Abstract
Objective:
To assess the axillary recurrence rate in breast cancer patients with negative sentinel lymph node (SLN) or SLN micrometastases (>0.2 mm to ≤2.0 mm) after breast surgery and SLN procedure without formal axillary lymph node dissection (ALND).
Summary Background Data:
Under controlled study conditions, the SLN procedure proved to be a reliable method for the evaluation of the axillary nodal status in patients with early-stage invasive breast cancer. Axillary dissection of levels I and II can thus be omitted if the SLN is free of macrometastases. The prognostic value and potential therapeutic consequences of SLN micrometastases, however, remain a matter of great debate. We present the follow-up data of our prospective SLN study, particularly focusing on the axillary recurrence rate in patients with negative SLN and SLN micrometastases.
Methods:
In this prospective study, 236 SLN procedures were performed in 234 patients with early-stage breast cancer between April 1998 and September 2002. The SLN were marked and identified with 99m technetium-labeled colloid and blue dye (Isosulfanblue 1%). The excised SLNs were examined by step sectioning and stained with hematoxylin and eosin and immunohistochemistry (cytokeratin antibodies Lu-5 or CK 22). Only patients with SLN macrometastases received formal ALND of levels I and II, while patients with negative SLN or SLN micrometastases did not undergo further axillary surgery.
Results:
The SLN identification rate was 95% (224/236). SLN macrometastases were found in 33% (74/224) and micrometastases (>0.2 mm to ≤2 mm) in 12% (27/224) of patients. Adjuvant therapy did not differ between the group of SLN-negative patients and those with SLN micrometastases. After a median follow-up of 42 months (range 12–64 months), 99% (222/224) of evaluable patients were reassessed. While 1 patient with a negative SLN developed axillary recurrence (0.7%, 1/122), all 27 patients with SLN micrometastases were disease-free at the last follow-up control.
Conclusions:
Axillary recurrences in patients with negative SLN or SLN micrometastases did not occur more frequently after SLN biopsy alone compared with results from the recent literature regarding breast cancer patients undergoing formal ALND. Based on a median follow-up of 42 months—one of the longest so far in the literature—the present investigation does not provide evidence that the presence of SLN micrometastases leads to axillary recurrence or distant disease and supports the theory that formal ALND may be omitted in these patients.
We prospectively investigated the rate of axillary recurrences of 234 breast cancer patients who underwent sentinel lymph node (SLN) biopsy. In the subset without formal axillary lymph node dissection, 1 axillary recurrence was observed in patients with negative SLN, none in patients with SLN micrometastases after a median follow-up of 42 months.
Axillary lymph node status is one of the most powerful prognostic factors of breast cancer and determines subsequent adjuvant treatment. Screening mammography often enables detection of breast cancer at an early stage and thus, the percentage of node-positive patients who benefit from routine ALND is constantly decreasing.1 Therefore, most patients undergoing formal ALND could be spared the considerable short- and long-term sequelae of axillary surgery.2,3 Sentinel lymph node (SLN) biopsy has become routine practice in the surgical therapy for breast cancer patients as the SLN accurately reflects the status of the remaining axillary lymph nodes.4–7 An increasing number of studies demonstrated the feasibility and accuracy of SLN biopsy in the past years. However, little is known regarding midterm follow-up results, and no long-term data are currently available. The objective of the present prospective study was to evaluate midterm follow-up data, focusing on axillary recurrences and outcome of breast cancer patients with negative SLN and SLN micrometastases undergoing SLN biopsy only.
PATIENTS AND METHODS
Between April 1998 and September 2002, 234 patients with early-stage breast cancer were prospectively enrolled in the present investigation. Two patients had bilateral breast cancer. Thus, a total of 236 SLN procedures were performed. Inclusion criteria for the present study were (1) presence of palpable breast cancer; (2) tumor size clinically equal to or less than 3 cm in diameter; and (3) absence of clinically palpable axillary lymph nodes. Written informed consent was obtained from all patients.
Lymphatic Mapping and Operative Technique
SLN mapping was performed by using a combination of a radiolabeled colloid and a vital blue dye. 99m Tc-labeled nanocolloid (Nanocol®; Nycomed AG, Wädenswil, Switzerland) at a dose of 4 × 20 MBq was injected, 3 × 20 MBq peritumorally and 1 × 20 MBq subdermally above the tumor site. Lymphoscintigraphy was performed preoperatively to identify lymphatic flow to axillary and/or parasternal lymph nodes. Hot spots were marked on the skin. The technique of SLN biopsy has been described previously.8 Briefly, SLNs were intraoperatively identified first by use of a handheld gamma probe (Navigator®; USSC, RMD Waterton, MA) to allow a precise and short incision of the overlying skin. Two to 5 mL of Isosulfanblue (Lymphazurin®; Ben Venue Labs Inc., Bedford, OH) were injected in the same fashion as the radioactive tracer 5 minutes prior to incision. All hot and/or blue lymph nodes were excised and labeled separately as SLNs. Dissection was continued until all hot and blue nodes had been removed and the background count of the axilla was less than 10% of the hottest lymph node.
