorylation remained upregulated for the duration of the treatments.
Hypoxia-induced phosphorylation of eIF2α correlates with, but is independent of, HIF-1α accumulation.
HIF-1α is a hypoxia-inducible protein component of HIF. HIF-1α protein has a very short half-life but accumulates rapidly under hypoxic conditions via the inactivation of a recently identified family of oxygen-dependent prolyl-hydroxylases. Under aerobic conditions, these enzymes hydroxylate proline residues on HIF-1α, resulting in recognition and ubiquitination via the VHL ubiquitin ligase and subsequent degradation in the 26S proteasome. Under hypoxic conditions, HIF is unhydroxylated and protected from degradation via VHL (7, 17, 32, 33, 75). HIF-1α activates the transcription of a variety of hypoxia-inducible genes, including vascular endothelial growth factor, metabolic enzymes like Glut-1, etc. (reviewed in reference 58). We therefore decided to utilize the accumulation and degradation of HIF-1α as a marker of hypoxia and reoxygenation in cells. Exposure of A549 cells to hypoxia (0.01% O2) for 2 and 4 h caused a time-dependent increase in eIF2α phosphorylation and HIF-1α accumulation (Fig. 3A). Reoxygenation of cells for 30 min after a 4-h hypoxia treatment caused eIF2α dephosphorylation and degradation of HIF-1α. Pretreatment of the cellular extract from the 4-h hypoxia treatment with calf intestinal alkaline phosphatase resulted in loss of the immunoreactive band corresponding to phosphorylated, but not total, eIF2α, demonstrating the specificity of the phospho-specific antibody for the phosphorylated form.
The correlation between HIF-1α accumulation and eIF2α phosphorylation, in conjunction with the demonstrated effects of HIF-1α on cellular metabolism, raised the possibility that induction of eIF2α phosphorylation is dependent on HIF-1α activity. To examine this possibility, we investigated the induction of eIF2α phosphorylation in MEFs from HIF-1α wild-type and knockout animals. HIF-1α−/− MEFs exhibit slower growth rates and a decreased glycolytic capacity under hypoxia compared to HIF-1α+/+ MEFs (56). When the induction of eIF2α phosphorylation was examined, no significant differences in the rate or magnitude of induction of phosphorylation was observed (Fig. 3B). These results demonstrate that hypoxia-induced eIF2α phosphorylation is readily reversible upon reoxygenation and follows the same kinetics as HIF-1α accumulation-degradation. However, induction of eIF2α phosphorylation does not require HIF-1α.
A dominant-negative, nonphosphorylatable mutant form of eIF2α inhibits phosphorylation of endogenous eIF2α and attenuates inhibition of protein synthesis under hypoxia.
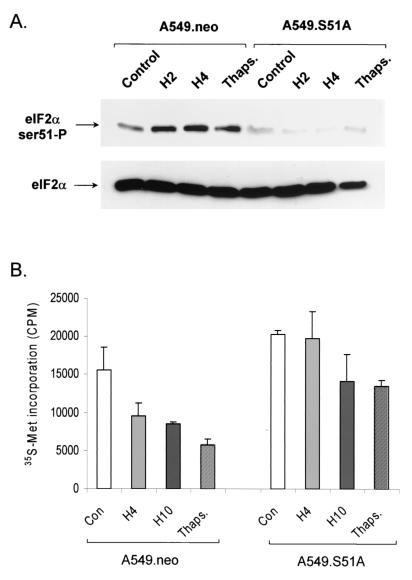
To investigate the role of eIF2α in the inhibition of translation under hypoxia, we used a mutant allele of eIF2α that encodes a protein with a single amino acid substitution at position 51 (Ser224Ala; S51A). This allele has been shown to act in a dominant-negative fashion to inhibit or delay the phosphorylation of endogenous eIF2α in response to stress or treatment with agents that induce eIF2α phosphorylation (16, 55, 65). A549 cells were transfected with this plasmid or with an empty vector plasmid expressing only the neomycin resistance gene (neo). Following selection with neomycin (G418) for 2 weeks, stable clones were obtained from both sets of transfections and further characterized for induction of eIF2α phosphorylation and inhibition of protein and mRNA synthesis. As shown in Fig. 4A, A549.neo cells exhibit increased phosphorylation of eIF2α in response to hypoxia and thapsigargin treatments. In contrast, the basal, hypoxia-induced, and thapsigargin-induced levels of eIF2α phosphorylation were dramatically reduced in the A549 dominant-negative eIF2α mutant cells. These results confirm that stable transfection of cells with the dominant-negative eIF2α allele can inhibit phosphorylation of both transfected and endogenous eIF2α protein.
FIG. 4.
Stable transfection of the dominant-negative eIF2α allele results in attenuation of phosphorylation of endogenous eIF2α and translational inhibition. (A) Immunoblot of phosphorylated (top) and total (bottom) eIF2α in A549 cells stably transfected with the empty vector plasmid (A549.neo) or with a plasmid expressing the S51A mutant form eIF2α (A549.S51A). (B) [35S]methionine (35S-Met) incorporation into TCA-precipitable material in response to hypoxia and thapsigargin (Thaps.) treatments. Labeling, collection, and [35S]methionine incorporation were performed as described in the legend to Fig. 1. Data are depicted as non-normalized counts per minute (CPM). Each experiment was performed three times. Error bars represent standard errors. Con, control.
As in the experiments with untransfected A549 cells shown in Fig. 1, increased levels of eIF2α phosphorylation in response to hypoxia and thapsigargin treatments in A549.neo cells correlated with decreased levels of translation, as determined by [35S]methionine incorporation into TCA-precipitable material (Fig. 4B). In contrast to the neomycin-transfected cells, inhibition of translation by hypoxia in the S51A-expressing cells was attenuated. After 4 h of incubation under hypoxia, no significant decrease in [35S]methionine incorporation was observed compared with that in control cells. A moderate (30.5%) decrease in [35S]methionine incorporation was detected after 10 h of hypoxia despite the lack of further changes in eIF2α phosphorylation. This effect is likely due to alternative mechanisms of protein translation regulation and/or reduced rates of mRNA synthesis (M. Koritzinsky et al., unpublished observations). A similar result was also observed in eIF2α wild-type cells after prolonged hypoxic incubation, where protein translation remained low even after levels of eIF2α phosphorylation began to decrease. Nevertheless, after 4 h of incubation under hypoxia, no decrease in the rate of mRNA synthesis was observed in the S51A-expressing cell line, suggesting that the dominant-negative effect of this allele on hypoxia-induced inhibition of protein synthesis is not a result of decreased mRNA levels. These results strongly suggest that eIF2α phosphorylation is required for rapid and optimal protein synthesis inhibition in response to hypoxic stress.
