Abstract
Objective:
To investigate the uses and limitations of cyclooxygenase- (COX) 2 inhibition using clinically relevant doses of oral rofecoxib in the treatment of murine models of non–small-cell lung cancer (NSCLC).
Summary Background Data:
Overexpression of COX-2 has been reported in lung cancer. Several studies have demonstrated that high doses of COX-2 inhibitors could inhibit the growth of rodent and human lung cancer cell lines. The potential uses and limitations of COX-2 inhibition at doses equivalent to those currently approved for use in humans have not been well studied.
Methods:
Three murine NSCLC cell lines were injected into the flanks of mice to establish tumor xenografts. Mice were treated orally with low doses of a COX-2 inhibitor (rofecoxib chow, 0.0075%). Mechanisms were evaluated by analysis of tumor-infiltrating lymphocytes. To study rofecoxib as adjuvant therapy, large established tumors (14–18 days after tumor inoculation) were surgically debulked and animals were treated with rofecoxib starting 3 days before surgery. Recurrence of the tumor after debulking was monitored.
Results:
Rofecoxib significantly slowed the growth of small (0-120 mm3) tumors (P < 0.01-0.05) in all 3 cell lines, with higher efficacy in the more immunogenic tumors. Minimal responses were noted in larger tumors. Rofecoxib appeared to augment CD8+ T cell infiltration in immunogenic tumors. Rofecoxib significantly reduced the recurrence rate after debulking (P < 0.01).
Conclusions:
Clinically relevant doses of the COX-2 inhibitor rofecoxib given orally were effective in inhibiting the growth of small (but not large) tumors in 3 murine NSCLC cell lines tested and in preventing recurrences after surgical debulking. Depending on the immunogenicity of human tumors, COX-2 inhibition might be useful as adjuvant therapy for surgically resectable NSCLC.
Clinically relevant doses of the cyclooxygenase-2 (COX-2) inhibitor rofecoxib given orally were effective in inhibiting the growth of small tumors and in preventing recurrence after debulking in non–small-cell lung cancer (NSCLC). These data suggest that COX-2 inhibition may be useful as adjuvant therapy for surgical resectable NSCLC.
Lung cancer is the leading cause of cancer death with non–small-cell lung cancer (NSCLC) accounting for approximately 80% of thoracic malignancies. The overall survival for NSCLC remains poor, with only 12% to 14% of patients surviving 5 years from diagnosis.1 Even in those patients who are eligible for surgery, more than one quarter of cases relapse, usually at distant sites.2,3 Novel approaches to the management of lung cancer are urgently required.
One potential therapeutic target in cyclooxygenase- (COX) 2. COX is a key enzyme in the conversion of arachidonic acid to prostaglandins (PGs) and other eicosanoids. COX-1 is a housekeeping gene expressed in most tissues. COX-2 usually is absent but is induced by inflammatory and mitogenic stimuli, resulting in increased synthesis of PGs in inflamed and neoplastic tissues. COX-2 mRNA and protein levels are overexpressed in several types of human malignancies,4,5 including lung cancer6, suggesting that COX-2 is mechanistically linked to the development of cancer. Clinical data in support of this hypothesis are recent studies showing that COX-2 overexpression is a marker of poor prognosis in stage I NSCLC7,8 and is also associated with invasion and metastasis in lung cancer.9,10
Experimental data have also been generated suggesting an important role of COX-2 overexpression in lung cancer pathogenesis. Several studies have demonstrated that selective COX-2 inhibitors could inhibit the in vitro growth of human and mouse lung cancer cell lines.11–13 The use of COX-2 inhibitors in combination with conventional anticancer agents has also demonstrated additive antigrowth effects in human lung cancer cell lines.14,15 The antitumor activities of COX-2 inhibitors have also been studied in selected mouse models of NSCLC. Administration of the selective COX-2 inhibitors celecoxib and SC-58263 resulted in decreased tumor growth of the Lewis lung carcinoma (LLC) line in C57/B6 mice.16–18 The COX-2 inhibitor JTE-522 decreased growth of a human lung cancer line in nude mice.15 Two chemoprevention studies in mice have been reported. Rioux and Castonguay19 showed prevention of NNK-induced lung tumorigenesis in A/J mice using the selective COX-2 inhibitor NS-398. In contrast, Kisley et al20 reported that celecoxib reduced inflammation but not tumorigenesis in mice in a protocol in which methylcholanthrene was followed by chronic butylated hydroxytoluene. Key limitations of the treatment studies mentioned above were that only very limited number of models were tested (ie, only the nonimmunogenic LLC mouse lung cancer cell line) and that very high doses of COX-2 inhibitors were used, doses that would be difficult to achieve in human patients.
The goal of this study was to evaluate the value and limitations of a specific COX-2 inhibitor, rofecoxib, using an oral dosing regimen that resulted in serum levels that were similar to those that could be achieved in a clinical trial. Because augmentation of antitumor immune responses is one of the key antitumor mechanisms through which COX-2 inhibition may work (21; also see Discussion), we felt it important to test oral rofecoxib in mouse lung cancer models with different levels of immunogenicity. Based on our findings that COX-2 inhibition was only effective in small tumors, we also investigated the potential value of rofecoxib as adjuvant therapy after debulking surgery.
MATERIALS AND METHODS
Animals and Tumor Cells
All mice used were pathogen free BALB/c, C57BL/6, or CB17-SCID females (6–8 weeks old; weight, ∼20–25 g) and were obtained from Taconic Laboratory (Germantown, NY). Each experiment used at least 5 mice per condition and was repeated at least once for confirmation of results. The Animal Use Committee of the University of Pennsylvania approved all protocols in compliance with the Guide for the Care and Use of Laboratory Animals.
The murine bronchoalveolar carcinoma cell line L1C222 and the LLC (Lewis lung cell carcinoma) cells (obtained from the American Type Culture Collection, Rockville, MD) were cultured in DMEM (Mediatech, Washington, DC) with 10% FBS, 100 units/mL penicillin G, 100 μg/mL streptomycin, and 2 mM glutamine. The TC-1 cell line was generated by transduction of C57BL/6 primary lung epithelial cells with a retroviral vector expressing HPV16 E6/E7 plus a retrovirus expressing activated c-Ha-ras23 and was grown and maintained in RPMI 1640 (Life Technologies, Inc.) supplemented with 10% FBS, 2 mM glutamine, and 100 units/mL penicillin G and 100 μg/mL streptomycin.
