Abstract
The MDM2 homolog MDMX is an important regulator of p53 activity during embryonic development. MDMX inactivation in mice results in embryonic lethality in a p53-dependent fashion. The expression level of MDMX is not induced by DNA damage, and its role in stress response is unclear. We show here that ectopically expressed MDMX is mainly localized in the cytoplasm. DNA damage promotes nuclear translocation of MDMX in cells with or without p53. Coexpression of MDM2 or p53 is sufficient to induce MDMX nuclear translocation, suggesting that activation of p53 and induction of MDM2 expression can contribute to this process. Stable transfection of MDMX into U2OS cells does not alter p53 level but results in reduced p53 DNA-binding activity and reduced MDM2 expression. The ability of ARF (alternate reading frame of INK4a) to activate p53 is also significantly inhibited by expression of MDMX. These results suggest that MDMX function may be regulated by DNA damage. Furthermore, MDMX may complement MDM2 in regulating p53 during embryonic development due to its ability to inhibit p53 in the presence of ARF.
Activation of the p53 tumor suppressor after DNA damage or oncogenic signaling is an important protective mechanism that allows repair of DNA and elimination of premalignant cells by apoptosis. P53 level and activity is mainly controlled by MDM2, which is induced by p53 and promotes p53 degradation by binding and promoting p53 ubiquitination (22). DNA strand breaks activate the ATM kinase, which phosphorylates p53 and blocks MDM2 binding, leading to p53 stabilization. UV irradiation also activates p53 by inducing phosphorylation at the N- and C-terminal phosphorylation sites, as well as direct downregulation of MDM2 expression (13, 19). MDM2 expression is strongly induced after DNA damage by p53-activated transcription, which may play a role in limiting the magnitude of p53 activation and protecting cells from unnecessary apoptosis after minor damage. MDM2 expression may also prepare cells to return to normal cycling by degrading p53 after the damage is repaired.
MDMX is a recently identified homolog of MDM2 (16). MDMX shares strong homology to MDM2 at the amino acid sequence level and can bind to p53 and inhibit its transcription function in transient transfection assays. However, unlike MDM2, MDMX does not promote p53 ubiquitination or degradation (17). Furthermore, expression of MDMX is not induced by DNA damage (16). Therefore, the role of MDMX in regulating p53 and cellular response to stress is unclear. MDM2 is well established as an important regulator of p53 activity during embryonic development. Knockout of MDM2 in mice results in embryonic lethality due to hyperactivation of p53 (11). However, recent studies showed that MDMX-null mouse also dies in utero in a p53-dependent fashion, which can be rescued by crossing into the p53-null background (2, 12). Therefore, MDMX is also an important regulator of p53 during embryonic development, having a function that cannot be substituted by endogenous MDM2.
MDM2 is a nuclear-cytoplasmic shuttling protein with well-defined nuclear localization signal (NLS; residues 179 to 184) and nuclear export signal (NES; residues 191 to 205) sequences (22). MDM2 mainly accumulates in the nucleoplasm in ARF (alternate reading frame of INK4a)-deficient tumor cells and is partly nucleolar in ARF-expressing cells. Expression of ARF targets MDM2 into the nucleolus due to presence of a nucleolar localization signal in ARF and a cryptic nucleolar localization signal in the MDM2 RING domain (residues 466 to 473) (9, 21). In contrast, MDMX does not have conserved NLS and NES sequences in the corresponding region compared to MDM2. The localization of MDMX has been described in several studies, and both nuclear and cytoplasmic distribution has been reported (7, 14, 20). Since some experiments were performed with green fluorescent protein-tagged MDMX, it is unclear whether the difference was the result of modifications or cell lines. In the present study, we examined MDMX localization in cells before and after stress treatment and found that its localization is regulated by DNA damage. Both MDM2/p53-dependent and independent mechanisms play a role in regulating MDMX nuclear translocation. Furthermore, MDMX inhibits the DNA-binding function of p53 in vivo and reduces the ability of ARF to activate p53 and induce growth arrest.
MATERIALS AND METHODS
Cell lines, plasmids, and recombinant viruses.
H1299 (lung carcinoma, p53-null), U2OS (osteosarcoma, p53 wild type), and Saos2 (osteosarcoma, p53-null) cells were provided by Arnold J. Levine. MDM2/p53 double-null 174.1 primary mouse embryo fibroblasts were provided by Guillermina Lozano. Adenovirus expressing human p14ARF was kindly provided by Yue Xiong. Adenovirus expressing p53 was described previously (8). Myc-tagged human MDMX cDNA and MDMX101-490 mutant were kindly provided by Donna George (15). Restriction digest was used to retrieve the MDMX coding region and cloned into pcDNA3 vector to generate an MDMX expression plasmid without the epitope tag. The MDMX1-393 deletion mutant was generated by truncating the 3′ region of the cDNA with EcoRI. To generate MDMX polyclonal and monoclonal antibodies, the entire MDMX coding region was expressed in Escherichia coli as a His6-tagged protein, purified and used to immunize rabbits and mice. The 8C6 monoclonal antibody reacts with residue 101 to 393 region of MDMX since it binds both 1-393 and 101-490 mutants and detects mainly full-length MDMX (80 kDa) in U2OS cells. This antibody also detects a 60 kd band in primary human skin fibroblasts and H1299 cells, possibly an alternative splice form or caspase cleavage product of MDMX (3).
Adenovirus infections.
Recombinant adenovirus expressing ARF or p53 was amplified by using 293 cells. The titer of the crude lysate was determined by serial dilution and detection of cytopathic effects on 293 cells in 96-well plates. Cells were infected with 12 to 50 PFU/cell. MDM2 protein levels were determined 18 to 24 h after addition of the viruses. Fluorescence-activated cell sorting (FACS) analysis was performed 16 to 24 h after viral infection.