PERK, a kinase that phosphorylates eIF2α in response to ER stress, becomes hyperphosphorylated under hypoxia.
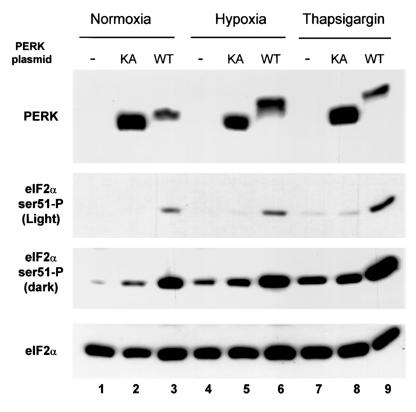
PERK, a kinase found in the lumen of the ER, phosphorylates eIF2α in response to various stimuli that induce ER stress, like glucose deprivation, ischemia-reperfusion, and treatment with thapsigargin (3, 24, 25, 41, 61, 62, 64). Activation of this kinase involves autophosphorylation, which results in a shift in the apparent molecular weight on a polyacrylamide gel (25, 26). Mutation of the lysine at position 618 to alanine, which lies close to the catalytic center, reduces the autocatalytic activity of the protein. Low expression levels of the endogenous protein did not allow a clear analysis of the effects of hypoxia or thapsigargin on its phosphorylation (results not shown). We therefore used transient transfections of plasmids expressing either wild-type mouse PERK or mutant K618A mouse PERK in A549 cells to examine these effects. As shown in Fig. 5 (top), under normoxic conditions, wt-PERK exhibits a slightly slower electrophoretic mobility than K618A-PERK, which has been postulated to arise from activation of wt-PERK by autophosphorylation (26). Such an autophosphorylation activity has been reported when PERK is expressed at high levels, presumably because of oligomerization. Also, overexpression of K618A-PERK moderately increased the levels of eIF2α phosphorylation because of the residual catalytic activity of this mutant protein (Fig. 5, two middle parts, lane 2). Expression of the wt-PERK protein, on the other hand, induced a significantly larger increase in eIF2α phosphorylation (lane 3). Upon treatments with hypoxia, there was an even larger shift in the electrophoretic mobility of wt-PERK compared to that of untreated cells, while the K618A-PERK protein failed to exhibit a similar shift (compare lanes 5 and 6). As expected, hypoxia increased the levels of eIF2α phosphorylation in untransfected cells, while in cells transfected with wt-PERK, the increase in eIF2α phosphorylation was 2.8-fold greater than that observed in cells transfected with the K618A-PERK mutant construct or nontransfected cells (lane 6 versus lanes 4 and 5). Similar results were obtained with cells treated with thapsigargin (lane 9 versus lanes 7 and 8). These findings suggest that PERK becomes phosphorylated and activated under hypoxia and may be involved in hypoxia-induced eIF2α phosphorylation.
FIG. 5.
Hypoxia induces a shift in the electrophoretic mobility of PERK. A549 cells were transfected with either wild-type (WT) or K618A (KA) mouse PERK and exposed to hypoxia or thapsigargin as indicated. Immunoblotting was performed with a rabbit polyclonal antibody raised against human PERK (top) or with the anti-phospho-eIF2α antibody (two middle parts; a light and a dark exposure of the same film are shown for easier comparison of levels of phosphorylated eIF2α). The same blot was stripped and reblotted with the anti-eIF2α (total eIF2α) antibody (bottom). The lower expression of wild-type mouse PERK can be attributed to the decreased efficiency of translation of the plasmid construct because of higher eIF2α phosphorylation. The upper band corresponds to hyperphosphorylated PERK, while the lower band corresponds to hypophosphorylated PERK.
PERK, but not PKR, is required for hypoxia-induced eIF2α phosphorylation.
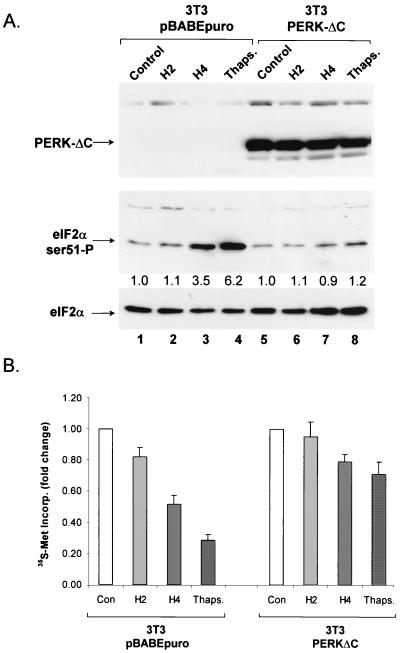
The results obtained with transfected PERK shown above suggest that PERK is activated by hypoxic stress. However, it is not known whether PERK is required for hypoxia-induced phosphorylation of eIF2α. To investigate the requirement of PERK for the phosphorylation of eIF2α by hypoxia, we utilized cells expressing a dominant-negative PERK allele and PERK knockout cells. A PERK allele with a truncated C terminus (PERKΔC) has been shown to act in a dominant-negative fashion to inhibit endogenous PERK activation (3). The dominant-negative allele was cloned into a retroviral expression vector as a fusion gene with the c-myc epitope. 3T3 cells were infected with retrovirus carrying the dominant-negative PERK allele or with the empty virus carrying only a selectable marker (pBABEpuro). Infected cells were tested for the ability to phosphorylate eIF2α in response to hypoxia or thapsigargin. As demonstrated in Fig. 6, cells infected with the retrovirus carrying the PERKΔC cDNA expressed significant amounts of the myc-tagged dominant-negative PERKΔC protein while expression of this protein was absent from 3T3 cells infected with the empty vector. Neither the basal levels of eIF2α phosphorylation nor the levels of total eIF2α differed significantly between the two cell lines. However, while the 3T3 pBABEpuro cells exhibited a substantial increase in eIF2α phosphorylation in response to hypoxia and thapsigargin treatments, no such increase was observed in the 3T3 PERKΔC cells. These results strongly suggest that abrogation of PERK function inhibits eIF2α phosphorylation in response to hypoxic stress.
FIG. 6.
