Abstract
This is the first study reporting the inactivation of a member of the mouse gene family of toxin-related ecto-ADP-ribosyltransferases (ARTs). Transfer of the ADP-ribose moiety from NAD onto extracellular arginine residues on T-cell membrane proteins is mediated by glycosylphosphatidylinositol-linked cell surface ARTs. Exposure of T cells to ecto-NAD blocks T-cell activation and induces T-cell apoptosis. To determine a possible role of ecto-ART2.1 and ART2.2 in these processes, we generated ART2.1/ART2.2 double-knockout mice. ART2-deficient mice were healthy and fertile and showed normal development of lymphoid organs. ART2-deficient T cells showed a dramatically reduced capacity to ADP-ribosylate cell surface proteins, indicating that most if not all ART activity on the T-cell surface can be attributed to the ART2s. Moreover, ART2-deficient T cells were completely resistant to NAD-induced apoptosis and partially resistant to NAD-mediated suppression of proliferation. These results demonstrate that the ART2 ectoenzymes are an essential component in the regulation of T-cell functions by extracellular NAD, e.g., following release of NAD upon lysis of cells in tissue injury and inflammation.
Posttranslational modification of proteins provides an important mechanism for regulating cellular functions. Protein phosphorylation mediated by protein kinases is well established as an essential component of intracellular signal transduction. Mounting evidence suggests that protein ADP-ribosylation mediated by ADP-ribosyltransferases (ARTs) may serve a similar regulatory function in the extracellular environment (10, 18, 32).
We have previously reported the molecular cloning and biochemical characterization of a family of glycosylphosphatidylinositol (GPI)-anchored and secretory mammalian cell surface mono(ADP-ribosyl)transferases (ART1 to ART5) which are related in structure and function to bacterial ADP-ribosylating toxins (20, 21). These enzymes catalyze the transfer of the ADP-ribose moiety of NAD onto a specific amino acid residue of a target protein, while the nicotinamide moiety is released.
In the context of the immune system, treatment of lymphocytes with ART inhibitors or the ART substrate NAD profoundly affects cellular functions, including clustering of T-cell receptors, proliferation, cytotoxicity, and in vivo homing (26, 29, 36). Recently, we and others have shown that extracellular NAD can induce apoptosis of naïve T cells in vitro and in vivo (1, 25). NAD-induced T-cell apoptosis shares certain features with the classic death receptor-induced pathway of apoptosis, including exposure of phosphatidylserine on the outer leaflet of the plasma membrane, followed by fragmentation of DNA and, ultimately, failure to exclude the DNA-staining dye propidium iodide (1). However, in contrast to death receptor-induced apoptosis, NAD-induced apoptosis cannot be blocked with caspase inhibitors and affects naïve cells more severely than activated T cells. Thus, exposure to NAD may constitute an alternative or complementary mechanism to triggering of death receptors for inducing apoptosis of distinct T-cell subsets.
A number of T-cell surface proteins, including LFA-1, CD8, CD44, and CD45, have been identified as target proteins of ADP-ribosylation (28, 29, 35). Removal of GPI-anchored surface proteins by treatment of cells with phosphatidylinositol-specific phospholipase C renders T cells resistant to the suppressive effects of ecto-NAD. Moreover, strong activation signals, e.g., stimulation of protein kinase C by treatment of cells with phorbol esters, induce metalloprotease-catalyzed shedding of ART activity from the T-cell surface (14, 27). Consequently, recently activated T cells are relatively resistant to the suppressive effects of ecto-NAD (14, 27).
So far, the molecular identity of the T-cell surface ART(s) has not been clarified. Mouse chromosome 7 contains a cluster of genes encoding four ecto-ARTs (ART1, ART2.1, ART2.2, and ART5), all of which have been implicated in affecting T-cell functions (6, 23, 26, 30, 31). The tandem Art2a and Art2b genes encode the GPI-anchored cell surface enzymes ART2.1 and ART2.2, respectively, which have 80% sequence identity. The overlapping Art1 and Art5 genes encode the GPI-anchored and secretory enzymes ART1 and ART5, respectively, which have 40% sequence identity to each other and 30% identity to the ART2s (2, 6, 12).
We have recently developed the ART2.2-specific monoclonal antibody Nika102 (19). With Nika102, we demonstrated that expression of ART2.2 is developmentally regulated during postnatal ontogeny and that cell surface ART2.2 is expressed by mature T cells but not by B cells (19). Moreover, reverse transcription (RT)-PCR analyses indicate that Art2a but not Art1 or Art5 is coexpressed with Art2b in peripheral T cells.
In this study, we used a genetic approach to inactivate ART2.1 and ART2.2. With our findings, we establish that the ART2 ectoenzymes are an essential component for the regulation of T-cell functions by extracellular NAD. The ART2-deficient animals described here provide important tools to further elucidate the function of these intriguing ectoenzymes.
MATERIALS AND METHODS
Mice.
Mice were obtained from the Animal Resources Units of the University of California at San Francisco and the University Hospital, Hamburg.
Construction of Art2a and Art2b targeting vectors and generation and genotyping of knockout mice.
PCR primers CVC (TTC CAT GGA ACR GCT TTA GTT C, where R denotes A or G) and CRC (CAT AGC CTG GAA TTA ACA CCT CCT C) derived from Art2a cDNA (17) were used to screen a 129/Sv mouse genomic library and isolate three positive genomic clones (Genome Systems, clones 3691, 3692, and 3693). Southern blot, subcloning, and sequence analysis demonstrated that one of these, 3691, contained only Art2b, and the other two contained both Art2 genes. A targeting vector for the Art2a locus was constructed in which the main coding exon of Art2a was replaced by the neomycin resistance gene under control of the phosphoglycerate kinase (PGK) promoter and in which the 3′ homology arm was flanked by the herpesvirus thymidine kinase gene under the control of the PGK promoter. As a 3′ homolog, a 2.4-kb fragment was flanked by EcoRI sites via PCR mutagenesis and was cloned in the EcoRI site of pKSNT (kindly provided by W. Wurst, Toronto) (34). The plasmid was opened by HindIII digestion, and a 2.7-kb SacI-HindIII fragment was inserted as a 5′-end homolog.
A targeting vector for the Art2b gene was constructed which replaced the main coding exon with the hygromycin resistance gene under the control of the PGK promoter. As a 5′ homolog, a 3.9-kb SalI-HindIII fragment was cloned into the respective sites of pPGKhygro. As a 3′ homolog, a 1.4-kb fragment was flanked by EcoRI sites via PCR mutagenesis and was cloned in the EcoRI site. The plasmid was reopened by XbaI digestion, and a 4-kb XbaI fragment was inserted to extend the 3′ arm.
