Abstract
Cox17p is essential for the assembly of functional cytochrome c oxidase (CCO) and for delivery of copper ions to the mitochondrion for insertion into the enzyme in yeast. Although this small protein has already been cloned or purified from humans, mice, and pigs, the function of Cox17p in the mammalian system has not yet been elucidated. In vitro biochemical data for mammalian Cox17p indicate that the copper binds to the sequence -KPCCAC-. Although mouse embryos homozygous for COX17 disruption die between embryonic days E8.5 and E10, they develop normally until E6.5. This phenotype is strikingly similar to embryos of Ctr1(−/−), a cell surface copper transporter, in its lethality around the time of gastrulation. COX17-deficient embryos exhibit severe reductions in CCO activity at E6.5. Succinate dehydrogenase activity and immunoreactivities for anti-COX subunit antibodies were normal in the COX17(−/−) embryos, indicating that this defect was not caused by the deficiency of other complexes and/or subunits but was caused by impaired CCO activation by Cox17p. Since other copper chaperone (Atox1 and CCS)-deficient mice show a more moderate defect, the disruption of the COX17 locus causes the expression of only the phenotype of Ctr1(−/−). We found that the activity of lactate dehydrogenase was also normal in E6.5 embryos, implying that the activation of CCO by Cox17p may not be essential to the progress of embryogenesis before gastrulation.
Copper is an essential trace element for aerobic organisms, acting as a cofactor for mitochondrial, cytosolic, and vesicular enzymes (27). In the past few years, three independent pathways for intracellular copper trafficking have been identified in yeast. Generally, copper in the form of Cu(I) is transported across the plasma membrane by the high-affinity Cu transporter Ctr1 and is then picked up and transported to each target organelle by cytosolic small proteins (18). Cox17p is one of these small proteins and is considered to be involved in copper recruitment to mitochondria and in the functional assembly of cytochrome c oxidase (CCO), the terminal enzyme of the mitochondrial respiratory chain (5, 6).
It was reported that a proline-rich 62-mer polypeptide was purified from the gel filtration fraction of a porcine heart extract (26). A structural analysis showed that it was a mammalian homologue of yeast Cox17p (yCox17p). Along with the porcine protein (4, 26), human (1), rat (12), and mouse (12, 17) Cox17p homologues have been identified to date. Although it was thought that yCox17p forms a homooligomer (8) and guides Cu to mitochondria for incorporation into CCO, its physiological role in mammals is unclear. Recently, it was reported that expression levels of Cox17p mRNA were high in the mouse heart, kidneys, and brain as well as in some endocrine cell lines but quite low in the small intestine, liver, and some fibroblast cell lines (12). Furthermore, COX17 genomic DNA has been isolated, and its genetic structure and the 5′-promoter function were determinated. Mouse COX17 is a single gene that spans ∼6 kb, consists of three exons, and is mapped to the center of chromosome 16 (25). Transcription factors Sp1 and NRF-1 (nuclear respiration factor 1) drive the basal transcription of this gene (24). The transcriptional mechanism of COX17 is similar to that of other COX subunits (21), which indirectly implies that the Cox17p is also involved in cellular respiration. Since our goal is to determine the physiological function of Cox17p in the mammalian system, we first examined the copper-binding activity of mammalian Cox17p in vitro and then generated mice carrying a null mutation of COX17 and analyzed them. Next, we present genetic evidence that COX17 is required for the transport of copper to the mitochondria and CCO activity. Several case of specific deficiencies of CCO in humans have been reported, with most of them being associated with severe neonatal or infantile lactic acidosis and early death. For example, patients with a fatal cardioencephalomyopathy or hypertrophic cardiomyopathy, marked by a severe CCO deficiency, harbor mutations in the SCO2 gene, which is a related CCO assembly gene that is thought to cooperate with Cox17p (10, 11, 20). A COX17-deficient mouse underwent embryonic death with severe reduction in CCO activity, which is consistent with the previous clinical evidence as in the case of the SCO2 mutation. Furthermore, we show here that Cox17p is not only indispensable for the activation of CCO but also essential for embryonic growth and development. We also report a marked reduction in CCO activity in 6.5-day viable embryos (E6.5), indicating that CCO-independent embryogenesis progressed up to this stage. Most recently, gene disruption of Ctr1 was also reported to result in embryonic death (13, 14). The relationship between this molecule and Cox17p is also discussed in light of the developmental mechanism.
MATERIALS AND METHODS
Preparation of recombinant mouse Cox17p and its analogs.
The coding region of the mouse Cox17p cDNA (12) was cloned into the vector pGEX4T-2 (Amersham Pharmacia). Site-directed mutagenesis was performed using a pair of oligonucleotide primers, which changes the Cys residues in the KPCCAC motif to Gly residues, the 5′- and 3′-terminal primer set, and the above-mentioned fragment as a template by a two-step PCR. A C-terminally deleted construct was generated using a 5′-terminal primer and 3′-nested primer to remove the pentadecapeptide IEAHKECMRALGFKI. These mutant fragments were also subcloned into pGEX4T-2. Standard cultivation and transformation conditions were used. Crude glutathione S-transferase (GST) fusion proteins harboring Cox17p, Cox17p(C23,24,26G), and Cox17p(1-48) were extracted from transformed BL21 bacteria and then purified using a glutathione-Sepharose 4B column (Amersham Pharmacia). The purified GST fusion proteins were digested with thrombin to generate an “N-terminally extended” Cox17p and its analogs. In all cases, the fidelity of the cDNA sequence, as well as the presence of the intended mutation, was confirmed by dideoxy nucleotide sequencing.