Prior to the initiation of the present investigation, the SLN procedure has been validated at our institution based on 44 breast cancer patients in whom both SLN and ALN dissection level I and II were performed. For that group, the SLN identification rate was 93%, sensitivity 94%, specificity 100%, and negative predictive value 95%.8
Pathologic Examination of Lymph Nodes
Frozen section was routinely performed intraoperatively. Lymph nodes larger than 5 mm in diameter were bisected, whereas lymph nodes less or equal to 5 mm in diameter were not bisected but totally submitted for frozen section analysis. The SLNs were intraoperatively examined at 3 levels with hematoxylin and eosin (H&E)-stained sections at a cutting interval of 150 μm. The remaining tissue of the SLNs was formalin-fixed and embedded in paraffin for histologic analysis. The residual tissue was then examined using step sectioning at a cutting interval of 250 μm. Step sections were stained with H&E. If no carcinoma cells were detected, immunohistochemistry with cytokeratin antibody Lu-5 or CK 22 using a standard immunoperoxidase method (ABC-Elite) was performed. Lu-5 (Bio Medicals, Augst, Switzerland) is a pan-cytokeratin monoclonal antibody that recognizes types I and II cytokeratin subfamilies of all epithelial and mesothelial cells.
Micrometastases are defined based on a size greater than 0.2 mm and less than or equal to 2 mm in diameter according to the AJCC classification.9 Therefore, isolated tumor cells or tumor cell clusters measuring less than 0.2 mm in diameter did not meet the definition of micrometastases.10 Patients with submicrometastases (≤0.2 mm; n = 3) were considered node negative in the present investigation. Patients with SLN macrometastases immediately underwent ALND of levels I and II. Conversely, no ALND was performed in patients with SLN micrometastases and tumor-free SLN.
Adjuvant Therapy
After breast-conserving surgery patients received postoperative radiation therapy with 45 Gy over 5 weeks with a boost of 10 Gy to the tumor site, which had been marked with a clip during operation. No radiation was given to the axilla. Adjuvant therapy consisted of hormonal treatment (tamoxifen for 5 years: 20 mg daily) and/or chemotherapy (Adriamycin + cyclophosphamide or epirubicin + cyclophosphamide) every 3 weeks for a total of 12 weeks. In low-risk patients, elderly patients, and patients with contraindications for anthracyclines, 6 cycles of CMF (cyclophosphamide + methotrexate + 5-FU) were given. The indication for adjuvant therapy was based on the recommendation of the St. Gallen Consensus Conference.11,12 Based on these recommendations, patients with SLN micrometastases were considered node-negative, and the decision to administer adjuvant therapy was based only on characteristics of the primary tumor.
Postoperative Follow-up
The follow-up diagnostic procedures were clinical examination of the breast and of the axillary lymph nodes every 4 months, as well as annual mammography. Additional ultrasound of the breast was performed to clarify suspicious mammographic findings.
Statistical Analyses
Mann-Whitney U test was used for comparisons of continuous outcomes, while Fisher exact test and χ2 test were used for comparisons of dichotomous and categorical variables. Statistical significance was defined below an α level of 0.05. All statistical tests were 2-sided. For compilation of data, Microsoft Access database software (Microsoft Corporation, Redmond, WA) was used. Statistical analyses were performed with GraphPad InStat software version 3.05 (GraphPad Software, San Diego, CA).