A dominant-negative allele of PERK inhibits eIF2α phosphorylation in response to hypoxia and thapsigargin (Thaps.). (A) 3T3 cells infected with a retrovirus expressing a c-myc epitope-tagged C-terminal deletion of PERK (PERKΔC) and 3T3 cells infected with the empty vector (pBABEpuro) were exposed to hypoxia for the indicated times or treated with 1 μM thapsigargin for 2 h. (Top) Immunoblotting with an antibody against a c-myc tag (9E10), showing the expression of dominant-negative PERK. (Middle) Immunoblotting with the anti-ser51-P eIF2α antibody. (Bottom) Immunoblotting with a polyclonal antibody that recognizes total eIF2α. (B) Effects of hypoxia and thapsigargin on protein synthesis rates in 3T3 cells expressing a dominant-negative PERK allele. 3T3 cells stably infected with PERKΔC or the empty vector expressing only the puromycin resistance gene were exposed to hypoxia (0.01%) for the indicated times or treated with thapsigargin (1 μM) for 2 h. During the last 20 min of the treatments, cells were labeled with [35S]methionine and TCA-precipitable counts were measured as described in Materials and Methods. The results shown are averages of three independent experiments ± the standard errors. Con, control; Incorp., incorporation.
With the same cell lines and treatments, we investigated the effects of hypoxia and thapsigargin on the rates of protein synthesis as shown in Fig. 6B. Exposure of cells to hypoxia (0.01% O2) for 2 h caused a modest (18%) inhibition of protein synthesis, while a longer treatment (4 h) caused a more substantial inhibition (57%). Treatment with thapsigargin (1 μM) for 2 h caused a 77% inhibition of protein synthesis. In contrast, in PERKΔC-expressing cells, the inhibition of protein synthesis was markedly reduced (11% for 4 h of hypoxia and 30% for thapsigargin treatment). The inability of PERKΔC expression to completely block the inhibitory effect of 4 h of hypoxia or thapsigargin on protein synthesis may reflect the presence of an additional, non-eIF2α-mediated mechanism of protein synthesis inhibition.
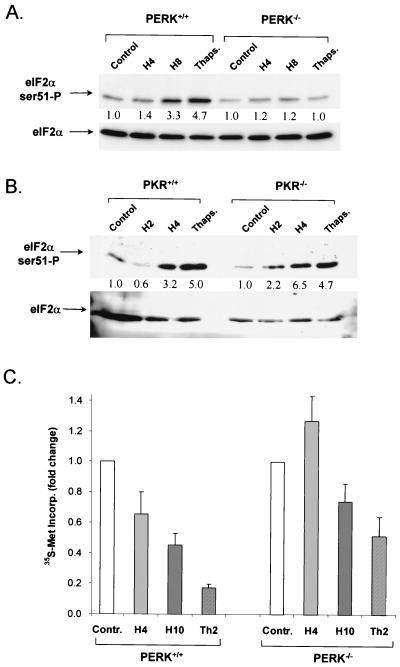
The availability of mice with a homozygous deletion of PERK allowed us to examine the role of PERK in a null background. With SV40 large-T-antigen-transformed MEFs derived from PERK knockout and wild-type mice (25), we examined the phosphorylation of eIF2α. Moderate hypoxia (0.05% O2) induced a time-dependent increase in phosphorylation of eIF2α in the PERK+/+ cells (1.4-fold at 4 h and 4.3-fold at 8 h), and thapsigargin also induced a strong (4.7-fold) phosphorylation (Fig. 7A). On the other hand, hypoxia induced a much smaller increase in eIF2α phosphorylation in PERK−/− cells (1.2-fold at 4 and 8 h), while thapsigargin failed to induce any increase in phosphorylation. This result is consistent with the results obtained with the dominant-negative PERK allele and strongly suggests that PERK is the kinase primarily responsible for eIF2α phosphorylation under hypoxia. The slight but consistent increase in eIF2α phosphorylation under moderate hypoxia in the PERK−/− cells suggests that an additional mechanism may exist that contributes (albeit to a much smaller extend) to phosphorylation of eIF2α under hypoxia. This mechanism appears to become more prominent under conditions of complete anoxia, where a small increase in phosphorylation of eIF2α is observed in PERK−/− cells (data not shown). One such mechanism may involve inhibition of a phosphatase that dephosphorylates eIF2α (42). However, under more physiological levels of hypoxia (0.05 to 1% O2), PERK activation appears to be the dominant mechanism.
FIG. 7.
Hypoxia fails to induce phosphorylation of eIF2α in PERK−/−, but not in PKR−/−, MEFs. PERK+/+ and PERK−/− MEFs immortalized with the SV40 large-T antigen (A) and PKR+/+ and PKR−/− MEFs (B) were exposed to moderate hypoxia (0.05% O2) for the indicated times or to 1 μM thapsigargin (Thaps.) for 2 h, and protein extracts were subjected to immunoblotting with a goat antibody raised against eIF2α phosphorylated on Ser51. The membrane was stripped and reprobed with a rabbit polyclonal antibody that recognizes both phosphorylated and total eIF2α. The values between the two parts represent the fold induction of phosphorylated eIF2α levels compared to control levels after normalization to total eIF2α levels. (C) Effects of hypoxia and thapsigargin on protein synthesis rates in PERK+/+ and PERK−/− MEFs. MEFs were exposed to hypoxia (0.05%) for the indicated times or treated with thapsigargin (1 μM) for 2 h. During the last 20 min of the treatments, cells were labeled with [35S]methionine and TCA-precipitable counts were measured as described in Materials and Methods. The results shown are averages of three independent experiments ± the standard errors. Incorp., incorporation; Contr., control.
To rule out the possibility that another eIF2α kinase is involved in hypoxia-induced eIF2α phosphorylation, we conducted similar experiments with wild-type and knockout cells derived from mice with a deletion of the PKR-encoding gene. The double-stranded-RNA-dependent serine/threonine kinase PKR is activated in virus-infected and interferon-treated cells by double-stranded RNA (34, 53, 69, 74). Overexpression of dominant-negative forms of PKR results in reduced levels of phosphorylation of eIF2α and transformation of NIH 3T3 cells in vitro and in vivo (16, 39). Upon activation, PKR phosphorylates a number of downstream targets, including eIF2α and p53 (12, 13). As shown in Fig. 7B, exposure of both PKR+/+ and PKR−/− cells to hypoxia resulted in a progressive increase in the levels of eIF2α phosphorylation. The increase in eIF2α phosphorylation in the PKR−/− cells suggests that PKR is not involved in eIF2α phosphorylation under hypoxia. Taken together, the data from the PERK−/− and PKR−/− cells indicate that phosphorylation of eIF2α by hypoxic stress requires PERK activity. To investigate the effect of hypoxia on protein synthesis regulation in the PERK+/+ and PERK−/− MEFs, we exposed these cells to hypoxia or treated them with thapsigargin as described above for assaying eIF2α phosphorylation. As shown in Fig. 7C, the inhibition of eIF2α phosphorylation was significantly higher in the PERK+/+ MEFs than in the PERK−/− MEFs in response to treatment with hypoxia or thapsigargin. These results, together with the results of the experiments with the PERKΔC construct, further underscore the critical role of PERK in the phosphorylation of eIF2α and protein synthesis inhibition following hypoxic stress.
