Abstract
Murine cells do not support efficient assembly and release of human immunodeficiency virus type 1 (HIV-1) virions. HIV-1-infected mouse cells that express transfected human cyclin T1 synthesize abundant Gag precursor polyprotein, but inefficiently assemble and release virions. This assembly defect may result from a failure of the Gag polyprotein precursor to target to the cell membrane. Plasma membrane targeting of the precursor is mediated by the amino-terminal region of polyprotein. To compensate for the assembly block, we substituted the murine leukemia virus matrix coding sequences into an infectious HIV-1 clone. Transfection of murine fibroblasts expressing cyclin T1 with the chimeric proviruses resulted in viruses that were efficiently assembled and released. Chimeric viruses, in which the cytoplasmic tail of the transmembrane subunit, gp41, was truncated to prevent potential interference between the envelope glycoprotein and the heterologous matrix, could infect human and murine cells. They failed to further replicate in the murine cells, but replicated with delayed kinetics in human MT-4 cells. These findings may be useful for establishing a murine model for HIV-1 replication.
Murine cells expressing hu-CD4, CCR5, and hu-cyclin T1 support the early steps in the retrovirus replication cycle, including entry, reverse transcription, and integration. Following integration, sufficient quantities of viral mRNA transcripts are synthesized, and the cells accumulate abundant quantities of virion structural proteins. Nevertheless, with the possible exception of Chinese hamster ovary cells, none of the cell lines tested supports efficient virus replication. Although entry, integration, and expression of virus proteins occur efficiently, virion assembly and Gag polyprotein processing are severely impaired, resulting in markedly reduced levels of virus release and the failure of the virus to spread (1, 9, 11). The assembly defect is unlikely to be due to the inability of the human immunodeficiency virus type 1 (HIV-1) protease to function in murine cells, because processing is not required for assembly (4) and protease activity in murine cells has been demonstrated (6). The observation that viral proteins accumulate in vesicular structures rather than at the plasma membrane (9) suggested that inappropriate targeting of the Gag polyprotein in murine cells may contribute to the assembly block. Gag precursor trafficking to the plasma membrane is mediated primarily by the amino-terminal myristylation of the polyprotein and the basic patch near the amino terminus of matrix (MA) (4). Mutations in MA that block membrane targeting or binding result in viruses that fail to process or assemble efficiently in human cells (5), a phenotype not unlike wild-type HIV-1 in murine cells (1, 9). It is plausible that the membrane targeting signals in HIV-1 MA do not function in murine cells.
In light of these findings, we attempted to restore HIV-1 assembly and release by substituting the MA region of HIV-1 with that of murine leukemia virus (MuLV), a divergent retrovirus that replicates efficiently in murine cells. Although the MuLV MA bears minimal sequence homology to the HIV-1 MA, they share functional similarities (2), including the essential myristylation site and the basic patch.
Construction of MuLV (MA)-HIV chimeras.