Agents
The COX-2 inhibitor rofecoxib (MK-0966) was obtained from Merck Frosst Canada & Co. (Quebec, Canada). Rofecoxib was incorporated into mouse chow by Test Diet (Richmond, IN) at a concentration of 0.0075%. Mice were fed this chow according to experimental design.
Measurement of COX-2 Expression by PCR
Total RNA was extracted from cells using guanidinium isothiocyanate via standard techniques. The concentration and purity of the RNA were determined by spectrophotometry and UV adsorption at wavelengths 260 nm and 280 nm. The quality and integrity of RNA were assessed by electrophoresis on 1% formaldehyde-agarose gel to confirm the presence of sharp 28 S and 18 S bands.
Complimentary DNA was synthesized from RNA that was free of DNA. To assess the integrity of the cDNA, regions of the β-actin and GAPDH mRNAs were amplified by PCR. The murine COX-2 cDNA was amplified using the forward (5′-cagcaaatccttgctgttcc-3′) and reverse (5′-tggagaaggcttcccagct-3′) primers. Amplification conditions were 5 minutes at 94°C, followed by 30 cycles of 94°C (21 s), 55°C (21 s) 72°C (30 s), and followed by 72°C 8 minutes. A product 338 bp long was confirmed by gel electrophoresis. Following confirmation of COX-2 expression in tumor cells, we next determined the differential COX-2 mRNA expression levels in all the mouse tumor cell lines by performing real-time PCR using the Smart Cycler System (Cephid, Sunnyvale, CA). The amount of cDNA between the cell lines was normalized using β-actin levels. Using normalized amounts of cDNA, COX-2 expression analysis was performed for each cell line in quadruplicate and the COX-2 expression level relative to β-actin was determined and plotted for each cell line.
Prophylactic Immunity Test
Protective immunity was tested by immunizing naive mice with 5 × 105 irradiated (5000 rad) tumor cells subcutaneously (s.c.) once followed by challenge with 5 × 105 live tumor cells on the opposite flank 3 weeks after immunization. Naive mice were injected as controls at the time of live tumor challenge.
Inhibition of Tumor Growth With Rofecoxib Treatment
Mice were injected with 1 × 106 L1C2 (grown in BALB/c mice), TC-1, and LLC (grown in C57BL/6 mice) cells s.c. into the right flank of each animal (Day 0). The groups treated with COX-2 inhibitor (n = 5 per group) were started on rofecoxib chow on Day 0, Days 6–7 (tumor volume; 90–120 mm3) and Days 12–14 (tumor volume; 250–350 mm3) after tumor inoculation. The tumor size was measured twice weekly and the volume calculated using the formula (3.14 × long axis × short axis × short axis)/6.
Debulking Surgery and Metastatic Disease Model
Mice were injected with 1 × 106 L1C2 and TC-1 cells s.c. on the right flank (“surgical site” tumor). Debulking of the surgical site tumor was undertaken (arbitrarily defined as day 0) when tumor volume reached large size (>500 mm3; 14–18 days after first tumor injection). A complete resection was attempted. All macroscopically visible tumors were removed and the wound was closed using silk suture. At the time of debulking, mice were also injected with 1 × 106 L1C2 or TC-1 cells into the left flank to create a metastatic disease (“distal site”). Treatment with rofecoxib was started 3 days before debulking and continued for the duration of the experiment. The tumor volume of distal site was calculated as above. Tumor recurrence was defined as the first day when a tumor was unambiguously visible or palpable (approximately 2 × 2 mm).
Tumor-Neutralizing Assay (Winn Assay)
To test the effect of rofecoxib on the generation of specific antitumor T cell-enriched cells, Winn assays were performed as previously described.24,25 Spleen cells were isolated and CD8+ T cells were purified using the MACs system (Miltenyi Biotec, Auburn, CA). This cell population contained greater than 90% CD8+ T cells by FACs (data not shown). The CD8+ T-cell enriched populations from tumor-bearing mice (treated with or without rofecoxib) were admixed with 1 × 106 viable tumor cells at a ratio of 3:1. The mixture was inoculated s.c. into naive BALB/c or C57BL/6 mice. Tumor growth was measured after 1 and 2 weeks.
WBC Isolation From Tumors
WBC isolation from tumors was performed as previously described.26 Briefly, tumors were harvested from mice treated with or without rofecoxib treatment at days 10–14 after tumor inoculation, cut into small pieces, and stirred in a digestion buffer bath containing collagenase type IV (0.1%; Sigma Chemical Co., St. Louis, MO) and DNase, type IV (Roche Co., Indianapolis, IN). The tumor and digestion media were incubated 1 hour at 37°C on a shaker. Digested samples and media were collected through a 70-μm filter (CellStrainer; BD Falcon, Franklin Lakes, NJ) and cells were separated on a Ficoll-Paque (Amersham Bioscience, Uppsala, Sweden) density gradient for 25 minutes at 400 × g at 4°C. The dense layer, enriched for lymphocytes, was collected for studies.
Flow Cytometric Analysis of Tumor-Infiltrating Lymphocytes
To investigate the percentage of CD4+ T cells or CD8+ T cells in the tumor infiltrating WBCs, we analyzed single cell tumor suspensions by flow cytometry on a Becton Dickinson FACS Calibur (Becton Dickinson, Mountain View, CA) using CellQuest analysis software. For each sample, we collected 10,000 events. We blocked nonspecific staining with anti-CD16/CD32 mAb (Fc block, 2.4G2; eBioscience San Diego, CA). We subsequently stained cells with the mAbs anti-CD45-FITC (30-F11), anti-CD4-PE (RM4-5), anti-CD8-PE (53–6.7), and anti-NK-1.1-PE (PK 136) from BD PharMingen (San Diego, CA).
Statistics
Statistical analyses of the tumor volume data at a given time point were performed using unpaired Student t test or 1-way ANOVA with appropriate post hoc testing (Fisher's PLSD). The percentage of tumor-infiltrating CD4+ T or CD8+ T cells were analyzed with unpaired Student t test. The percentage of tumor-free curves was analyzed with the χ2 test. Calculations were made using StatView (Cary, NC). Statistical significance was set at P < 0.05. Results are expressed as mean ± SEM.
RESULTS
Characterization of Three Murine NSLCL Cell Lines
Since the effects of COX-2 inhibition may be influenced by the expression levels of COX-2 and/or the immunogenicity of the tumors, we first studied these features in 3 different murine lung cancer cell lines: L1C2 (grown in Balb/C mice), TC-1 (grown in C57BL/6 mice), and LLC (grown in C57BL/6 mice).