Loss of PERK reduces cellular survival levels under prolonged hypoxia.
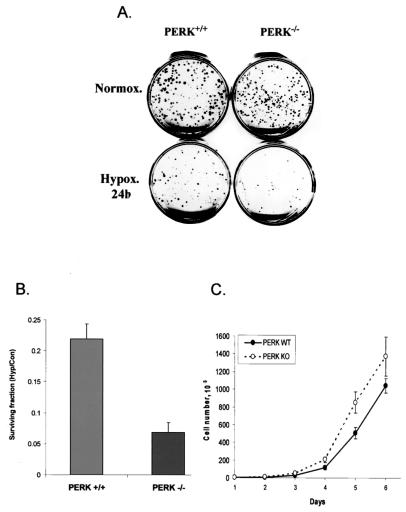
Loss of PERK activity has been shown to increase the sensitivity of cells to ER stresses like treatment with thapsigargin or the glycosylation blocker tunicamycin, presumably because of the accumulation of misfolded proteins in the ER (proteotoxicity) (25). To investigate whether PERK influences cellular survival under hypoxic conditions, we exposed PERK+/+ and PERK−/− cells to prolonged moderate hypoxia (0.05% O2) for 24 h and assayed for the ability of the cells to survive the stress and proliferate by performing clonogenic-survival assays. As shown in Fig. 8A and B, exposure of both cell types to hypoxia resulted in significant loss of viability. However, in this otherwise isogenic system, the PERK+/+ cells consistently displayed approximately threefold higher levels of clonogenic survival than did PERK−/− cells, indicating that the presence of PERK confers a survival advantage under conditions of prolonged exposure to moderate hypoxia. To rule out the possibility that the difference in clonogenic survival could be due to different growth rates between the two cell lines, we assayed the proliferation rates of these two cell lines under normoxic conditions. Cells were plated at equal numbers, and every 24 h, the number of viable cells was determined by counting cells that excluded trypan blue dye. As the results in Fig. 8C show, the PERK+/+ cells actually grew at a slightly (approximately 20%) slower rate than the PERK−/− MEFs over a 6-day period. Therefore, the lower survival of PERK−/− cells following exposure to hypoxia is not due to slower growth of these cells but reflects the lower number of cells that survive the stress to form colonies, compared with that of PERK+/+ cells
FIG. 8.
Loss of PERK reduces cell survival following hypoxic stress. (A) Clonogenic survival of PERK+/+ and PERK−/− MEFs after a 24-h exposure to hypoxia (Hypox.). Following hypoxic stress, 300 cells were plated into dishes and allowed to grow under normoxia (Normox.) for 12 days. Experiments were performed in triplicate. (B) Quantitation of cell survival following hypoxic stress. Colonies representing >50 cells were counted, and cell survival fractions were calculated for control (untreated) and treated (exposed to hypoxia) cells. Average cell survival fractions (hypoxia/control [Hyp/Con]) from three independent experiments are reported for each cell line, along with the standard deviations. (C) Proliferation of PERK+/+ and PERK−/− MEFs under normoxic conditions. Five thousand cells were plated in 24-well plates, and the viable cells (cells excluding trypan blue) were counted with a hemocytometer every 24 h. WT, wild type; KO, knockout.
DISCUSSION
Cellular adaptation to hypoxic stress is a multifaceted process that involves both a shift of cellular metabolism toward anaerobic glycolysis and an inhibition of energy-consuming processes like cell proliferation and macromolecular synthesis (30, 54, 57, 70). At the multicellular or organ level, additional mechanisms like neoangiogenesis and increased synthesis of erythropoietin are also employed in an attempt to increase the oxygen supply to oxygen-starved tissue (4, 58, 59). Abnormalities in oxygen homeostasis are associated with pathological disease states, including brain and cardiac muscle ischemia-reperfusion injury and tumor hypoxia (2, 14, 18, 46, 73). Understanding the molecular events in these types of stress responses is crucial for identifying strategies for therapeutic intervention. In the case of tumor hypoxia, elucidation of adaptive mechanisms of tumor cells to hypoxia-anoxia may provide a mechanism for selective targeting of tumor cells (20).
In this report, we demonstrate that translation initiation factor eIF2α plays a critical role in the downregulation of protein synthesis in response to hypoxic stress in normal and transformed cells. The increase in eIF2α phosphorylation is both time and oxygen dependent and shows a strong correlation with decreased rates of protein synthesis. Reoxygenation of hypoxic cells has been previously shown to result in recovery of protein synthesis to levels similar to that found in normoxic cells, and we find that eIF2α becomes dephosphorylated within 30 to 60 min of reoxygenation. This rapid and reversible modification of eIF2α could allow the cell to adapt to the fluctuating O2 concentrations that are known to occur in tumors. While the model of static, chronic hypoxia appears to apply to certain cases of solid tumors and to spheroids, recent evidence suggests that a more dynamic and fluctuating hypoxia model may apply to most solid tumors (35). This form of transient or intermittent hypoxia has been attributed to the irregular vasoconstriction and vasodilation of newly formed blood vessels, as well as to the leakiness of the vasculature in tumors (5, 6, 9, 10). In this situation, a rapid on-off mechanism to regulate energy consumption, like eIF2α phosphorylation, would be more advantageous than regulation of gene transcription.
The fast kinetics of phosphorylation-dephosphorylation of eIF2α and inhibition and recovery of protein synthesis are similar to the regulation of other cellular processes during hypoxia-reoxygenation. In addition to the rapid inhibition of firing of replicon initiation in Ehrlich ascites and HeLa cells, a recent report describes the remarkable property of zebra fish embryos to enter a state of suspended animation under anaerobic conditions (44). The embryos are able to survive hypoxic treatments of up to 24 h by suspending processes like cell cycle progression and cardiac function. These processes resumed and the embryos appeared to function normally when they were returned to normoxic conditions. Therefore, exposure of a cell to a hypoxic environment initiates a fast-acting, reversible program of adaptation that involves downregulation of protein synthesis, DNA replication, and cell cycle arrest. The decrease in protein synthesis rates has been postulated to contribute to energy conservation under a reduced energy supply because of decreased oxidative phosphorylation. This strategy appears to be also employed in other cases of cellular adaptation to stress. For example, Frerichs et al. demonstrated that during mammalian hibernation, protein synthesis in the brain is suppressed to 0.04% of the level in active animals (19). This remarkable repression is not only compatible with normal brain function and with the absence of any evidence of measurable cell death but is also completely reversible at the end of hibernation. More importantly, phosphorylation of eIF2α was shown to correlate with this marked depression in protein synthesis. Thus, by reducing protein synthesis and energy consumption to a minimum, the cells in the hibernating brain are able to survive under conditions of limited energy supply.
