Abstract
A calcineurin-nuclear factor of activated T cells (NFAT) regulatory pathway has been implicated in the control of cardiac hypertrophy, suggesting one mechanism whereby alterations in intracellular calcium handling are linked to the expression of hypertrophy-associated genes. Although recent studies have demonstrated a necessary role for calcineurin as a mediator of cardiac hypertrophy, the potential involvement of NFAT transcription factors as downstream effectors of calcineurin signaling has not been evaluated. Accordingly, mice with targeted disruptions in NFATc3 and NFATc4 genes were characterized. Whereas the loss of NFATc4 did not compromise the ability of the myocardium to undergo hypertrophic growth, NFATc3-null mice demonstrated a significant reduction in calcineurin transgene-induced cardiac hypertrophy at 19 days, 26 days, 6 weeks, 8 weeks, and 10 weeks of age. NFATc3-null mice also demonstrated attenuated pressure overload- and angiotensin II-induced cardiac hypertrophy. These results provide genetic evidence that calcineurin-regulated responses require NFAT effectors in vivo.
Cardiac hypertrophy is defined by an increase in ventricular wall thickness accompanied by an increase in cardiomyocyte cell volume. Hypertrophic enlargement is precipitated by increased workload or by decreased efficiency within the heart, conditions that are associated with hypertension, ischemic heart disease, valvular insufficiency, neuroendocrine disruptions, or intrinsic defects in contractile proteins (reviewed in reference 30). Although initially compensatory, sustained cardiac hypertrophy predisposes an individual to sudden death, arrhythmias, functional decompensation, and overt heart failure (30).
Numerous regulatory pathways have been implicated in the transduction of hypertrophic signaling, linking neuroendocrine and mechanical stress stimuli to altered cardiac gene expression (reviewed in reference 40). Although numerous hypertrophic regulatory pathways have been identified, the recent characterization of the calcium-regulated phosphatase calcineurin as an important signaling factor in the heart has generated considerable interest. Transgenic mice expressing an activated form of calcineurin in the heart developed robust hypertrophy that quickly transitioned to dilation and failure (41). Subsequently, the calcineurin inhibitory drugs cyclosporine (Cs) and FK506 were shown to inhibit or attenuate cardiac hypertrophy or cardiomyopathy in most, but not all, rodent models of heart disease, suggesting a necessary regulatory role for this signaling pathway in the heart (reviewed in reference 39). More recently, transgenic mice expressing either the calcineurin inhibitory domains of Cain, AKAP79, MCIP1, or dominant-negative calcineurin were shown to have attenuated cardiac hypertrophy in response to pathophysiologic stimulation (7, 52, 72).
Perhaps the best-characterized target of calcineurin is the nuclear factor of activated T cells (NFAT) transcription factor family. Calcineurin directly dephosphorylates NFAT transcription factors, permitting their nuclear translocation and participation in transcriptional regulatory complexes (13, 51). Five members of the NFAT family have been identified in mammals, including NFATc/2/c1, NFATp/1/c2, NFAT3/c4, NFAT4/c3, and NFAT5 (29, 51). Calcineurin-mediated nuclear import of NFAT upregulates the expression of immune response genes in T lymphocytes, including interleukin 2 (IL-2), IL-3, IL-4, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor alpha, and Fas ligand (5, 51).
Genes encoding the five NFAT proteins are expressed in semirestricted patterns throughout the body. NFATc1, -c2, and -c3 are most highly expressed in immune cells and skeletal muscle, as well as weakly expressed in many other cell types, whereas NFATc4 and NFAT5 are more evenly expressed throughout the body (29, 51). Targeted disruption of NFATc1, -c2, and -c3 genes has identified critical roles for these factors in immune cell function and/or survival (19, 44, 46, 50, 67). Disruption of the NFATc1 gene resulted in embryonic lethality due to aberrant heart valve formation and cardiac insufficiency (6, 48). More recently, NFATc2- and NFATc3-null mice were shown to have defects in skeletal muscle fiber number or size (21, 25), while NFATc2-null mice were also shown to undergo aberrant chondrogenesis (49). Lastly, the combinatorial disruption of NFATc3/NFATc4 in mice resulted in embryonic lethality due to vascular insufficiency, demonstrating a role for NFAT factors in developmental patterning (15). Collectively, NFAT factors are expressed in multiple cell types and at different developmental times, where they perform diverse functions.
While heart-specific activation of NFATc4 is sufficient to induce robust hypertrophy in transgenic mice (41), it is unknown whether NFAT factors are direct mediators of calcineurin-regulated cardiac hypertrophy. Here we show that NFATc3-null mice are partially deficient in their ability to undergo cardiac hypertrophy in response to calcineurin activation, providing genetic evidence that NFATc3 is a necessary and direct downstream effector of calcineurin in the heart.
MATERIALS AND METHODS
NFATc4 gene targeting.
A genomic clone was isolated from a sv/129 phage library and mapped for construction of the NFATc4 targeting vector. The three exons encoding the DNA-binding domain were chosen for targeted replacement. The targeting arms were generated by PCR by using Expand high-fidelity polymerase (Boehringer Mannheim). Methods for electroporation of AB2.2 embryonic stem (ES) cells with the linearized targeting vector, growth of ES cells on STO feeder fibroblast cells, and culturing conditions for G418 and FIAU resistance were described earlier in detail (38, 47). Two correctly targeted clones, D10 and D11, were used for injection into C57BL/6 blastocysts to generate chimeric mice, which were bred with C57BL/6 females, resulting in germ line transmission for both ES cell clones. All experimental protocols were approved by the Institutional Animal Care and Use Committee. The NFATc3 gene-targeted mice were a gift from Laurie Glimcher (44).
Animal models.
Abdominal aortic banding was performed on 8- to 12-week-old animals anesthetized with 2% isoflurane-70% O2. The abdominal aorta was exposed with a left medial ventral incision caudal to the diaphragm and 7-0 prolene ligature was tied around a blunted 27-gauge needle just superior to the celiac artery to create a defined constriction upon removal of the needle. Alzet 2002 osmotic minipumps were implanted in 8- to 12-week-old mice anesthetized as described above and placed through a small dorsal incision into the subcutaneous space lateral to the spine. Pumps were filled with angiotensin II (432 μg kg−1 day−1 in 150 mM NaCl-0.01 N acetic acid).
Nuclear extraction and Western blotting.
Mouse, rat, and human heart protein extracts were prepared by a modification on the method of Liew and colleagues (26). Briefly, crude nuclear extract was spun on a sucrose cushion for 1 h at 4°C at 112,000 × g to band nuclei. Nuclei were then collected and lysed in lysis buffer (10 mM Tris base, 5 mM EDTA, 50 mM NaCl, 30 mM sodium pyrophosphate, 50 mM NaF, 100 μM sodium orthovanadate, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and 0.5 μg of pepstatin, leupeptin, and aprotinin ml−1; final pH, 7.6) and spun to clear the nuclear debris. Western blotting was performed as previously described (8). Antibodies used for blotting are described in Table 1.
TABLE 1.
Summary of the antibodies used in this study
| Isoform | Sourcea (catalog no.) | Successful EMSA | Successful Western blot analysis |
|---|---|---|---|
| NFATc1 | Santa Cruz (1789X) | ||
| NFATc1 | Santa Cruz (1149X) | ||
| NFATc1 | Santa Cruz (7294) | ||
| NFATc1 | Orbigen (10195) | Yes | |
| NFATc1 | PharMingen (556602) | Yes | |
| NFATc2 | Santa Cruz (7295) | Yes | Yes |
| NFATc2 | Noncommercial (1777) | ||
| NFATc3 | Santa Cruz (8321) | Yes | Yes |
| NFATc3 | Santa Cruz (1154) | ||
| NFATc3 | Santa Cruz (8405) | ||
| NFATc4 | Santa Cruz (1153) | ||
| NFATc4 | Santa Cruz (13036X) | Yes | |
| NFATc4 | Oncogene (PC625) | Yes | |
| NFATc4 | Noncommercial (889) | ||
| NFATc4 | Noncommercial (890) | ||
| NFAT5 | Santa Cruz (13035X) | Yes | |
| NFAT5 | Oncogene (PC626) |
The noncommercial NFAT antibodies were a generous gift of Nancy R. Rice (Harvard). All other commercial antibodies were used at the recommended concentrations.
Dot blot analysis of hypertrophic markers.
Total RNA was isolated from the ventricular tissue of mice by using Trizol reagent (Gibco-BRL) according to the manufacturer's protocol. The RNA was resuspended in water, quantified, and denatured, and 2 μg was blotted onto nitrocellulose filters by using a dot blot filtration manifold (Bio-Rad, Melville, N.Y.). After the blotting step, the filters were baked at 80°C for 2 h, prehybridized, hybridized, and washed as described previously (23). The sequences of the oligonucleotide DNA probes were also described previously (23). Hybridization signals were quantified by using a Storm 860 PhosphorImager and ImageQuant software (Molecular Dynamics) and then normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase).
RT-PCR.