RESULTS
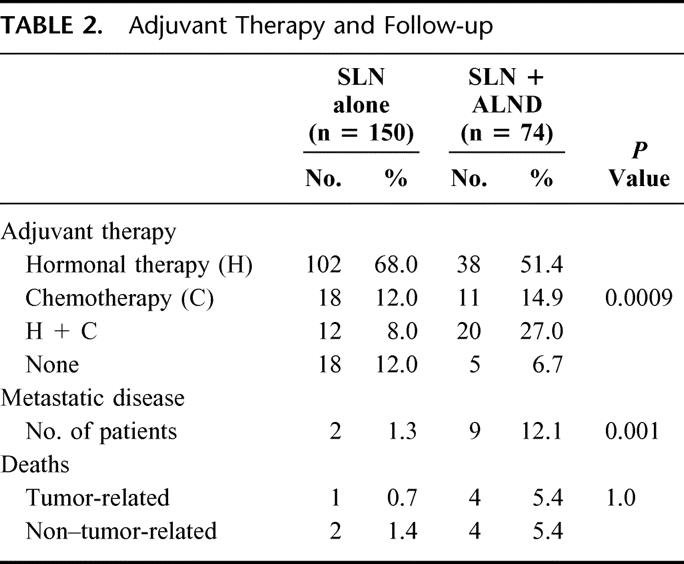
The characteristics of the groups without and with completion ALND after SLN biopsy are listed in Table 1. Mean age was 59.9 (± 11.7) years and 63.5 (± 12.0) years, respectively. The patients were predominantly postmenopausal. Mean tumor size was 16.5 mm for the “SLN alone” group and 26.9 mm for the “SLN + ALND” group. The “SLN alone” group contained more pT1 stages (72%) compared with the “SLN + ALND” group (36.4%). In 3 patients, tumor size, measured clinically as 3 cm, reached a pT3 stage with a maximum histologic tumor diameter of 55 mm. The pT4 stages were due to infiltration of the skin or pectoralis muscle. No statistical difference between the subsets undergoing SLN biopsy only versus formal ALND was seen for menopausal status, histologic type and grading, localization of the primary tumor, estrogen and progesterone receptor status and mean number of removed SLNs. Significantly more mastectomies were performed in the “SLN + ALND” group compared with the subset undergoing only SLN biopsy due to the more advanced stages. Similarly, in patients having formal ALND, adjuvant therapy was administered more frequently in this subset. Eighty-eight percent of patients with SLN biopsy alone received either hormonal and/or chemotherapy compared with 93% in the “SLN + ALND” group (Table 2). There were no significant differences between patients with negative SLN and SLN micrometastases regarding the frequency of adjuvant therapy regimens applied (P = 0.2).
TABLE 1. Patients and Tumor Characteristics of 224 Successful Mappings
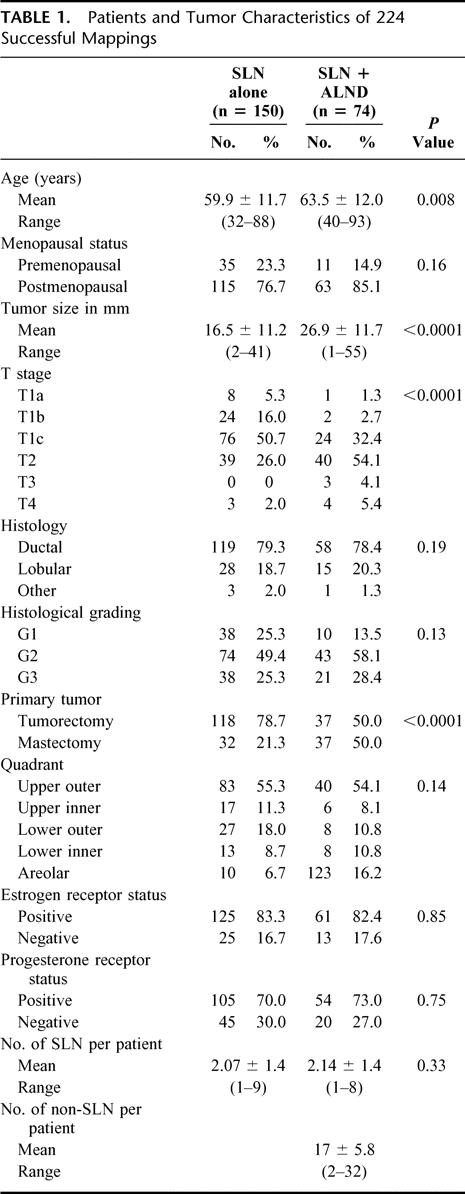
TABLE 2. Adjuvant Therapy and Follow-up
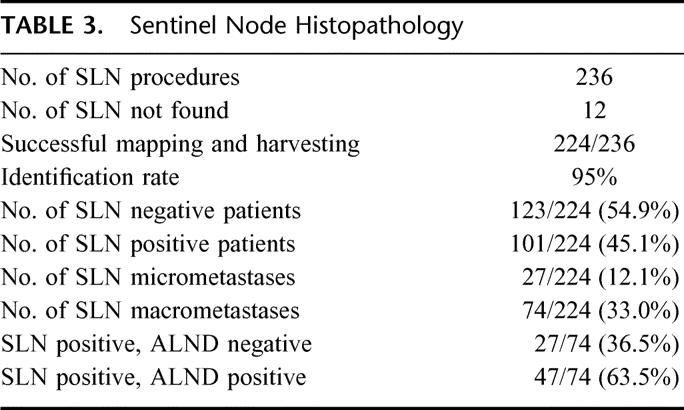
Between April 1998 and September 2002, 236 SLN biopsies were performed on 234 patients with breast cancer. Two hundred twenty-four SLN procedures were successfully completed (identification rate of 95% [224/236]). Preoperative lymphoscintigraphy was performed in 210 patients. Lymphoscintigraphy revealed a pure axillary lymphatic flow in 172 of 210 cases (82%) and an additional flow to parasternal lymph nodes in 19 of 210 cases (9%). In another 19 of 210 patients (9%), lymphatic drainage was not visualized in the scintigram. Parasternal drainage without axillary involvement was not observed in any of the patients. A mean number of 2.1 (± 1.4) SLNs per patient were harvested. The SLNs were tumor-free in 123 of 224 patients (54.9%) and contained micrometastases in 27 of 224 patients (12.1%). Micrometastases were detected by step sectioning with H&E staining in 14 of 27 cases and by additional immunohistochemistry in 13 of 27 patients. The identification of micrometastases led to a formal upstaging in 18% (27 of 150) of patients.
