Abstract
Objectives:
The objectives of this study were to analyze the actual long-term outcome after the surgical resection of extrahepatic bile duct cancer and to identify the characteristics shared by long-term survivors (5 years or longer).
Summary Background Data:
Although reported 5-year survival rates of extrahepatic bile duct cancer lie between 20% and 30%, these data are not reflecting the actual cure rate. Some patients survive longer than 5 years with recurrent disease. In some patients, recurrence is detected after 5 years. Accordingly, true cure rate is probably substantially lower than the 5-year survival rate.
Methods:
One hundred fifty-one patients from a total of 282 patients with extrahepatic bile duct cancer (excluding ampulla of Vater cancer) underwent surgical resection between 1986 and 1997. We analyzed the actual survival outcome and postresection prognostic factors after resection, which included hepatobiliary resection (HBR; extended either right or left hepatectomy, caudate lobectomy, and hilar bile duct resection, n = 23), bile duct resection (BDR; n = 25), and pancreatoduodenectomy (PD; n = 103). We also compared the clinicopathologic characteristics of actual long-term survivors (n = 49) with those who survived longer than 5 years and with short-term (<5 years) survivors.
Results:
Forty-nine of the 151 resection cases (32.5%) survived 5 years or longer; there was no 5-year survivor in the nonresected cases. The actual 5-year survival rate was 47.8% after HBR (11 of 23), 28.0% after BDR (7 of 25), and 30.1% after PD (31 of 103) (P = 0.083). Tumor histology and lymph node metastasis were identified as independent prognostic factors by multivariate analysis. Some long-term survivors had poor postoperative prognostic factors such as T3, lymph node metastasis, or microscopic margin involvement, but none with a poorly differentiated tumor. Seven long-term survivors had recurrent disease at 5 years, and recurrence was detected after 5 years in 8 more patients. Therefore, the actual cure rate (<19.2%) was substantially less than the 5-year survival rate.
Conclusions:
In cases of extrahepatic bile duct cancer, resection should be considered and efforts should be made to obtain a tumor-free margin. An aggressive surgical approach will give some survival benefit to the patients with even advanced disease. Long-term follow up is needed before declaring “a cure,” because late recurrence after 5 years is detected not infrequently. Adjuvant therapy, local and systemic, needs to be further developed.
The authors present the experiences of a single institution on the topic of extrahepatic bile duct cancer in an effort to better define the natural history of this disease and to identify those factors affecting long-term survival. Some long-term survivors were found to have risk factors associated with a poor prognosis such as lymph node metastasis or margin involvement. The authors suggest that long-term follow ups of more than 5 years are requisite for determining cure in patients with bile duct cancer and that surgical resection should be actively applied in those with advanced disease.
Although reported 5-year survival rates of extrahepatic bile duct cancer lie between 20% and 30%, these data do not reflect the actual cure rate. Some patients survive longer than 5 years with recurrent disease. In some patients, recurrence is detected after 5 years. Accordingly, true cure rate is probably substantially lower than the 5-year survival rate reported in curatively resected cases.
Sometimes patients survive a few years after a drainage procedure only, and others who undergo resection with microscopic tumor involvement of the bile duct margin survive longer than expected. Such rather unusual outcomes probably stem from the slow-growing characteristics of the tumor. Moreover, the majority of studies that have reported prognostic factors and survival outcome1–7 have been limited because few studies have been large enough in scope, with respect to patient number and/or long term follow up to properly determine the actual long-term clinical course.
Extrahepatic bile duct cancer is usually classified as upper, middle, or distal bile duct cancer according to the anatomic location8; however, tumors are rarely confined to 1 segment because bile duct cancer tends to spread along the bile duct wall longitudinally. Pancreatoduodenectomy is generally performed in cases of distal and mid third cancer (common bile duct cancer) and bile duct resection, without or with hepatectomy (hepatobiliary resection), for proximal third cancer (common hepatic duct and Klatskin tumor). Moreover, it seems reasonable that survival analysis based on the type of resection would be more practical and helpful to surgeons rather than the poorer definable location-based system currently used.
The purpose of this study was to determine actual survival in patients with extrahepatic bile duct cancer according to resection type, at least to the postoperative 5-year stage, and to identify those factors associated with long-term survival. We also investigated the status of patients at the 5-year stage to include late recurrence and recurrence pattern in our analysis of long-term outcome.