129/Ola-derived J1 embryonic stem (ES) cells were transfected with the Art2a targeting vector by electroporation (25 μg of SalI-linearized plasmid/2 × 107 cells), grown under double selection (200 μg of G418 and 0.5 μg of ganciclovir per ml) and screened by Southern blot analyses with standard methods (13). Art2a-targeted ES cells were expanded, retransfected with the Art2b construct (25 μg of NotI-linearized plasmid/2 × 107 cells), and grown under double selection (200 μg of G418 and 150 μg of hygromycin per ml). Targeted ES cells and mice carrying the mutant alleles were identified by Southern blot analyses with probes specific to genomic regions up- or downstream of the targeted regions (12, 20).
Chimeric mice were produced by injection of targeted ES cells into 3.5-day-old blastocysts according to standard techniques (11). Germ line transmission of mutant alleles was confirmed by Southern blot analyses, and mice carrying the Art2 mutations were mated with C57BL/6J or BALB/c/ByJ mice. Inheritance of wild-type and mutant alleles was followed by PCR analyses on genomic DNA obtained from tail biopsies with pairs of primers specific for wild-type or mutant alleles: for Art2a, F13 (CAT CCA CAG AGC CTT AAT GAG) × F43 (CTA AGC TGC TAA CGT TGT CTG C) (wild type), and F12b (CGG TAT CGC CGC TCC CGA TTC) × F43 (mutant), and for Art2b, F14 (TTG GAA AGA GAT CAA AAA CAG TAC) × F44 (CTC TCT TTG TTA AAG ATG AAG AAC T) (wild type), and Hy1f (GTG CTT TCA GCT TCG ATG TAG G) and Hy2r (CAC AGC CAT CGG TCC AGA CG) (ART2−/−). All experiments were performed with ART2-deficient (ART2−/−) and wild-type (+/+) littermates obtained from brother-sister matings of +/− animals.
RT-PCR analyses.
RNA was isolated from lymph node cells with RNeasy columns (Qiagen, Hilden, Germany) and subjected to RT-PCR analyses with primers CVA and CRE as described previously (22).
Histological analyses.
Tissue specimens were fixed in 4% formalin containing 1% acetic acid and embedded in paraffin. Deparaffinated sections were stained with hematoxylin and eosin, periodic acid-Schiff reaction, and Giemsa staining according to standard protocols.
Antibodies and immunofluorescence analysis.
Single-cell suspensions from thymus, spleen, and lymph nodes were prepared and processed for flow cytometry on a FACScan (Becton Dickinson) as described previously (9, 19). ART2.2-specific monoclonal antibody Nika102 (rat immunoglobulin G2a [IgG2a]) was produced from a gene gun-immunized rat as described previously (19). Mouse monoclonal antibody 1G4 (IgG2a) specific for etheno-adenosine (37) was kindly provided by Regina Santella (Columbia University, New York, N.Y.). Other monoclonal antibodies used in this study for immunofluorescence staining and activation assays include anti-CD3e (145-2C11), anti-CD4 (GK1.5), anti-CD8 (53-6.72), anti-CD25 (3C7), anti-CD28 (37.51) anti-CD38 (90), and anti-CD69 (H1.2F3). Biotin, phycoerythrin, and fluorescein isothiocyanate conjugates were purchased from PharMingen. GK1.5 (anti-CD4) was labeled with indocarbocyanine as described previously (19). Apoptotic and necrotic cells were stained with annexin V-fluorescein isothiocyanate conjugate and propidium iodide as described previously (1).
Stimulation of T cells and proliferation assays.
T cells were prepared from lymph node cell suspensions by depleting B cells by magnetic cell separation with Dynabead-immobilized goat anti-mouse IgG (Dynal, Hamburg, Germany) (4 to 6 beads/cell). T cells (5 × 105 cells/ml) were then cultured in 96- or 24-well plates coated with anti-CD3 (at 1 μg/ml in phosphate-buffered saline) in RPMI medium containing the indicated amounts of soluble anti-CD28 or interleukin-2. Cells were incubated at 37°C for the indicated times and then subjected either to immunofluorescence analyses or incubated with [3H]thymidine for 8 h prior to harvesting and determination of incorporated label (TopCount; Packard).
RESULTS
Generation of ART2.1/ART2.2−/− mice.
The ART2.1 and ART2.2 ecto-ADP-ribosyltransferases exhibit 80% amino acid sequence identity and are encoded by two closely linked genes on mouse chromosome 7 (12, 34). Since the two ectoenzymes are coordinately expressed by T cells and could be functionally redundant, we sequentially inactivated both genes in ES cells with the strategy schematically illustrated in Fig. 1. Integration of the targeting vectors into the mouse genome by homologous recombination resulted in replacement of the major coding exons of Art2a and Art2b by the neomycin and hygromycin resistance genes, respectively (Fig. 1 and 2A). ES cells carrying the mutant alleles were identified by Southern blot analyses (Fig. 2B), and cells from clone 8 were used to generate the ART2.1/ART2.2 double-knockout mouse line. Homozygous null animals were recovered in the F2 generation with the expected Mendelian frequencies. ART2-deficient mice were viable and fertile and did not show any overt phenotype. Since ART2-deficient animals could not be identified among littermates by observation alone, we developed a PCR screening assay which permitted genotyping from tail biopsies (Fig. 2C).
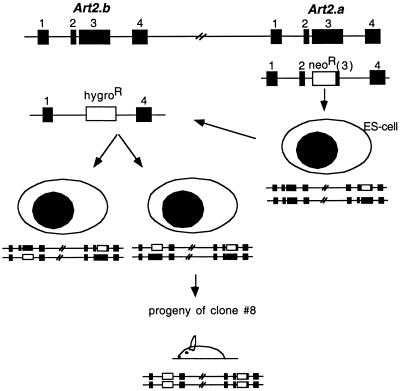
FIG. 1.