Reverse transcription-PCR (RT-PCR) was performed by using a Titan One-Tube RT-PCR kit (Boehringer Mannheim) according to the manufacturer's protocol. Cycling conditions were as follows: 95°C for 25 s, 54°C for 30 s, and 68°C for 45 s for 31 cycles. Approximately 8 μl of product was run on a 0.5× Tris-borate-EDTA-6% polyacrylamide gel. Primer sets were as follows: NFATc1 (5′-CCTTCGGAAGGGTGCCTTTT-3′ and 5′-AGGCGTGGGGCCTCAGCAGG-3′), NFATc2 (5′-TGGCCCGCGACATCTACCCT-3′ and 5′-TGGTAGAAGGCGTGCGGCTT-3′), NFATc3 (5′-TGGATCTCAGTATCCTTTAA-3′ and 5′-CACACGAAATACAAGTCGGA-3′), NFATc4 (5′-CATTGGCACTGCAGATGAG-3′ and 5′ CGTAGCTCAATGTCTGAAT-3′), and L7 (5′-GAAGCTCATCTATGAGAAGGC-3′ and 5′-AAGACGAAGGAGCTGCAGAAC-3′). Products were visualized by using a Storm 820 PhosphorImager (Molecular Dynamics).
EMSA.
Electrophoretic mobility shift assays (EMSAs) were performed by using a double-stranded oligonucleotide containing a consensus NFAT binding site from the IL-4 gene promoter as previously described (41). Briefly, 25 μg of neonatal rat heart nuclear protein extract or cultured cardiomyocyte whole-cell extracts were incubated with 100,000 cpm of 32P-labeled double-stranded oligonucleotide, 1.25 μg of poly(dI-dC)-poly(dI-dC), EMSA buffer (12 mM HEPES [pH 7.9], 4 mM Tris [pH 7.9], 50 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 12% glycerol, and 2 μg of aprotinin, leupeptin, and pepstatin/ml) on ice for 20 min in 20 μl. A nondenaturing 5% polyacrylamide gel with 0.5× Tris-borate-EDTA was used to resolve the bound protein complexes from the free probe. Antibodies used in the EMSAs are described in Table 1.
Statistical analysis.
Differences between experimental groups were analyzed by using either a two-tailed Student t test or one-way analysis of variance, followed by Bonferroni's post-test when appropriate by using InStat 3.0 software (GraphPad Software, Inc., San Diego, Calif.).
RESULTS
Targeted deletion of NFATc4 does not attenuate calcineurin-induced cardiac hypertrophy.
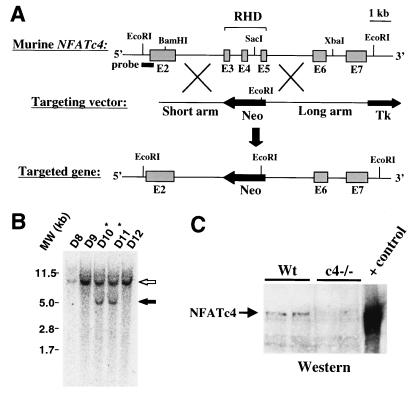
We began our investigation of NFAT factors as cardiac hypertrophic effectors by generating mice carrying a null mutation in NFATc4, which is the isoform originally described to promote hypertrophy in the hearts of transgenic mice (41). A NFATc4 genomic clone was isolated and a targeting vector was designed to disrupt exons 3 to 5 encoding the Rel homology domain (Fig. 1A). By using a probe external to the short arm, Southern blotting of EcoRI-digested ES cell DNA revealed two clones that had undergone correct homologous recombination, each of which was used to generate independent gene targeted mice (Fig. 1B). NFATc4-null mice lacked NFATc4 mRNA (see Fig. 7) and were viable well into adulthood without detectable phenotypic abnormalities. Western blotting was also performed with cardiac nuclear protein extracts, which showed low levels of NFATc4 protein expression in the hearts of wild-type mice, but no expression in the hearts of null mice (Fig. 1C). NFATc4-null mice were also recently generated by another group and shown to be overtly normal (15).
FIG. 1.
Targeted disruption of the murine NFATc4 gene. (A) Genomic structure of the NFATc4 gene, the targeting vector, and the theoretical targeted allele which deletes the Rel homology domain (RHD) of NFATc4. (B) EcoRI-digested genomic Southern blot analysis of targeted ES cell clones D10 and D11 shows an endogenous 10-kb fragment (open arrow) and a targeted 5-kb fragment (solid arrow). (C) NFATc4 Western blot from wild-type and NFATc4−/− adult hearts. A positive control for NFATc4 protein migration was generated by the transfection of Cos cells.
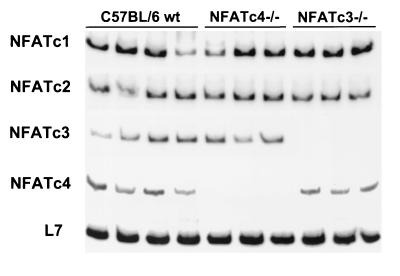
FIG. 7.
Semiquantitative RT-PCR analysis of NFATc1 to -c4 mRNA levels from wild-type hearts (n = 4), NFATc4−/− hearts (n = 3), and NFATc3−/− hearts (n = 3) at 6 weeks of age.
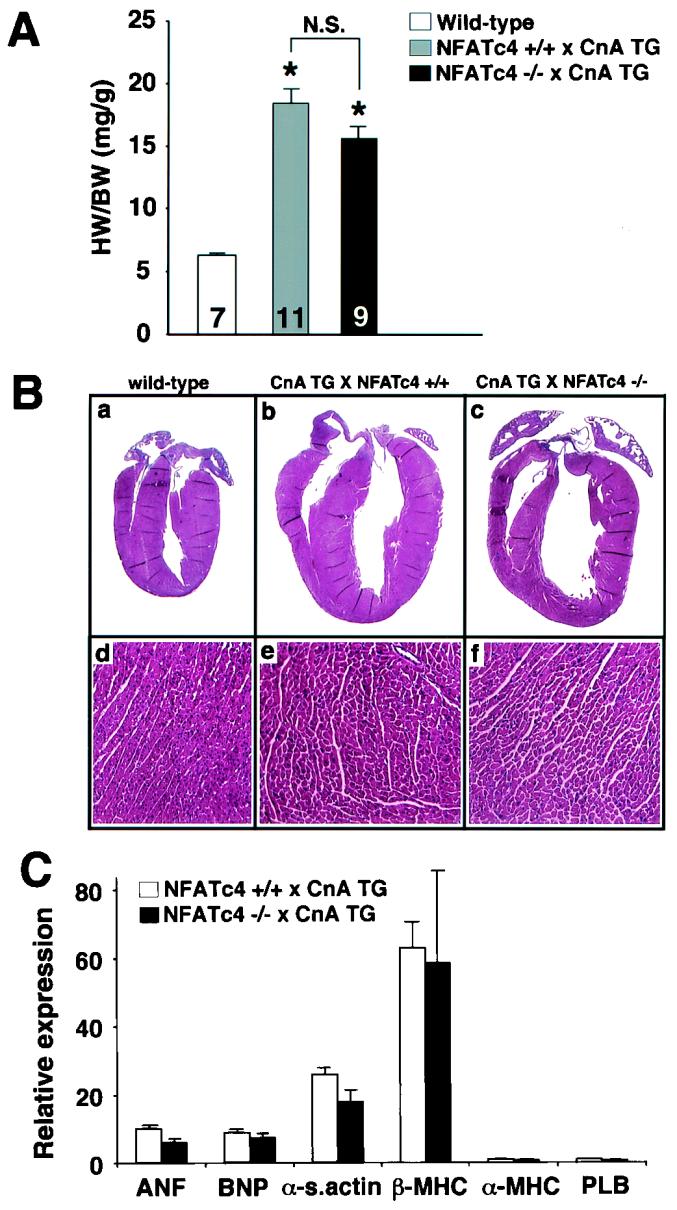
To test the hypothesis that NFATc4 is a critical mediator of calcineurin-induced cardiac hypertrophy, NFATc4-null mice were crossed with cardiac-specific calcineurin-transgenic mice. Calcineurin-transgenic mice express a truncated mouse cDNA which encodes amino acids 1 to 398 of calcineurin Aα under the control of the alpha type of myosin heavy chain (α-MHC) promoter, resulting in profound cardiac hypertrophy (41). The data demonstrated that the loss of NFATc4 did not diminish cardiac hypertrophy directed by the calcineurin transgene at 18 days of age (Fig. 2A). NFATc4-null mice, and control wild-type mice of the same genetic background were each characterized by an ∼3-fold increase in heart-to-body-weight ratio in the presence of the calcineurin transgene (P < 0.05) (Fig. 2A). Examination of younger mice (14 days) and older mice (28 days) from either genotype also failed to reveal any reduction in calcineurin-mediated cardiac hypertrophy in NFATc4-null mice (data not shown).
FIG. 2.