Western blot and immunoprecipitation.
Cells were lysed in radioimmunoprecipitation assay buffer (1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100, 50 mM Tris [pH 7.4], 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride), and 10 to 50 μg of protein was fractionated by SDS-polyacrylamide gel electrophoresis and transferred to Immobilon-P filters (Millipore). The filter was blocked for 1 h with phosphate-buffered saline (PBS) containing 5% nonfat dry milk, 0.1% Tween 20 and then incubated for 1 h with 3G9 (MDM2), DO-1 (p53), and 8C6 (MDMX) in PBS containing 5% nonfat dry milk. Bound primary antibody was detected by incubating for 1 h with horseradish peroxidase-goat anti-mouse immunoglobulin G (IgG). The filter was developed by using the ECL-Plus reagent (Amersham). For immunoprecipitation-Western blot analysis, cells were lysed in lysis buffer (50 mM Tris-HCl [pH 8.0], 5 mM EDTA, 150 mM NaCl, 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride), and 300 to 1,000 μg of protein was immunoprecipitated with p53 or MDM2 antibodies and protein A-Sepharose beads (Sigma) for 4 h at 4°C. The beads were washed with lysis buffer, and the immunoprecipitate was fractionated by SDS-polyacrylamide gel electrophoresis. MDMX was detected by Western blot with 8C6.
Immunofluorescence staining.
Cells cultured on chamber slides were fixed with acetone-methanol (1:1) for 3 min at room temperature, blocked with PBS plus 10% normal goat serum (PBS+10% NGS) for 20 min, and incubated with anti-p53 Pab1801 hybridoma supernatant (1:10 dilution), anti-MDM2 2A9 hybridoma supernatant (1:100 dilution), and anti-MDMX 8C6 hybridoma supernatant (1:100 dilution) in PBS+10%NGS for 2 h. The slides were washed with PBS+0.1% Triton X-100, incubated with fluorescein isothiocyanate-goat anti-mouse IgG in PBS+10% NGS for 1 h, washed with PBS+0.1% Triton X-100, and mounted. For double staining of MDMX and MDM2, cells were incubated with a mixture of 1:5,000 rabbit-anti-MDMX serum and 1:100 2A9 anti-MDM2 antibody, followed by incubation with fluorescein isothiocyanate-goat anti-rabbit IgG and rhodamine-goat anti-mouse IgG. For FACS analysis of MDMX-expressing cells, cells were treated with trypsin, fixed, and stained in suspension by using 8C6 antibody and propidium iodide.
Chromatin immunoprecipitation and quantitative PCR.
Chromatin immunoprecipitation was carried out by using a published procedure (1). P53 immunoprecipitation was performed with DO-1 antibody, and MDMX immunoprecipitation was performed with a mixture of two monoclonal antibodies, 8C6 and 10G11. Coprecipitated DNA was analyzed by real-time PCR for the presence of p53-binding element from MDM2 intron 1 with the following primers and probes: human MDM2 forward primer (5′-GTGGCGATTGGAGGGTAGAC), human MDM2 reverse primer (5′-GTCGGTGCTTACCTGGATCAG), human MDM2 probe (6FAM-CACGGACGCACGCC-MGBNFQ; Applied Biosystems), DHFRF forward primer (5′-TCGCCTGCACAAATAGGGAC), DHFRR reverse primer (5′-AGAACGCGCGGTCAAGTTT), and DHFR probe (6FAM-GGGCGGCCACAATTTCGCG-MGBNFQ). Real-time PCR analysis was carried out by the Molecular Biology Core of H. Lee Moffitt Cancer Center.
RESULTS
Nuclear translocation of MDMX after DNA damage.
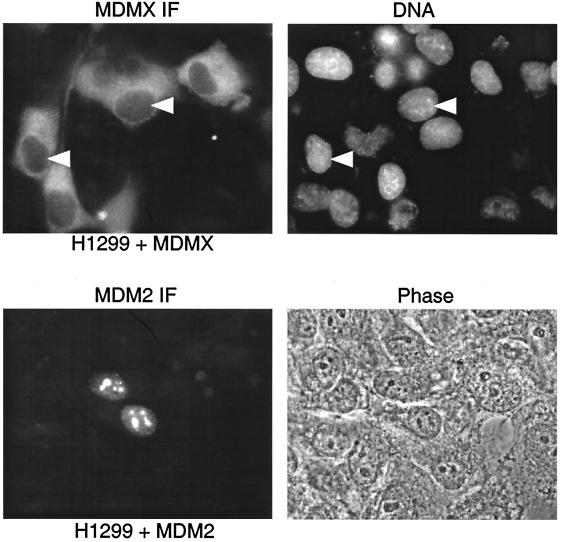
Although MDMX has significant sequence homology to MDM2, it does not have the characteristic NLS at the same region as MDM2. Different laboratories have described localization of MDMX in the cytoplasm as M2-tagged protein (14) or in the nucleus as V5 or green fluorescent protein-tagged proteins (6, 7). We examined the localization of MDMX in several cell lines after transient or stable transfection. We observed predominantly cytoplasmic localization of myc-tagged MDM2 in H1299, U2OS, and Saos2 cells. For example, in H1299 cells, transient transfection of myc-MDMX resulted in strong cytoplasmic staining of MDMX with anti-myc antibody, whereas transfected MDM2, as well as endogenous MDM2, was mainly localized in the nucleolus (Fig. 1).
FIG. 1.