Despite the rapid and relatively extended phosphorylation of eIF2α by hypoxic stress, this phosphorylation is not sustained indefinitely. Longer anoxia incubation times result in decreased eIF2α phosphorylation levels. Sustained eIF2α phosphorylation (and subsequently general translational inhibition) may be incompatible with cell viability. Therefore, it appears that under hypoxic stress, eIF2α phosphorylation levels may be tightly regulated to ensure cellular survival in a low-oxygen environment. This reduction in eIF2α phosphorylation under prolonged hypoxia may be achieved via activation of a negative feedback pathway, and such a pathway may also be involved in the rapid dephosphorylation of eIF2α upon reoxygenation. Recently, it has been reported that the product of the GADD34 gene participates in such a negative feedback loop to dephosphorylate eIF2α following stress (42). Although there is no evidence that hypoxic stress upregulates GADD34, tumor hypoxia has been shown to induce two other GADD family members, GADD45 and GADD153 (49). It would be interesting to examine whether hypoxia or hypoxia-reoxygenation induces GADD34 and whether overexpression of this protein can antagonize hypoxia-induced phosphorylation of eIF2α.
The reduction in eIF2α phosphorylation after sustained periods of hypoxia did not correlate with an increase in overall levels of protein translation at those time points. Similarly, protein synthesis remains inhibited following prolonged hypoxia in cells that express the dominant-negative S51A eIF2α protein and in cells that express dominant-negative PERKΔC. This occurs despite no observable increase in the phosphorylation of eIF2α in both of these mutant cell lines. What mechanisms could be responsible for this phenomenon? First, reduced rates of mRNA synthesis have been observed under prolonged hypoxia and may, in part, explain the reduced rates of protein synthesis. Alternatively, other translational control mechanisms may be used during hypoxia, such as those that regulate the assembly mRNA cap-binding complex eIF4F. Indeed, under conditions of prolonged hypoxia, we have observed changes in the regulation of several factors that result in reduced translation initiation, including eIF4E, eIF4G, and the eIF4E binding proteins (B. G. Wouters et al., unpublished observations). The transition from regulation of translation from eIF2α to eIF4F during prolonged hypoxia may facilitate the translation of specific mRNAs that are critical for the phenotypic changes that occur during hypoxia.
The switch of cellular metabolism from mostly aerobic to mostly anaerobic is regulated by several gene products under the control of HIF-1α (58). Cells with homozygous deletions of this gene are unable to adapt their metabolism to anaerobic glycolysis and are less able to adapt to the new hypoxic environment. Under these conditions, the cell can deplete glucose faster and run out of energy (56). Recently, eIF2α phosphorylation was shown to be critical for glucose homeostasis. Mice homozygous for a mutant, nonphosphorylatable allele of eIF2α or homozygous for deletion of the PERK gene displayed a deficiency in pancreatic β cells and in the unfolded-protein response (24, 55). Therefore, a reasonable hypothesis is that HIF-1α might regulate eIF2α phosphorylation directly or indirectly under hypoxic stress via glucose deprivation. However, our data demonstrate that this is not the case. eIF2α was found to be phosphorylated in response to hypoxia in both HIF-1α+/+ and HIF-1α−/− MEFs, with similar kinetics. This result indicates that the signal for eIF2α phosphorylation under hypoxia is independent of HIF-1α accumulation and subsequent downstream events and further suggests that hypoxia-induced eIF2α phosphorylation does not involve changes in glucose homeostasis (Fig. 9C). Rather, coupled with the data showing activation of PERK, the results described above are compatible with an ER-generated signal for activation of eIF2α phosphorylation by hypoxia.
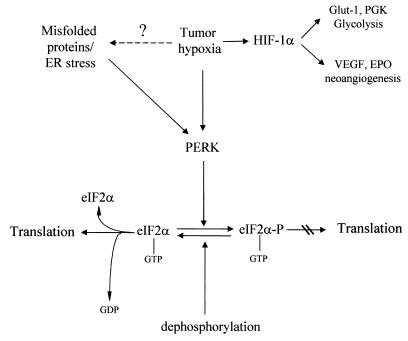
FIG. 9.
Model for the regulation of protein synthesis by hypoxia. Hypoxia activates PERK either directly or through the induction of misfolded proteins in the ER. PERK then phosphorylates eIF2α, which leads to translational inhibition and increased cell survival. VEGF, vascular endothelial growth factor.
Although the mechanism of PERK activation by hypoxia remains unknown, a possible mechanism is activation of the unfolded-protein response (Fig. 9). Hypoxic stress is known to activate a number of ER-resident proteins, in addition to PERK, like the chaperone protein BiP (GRP78) (37, 38, 49, 63), the 150-kDa ORP protein (43), and the stress response protein CHOPP (GADD153) (49). Hypoxic stress induces BiP accumulation in a number of tumor cells, and this activation was shown to be critical for cell survival, since expression of an antisense BiP construct enhanced the cytotoxic effect of hypoxia on these tumor cells (38). The increase in BiP levels is postulated to confer a cytoprotective effect on stressed cells via its chaperonin role and inhibition of protein misfolding in the ER. More recently, BiP was shown to bind to PERK and another ER-resident kinase, IRE1, in unstressed cells and to negatively regulate their function, presumably by inhibiting their oligomerization (26). Upon ER stress, BiP dissociates from PERK and IRE1, relieving its negative regulation, which leads to their activation. BiP levels increase under hypoxic stress and in response to other ER stressors, but this accumulated BiP may not be able to interact with PERK because of competitive binding to misfolded proteins, leading to PERK activation.