To test the immunogenicity of each NSLCL cell line, we injected mice with irradiated tumor cells, waited 3 weeks, and rechallenged the animals with 5 × 105 live tumor cells into the flank. As shown in Figure 1, the immunogenicity of each cell line was markedly different. Mice immunized with irradiated L1C2 cells were 100% protected against a subsequent live tumor challenge. Mice injected with irradiated TC-1 cells showed an intermediate response. Mice immunized with irradiated LLC cells showed virtually no protection against a subsequent live tumor challenge. Using this assay, L1C2 would be classed as immunogenic, LLC1 as nonimmunogenic, and TC-1 as intermediate.
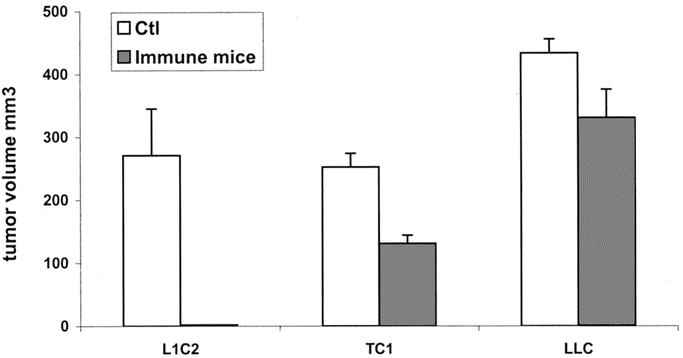
FIGURE 1. Immunogenicity of three lung cancer cell lines. Three weeks after injection with irradiated cells, immunized animals (immune mice) or naive mice (control) were injected with 5 × 105 live tumor cells.
To estimate the relative levels of expression of COX-2 in each tumor cell line, cDNA from each tumor was obtained, real-time PCR using COX-2 and β-actin-specific primers was performed, standard curves were generated, and the relative amount of message was normalized to actin levels. The ratio of COX-2 expression to actin was 0.57 for LLC cells, 0.39 for L1C2 cells, and 0 for the TC1 (ie, no detectable COX-2 message).
The Effect of Rofecoxib on the Development of Lung Cancer Tumor Growth
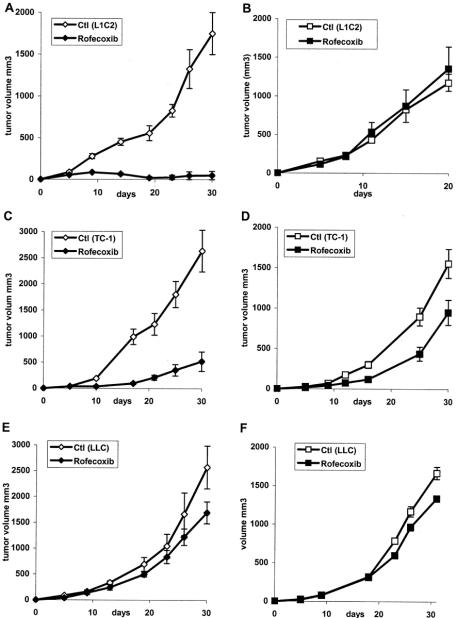
We have previously shown that oral administration of 0.0075% rofecoxib chow leads to serum levels of approximately 0.1 μg/mL, a level similar to that seen in patients taking rofecoxib (Vioxx).27 We first tested the ability of this dose of rofecoxib to inhibit the development of each tumor line. Mice were placed on control chow or rofecoxib chow on day 0. At this time, 1 million tumor cells were injected into 1 flank of each animal, and tumor size was followed over time. As shown in Figure 2, continuous treatment with the COX-2 inhibitor at the time of tumor cell injection almost completely inhibited the growth of L1C2 cells (Fig. 2A). At 30 days, the average tumor size in the control group was 1500 mm3 compared with only 50 mm3 in the COX-2-inhibited group (P < 0.001). TC1 cell growth was also markedly inhibited (Fig. 2C). At 30 days, the tumor size in the control group was 2500 mm3 compared with 500 mm3 in the COX-2-inhibited group (P < 0.05). In contrast, treatment of LLC with rofecoxib had a significant but much smaller effect (Fig. 3E). At 30 days, the average size of tumors in the control group was 2500 mm3 compared with 1600 mm3 in the COX-2-inhibited group (P < 0.05).
FIGURE 2. Effect of rofecoxib treatment on the development of tumors in normal and immunodeficient mice. Mice were placed on control (Ctl) chow or rofecoxib-containing (Rofecoxib) chow and injected s.c. with 106 L1C2 tumor cells (A, B), TC-1 tumor cells (C, D), or LLC cells (E, F) tumor cells. Tumor growth was measured in both immunocompetent mice (A, C, E) or SCID mice (B, D, F) lacking B and T cells.
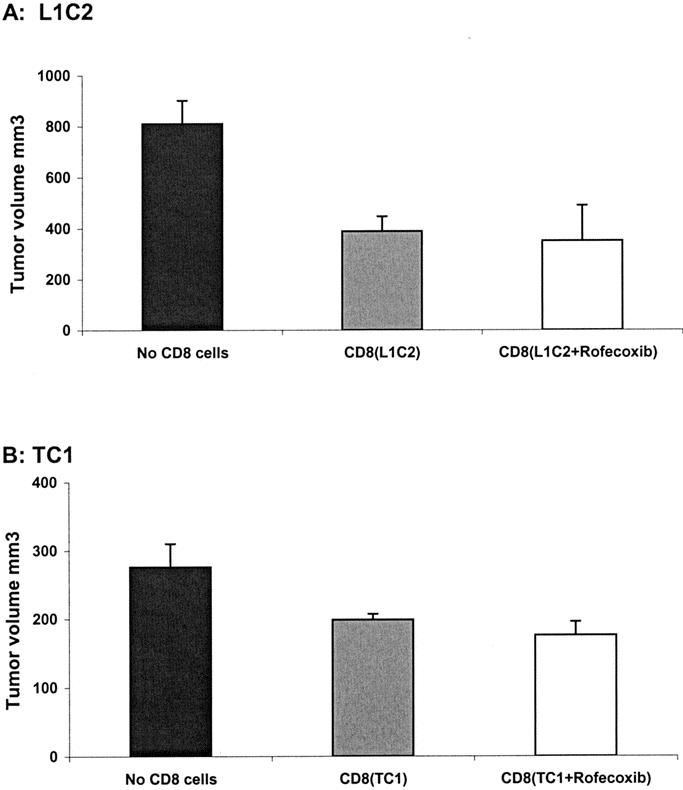
FIGURE 3. COX-2 inhibition does not augment the activity of cytotoxic CD8+ T-cells (Winn assay). CD8+ T cells were isolated from the spleens of tumor-bearing L1C1 or TC-1 mice that had been fed regular chow (CD8(L1C2) or CD8(TC-1)) or rofecoxib chow (CD8(L1C2 + Rofecoxib) or CD8 (TC-1 + Rofecoxib)). These isolated CD8+ T lymphocytes were mixed ex vivo with 106 L1C2 or TC-1 tumor cells at ratio of 3:1 and injected into naive mice. Tumor growth was assessed after 15 days. Mice injected with tumor cells alone were used control (No CD8 cells). A, Injection of mixture of 3 × 106 T cells from CD8 (L1C2) or CD8 (L1C2 + Rofecoxib) admixed with 106 L1C2 tumor cells led to almost 50% inhibition of tumor growth compared with 106 L1C2 tumor cell injection (no CD8 cells) (P < 0.01 and P < 0.05). B, Injection of mixture of 3 × 106 T cells from CD8 (TC-1) or CD8 (TC-1 + Rofecoxib) admixed with 106 TC-1 tumor cells led to almost 30% inhibition of tumor growth compare with 106 TC-1 tumor cell injection (no CD8 cells) (P < 0.05 and P < 0.01). However, there were no significant differences between T cells treated with and without rofecoxib in either tumor.