PATIENTS AND METHODS
Operative Procedures and Groups
Survival data was analyzed according to the type of resection. Distant metastasis, extensive lymph node metastasis, bilateral extensive intrahepatic duct infiltration, bilateral branch involvement of major vessels, and other systemic poor operative risk factors were contraindications of curative resection. The decision concerning resectability was made preoperatively and was mainly based on computed tomography, cholangiography, and sometimes choledochoscopic and angiographic findings.
Resective procedures were classified into 3 groups: a pancreatoduodenectomy with or without pylorus preservation (PD) and a segmental bile duct resection (BDR) without or with hepatectomy (more than hemihepatectomy) (HBR). BDR was preserved for common hepatic duct cancer or Bismuth type I or II Klatskin tumors. Resection type determination was dependent on the location and extent of the tumor. For bile duct, at least 1 cm gross tumor margins and negative microscopic margins on frozen section were the aims of curative surgery. If a microscopic free margin could not be obtained, additional resection was attempted. In this classification, we excluded all cases that underwent resection for palliation with gross residual tumor (R2) (n = 12). In all patients, regional lymph nodes were removed down to the right side of the celiac and superior mesenteric arteries, and all tissues in the hepatoduodenal ligament, except the portal vein and the hepatic artery, were removed (skeletization of the hepatoduodenal ligament). For curative surgery in HBR, we resected more than the hemiliver with the caudate lobe. Since 1991, pylorus preservation has been attempted in all PD cases, except when duodenal ischemia, duodenal ulcer, or duodenal tumor infiltration was present.
Patients and Data Collection
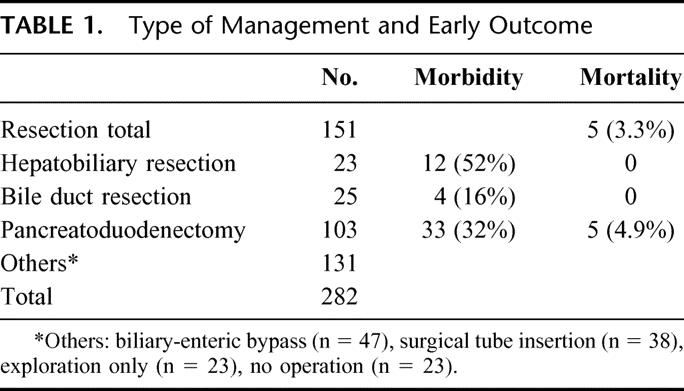
To obtain the actual survival data, we confined the study period from January 1, 1986, to December 31, 1997, which allowed the latest case to achieve 5 postoperative years. Patient data was obtained by a retrospective review of medical records or by direct or indirect contact. During the study period, a total of 282 patients were admitted and diagnosed as having extrahepatic bile duct cancer. Mean patient age was 58 years and there were 197 men and 85 women. Among these 282 patients, 151 (53.5%) underwent resection (Table 1); 103 patients received PD, 25 BDR, and 23 HBR. Combined portal vein resection was performed in 5 patients (2 in HBR and 3 in PD).
TABLE 1. Type of Management and Early Outcome
Prognostic Factors and the Characteristics of Long-term Survivors
Patient demographics, intraoperative factors, pathologic factors, and postoperative adjuvant treatment were evaluated to determine the prognostic factors after the resection for extrahepatic bile duct cancer. Except the patients who had T1,2N0 lesions with no residual tumor, or poor performance status or refused the chemoradiation, we performed chemoradiotherapy. The patients received external-beam radiation therapy (EBRT) with megavoltage equipment (6–10 MV x-ray or 60Co gamma ray). The patients received 40 Gy of radiation delivered as a split course of 20 Gy in 10 fractions over 2 weeks with a 2-week break between courses. The 5-FU was added during days 1 through 3 of each course of radiotherapy at a dose of 500 mg/m2 per day. After radiotherapy, patients received 5-FU monthly (500 mg/m2 per day for 5 days) during 1 year.
After analyzing the actual 5-year outcomes of surgical treatment of extrahepatic bile duct cancer, we divided the patients into 2 groups: the long-term survivors’ group, who survived more than 5 years, and the short-term survivors’ group, who died within 5 years. We compared the clinicopathologic features of these groups to explore the possibility of there being significant differences. We also investigated the detailed status of long-term survivors to quantify late recurrence and characterize recurrence patterns.
Statistical Analysis
Survival data were processed using the Kaplan-Meier method and were compared using the log-rank test. A P value of less than 0.05 was considered statistically significant. Only variables that were statistically significant by univariate analysis were included in the multivariate analysis, which was performed using Cox proportional hazards regression. To identify differences between the actual long-term survivors and short-term survivors groups, the Student t test and the chi-squared test were used.