Gravimetrical, histochemical, and molecular analysis of calcineurin-transgenic mice crossed into the NFATc4-null background. (A) Heart weight/body weight ratios of 18-day-old wild-type, CnTG NFATc4+/+, and CnTG NFATc4−/− mice. The number of mice analyzed in each group is shown within each bar. (B) Representative gross and microscopic (magnification, ×140) histologic heart sections stained with hematoxylin and eosin. (C) Induction of hypertrophy-associated mRNA transcripts in CnTG NFATc4+/+ and CnTG NFATc4−/− mice as measured by RNA dot blot quantitation (i.e., the fold increase over wild-type normalized to GAPDH; n = 4 for all groups). HW, heart weight; BW, body weight; CnA TG, calcineurin transgenic.
Histologic analysis of NFATc4-null mice and wild-type littermate mice, each with the calcineurin transgene, demonstrated identical cardiac pathology and morphologic hypertrophy (Fig. 2B). A similar degree of wall thickening, dilation, and myofibrillar hypertrophy was observed at 18 days of age (Fig. 2B). Total RNA was collected for analysis of hypertrophic gene expression by quantitative dot blotting. No significant expression differences in b-type natriuretic peptide (BNP), α-skeletal actin, α-MHC, β-MHC, atrial natriuretic factor (ANF), or phospholamban mRNA levels were observed between calcineurin transgene-containing NFATc4-null or wild-type mice at 18 days of age (Fig. 2C). These data suggest that NFATc4 is not a downstream effector of calcineurin in the postnatal heart or that loss of NFATc4 can be compensated for by another cardiac-expressed NFAT factor (see Discussion).
Loss of NFATc4 does not compromise pathophysiologic cardiac hypertrophy.
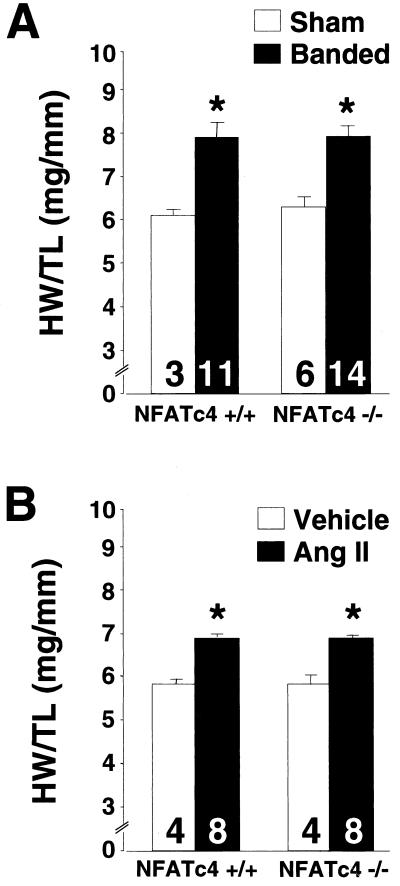
To investigate the role of NFATc4 in regulating cardiac hypertrophy due to physiologic stimuli, 8- to 12-week-old NFATc4-null mice were subjected to 14 days of abdominal aortic constriction or continuous infusion of angiotensin II. Aortic banding in mice and rats and angiotensin II stimulation of cultured cardiomyocytes have previously been shown to activate calcineurin signaling (7, 27, 61). At 2 weeks after aortic banding, NFATc4 wild-type mice demonstrated a significant increase in heart weight/tibia length ratios compared to sham operated wild-type mice (P < 0.05) (Fig. 3A). An identical increase in cardiac hypertrophy was observed in aortic-banded NFATc4-null mice, indicating that NFATc4 is not required for the pressure overload response or that its function is compensated for by another heart-expressed NFAT factor (P < 0.05) (Fig. 3A).
FIG. 3.
Heart weight/tibia length ratios of 8- to 12-week-old NFATc4−/− and NFATc4+/+ mice subjected to 14 days of abdominal aortic constriction (A) or angiotensin II infusion (432 μg kg−1 day−1) (B). (∗P < 0.05 versus sham- or vehicle-treated controls). The number of mice analyzed in each group is shown within each bar. HW, heart weight; TL, tibia length.
NFATc4-null mice were also evaluated for the magnitude of hypertrophy in response to continuous angiotensin II infusion over a period of 2 weeks. Consistent with the aortic banding data, angiotensin II infusion resulted in a nearly identical profile of cardiac hypertrophy between wild-type littermate controls and NFATc4-null mice (P < 0.05) (Fig. 3B). Quantitation of the expression of ANF, skeletal α-actin, and β-MHC mRNA levels demonstrated similar increases in both genotypes in response to angiotensin II infusion or aortic banding (data not shown).
NFATc3 functions downstream of calcineurin in the heart.
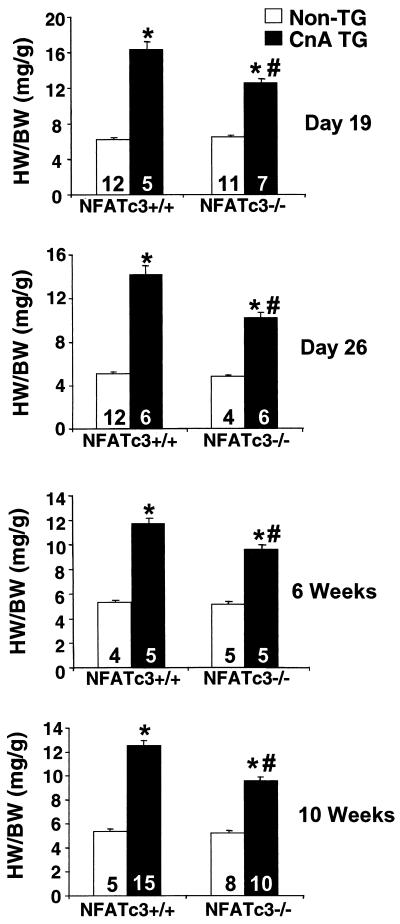
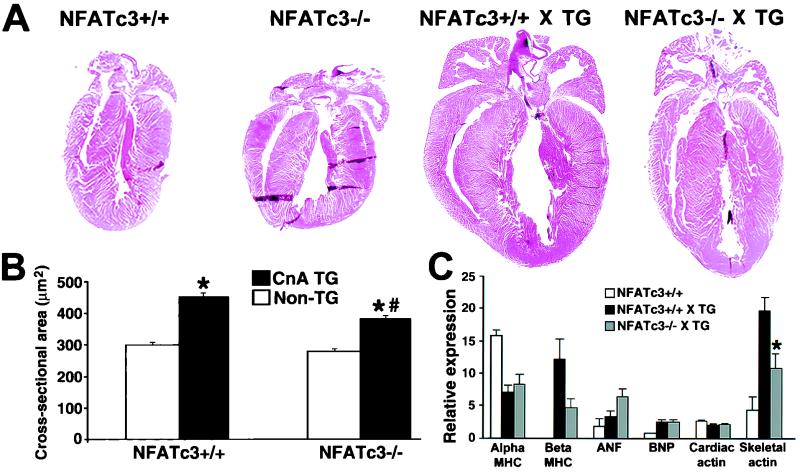
Whereas NFATc4 was shown to be somewhat dispensable as a downstream calcineurin effector in the heart, we hypothesized that another NFAT family member might be involved. NFATc3, the closest structural homolog to NFATc4, had previously been shown to be expressed in the heart by Northern blotting (28). Therefore, NFATc3 gene targeted mice were obtained (44) and crossed with calcineurin-transgenic mice to determine the necessity of this NFAT factor as a potential downstream effector of calcineurin in the heart. In contrast to the NFATc4-null mice, calcineurin transgene-induced hypertrophy was attenuated by 42, 38, 28, 20, and 44% at 19 days, 26 days, 6 weeks, 8 weeks, and 10 weeks age, respectively, in mice lacking NFATc3 (P < 0.05) (Fig. 4) (8-week time point [not shown]). Histologic analysis of NFATc3-null calcineurin-transgenic hearts showed a noticeable reduction in gross heart size compared to their wild-type transgenic counterparts at 6 weeks of age (Fig. 5A). As a more quantitative evaluation of the hypertrophy, cardiomyocyte cross-sectional areas were measured at both 6 (Fig. 5B) and 10 weeks (data not shown) of age. The data demonstrate a significant reduction in calcineurin-induced cellular hypertrophy, suggesting that the reduction in heart size is not due to differences in cell number (P < 0.05) (Fig. 5B). Finally, cardiac hypertrophy and chamber dilation were measured by echocardiography, which identified a significant reduction in calcineurin-induced left ventricular wall and septal thicknesses, as well as left ventricular chamber dimensions, in NFATc3-null hearts (P < 0.05) (Table 2). Despite the reduction in calcineurin-induced cardiac hypertrophy discussed here, the loss of NFATc3 did not produce a statistically significant rescue of fractional shortening. Collectively, these data indicate that whereas the loss of NFATc3 is sufficient to partially disrupt the hypertrophic response, it is not sufficient to obviate the progression to dilated heart failure (Table 2).
FIG. 4.
Heart weight/body weight ratios of NFATc3+/+ and NFATc3−/− mice in the absence or presence of a heart-restricted activated calcineurin transgene at 19 days, 26 days, 6 weeks, and 10 weeks of age. ✽, P < 0.05 versus NFATc3+/+ mice; #, P < 0.05 versus CnTG NFATc3+/+ mice. The number of mice analyzed in each group is shown within each bar. HW, heart weight; BW, body weight; CnA TG, calcineurin transgenic.