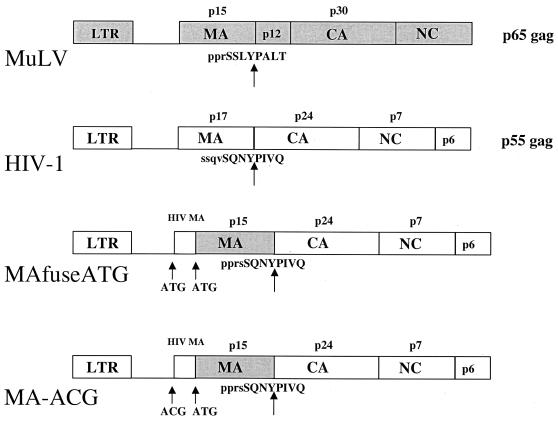
Chimeric proviruses were generated by substituting the MA coding region of the infectious HIV-1 dualtropic clone DH-12 (15) with the corresponding region of Moloney (Mo) MuLV. The chimeras maintained the HIV protease cleavage sites at the MuLV-HIV junctions. The HIV sequence extending from the 5′-untranslated region through the first 42 nucleotides of the HIV-1 MA was left intact to retain the predicted RNA packaging signals (10). Two different chimeras were constructed (Fig. 1). In MAfuseATG, the translation initiation codon of HIV-1 MA sequence was preserved, resulting in a Gag polyprotein containing the amino-terminal 14 residues of HIV-1 MA fused to full-length MuLV MA. In the second chimera, MA-ACG, the translation initiation codon of HIV-1 MA was changed to ACG, resulting in a Gag polyprotein that initiates at the MuLV MA initiation codon and contains no HIV-1 MA residues. Versions of both chimeras were constructed in which a premature stop codon was introduced in the gp41 coding sequence to remove the final 143 residues of the cytoplasmic tail. This was done to prevent potential interference between the cytoplasmic tail of gp41 and the MuLV MA. The truncation allows efficient pseudotyping of MuLV cores with HIV-1 envelope glycoproteins (Env), but does not reduce particle infectivity (7, 14). Preliminary experiments in which HEK 293 cells were transfected with MuLV-HIV chimeras containing full-length (FL) or truncated (TE) HIV-1 Env showed that the TE Envs were incorporated into virions more efficiently than FL Env (data not shown), consistent with the earlier reports.
FIG. 1.
Structure of the Gag and 5′ untranslated regions of the MuLV (MA)-HIV chimeras. The HIV-1 protease cleavage site at the MuLV-HIV junction and translation initiation sites are shown. The HIV-1 provirus used was a DH-12 clone in which a region between a conserved BssH-II site in the 5′ leader and an SphI site in CA had been replaced by the corresponding region from NL4-3.
Processing and release of chimeric viruses from human and mouse cells.
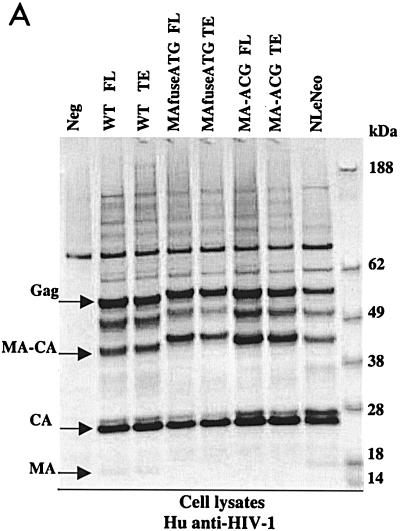
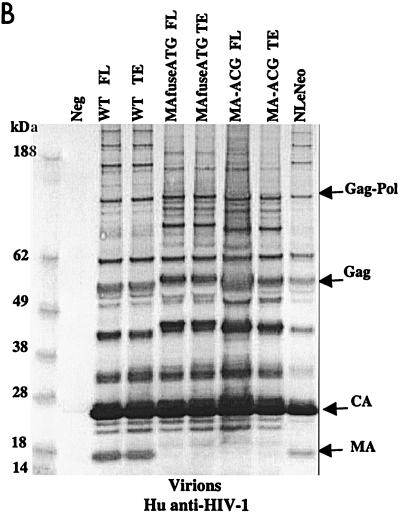
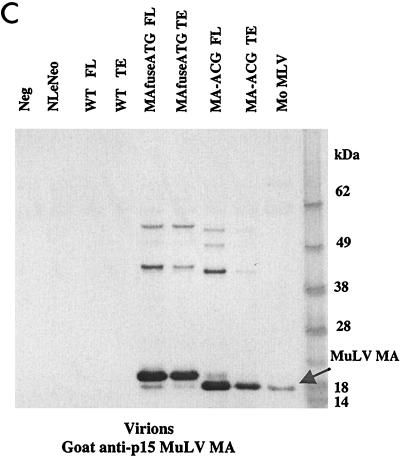
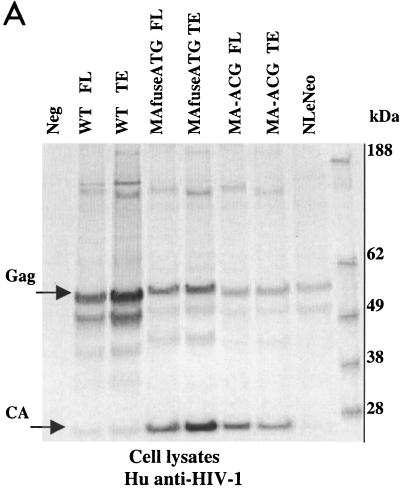
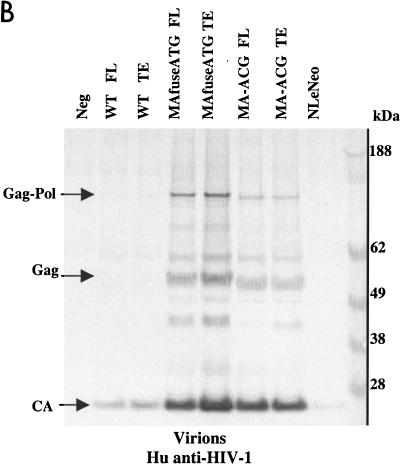
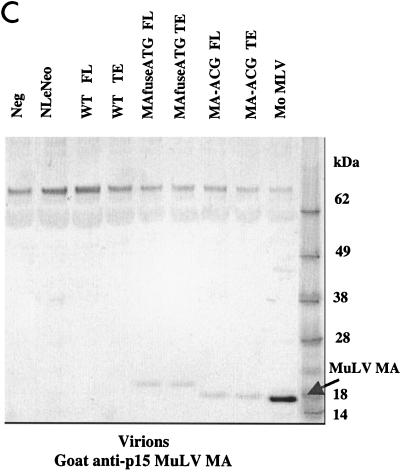