RESULTS
Operative Mortality and Morbidity
Operative mortality rate was 3.3% among patients that were treated by resection. The operative mortality for PD was 4.9%; there was no operative mortality for BDR or HBR. Table 1 shows the overall operative mortality and morbidity according to the types of resection. The operative morbidity of BDR (16%) was lower than for the other resection types (52% for HBR and 32% for PD) (P = 0.028). The most frequent complication was an abdominal fluid collection or abscess (30%) in HBR and pancreatic leakage (18.4%) in PD.
Survival
The actual survival rate for all patients was 55.5% at 1 year, 22.9% at 3 years, and 17.6% at 5 years. The 1-, 3-, and 5-year survival rates of resection cases were 72.9%, 41.1%, and 32.5%, respectively, and those of nonresection cases were 35.4%, 1.6%, and 0%, respectively (Fig. 1) (P <0.001). Of the 151 patients who underwent resection, 49 patients (32.5%) have been living for 5 years or more and 42 patients (29.1%) were disease-free at the 5-year point. There was no 5-year survivor among those who did not undergo resection (n = 131).
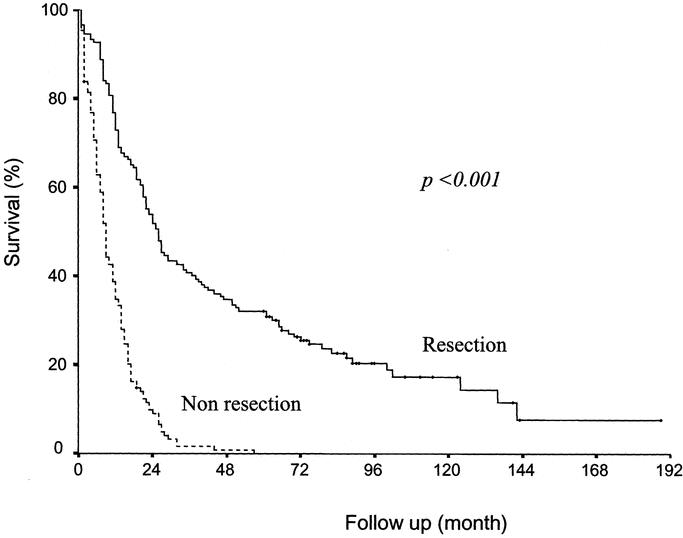
FIGURE 1. Comparison of the survival of patients with and without resection. Significant differences were found between those who underwent resection (n = 151) and those who did not (n = 131) (P <0.001).
Figure 2 shows survival results among those who underwent resection according to the type of resection. Actual 5-year survival rates were 47.8% (11 of 23) for HBR, 28.0% (7 of 25) for BDR, and 30.1% (31 of 103) for PD. Differences between survival rates were not significant (P = 0.083).
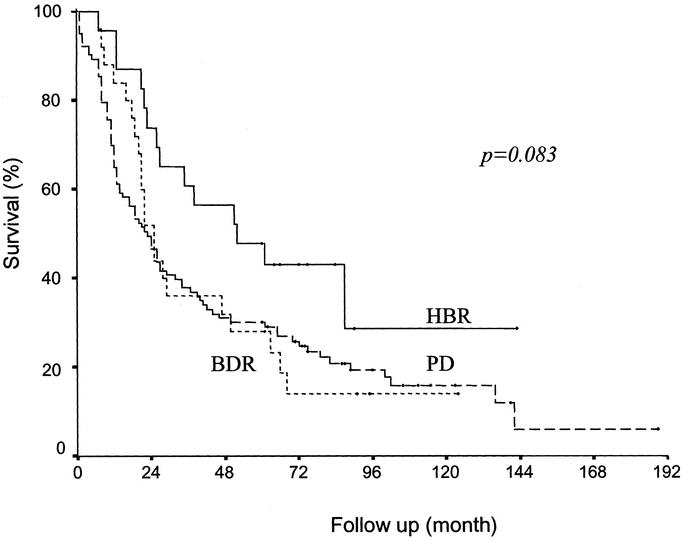
FIGURE 2. Comparison of survival according to the type of resection. No survival differences were observed according to the type of resection (P = 0.083). HBR, hepatobiliary resection (n = 23); BDR, bile duct resection (n = 25); PD, pancreatoduodenectomy (n = 103).