FIG. 5.
(A) Representative hematoxylin-and-eosin-stained gross histology from 6-week-old mice crossed with the heart-restricted calcineurin transgene. (B) Myofibrillar cross-sectional areas measured by wheat germ agglutinin-TRITC (tetramethyl rhodamine isothiocyanate) staining of histologic heart sections as previously described (27) (n = 250 fibers in each group). ✽, P < 0.05 versus nontransgenic mice; #, P < 0.05 versus CnTG NFATc3+/+ mice. (C) Quantitation of RNA dot blot analysis of hypertrophy-associated transcripts from wild-type (n = 3), CnTG NFATc3+/+ (n = 5), and CnTG NFATc3−/− (n = 4) mice (i.e., the fold increase versus GAPDH × 10). ✽, P < 0.05 versus CnTG NFATc3+/+ mice.
TABLE 2.
Echocardiographic measurements in calcineurin-transgenic mice crossed into the NFATc3-null background at 10 weeks of agea
| Parameter | Mean ± SEM in:
|
|||
|---|---|---|---|---|
| NFATc3+/+ mice (n = 5) | NFATc3−/− mice (n = 9) | CnTG NFATc3+/+ mice (n = 10) | CnTG NFATc3−/− mice (n = 7) | |
| Septum (mm) | 0.68 ± 0.05 | 0.66 ± 0.04 | 0.79 ± 0.04∗ | 0.68 ± 0.04∗∗ |
| LV free wall (mm) | 0.59 ± 0.05 | 0.62 ± 0.04 | 0.71 ± 0.04∗ | 0.56 ± 0.05∗∗ |
| LVEDD (mm) | 3.68 ± 0.12 | 3.42 ± 0.12 | 4.41 ± 0.24∗ | 3.83 ± 0.28∗∗ |
| LVESD (mm) | 2.34 ± 0.14 | 2.13 ± 0.12 | 3.30 ± 0.17∗ | 2.95 ± 0.20∗∗ |
| FS (%) | 36.5 ± 2.6 | 37.6 ± 2.5 | 24.7 ± 2.2∗ | 22.5 ± 2.5∗ |
NFATc3 wild-type mice and NFATc3-null mice were compared at baseline or when crossed into the calcineurin-transgenic background by echocardiography. Each mouse was measured three times (5 to 10 mice in each group). LV, left ventricle measured in diastole; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension. Fractional shortening (FS) was calculated as (LVEDD − LVESD)/LVEDD × 100. ∗, P < 0.05 versus NFATc3+/+ mice; ∗∗, P < 0.05 versus CnTG NFATc3+/+ mice. n = number of mice.
The loss of NFATc3 partially diminished the hypertrophic transcriptional program induced by the calcineurin transgene at 10 weeks of age (Fig. 5C). Whereas the loss of NFATc3 did not diminish expression of β-MHC, ANF, or BNP, it did result in a significant decrease of α-skeletal actin mRNA levels (Fig. 5C). These results demonstrate a subtle and yet definable defect in the molecular program of cardiac hypertrophy in the absence of NFATc3.
NFATc3 plays a role in pathophysiologic hypertrophy.
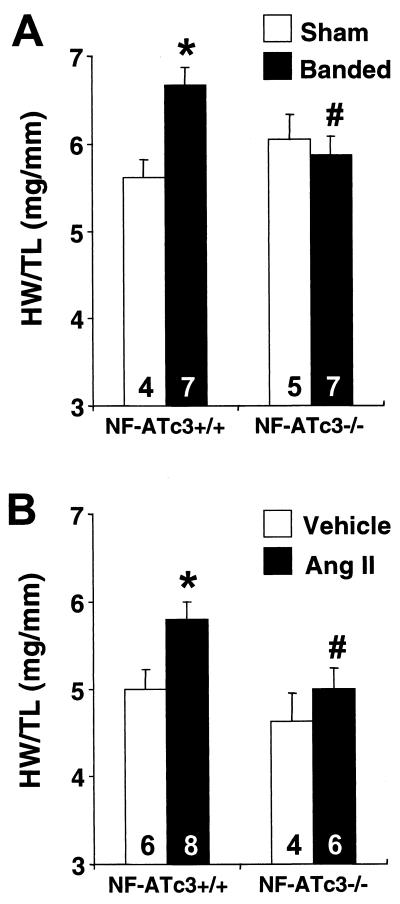
Although the activated calcineurin transgene functions as a robust and myocyte-autonomous hypertrophic stimulus, it was of interest to determine whether NFATc3 also regulated hypertrophy in response to more physiological stimuli. In this regard, 8- to 12-week-old NFATc3-null mice and wild-type controls were subjected to abdominal aortic banding and angiotensin II infusion. Unlike NFATc4-null mice, which showed no defect in cardiac hypertrophy, NFATc3-null mice displayed a significant reduction in heart weight/tibia length ratios in response to aortic banding or angiotensin II infusion compared to wild-type controls (Fig. 6). These results demonstrate the requirement of NFATc3 for mediating pressure overload- or angiotensin II-induced hypertrophy.
FIG. 6.
Heart weight/tibia length ratios of wild-type and NFATc3−/− mice subjected to 14 days of abdominal aortic constriction (A) or angiotensin II infusion (432 μg kg−1 day−1) (B). ✽, P < 0.05 versus NFATc3+/+ mice untreated; #, P < 0.05 versus NFATc3+/+ mice treated. The number of mice analyzed in each group is shown within each bar. HW, heart weight; TL, tibia length.
It should be noted that heart weights were normalized to tibia lengths for aortic banding and agonist infusion studies, and yet they were normalized to body weights in the calcineurin transgene-crossed mice. While either normalization strategy is acceptable, NFATc3-null mice displayed a variably penetrant defect in skeletal muscle mass that generated greater variability in body weights (for example, see reference 25). This increased variability was more problematic for normalizing the small 20 to 30% alterations in heart size associated with aortic banding and agonist infusion, whereas the greater 200 to 300% increases in heart size associated with the calcineurin transgene were easily normalized to either standard.
Analysis of NFAT mRNA isoforms in the heart.
The differential hypertrophic regulatory roles of NFATc3 and NFATc4 suggested either a difference in gene function, a difference in expression levels in the heart, or that one isoform might compensate for the loss of another. We first examined the possibility that the loss of NFATc4 might promote active compensation through increased expression of another NFAT gene in the heart. By using RT-PCR, transcripts of all four calcineurin-regulated NFAT genes were detected in the adult mouse heart (Fig. 7). However, loss of either NFATc3 or NFATc4 did not cause a change in the mRNA levels of the remaining NFAT genes in the heart. Whereas such data do not rule out a compensation beyond the transcriptional level, they at least suggest a lack of transcriptional dependency between NFATc3 and NFATc4 in the heart.
Analysis of NFAT protein isoforms in the heart.
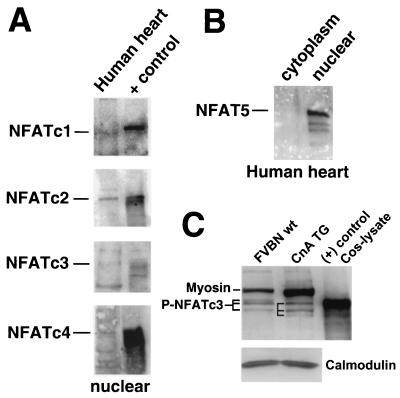
Although mRNA transcripts were detected for NFATc1, -c2, -c3, and c4 in the heart (Fig. 7 and references 20, 28, and 33), the presence of each protein in the heart has not been reported. To address this issue, nucleus-enriched protein extracts were generated from normal adult human hearts and subjected to Western blotting with a large array of commercially available antibodies. Human hearts were chosen because nearly all commercially available NFAT isoform-specific antibodies are made against human epitopes. Since NFAT isoforms are expressed at relatively low levels in the heart and since many of the purchased antibodies proved ineffective (Table 1), NFAT migration standards were also employed (Fig. 8A). The data demonstrate a definable band of the correct migration for NFATc2, NFATc3, and NFATc4 in the heart (compared to lysates of NFATc2- to -c4-transfectioned Cos cells), whereas the migration of NFATc1 was slightly faster in the human heart compared to a control extract of thymocytes (Fig. 8A). This subtle difference in NFATc1 migration may be due to differential splicing of the heart-expressed isoform versus the NFATc1 isoform that is predominant in thymocytes or differential phosphorylation. Collectively, these results are consistent with the RT-PCR analysis and suggest that all four NFATc gene isoforms are probably expressed in the myocardium.
FIG. 8.
Western blots of NFAT protein expression in the heart. (A) Nuclear protein extracts from adult human hearts were subjected to Western blotting for NFATc1 to -c4. The positive control consisted of Cos cells transfected with an expression vector for NFATc2 to -c4 or thymocyte extract for NFATc1. (B) Western blot for NFAT5 from human cytoplasmic or nuclear extracts. (C) Protein extracts from wild-type hearts (pool of seven) or α-MHC-CnA-transgenic hearts (pool of six) were Western blotted for NFATc3. The various phosphorylation states are designated by horizontal lines. Myosin, myosin protein that cross-reacts with the NFATc3 primary antibody from the crude mouse heart protein extracts.