To examine the synthesis and processing of virion proteins by the chimeric proviruses, human embryonal kidney cells (HEK 293 EBNA) and murine fibroblasts expressing hu-CD4, CCR5, and cyclin T1 (MGT5.CyT) (9) were transfected with both FL and TE chimeric proviral DNA. MGT5.cyT cells contain an enhanced green fluorescent protein (EGFP) reporter gene under the control of HIV-1 LTR. Cell lysates and supernatant virions were prepared and analyzed on immunoblots as detailed in the legend to Fig. 2. Briefly, the material was fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. The membranes were then probed with anti-HIV antisera or anti-p15 MuLV MA antibody and visualized by incubation with alkaline phosphatase-conjugated secondary antibodies followed by 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (BCIP/NBT) substrate. The EGFP fluorescence in the MGT5.cyT cells transfected with HIV proviruses served as an internal control for transfection efficiency. A noninfectious proviral DNA, NLeNeo, in which the env gene of NL4-3 was replaced by a neomycin resistance gene, was also included as a control. This provirus expresses full-length Gag polyprotein, but no Env.
FIG. 2.
Chimeric viruses are processed in human cells. Immunoblot analyses of cell lysates and viruses from HEK 293 EBNA transfected with wild-type (WT) HIV-1, MAfuseATG, and MA-ACG proviral DNA. HEK 293 EBNA cells were transfected with 2 μg of each proviral DNA by lipofection with FuGENE (Roche Diagnostics), according to the manufacturer’s instructions. Culture medium was replaced the next day, and supernatants were collected 3 days posttransfection. (A) Cell lysates were separated by SDS-PAGE. The proteins were transferred to nitrocellulose membranes, probed with human antisera to HIV-1, incubated with alkaline phosphatase-conjugated secondary antibody, and developed with BCIP/NBT substrate. (B and C) Virions were pelleted by ultracentrifugation through a 20% sucrose cushion at 100,000 × g for 2 h at 4°C. Pellets were resuspended in lysis buffer and analyzed by Western blotting with human antisera to HIV-1 (B) or goat anti-p15 MuLV MA antibody (C). FL, provirus containing full-length HIV-1 envelope; TE, provirus containing truncated HIV-1 envelope. A GFP expression vector served as the negative control; NLeNeo is a NL4-3 clone in which the envelope has been replaced by the neomycin resistance marker.