Localization of MDMX and MDM2. H1299 cells were transiently transfected with human MDM2 or myc epitope-tagged human MDMX and stained with anti-MDM2 antibody or anti-myc antibody. Nuclei were indicated by DAPI (4′,6′-diamidino-2-phenylindole) staining of DNA. Nucleoli of MDM2-transfected cells were identified by phase-contrast photography.
To eliminate the possibility that MDMX localization may be affected by the attached epitope, we generated rabbit polyclonal antiserum and monoclonal antibody against full-length MDMX. An untagged version of MDMX was expressed in H1299 and U2OS cells by transient or stable transfection and stained with the MDMX-specific antibodies. The results were identical with the myc-tagged MDMX (see Fig. 5 and 7). Therefore, our results show that MDMX is a protein that mainly localized in the cytoplasm of unstressed cells, a finding distinct from that of MDM2.
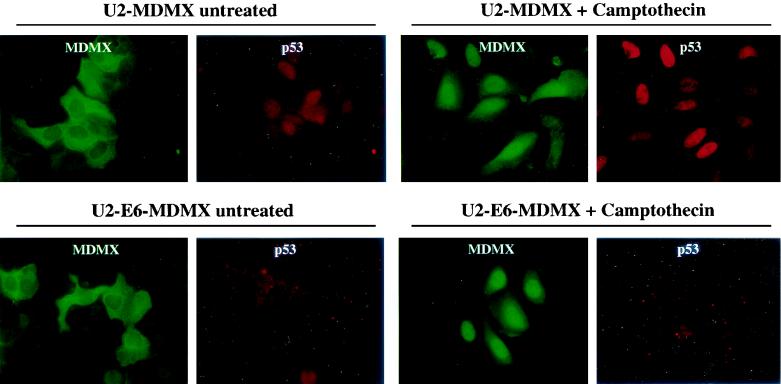
FIG. 5.
Nuclear translocation of MDMX in U2OS independent of p53 and MDM2 induction. U2OS cells expressing HPV16 E6 were stably transfected with untagged MDMX plasmid. Pooled MDMX transfectants were treated with 0.5 μM camptothecin for 18 h and stained for p53 and MDMX expression by double fluorescence staining with Pab1801 anti-p53 antibody and rabbit anti-MDMX serum.
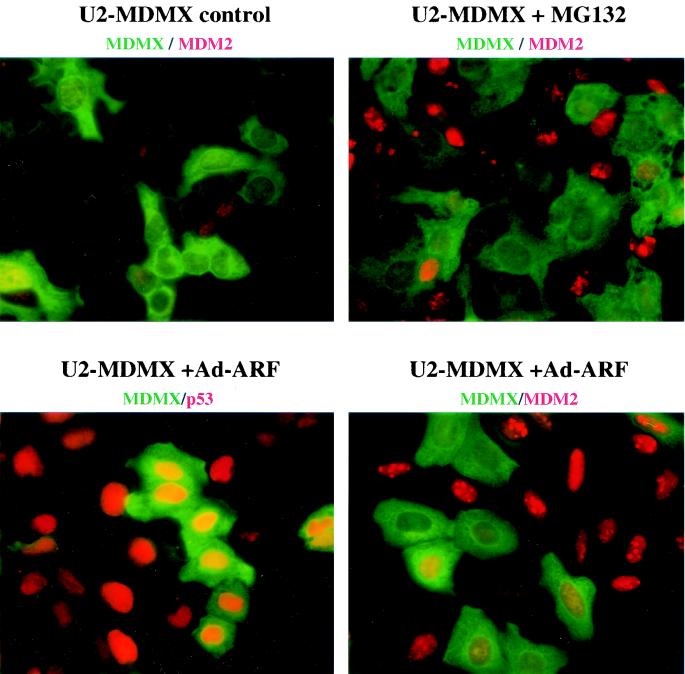
FIG. 7.
Expression of MDMX inhibits p53 activation and MDM2 induction by ARF. Pooled U2OS cells stably transfected with un-tagged MDMX were infected with 50 PFU/cell of Ad-ARF for 24 h or treated with 30 μM MG132 for 4 h to inhibit MDM2 degradation. Cells were double stained with rabbit anti-MDMX serum and monoclonal antibodies against p53 or MDM2. MDMX was stained green, and MDM2 or p53 were stained red.
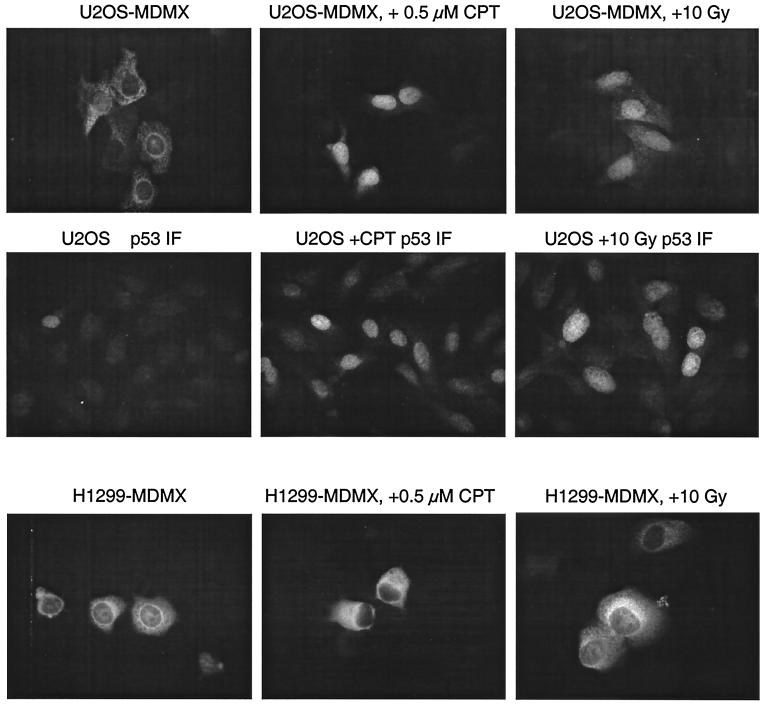
MDMX expression is not induced by DNA damage (16). To determine whether MDMX localization is regulated by DNA damage response, we treated cell lines stably transfected with MDMX with DNA-damaging drugs camptothecin, etoposide, and gamma radiation. Immunofluorescence staining of MDMX showed that in p53-null H1299 cells, MDMX remained mostly in the cytoplasm after DNA damage. However, in p53-wild-type U2OS cells, there was significant nuclear transfer of MDMX (Fig. 2). Staining of treated cells with anti-p53 antibody Pab1801 showed induction of p53 accumulation, confirming the DNA damage response induced by the treatments.