The NFAT5 gene was recently cloned and determined to be distantly related to NFATc1 to -c4. However, NFAT5 does not contain many of the conserved regulatory domains present within NFATc1 to -c4 (29). Indeed, NFAT5 was reported to be constitutively nuclear in T cells, HeLa cells, and C2C12 myoblasts and not subject to calcineurin regulation or Cs inhibition (29). Whereas NFAT5 mRNA is present in the heart (29), its protein expression and potential localization has not been described. Accordingly, Western blotting identified NFAT5 protein of the predicted molecular weight in the nuclear protein fraction of the human heart but not in the cytoplasmic fraction (Fig. 8B). However, NFAT5 functions distinct from NFATc1 to -c4 and, hence, is unlikely to alter calcineurin-NFATc1 to -c4 signaling in cardiac myocytes. For example, unlike NFATc1 to -c4, NFAT5 binds DNA as a homodimer to a unique asymmetric TonE DNA site involved in hypertonicity responses (58).
Although the Western data discussed above suggest that as many as five different NFAT factors are expressed in the heart, the exact cell type of expression is uncertain. The heart contains fibroblasts, endothelial cells, and lymphocytes that are each represented in the cardiac protein extracts used here. To verify whether NFATc3 is expressed in cardiac myocytes, which is the isoform shown to affect the hypertrophic response in gene-targeted mice, we pooled 6-week-old calcineurin-transgenic hearts (n = 6) and wild-type control hearts (n = 7) to make total protein extracts for Western blotting. Since the activated calcineurin transgene is cardiomyocyte autonomous (α-MHC promoter), any alterations in NFAT levels or phosphorylation state likely reflect NFAT protein from cardiomyocytes and not another cell type. Indeed, the data show detectable NFATc3 protein in the adult heart from both wild-type and calcineurin-transgenic mice (Fig. 8C). However, NFATc3 protein from calcineurin-transgenic hearts showed one isoform that migrated more quickly compared to the isoforms detected in the wild-type hearts, suggesting less phosphorylation. In addition, the relative amount of NFATc3 protein present in the total cardiac protein extract was higher in calcineurin-transgenic hearts (24% increase), whereas control blotting for calmodulin levels showed no change (Fig. 8C). The NFATc3 antibody also weakly cross-reacted with total myosin protein as confirmed with a myosin standard (data not shown) (myosin protein is also upregulated in the calcineurin-transgenic heart). In summary, the data described here indicate that NFATc3 protein is expressed in cardiac myocytes and subject to calcineurin regulation.
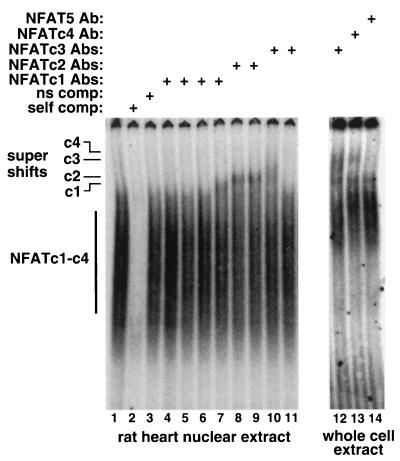
Given the relatively faint Western blot signals shown above, we sought to further verify the expression of each isoform by using an independent assay that was distinct from Western blotting. EMSAs were performed by using neonatal rat heart nuclear protein extracts or cultured cardiomyocyte protein extracts in the presence of antibodies for each NFAT isoform. The shifted complex, which competes with self (lane 2) but not a nonspecific site (lane 3), is somewhat diffuse given that it contains multiple NFAT isoforms, each of which is differentially phosphorylated and spliced. In preliminary experiments, a large panel of NFAT antibodies were employed to identify those capable of supershifting in this assay (Table 1). In analyses with selected antibodies, the data demonstrate the presence of NFATc1 (lane 7), NFATc2 (lanes 8 and 9), NFATc3 (lanes 10 and 12), and NFATc4 (lane 13) in the heart by the appearance of a more slowly migrating species (Fig. 9). These analyses support the RT-PCR data and the Western data discussed above that collectively suggest expression all five NFAT factors in the mammalian heart.
FIG. 9.
EMSA for NFAT protein expression in the heart. Lanes 1 to 11 consisted of rat heart nuclear protein extracts that were incubated with a consensus NFAT binding site from the IL-4 promoter. Lanes 12 to 14 consisted of protein extracts from cultured neonatal rat cardiomyocytes. Although not all antibodies employed were capable of “supershifting” the NFAT-DNA complexes, certain antibodies successfully shifted NFATc1 to -c4 but not NFAT5.
DISCUSSION
The initial report describing calcineurin and NFAT factors as regulators of cardiac hypertrophy extended the hypothesis that alterations in calcium handling underlie the initiation and progression of certain forms of heart disease (41). However, the downstream mechanism whereby calcium-induced calcineurin activation promotes or maintains the hypertrophic state of the heart is currently uncharacterized. We show here that NFATc3, but not NFATc4, is a critical downstream effector of calcineurin-mediated hypertrophy. Given the side effects currently associated with traditional calcineurin inhibitory agents, our results suggest a more targeted approach for future drug design aimed at either NFATc3 or its ability to interact with calcineurin.
Calcineurin inhibitory agents and animal models of cardiac hypertrophy.
The hypothesis that calcineurin is a necessary mediator of cardiac hypertrophy was based on the ability of Cs to block agonist-induced cardiomyocyte hypertrophy in vitro (41, 68). However, the effectiveness of Cs and FK506 as hypertrophic inhibitory agents in animal models of disease was initially controversial. Whereas Cs and FK506 were first shown to prevent hypertrophic and dilated cardiomyopathy in three separate transgenic mouse models of intrinsic heart disease and in a rat model of pressure overload hypertrophy (60), four subsequent studies concluded that calcineurin inhibitors did not reduce pressure overload hypertrophy in mice or rats (10, 31, 71; J. G. Müller, S. Nemoto, M. Laser, B. A. Carabello, and D. R. Menick, Letter, Science 282:1007, 1998). More recently, Cs treatment of αMyHC403 mutant mice was shown to actually induce greater disease manifestation through further alterations in intracellular calcium handling (12). In contrast, 17 separate reports have shown that inhibition of calcineurin with either Cs or FK506 does, in fact, diminish cardiac hypertrophy or disease progression in diverse rodent models (9, 11, 14, 18, 24, 27, 34, 42, 53-57, 60, 62, 64, 69). Cs also prevented exercise-induced cardiac hypertrophy in the rat (11), attenuated hypertrophy and histopathology in renin and angiotensinogen-transgenic rats (36), attenuated myocardial-infarction-induced cardiac hypertrophy in rats (43), and reduced cardiac hypertrophy in activated Gαq-transgenic mice (35).
While most pharmacologic studies support the hypothesis that calcineurin is a necessary regulatory of cardiac hypertrophy, the specificity of Cs and FK506 in the heart remains an open question (12, 22). To address the issue of specificity, three independent groups have recently reported heart-specific transgenic mice with inhibited calcineurin activity in the heart. Transgenic mice expressing the noncompetitive calcineurin inhibitory domains from Cain/Cabin-1 and AKAP79 in the heart showed reduced hypertrophy in response to pressure overload stimulation and agonist infusion (7). In addition, transgenic mice expressing the calcineurin-negative regulatory protein MCIP1 in the heart demonstrated reduced cardiac hypertrophy in response to stress stimulation or aortic banding (17, 52). Finally, transgenic mice expressing a dominant-negative mutant of calcineurin in the heart also showed reduced cardiac hypertrophy after aortic banding (72). Collectively, these four different transgenic models have extended the hypothesis that calcineurin is a necessary regulator of pathophysiologic hypertrophy and suggest that the inhibitory effects of Cs and FK506 are largely attributable to calcineurin blockade.
More recently, calcineurin Aβ-null mice were generated and characterized. calcineurin Aβ-null mice are overtly normal but display a significant reduction in heart weight in unstimulated mice, suggesting that calcineurin might also regulate homeostatic heart size at baseline (4). More significantly, calcineurin Aβ-null mice failed to undergo cardiac hypertrophy in response to pressure overload, isoproterenol infusion, or angiotensin II infusion (4).
NFAT factors as downstream effectors of calcineurin signaling in the heart.
NFATc4 (NFAT3) was originally implicated as a regulator of hypertrophic gene expression in the heart based on its ability to physically interact with GATA4 and to regulate expression of the BNP gene promoter (41). This initial observation was extended in transgenic mice whereby overexpression of a constitutively nuclear mutant of NFATc4 induced cardiac hypertrophy (41). However, overexpression of activated NFATc4 likely mimics the effects of any of the NFAT factors given the relatively high degree of homology within the DNA-binding domain of these factors (51). In addition, mRNA and protein for all four calcineurin-regulated NFAT genes has been detected in the heart, suggesting that any or multiple NFAT factors might participate in regulating cardiac gene expression (Fig. 7 and 8) (20, 28, 29, 33).