The subset of node negative patients (n = 123) or patients with SLN micrometastases (n = 27) did not undergo formal ALND. In none of these patients, additional SLN macrometastases were discovered in permanent sections. Thus, no secondary ALND was necessary for any of the patients. In 63.5% of patients (47/74) with SLN macrometastases, additional metastases were found in non-SLNs. In the remaining 36.5% (27/47), the SLN was the only site of macrometastases (Table 3).
TABLE 3. Sentinel Node Histopathology
Complications significantly increased when an additional ALND was performed compared with patients undergoing SLN biopsy alone. Fifteen (20.3%) of 74 patients who underwent ALND developed axillary complications including seroma (n = 8), axillary wound infection (n = 2), and chronic, clinically apparent lymphedema (n = 5). In contrast, only 1 of 150 patients in the group having SLN biopsy alone suffered from a hematoma. No patient in the latter group experienced lymphedema, numbness, or paresthesia of the upper arm.
Median follow-up was 42.0 months, ranging from 12 to 64 months in the entire patient population. Median follow-ups in the subsets of patients with negative SLN, micrometastatic SLN, and SLN with macrometastases were 42.4 (range, 12 to 64), 42.7 (range, 14 to 64) and 39.0 (range, 12 to 64) months, respectively. There were no statistically significant differences between the 3 groups (P = 0.8). Two patients were lost to follow-up; 11 patients died. The deaths were related to metastatic breast cancer in 5 of 11 cases. The other 6 patients died of other causes than tumor disease. Follow-up examinations were performed for a total of 222 of 224 (99%) patients, 149 of 150 (99%) in the “SLN alone” group and 73 of 74 (99%) in the “SLN + ALND” group. Local recurrences in the breast occurred in 10 of 222 patients (4.5%), 6 of them in the group of SLN biopsy alone. Axillary recurrences were observed in 1 patient after formal ALND and in 1 patient with a negative SLN. The first patient was a 60-year-old postmenopausal woman who suffered an axillary recurrence 12 months after mastectomy, SLN biopsy, and formal ALND for a pT2 invasive, poorly differentiated lobular carcinoma, estrogen- and progesterone-receptor positive, with a tumor size of 25 mm. The SLN contained a macrometastasis, and another 14 out of 23 axillary lymph nodes were involved. Postoperative adjuvant therapy consisted of a combination of tamoxifen and chemotherapy. No radiotherapy had been applied. The second patient was a 47-year-old premenopausal woman who developed axillary recurrence 14 months after tumorectomy and SLN biopsy only for a pT2 invasive, moderately differentiated lobular carcinoma, estrogen- and progesterone-receptor positive, with a tumor size of 41 mm and a tumor-free SLN. Postoperatively, radiotherapy to the breast and tamoxifen plus chemotherapy were administered. The axillary lymph node metastases, as well as the remaining level I and II lymph nodes, were then removed. None of the patients with SLN micrometastases has so far developed axillary recurrences. Therefore, the axillary recurrence rate in the subset with negative SLN was 0.8% (1/122), in patients with SLN micrometastases 0% (0/27), and in patients with SLN macrometastases 1.4% (1/73). Distant metastases were detected in 10 of 222 patients (4.5%). Seven of those had SLN macrometastases and developed distant disease in bone (n = 4), lung (n = 2), and brain (n = 1). Three SLN-negative patients developed distant metastases in bone (n = 2) and/or lung (n = 2).
DISCUSSION
The present prospective investigation shows that axillary recurrences in patients with negative SLN or SLN micrometastases (>0.2 mm to ≤2.0 mm) do not occur more frequently after SLN biopsy alone when compared with results in breast cancer patients undergoing formal ALND as reported in the recent literature. Indeed, based on a median follow-up of 42 months, our study does not provide evidence that the presence of SLN micrometastases leads to axillary recurrence or distant disease and supports the theory that formal axillary dissection may be omitted in these patients.