FIG. 2.
Induction of MDMX nuclear translocation by DNA damage. U2OS and H1299 cells stably transfected with myc-tagged MDMX were treated with 0.5 μM camptothecin or 10 Gy of gamma radiation for 18 h. The localization of MDMX was determined by staining with anti-myc antibody. P53 was stained with Pab1801 antibody to confirm response to DNA damage.
MDM2 and p53 expression induces MDMX nuclear entry.
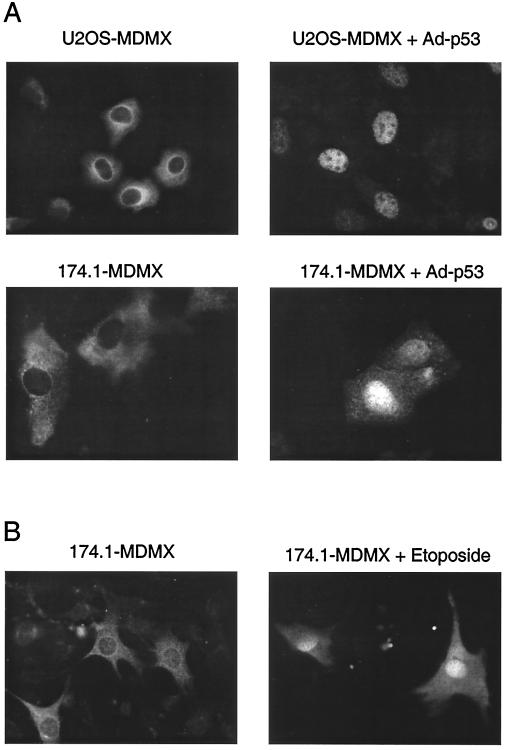
Since MDMX in the p53-null H1299 cells did not translocate to the nucleus after DNA damage, we examined whether increased MDM2 and p53 expression after DNA damage was responsible for the nuclear targeting of MDMX by cotransfecting MDMX with MDM2 or p53 in H1299 cells. The result showed that coexpression of MDM2 efficiently targeted MDMX into the nucleus independent of p53 (Fig. 3A). Transfection with p53 also targeted MDMX into the nucleus in H1299 cells (data not shown). Infection of U2OS cells stably expressing MDMX with adenovirus expressing wild-type p53 also induced MDMX nuclear entry (Fig. 4A). To determine whether the effect of p53 expression also requires induction of MDM2, the experiment was performed with 174.1 cells null for both p53 and MDM2. P53 was still capable of inducing MDMX nuclear entry in this cell line (Fig. 4A), suggesting that p53 alone can mediate MDMX nuclear entry. In 174.1 cells, the p53-14Q19S mutant deficient for MDM2 binding did not induce MDMX nuclear entry (8) (data not shown), indicating that complex formation between MDMX and p53 was required for MDMX nuclear entry in the absence of MDM2. Therefore, MDM2 and p53 can each target MDMX nuclear translocation.
FIG. 3.
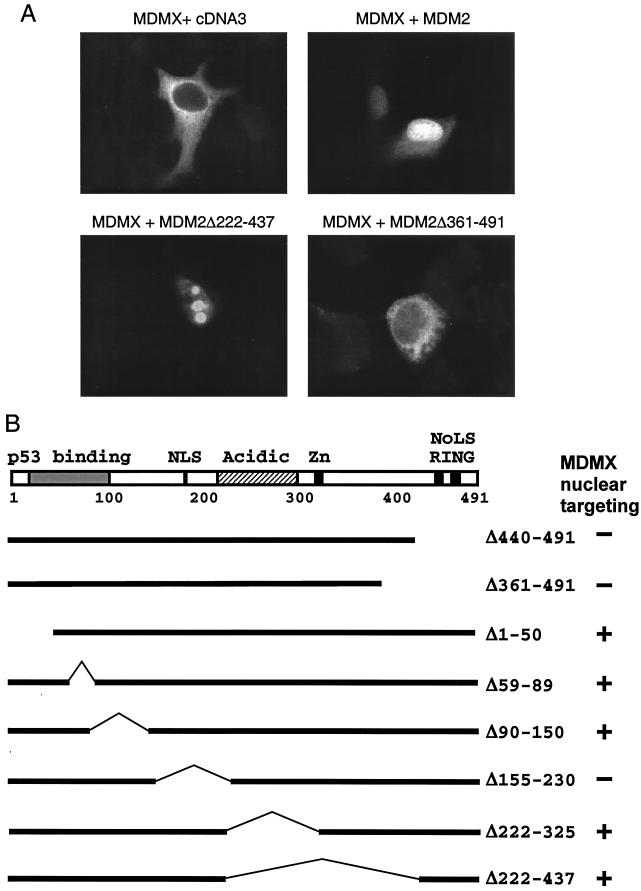
Induction of MDMX nuclear translocation by MDM2. (A) H1299 cells were transiently cotransfected with MDMX and MDM2 mutants. At 48 h after transfection, cells were stained for MDMX localization with anti-myc antibody. (B) Diagram of MDM2 mutants tested and summary of results.