In vitro copper-binding assay.
The in vitro copper-binding assay was performed as previously described (2). In cells, copper is converted to the Cu(I) form rather than to the Cu(II) form and most probably is presented to Cox17p in a reduced form. To test whether mouse Cox17p (mCox17p) can bind to copper specifically under reducing conditions, about 25 nmol each of purified Cox17p, Cox17p(C23,24,26G), and Cox17p(1-48) was reduced with 150 mM dithiothreitol and incubated for 20 min at room temperature with 6 molar equivalents of CuCl2 under a nitrogen gas flow. Under this condition, copper existed in the Cu(I) form. These reaction mixtures were then loaded onto a Sephadex G-25 (Amersham Pharmacia) gel filtration column (1.6 by 5.0 cm), and the eluates were collected as 1-ml fractions. The amount of protein in each fraction was assayed by the Bradford method and by amino acid composition analysis using a portion of each fraction (20 μl each). The copper concentration was analyzed using an atomic absorption spectrometer (15) after wet digestion with HNO3.
Targeted disruption of COX17.
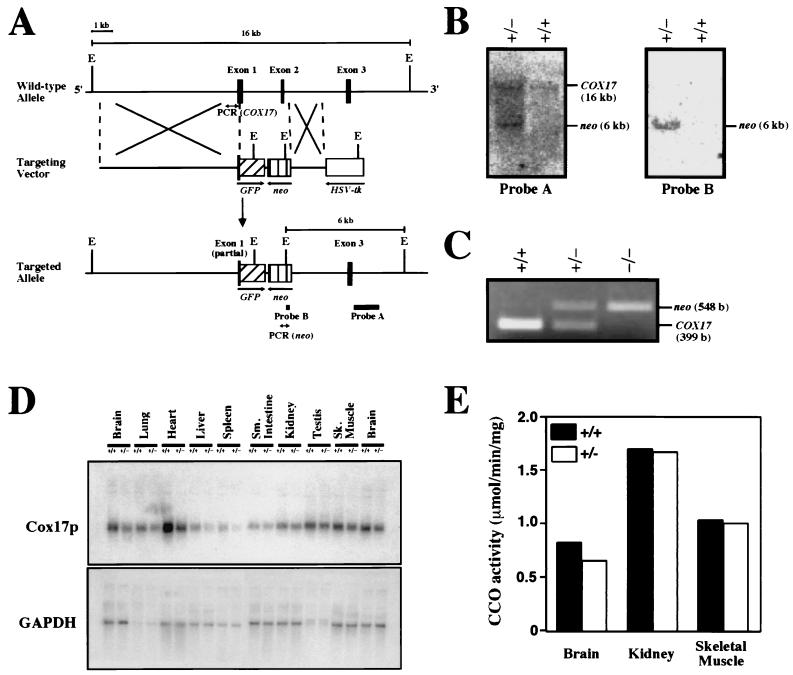
To target the COX17 gene in embryonic stem (ES) cells, COX17 genomic clones were isolated from the 129/SvJ strain of the mouse lambda FIX II library (Stratagene) (19) using mouse Cox17p cDNA (12) as a probe. We replaced a 2.7-kb SacI-HindIII fragment containing part of the first exon (5′ end of the open reading frame) up to the 3′ end of the second exon with green fluorescence protein (GFP) (with the NcoI site fused to the first ATG of COX17) and the neo (opposite direction) gene. GFP and neo were driven under the control of the endogenous COX17 promoter and the pCMV promoter, respectively. Introduction of a negative selection marker, the herpes simplex virus thymidine kinase gene, at the 3′ end of the construct (opposite direction) enabled the use of a positive and negative selection scheme (15). The targeting vector was linearized at a unique SalI site before being transfected into 129/SvJ ES cells, which were then subjected to double selection. Doubly resistant colonies were screened by PCR using a COX17 primer set (5′-ATGGCTTCGAAGTCGGGG-3′ and 5′-CCTTTCA-GGGTCTTGTGC-3′) and a neo primer set (5′-CCATTGCTCAGCGGTGCTG-3′ and 5′-GCCAAGGAGATGGTA-TGTATGTATG-3′). PCR-positive clones were confirmed by Southern blot using probes A and B (see Fig. 2A). Targeted frozen cells were expanded and injected into C57BL/6J blastocysts to produce highly chimeric male mice that transmitted the targeted COX17 allele in the germ line. The COX17(+/−) mice were generated by breeding between male chimeric mice and female C57BL/6 mice. Offspring were genotyped by Southern blotting and PCR analysis with primer sets specific to the COX17 gene and the neo gene, as mentioned above.
FIG. 2.