The prognostic and therapeutic implications of SLN micrometastases remain a matter of great debate.13–21 Unidentified micrometastases have been held responsible for the occurrence of up to 30% distant metastases of breast cancer patients with negative axillary lymph nodes after ALND.22,23 Some of these patients might benefit from additional adjuvant therapy. Various retrospective studies reported a significant disease-free and overall survival disadvantage in breast cancer patients with micrometastases24; others, however, failed to find any significant association.25–30
In our prospective study, 26.7% (27/101) of all SLN metastases were micrometastases. Due to their unknown prognostic significance, no formal ALND was performed in patients with SLN micrometastases. Only 2 of 27 SLN micrometastases were detected in frozen sections, whereas the remaining 25 were identified in permanent sections, and most notably half of them exclusively with immunohistochemistry. In the subset of node-negative patients (n = 123), or patients with SLN micrometastases (n = 27), no additional SLN macrometastases were discovered in permanent sections. Thus, no secondary ALND was necessary for any of these patients. After a median follow-up of 42 months, no axillary recurrence or distant metastases were observed in patients with SLN micrometastases, which were spared a formal ALND and potentially associated short- and long-term complications.3,31–36 Our findings confirm the results of a recent single-institutional investigation, which reported on 167 early-stage breast cancer patients who underwent SLN dissection only and did not have any axillary recurrences during median-term follow-up.37
Since the introduction of the SLN procedure in clinical practice, axillary lymph node micrometastases are more likely to be detected,5,6,38–45 the reason being that pathologists can focus on a few lymph nodes only, for which more thorough analysis such as step sectioning and immunohistochemistry can be applied. The systematic use of step sectioning and immunohistochemistry is not feasible in the assessment of all the nodes in ALND specimens, as these procedures are prohibitively time consuming and costly. Conversely, SLN biopsy offers the advantage that these efforts can be focused on a small number of lymph nodes.24 Current literature reports that 15% to 48% of all SLN metastases are micrometastases, leading to an upstaging of node-negative patients in 9% to 25%.5,6,38–45 Some studies examined the correlation of SLN- and non-SLN metastases. Some investigations in which standard histologic examinations of non-SLNs (bisection, H&E staining) were performed found that non-SLNs contained metastases in 7% to 53% in patients with SLN micrometastases.44,46 Increasing size of the primary tumor, presence of peritumoral lymphatic invasion, and larger diameter of SLN micrometastases significantly correlated with the presence of non-SLN metastases in multivariate analyses.46 Other studies found that the percentage of detected metastases in lymph nodes increases using step sectioning and IHC as compared with standard pathology protocols.43,45 In the present investigation, micrometastases were detected by step sectioning with H&E staining in 14 cases and by immunohistochemistry in 13 patients. The identification of micrometastases led to a formal upstaging in 18% of patients (27/150).
ALND provides excellent regional control, with axillary recurrence rates ranging from 0% to 2%.15,47–49 In our own recent retrospective study, we found an axillary recurrence rate of 1.3% after standard ALND level I and II in 390 patients operated between 1986 and 1996.50 As it is well known that axillary recurrences worsen the prognosis of breast cancer patients,47–49 it is crucial to achieve similar results regarding axillary recurrences after ALND and SLN procedure alone. The a priori hypothesis of the present investigation was that this could be achieved despite a known and accepted false-negative rate of 5% of patients undergoing SLN biopsy.7 Axillary recurrences usually occur after a median time of 24 months after surgery.15,47,49,51 Based on a median follow-up of 42 months, our study indicates that axillary recurrence after SLN biopsy is a rare phenomenon in patients with negative SLN or SLN micrometastases. There are few studies addressing axillary recurrence after SLN biopsy alone for negative SLNs.6,31,32,37,52–58 None of these studies reported an axillary recurrence rate exceeding 1.4% after a median follow-up ranging from 16 to 46 months (Table 4).
TABLE 4. Comparison of Axillary Recurrence Rates After SLN Biopsy Alone
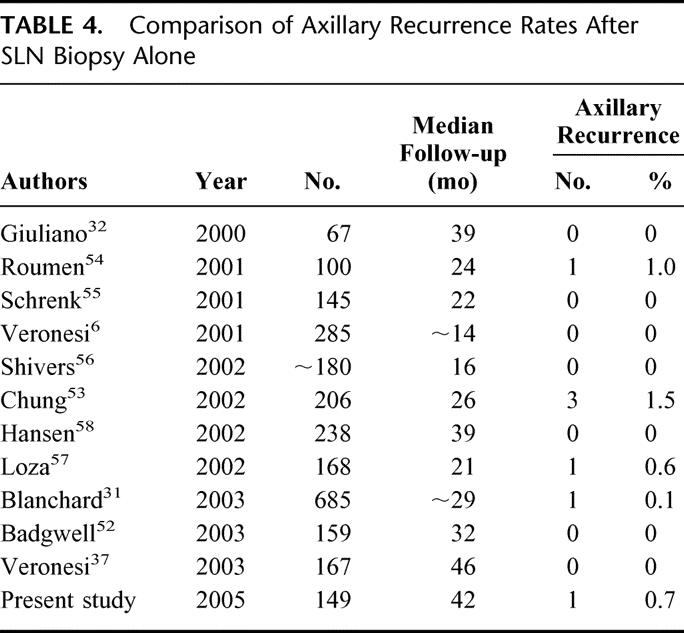
Recent retrospective analyses and studies on selected patients with SLN micrometastases without further ALND suggest that this subset of patients will not suffer from a higher incidence of regional recurrences.59–61 The findings in our prospective trial of 0.7% (1/149) axillary recurrences in SLN-negative patients and 0% in patients with SLN micrometastases after a median follow-up of 42 months—one of the longest so far published in medical literature—are in line with previous literature.