Schematic illustration of the targeting strategy to generate ART2.1/ART2.2 double-knockout mice. The Art2 gene locus is shown on top, with exons depicted as boxes. ES cells were first transfected with a targeting construct in which the major coding exon of Art2a was replaced with a neomycin resistance cassette. Homologous recombination at the target locus resulted in ES cells heterozygous for the wild-type and Art2a mutant alleles (right). These cells were retransfected with a targeting construct in which the major coding exon of Art2b was replaced with a hygromycin resistance cassette. Homologous recombination at the target locus resulted either in ES cells heterozygous for wild-type and Art2a/Art2b double mutant alleles (center) or in ES cells heterozygous for Art2a and Art2b single mutant alleles (left). Progeny from matings of chimeric mice derived from doubly targeted ES clone 8 with C57BL/6J mice revealed cosegregation of the mutant alleles, yielding the ART2.1/ART2.2 double-knockout line.
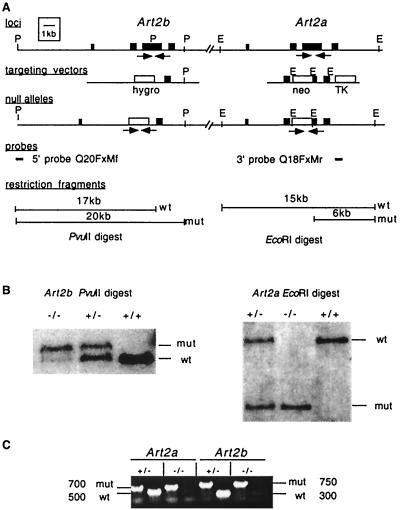
FIG. 2.
Disruption of the Art2a and Art2b genes in ES cells and mice. (A) Schematic representation of the Art2 locus, the Art2a and Art2b targeting vectors, and the targeted Art2 alleles. Filled boxes indicate exons, and labeled boxes indicate the neomycin (neo) resistance, hygromycin (hygro) resistance, and herpesvirus thymidine kinase (TK) cassettes. Homologous recombination of the targeting vectors with the endogenous Art2a and Art2b genes deletes most of the main coding exons. Relevant restriction sites are abbreviated as follows: P, PvuII; E, EcoRI. (B) Detection of targeted and wild-type Art2a and Art2b alleles by Southern blot analyses of genomic DNAs obtained from progeny derived from a heterozygous mating. DNA (5 μg per lane) was digested with PvuII (left panel) or EcoRI (right panel), blotted onto nitrocellulose membranes, and hybridized with the probes indicated by solid lines in panel A. The Art2b 5′ probe detects a 17-kb PvuII fragment from the endogenous Art2b locus and a 20-kb fragment from the targeted locus; the Art2a 3′ probe detects a 15-kb EcoRI fragment from the endogenous Art2a locus and a 6-kb fragment from the targeted locus. (C) Detection of targeted and wild-type Art2a and Art2b alleles by PCR analyses of genomic DNAs obtained from tail biopsies of progeny derived from a heterozygous mating. DNA (50 to 200 ng per lane) was subjected to PCR amplification with pairs of primers as indicated by arrows in panel A. These primers amplify a 500-bp fragment from the endogenous Art2a locus and a 700-bp fragment from the targeted locus, and a 350-bp fragment from the Art2b locus and a 750-bp fragment from the targeted locus.
ART2−/− mice show normal development of the major lymphocyte subsets.
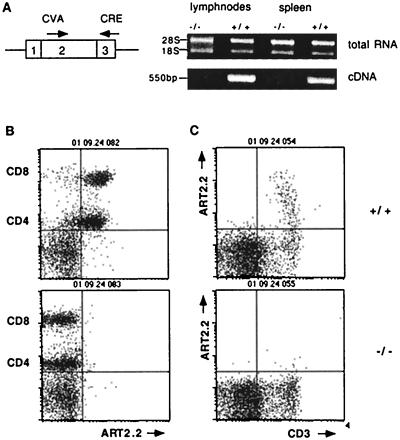
Reverse transcription-PCR analyses of RNA isolated from purified lymph node T cells confirmed that transcripts for Art2.1 and Art2.2 were present in wild-type but not ART2-deficient mice (Fig. 3A). To further demonstrate that the mutations introduced into the Art2.1 and Art2.2 genes resulted in loss of expression of these genes, thymus and lymph node cells obtained from wild-type and ART2-deficient mice were compared by fluorescence-activated cell sorting analyses for expression of cell surface ART2.2 with monoclonal antibody Nika102 (19) (Fig. 3B and C). In wild-type mice, expression of ART2.2 is developmentally regulated; in the thymus, this antigen is expressed only on a small subset of mature (CD3hi) thymocytes (Fig. 3C), whereas in lymph nodes, it is expressed on the majority of both CD4+ and CD8+ T-cell subsets (Fig. 3B). The results confirm that ART2 double-knockout mice lack expression of ART2.1 and ART2.2.
FIG. 3.
T cells from ART2 double-knockout mice do not express Art2a or Art2b. (A) RNA was prepared from lymph node cells obtained from 8-week-old wild-type, ART2.1/ART2.2−/−, and heterozygous (+/−) mice and assessed for the presence of Art2 gene transcripts by RT-PCR analyses. The pair of primers used in these analyses were derived from separate exons and amplify both Art2 loci. Lymph node cells (B) and thymocytes (C) were obtained from ART2.1/ART2.2−/− mice and wild-type littermates. Thymocytes were assessed for cell surface expression of ART2.2 and CD3 by fluorescence-activated cell sorting analyses with antibodies labeled with appropriate fluorochromes. Lymph node cells were triple stained for expression of CD4, CD8, and ART2.2. Note that use of indodicarbocyanine-conjugated anti-CD4 and brighter staining phycoerythrin-conjugated anti-CD8 permits simultaneous analysis of CD4+ T-helper and CD8+ cytotoxic T cells (19). The results shown were obtained with 6-week-old female mice from the first backcross to B6. Results shown are representative of four separate and independent experiments.
Histological analyses showed normal development of brain, lung, liver, kidney, intestine, pancreas, thyroid, and salivary glands. ART2-deficient mice had thymuses, spleens, lymph nodes, and Peyer's patches of normal size and architecture and did not show any detectable histological abnormalities. Fluorescence-activated cell sorting analyses did not show any significant differences between wild-type and ART2-deficient counterparts in the major lymphocyte subsets of thymus, spleen, and lymph nodes (Table 1).
TABLE 1.