Prognostic Factors
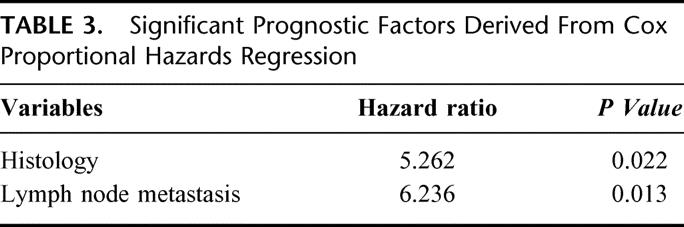
Table 2 shows the prognostic factors of patients who underwent surgical resection. Tumor histology, lymph node metastasis, and 5th AJCC (American Joint Committee on Cancer) stage were significant prognostic factors. Depth of invasion had only marginal significance (P = 0.077). In the Cox proportional hazard regression for multivariate analysis (Table 3), tumor histology (P = 0.022) and lymph node metastasis (P = 0.013) only were identified as the 2 independent prognostic factors.
TABLE 2. Univariate Analysis of Prognostic Factors After Surgical Resection
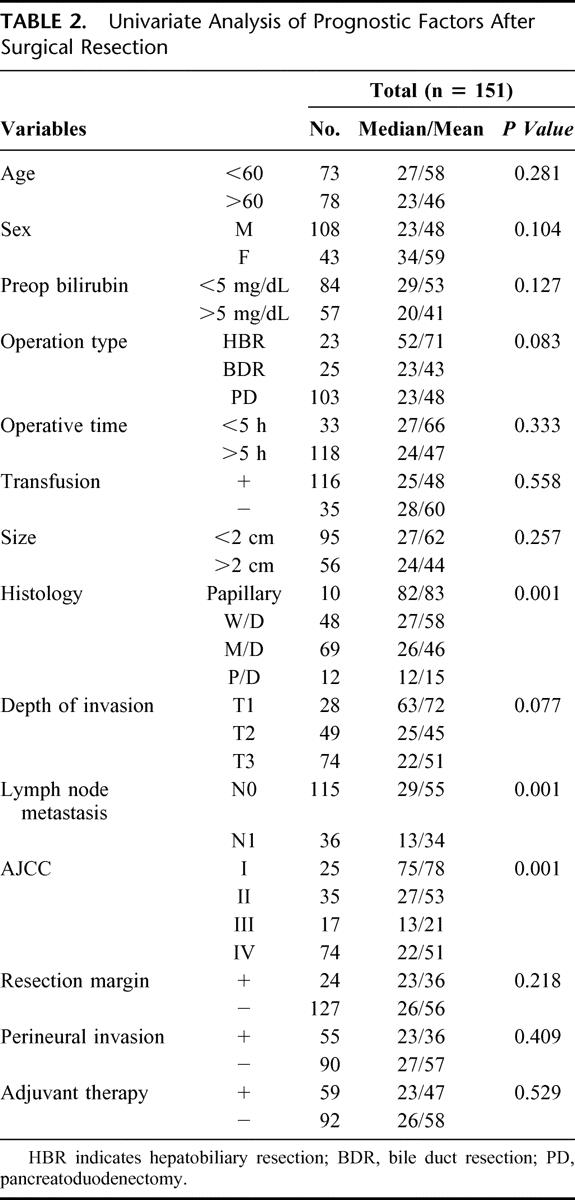
TABLE 3. Significant Prognostic Factors Derived From Cox Proportional Hazards Regression
Characteristics of Long-term Survivors and Comparison Between Long-term and Short-term Survivors
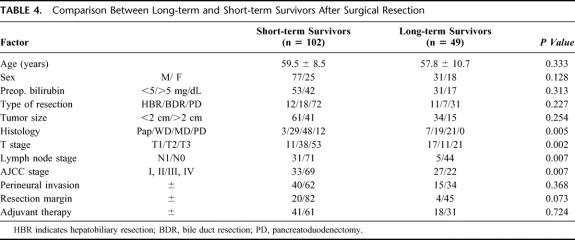
In our series, 49 patients survived longer than 5 years after surgical resection. The characteristics of these patients are summarized in Table 4. The 49 patients included 31 males and 18 females (mean age, 57.8 years). Tumor size (mean, 2.0 cm) varied from 0.5 to 5.0 cm. There were 11 long-term survivors in HBR, 7 in BDR, and 31 in PD. Among the long-term survivors, no patient had a poorly differentiated adenocarcinoma; 57% of the patients had papillary or well-differentiated adenocarcinoma. Five (10.2%) patients who had lymph node metastasis and 4 (8.2%) patients who had tumoral involvement of resection margin survived longer than 5 years. Twenty-two (44.9%) patients of the long-term survivors had advanced AJCC stage (1 was stage III and 21 were stage IV).