In summary, axillary recurrences in patients with negative SLN or SLN micrometastases did not occur more frequently after SLN biopsy alone compared with results from recent literature regarding breast cancer patients undergoing formal ALND. However, the morbidity of formal ALND can be avoided in patients undergoing SLN biopsy alone. Our investigation does not provide evidence that the presence of SLN micrometastases leads to axillary recurrence or distant disease and supports the theory that formal axillary dissection may be omitted in these patients. Ongoing prospective trials from the American College of Surgeons Oncology Group, National Surgical Adjuvant Breast and Bowel Project, and International Breast Cancer Study Group are hoped to provide a definite answer regarding prognostic and therapeutic implications of micrometastases in breast cancer patients.
Footnotes
Address correspondence to: Igor Langer, MD, Department of Surgery, University Hospital Basel, Spitalstrasse 21, CH-4031 Basel, Switzerland. E-mail: ilanger@uhbs.ch.
REFERENCES
- 1.Gambazzi F, Zuber M, Oertli D, et al. [Small breast carcinomas: less axillary surgery?]. Swiss Surg. 2000;6:116–120. [DOI] [PubMed] [Google Scholar]
- 2.Warmuth MA, Bowen G, Prosnitz LR, et al. Complications of axillary lymph node dissection for carcinoma of the breast: a report based on a patient survey. Cancer. 1998;83:1362–1368. [DOI] [PubMed] [Google Scholar]
- 3.Burak WE, Hollenbeck ST, Zervos EE, et al. Sentinel lymph node biopsy results in less postoperative morbidity compared with axillary lymph node dissection for breast cancer. Am J Surg. 2002;183:23–27. [DOI] [PubMed] [Google Scholar]
- 4.Bass SS, Cox CE, Ku NN, et al. The role of sentinel lymph node biopsy in breast cancer. J Am Coll Surg. 1999;189:183–194. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano AE, Jones RC, Brennan M, et al. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997;15:2345–2350. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, Galimberti V, Zurrida S, et al. Sentinel lymph node biopsy as an indicator for axillary dissection in early breast cancer. Eur J Cancer. 2001;37:454–458. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz GF, Giuliano AE, Veronesi U. Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast, April 19–22, 2001, Philadelphia, Pennsylvania. Cancer. 2002;94:2542–2551. [DOI] [PubMed] [Google Scholar]
- 8.Langer I, Zuber M, Kochli OR, et al. [Validation study of the sentinel lymph node (SLN) method in invasive breast carcinoma: personal data and review of the literature]. Swiss Surg. 2000;6:128–136. [DOI] [PubMed] [Google Scholar]
- 9.Singletary SE, Allred C, Ashley P, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–3636. [DOI] [PubMed] [Google Scholar]
- 10.Hermanek P, Hutter RV, Sobin LH, et al. International Union Against Cancer: classification of isolated tumor cells and micrometastasis. Cancer. 1999;86:2668–2673. [PubMed] [Google Scholar]
- 11.Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. J Natl Cancer Inst. 1998;90:1601–1608. [DOI] [PubMed] [Google Scholar]
- 12.Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer: Seventh International Conference on Adjuvant Therapy of Primary Breast Cancer. J Clin Oncol. 2001;19:3817–3827. [DOI] [PubMed] [Google Scholar]
- 13.Bland KI, Scott-Conner CE, Menck H, et al. Axillary dissection in breast-conserving surgery for stage I and II breast cancer: a National Cancer Data Base study of patterns of omission and implications for survival. J Am Coll Surg. 1999;188:586–595. [DOI] [PubMed] [Google Scholar]
- 14.Brenin DR, Morrow M, Moughan J, et al. Management of axillary lymph nodes in breast cancer: a national pattern of care study of 17,151 patients. Ann Surg. 1999;230:686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher B, Redmond C, Fisher ER, et al. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med. 1985;312:674–681. [DOI] [PubMed] [Google Scholar]
- 16.Kjaergaard J, Blichert-Toft M, Andersen JA, et al. Probability of false negative nodal staging in conjunction with partial axillary dissection in breast cancer. Br J Surg. 1985;72:365–367. [DOI] [PubMed] [Google Scholar]