Mechanisms of COX-2 Inhibition on Tumor Development
Antitumor Effect of Rofecoxib in SCID Mice
The data above indicate that the antitumor effect of rofecoxib was stronger in immunogenic tumors (L1C2, and TC1) than a nonimmunogenic tumor (LLC).
To investigate if the mechanisms of this antitumor effect were lymphocyte-dependent, studies were repeated in immunodeficient SCID mice that lack B and T cells. As shown in Figure 2B, the antitumor effects of rofecoxib were completely lost in L1C2 tumor-bearing SCID mice. In TC1 tumor-bearing SCID mice, a large portion of the antitumor effect was lost; however, there was still a significant difference in tumor size at 30 days (Fig. 2D; P < 0.05). The small effects seen in the LLC1 models were similar in immunocompetent versus SCID mice (Fig. 2F; P < 0.01).
These findings show that, at the doses administered, the major antitumor effect of rofecoxib was immunologic in nature (versus direct antiproliferative activity, macrophage or NK cell effects, or direct antiangiogenic properties) and dependent on T and/or B lymphocytes.
Rofecoxib Does Not Increase the Formation of Cytotoxic T Lymphocytes
One proposed immunologic mechanism by which COX-2 inhibition might work is by augmenting dendritic cell activity leading to increased activity or numbers of cytotoxic T cells.28 To test whether COX-2 inhibition increased the total number of CD8+ T cells, mice bearing L1C2, TC-1, or LLC tumors were fed rofecoxib or regular chow. After 10 days, these mice were killed and CD8+ T cells were isolated from the spleens and counted. COX-2 inhibition with rofecoxib did not change the number of CD8+ T cells present (data not shown).
To test whether increased cytotoxic T-cell activity was operative in our models, CD8+ T cells were isolated from the spleens of tumor-bearing L1C2, TC-1, LLC mice that had been fed normal or rofecoxib chow. These T-cells were then used in Winn assays (ie, coinjected with tumor cells into the flanks of naive animals). A CD8+ T cell:tumor cell ratio of 3:1 was chosen to give partial tumor inhibition, so that augmentation or decrement of function could be quantified. Injection of a mixture of 3 × 106 T cells from non–tumor-bearing animals admixed with 106 L1C2 or 106 TC1 cells caused no inhibition of tumor growth (data not shown). As shown in Figure 3A, injection of a mixture of 3 × 106 T cells admixed with 106 L1C2 tumor cells led to a 50% inhibition of tumor growth (P < 0.05), regardless of whether the donor animals had been fed with rofecoxib or not. Injection of a mixture of 3 × 106 T cells admixed with 106 TC-1 tumor cells led to an approximate 30% inhibition of tumor growth (Fig. 4B; P < 0.05). Again, COX-2 inhibition did not increase CTL activity. As might be expected from the nonimmunogenic nature of the tumor, no CTL activity was detected in splenocytes from LLC-bearing mice in either control or rofecoxib-treated animals (data not shown). These data suggest that COX-2 inhibition did not lead to increased numbers or activity of CTLs.
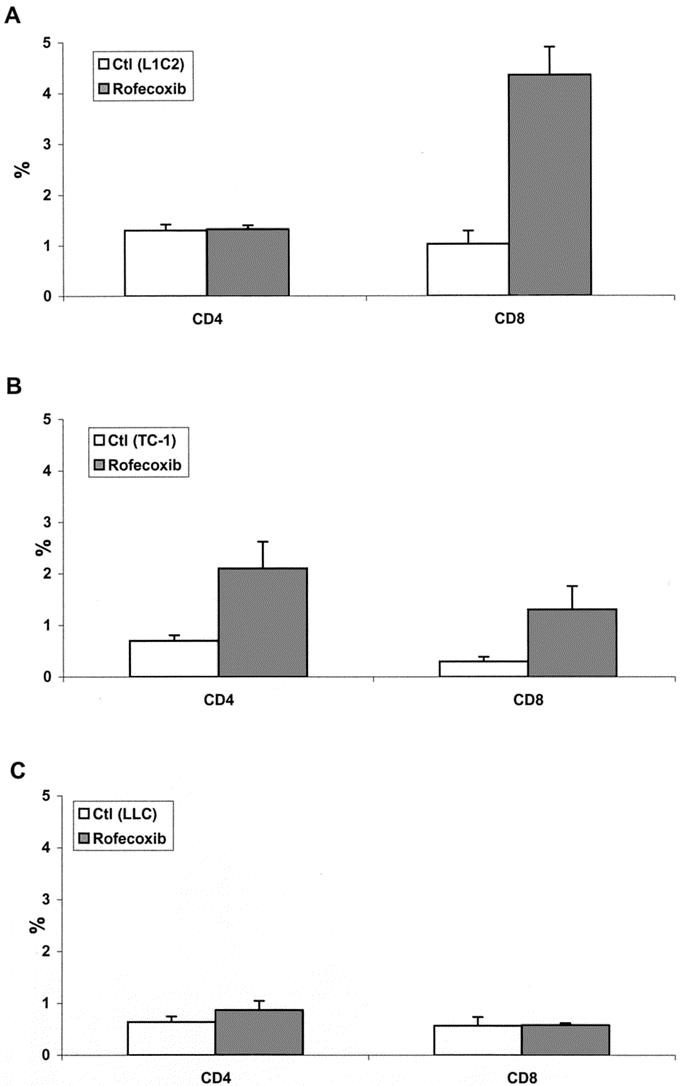
FIGURE 4. COX-2 inhibition increases T-cell infiltration into immunogenic tumors. Tumors were digested and the tumor infiltrating WBCs (CD45+) cells were identified by flow cytometric analysis. The fraction of CD4+ T cells or CD8+ T cells as a percentage of the total number of CD45+ cells harvested was determined. A, L1C2 tumors; B, TC-1 tumors; C, LLC1 tumors.