Targeted disruption of the mouse COX17 gene. (A) Maps of the wild-type COX17 allele, the targeting vector, and the targeted COX17 locus. The solid boxes represent COX17 exons. A promoterless GFP gene cassette (hatched box) was inserted 3 bp downstream of the ATG codon of COX17. neo, neomycin phosphotransferase; HSV-tk, herpes simplex virus thymidine kinase; E, EcoRI restriction sites. The positions of the probes used for genotyping by Southern blot analysis and the primers used for genotyping by PCR are indicated. (B) Southern blot analysis was performed to screen ES clones (right panel) and genotype the progenies from heterozygous matings (left panel). (C) PCR genotyping of progenies from heterozygous matings. (D) Analysis of Cox17p mRNA from organs from wild-type and COX17(+/−) mice. GAPDH mRNA levels were used as a loading control. COX17(+/−) mice expressed ∼50% of the levels of the Cox17p mRNA in the brain, heart, kidneys, and skeletal muscles as those in their wild-type littermates. (E) CCO activities in wild-type and COX17(+/−) mice. Activities of CCO from whole-tissue homogenates were analyzed for 5- to 6-week-old wild-type mice and their COX17(+/−) littermates. The CCO activity is slightly reduced in the brains of the heterozygotes compared with their wild-type littermates, and no significant difference is observed in the kidneys or skeletal muscle.
RNA analysis.
Total RNA was isolated from organs as previously described (12). Total RNA (20 μg) was separated by electrophoresis on a 1.5% agarose gel, and separated products were transferred to a nylon membrane; Cox17p and glyceraldehyde-3-phosphate dehydrogenase mRNAs were detected using radiolabeled mouse Cox17p- and GAPDH coding sequences as probes (12).
Biochemical analysis of CCO.
Mouse tissues were homogenized in 0.25 M sucrose buffer containing 3 mg of digitonin per ml (final concentration). CCO activity was measured by the method of Capaldi et al. (3) with slight modifications. Bovine cytochrome c was reduced with ascorbate and then desalted using a Sephadex G-25 column (Amersham). The concentration of the reduced substrate was determined spectrophotometrically at a wavelength of 550 nm. All spectrophotometric measurements were performed using a UV-160A spectrophotometer (Shimadzu).
Immunohistochemistry.
Fresh frozen sections (8 μm) of embryos in the decidua were prepared using a CM30503 cryostat (Leica). Sections were immunostained with anti-COXI, anti-COXII, and anti-COXIV monoclonal antibodies (Molecular Probe) and then with Alexa Fluor 488 anti-mouse immunoglobulin G (Molecular Probes) as the secondary antibody by standard procedures (22). Histological sections were stained with Meyer's hematoxylin (Sigma) and 0.6% eosin Y (Sigma).
Histological analyses.
We used fresh frozen embryonic sections (8 μm) from COX17(−/−) mice and from normal controls for CCO, succinate dehydrogenase (SDH) and lactate dehydrogenase (LDH) histochemistry, as previously described (22).
RESULTS
Copper-binding activity of mammalian Cox17p.
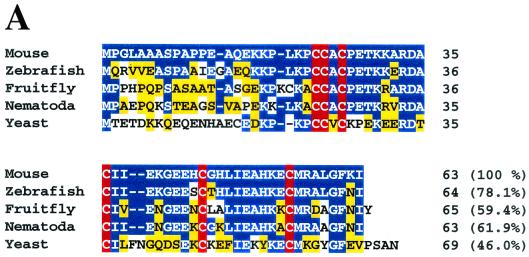
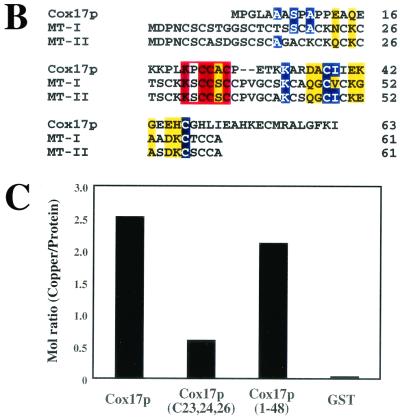
yCox17p contains seven cysteinyl residues, of which six are conserved in other homologues (1, 4, 12, 26). In particular, three-fourths of the C-terminal portions are mutually highly homologous among nematodes and mammals (Fig. 1A). A comparison of mouse Cox17p with mouse metallothionein I and II reveals a short common domain with the sequence -KXCCXC- (Fig. 1B) (1). This motif is a putative copper-binding site of yCox17p and human Cox17p (1). Furthermore, the original respiration-deficient yeast cox17 mutant had a point mutation of Cys57 → Tyr57 (5). This implies that the C-terminal structure of Cox17p is involved in its function. Therefore, we first generated mutants whose cysteine residues were altered to examine the metal-binding properties of mammalian Cox17p. The recombinant wild-type Cox17p protein was purified as a GST fusion protein, and the tag was cleaved using thrombin. Cox17p(C23, 24, 26G), a triple mutant mutated in the above-mentioned metal-binding motif, and Cox17p(1-48), a C-terminally truncated mutant, were also prepared. The wild type, Cox17p(C23, 24, 26G), and Cox17p(1-48) were incubated with CuCl 2 in the presence of an excess amount of a reducing agent. In these studies, GST, a non-copper-binding protein, served as a control for nonspecific binding. As can be seen in Fig. 1C, wild-type mouse Cox17p specifically bound to copper ions, and this binding was abrogated not by the C-terminal deletion but by mutation of the cysteinyl residues in the putative metal-binding motif.