- 17.Morrow M. A survival benefit from axillary dissection: was Halsted correct? Ann Surg Oncol. 1999;6:17–18. [DOI] [PubMed] [Google Scholar]
- 18.Orr RK. The impact of prophylactic axillary node dissection on breast cancer survival: a Bayesian meta-analysis. Ann Surg Oncol. 1999;6:109–116. [DOI] [PubMed] [Google Scholar]
- 19.Parmigiani G, Berry DA, Winer EP, et al. Is axillary lymph node dissection indicated for early-stage breast cancer? a decision analysis. J Clin Oncol. 1999;17:1465–1473. [DOI] [PubMed] [Google Scholar]
- 20.Senofsky GM, Moffat FL Jr, Davis K, et al. Total axillary lymphadenectomy in the management of breast cancer. Arch Surg. 1991;126:1336–1341. [DOI] [PubMed] [Google Scholar]
- 21.van der Wal BC, Butzelaar RM, van der MS, et al. Axillary lymph node ratio and total number of removed lymph nodes: predictors of survival in stage I and II breast cancer. Eur J Surg Oncol. 2002;28:481–489. [DOI] [PubMed] [Google Scholar]
- 22.Rosen PR, Groshen S, Saigo PE, et al. A long-term follow-up study of survival in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma. J Clin Oncol. 1989;7:355–366. [DOI] [PubMed] [Google Scholar]
- 23.De Vita VT Jr. Breast cancer therapy: exercising all our options. N Engl J Med. 1989;320:527–529. [DOI] [PubMed] [Google Scholar]
- 24.Dowlatshahi K, Fan M, Snider HC, et al. Lymph node micrometastases from breast carcinoma: reviewing the dilemma. Cancer. 1997;80:1188–1197. [DOI] [PubMed] [Google Scholar]
- 25.Prognostic importance of occult axillary lymph node micrometastases from breast cancers: International (Ludwig) Breast Cancer Study Group. Lancet. 1990;335:1565–1568. [PubMed] [Google Scholar]
- 26.Cote RJ, Peterson HF, Chaiwun B, et al. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer: International Breast Cancer Study Group. Lancet. 1999;354:896–900. [DOI] [PubMed] [Google Scholar]
- 27.Hainsworth PJ, Tjandra JJ, Stillwell RG, et al. Detection and significance of occult metastases in node-negative breast cancer. Br J Surg. 1993;80:459–463. [DOI] [PubMed] [Google Scholar]
- 28.McGuckin MA, Cummings MC, Walsh MD, et al. Occult axillary node metastases in breast cancer: their detection and prognostic significance. Br J Cancer. 1996;73:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasser IA, Lee AK, Bosari S, et al. Occult axillary lymph node metastases in “node-negative” breast carcinoma. Hum Pathol. 1993;24:950–957. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson EJ, Hause LL, Hoffman RG, et al. Occult axillary lymph node metastases in invasive breast carcinoma: characteristics of the primary tumor and significance of the metastases. Pathol Annu. 1982;17(pt 2):67–91. [PubMed] [Google Scholar]
- 31.Blanchard DK, Donohue JH, Reynolds C, et al. Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg. 2003;138:482–487. [DOI] [PubMed] [Google Scholar]
- 32.Giuliano AE, Haigh PI, Brennan MB, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol. 2000;18:2553–2559. [DOI] [PubMed] [Google Scholar]
- 33.Haid A, Kuehn T, Konstantiniuk P, et al. Shoulder-arm morbidity following axillary dissection and sentinel node only biopsy for breast cancer. Eur J Surg Oncol. 2002;28:705–710. [DOI] [PubMed] [Google Scholar]
- 34.Schrenk P, Rieger R, Shamiyeh A, et al. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer. 2000;88:608–614. [DOI] [PubMed] [Google Scholar]
- 35.Swenson KK, Nissen MJ, Ceronsky C, et al. Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer. Ann Surg Oncol. 2002;9:745–753. [DOI] [PubMed] [Google Scholar]
- 36.Temple LK, Baron R, Cody HS III, et al. Sensory morbidity after sentinel lymph node biopsy and axillary dissection: a prospective study of 233 women. Ann Surg Oncol. 2002;9:654–662. [DOI] [PubMed] [Google Scholar]
- 37.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–553. [DOI] [PubMed] [Google Scholar]
- 38.Chu KU, Turner RR, Hansen NM, et al. Sentinel node metastasis in patients with breast carcinoma accurately predicts immunohistochemically detectable nonsentinel node metastasis. Ann Surg Oncol. 1999;6:756–761. [DOI] [PubMed] [Google Scholar]