Consistent with the protective role of BiP in hypoxic cells is our finding of a protective role for PERK under conditions of prolonged hypoxic stress. The differential survival levels between PERK+/+ and PERK−/− cells under extreme hypoxia (∼3-fold) are not as dramatic as those observed under conditions of pharmacologically induced acute ER stress (10- to 100-fold), but it is unlikely that such extreme conditions of ER stress occur physiologically in organisms (25). The difference in clonogenic survival observed under hypoxia in vitro may also extend to in vivo tumor models and may have important ramifications for tumor growth in vivo. In this respect, it will be important to investigate the role of PERK in tumor growth in vivo with xenograft tumor models and correlate any differences in growth rates with hypoxic areas of the tumor. If eIF2α phosphorylation and PERK activity are also found to play critical roles in tumor growth rates, these findings may lead to the design of therapeutic modalities that inhibit this adaptive response and thereby target hypoxic cells within a tumor.
Acknowledgments
We thank Heather P. Harding and David Ron, Skirball Institute, NYU School of Medicine, for the generous gift of PERK+/+ and PERK−/− MEFs and the expression plasmids for wild-type and K618A mutant PERK. We also thank Randy Johnson, UCSD, for the gift of the HIF-1α+/+ and HIF-1α−/− MEFs. We are grateful to Amato Giaccia of Stanford University for helpful and stimulating discussions relating to this project.
This work was supported by U.S. Public Health Service grant CA94214 from the NCI, National Institutes of Health, to C.K. and by funds from the Department of Radiation Oncology, Wake Forest University School of Medicine.
REFERENCES
- 1.Abraham, N., D. F. Stojdl, P. I. Duncan, N. Methot, T. Ishii, M. Dube, B. C. Vanderhyden, H. L. Atkins, D. A. Gray, M. W. McBurney, A. E. Koromilas, E. G. Brown, N. Sonenberg, and J. C. Bell. 1999. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J. Biol. Chem. 274:5953-5962. [DOI] [PubMed] [Google Scholar]
- 2.Allen, D. G., and C. H. Orchard. 1987. Myocardial contractile function during ischemia and hypoxia. Circ. Res. 60:153-168. [DOI] [PubMed] [Google Scholar]
- 3.Brewer, J. W., and J. A. Diehl. 2000. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc. Natl. Acad. Sci. USA 97:12625-12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, J. M. 1990. Tumor hypoxia, drug resistance, and metastases. J. Natl. Cancer Inst. 82:338-339. [DOI] [PubMed] [Google Scholar]
- 5.Brown, J. M., and A. J. Giaccia. 1994. Tumour hypoxia: the picture has changed in the 1990s. Int. J. Radiat. Biol. 65:95-102. [DOI] [PubMed] [Google Scholar]
- 6.Brown, J. M., and A. J. Giaccia. 1998. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 58:1408-1416. [PubMed] [Google Scholar]
- 7.Bruick, R. K., and S. L. McKnight. 2001. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337-1340. [DOI] [PubMed] [Google Scholar]
- 8.Burda, J., M. E. Martin, M. Gottlieb, M. Chavko, J. Marsala, A. Alcazar, M. Pavon, J. L. Fando, and M. Salinas. 1998. The intraischemic and early reperfusion changes of protein synthesis in the rat brain. eIF-2α kinase activity and role of initiation factors eIF-2α and eIF-4E. J. Cereb. Blood Flow Metab. 18:59-66. [DOI] [PubMed] [Google Scholar]
- 9.Chaplin, D. J., R. E. Durand, and P. L. Olive. 1987. Acute hypoxia in tumors: implications for modifiers of radiation effects. Int. J. Oncol. Biol. Phys. 12:1279-1282. [DOI] [PubMed] [Google Scholar]
- 10.Chaplin, D. J., and S. A. Hill. 1995. Temporal heterogeneity in microregional erythrocyte flux in experimental solid tumours. Br. J. Cancer 71:1210-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemens, M. J., and U. A. Bommer. 1999. Translational control: the cancer connection. Int. J. Biochem. Cell Biol. 31:1-23. [DOI] [PubMed] [Google Scholar]
- 12.Cuddihy, A. R., S. Li, N. W. Tam, A. H. Wong, Y. Taya, N. Abraham, J. C. Bell, and A. E. Koromilas. 1999. Double-stranded-RNA-activated protein kinase PKR enhances transcriptional activation by tumor suppressor p53. Mol. Cell. Biol. 19:2475-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuddihy, A. R., A. H. Wong, N. W. Tam, S. Li, and A. E. Koromilas. 1999. The double-stranded RNA activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro. Oncogene 18:2690-2702. [DOI] [PubMed] [Google Scholar]
- 14.Denko, N. C., and A. J. Giaccia. 2001. Tumor hypoxia, the physiological link between Trousseau's syndrome (carcinoma-induced coagulopathy) and metastasis. Cancer Res. 61:795-798. [PubMed] [Google Scholar]
- 15.Dever, T. E. 1999. Translation initiation: adept at adapting. Trends Biochem. Sci. 24:398-403. [DOI] [PubMed] [Google Scholar]
- 16.Donze, O., R. Jagus, A. E. Koromilas, J. W. Hershey, and N. Sonenberg. 1995. Abrogation of translation initiation factor eIF-2 phosphorylation causes malignant transformation of NIH 3T3 cells. EMBO J. 14:3828-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein, A. C., J. M. Gleadle, L. A. McNeill, K. S. Hewitson, J. O'Rourke, D. R. Mole, M. Mukherji, E. Metzen, M. I. Wilson, A. Dhanda, Y. M. Tian, N. Masson, D. L. Hamilton, P. Jaakkola, R. Barstead, J. Hodgkin, P. H. Maxwell, C. W. Pugh, C. J. Schofield, and P. J. Ratcliffe. 2001. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43-54. [DOI] [PubMed] [Google Scholar]
- 18.Fine, L. G., C. Orphanides, and J. T. Norman. 1998. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int. Suppl. 65:S74-S78. [PubMed] [Google Scholar]
- 19.Frerichs, K. U., C. B. Smith, M. Brenner, D. J. DeGracia, G. S. Krause, L. Marrone, T. E. Dever, and J. M. Hallenbeck. 1998. Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc. Natl. Acad. Sci. USA 95:14511-14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giaccia, A. J. 1996. Hypoxic stress proteins: survival of the fittest. Semin. Radiat. Oncol. 6:46-58. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein, E. N., C. R. Owen, B. C. White, and J. A. Rafols. 1999. Ultrastructural localization of phosphorylated eIF2α [eIF2α(P)] in rat dorsal hippocampus during reperfusion. Acta Neuropathol. 98:493-505. [DOI] [PubMed] [Google Scholar]