Distribution of lymphocyte subsetsa
| Cells | Marker(s) | Mean % positive ± SD
|
|
|---|---|---|---|
| Wild-type mice | ART2-deficient mice | ||
| Thymocytes | CD4− CD8− | 2 ± 1 | 2 ± 1 |
| CD4+ CD8+ | 83 ± 6 | 81 ± 4 | |
| CD4+ CD8− | 7 ± 2 | 8 ± 3 | |
| CD4− CD8+ | 3 ± 2 | 2 ± 1 | |
| Lymph node | CD4+ | 40 ± 10 | 43 ± 3 |
| CD8+ | 28 ± 2 | 28 ± 2 | |
| CD3+ | 73 ± 8 | 77 ± 3 | |
| sIg+ | 22 ± 6 | 19 ± 3 | |
| CD25+ | 2 ± 0 | 3 ± 1 | |
| CD38+ | 27 ± 5 | 30 ± 4 | |
| CD69+ | 3 ± 1 | 4 ± 1 | |
Thymocytes and lymph node cells were obtained from ART2.1/ART2.2−/− mice and wild-type littermates and assessed for cell surface expression of CD3, CD4, CD8, CD25, CD38, CD69, and surface immunoglobulin (sIg). Values are mean percentages of marker-positive cells with standard deviations for three mice at 6 to 10 weeks of age.
Unperturbed upregulation of activation antigens following activation of ART2-deficient T cells.
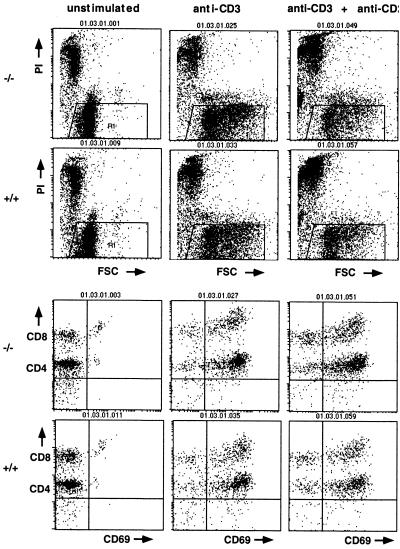
Since expression of ART2 is restricted to T cells, we tested the response of purified ART2-deficient T cells and their wild-type counterparts to traditional T-cell activation regimens, i.e., T-cell receptor triggering by immobilized anti-CD3 antibodies and further cultivation in the absence or presence of anti-CD28 antibodies or interleukin-2. The results shown in Fig. 4 and Table 2 demonstrate that ART2-deficient T cells responded normally to these treatments. Like their wild-type counterparts, ART2-deficient T cells underwent blast transformation, as evidenced by an increased forward scatter (Fig. 4). Moreover, they upregulated cell surface expression of the activation antigens CD69 and CD25 (Fig. 4 and Table 3).
FIG. 4.
Peripheral T cells from ART2.1/ART2.2−/− mice respond normally to T-cell receptor triggering with blast transformation and upregulation of activation antigens. T cells were obtained from ART2.1/ART2.2−/− mice and wild-type littermates and cultured for 24 h on plates lacking (left) or containing immobilized anti-CD3 antibodies alone (center) or in combination with soluble anti-CD28 antibodies (right). Blast transformation was assessed by forward side scatter analyses and upregulation of CD69 activation antigens. The results shown were obtained with 13-week-old female mice from the first backcross to BALB/c mice. Results shown are representative of three separate and independent experiments.
TABLE 2.
ART-deficient T cells efficiently upregulate activation antigens CD69 and CD25 upon T-cell receptor triggering even in the presence of added NADa
| Cells | NAD added | % Positive cells
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Unstimulated
|
Anti-CD3
|
Anti-CD3 + anti-CD28
|
Anti-CD3 + interleukin-2
|
||||||
| +/+ | −/− | +/+ | −/− | +/+ | −/− | +/+ | −/− | ||
| CD69+ | − | 3.5 | 5.5 | 84.9 | 82.1 | 78.1 | 80.1 | 88.6 | 79.8 |
| + | 8.1 | 7.0 | 36.7 | 58.9 | 32.6 | 58.5 | 38.5 | 63.1 | |
| CD25+ | − | 7.7 | 8.8 | 62.2 | 61.7 | 57.2 | 62.2 | 69.2 | 52.2 |
| + | 8.0 | 8.1 | 9.1 | 14.6 | 13.2 | 17.6 | 17.0 | 31.0 | |
T cells (5 × 105/ml of RPMI medium) were incubated for 24 h in the presence or absence of 50 μM NAD before staining as in Fig. 4. Numbers indicate percentages of marker-positive cells. +/+, wild-type mice; −/−, ART2-deficient mice.
TABLE 3.
Enhanced survival of ART2-deficient T cells in the presence of exogenous NADa
| NAD added | % Vital cells
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Unstimulated
|
Anti-CD3
|
Anti-CD3 + anti-CD28
|
Anti-CD3 + interleukin-2
|
|||||
| +/+ | −/− | +/+ | −/− | +/+ | −/− | +/+ | −/− | |
| − | 65.8 | 73.1 | 60.4 | 72.1 | 25.4 | 47.2 | 18.9 | 20.4 |
| + | 23.9 | 56.6 | 27.5 | 68.6 | 11.7 | 46.9 | 8.9 | 30.9 |
Cells (5 × 105/ml of RPMI medium) were incubated for 24 h in the presence or absence of 50 μM NAD before staining as in Fig. 4. Dead cells were stained with propidium iodide. Numbers indicate percentages of vital cells. +/+, wild-type mice; −/−, ART2-deficient mice.
Lack of cell surface ADP-ribosylation activity on ART2-deficient T cells.
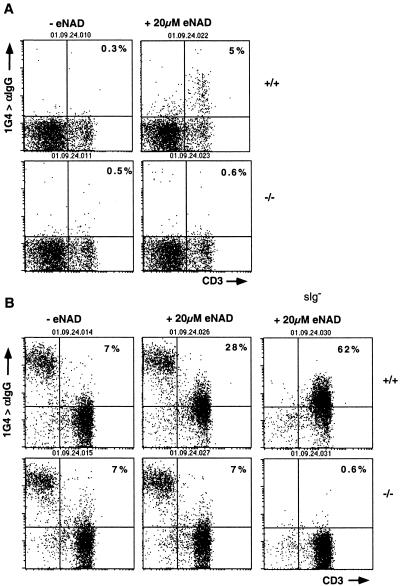
A hallmark of the ART2.1 and ART2.2 ectoenzymes is their capacity to catalyze ADP-ribosylation of other cell surface proteins upon exposure of cells to extracellular NAD (1, 14). We have recently developed a convenient method for monitoring ADP-ribosylation of T-cell surface proteins with etheno-NAD, an NAD analogue which carries an etheno group on the adenosine moiety, and 1G4, a monoclonal antibody specific for etheno-adenosine (14). We used this assay to assess cell surface ADP-ribosylation activity on T cells of wild-type and ART2-deficient animals (Fig. 5). The results revealed a dramatic reduction in cell surface ADP-ribosyltransferase activity to background levels on both ART2-deficient thymocytes (Fig. 5A) and ART2-deficient peripheral T cells (Fig. 5B) compared to their wild-type counterparts. These findings indicate that the ART2 ectoenzymes represent the major ART activity on T cells.