Immunoblot analysis showed that the transfected human cells synthesized abundant chimeric pr55gag and that this was processed to the mature virion proteins as efficiently as native HIV Gag (Fig. 2A). Similarly, the chimeric virions contained the wild-type complement of virion proteins (Fig. 2B), with the exception of the HIV-1 p17 MA, which, as expected, was absent. p24 capsid (CA) was the predominant protein component of all virions. The slightly larger size of the pr55gag polyprotein and its processed intermediates in the MAfuseATG samples was an expected consequence of the extra 14 amino-terminal MA residues in this chimera. Probing the virion blots with anti- p15 MuLV MA antibody confirmed the incorporation of the MuLV MA (Fig. 2C). MA found in the MAfuseATG virions was larger than that observed for the MA-ACG virions due to the presence of the additional 14 amino-terminal residues. MA found in MA-ACG virions possessed the same mobility as wild-type MuLV MA, as expected. MuLV MA was not detected in the wild-type viruses. Both chimeric viruses were efficiently released into the culture supernatant of transfected human cells, reaching p24 CA concentrations of 200 to 400 ng per ml, similar to those of wild-type DH-12.
In the murine fibroblast cell line, consistent with earlier findings (1, 9), wild-type HIV-1 pr55gag polyprotein present in cell lysates was inefficiently processed to p24 CA (Fig. 3A). Very few virions were released from the transfected cells (Fig. 3B), and culture supernatant p24 CA levels reached only 3 ng/ml. Importantly, both chimeric viruses showed dramatically improved processing of pr55gag in the cell lysates and were released from transfected cells in substantially greater amounts (Fig. 3B). Chimeric viruses achieved supernatant p24 CA concentrations of 15 to 50 ng per ml, a level 5- to 16-fold greater than that of wild-type HIV-1. The Western blot protein profiles in the lysates and the viral pellet of the chimeric constructs were similar to those derived from transfected human cells. The chimeric viruses released from murine cells also incorporated MuLV p15 MA efficiently (Fig. 3C). While the HEK 293 EBNA cells generally released greater amounts of virions than the MGT5.CyT cells, this was due largely to the higher transfectability of the HEK 293 EBNA cells.
FIG. 3.
Chimeric viruses are processed and released efficiently from murine cells. Immunoblot analyses of cell lysates and viruses from MGT5.cyT transfected with wild-type (WT) HIV-1, MAfuseATG, and MA-ACG proviral DNA. Samples were processed as described earlier. (A) MGT5.cyT cell lysates probed with human antisera to HIV-1. (B and C) Pelleted virions probed with human antisera to HIV-1 (B) or goat anti-p15 MuLV MA antibody (C).
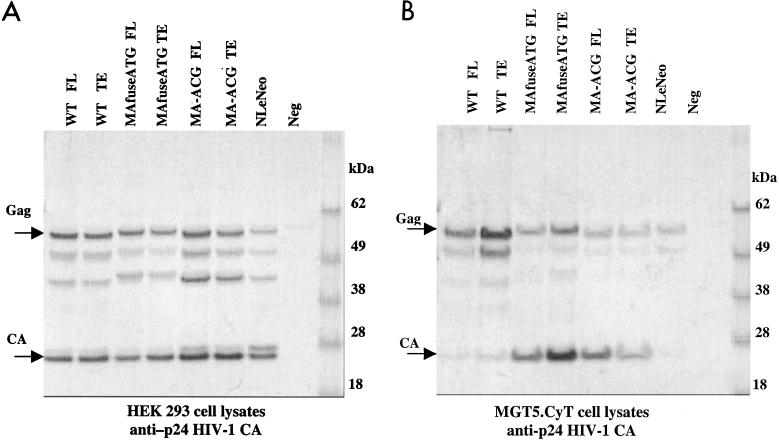
To assess the relative efficiency with which wild-type and chimeric Gag polyproteins were processed, the ratio of pr55gag to p24 CA for each construct was determined. Western blots of transfected cell lysates were probed with anti-p24 CA, and the intensities of the pr55gag and p24 CA bands were quantitated (Fig. 4 and Table 1). In human cells, the pr55gag/p24 CA ratios of the wild-type HIV-1 and chimeric constructs were similar and ranged from 0.7 to 1.1. A side-by-side quantitation of identical samples with alkaline phosphatase-conjugated antibodies and 35S-labeled streptavidin for the Western blots yielded similar results. In contrast to HEK-transfected lysates, in the murine fibroblasts, the ratio for wild-type HIV-1 was significantly greater, ranging from 9.1 to 9.9, reflecting the inefficiency of pr55gag processing. Importantly, the ratio for both chimeric Gag proteins in the murine cells ranged from 0.6 to 0.9, reflecting an efficiency of processing similar to that in the human cells. Thus, the chimeras appeared to have largely restored HIV-1 assembly and release in the murine cells. Further characterization of the chimeric viruses was performed with the TE versions.