FIG. 4.
Induction of MDMX nuclear translocation by p53 and p53-independent mechanisms. (A) U2OS (p53-wild type) and 174.1 (MDM2/p53-null mouse embryo fibroblasts) stably transfected with MDMX were infected with adenovirus expressing wild-type p53. At 24 h after infection, MDMX was stained with anti-myc antibody. (B) MDM2/p53-null 174.1 cells stably transfected with MDMX were treated with 70 μM etoposide for 24 h, and MDMX was stained with anti-myc antibody.
MDMX interacts with the N terminus of p53 and the RING finger of MDM2 (15, 18). To determine whether complex formation with MDM2 or p53 is required for nuclear entry, MDM2 mutants were coexpressed with MDMX in H1299 cells. As expected, MDM2 mutants deficient for nuclear entry due to deletion of the NLS signal or without the RING finger failed to target MDMX into the nucleus (Fig. 3). Although a significant amount of transfected MDM2 localized to the nucleolus in transfected H1299 cells, we did not observe MDMX in the nucleolus in cotransfection with full-length MDM2, suggesting that MDM2 does not target MDMX into the nucleolus. The only exception is the Δ222-437 MDM2 mutant, which localized to the nucleolus without ARF and also targeted MDMX into the nucleolus (Fig. 3A). These results suggest that MDMX is targeted to the nucleus by forming complexes with MDM2 or p53. The nuclear localization function of MDM2 is important for mediating nuclear entry of the MDM2-MDMX complex.
MDMX nuclear accumulation after DNA damage or by MDM2 and p53 expression may be due to increased nuclear import or reduced nuclear export of shuttling MDMX. When H1299 or U2OS transfected with MDMX was treated with leptomycin B to block nuclear export, no nuclear accumulation of MDMX was observed (data not shown), suggesting that it did not shuttle into the nucleus at a significant rate. Therefore, nuclear accumulation after DNA damage or by MDM2 and p53 expression is due to nuclear targeting by forming complexes with nuclear-entry-competent p53 and MDM2.
MDMX nuclear translocation independent of p53 and MDM2.
The behavior of MDMX in H1299 and U2OS cells described above appeared to indicate that p53 is required for MDMX nuclear translocation. However, transfection of MDMX into p53/MDM2 double-null 174.1 cells, which were a mixture of primary embryo fibroblasts revealed that a small fraction of transfected cells contained nuclear MDMX (data not shown). DNA damage treatment also induced significant nuclear translocation of MDMX in 174.1 cells (Fig. 4B). This suggested that there is also cell type-dependent, p53/MDM2-independent mechanism of MDMX nuclear entry. Therefore, we wanted to determine whether nuclear translocation of MDMX in U2OS after DNA damage is completely dependent on p53.
We created U2OS cell lines stably expressing human papillomavirus type 16 (HPV16) E6 protein by infection with an E6 retrovirus expression vector. An U2OS-E6 cell line that showed greatly reduced p53 expression and no induction of p53 and MDM2 after DNA damage was then stably transfected with MDMX expression vector. Treatment of the U2OS-E6-MDMX cells showed that MDMX nuclear translocation occurred in this cell line as efficiently as in U2OS-MDMX cells, despite lack of p53 and MDM2 induction after DNA damage (Fig. 5). Therefore, in addition to p53 and MDM2, MDMX nuclear transport after DNA damage can be mediated by other factors in certain cell types.
MDMX inhibits p53 DNA binding and MDM2 expression.
MDMX has been shown to inhibit p53 transcription activity, stabilize MDM2, and inhibit p53 degradation by MDM2 (6, 15, 16). These observations were made by using transient coexpression of MDMX with MDM2 and p53. To determine whether expression of MDMX in stably transfected cells affect p53 and MDM2 expression, clonal cell lines of U2OS stably expressing MDMX were established.
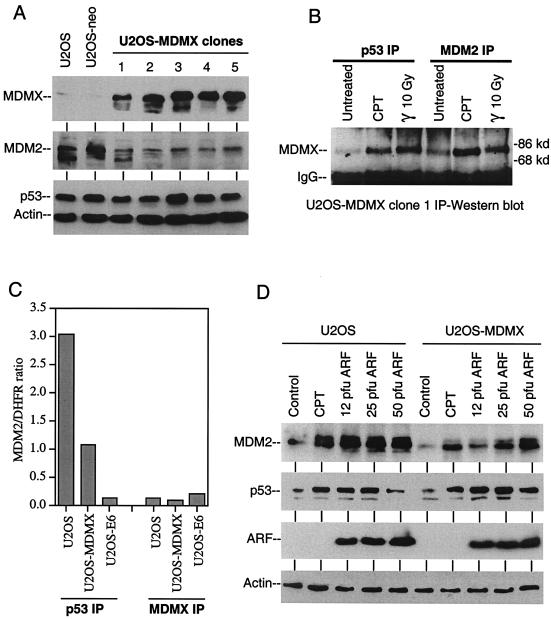
Although MDMX has been shown to stabilize MDM2 and p53, we did not detect increased MDM2 and p53 levels in cells expressing MDMX (Fig. 6A). On the contrary, MDMX-positive cell lines expressed significantly lower levels of MDM2, suggesting that the transcription function of p53 may be inhibited by MDMX. Consistent with this interpretation, immunoprecipitation of p53 from the cell lines also coprecipitated MDMX (Fig. 6B). The amount of MDMX-p53 complex also increased after DNA damage possibly due to increase in p53 level, suggesting that MDMX can interfere with p53 function by forming complexes in the absence or presence of stress.