FIG. 5.
T cells from ART2.1/ART2.2−/− mice show a dramatically reduced capacity to ADP-ribosylate cell surface proteins. Thymic (A) and lymph node (B) cells were obtained from ART2.1/ART2.2−/− mice and wild-type littermates. Cells were incubated for 30 min without (left) or with 20 μM etheno-NAD (center and right). Cells were then washed and stained with etheno-adenosine-specific mouse monoclonal antibody 1G4, followed by phycoerythrin-conjugated anti-mouse IgG and phycoerythrin-conjugated anti-CD3. Note that lymph node B cells are stained by the anti-mouse IgG antibody even in the absence of etheno-NAD. As observed previously (19), B cells do not show any increased staining with 1G4 after treatment with etheno-NAD. Panels on the right in B show staining obtained with surface Ig-depleted lymph node cells. Numbers indicate the percentage of cells in the upper right quadrant. The results shown were obtained with 6-week-old female mice from the first backcross to B6 mice. Results shown are representative of six separate and independent experiments.
ART2-deficient T cells are less sensitive to the suppressive effects of extracellular NAD.
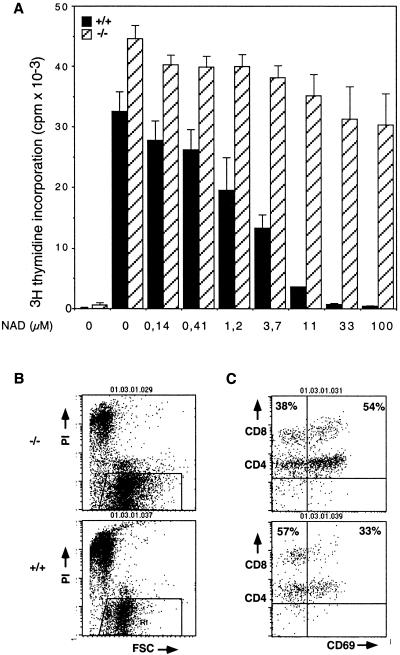
Exposure of T cells in vitro to extracellular NAD has previously been shown to suppress T-cell functions, including cell activation and proliferation (1, 25, 29). We therefore analyzed the effects of exposing wild-type T cells and their ART2-deficient counterparts to increasing amounts of extracellular NAD. As illustrated in Fig. 6, the proliferation of wild-type T cells in response to T-cell receptor triggering was markedly suppressed in the presence of increasing concentrations of exogenous NAD. In contrast, ART2-deficient T cells were much less sensitive to inhibition of proliferation by ecto-NAD (Fig. 6A). Moreover, ecto-NAD strongly inhibited upregulation of the activation antigens CD69 and CD25 by wild-type T cells in response to T-cell receptor engagement, but much less so in the case of ART2-deficient T cells (Fig. 6B and Table 2).
FIG. 6.
T cells from ART2.1/ART2.2−/− mice are less sensitive to suppressive effects of NAD on proliferation (A), blast transformation (B), and upregulation of cell surface antigens (C). (A) ART2−/− and wild-type T cells were cultured for 48 h on plates containing immobilized anti-CD3 antibodies (1 μg/ml), soluble anti-CD28 antibodies (1 μg/ml), and the indicated concentrations of NAD. Cells were then incubated with [3H]thymidine for 8 h, harvested, and assayed for DNA-incorporated radioactivity as an indicator of cell proliferation. Columns on the very left represent control cells which were cultured without stimulating antibodies. (B and C) ART2−/− and wild-type T cells were cultured for 24 h with antibodies in the presence of 50 μM NAD on plates containing immobilized anti-CD3. Blast transformation and expression of CD69 were assessed as in Fig. 4. Numbers indicate the percentage of cells in the upper left and right quadrants. The results shown were obtained with 8-week-old mice from the third backcross to BALB/c mice (A) and with 13-week-old mice from the first backcross to BALB/c mice (B and C). Results shown are representative of four separate and independent experiments.
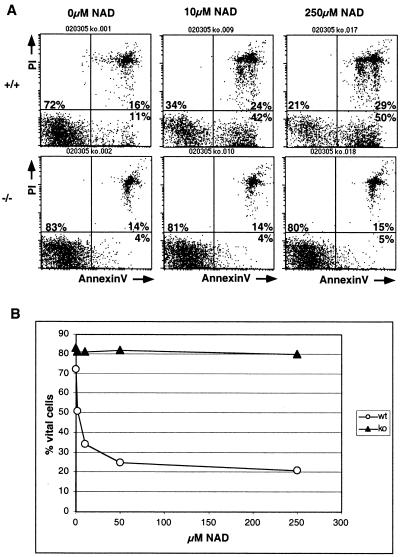
Comparison of living and dead cells in NAD-treated cultures revealed that ART2-deficient T cells survived much better in the presence of NAD than their wild-type counterparts (Table 3). The finding that ART2−/− T cells were better protected at higher NAD concentrations from the suppressive effects of NAD than at lower concentrations (Fig. 7) and that the induction of CD69 and of CD25 by stimulation with anti-CD3, anti-CD28, and interleukin-2 is also inhibited in ART2−/− T cells (Table 3) could point to an acceptor for NAD in addition to ART2.
FIG. 7.
T cells from ART2.1/ART2.2−/− mice are resistant to NAD-induced apoptosis. ART2.1/ART2.2−/− and wild-type T cells were treated with the indicated concentrations of extracellular NAD for 30 min at 37°C. Cells were then washed and stained with annexin V-fluorescein isothiocyanate and propidium iodide. Numbers in A indicate the percentage of cells in the lower left, lower right, and upper right quadrants. (B) Plot of vital cells (lower left quadrant) versus NAD concentration. The results shown were obtained with 6-week-old male mice from the fourth backcross to BALB/c mice. Results shown are representative of six separate and independent experiments.
ART2-deficient T cells are resistant to NAD-induced apoptosis.