TABLE 4. Comparison Between Long-term and Short-term Survivors After Surgical Resection
We compared the clinicopathologic factors of long- and short-term (≥ or <5 years) survivors after resection for extrahepatic bile duct cancer (Table 4). Significant differences were found with regard to tumor histology, depth of invasion, lymph node metastasis, and AJCC stage. Multivariate analysis showed that tumor histology (P = 0.004) and lymph node metastasis (P = 0.029) were statistically significant indicators of survival.
Outcome of Long-term Survivors With Known Risk Factors
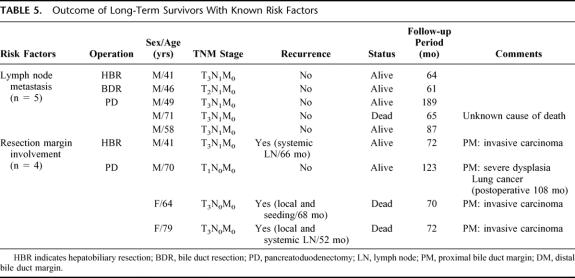
Among the long-term survivors (n = 49), there were some patients with poor prognostic factors such as lymph node metastasis. Table 5 shows the details of these patients and their outcomes. In cases with lymph node metastasis (n = 36), there were 5 long-term survivors (13.9%). Of these 5 patients, 4 patients lived for 61, 64, 87, and 189 months with a disease free status and 1 patient died at 65 months as a result of an unknown cause. Although patients with lymph node metastasis had an earlier mean recurrence than those without lymph node metastasis, some patients achieved cure after resection.
TABLE 5. Outcome of Long-Term Survivors With Known Risk Factors
Four long-term survivors had microscopic tumor involvement (carcinoma or severe dysplasia)9 of the bile duct margin, as histologically reconfirmed by a gastroenterologic pathologist. Three had invasive carcinoma at the resection margin and 1 had severe dysplasia (Table 5). Two of these 4 patients (excluding 2 patients in their 70s) received postoperative chemoradiotherapy. All 3 patients with an invasive carcinoma at the resection margin experienced late recurrence, and 2 of the 3 died at 70 and 72 months after surgery.
In patients who underwent a combined portal vein resection (n = 5), only 1 who received HBR survived over 5 years. The tumor involved the adventitia layer of the portal vein. The other 4 patients had tumor invasion of the muscle or intima layer, and all died within 2 years.
Clinical Status of Long-term Survivors and Detailed Follow-up Results
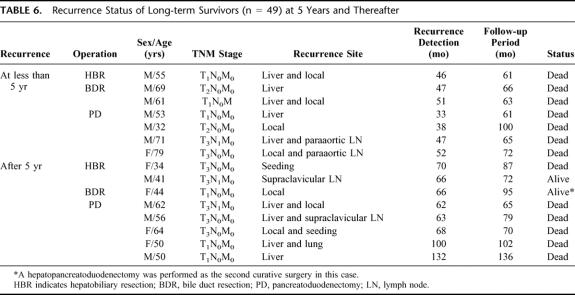
Table 6 summarizes the recurrence status of long-term survivors. Of 49 long-term survivors, 7 patients had disease recurrence at less than 5 years and all eventually died. Late recurrence was detected after 5 years in 8 long-term survivors. Six of these 8 patients died of recurrence, and 1 44-year-old female patient, who underwent segmental bile duct resection for T1 papillary adenocarcinoma, received a second curative resection (right extended hepatectomy and pancreatoduodenectomy) because of local recurrence at the right intrahepatic and intrapancreatic distal bile duct. This patient continues to be disease-free after almost 8 years of follow up.
TABLE 6. Recurrence Status of Long-term Survivors (n = 49) at 5 Years and Thereafter
Figure 3 summarizes the outcome of all extrahepatic bile duct cancers at our institute over the study period. Of the 151 resection patients, 49 patients survived for more than 5 years (actual 5-year survival rate 32.5%). However, late recurrence was frequently detected, and only 29 patients (34 patients if long-term survivors who died of bile duct cancer-unrelated causes are included) showed no evidence of disease after long-term follow up (over 5 years). This means that the actual cure rate for extrahepatic bile duct cancer is less than 19.2% (22.5%) after surgical resection and 10.3% (12.1%) for all extrahepatic bile duct cancer cases.