Rofecoxib Increases CD8+ T-Cell Infiltration Into Immunogenic Tumors
An alternative explanation for our observed results was that rofecoxib augmented CTL trafficking, persistence, or activity within the tumors. To investigate these mechanisms, we digested tumors harvested from mice treated with rofecoxib or control chow killed at 10 to 14 days after inoculation and analyzed the tumor infiltrating lymphocytes by FACS analysis.
The fraction of CD4+ T cells or CD8+ T cells as a percentage of the total number of WBCs (CD45+) harvested was determined. As shown in Figure 4, there were marked differences in the percentage of CD4+ T cells or CD8+ T cells in 3 cell lines. L1C2 tumor treated with rofecoxib showed significantly increased CD8+ T cell infiltration (4.5% of CD45 cells) versus control (0.9%) (Fig. 4A; P < 0.001). TC-1 tumor treated with rofecoxib showed a smaller but significant increase in both CD4+ (2.2% versus 0.7%) and CD8+ (1.5% versus 0.2%) T-cell infiltration (Fig. 4B; P < 0.05 and P < 0.05). In contrast, LLC showed minimal CD4+ and CD8+ T-cell infiltration, with no change after rofecoxib treatment (Fig. 4C). These findings showed that rofecoxib increased CD8+ T-cell infiltration into immunogenic tumors (L1C2 and TC-1) but not into nonimmunogenic tumors (LLC).
The Effect of Rofecoxib Treatment on Established Tumors
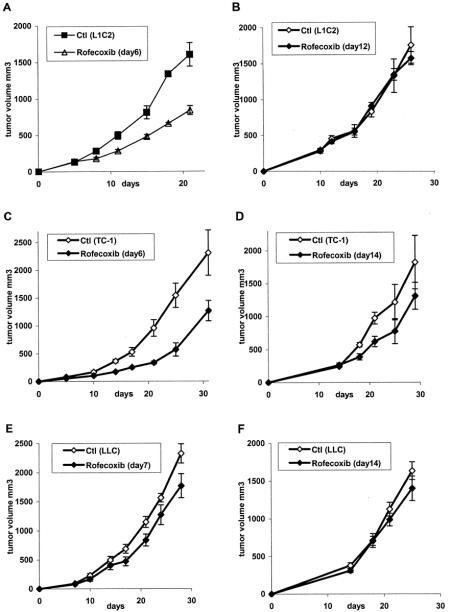
We next tested the effect of rofecoxib on established tumors. In one set of experiments, mice were changed to rofecoxib chow 5 to 7 days after tumor inoculation when the average tumor volume was approximately 100 mm3. In a second set of mice, rofecoxib chow was begun 14 to 16 days after tumor inoculation when the average tumor volume was 250 to 350 mm.3 Treatment of small L1C2 or TC1 tumors with rofecoxib significantly inhibited tumor growth (Figs. 5A, C; P < 0.001 and P < 0.05). And the growth of small LLC1 tumors was slightly inhibited, but not significantly (Fig. 5E; P = 0.14). In contrast, significant inhibition of growth was not detected when treatment of larger tumors was attempted (Fig. 5B, D, and F).
FIGURE 5. Effect of rofecoxib treatment on established tumors. Mice were injected with 106 L1C2 (A, B), TC-1 (C, D), and LLC (E, F) tumor cells and allowed to grow. After 6 to 7 days (tumor volume: 90–120 mm3, panels A, C, E) or 12 to 14 days (tumor volume: 250–350 mm3, panels B, D, F), treated mice were changed to rofecoxib chow or continued on control (Ctl) chow.
The Effect of Rofecoxib as Adjuvant Therapy After Surgical Debulking
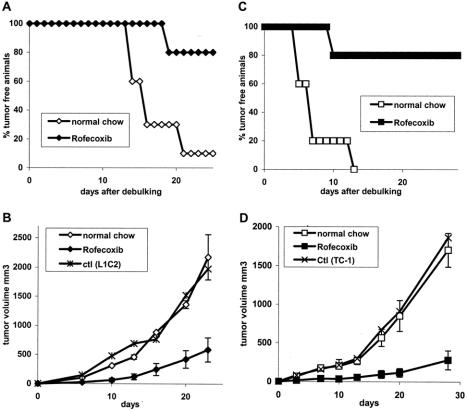
Since most lung-cancer patients present with large tumor burdens, our data suggest that COX-2 inhibitory therapy is not likely to be effective. However, since over 25% of all surgically resected patients relapse (with more than a 50% relapse rate in stage IIb patients), we postulated that COX-2 inhibition could be valuable as adjuvant therapy after surgery when tumor burden is minimal. This clinical scenario was modeled by testing the effect of rofecoxib after surgically debulking flank tumors. Accordingly, we injected tumor cells into the right flanks of mice, waited for 14 to 18 days until the tumors reached large size, and then surgically debulked the tumor. Adjuvant treatment with rofecoxib was started at 3 days before debulking. In addition to monitoring recurrence of tumor, a focus of “metastatic disease” was modeled by injecting tumor cells into the contralateral side (left flank). We were unable to perform debulking studies in LLC mice due to the high production of angiogenesis inhibitors by this tumor that markedly stimulate the growth of any residual disease after removal of the primary tumor.29,30
After debulking of large L1C2 tumors (Fig. 6A), 90% of tumors recurred after 20 days. In contrast, only 20% of the tumors recurred in animals treated with rofecoxib (P < 0.01). Consistent with these findings, the growth of tumor cells implanted as a metastatic focus was significantly inhibited (Fig. 6B; P < 0.01). In a similar fashion, after debulking of large TC1 tumors (Fig. 6C), 100% of tumors recurred after 13 days. In contrast, only 20% of tumors recurred in animals treated with rofecoxib (P < 0.001). Metastatic tumor growth was significantly inhibited (Fig. 6D; P < 0.001) by rofecoxib.