The suppressive effects of ecto-NAD on T cells are mediated at least in part by NAD-induced T-cell apoptosis (1, 25). As an early sign of NAD-triggered apoptosis, wild-type T cells expose phosphatidylserine within minutes after exposure to ecto-NAD and thus can be stained with annexin V (Fig. 7A, top panels). In striking contrast to their wild-type counterparts, ART2-deficient T cells did not expose phosphatidylserine even upon prolonged exposure to high concentrations of ecto-NAD (Fig. 7A, lower panels). These results demonstrate that ART2-deficient T cells are completely resistant to NAD-induced exposure to phosphatidylserine and uptake of propidium iodide. Note that cell death was not demonstrated in the assays used here. Also note that cell preparations from wild-type animals consistently contained a small number of annexin V-binding cells even in the absence of added exogenous NAD, whereas preparations from ART2-deficient animals contained few if any annexin V-binding cells (e.g., Fig. 7A, left panels).
DISCUSSION
This is the first study reporting the inactivation of a member of the mouse gene family for the toxin-related ecto-ADP-ribosyltransferases. The results reported here provide new insights into the role of these ectoenzymes in regulating T-cell functions. Moreover they establish an important experimental model to further elucidate the function of these intriguing ectoenzymes.
ART2-deficient animals are vital and fertile and show normal development of the major lymphocyte subsets (Table 1). In these aspects, ART2-deficient mice resemble a growing number of mouse lines made genetically deficient for other lymphocyte cell surface proteins, such as CD2, CD38, CD134, and BP-1 (4, 15, 16, 24, 33). Interestingly, the responses of ART2-deficient T cells to NAD differ strikingly from those of their wild-type counterparts (Tables 2 and 3, Fig. 5 to 7).
A number of previous reports demonstrated that exposure of murine T cells to exogenous NAD suppresses T-cell functions and that these effects are mediated at least in part by ecto-ADP-ribosyltransferases (1, 29, 35). The results of the present study (Fig. 5) demonstrate that ART2.1 and ART2.2 account for most if not all of the ART activity on the surface of thymic and peripheral lymph node T cells.
Recent studies have provided evidence that the suppressive effects of extracellular NAD on T-cell functions are mediated at least in part by apoptosis induced upon ADP-ribosylation of cell surface proteins, raising the hypothesis that ecto-NAD may provide an alternative to traditional mechanisms for inducing T-cell apoptosis (1, 25). The results of this study lend support to this notion. Moreover, the finding that ART2-deficient T cells are resistant to NAD-induced exposure of phosphatidylserine, uptake of propidium iodide (Fig. 7), and fragmentation of DNA (results not shown) demonstrates that these effects of NAD are mediated by ART2.1 and/or ART2.2. Since cell death was not demonstrated in the assays used here, our findings do not necessarily imply that NAD is not able to induce cell death in ART2−/− T cells, and further studies are necessary to prove this point.
In contrast to NAD-triggered T-cell apoptosis, some of the other suppressive effects of ecto-NAD, i.e., on T-cell proliferation (Fig. 6A) and upregulation of activation antigens (Fig. 6B, Tables 2 and 3), cannot be attributed entirely to ART2.1 and ART2.2, since these T-cell functions are still affected to some extent by NAD in ART2-deficient animals. It is conceivable that these residual effects of ecto-NAD on T-cell proliferation and activation antigen expression in ART2 knockout mice is mediated by another acceptor for NAD, e.g., other ARTs or other NAD-catabolizing ectoenzymes.
Related gene family members can sometimes compensate fully or in part for the loss of a paralogous gene(s). The other known Art gene family members (Art1, Art3, Art4, and Art5) are preferentially expressed in other nonlymphoid tissues. Their gene products show only 28 to 40% amino acid sequence identity to ART2.1 and ART2.2 (2, 6, 30, 31, 38). The lack of detectable ART activity on thymocytes and lymph node cells of ART2-deficient mice (Fig. 5) indicates that these cells do not express significant levels of other ARTs. However, it is conceivable that other ARTs might become activated in specialized lymphoid compartments or during stages of T-cell differentiation not assessed in our experiments. Furthermore, it is not unlikely that ART2.1 and ART2.2, which exhibit 80% amino acid sequence identity, are functionally redundant. ART2.1 and ART2.2 single-knockout mice and mice deficient in other ARTs may shed light on these issues.
It is important to note that other lymphocyte cell surface enzymes catabolizing extracellular NAD have been described. These include the CD38 family of NAD-dependent ADP-ribosylcyclases and the PC-1 family of phosphodiesterases, both of which can hydrolyze NAD (5, 7, 8). It is possible that the residual suppressive effects of NAD on ART2-deficient T cells (Fig. 6) are mediated by NAD-catabolites of these enzymes (e.g., nicotinamide and ADP-ribose or cyclic ADP-ribose in the case of CD38, AMP and nicotinamide mononuculeotide in the case of PC-1). ART2 knockout mice can now be intercrossed with available knockout mice for other NAD-metabolizing ectoenzymes to address this question.
It still remains to be established under what conditions NAD is released from cells in vivo in order to serve as substrate for ecto-ARTs. While intracellular NAD levels are in the range of 0.1 to 0.2 mM, the concentration of NAD in extracellular fluids, e.g., in serum, is 100-fold lower, i.e., in the lower micromolar range. The controlled release of NAD from cells expressing connexin 43, a member of the gap junction protein family which can form NAD-specific channels (3), could cause extracellular NAD concentrations to rise locally. Moreover, bursts of high local extracellular NAD levels presumably occur upon lysis of cells, e.g., in areas of inflammation and tissue injury.
The results of our experiments indicate that wild-type T cells are exquisitely sensitive to such perturbations in the levels of extracellular NAD, with membrane-bound ART2 ectoenzymes acting as a kind of NAD sensor. These enzymes catalyze ADP-ribosylation of other cell surface proteins upon exposure of cells to ecto-NAD, resulting in an NAD concentration-dependent inhibition of cell proliferation and an NAD concentration-dependent activation of cellular apoptosis. ART2-deficient cells, in contrast, evidently lack these control mechanisms.
It will be of great interest to determine how this regulatory defect affects immune functions in vivo, e.g., upon challenge of the immune system by infection, tissue damage, and inflammation, as well as in the control of autoimmune reactions. The ART2-deficient mouse line described here provides a unique experimental model with which to address these questions. In particular, T-cell responses during trauma and inflammatory situations should be investigated in ART2−/− mice and compared to those in wild-type mice.