FIG. 6.
Inhibition of MDM2 expression and p53 DNA binding by MDMX. (A) Clonal cell lines of U2OS expressing MDMX were analyzed for MDMX, MDM2, and p53 expression by Western blot. (B) Complex formation between MDMX, p53, and MDM2 were detected by immunoprecipitation (IP) with p53 antibody Pab1801 and MDM2 antibody 2A9, followed by Western blotting with MDMX antibody 8C6. (C) Chromatin immunoprecipitation (IP) analysis of p53 DNA binding. Cells were cross-linked with formaldehyde and chromatin fragments were precipitated with anti-p53 or anti-MDMX antibodies. The amount of precipitated p53 binding site in MDM2 intron 1 was quantitated by real-time PCR and normalized to the amount of nonspecifically precipitated DHFR promoter in the same sample. The results were averages of triplicate reactions. (D) Effects of DNA damage (0.5 μM camptothecin, 18 h) and ARF expression (Ad-ARF infection for 18 h at indicated PFU/cell) on MDM2 and p53 levels in U2OS and an U2OS-MDMX stable cell line.
Since the p53 protein level did not change in MDMX-expressing cells, we tested whether MDMX inhibited the ability of p53 to bind DNA in vivo by using chromatin immunoprecipitation assay. The ability of p53 to interact with the p53 binding site in MDM2 intron 1 was tested by quantitative TaqMan PCR of immunoprecipitates with p53 antibody and MDMX antibody. The results showed that expression of MDMX caused a threefold inhibition of p53 DNA-binding activity (Fig. 6C). As a positive control, expression of HPV E6 caused a 30-fold reduction in p53 DNA binding due to degradation of p53. Immunoprecipitation of MDMX did not result in the specific capture of p53 DNA-binding site, suggesting that the MDMX-p53 complex did not bind DNA. Therefore, the expression of MDMX may inhibit p53 by forming complexes that do not bind DNA.
Western blot analysis also showed that MDMX expression did not prevent p53 accumulation after DNA damage or ectopic expression of ARF by recombinant adenovirus infection. However, the ability of these treatments to induce MDM2 expression was reduced in MDMX-transfected cells (Fig. 6D). Therefore, MDMX expression may inhibit the activation of p53 after stress despite its normal accumulation. This is further tested below.
MDMX prevents p53 activation by ARF.
MDMX-expressing cells showed normal ability to accumulate p53 but reduced MDM2 expression after DNA damage (Fig. 6D), suggesting that it is able to inhibit p53 function after DNA damage. However, we did not observe changes in cell cycle distribution after gamma irradiation or camptothecin treatment when we compared cells that were positive or negative for MDMX expression (data not shown). It is possible that these DNA damage treatments affect cell cycle progression through multiple mechanisms, which obscure the effect of MDMX.
To test whether MDMX regulates another important p53 response pathway mediated by ARF, U2OS cells stably expressing MDMX were infected with recombinant adenovirus expressing ARF at concentrations sufficient to achieve ARF expression in >90% cells. At 24 h after infection, cells were analyzed by double fluorescence staining to determine the level of p53 and MDM2 in MDMX-positive and MDMX-negative cells. The results showed that MDMX expression did not prevent induction of p53 by ARF (Fig. 7), which was consistent with Western blot results (Fig. 6D). However, the ability of ARF to induce MDM2 was significantly reduced in MDMX-positive cells compared to adjacent MDMX-negative cells. Staining of U2OS-MDMX pools also confirmed the reduced expression level of MDM2 in MDMX-expressing cells in the absence of stress. Although the staining of basal level MDM2 was too weak to determine whether there was a difference between MDMX-positive and negative cells, inhibition of MDM2 degradation by MG132 for 4 h revealed significantly less MDM2 accumulation in MDMX-positive cells (Fig. 7). Therefore, the rate of MDM2 synthesis was reduced in MDMX-positive cells, which was consistent with results described in Fig. 6.
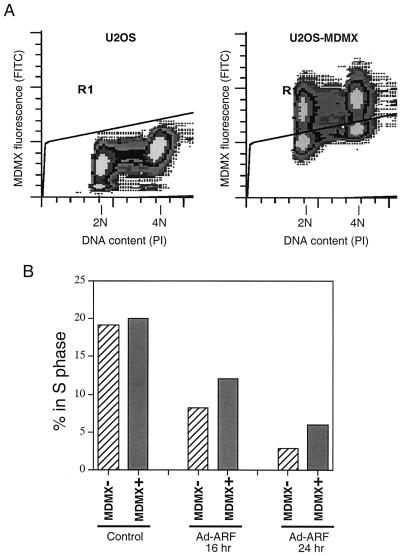
To determine whether MDMX expression reduced the ability of ARF to induce growth arrest, pooled U2OS-MDMX stable transfectants were infected with ARF virus and stained for MDMX expression and DNA. The cell cycle profiles were determined for the MDMX-positive cells and MDMX-negative cells in the same infected population. The results show that infection with ARF virus caused strong reduction of S phase population in MDMX-negative cells. MDMX-positive cells also had a reduction of S cells, but this population was still 50 to 100% higher than that of the MDMX-negative cells (Fig. 8). Therefore, MDMX prevents ARF activation of p53 and partially prevents inhibition of cell proliferation induced by ARF.