FIG. 1.
Binding of copper to mouse Cox17p. (A) Comparison of the amino acid sequences of mouse, zebrafish (accession no. BE201771), fruit fly (AI062275), nematode (AF099919), and yeast Cox17ps. Identical residues and conserved substitutions are indicated in blue and yellow, respectively. The highly conservative cysteine residues are highlighted in red. (B) Amino acid comparison among mouse Cox17p and metallothionein I and II (MT-I, MT-II). Identical residues and conserved substitutions are indicated by blue and yellow boxes, respectively. The putative copper-binding motif (KXCCXC) of Cox17p is boxed in red. (C) Molar copper contents of GST, wild-type Cox17p, Cox17p(C23,24,26G), and Cox17p(1-48). Significant reduction of copper binding was observed using the Cox17p (C23,24,26G) mutant.
Generation and effect of Cox17p null mutations.
To disrupt the gene, a targeting vector was constructed in which the COX17 sequence coding from halfway through exon 1 to the end of exon 2 was replaced by a GFP-neo cassette (Fig. 2A). This construct was electroporated into 129SvJ ES cells, and correctly targeted ES cells were discerned by Southern blot (Fig. 2B) and PCR (Fig. 2C) analyses. The homologous recombinants were injected into blastocysts, and the disrupted COX17 allele was transmitted through the germ line to the next generation. F1 heterozygotes were generated by crossing the chimeras with C57BL/6J females. The heterozygous mice appeared to be healthy; they were fertile and of normal size. Northern blotting experiments demonstrated that the COX17(+/−) mice expressed ∼50% of the levels of the Cox17p mRNA of their wild-type littermates in the brain, heart, kidneys, and skeletal muscle (Fig. 2D). Then we asked whether any effect on CCO activity could be discerned in the heterozygous animals. While CCO activity was reduced in the brains of the heterozygotes by less than 20% compared with wild-type littermates, no significant difference was observed in the kidneys or skeletal muscle (Fig. 2E). The perturbations in the levels of Cox17p expression, through the inactivation of one COX17 allele, do not result in pronounced effects in most of the tissues apart from the brain.
Progenies from intercrosses of these heterozygous mice were genotyped 3 weeks after birth (Table 1). The ratio of wild-type to heterozygous littermates was 1.0:1.9. This indicated an embryonic lethal phenotype associated with the COX17(−/−) genotype. The stage of embryonic death associated with COX17 loss of function was investigated by determining the genotypes of embryos between E14 and E10. No homozygous COX17(−/−) fetuses were found in this period, and the ratio of COX17(+/+) to COX17(+/−) fetuses was 1.0:2.5 (Table 1). Therefore, we performed PCR to genotype the pregastrulation stage embryos. Of 41 fetuses isolated at E6.5, 9 were COX17(+/+), 22 were COX17(+/−), and 10 were COX17(−/−). This ratio is maintained until E8.5 and is in close agreement with the expected ratio for Mendelian inheritance (Table 1). However, no homozygous mutant embryos were found beyond E8.5. Thus, homozygous null mutations in COX17 result in death between E8.5 and E10.
TABLE 1.
Genotypes of progenies of heterozygous matings
| Stage | No. of progeny (%) with genotype:
|
Total no. | ||
|---|---|---|---|---|
| +/+ | +/− | −/− | ||
| Newborna | 32 (34) | 61 (66) | 0 (0) | 93 |
| E13-14 | 10 (29) | 25 (71) | 0 (0) | 35 |
| E10 | 4 (29) | 10 (71) | 0 (0) | 14 |
| E8.5 | 4 (22) | 10 (56) | 4 (22) | 18 |
| E7.5 | 6 (30) | 9 (45) | 5 (25) | 20 |
| E6.5 | 9 (22) | 22 (54) | 10 (24) | 41 |
Newborn-mouse DNA was isolated from 1-day- to 4-week-old animals.
Morphology and histochemistry of COX17(−/−) embryos.