Acknowledgments
F.K.-N. and N.K., who designed and supervised this study, share senior authorship.
We thank Martina Matthes, Maren Kühl, Marion Nissen, Vivienne Welge, Katrin Bartels, Dunja Freese, Felix Scheuplein, UKE, Hamburg; Christiane Steeg, BNI, Hamburg; and Jason Dietrich, Ling Ling Wang, and Leonid Pravoverov, University of California at San Francisco, for technical assistance. F.K.-N. and W.O. thank the laboratory staffs of the Litmann and Killeen laboratories for generous hospitality during their stays as visiting scientists at the University of California at San Francisco. F.K.-N. cloned the targeting constructs and isolated the targeted ES cells, N.K. performed the blastocyst injections, J.L. performed the histological examinations, and W.O. performed the RT-PCR, fluorescence-activated cell sorting analyses, and proliferation assays. F.H., J.L., M.S., and D.L. made essential contributions to the study design. F.K.-N. wrote the paper. Parts of the work described in this study represent the partial fulfillment of the requirements for the graduate thesis of W.O. We thank Bernhard Fleischer, Hamburg, and Edward Leiter, Bar Harbor, Maine, for helpful discussions and critical reading of the manuscript.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 545 B9 to F.K.-N. and F.H.).
REFERENCES
- 1.Adriouch, S., W. Ohlrogge, F. Haag, F. Koch-Nolte, and M. Seman. 2001. Rapid induction of naive T-cell apoptosis by ecto-nicotinamide adenine dinucleotide: requirement for mono(ADP-ribosyl)transferase 2 and a downstream effector. J. Immunol. 167:196-203. [DOI] [PubMed] [Google Scholar]
- 2.Braren, R., G. Glowacki, M. Nissen, F. Haag, and F. Koch-Nolte. 1998. Molecular characterization and expression of the gene for mouse NAD+:arginine ecto-mono(ADP-ribosyl)transferase, Art1. Biochem. J. 336:561-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruzzone, S., L. Guida, E. Zocchi, L. Franco, and A. De Flora. 2001. Connexin 43 hemichannels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 15:10-12. [DOI] [PubMed] [Google Scholar]
- 4.Cockayne, D. A., T. Muchamuel, J. C. Grimaldi, H. Muller-Steffner, T. D. Randall, F. E. Lund, R. Murray, F. Schuber, and M. C. Howard. 1998. Mice deficient for the ecto-nicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses. Blood 92:1324-1333. [PubMed] [Google Scholar]
- 5.Ferrero, E., F. Saccucci, and F. Malavasi. 2000. The making of a leukocyte receptor: origin, genes and regulation of human CD38 and related molecules. Chem. Immunol. 75:1-19. [DOI] [PubMed] [Google Scholar]
- 6.Glowacki, G., R. Braren, M. Cetkovic-Cvrlje, E. H. Leiter, F. Haag, and F. Koch-Nolte. 2001. Molecular cloning, chromosomal mapping, and expression of the gene for mouse ecto-mono(ADP-ribosyl)transferase Art5. Gene 275:267-277. [DOI] [PubMed] [Google Scholar]
- 7.Goding, J. W., and M. C. Howard. 1998. Ecto-enzymes of lymphoid cells. Immunol. Rev. 161:5-10. [DOI] [PubMed] [Google Scholar]
- 8.Goding, J. W., R. Terkeltaub, M. Maurice, P. Deterre, A. Sali, and S. I. Belli. 1998. Ecto-phosphodiesterase/pyrophosphatase of lymphocytes and nonlymphoid cells: structure and function of the PC-1 family. Immunol. Rev. 161:11-26. [DOI] [PubMed] [Google Scholar]
- 9.Haag, F., V. Andresen, S. Karsten, F. Koch-Nolte, and H. Thiele. 1995. Both allelic forms of the rat T-cell differentiation marker RT6 display nicotinamide adenine dinucleotide (NAD)-glycohydrolase activity, yet only RT6.2 is capable of automodification upon incubation with NAD. Eur. J. Immunol. 25:2355-2361. [DOI] [PubMed] [Google Scholar]
- 10.Haag, F., and F. Koch-Nolte. 1997. ADP-ribosylation in animal tissues: structure, function and biology of mono(ADP-ribosyl)transferases and related enzymes. Plenum Press, New York, N.Y. [DOI] [PubMed]
- 11.Hogan, B. 1994. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 12.Hollmann, C., F. Haag, M. Schlott, A. Damaske, H. Bertuleit, M. Matthes, M. Kühl, H. G. Thiele, and F. Koch-Nolte. 1996. Molecular characterization of mouse T-cell ecto-ADP-ribosyltransferase Rt6: cloning of a second functional gene and identification of the Rt6 gene products. Mol. Immunol. 33:807-817. [DOI] [PubMed] [Google Scholar]
- 13.Joyner, A. L. 1993. Gene targeting: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 14.Kahl, S., M. Nissen, R. Girisch, T. Duffy, E. H. Leiter, F. Haag, and F. Koch-Nolte. 2000. Metalloprotease-mediated shedding of enzymatically active mouse ecto-ADP-ribosyltransferase ART2.2 upon T-cell activation. J. Immunol. 165:4463-4469. [DOI] [PubMed] [Google Scholar]
- 15.Killeen, N., S. G. Stuart, and D. R. Littman. 1992. Development and function of T cells in mice with a disrupted CD2 gene. EMBO J. 11:4329-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knobeloch, K. P., M. D. Wright, A. F. Ochsenbein, O. Liesenfeld, J. Löhler, R. M. Zinkernagel, I. Horak, and Z. Orinska. 2000. Targeted inactivation of the tetraspanin CD37 impairs T-cell-dependent B-cell response under suboptimal costimulatory conditions. Mol. Cell. Biol. 20:5363-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch, F., F. Haag, and H. G. Thiele. 1990. Nucleotide and deduced amino acid sequence for the mouse homologue of the rat T-cell differentiation marker RT6. Nucleic Acids Res. 18:3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch-Nolte, F., M. Abromeit, A. Gerber, S. Kahl, W. Ohlrogge, G. Glowacki, R. Braren, J. Fang, S. Rothenburg, and F. Haag. 2000. Molecular characterization of NAD+-metabolizing ectoenzymes in mice and men: endogenous relatives of ADP-ribosylating bacterial toxins, p. 221-233. In L. Vanduffel and R. Lemmens (ed.), Ecto-ATPases and related ectonucleotidases. Shaker Publishing B.V., Mastricht, The Netherlands.