Although the mRNAs for NFATc1 to -c4 were readily detected in the mouse heart, two issues complicate the validation of the relevant calcineurin-dependent isoform(s). First, definitive assessment of NFATc1 to -c4 protein expression in the adult heart has not been previously described. Second, cell types other than cardiac myocytes are present within the heart, including fibroblasts, endothelial cells, and lymphocytes. We were able to detect all four calcineurin-regulated NFATc factors, as well as NFAT5, by Western blot analysis of total human heart homogenates (either nuclear or cytoplasmic fractions). Our attempts to characterize which NFAT proteins are expressed in the heart were initially unsuccessful, given that most commercially available antibodies proved ineffective in detecting the low levels of NFAT isoforms in the adult heart (17 antibodies were examined). However, sensitive antibodies were identified (Table 1) that reliably detected each NFAT isoform in the heart (Fig. 8). This conclusion is further supported by the EMSA analysis, which showed detection of NFATc1 to -c4 in rat heart protein extracts. Although NFAT5 protein was detected in the human heart within the nuclear protein fraction, the NFAT oligonucleotide probe used in the EMSA analysis is not recognized by NFAT5 (58). Indeed, NFAT5 is of a divergent class of Rel homology domain-containing factors that functions distinctly from NFATc1 to -c4 and is not subject to significant calcineurin regulation (29, 58).
Although targeted disruption of NFATc4 did not diminish the magnitude of calcineurin-dependent hypertrophy, NFATc3-null mice showed a significant and long-standing reduction in calcineurin-induced hypertrophy as far out as 10 weeks of age. By this time in the calcineurin-transgenic mouse, cardiac hypertrophic growth has ceased and severe dilated heart failure is manifested. These results indicate that loss of NFATc3 produces a fundamental deficit in the ability of these hearts to maximally hypertrophy in response to calcineurin activation. These data further establish NFATc3 as a critical downstream mediator of calcineurin-regulated hypertrophy in the heart and validate the original hypothesis that calcineurin mediates myocyte hypertrophy, in part, through NFAT transcription factors.
The hypothesis that NFAT is a critical mediator of calcineurin signaling is also supported by two recent studies in which cardiac myocyte hypertrophic growth was reduced by overexpression of glycogen synthase kinase 3β (GSK3β). GSK3β was previously shown to directly phosphorylate the N-terminal regulatory domain of NFATc1, thus antagonizing the action of calcineurin and inhibiting nuclear shuttling of NFAT (2). Overexpression of GSK3β in cultured cardiomyocytes attenuated agonist-induced hypertrophy, in part, by blocking NFAT nuclear translocation (16). More recently, transgenic mice with GSK3β expression in the heart had reduced cardiac hypertrophy in response to the activated calcineurin transgene and in response to isoproterenol infusion (1). Taken together with the results presented in the present study, multiple lines of evidence implicate NFAT factors as necessary mediators of calcineurin-regulated hypertrophic signaling.
Other effectors of calcineurin-regulated cardiac hypertrophy.
Although NFATc3-null mice showed reduced hypertrophy in response to the activated calcineurin transgene, the effect was only partial, suggesting the necessity of other calcineurin-dependent regulatory factors in the heart. Indeed, NFATc3-null mice containing the calcineurin transgene still showed an approximately twofold increase in cardiac hypertrophy at each time point examined. Cardiac hypertrophy was also not completely eliminated in NFATc3-null mice infused with angiotensin II, further suggesting the involvement of other regulatory factors. The most straightforward interpretation of these data are that NFATc1, NFATc2, or NFATc4 compensate, in part, for the loss of NFATc3 in the heart. All four NFATc factors are detectable in the heart and could easily participate in mediating the residual twofold hypertrophic response observed in the NFATc3-null mice crossed with the calcineurin transgene. Even though deletion of NFATc3 did not completely block calcineurin-induced hypertrophy or its progression to dilated heart failure, NFATc3-null mice displayed a reproducible reduction in hypertrophic growth of ≈35% at multiple time points. These data indicate that NFATc3 plays a more pivotal role in regulating the hypertrophic program. In lymphocytes, NFATc3-null mice appear to display a phenotype most consistent with calcineurin gene-targeted mice (44), collectively suggesting NFATc3 as a more critical calcineurin effector in multiple cell types.
Recent evidence has also implicated the myocyte enhancer factor 2 (MEF-2) transcription factor family as downstream transducers of calcineurin signaling in cardiac myocytes, skeletal muscle cells, neurons, and T lymphocytes (32, 45, 65, 70). Specifically, the phosphorylation status of MEF2A and its DNA-binding efficiency were shown to be Cs sensitive (32). Calcineurin was also shown to directly complex with MEF2A and to dephosphorylate it in skeletal muscle cells (66). Further support for a MEF-2-calcineurin regulatory pathway was provided by the observation that cardiac- and skeletal muscle-specific calcineurin-transgenic mice showed activation of a MEF-2-dependent Lac-Z reporter transgene, directly demonstrating that MEF-2 transcriptional responses are modified by calcineurin in vivo (45, 65). Lastly, MEF-2 was reported to directly interact with NFAT factors in T lymphocyte hybridoma cells, suggesting the coregulation of two distinct calcineurin effector proteins (3). Given these reports, the remaining calcineurin-dependent hypertrophic response observed in NFATc3-null mice might reflect the activity of MEF-2 factors in the heart, NFATc1, NFATc2, NFATc4, or other regulated factors such as NF-κB and Elk-1 (37, 59, 63).
Whereas multiple regulatory factors are undoubtedly influenced by calcineurin in the heart, the present study establishes the importance of NFAT factors as necessary transducers of calcineurin-regulated gene expression. Given the known toxicity associated with traditional calcineurin inhibitory agents, this study suggests the design of novel agents that are directed at NFATc3 or its interaction with calcineurin in affecting certain biologic responses that depend on calcineurin or NFAT signaling.
Acknowledgments
We thank Laurie Glimcher for supplying NFATc3 gene targeted mice. B.J.W. was supported by an M.D./Ph.D. scholar award from the University of Cincinnati Physician Scientist Training Program.
O.F.B. was supported by a National Institutes of Health Individual Research Service Award HL10336, and J.C.B. was supported by a National Institutes of Health training grant 5T32 HL07382. This work was also supported by National Institutes of Health grants and a Scholar award from the Pew Foundation (J.D.M.).
REFERENCES
- 1.Antos, C. L., T. A. McKinsey, N. Frey, W. Kutschke, J. McAnally, J. M. Shelton, J. A. Richardson, J. A. Hill, and E. N. Olson. 2002. Activated glycogen synthase-3β suppresses cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 99:907-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930-1934. [DOI] [PubMed] [Google Scholar]
- 3.Blaeser, F., H. Ho, R. Prywes, and T. A. Chatila. 2000. Ca2+-dependent gene expression mediated by MEF2 transcription factors. J. Biol. Chem. 275:197-209. [DOI] [PubMed] [Google Scholar]
- 4.Bueno, O. F., B. J. Wilkins, K. M. Tymitz, B. J. Glascock, T. F. Kimball, J. N. Lorenz, and J. D. Molkentin. 2002. Impaired cardiac hypertrophic response in calcineurin Aβ-deficient mice. Proc. Natl. Acad. Sci. USA 99:4586-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crabtree, G. R. 1999. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell 96:611-614. [DOI] [PubMed] [Google Scholar]
- 6.de la Pompa, J. L., L. A. Timmerman, H. Takimoto, H. Yoshida, A. J. Elia, E. Samper, J. Potter, A. Wakeham, L. Marengere, B. L. Langille, G. R. Crabtree, and T. W. Mak. 1998. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 392:182-186. [DOI] [PubMed] [Google Scholar]