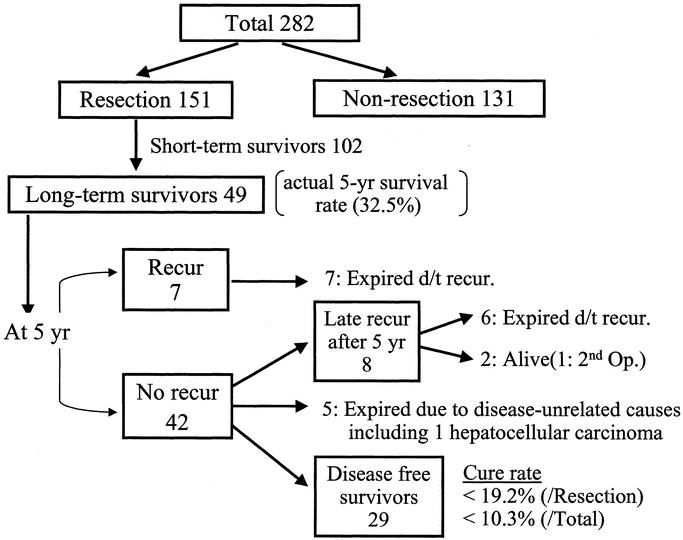
FIGURE 3. Natural history of extrahepatic bile duct cancer.
DISCUSSION
In our study, we analyzed the clinical results of extrahepatic bile duct cancer cases according to resection type, because this represents an easier way of comparing survival data and identifying prognostic factors. Usually surgeons perform pancreatoduodenectomy in cases of middle and distal bile duct cancer. In upper extrahepatic bile duct cancer, bile duct resection with major hepatectomy is widely used and offers improved survival.10–13 BDR barely provides a margin-free resection because of the tendency for longitudinal tumor spread along the bile duct. At our hospital, HBR was first performed in 1993; over recent years, BDR has decreased and HBR has increased. The resectability of proximal cancer was low (35%) and that of distal cancer was high (70%), which is why the number of HBR and BDR cases were small compared with PD.
The majority of reports show actuarial survival rates and associated prognostic factors. However, as a result of the extrapolated calculation method used, actuarial survival has a tendency to overstate true survival.14 In our study, the actual 5-year survival rate in whole extrahepatic bile duct cancer was 17.6% and that of the resection group was 32.5%. Compared with our previous data spanning 1977 through 1985 (the 5-year survival rate for all patients and the resection group was 8% and 24%, respectively),15 the present study shows a marked improvement in survival. Resectability also improved from 19.2% to 53.5%. The causes of these improvements in survival and resectability were various and included factors like the introduction of major hepatobiliary resection for Klatskin tumor during this study period.16
In the case of extrahepatic bile duct cancer, the survival rate of the distal bile duct is generally higher than that of the proximal bile duct. We found no differences with respect to survival according to tumor location or resection type, which suggests that once the tumor has been removed curatively, the prognoses of different locational types are similar.
However, the prognostic factors postsurgical resection for extrahepatic bile duct cancer are not well known as a result of the relatively small number of cases. Depth of invasion,12 perineural invasion,17 AJCC stage,18 tumoral margin involvement,13 tumor histology and differentiation grade,19 and lymph node metastasis20 are known prognostic factors in extrahepatic bile duct cancer. In the present study, multivariate analysis showed that histologic differentiation and lymph node metastasis were independent prognostic factors, and a comparison of the clinicopathologic features of short-term and long-term survivors revealed similar results.
However, some patients with poor prognostic factors who underwent surgical resection were among the 5-year survivors. Approximately 30% of the T3 cases survived for 5 years or more, which means that even T3 disease can have a good outcome if the disease is localized. Moreover, 14% of the lymph node-positive cases survived 5 years or longer. Our data shows that lymph node-positive cases usually develop early recurrence, but that once patients have survived 5 years, late recurrence is rare. However, in cases of poorly differentiated cancer, recurrence invariably developed and patients died within 5 years, without exception. In view of the fact that histologic differentiation is an independent prognostic factor, we are tempted to conclude that poorly differentiated cancer has poorly prognosis regardless of stage.
The attainment of a microscopically tumor-negative margin is important in the resection of bile duct cancer, the infiltrating nature of the tumor, and the complexity of the biliary tract frequently prevent with surgeons achieving sufficient resection margin. The University of California–San Francisco group reported that only 22% of grossly resected tumors had microscopically negative margins.21 In the present study, 15.9% (24 of 151) of resected cases had microscopic tumor involvement in the bile duct resection margin. By resection type, the margin-positive rates were 21.7% (HBR), 20% (BDR), and 13.6% (PD), and there was no statistical difference between these groups. Four of 24 with a positive resection margin survived 5 years or longer, but 3 of them, who had an invasive carcinoma at the resection margin, eventually showed late recurrence, which means that patients without clinical recurrence during the early follow-up period, despite a positive resection margin, have a real risk of late recurrence and need to be closely followed up, even beyond 5 years.