- 39.Giuliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222:394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giuliano AE, Barth AM, Spivack B, et al. Incidence and predictors of axillary metastasis in T1 carcinoma of the breast. J Am Coll Surg. 1996;183:185–189. [PubMed] [Google Scholar]
- 41.Hill AD, Tran KN, Akhurst T, et al. Lessons learned from 500 cases of lymphatic mapping for breast cancer. Ann Surg. 1999;229:528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds C, Mick R, Donohue JH, et al. Sentinel lymph node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol. 1999;17:1720–1726. [DOI] [PubMed] [Google Scholar]
- 43.Turner RR, Chu KU, Qi K, et al. Pathologic features associated with nonsentinel lymph node metastases in patients with metastatic breast carcinoma in a sentinel lymph node. Cancer. 2000;89:574–581. [DOI] [PubMed] [Google Scholar]
- 44.Veronesi U, Paganelli G, Viale G, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;91:368–373. [DOI] [PubMed] [Google Scholar]
- 45.Weaver DL, Krag DN, Ashikaga T, et al. Pathologic analysis of sentinel and nonsentinel lymph nodes in breast carcinoma: a multicenter study. Cancer. 2000;88:1099–1107. [PubMed] [Google Scholar]
- 46.Chu KU, Turner RR, Hansen NM, et al. Do all patients with sentinel node metastasis from breast carcinoma need complete axillary node dissection? Ann Surg. 1999;229:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fredriksson I, Liljegren G, Arnesson LG, et al. Consequences of axillary recurrence after conservative breast surgery. Br J Surg. 2002;89:902–908. [DOI] [PubMed] [Google Scholar]
- 48.Ivens D, Hoe AL, Podd TJ, et al. Assessment of morbidity from complete axillary dissection. Br J Cancer. 1992;66:136–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris JR, Osteen RT. Patients with early breast cancer benefit from effective axillary treatment. Breast Cancer Res Treat. 1985;5:17–21. [DOI] [PubMed] [Google Scholar]
- 50.Harder L, Harder F. Outcome and complications after standard axillary lymph node dissection in breast cancer. Thesis, 2002. University of Basel, Switzerland. [Google Scholar]
- 51.Newman LA, Hunt KK, Buchholz T, et al. Presentation, management and outcome of axillary recurrence from breast cancer. Am J Surg. 2000;180:252–256. [DOI] [PubMed] [Google Scholar]
- 52.Badgwell BD, Povoski SP, Abdessalam SF, et al. Patterns of recurrence after sentinel lymph node biopsy for breast cancer. Ann Surg Oncol. 2003;10:376–380. [DOI] [PubMed] [Google Scholar]
- 53.Chung MA, Steinhoff MM, Cady B. Clinical axillary recurrence in breast cancer patients after a negative sentinel node biopsy. Am J Surg. 2002;184:310–314. [DOI] [PubMed] [Google Scholar]
- 54.Roumen RM, Kuijt GP, Liem IH, et al. Treatment of 100 patients with sentinel node-negative breast cancer without further axillary dissection. Br J Surg. 2001;88:1639–1643. [DOI] [PubMed] [Google Scholar]
- 55.Schrenk P, Hatzl-Griesenhofer M, Shamiyeh A, et al. Follow-up of sentinel node negative breast cancer patients without axillary lymph node dissection. J Surg Oncol. 2001;77:165–170. [DOI] [PubMed] [Google Scholar]
- 56.Shivers S, Cox C, Leight G, et al. Final results of the Department of Defense multicenter breast lymphatic mapping trial. Ann Surg Oncol. 2002;9:248–255. [DOI] [PubMed] [Google Scholar]
- 57.Loza J, Colo F, Nadal J, et al. Axillary recurrence after sentinel node biopsy for operable breast cancer. Eur J Surg Oncol. 2002;28:897–898. [DOI] [PubMed] [Google Scholar]
- 58.Hansen NM, Grube BJ, Giuliano AE. The time has come to change the algorithm for the surgical management of early breast cancer. Arch Surg. 2002;137:1131–1135. [DOI] [PubMed] [Google Scholar]
- 59.Fant JS, Grant MD, Knox SM, et al. Preliminary outcome analysis in patients with breast cancer and a positive sentinel lymph node who declined axillary dissection. Ann Surg Oncol. 2003;10:126–130. [DOI] [PubMed] [Google Scholar]
- 60.Guenther JM, Hansen NM, DiFronzo LA, et al. Axillary dissection is not required for all patients with breast cancer and positive sentinel nodes. Arch Surg. 2003;138:52–56. [DOI] [PubMed] [Google Scholar]
- 61.Liang WC, Sickle-Santanello BJ, Nims TA. Is a completion axillary dissection indicated for micrometastases in the sentinel lymph node? Am J Surg. 2001;182:365–368. [DOI] [PubMed] [Google Scholar]