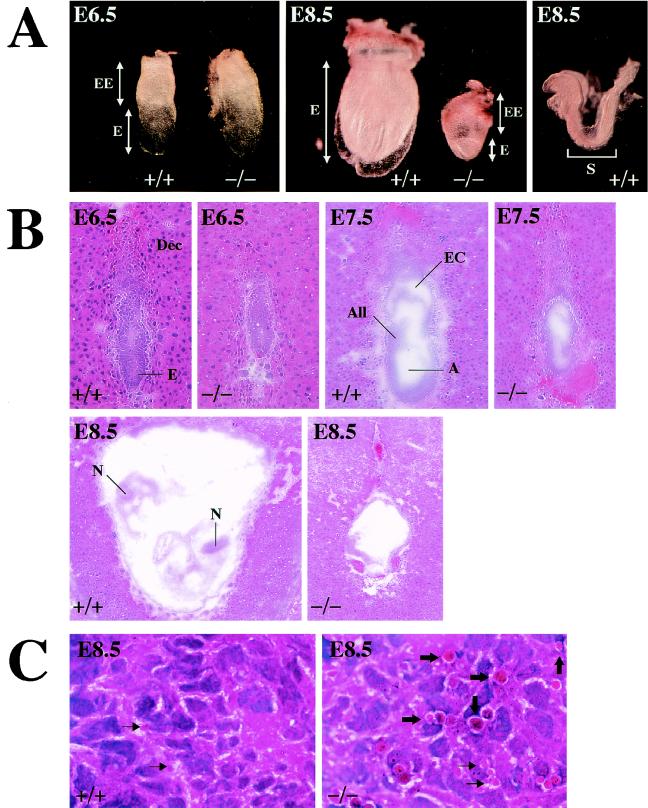
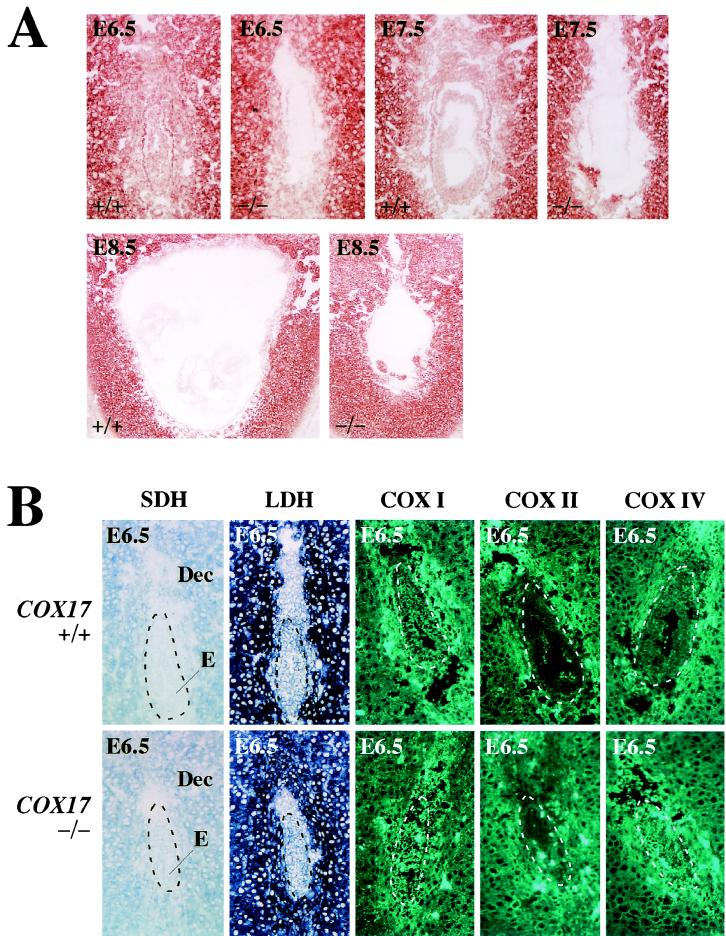
The development of the embryo appeared to advance by E6.5 in all progenies of heterozygous matings (Fig. 3A, left panel). Whereas wild-type littermates at E7.5 proceeded gastrulation, COX17(−/−) embryos were severely retarded and appeared more fragile on dissection of the decidua (Fig. 3B). At E8.5, the wild-type embryos further proceeded to form a neural tube and somite (Fig. 3A and B); the embryonic region was smaller than the extraembryonic region and the embryonic cavity had shrunk in mutant embryos (Fig. 3B). Furthermore, at E8.5, many embryonic cells became smaller than those of the wild-type littermates and condensed nuclei were observed in some of these cells (Fig. 3C).
FIG. 3.
Morphological and histological analysis of wild-type and mutant embryos. (A) Whole-mount gross morphology of wild-type and COX17(−/−) embryos at E6.5 and E8.5. The embryos are oriented such that the embryonic region is located at the bottom and the extraembryonic structures are on the top. All embryos were genotyped by PCR after photography. Morphologically, wild-type embryos were indistinguishable from heterozygotes. No significant differences were observed between wild-type and mutant embryos at E6.5 (left panel; magnification, ×50). Whereas wild-type embryos at E8.5 have proceeded into organogenesis, E8.5 mutant embryos retain large extraembryonic regions and the embryonic regions have atrophied (middle panel; magnification, ×25). The right panel reveals a dissected wild-type embryo (magnification, ×25), and organogenesis has already started. E, embryonic region; EE, extraembryonic region; S, somites. (B) Hematoxylin and eosin staining of sagittal sections of wild-type and COX17(−/−) E6.5 (magnification, ×100), E7.5 (magnification, ×50) and E8.5 (magnification, ×25) embryos. A, amniotic cavity; All, allantois; Dec, decidua; E, embryo; EC, exocoelomic cavity; N, neural tube. (C) Magnified images of hematoxylin-and-eosin-stained embryos at E8.5 (magnification, ×1,000). In the mutant embryonic region, many atrophied cells (thin arrows) and nuclear condensation (thick arrows) can be seen (right panel). Conversely, in the wild-type embryo, few apoptic cells can be observed (left panel).
Oxidative phosphorylation and glycolysis of COX17(−/−) embryos.
Whereas the wild-type embryos isolated at E6.5 retained the CCO activity, COX17(−/−) embryos isolated at the same stage have already shown severe reduction in the CCO activity in embryonic and extraembryonic tissues. This defect was also observed in later stages (Fig. 4A).
FIG. 4.