FIGURE 6. Effect of COX-2 inhibition on tumor recurrence after surgery. Mice were injected with 106 tumor cells into the right flank and, when tumors reached a size of approximately 500 mm3, had their tumors surgically removed. At the same time, they were injected with 106 tumor cells into left flank as metastatic focus. Three days prior to surgery, mice were fed with regular or rofecoxib chow. Recurrence rate was determined in L1C2 tumor cells (A) and L1C2 tumor cells (C). Growth of the metastatic focus was determined in L1C2 tumor cells (B) and L1C2 tumor cells (D).
These data demonstrate that adjuvant therapy with COX-2 inhibitor markedly reduces the extent of tumor recurrence after surgery.
DISCUSSION
COX-2 can affect multiple mechanisms that are important in carcinogenesis. Nonimmune mechanisms include (i) a function in xenobiotic metabolism where it can activate a variety of carcinogens including chemicals found in tobacco smoke31; (ii) a role in angiogenesis32,33; levels of COX-2 were found to correlate with both VEGF expression and tumor vascularization15–17; (iii) inhibition of apoptosis, an effect that could also predispose to cancer34–36; and (iv) regulation of matrix metalloproteinase-2 and CD44, thus facilitating with tumor invasion.37 Immune mechanisms are also important. COX-2 is central to the generation of PGE2 that then mediates immunosuppressive effects at multiple levels. PGE2 is a potent inhibitor of IL12 and an inducer of IL-10.38 It has been shown that abrogation of COX-2 expression promotes antitumor activity and lymphocyte infiltration by restoring a balance between 1L-10 and IL-12 in the LLC model of lung cancer.18 In addition, COX-2 may contribute to immunosuppression by inhibiting dendritic cell function, in part through PGE2-mediated IL-10 production.39–41
The results of our study suggest that majority of the effects of oral rofecoxib were due to immunologic effects. This is supported by the observation that the efficacy of rofecoxib was highest in the most immunogenic tumor (L1C2) and lowest in the least immunogenic tumor (LLC). More direct evidence was provided in experiments performed in SCID mice lacking B and T cells. In these studies, virtually all of the antitumor effects of rofecoxib were lost in SCID mice injected with L1C2 cells and are thus likely due to T-cell-mediated effects. Since one of the potential effects of COX-2 inhibition is augmentation of dendritic cell function leading to the increased generation of cytotoxic T lymphocytes, we determined if rofecoxib treatment led to increased CTL activity as measured in the Winn assay. As shown in Figure 3A and B, splenocytes from both L1C2- and TC1-bearing animals had antitumor activity; however, this was not enhanced by COX-2 inhibition. Since peripheral CTL activity was not altered, we hypothesized that that COX-2 inhibition might decrease immunosuppression within the tumors, leading to augmented CTL trafficking, persistence, or activity within the tumors. To investigate this possibility, we analyzed the type of tumor infiltrating lymphocytes by flow cytometric analysis. As shown in Figure 4, there was a clear difference between immunogenic (L1C2 and TC1) and nonimmunogenic (LLC) tumors. L1C2 tumors treated with rofecoxib showed a significantly increased percentage of infiltrating CD8+ T cells (Fig. 4A). TC-1 tumors treated with rofecoxib showed a smaller but significant increase in both CD4+ and CD8+ T-cell infiltration (Fig. 4B). Interestingly, TC-1 tumors treated with rofecoxib also showed increased NK-cell infiltration (data not shown). In contrast, LLC tumors had minimal CD4+ and CD8+ T-cell infiltration and had no increases after rofecoxib treatment (Fig. 4C). These findings show that low-dose oral rofecoxib treatment increases CD8+ T-cell infiltration into immunogenic tumors. The activity of these cells might also be enhanced; however, we did not specifically examine this issue.
A small, but significant, effect was seen in the TC1/SCID model, suggesting a nonimmunologic component. It is of interest that a clear effect was seen in the TC1 tumors, even though these cells (unlike most lung cancer cell lines) do not have detectable mRNA for COX-2 at baseline. This finding is likely explained by the induction of stromal-derived COX-2 production. Using COX-2 knockout mice, Williams et al17 showed that tumor growth was highly dependent on COX-2 production by stromal fibroblasts.
One key question surrounding the use of COX-2 inhibition in the treatment of lung cancer relates to effects in dose ranges that would be achievable in patients. Pharmacokinetic studies in normal subjects indicate that after administration of a 50-mg dose of rofecoxib (usual recommended dose 25 mg once per day) the peak plasma concentration was 0.4 μg/mL (1.2 μM) and the level at 24 hours was approximately 0.1 μg/mL (0.32 μM).42 In a previous study, using 0.01% rofecoxib chow, Yao et al43 measured serum levels of 0.26 μg/mL. In our previous study of 0.0075% rofecoxib chow in the treatment of mesothelioma, we measured similar steady state levels of 0.077 (± 0.009) μg/mL (0.25 μM).27 Thus, doses of rofecoxib administered in this study should result in serum levels similar to that seen in humans taking therapeutically recommended doses.
We are aware of 3 other studies in which LLC tumors have been treated with COX-2 inhibitors. Given the caveat that different COX-2 inhibitors were been administered via different routes, our data seem consistent with these other results. Two previous studies used the COX-2 inhibitor celecoxib (SC-58125, Celebrex). The half-life of this drug is shorter. In humans, it is administered at a usual dose of 200 mg twice daily. In humans, a single dose of 300 mg resulted in a peak plasma level of 2.7 μg/mL (7 μM) and a level of 0.5 μg/mL (0.13 μM) at 8 hours postdose.44 Masferrer et al16 treated mice injected with LLC cells from the day of implantation with celecoxib in the diet at doses ranging from 160 to 3200 parts per million (ppm). Celecoxib peak plasma levels were between 0.2 μg/mL (0.5 μM) and 9.0 μg/mL (23.58 μM) for the 160- and 3200-ppm doses. They observed an inhibition of tumor growth in a dose-responsive relationship. The relatively small degree of tumor inhibition (40%) using 160 ppm was similar to our results. At higher doses, more impressive tumor growth inhibition was seen. Williams et al17 pretreated C57BL/6 mice with celecoxib chow at a dose of 1500 mg/kg (0.15%) chow (about 10 times higher than in our study). Celecoxib inhibited the growth of LLC cells by about 50% at 17 days after implantation. Although plasma levels were not measured, Kisley et al20 showed that in Balb/C mice, doses of 1000 mg/kg led to very high plasma levels of about 18 μM. Stolina et al18 showed marked inhibition of LLC tumor growth using pretreatment with the COX-2 inhibitor SC58236 at a dose of 3 mg/kg administered intraperitoneally 3 times per week. No serum levels were obtained, making comparisons impossible.