A free bile duct margin is sometimes very difficult to achieve because of the rather short length of the bile duct and because the extent of microscopic spread is very variable.9 Moreover, the interpretation of frozen sections of the bile duct is very difficult even for experienced pathologists. Both early and late recurrence occurred in patients with a positive bile duct margin. Recurrence appears to be a matter of time, especially in cases of overt adenocarcinoma at the resection margin. The timing of recurrence, in margin-positive cases, is probably dependent on the rate of tumor growth. Therefore, in such cases, although long-term survival might be extended, complete cure appears to be almost impossible. However, in cases of dysplasia at the margin, in view of the combined presence of an inflammatory condition and the difference in histologic criteria, more observation is needed to draw any conclusions.9,22
Although adjuvant radiotherapy and/or chemotherapy have not had usually survival benefit in extrahepatic bile duct cancer, some have reported that postoperative radiotherapy or chemoradiotherapy might have benefit, especially in the management of patients with a positive microscopic margin.22,23
Although we were unable to find a statistically significant survival gain in patients who received radio- or chemoradiotherapy, some patients with a positive resection margin survived over than 5 years after chemoradiotherapy. However, because the number of patients was small, this result is without statistical meaning. Nevertheless, we believe that some subset of patients with a positive margin have a better chance of cure if radio- or chemoradiotherapy is combined with surgical resection.24
These days, many surgeons believe that segmental bile duct resection is insufficient for curative resection.25 We found that long-term survival may be expected even after bile duct resection, especially in patients with a T1 lesion and a papillary or well-differentiated tumor histology. Considering it has the lowest operative morbidity, bile duct resection with regional lymph node dissection can be recommended in patients with a mucosal lesion with a favorable histology and a poor general condition.
Curative surgery for recurrent disease is usually impossible, but we experienced 2 cases (1 case in this study and another more recent case). Both of these cases were of papillary carcinoma without lymph node metastasis. One patient received right hepatectomy and pylorus-preserving pancreatoduodenectomy (PPPD) for a proximal and distal bile duct recurrence 6 years after BDR and the second patient received PPPD for a distal bile duct recurrence 3 years after right HBR. These 2 patients are disease-free 4 years and 1 year after a second curative surgery, respectively.
We believe that actual long-term survival after surgical resection for extrahepatic bile duct cancer is lower than that estimated as a result of its frequent late recurrence. Fifteen patients among the 49 long-term survivors showed disease recurrence before 5 years or thereafter. The 5-year actual survival rate after surgical resection for extrahepatic bile duct cancer was found to be 32.5% in this study; however, our estimated cure rate is less than 19.2%. Although late recurrence has not occurred so uncommonly, some cases of late recurrence may be cured by a secondary curative operation.26–28
In conclusion, resection should be considered a first option, and every effort should be made to obtain a tumor-free margin in cases of extrahepatic bile duct cancer. Microscopic tumor involvement in the resection margin in attempted curative surgery does not always mean early recurrence. An aggressive surgical approach will give some survival benefit to those with even advanced disease. Moreover, long-term follow up is needed before declaring cure, because late recurrence after 5 years is not infrequent. Adjuvant therapeutic modalities, both local and systemic, need to be developed.
Footnotes
Reprints: Sun-Whe Kim, MD, PhD, FACS, Professor, Department of Surgery, Seoul National University College of Medicine, 28 Yongon-dong, Chongno-gu, Seoul, 110-744, Korea. E-mail: sunkim@plaza.snu.ac.kr.