Histochemical examination of sectioned embryos. (A) CCO activities in the wild-type and COX17(−/−) embryos at E6.5. COX17(−/−) embryos at this stage have already shown a severe reduction in CCO activity in embryonic and extraembryonic tissues. This defect was also observed in later stages. Series of sections identical to those shown in Fig. 3A were used. (B) Activities of SDH and LDH and immunohistochemical staining to detect COXI, COXII, and COXIV at E6.5 (magnification, ×100). Only the CCO activity completely disappeared, while other enzyme activities were normal in mutant embryos. No significant differences in immunoreactivities of COX subunits were observed between wild-type and mutant embryos. E, embryonic region; Dec, decidua. All sections were derived from those shown in Fig. 3B (E6.5). The embryonic regions are also indicated by dotted lines.
The CCO-defective phenotype of COX17(−/−) indicates the possibility that gene disruption of the COX17 allele may affect not only the transport of copper to the mitochondria but also other steps of oxidative phosphorylation. COXI, COXII, and COXIV are subunits of complex IV (CCO), and SDH participates in the oxidative phosphorylation as complex II. No differences in SDH activity between COX17(−/−) mice and their wild-type littermates were observed (Fig. 4B, SDH). The immunoreactivity of the COXI, COXII, and COXIV subunits also appeared normal (Fig. 4B, COXI, COXII, COXIV). Furthermore, strong signals for LDH, the terminal enzyme of glycolysis, were detected in all types of embryo (Fig. 4B, LDH).
DISCUSSION
-KPCCXC- is a conserved motif for the binding of copper ions.
Although recent studies have revealed that wild-type yCox17p binds to three copper ions per molecule and that a Cys → Ser substitution at two of these three residues in the putative metal-binding motif (-KPCCXC-) markedly inhibits copper binding (1), it was not known whether copper binds to the mammalian Cox17p. Srinivasan et al. showed that it binds to two copper ions per molecule by using the GST-yCox17p fusion protein and a cleaved hybrid (23), but an experiment using untagged purified yCox17p indicated that the molar copper content is 3.0 (9). Our results show that mouse Cox17p, prepared as a GST fusion protein and then by cleaving the hybrid, binds to 2.5 copper ions per molecule (Fig. 1C). However, the untagged human Cox17p binds to 3.3 copper ions per molecule (unpublished data), and this value is in good agreement with the data for the untagged yCox17p. Copper binding was significantly abolished not by the C-terminal deletion but by a triple mutation of the cysteine residues in -KPCCXC-. As suggested by the authors of a recent study, the C-terminal structure including Cys57 is not essential for copper binding but may be involved in interactions with other molecules and/or in translocation of holo-Cox17p the mitochondria (9).
Cox17p is essential for CCO activation and early embryogenesis.
Yeast cox17 null mutants failed to grow on nonfermentable carbon sources and have no CCO activity in isolated mitochondria (5). Therefore, we wondered whether mammalian Cox17p plays the same role. To investigate this, we inactivated the COX17 gene in mice by targeted mutagenesis. A targeting vector was designed in which the COX17 sequence coding from halfway along exon 1 to the end of exon 2 was replaced by a GFP-neo cassette (Fig. 2A). GFP was expected to be expressed by the endogenous COX17 promoter, enabling us to analyze the expression pattern of Cox17p. The heterozygous pups grew normally and appeared to be healthy; they were fertile and of normal size. Contrary to expectation, the basal expression level of COX17 was too low for us to detect the expression of GFP in COX17(+/−) tissues. Since CCO activity was reduced by less than 20% in the total-brain homogenates from COX17(+/−) mutants compared with wild-type littermates, no significant differences were observed in the other tissue (Fig. 2E). Based on these observations, we concluded that the heterozygosity of COX17 is phenotypically normal. In contrast, homozygous disruption of the mouse COX17 gene leads to CCO deficiency followed by embryonic death (Fig. 4A). Since W303DCOX17 strains, a deletion mutant of with deletion of the yCox17p gene, exhibit a specific CCO deficiency in the presence of nonfermentable carbon sources (5), the phenotype of the COX17(−/−) embryo is similar to that of the yeast cox17 null mutant.
In addition to the CCO deficiency, severe developmental defects were observed in the COX17(−/−) mutant. The phenotype of the COX17(−/−) embryo is strikingly similar to that of the CTR1(−/−) embryo in its death around the time of gastrulation (13, 14). Furthermore, an incomplete embryonic cavity and apoptosis in the embryonic region were common to both COX17(−/−) and CTR1(−/−) embryos (Figs. 3A and B). To date, three different proteins that transport Cu from CTR1 to three different cellular locations have been identified: Cox17p guides Cu to the mitochondria for insertion into CCO; CCS (copper chaperone for SOD) targets Cu to SOD1, a primary antioxidant enzyme in the cytosol; and Atox1 (anti-oxidant 1) directs Cu to a trans-Goligi network of cells and interacts with the Menkes and Wilson copper-transporting ATPases. When Atox1 and CCS were knocked out, both phenotypes were normal during embryogenesis; Atox1(−/−) mice died perinatally (7) and CCS(−/−) mice were born as mature infants (28). To the best of our knowledge, Cox17p is a prominent candidate as the copper chaperone involved in the early embryogenesis downstream of CTR1.
Activation of CCO by Cox17p may not be essential for early embryogenesis until E6.5 but is necessary for gastrulation.