FIG. 8.
MDMX expression reduces growth arrest by ARF. U2OS cells stably transfected with untagged MDMX were infected with Ad-ARF virus at 50 PFU/cell. At indicated times after infection, cells were stained for MDMX expression and DNA content and analyzed by FACS. (A) FACS profile of U2OS and U2OS-MDMX pool. Cells above (MDMX positive) and below (MDMX negative) the artificial gate were quantitated for cell cycle distribution. (B) The percentage of cells in the S phase in MDMX-expressing cells were compared to MDMX-negative cells after Ad-ARF infection. Expression of MDMX partially protected cells from growth arrest by ARF.
DISCUSSION
Although MDMX shares sequence homology to MDM2 and plays an important role in regulating p53, the signals and molecules that regulate MDMX are still poorly defined. The results described above show that the subcellular localization of MDMX is regulated by DNA damage from inhibitors of topoisomerase 1 and 2 and from gamma irradiation. Since MDMX expression level is not induced by DNA damage, change of localization may be an important mechanism by which MDMX is regulated in the cell. Nuclear accumulation of MDMX after DNA damage appears to be due to de novo nuclear entry. Inhibition of nuclear export with leptomycin B in the absence of damage does not lead to nuclear accumulation of MDMX, suggesting that MDMX does not shuttle between the nucleus and cytoplasm in the absence of stress.
MDMX does not have conserved NLS or NES sequence, this is consistent with its largely cytoplasmic localization in unstressed cells. Nuclear translocation after DNA damage would require interaction with other nuclear proteins. Our results show that both MDM2 and p53 can induce MDMX nuclear entry through complex formation with MDMX. Two studies published during the course of this work also showed that MDM2 induces MDMX nuclear translocation (4, 10). This may be part of the mechanism by which MDMX is targeted to the nucleus, since DNA damage induces significant expression of MDM2 and p53. However, MDMX nuclear translocation after DNA damage can occur in MDM2/p53 double-null mouse embryo fibroblasts and in U2OS cells after p53 and MDM2 induction is eliminated by expression of HPV E6. Therefore, proteins other than MDM2 and p53 may also bind to MDMX after DNA damage and mediate its nuclear translocation.
MDMX has been shown to inhibit the ubiquitin ligase function of MDM2, which leads to stabilization of MDM2 and p53 in transient-transfection experiments (5, 15, 17). However, we did not observe stabilization of MDM2 or p53 in U2OS cells stably expressing ectopic MDMX. The reason for this discrepancy is still unclear. We speculate that this may be due to the establishment of a new balance between MDM2 and MDMX in stable cell lines. MDMX appears to stimulate the ability of MDM2 to degrade p53 under certain conditions (4), which may be responsible for maintaining normal p53 turnover in U2OS-MDMX cells expressing a reduced level of MDM2. Expression of MDMX did not prevent p53 accumulation after DNA damage but appeared to prevent its full activation. Therefore, MDMX overexpression and nuclear translocation should also have an impact on the function of p53 pathway after DNA damage, although further analysis will be required to identify a clear biological effect due to MDMX expression.
Previous studies showed that MDMX inhibits p53 transcription function in transient-transfection and reporter assays (16). We found that this activity of MDMX is very weak compared to MDM2 in transient-transfection assays. However, stable expression of MDMX in U2OS cells clearly showed a decrease in MDM2 expression, which correlated with decreased p53 binding to DNA in vivo. Furthermore, MDMX-expressing cells had reduced ability to activate p53 after expression of ARF, although p53 level rises normally after ARF expression. The ability of ARF to induce growth arrest was also partially inhibited in MDMX-expressing cells. These results suggest that MDMX may be relatively resistant to the inhibitory effect of ARF. This may be due to the fact that MDMX does not function as an E3 ligase but by directly binding and inhibiting p53 DNA binding. This inhibitory effect may not be directly reversed by ARF, since ARF mainly acts by inhibiting p53 ubiquitination by MDM2. This difference may allow MDMX to play an important role in controlling p53 activity during embryonic development, when MDM2 is sensitive to ARF due to strong mitogenic signaling and cell proliferation.
The MDMX knockout mouse can be rescued by inactivation of p53, suggesting that the major function of MDMX during development is to regulate p53. However, its ability to translocate to the nucleus in the absence of p53 and MDM2 induction suggests that it interacts with other molecules after DNA damage and may have additional roles in DNA damage response. Identification of such putative proteins may shed light on the mechanism and function of MDMX regulation. The ability of MDMX to inhibit p53 activation by ARF may also contribute to tumor development by attenuating p53 response to abnormal mitogenic signaling.
Acknowledgments
We thank Donna George for the MDMX constructs which made our work possible. We also thank Martha Marlow of Princeton University for generating MDMX monoclonal antibodies and the Molecular Biology Core and Flow Cytometry Core of Moffitt Cancer Center for real-time PCR and FACS analyses.
This work was supported by grants from the American Cancer Society (RSG CNE-102445) and National Institutes of Health (CA88406) to J.C.