- 7.De Windt, L. J,. H. W. Lim, O. F. Bueno, Q. Liang, J. C. Braz, B. J. Glascock, T. F. Kimball, F. del Monte, R. J. Hajjar, and J. D. Molkentin. 2001. Targeted inhibition of calcineurin attenuates cardiac hypertrophy invivo. Proc. Natl. Acad. Sci. USA 98:3322-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Windt, L. J., H. W. Lim, S. Haq, T. Force, and J. D. Molkentin. 2000. Calcineurin promotes protein kinase C and c-Jun NH2-terminal kinase activation in the heart: cross-talk between cardiac hypertrophic signaling pathways. J. Biol. Chem. 275:13571-13579. [DOI] [PubMed] [Google Scholar]
- 9.Deng, L., B. Huang, D. Qin, K. Ganguly, and N. El-Sherif. 2001. Calcineurin inhibition ameliorates structural, contractile, and electrophysiologic consequences of postinfarction remodeling. J. Cardiovasc. Electrophysiol. 12:1055-1061. [DOI] [PubMed] [Google Scholar]
- 10.Ding, B., R. L. Price, T. K. Borg, E. O. Weinberg, P. F. Halloran, and B. H. Lorell. 1999. Pressure overload induces severe hypertrophy in mice treated with cyclosporine, an inhibitor of calcineurin. Circ. Res. 84:729-734. [DOI] [PubMed] [Google Scholar]
- 11.Eto, Y., K. Yonekura, M. Sonoda, N. Arai, M. Sata, S. Sugiura, K. Takenaka, A. Gualberto, M. L. Hixon, M. W. Wagner, and T. Aoyagi. 2000. Calcineurin is activated in rat hearts with physiological left ventricular hypertrophy induced by voluntary exercise training. Circulation 101:2134-2137. [DOI] [PubMed] [Google Scholar]
- 12.Fatkin, D., B. K. McConnell, J. O. Mudd, C. Semsarian, I. G. Moskowitz, F. J. Schoen, M. Giewat, C. E. Seidman, and J. G. Seidman. 2000. An abnormal Ca2+ response in mutant sarcomere protein-mediated familial hypertrophic cardiomyopathy. J. Clin. Investig. 106:1351-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flanagan, W. M., B. Corthesy, R. J. Bram, and G. R. Crabtree. 1991. Nuclear association of a T-cell transcription factor blocked by FK506 and cyclosporin A. Nature 352:803-807. [DOI] [PubMed] [Google Scholar]
- 14.Goldspink, P. H., R. D. McKinney, V. A. Kimball, D. L. Geenen, and P. M. Buttrick. 2001. Angiotensin II induced cardiac hypertrophy in vivo is inhibited by cyclosporin A in adult rats. Mol. Cell. Biochem. 226:83-88. [DOI] [PubMed] [Google Scholar]
- 15.Graef, I. A., F. Chen, L. Chen, A. Kuo, and G. R. Crabtree. 2001. Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell 105:863-875. [DOI] [PubMed] [Google Scholar]
- 16.Haq, S., G. Choukroun, Z. B. Kang, H. Ranu, T. Matsui, A. Rosenzweig, J. D. Molkentin, A. Alessandrini, J. Woodgett, R. Hajjar, A. Michael, and T. Force. 2000. Glycogen synthase kinase-3β is a negative regulator of cardiomyocyte hypertrophy. J. Cell Biol. 151:117-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill, J. A., B. Rothermel, K. D. Yoo, B. Cabuay, E. Demetroulis, R. M. Weiss, W. Kutschke, R. Bassel-Duby, and R. S. Williams. 2002. Targeted inhibition of calcineurin in pressure-overload cardiac hypertrophy: preservation of systolic function. J. Biol. Chem. 277:10251-10255 [DOI] [PubMed] [Google Scholar]
- 18.Hill, J. A., M. Karimi, W. Kutschke, R. L. Davisson, K. Zimmerman, Z. Wang, R. E. Kerber, and R. M. Weiss. 2000. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation 101:2863-2869. [DOI] [PubMed] [Google Scholar]
- 19.Hodge, M. R., A. M. Ranger, F. C. de la Brousse, T. Hoey, M. J. Grusby, and L. H. Glimcher. 1996. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity 4:397-405. [DOI] [PubMed] [Google Scholar]
- 20.Hoey, T., Y. L. Sun, K. Williamson, and X. Xu. 1995. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity 2:461-472. [DOI] [PubMed] [Google Scholar]
- 21.Horsley, V., B. B. Friday, S. Matteson, K. M. Kegley, J. Gephart, and G. Pavlath. 2001. Regulation of the growth of multinucleated muscle cells by an NFATc2-dependent pathway. J. Cell Biol. 153:329-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen, P. M., O. Zeitz, B. Keweloh, U. Siegel, L. S. Maier, P. Barckhausen, B. Pieske, J. Prestle, S. E. Lehnart, and G. Hasenfuss. 2000. Influence of cyclosporine A on contractile function, calcium handling, and energetics is isolated human and rabbit myocardium. Cardiovasc. Res. 47:99-107. [DOI] [PubMed] [Google Scholar]
- 23.Jones, W. K., I. L. Grupp, T. Doetschman, G. Grupp, H. Osinska, T. E. Hewett, G. Boivin, J. Gulick, W. A. Ng, and J. Robbins. 1996. Ablation of the murine alpha myosin heavy chain gene leads to dosage effects and functional deficits in the heart. J. Clin. Investig. 98:1906-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamiya, H., K. Okumura, M. Ito, Y. Saburi, T. Tomida, K. Hayashi, H. Matsui, and T. Hayakawa. 2001. Calcineurin inhibitor attenuates cardiac hypertrophy due to energy metabolic disorder. Can. J. Cardiol. 17:1292-1298. [PubMed] [Google Scholar]
- 25.Kegley, K. M., J. Gephart, G. L. Warren, and G. K. Pavlath. 2001. Altered primary myogenesis in NFATc3−/− mice leads to decreased muscle size in the adult. Dev. Biol. 232:115-126. [DOI] [PubMed] [Google Scholar]
- 26.Liew, C. C., G. Jackowski, T. Ma, and M. J. Sole. 1983. Nonenzymatic separation of myocarial cell nuclei from whole heart tissue. Am. J. Physiol. 244:C3-C10. [DOI] [PubMed] [Google Scholar]
- 27.Lim, H. W., L. J. De Windt, L. Steinberg, T. Taigen, S. A. Witt, T. R. Kimball, and J. D. Molkentin. 2000. Calcineurin expression, activation, and function in cardiac pressure-overload hypertrophy. Circulation 101:2431-2437. [DOI] [PubMed] [Google Scholar]
- 28.Liu, J., N. Koyano-Nakagawa, Y. Amasaki, F. Saito-Ohara, T. Ikeuchi, S. Imai, T. Takano, N. Arai, T. Yokota, and K. Arai. 1997. Calcineurin-dependent nuclear translocation of a murine transcription factor NFATx: molecular cloning and functional characterization. Mol. Biol. Cell 8:157-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopéz-Rodríguez, C., J. Aramburu, A. S. Rakeman, and A. Rao. 1999. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc. Natl. Acad. Sci. USA 96:7214-7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorell, B. H., and B. A. Carabello. 2000. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 102:470-479. [DOI] [PubMed] [Google Scholar]
- 31.Luo, Z., K. G. Shyu, A. Gualberto, and K. Walsh. 1998. Calcineurin and cardiac hypertrophy. Nat. Med. 10:1092-1093. [DOI] [PubMed] [Google Scholar]
- 32.Mao, Z., and M. Wiedmann. 1999. Calcineurin enhances MEF2 DNA binding activity in calcium-dependent survival of cerebellar granule neurons. J. Biol. Chem. 274:31102-31107. [DOI] [PubMed] [Google Scholar]
- 33.Masuda, E. S., Y. Naito, H. Tokumitsu, D. Campbell, F. Saito, C. Hannum, K. Arai, and N. Arai. 1995. NFATx, a novel member of the nuclear factor of activated T cells family that is expressed predominantly in the thymus. Mol. Cell. Biol. 15:2697-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meguro, T., C. Hong, K. Asai, G. Takagi, M. T. A. cKinsey, E. N. Olson, and S. F. Vatner. 1999. Cyclosporine attenuates pressure-overload hypertrophy in mice while enhancing susceptibility to decompensation and heart failure. Circ. Res. 84:735-740. [DOI] [PubMed] [Google Scholar]
- 35.Mende, U., A. Kagen, A. Cohen, J. Aramburu, F. J. Schoen, and E. J. Neer. 1998. Transient cardiac expression of constitutively active Gαq leads to hypertrophy and dilated cardiomyopathy by calcineurin-dependent and independent pathways. Proc. Natl. Acad. Sci. USA 95:13893-13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mervaala, E., D. N. Muller, J. K. Park, R. Dechend, F. Schmidt, A. Fiebeler, M. Bieringer, V. Breu, D. Ganten, H. Haller, and F. C. Luft. 2000. Cyclosporin A protects against angiotensin II-induced end-organ damage in double transgenic rats harboring human renin and angiotensinogen genes. Hypertension 35(Pt. 2):360-366. [DOI] [PubMed] [Google Scholar]
- 37.Meyer, S., N. G. Kohler, and A. Joly. 1997. Cyclosporine A is an uncompetitive inhibitor of proteasome activity and prevents NF-κB activation. FEBS Lett. 413:354-358. [DOI] [PubMed] [Google Scholar]
- 38.Molkentin, J. D., Q. Lin, S. A. Duncan, and E. N. Olson. 1997. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 11:1061-1072. [DOI] [PubMed] [Google Scholar]
- 39.Molkentin, J. D. 2000. Calcineurin and beyond: cardiac hypertrophic signaling. Circ. Res. 87:731-738. [DOI] [PubMed] [Google Scholar]