In all of the previous studies, animals were either pretreated with COX-2 inhibitor before tumor injection or treated at the time of inoculation. We also examined the effects of COX-2 inhibition on established tumors, a much more clinically relevant scenario. We found that rofecoxib was still able to markedly decrease the growth rate of small (100 mm3) tumors, but was much less effective in treating larger (>250 mm3) tumors. The reason for this loss of efficacy in larger tumors is not yet known; however, it does define potential limitations of this therapy and help to frame potential clinical applications.
The generalizability of mouse models should be clearly recognized. It is not known for certain how these findings will translate to patients with lung cancer whose tumors are not usually considered highly immunogenic. Although immune-based therapies have proven more effective in “immunogenic” tumors like renal cell carcinoma or melanoma, there are many studies to suggest that lung cancer may also be amenable to immune modulation (reviewed in45). For example, a recent study using vaccination with autologous tumor cells secreting granulocyte-macrophage colony-stimulating factor46 has shown promise, as has a randomized trial of adoptive immunotherapy.47 As an increasing number of tumor antigens are being discovered on lung cancer cells, newer vaccination strategies hold promise.44 Although not yet well understood, human lung cancers likely vary widely in their ability to induce an effective immune response. A high number of tumor-infiltrating lymphocytes has been associated with favorable prognosis in lung and other tumors.48 Our data would suggest that COX-2 inhibition will be more effective in patients with these more “immunogenic tumors.” How many human lung cancers fall into this category will have to be examined in clinical trials.
An important conclusion of our study is that COX-2 inhibition would likely be effective only in small tumors. One application of this therapy could thus be in patients with minimal residual tumor (ie, after surgery). We tested this hypothesis by examining the efficacy of rofecoxib in animals after “debulking” surgery in which large tumors were “entirely” removed. Consistent with our previous results, we demonstrated that rofecoxib treatment markedly reduced the incidence of tumor recurrence and significantly inhibited the growth of an artificial “metastatic focus.” Approximately 30% of patients with early-stage NSCLC present with a tumor confined to the lung and locoregional lymph nodes (stages I and II disease) are candidates for curative surgery. Currently, this group of patients does not receive adjuvant therapy, despite the fact that after apparent complete resection of disease, the 5-year survival for patients with stage I NSCLC is 57% to 67%, and stage II, 39% to 55%, with the majority of patients relapsing within the first 2 years of surgery.49 Our data indicate that COX-2 inhibition at doses of rofecoxib that are well tolerated with current dosing regimens could be an efficacious, safe, and relatively inexpensive approach to adjuvant therapy for lung cancer. It is also possible that COX-2 adjuvant therapy could allow more aggressive “debulking” in patients now considered marginal for surgery (ie, stage IIIA or IIIb).
In summary, we report that relatively low-dose rofecoxib treatment can inhibit the development of NSCLC tumors in early- but not in late-stage tumors. The mechanisms of this tumor growth inhibition were largely due to immune effects. Rofecoxib prevented tumor recurrence and inhibited metastatic tumor growth after debulking surgery. Clinical trials will be necessary to assess the utility of COX-2 inhibitor as adjuvant therapy for patients with early-stage (stages I and II), surgically resectable tumors.
ACKNOWLEDGMENTS
The authors would like to thank Ian Roger and Pauline Luk of Merck and Merck-Frosst Canada for the gift of Rofecoxib chow.
Footnotes
Supported by NCI PO1 CA 66726.
Reprints: Steven M. Albelda, MD, 856 BRB II/III, University of Pennsylvania Medical Center, 421 Curie Blvd., Philadelphia, PA 19104. E-mail: albelda@mail.med.upenn.edu.
REFERENCES
- 1.Greenlee RT, Hill-Harmon MB, Murray T, et al. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. [DOI] [PubMed] [Google Scholar]
- 2.Asamura H, Nakayama H, Kondo H, et al. Lymph node involvement, recurrence, and prognosis in resected small, peripheral, non-small-cell lung carcinomas: are these carcinomas candidates for video-assisted lobectomy? J Thorac Cardiovasc Surg. 1996;111:1125–1134. [DOI] [PubMed] [Google Scholar]
- 3.Feld R, Rubinstein LV, Weisenberger TH. Site of recurrence in resected stage I non-small-cell lung cancer: a guide for future studies. J Clin Oncol. 1984;2:1352–1358. [DOI] [PubMed] [Google Scholar]
- 4.Ristimaki A, Honkanen N, Jankala H, et al. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276–1280. [PubMed] [Google Scholar]
- 5.Tucker ON, Dannenberg AJ. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- 6.Wolff H, Saukkonen K, Anttila S, et al. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58:4997–5001. [PubMed] [Google Scholar]
- 7.Khuri FR, Wu H, Lee JJ, et al. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res. 2001;7:861–867. [PubMed] [Google Scholar]
- 8.Brabender J, Park J, Metzger R, et al. Prognostic significance of cyclooxygenase 2 mRNA expression in non-small cell lung cancer. Ann Surg. 2002;235:440–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achiwa H, Yatabe Y, Hida T, et al. Prognostic significance of elevated cyclooxygenase 2 expression in primary, resected lung adenocarcinomas. Clin Cancer Res. 1999;5:1001–1005. [PubMed] [Google Scholar]
- 10.Hida T, Yatabe Y, Achiwa H, et al. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- 11.Tsubouchi Y, Mukai S, Kawahito Y, et al. Meloxicam inhibits the growth of non-small cell lung cancer. Anticancer Res. 2000;20:2867–2872. [PubMed] [Google Scholar]
- 12.Hida T, Kozaki K-I, Muramatsu H, et al. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res. 2000;6:2006–2011. [PubMed] [Google Scholar]
- 13.Qadri SSA, Wang JH, Redmond KC, et al. The role of COX-2 inhibitors in lung cancer. Ann Thorac Surg. 2002;74:1648–1652. [DOI] [PubMed] [Google Scholar]