REFERENCES
- 1.Boyd, K. E., J. Wells, J. Gutman, S. M. Bartley, and P. J. Farnham. 1998. c-Myc target gene specificity is determined by a post-DNA binding mechanism. Proc. Natl. Acad. Sci. USA 95:13887-13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finch, R. A., D. B. Donoviel, D. Potter, M. Shi, A. Fan, D. D. Freed, C. Y. Wang, B. P. Zambrowicz, R. Ramirez-Solis, A. T. Sands, and N. Zhang. 2002. mdmx is a negative regulator of p53 activity in vivo. Cancer Res. 62:3221-3325. [PubMed] [Google Scholar]
- 3.Gentiletti, F., F. Mancini, M. D'Angelo, A. Sacchi, A. Pontecorvi, A. G. Jochemsen, and F. Moretti. 2002. MDMX stability is regulated by p53-induced caspase cleavage in NIH 3T3 mouse fibroblasts. Oncogene 21:867-877. [DOI] [PubMed] [Google Scholar]
- 4.Gu, J., H. Kawai, L. Nie, H. Kitao, D. Wiederschain, A. G. Jochemsen, J. Parant, G. Lozano, and Z. M. Yuan. 2002. Mutual dependence of MDM2 and MDMX in their functional inactivation of p53. J. Biol. Chem. 277:19251-19254. [DOI] [PubMed] [Google Scholar]
- 5.Jackson, M. W., and S. J. Berberich. 2000. MdmX protects p53 from Mdm2-mediated degradation. Mol. Cell. Biol. 20:1001-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson, M. W., and S. J. Berberich. 2002. MdmX protects p53 from Mdm2-mediated degradation. Mol. Cell. Biol. 20:1001-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson, M. W., M. S. Lindstrom, and S. J. Berberich. 2002. MdmX binding to ARF affects Mdm2 protein stability and p53 transactivation. J. Biol. Chem. 276:25336-25341. [DOI] [PubMed] [Google Scholar]
- 8.Lin, J., X. Jin, C. Page, V. K. Sondak, G. Jiang, and R. K. Reynolds. 2001. A modified p53 overcomes mdm2-mediated oncogenic transformation: a potential cancer therapeutic agent. Cancer Res. 60:5895-5901. [PubMed] [Google Scholar]
- 9.Lohrum, M. A., M. Ashcroft, M. H. Kubbutat, and K. H. Vousden. 2001. Identification of a cryptic nucleolar-localization signal in MDM2. Nat. Cell Biol. 2:179-181. [DOI] [PubMed] [Google Scholar]
- 10.Migliorini, D., D. Danovi, E. Colombo, R. Carbone, P. G. Pelicci, and J. C. Marine. 2002. Hdmx recruitment into the nucleus by Hdm2 is essential for its ability to regulate p53 stability and transactivation. J. Biol. Chem. 277:7318-7723. [DOI] [PubMed] [Google Scholar]
- 11.Oca Luna, R. M., D. S. Wagner, and G. Lozano. 1995. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378:203-206. [DOI] [PubMed] [Google Scholar]
- 12.Parant, J., A. Chavez-Reyes, N. A. Little, W. Yan, V. Reinke, A. G. Jochemsen, and G. Lozano. 2002. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat. Genet. 29:92-95. [DOI] [PubMed] [Google Scholar]
- 13.Prives, C., and P. A. Hall. 1999. The p53 pathway. J. Pathol. 187:112-126. [DOI] [PubMed] [Google Scholar]
- 14.Rallapalli, R., G. Strachan, B. Cho, W. E. Mercer, and D. J. Hall. 1999. A novel MDMX transcript expressed in a variety of transformed cell lines encodes a truncated protein with potent p53 repressive activity. J. Biol. Chem. 274:8299-8308. [DOI] [PubMed] [Google Scholar]
- 15.Sharp, D. A., S. A. Kratowicz, M. J. Sank, and D. L. George. 1999. Stabilization of the MDM2 oncoprotein by interaction with the structurally related MDMX protein. J. Biol. Chem. 274:38189-38196. [DOI] [PubMed] [Google Scholar]
- 16.Shvarts, A., W. T. Steegenga, N. Riteco, T. van Larr, P. Dekker, M. Bazuine, R. C. A. van Ham, W. V. D. H. van Oordt, G. Hateboer, A. J. van der Eb, and A. G. Jochemsen. 1996. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J. 15:5349-5357. [PMC free article] [PubMed] [Google Scholar]
- 17.Stad, R., N. A. Little, D. P. Xirodimas, R. Frenk, A. J. van der Eb, D. P. Lane, M. K. Saville, and A. G. Jochemsen. 2002. Mdmx stabilizes p53 and Mdm2 via two distinct mechanisms. EMBO J. 2:1029-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanimura, S., S. Ohtsuka, K. Mitsui, K. Shirouzu, A. Yoshimura, and M. Ohtsubo. 1999. MDM2 interacts with MDMX through their RING finger domains. FEBS Lett. 447:5-9. [DOI] [PubMed] [Google Scholar]
- 19.Wu, L., and A. J. Levine. 1997. Differential regulation of the p21/WAF-1 and mdm2 genes after high-dose UV irradiation: p53-dependent and p53-independent regulation of the mdm2 gene. Mol. Med. 3:441-451. [PMC free article] [PubMed] [Google Scholar]
- 20.Yam, C. H., W. Y. Siu, T. Arooz, C. H. Chiu, A. Lau, X. Q. Wang, and R. Y. Poon. 1999. MDM2 and MDMX inhibit the transcriptional activity of ectopically expressed SMAD proteins. Cancer Res. 59:5075-5078. [PubMed] [Google Scholar]
- 21.Zhang, Y., and Y. Xiong. 1999. Mutations in human ARF exon 2 disrupt its nucleolar localization and impair its ability to block nuclear export of MDM2 and p53. Mol. Cell 3:579-591. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, Y., and Y. Xiong. 2001. Control of p53 Ubiquitination and nuclear export by MDM2 and ARF. Cell Growth Differ. 12:175-186. [PubMed] [Google Scholar]