- 19.Koch-Nolte, F., T. Duffy, M. Nissen, S. Kahl, V. Ablamunits, E. H. Leiter, and F. Haag. 1999. A new monoclonal antibody detects a developmentally regulated mouse ecto ADP-ribosyltransferase on T cells: subset distribution, inbred strain variation, and modulation upon T-cell activation. J. Immunol. 163:6014-6022. [PubMed] [Google Scholar]
- 20.Koch-Nolte, F., and F. Haag. 1997. Mono(ADP-ribosyl)transferases and related enzymes in animal tissues. Emerging gene families. Adv. Exp. Med. Biol. 419:1-13. [DOI] [PubMed] [Google Scholar]
- 21.Koch-Nolte, F., F. Haag, R. Kastelein, and F. Bazan. 1996. Uncovered: the family relationship of a T-cell-membrane protein and bacterial toxins. Immunol. Today 17:402-405. [DOI] [PubMed] [Google Scholar]
- 22.Koch-Nolte, F., J. Klein, C. Hollmann, M. Kühl, F. Haag, H. R. Gaskins, E. H. Leiter, and H. G. Thiele. 1995. Defects in the structure and expression of the genes for the T-cell marker Rt6 in NZW and (NZB × NZW)F1 mice. Int. Immunol. 7:883-890. [DOI] [PubMed] [Google Scholar]
- 23.Koch-Nolte, F., D. Petersen, S. Balasubramanian, F. Haag, D. Kahlke, T. Willer, R. Kastelein, F. Bazan, and H. G. Thiele. 1996. Mouse T-cell membrane proteins Rt6-1 and Rt6-2 are arginine/protein mono(ADPribosyl)transferases and share secondary structure motifs with ADP-ribosylating bacterial toxins. J. Biol. Chem. 271:7686-7693. [DOI] [PubMed] [Google Scholar]
- 24.Lin, Q., I. Taniuchi, D. Kitamura, J. Wang, J. F. Kearney, T. Watanabe, and M. D. Cooper. 1998. T and B cell development in BP-1/6C3/aminopeptidase A-deficient mice. J. Immunol. 160:4681-4687. [PubMed] [Google Scholar]
- 25.Liu, Z. X., O. Azhipa, S. Okamoto, S. Govindarajan, and G. Dennert. 2001. Extracellular nicotinamide adenine dinucleotide induces T-cell apoptosis in vivo and in vitro. J. Immunol. 167:4942-4947. [DOI] [PubMed] [Google Scholar]
- 26.Liu, Z. X., Y. Yu, and G. Dennert. 1999. A cell surface ADP-ribosyltransferase modulates T-cell receptor association and signaling. J. Biol. Chem. 274:17399-17401. [DOI] [PubMed] [Google Scholar]
- 27.Nemoto, E., S. Stohlman, and G. Dennert. 1996. Release of a glycosylphosphatidylinositol-anchored ADP-ribosyltransferase from cytotoxic T cells upon activation. J. Immunol. 156:85-92. [PubMed] [Google Scholar]
- 28.Nemoto, E., Y. Yu, and G. Dennert. 1996. Cell surface ADP-ribosyltransferase regulates lymphocyte function-associated molecule-1 (LFA-1) function in T cells. J. Immunol. 157:3341-3349. [PubMed] [Google Scholar]
- 29.Okamoto, S., O. Azhipa, Y. Yu, E. Russo, and G. Dennert. 1998. Expression of ADP-ribosyltransferase on normal T lymphocytes and effects of nicotinamide adenine dinucleotide on their function. J. Immunol. 160:4190-4198. [PubMed] [Google Scholar]
- 30.Okazaki, I. J., H. J. Kim, N. G. McElvaney, E. Lesma, and J. Moss. 1996. Molecular characterization of a glycosylphosphatidylinositol-linked ADP-ribosyltransferase from lymphocytes. Blood 88:915-921. [PubMed] [Google Scholar]
- 31.Okazaki, I. J., H. J. Kim, and J. Moss. 1996. Cloning and characterization of a novel membrane-associated lymphocyte NAD:arginine ADP-ribosyltransferase. J. Biol. Chem. 271:22052-22053. [DOI] [PubMed] [Google Scholar]
- 32.Okazaki, I. J., and J. Moss. 1998. Glycosylphosphatidylinositol-anchored and secretory isoforms of mono-ADP-ribosyltransferases. J. Biol. Chem. 273:23617-23620. [DOI] [PubMed] [Google Scholar]
- 33.Pippig, S. D., C. Pena-Rossi, J. Long, W. R. Godfrey, D. J. Fowell, S. L. Reiner, M. L. Birkeland, R. M. Locksley, A. N. Barclay, and N. Killeen. 1999. Robust B cell immunity but impaired T-cell proliferation in the absence of CD134 (OX40). J. Immunol. 163:6520-6529. [PubMed] [Google Scholar]
- 34.Prochazka, M., H. R. Gaskins, E. H. Leiter, F. Koch-Nolte, F. Haag, and H. G. Thiele. 1991. Chromosomal localization, DNA polymorphism, and expression of Rt-6, the mouse homologue of rat T-lymphocyte differentiation marker RT6. Immunogenetics 33:152-156. [DOI] [PubMed] [Google Scholar]
- 35.Wang, J., E. Nemoto, and G. Dennert. 1996. Regulation of CTL by ecto-nictinamide adenine dinucleotide (NAD) involves ADP-ribosylation of a p56lck-associated protein. J. Immunol. 156:2819-2827. [PubMed] [Google Scholar]
- 36.Wang, J., E. Nemoto, A. Y. Kots, H. R. Kaslow, and G. Dennert. 1994. Regulation of cytotoxic T cells by ecto-nicotinamide adenine dinucleotide (NAD) correlates with cell surface GPI-anchored/arginine ADP-ribosyltransferase. J. Immunol. 153:4048-4058. [PubMed] [Google Scholar]
- 37.Young, T. L., and R. M. Santella. 1988. Development of techniques to monitor for exposure to vinyl chloride: monoclonal antibodies to ethenoadenosine and ethenocytidine. Carcinogenesis 9:589-592. [DOI] [PubMed] [Google Scholar]
- 38.Yu, Y., S. Okamoto, E. Nemoto, and G. Dennert. 1997. Molecular cloning of a functional murine arginine-specific mono-ADP-ribosyltransferase and its expression in lymphoid cells. DNA Cell. Biol. 16:235-244. [DOI] [PubMed] [Google Scholar]