The most exciting finding of the present study is that normal embryogenesis of COX17(−/−) mutants proceeds until at least E6.5 without CCO activity (Fig. 3A). A severe reduction in CCO activity was also observed in the case of fatal infantile cardioencephalomyopathy with mutations in SCO2 (10, 11, 20). Human SCO2 is required for the assembly of COXI and COXII into the holoprotein and for the incorporation of copper into CCO (11). The histochemistry of muscles from patients with cardioencephalomyopathy revealed reductions in CCO activity in all fibers, but SDH activity appeared normal. Immunohistochemistry showed a severe reduction in the number of mitochondrial DNA-encoded COXI and COXII subunits (20). The major difference between COX17(−/−) and the SCO2 mutant is the existence of these subunits in the mitochondria. Since immunoreactivity for the subunits was confirmed to exist in COX17(−/−) embryos (Fig. 4B, COXI, COXII, and COXIV), we speculated that the CCO deficiency was caused solely by failure of copper transport to the mitochondria. This severe defect is observed at E6.5, when cell division proceeds vigorously in embryonic and extraembryonic regions. Since the activity of the terminal glycolytic enzyme LDH was detected not only in the wild type and heterozygote but also in the homozygote (Fig. 4B, LDH), glycolysis occurred normally at this stage. Although this finding seemed controversial, if one considers the minimal energy (generated by glycolysis) requirement in early embryogenesis, CCO activation may not be required.
On the other hand, why did the normal development of COX17(−/−) embryos stop after E6.5? Since many atrophied cells and nuclear condensation (Fig. 3C) were observed in the COX17(−/−) embryo, a possible mechanism of developmental retardation is hypothesized as follows. CCO inactivation by disruption of the COX17 allele might cause embryonic cell death. Since gastrulation does not proceed without the migration of mesodermal cells, apoptosis of these cells due to CCO deficiency leads to developmental retardation and embryonic death. In general, formation of the brain (which rises from the neural tube) and the heart, skeletal muscle, and kidneys (which are all derived from the mesoderm) starts after gastrulation. Since Cox17p mRNA is also highly expressed in these organs (12), the mesodermal cell death in COX17(−/−) mutants may be associated with this expression pattern. The finding of embryonic death in COX17 mutants may provide an explanation for the lack of obvious candidates for human diseases attributable to the severe CCO deficiency in the heart, brain, and skeletal muscles, such as the case of the SCO2 mutation. Such a significant function of this Cox17p in cellular respiration may be the basis for treating some types of fatal cardioencephalomyopathy or hypertrophic cardiomyopathy.
Acknowledgments
Yoshinori Takahashi and Koichiro Kako contributed equally to this work.
We thank Akiyoshi Fukamizu and Keiji Tanimoto of the University of Tsukuba for their advice on mouse histological analysis.
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science, Sports and Technology of Japan, and the University of Tsukuba Research Projects.
REFERENCES
- 1.Amaravadi, R., D. M. Glerum, and A. Tzagoloff. 1997. Isolation of a cDNA encoding the human homolog of COX17, a yeast gene essential for mitochondrial copper recruitment. Hum. Genet. 99:329-333. [DOI] [PubMed] [Google Scholar]
- 2.Beers, J., D. M. Glerum, and A. Tzagoloff. 1997. Purification, characterization, and localization of yeast Cox17p, a mitochondrial copper shuttle. J. Biol. Chem. 272:33191-33196. [DOI] [PubMed] [Google Scholar]
- 3.Capaldi, R. A., M. F. Marusich, and J. W. Taanman. 1995. Mammalian cytochrome c oxidase: characterization of enzyme and immunological detection of subunits in tissue extracts and whole cells. Methods Enzymol. 260:117-132. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Z. W., T. Bergman, C. G. Östenson, S. Efendic, V. Mutt, and H. Jörnvall. 1997. Characterization of dopuin, a polypeptide with special residue distributions. Eur. J. Biochem. 249:518-522. [DOI] [PubMed] [Google Scholar]
- 5.Glerum, D. M., A. Shtanko, and A. Tzagoloff. 1996. Characterization of COX17, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. J. Biol. Chem. 271:14504-14509. [DOI] [PubMed] [Google Scholar]
- 6.Glerum, D. M., A. Shtanko, and A. Tzagoloff. 1996. SCO1 and SCO2 act as high copy suppressers of a mitochondrial copper recruitment defect in Saccharomyces cerevisiae. J. Biol. Chem. 271:20531-20535. [DOI] [PubMed] [Google Scholar]
- 7.Hamza, I., A. Faisst, J. Prohaska, J. Chen, P. Gruss, and J. D. Gitlin. 2001. The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis. Proc. Natl. Acad. Sci. USA 98:6848-6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heaton, D. N., G. N. George, G. Garrison, and D. R. Winge. 2001. The mitochondrial copper metallochaperone Cox17 exists as an oligomeric, polycopper complex. Biochemistry 40:743-751. [DOI] [PubMed] [Google Scholar]