- 22.Graeber, T. G., J. F. Peterson, M. Tsai, K. Monica, A. J. Fornace, Jr., and A. J. Giaccia. 1994. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol. Cell. Biol. 14:6264-6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green, S. L., and A. J. Giaccia. 1998. Tumor hypoxia and the cell cycle: implications for malignant progression and response to therapy. Cancer J. Sci. Am. 4:218-223. [PubMed] [Google Scholar]
- 24.Harding, H. P., H. Zeng, Y. Zhang, R. Jungries, P. Chung, H. Plesken, D. D. Sabatini, and D. Ron. 2001. Diabetes mellitus and exocrine pancreatic dysfunction in PERK−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7:1153-1163. [DOI] [PubMed] [Google Scholar]
- 25.Harding, H. P., Y. Zhang, A. Bertolotti, H. Zeng, and D. Ron. 2000. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5:897-904. [DOI] [PubMed] [Google Scholar]
- 26.Harding, H. P., Y. Zhang, and D. Ron. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271-274. (Erratum, 398:6722, 1999.) [DOI] [PubMed] [Google Scholar]
- 27.Hochachka, P. W., L. T. Buck, C. J. Doll, and S. C. Land. 1996. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc. Natl. Acad. Sci. USA 93:9493-9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hockel, M., C. Knoop, K. Schlenger, B. Vorndran, E. Baussmann, M. Mitze, P. G. Knapstein, and P. Vaupel. 1993. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother. Oncol. 26:45-50. [DOI] [PubMed] [Google Scholar]
- 29.Hockel, M., K. Schlenger, B. Aral, M. Mitze, U. Schaffer, and P. Vaupel. 1996. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 56:4509-4515. [PubMed] [Google Scholar]
- 30.Hockel, M., and P. Vaupel. 2001. Biological consequences of tumor hypoxia. Semin. Oncol. 28:36-41. [PubMed] [Google Scholar]
- 31.Hu, B. R., and T. Wieloch. 1993. Stress-induced inhibition of protein synthesis initiation: modulation of initiation factor 2 and guanine nucleotide exchange factor activities following transient cerebral ischemia in the rat. J. Neurosci. 13:1830-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivan, M., K. Kondo, H. Yang, W. Kim, J. Valiando, M. Ohh, A. Salic, J. M. Asara, W. S. Lane, and W. G. Kaelin, Jr. 2001. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464-468. [DOI] [PubMed] [Google Scholar]
- 33.Jaakkola, P., D. R. Mole, Y. M. Tian, M. I. Wilson, J. Gielbert, S. J. Gaskell, A. Kriegsheim, H. F. Hebestreit, M. Mukherji, C. J. Schofield, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. 2001. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468-472. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman, R. J. 2000. Double-stranded RNA-activated protein kinase PKR, p. 503-527. In N. Sonenberg, J. W. B. Hershey, and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Kimura, H., R. D. Braun, E. T. Ong, R. Hsu, T. W. Secomb, D. Papahadjopoulos, K. Hong, and M. W. Dewhirst. 1996. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res. 56:5522-5528. [PubMed] [Google Scholar]
- 36.Koch, C. J. 1982. Oxygen effects in radiobiology. Adv. Exp. Med. Biol. 157:123-144. [DOI] [PubMed] [Google Scholar]
- 37.Koong, A. C., E. A. Auger, E. Y. Chen, and A. J. Giaccia. 1994. The regulation of GRP78 and messenger RNA levels by hypoxia is modulated by protein kinase C activators and inhibitors. Radiat. Res. 138:S60-S63. [PubMed] [Google Scholar]
- 38.Koong, A. C., E. Y. Chen, A. S. Lee, J. M. Brown, and A. J. Giaccia. 1994. Increased cytotoxicity of chronic hypoxic cells by molecular inhibition of GRP78 induction. Int. J. Radiat. Oncol. Biol. Phys. 28:661-666. [DOI] [PubMed] [Google Scholar]
- 39.Koromilas, A. E., S. Roy, G. N. Barber, M. G. Katze, and N. Sonenberg. 1992. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science 257:1685-1689. [DOI] [PubMed] [Google Scholar]
- 40.Kozak, M. 1999. Initiation of translation in prokaryotes and eukaryotes. Gene 234:187-208. [DOI] [PubMed] [Google Scholar]
- 41.Kumar, R., S. Azam, J. M. Sullivan, C. Owen, D. R. Cavener, P. Zhang, D. Ron, H. P. Harding, J. J. Chen, A. Han, B. C. White, G. S. Krause, and D. J. DeGracia. 2001. Brain ischemia and reperfusion activates the eukaryotic initiation factor 2α kinase, PERK. J. Neurochem. 77:1418-1421. [DOI] [PubMed] [Google Scholar]
- 42.Novoa, I., H. Zeng, H. P. Harding, and D. Ron. 2001. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153:1011-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozawa, K., Y. Tsukamoto, O. Hori, Y. Kitao, H. Yanagi, D. M. Stern, and S. Ogawa. 2001. Regulation of tumor angiogenesis by oxygen-regulated protein 150, an inducible endoplasmic reticulum chaperone. Cancer Res. 61:4206-4213. [PubMed] [Google Scholar]
- 44.Padilla, P. A., and M. B. Roth. 2001. Oxygen deprivation causes suspended animation in the zebrafish embryo. Proc. Natl. Acad. Sci. USA 98:7331-7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paschen, W. 1996. Disturbances of calcium homeostasis within the endoplasmic reticulum may contribute to the development of ischemic-cell damage. Med. Hypotheses 47:283-288. [DOI] [PubMed] [Google Scholar]
- 46.Peacock, A. 1990. Pulmonary hypertension due to chronic hypoxia. Br. Med. J. 300:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pettersen, E. O., N. O. Juul, and O. W. Ronning. 1986. Regulation of protein metabolism of human cells during and after acute hypoxia. Cancer Res. 46:4346-4351. [PubMed] [Google Scholar]
- 48.Preiss, T., and M. W. Hentze. 1999. From factors to mechanisms: translation and translational control in eukaryotes. Curr. Opin. Genet. Dev. 9:515-521. [DOI] [PubMed] [Google Scholar]