- 14.Soriano AF, Helfrich B, Chan DC, et al. Syngergistic effects of new chemopreventive agents and conventional cytotoxic agents against human lung cancer cell lines. Cancer Res. 1999;59:6178–6184. [PubMed] [Google Scholar]
- 15.Hida T, Kozaki K-I, Ito H, et al. Significant growth inhibition of human lung cancer cells both in vitro and in vivo by the combined use of a selective cyclooxygenase 2 inhibitor, JTE-522, and conventional anticancer agents. Clin Cancer Res. 2002;8:2443–2447. [PubMed] [Google Scholar]
- 16.Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 17.Williams CS, Tsujii M, Reese J, et al. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105:1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stolina M, Sharma S, Lin Y, et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. [DOI] [PubMed] [Google Scholar]
- 19.Rioux N, Castonguay A. Prevention of NNK-induced lung tumorigenesis in A/J mice by acetylsalicylic acid and NS-398. Cancer Res. 1998;58:5354–5360. [PubMed] [Google Scholar]
- 20.Kisley LR, Barrett BS, Dwyer-Nield LD, et al. Celecoxib reduces pulmonary inflammation but not lung tumorigenesis in mice. Carcinogenesis. 2002;23:1653–1660. [DOI] [PubMed] [Google Scholar]
- 21.Richardson CM, Sharma RA, COX G, et al. Epidermal growth factor receptors and cyclooxygenase-2 in the pathogenesis of non-small cell lung cancer: potential targets for chemoprevention and systemic therapy. Lung Cancer. 2003;39:1–13. [DOI] [PubMed] [Google Scholar]
- 22.Dubinett SM, Patrone L, Tobias J, et al. Intratumoral interleukin-2 immunotherapy: activation of tumor infiltrating and specific lymphocytes in vivo. Cancer Immunol Immunother. 1993;36:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigens. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 24.Winn HJ. In vivo methods for the assessment of antibody-mediated tumor immunity. J Natl Cancer Inst Monographs. 1972;35:13–18. [PubMed] [Google Scholar]
- 25.Nakajima C, Uekusa Y, Iwasaki M, et al. A role of interferon-γ (IFN-γ) in tumor immunity: T cells with the capacity to reject tumor cells are generated but fail to migrate to tumor sites in IFN-γ-deficient mice. Cancer Res. 2001;61:3399–3405. [PubMed] [Google Scholar]
- 26.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4+ CD8+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 27.DeLong AP, Tanaka T, Kruklitis R, et al. Use of cyclooxygenase-2 inhibition to enhance the efficacy of immunotherapy. Cancer Res. 2003;63:7845–7852. [PubMed] [Google Scholar]
- 28.Sharma S, Stolina M, Yang S-C, et al. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin Cancer Res. 2003;9:961–968. [PubMed] [Google Scholar]
- 29.O'Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. [DOI] [PubMed] [Google Scholar]
- 30.Camphausen K, Moses MA, Beecken W-D, et al. Radiation therapy to a primary tumor accelerates metastatic growth in mice. Cancer Res. 2001;61:2207–2211. [PubMed] [Google Scholar]
- 31.Wiese FW, Thompson PA, Kadlubar FF. Carcinogen substrate specificity of human COX-1 and COX-2. Carcinogenesis. 2001;21:5–10. [DOI] [PubMed] [Google Scholar]
- 32.Tsujii M, Kawano S, Tsuji S, et al. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. [DOI] [PubMed] [Google Scholar]
- 33.Uefuji K, Ichikura T, Mochizuki H. Cyclooxygenase-2 expression is related to prostaglandin biosynthesis and angiogenesis in human gastric cancer. Clin Cancer Res. 2000;6:135–138. [PubMed] [Google Scholar]
- 34.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. [DOI] [PubMed] [Google Scholar]
- 35.Sawaoka H, Kawano S, Tsuji S, et al. Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol Gastrointest Liver Physiol. 1998;274:1061–1167. [DOI] [PubMed] [Google Scholar]
- 36.Leahy KM, Ornberg RL, Wang Y, et al. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and Induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625–631. [PubMed] [Google Scholar]
- 37.Dohadwala M, Batra RK, Luo J, et al. Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem. 2002;277:50828–50833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang M, Stolina M, Sharma S, et al. Non-small cell lung cancer cyclooxygenase dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58:1208–1216. [PubMed] [Google Scholar]
- 39.Yang L, Yamagata N, Yadav R, et al. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest. 2003;111:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harizi H, Juzan M, Pitard V, et al. Cyclooxygenase-2-induced prostaglandin E2 enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–2263. [DOI] [PubMed] [Google Scholar]
- 41.Whittaker DS, Bahjat KS, Moldawer LL, et al. Autoregulation of human monocyte-derived dendritic cell maturation and IL-12 production by cyclooxygenase-2-mediated prostanoid production. J Immunol. 2000;165:4298–4304. [DOI] [PubMed] [Google Scholar]
- 42.Halpin RA, Porras AG, Geer LA, et al. The disposition and metabolism of rofecoxib, a potent and selective cyclooxygenase-2 inhibitor, in human subjects. Drug Metabol Dispos. 2002;30:684–693. [DOI] [PubMed] [Google Scholar]
- 43.Yao M, Kargman S, Lam EC, et al. Inhibition of cyclooxygenase-2 by rofecoxib attenuates the growth and metastatic potential of colorectal carcinoma in mice. Cancer Res. 2003;63:586–592. [PubMed] [Google Scholar]
- 44.Paulson SK, Hribar JD, Liu NK, et al. Metabolism and excretion of [14C]celecoxib in healthy male volunteers. Drug Metab Disp. 2000;28:308–314. [PubMed] [Google Scholar]
- 45.Dubinett SM, Batra RK, Miller P, et al. Tumor antigens in thoracic malignancy. Am J Respir Cell Mol Biol. 2000;22:524–527. [DOI] [PubMed] [Google Scholar]
- 46.Salgia R, Lynch T, Skarin A, et al. Vaccination with irradiated autologous tumor cells engineered to secrete GM-CSF augments antitumor immunity in some patients with metastatic non-small cell lung carcinoma. J Clin Oncol. 2003;21:624–630. [DOI] [PubMed] [Google Scholar]
- 47.Ratto G, Zino P, Mirabelli S. A randomized trial of adoptive immunotherapy with tumor-infiltrating lymphocytes and interleukin-2 versus standard therapy in the postoperative treatment of resected nonsmall cell lung carcinoma. Cancer. 1996;78:244–251. [DOI] [PubMed] [Google Scholar]
- 48.Eerola A-K, Soini Y, Paakko P. A high number of tumor-infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung cancer. Clin Cancer Res. 2000;6:1875–1881. [PubMed] [Google Scholar]
- 49.Mountain CF. Revisions in the International System for Staging. Chest. 1997;111:1710–1717. [DOI] [PubMed] [Google Scholar]