- 9.Heaton, D., T. Nittis, C. Srinivasan, and D. R. Winge. 2000. Mutational analysis of the mitochondrial copper metallochaperone Cox17. J. Biol. Chem. 275:37582-37587. [DOI] [PubMed] [Google Scholar]
- 10.Jaksch, M., I. Ogilvie, J. Yao, G. Kortenhaus, H. G. Bresser, K. D. Gerbitz, and E. A. Shoubridge. 2000. Mutations in SCO2 are associated with a distinct form of hypertrophic cardiomyopathy and cytochrome c oxidase deficiency. Hum. Mol. Genet. 9:795-801. [DOI] [PubMed] [Google Scholar]
- 11.Jaksch, M., C. Paret, R. Stucka, N. Horn, J. Müller-Höcker, R. Horvath, N. Trepesch, G. Stecker, P. Freisinger, C. Thirion, J. Müller, R. Lunkwitz, G. Rödel, E. A. Shoubridge, and H. Lochmüller. 2001. Cytochrome c oxidase deficiency due to mutations in SCO2, encoding a mitochondrial copper-binding protein, is rescued by copper in human myoblasts. Hum. Mol. Genet. 10:3025-3035. [DOI] [PubMed] [Google Scholar]
- 12.Kako, K., K. Tsumori, Y. Ohmasa, Y. Takahashi, and E. Munekata. 2000. The expression of Cox17p in rodent tissues and cells. Eur. J. Biochem. 267:6699-6707. [DOI] [PubMed] [Google Scholar]
- 13.Kuo, Y. M., B. Zhou, D. Cosco, and J. Gitschier. 2001. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc. Natl. Acad. Sci. USA 98:6836-6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, J., J. R. Prohaska, and D. J. Thiele. 2001. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc. Natl. Acad. Sci. USA 98:6842-6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansour, S. L., K. R. Thomas, and M. R. Capecchi. 1988. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature (London) 336:348-352. [DOI] [PubMed] [Google Scholar]
- 16.Murata, Y., E. Yamakawa, T. Iizuka, H. Kodama, T. Abe, Y. Seki, and M. Kodama. 1995. Failure of copper incorporation into ceruloplasmin in the Golgi apparatus of LEC rat hepatocytes. Biochem. Biophys. Res. Commun. 209:349-355. [DOI] [PubMed] [Google Scholar]
- 17.Nishihara, E., T. Furuyama, S. Yamashita, and N. Mori. 1998. Expression of copper trafficking genes in the mouse brain. Neuroreport 9:3259-3263. [DOI] [PubMed] [Google Scholar]
- 18.O'Halloran, T. V., and V. C. Culotta. 2000. Metallochaperones, an intracellular shuttle service for metal ions. J. Biol. Chem. 275:25057-25060. [DOI] [PubMed] [Google Scholar]
- 19.Ohmura, K., N. Kohno, Y. Kobayashi, K. Yamagata, S. Sato, S. Kashiwabara, and T. Baba. 1999. A homologue of pancreatic trypsin is localized in the acrosome of mammalian sperm and is released during acrosome reaction. J. Biol. Chem. 274:29426-29432. [DOI] [PubMed] [Google Scholar]
- 20.Papadopoulou, L. C., C. M. Sue, M. M. Davidson, K. Tanji, I. Nishino, J. E. Sadlock, S. Krishna, W. Walker, J. Selby, D. M. Glerum, R. V. Coster, G. Lyon, E. Scalais, R. Lebel, P. Kaplan, S. Shanske, D. C. De Vivo, E. Bonilla, M. Hirano, S. DiMauro, and E. A. Schon. 1999. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat. Genet. 23:333-337. [DOI] [PubMed] [Google Scholar]
- 21.Scarpulla, R. C. 2002. Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene 286:81-89. [DOI] [PubMed] [Google Scholar]
- 22.Sciacco, M., and E. Bonilla. 1996. Cytochemistry and immunocytochemistry of mitochondria in tissue sections. Methods Enzymol. 264:509-521. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan, C., M. C. Posewitz, G. N. George, and D. R. Winge. 1998. Characterization of the copper chaperone Cox17 of Saccharomyces cerevisiae. Biochemistry 37:7572-7577. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi, Y., K. Kako, H. Arai, T. Ohishi, A. Takehara, A. Fukamizu, and E. Munekata. 2002. Characterization and identification of promoter elements in mouse COX17 gene. Biochim. Biophys. Acta 1574:359-364. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi, Y., K. Kako, K. Ohmura, K. Tsumori, Y. Ohmasa, S. I., Kashiwabara, T. Baba, and E. Munekata. 2001. Genomic structure of mouse copper chaperone, COX17. DNA Seq. 12:305-318. [DOI] [PubMed] [Google Scholar]
- 26.Takenouchi, T., M. Fujimoto, A. Shimamoto, and E. Munekata. 1999. Isolation and characterization of Cox17p from porcine heart by determining its survival-promoting activity in NIH3T3 cells. Biochim. Biophys. Acta 1472:498-508. [DOI] [PubMed] [Google Scholar]
- 27.Valentine, J. S., and E. B. Gralla. 1997. Delivering copper inside yeast and human cells. Science 278:817-818. [DOI] [PubMed] [Google Scholar]
- 28.Wong, P. C., D. Waggoner, J. R. Subramaniam, L. Tessarollo, T. B. Bartnikas, V. C. Culotta, D. L. Price, J. Rothstein, and J. D. Gitlin. 2000. Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc. Natl. Acad. Sci. USA 97:2886-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]