- 49.Price, B. D., L. A. Mannheim-Rodman, and S. K. Calderwood. 1992. Brefeldin A, thapsigargin, and AIF4 stimulate the accumulation of GRP78 mRNA in a cycloheximide dependent manner, whilst induction by hypoxia is independent of protein synthesis. J. Cell. Physiol. 152:545-552. [DOI] [PubMed] [Google Scholar]
- 50.Probst, G., H. J. Riedinger, P. Martin, M. Engelcke, and H. Probst. 1999. Fast control of DNA replication in response to hypoxia and to inhibited protein synthesis in CCRF-CEM and HeLa cells. Biol. Chem. 380:1371-1382. [DOI] [PubMed] [Google Scholar]
- 51.Probst, H., K. Hamprecht, and V. Gekeler. 1983. Replicon initiation frequency and intracellular levels of ATP, ADP, AMP and of diadenosine 5′,5′"-P1,P4-tetraphosphate in Ehrlich ascites cells cultured aerobically and anaerobically. Biochem. Biophys. Res. Commun. 110:688-693. [DOI] [PubMed] [Google Scholar]
- 52.Probst, H., H. Schiffer, V. Gekeler, H. Kienzle-Pfeilsticker, U. Stropp, K. Stotzer, and I. Frenzel-Stotzer. 1988. Oxygen dependent regulation of DNA synthesis and growth of Ehrlich ascites tumor cells in vitro and in vivo. Cancer Res. 48:2053-2060. [PubMed] [Google Scholar]
- 53.Proud, C. G. 1995. PKR: a new name and new roles. Trends Biochem. Sci. 20:241-246. [DOI] [PubMed] [Google Scholar]
- 54.Richard, D. E., E. Berra, and J. Pouyssegur. 1999. Angiogenesis: how a tumor adapts to hypoxia. Biochem. Biophys. Res. Commun. 266:718-722. [DOI] [PubMed] [Google Scholar]
- 55.Scheuner, D., B. Song, E. McEwen, C. Liu, R. Laybutt, P. Gillespie, T. Saunders, S. Bonner-Weir, and R. J. Kaufman. 2001. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7:1165-1176. [DOI] [PubMed] [Google Scholar]
- 56.Seagroves, T. N., H. E. Ryan, H. Lu, B. G. Wouters, M. Knapp, P. Thibault, K. Laderoute, and R. S. Johnson. 2001. Transcription factor HIF-1 is a necessary mediator of the Pasteur effect in mammalian cells. Mol. Cell. Biol. 21:3436-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Semenza, G. L. 2000. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit. Rev. Biochem. Mol. Biol. 35:71-103. [DOI] [PubMed] [Google Scholar]
- 58.Semenza, G. L. 1999. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 15:551-578. [DOI] [PubMed] [Google Scholar]
- 59.Semenza, G. L., S. T. Koury, M. K. Nejfelt, J. D. Gearhart, and S. E. Antonarakis. 1991. Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc. Natl. Acad. Sci. USA 88:8725-8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheikh, M. S., and A. J. Fornace, Jr. 1999. Regulation of translation initiation following stress. Oncogene 18:6121-6128. [DOI] [PubMed] [Google Scholar]
- 61.Shi, Y., J. An, J. Liang, S. E. Hayes, G. E. Sandusky, L. E. Stramm, and N. N. Yang. 1999. Characterization of a mutant pancreatic eIF-2α kinase, PEK, and co-localization with somatostatin in islet delta cells. J. Biol. Chem. 274:5723-5730. [DOI] [PubMed] [Google Scholar]
- 62.Shi, Y., K. M. Vattem, R. Sood, J. An, J. Liang, L. Stramm, and R. C. Wek. 1998. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 18:7499-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song, M. S., Y. K. Park, J. H. Lee, and K. Park. 2001. Induction of glucose-regulated protein 78 by chronic hypoxia in human gastric tumor cells through a protein kinase C-ε/ERK/AP-1 signaling cascade. Cancer Res. 61:8322-8330. [PubMed] [Google Scholar]
- 64.Sood, R., A. C. Porter, K. Ma, L. A. Quilliam, and R. C. Wek. 2000. Pancreatic eukaryotic initiation factor-2α kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to endoplasmic reticulum stress. Biochem. J. 346(Pt. 2):281-293. [PMC free article] [PubMed] [Google Scholar]
- 65.Srivastava, S. P., K. U. Kumar, and R. J. Kaufman. 1998. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J. Biol. Chem. 273:2416-2423. [DOI] [PubMed] [Google Scholar]
- 66.Sutherland, R., J. Freyer, W. Mueller-Klieser, R. Wilson, C. Heacock, J. Sciandra, and B. Sordat. 1986. Cellular growth and metabolic adaptations to nutrient stress environments in tumor microregions. Int. J. Radiat. Oncol. Biol. Phys. 12:611-615. [DOI] [PubMed] [Google Scholar]
- 67.Sutherland, R. M., W. A. Ausserer, B. J. Murphy, and K. R. Laderoute. 1996. Tumor hypoxia and heterogeneity: challenges and opportunities for the future. Semin. Radiat. Oncol. 6:59-70. [DOI] [PubMed] [Google Scholar]
- 68.Tannock, I. 1976. Oxygen distribution in tumours: influence on cell proliferation and implications for tumour therapy. Adv. Exp. Med. Biol. 75:597-603. [DOI] [PubMed] [Google Scholar]
- 69.Thomis, D. C., and C. E. Samuel. 1993. Mechanism of interferon action: evidence for intermolecular autophosphorylation and autoactivation of the interferon-induced, RNA-dependent protein kinase PKR. J. Virol. 67:7695-7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vanden Hoek, T. L., L. B. Becker, Z. Shao, C. Li, and P. T. Schumacker. 1998. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J. Biol. Chem. 273:18092-18098. [DOI] [PubMed] [Google Scholar]
- 71.Vaupel, P., K. Schlenger, C. Knoop, and M. Hockel. 1991. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 51:3316-3322. [PubMed] [Google Scholar]
- 72.Vaupel, P., O. Thews, D. K. Kelleher, and M. Hoeckel. 1998. Oxygenation of human tumors: the Mainz experience. Strahlenther. Onkol. 174(Suppl. 4):6-12. [PubMed] [Google Scholar]
- 73.Weinachter, S. N., N. Blavet, R. A. O'Donnell, E. T. MacKenzie, and J. R. Rapin. 1990. Models of hypoxia and cerebral ischemia. Pharmacopsychiatry 23(Suppl. 2):94-98. [DOI] [PubMed] [Google Scholar]
- 74.Wu, S., and R. J. Kaufman. 1997. A model for the double-stranded RNA (dsRNA)-dependent dimerization and activation of the dsRNA-activated protein kinase PKR. J. Biol. Chem. 272:1291-1296. [DOI] [PubMed] [Google Scholar]
- 75.Yu, F., S. B. White, Q. Zhao, and F. S. Lee. 2001. HIF-1α binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc. Natl. Acad. Sci. USA 98:9630-9635. [DOI] [PMC free article] [PubMed] [Google Scholar]