REFERENCES
- 1.Nakayama F, Miyazaki K, Nagafuchi K. Radical surgery for middle and distal thirds bile duct cancer. World J Surg. 1988;12:60–63. [DOI] [PubMed] [Google Scholar]
- 2.Wade TP, Prasad CN, Virgo KS, et al. Experience with distal bile duct cancers in US Veterans Affairs hospitals: 1987–1991. J Surg Oncol. 1997;64:242–245. [DOI] [PubMed] [Google Scholar]
- 3.Nagorney DM, Donohue JH, Farnell MB, et al. Outcomes after curative resections of cholangiocarcinoma. Arch Surg. 1993;128:871–877. [DOI] [PubMed] [Google Scholar]
- 4.Boerma EJ. Research into the results of resection of hilar bile duct cancer. Surgery. 1990;108:572–580. [PubMed] [Google Scholar]
- 5.Burke EC, Jarnagin WR, Hochwald SN, et al. Hilar cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong Y, Blumgart LH, Lin E, et al. Outcome of treatment for distal bile duct cancer. Br J Surg. 1996;83:1712–1715. [DOI] [PubMed] [Google Scholar]
- 7.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longmire WP, McArthur MS, Bastounis EA, et al. Carcinoma of the extrahepatic biliary tract. Ann Surg. 1973;178:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebata T, Watanabe H, Ajioka Y, et al. Pathological appraisal of lines of resection for bile duct carcinoma. Br J Surg. 2002;89:1260–1267. [DOI] [PubMed] [Google Scholar]
- 10.Saldinger PF, Blumgart LH. Resection of hilar cholangiocarcinoma—a European and United States experience. J Hepatobiliary Pancreat Surg. 2000;7:111–114. [DOI] [PubMed] [Google Scholar]
- 11.Kosuge T, Yamamoto J, Shimada K, et al. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizumoto R, Ogura Y, Kusuda T. Definition and diagnosis of early cancer of the biliary tract. Hepatogastroenterology. 1993;40:69–77. [PubMed] [Google Scholar]
- 13.Pichlmayr R, Weimann A, Klempnauer J, et al. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg. 1996;224:628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsiotos GG, Farnell MB, Sarr MG. Are the results of pancreatectomy for pancreatic cancer improving? World J Surg. 1999;23:913–919. [DOI] [PubMed] [Google Scholar]
- 15.Choi JW, Kim SW, Park YH. Treatment of extrahepatic bile duct cancer. Korean J Gastroenterol. 1989;21:389–397. [Google Scholar]
- 16.Jang JY, Kim SW, Park SJ, et al. Clinical outcome of major hepatobiliary resections for malignant tumor of the extrahepatic biliary tree. J Korean Surg Soc. 2000;58:551–559. [Google Scholar]
- 17.Bhuiya MR, Nimura Y, Kamiya J, et al. Clinicopathologic factors influencing survival of patients with bile duct carcinoma: multivariate statistical analysis. World J Surg. 1993;17:653–657. [DOI] [PubMed] [Google Scholar]
- 18.Reding R, Buard JL, Lebeau G, et al. Surgical management of 552 carcinomas of the extrahepatic bile ducts (gallbladder and periampullary tumors excluded). Results of the French Surgical Association Survey. Ann Surg. 1991;213:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouchi K, Matsuno S, Sato T. Long-term survival in carcinoma of the biliary tract. Analysis of prognostic factors in 146 resections. Arch Surg. 1989;124:248–252. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki R, Takahashi M, Funato O, et al. Prognostic significance of lymph node involvement in middle and distal bile duct cancer. Surgery. 2001;129:677–683. [DOI] [PubMed] [Google Scholar]
- 21.Schoenthaler R, Phillips TL, Castro J, et al. Carcinoma of the extrahepatic bile ducts. The University of California at San Francisco experience. Ann Surg. 1994;219:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albores-Saavedra J, Henson DE, Klimstra DS. Tumors of the Gallbladder, Extrahepatic Bile Ducts, and Ampulla of Vater, 3rd ed. Washington, DC: Armed Forces Institute of Pathology; 2000. [Google Scholar]
- 23.Todoroki T, Ohara K, Kawamoto T, et al. Benefits of adjuvant radiotherapy after radical resection of locally advanced main hepatic duct carcinoma. Int J Radiat Oncol Biol Phys. 2000;46:581–587. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Kim SW, Bang YJ, et al. Role of postoperative radiotherapy in the management of extrahepatic bile duct cancer. Int J Radiat Oncol Biol Phys. 2002;54:414–419. [DOI] [PubMed] [Google Scholar]
- 25.Ouchi K, Suzuki M, Hashimoto L, et al. Histologic findings and prognostic factors in carcinoma of the upper bile duct. Am J Surg. 1989;157:552–556. [DOI] [PubMed] [Google Scholar]
- 26.Fujii K, Yamamoto J, Shimada K, et al. Resection of liver metastases after pancreatoduodenectomy: report of seven cases. Hepatogastroenterology. 1999;46:2429–2433. [PubMed] [Google Scholar]
- 27.Tanaka N, Nobori M, Kohzuma T, et al. Anastomotic recurrence at hepaticojejunostomy in a long-term survivor of bile duct carcinoma: report of a case. Surg Today. 1994;24:280–284. [DOI] [PubMed] [Google Scholar]
- 28.Targarona EM, Zografos G, Habib NA. Liver resection for recurrent hilar cholangiocarcinoma. Br J Surg. 1993;80:1433. [DOI] [PubMed] [Google Scholar]