- 40.Molkentin, J. D., and G. W. Dorn II. 2001. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol. 63:391-426. [DOI] [PubMed] [Google Scholar]
- 41.Molkentin, J. D., J. R. Lu, C. L. Antos, B. Markham, J. Richardson, J. Robbins, S. R. Grant, and E. N. Olson. 1998. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murat, A., C. Pellieux, H. R. Brunner, and T. Pedrazzini. 2000. Calcineurin blockade prevents cardiac mitogen-activated protein kinase activation and hypertrophy in renovascular hypertension. J. Biol. Chem. 275:40867-40873. [DOI] [PubMed] [Google Scholar]
- 43.Øie, E. B., O. P. Reidar, F. Clausen, and H. Attramadal. 2000. Cyclosporin A inhibits cardiac hypertrophy and enhances cardiac dysfunction during postinfarction failure in rats. Am. J. Physiol. Heart Circ. Physiol. 278:2115-2123. [DOI] [PubMed] [Google Scholar]
- 44.Oukka, M., I. C. Ho, F. C. de la Brousse, T. Hoey, M. J. Grusby, and L. H. Glimcher. 1998. The transcription factor NFAT4 is involved in the generation and survival of T cells. Immunity 9:295-304. [DOI] [PubMed] [Google Scholar]
- 45.Passier, R., H. Zeng, N. Frey, F. J. Naya, R. L. Nicol, T. A. McKinsey, P. Overbeek, J. A. Richardson, S. R. Grant, and E. N. Olson. 2000. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J. Clin. Investig. 10:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng, S. L., A. J. Gerth, A. M. Ranger, and L. H. Glimcher. 2001. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity 14:13-20. [DOI] [PubMed] [Google Scholar]
- 47.Ramirez-Solis, R., R. Davis, and A. Bradley. 1993. Gene targeting in embryonic stem cells. Methods Enzymol. 225:855-878. [DOI] [PubMed] [Google Scholar]
- 48.Ranger, A. M., M. J. Grusby, M. R. Hodge, E. M. Gravallese, F. C. de la Brousse, T. Hoey, C. Mickanin, H. S. Baldwin, and L. H. Glimcher. 1998. The transcription factor NF-ATc is essential for cardiac valve formation. Nature 392:186-190. [DOI] [PubMed] [Google Scholar]
- 49.Ranger, A. M., L. C. Gerstenfeld, J. Wang, T. Kon, H. Bae, E. M. Gravallese, M. J. Glimcher, and L. H. Glimcher. 2000. The nuclear factor of activated T cells (NFAT) transcription factor NFATp (NFATc2) is a repressor of chondrogenesis. J. Exp. Med. 191:9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ranger, A. M., M. Oukka, J. Rengarajan, and L. H. Glimcher. 1998. Inhibitory function of two NFAT family members in lymphoid homeostasis and Th2 development. Immunity 9:627-635. [DOI] [PubMed] [Google Scholar]
- 51.Rao, A., C. Luo, and P. G. Hogan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707-747. [DOI] [PubMed] [Google Scholar]
- 52.Rothermel, B. A., T. A. McKinsey, R. B. Vega, R. L. Nicol, P. Mammen, J. Yang, C. L. Antos, J. M. Shelton, R. Bassel-Duby, E. N. Olson, and R. S. Williams. 2001. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 98:3328-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sah, R., G. Y. Oudit, T. T. Nguyen, H. W. Lim, A. D. Wickenden, G. J. Wilson, J. D. Molkentin, and P. H. Backx. 2002. Inhibition of calcineurin and sarcolemmal Ca2+ influx protects cardiac morphology and ventricular function in K(v)4.2N transgenic mice. Circulation 105:1850-1856. [DOI] [PubMed] [Google Scholar]
- 54.Sakata, Y., T. Masuyama, K. Yamamoto, N. Nishikawa, H. Yamamoto, H. Kondo, K. Ono, K. Otsu, T. Kuzuya, T. Miwa, H. Takeda, E. Miyamoto, and M. Hori. 2000. Calcineurin inhibitor attenuates left ventricular hypertrophy, leading to prevention of heart failure in hypertensive rats. Circulation 102:2269-2275. [DOI] [PubMed] [Google Scholar]
- 55.Shimoyama, M., D. Hayashi, Y. Zou, E. Takimoto, M. Mizukami, K. Monzen, S. Kudoh, Y. Hiroi, Y. Yazaki, R. Nagai, and I. Komuro. 2000. Calcineurin inhibitor attenuates the development and induces the regression of cardiac hypertrophy in rats with salt-sensitive hypertension. Circulation 102:1996-2004. [DOI] [PubMed] [Google Scholar]
- 56.Shimoyama, M., D. Hayashi, E. Takimoto, Y. Zou, T. Oka, H. Uozumi, S. Kudoh, F. Shibasaki, Y. Yazaki, R. Nagai, and I. Komuro. 1999. Calcineurin plays a critical role in pressure overload-induced cardiac hypertrophy. Circulation 100:2449-2454. [DOI] [PubMed] [Google Scholar]
- 57.Shimoyama, M., D. Hayashi, Y. Zou, E. Takimoto, M. Mizukami, K. Monzen, Y. Yazaki, R. Nagai, and I. Komuro. 2001. Calcineurin inhibitor attenuates the development and induces the regression of cardiac hypertrophy in rats with salt-sensitive hypertension. J. Cardiol. 37:114-118. [PubMed] [Google Scholar]
- 58.Stroud, J. C., C, Lopez-Rodriguez, A. Rao, and L. Chen. 2002. Structure of a TonEBP-DNA complex reveals DNA encircled by a transcription factor. Nat. Struct. Biol. 9:90-94. [DOI] [PubMed] [Google Scholar]
- 59.Sugimoto, T., S. Stewart, and K. L. Guan. 1997. The calcium-dependent protein phosphatase calcineurin is the major Elk-1 phosphatase. J. Biol. Chem. 272:29415-29418. [DOI] [PubMed] [Google Scholar]
- 60.Sussman, M. A., H. W. Lim, N. Gude, T. Taigen, E. N. Olson, J. Robbins, M. C. Colbert, A. Gualberto, D. F. Wieczorek, and J. D. Molkentin. 1998. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science 281:1690-1693. [DOI] [PubMed] [Google Scholar]
- 61.Taigen, T., L. J. De Windt, H. W. Lim, and J. D. Molkentin. 2000. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. USA 97:1196-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takeda, Y., T. Yoneda, M. Demura, M. Usukura, and H. Mabuchi. 2002. Calcineurin inhibition attenuates mineralocorticoid-induced cardiac hypertrophy. Circulation 105:677-679. [DOI] [PubMed] [Google Scholar]
- 63.Tian, J., and M. Karin. 1999. Stimulation of Elk1 transcriptional activity by mitogen-activated protein kinases is negatively regulated by protein phosphatase 2B (calcineurin). J. Biol. Chem. 274:15173-15180. [DOI] [PubMed] [Google Scholar]
- 64.Wang, Z., B. Nolan, W. Kutschke, and J. A. Hill. 2001. Na+-Ca2+ exchanger remodeling in pressure overload cardiac hypertrophy. J. Biol. Chem. 276:17706-17711. [DOI] [PubMed] [Google Scholar]
- 65.Wu, H., F. J. Naya, T. A. McKinsey, B. Mercer, J. M. Shelton, E. R. Chin, A. R. Simard, R. N. Michel, R. Bassel-Duby, E. N. Olson, and R. S. Williams. 2000. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber-type. EMBO J. 19:1963-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu, H., B. Rothermel, S. Kanatous, P. Rosenberg, F. J. Naya, J. M. Shelton, K. A. Hutcheson, J. M. DiMaio, E. N. Olson, R. Bassel-Duby, and R. S. Williams. 2001. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 20:6414-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xanthoudakis, S., J. P. Viola, K. T. Shaw, C. Luo, J. D. Wallace, P. T. Bozza, D. C. Luk, T. Curran, and A. Rao. 1996. An enhanced immune response in mice lacking the transcription factor NFAT1. Science 272:892-895. [DOI] [PubMed] [Google Scholar]
- 68.Xia, Y., J. B. McMillin, A. Lewis, M. Moore, W. G. Zhu, R. S. Williams, and R. E. Kellems. 2000. Electrical stimulation of neonatal cardiac myocytes activates the NFAT3 and GATA4 pathways and upregulates the adenylosuccinate synthetase 1 gene. J. Biol. Chem. 275:1855-1863. [DOI] [PubMed] [Google Scholar]
- 69.Yang, G., T. Meguro, C. Hong, K. Asai, G. Takagi, V. L. Karoor, J. Sadoshima, D. E. Vatner, S. P. Bishop, and S. F. Vatner. 2001. Cyclosporine reduces left ventricular mass with chronic aortic banding in mice, which could be due to apoptosis and fibrosis. J. Mol. Cell. Cardiol. 33:1505-1514. [DOI] [PubMed] [Google Scholar]
- 70.Youn, H.-D., T. A. Chatila, and J. O. Liu. 2000. Integration of calcineurin and MEF2 signals by the coactivator p300 during T-cell apoptosis. EMBO J. 19:4323-4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang, W., R. C. Kowal, F. Rusnak, R. A. Sikkink, E. N. Olson, and R. G. Victor. 1999. Failure of calcineurin inhibitors to prevent pressure-overload left ventricular hypertrophy in rats. Circ. Res. 84:722-728. [DOI] [PubMed] [Google Scholar]
- 72.Zou, Y., Y. Hiroi, H. Uozumi, E. Takimoto, H. Toko, W. Zhu, S. Kudoh, M. Mizukami, M. Shimoyama, F. Shibasaki, and I. Komuro. 2001. Calcineurin plays a critical role in the development of pressure overload-induced cardiac hypertrophy. Circulation 104:97-101. [DOI] [PubMed] [Google Scholar]