FIG. 4.
Quantitation of chimeric Gag processing. pr55gag/p24 CA ratios in cell lysates of HEK 293 EBNA (A) and murine MGT5.cyT (B) cells transfected with wild-type (WT) and chimeric proviral DNA. Immunoblot analysis was performed with anti-p24 CA antibody. The intensities of the pr55gag and p24 CA bands were digitized and quantitated by densitometry, and the ratios were calculated (Table 1).
TABLE 1.
pr55gag/p24 CA ratios in HEK 293 EBNA and murine MGT5.cyT cellsa
| Cell type | Relative intensity
|
||
|---|---|---|---|
| pr55gag | p24 CA | pr55/p24 CA | |
| HEK 293 EBNA | |||
| Wild-type FL | 986 | 947 | 1.0 |
| Wild-type TE | 904 | 989 | 0.9 |
| MAfuseATG FL | 871 | 826 | 1.0 |
| MAfuseATG TE | 744 | 1,075 | 0.69 |
| MA-ACG-FL | 1,217 | 1,330 | 0.92 |
| MA-ACG-TE | 875 | 1,235 | 0.71 |
| MGT5.cyT | |||
| Wild-type FL | 1,857 | 203 | 9.1 |
| Wild-type TE | 2,953 | 299 | 9.9 |
| MAfuseATG FL | 1,127 | 1,299 | 0.9 |
| MAfuseATG TE | 1,677 | 2,541 | 0.7 |
| MA-ACG-FL | 867 | 1,431 | 0.6 |
| MA-ACG-TE | 650 | 777 | 0.8 |
Band intensities were quantitated by densitometry from Western blots of transfected cell lysates shown in Fig. 4.
Physical characterization of chimeric TE virions.
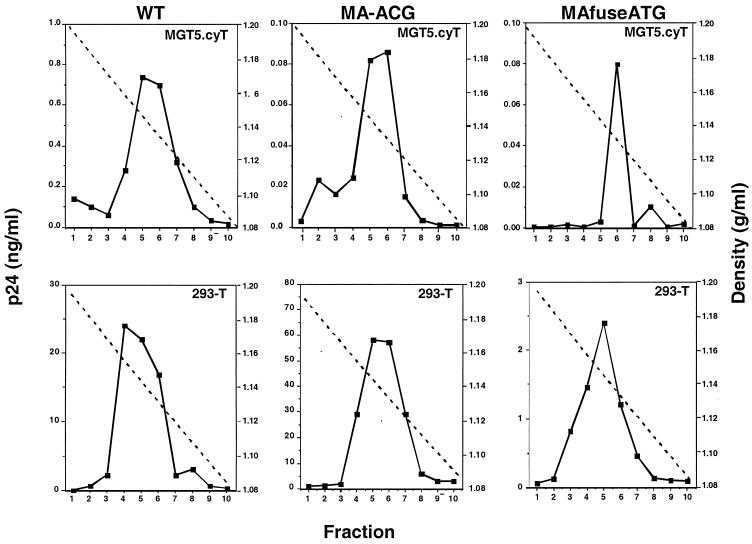
To determine whether the p24 CA released by chimera-transfected cells was in the form of virions or virus-like particles, the density of the particles released by the human and murine cells was measured on sucrose density gradients. This analysis showed that wild-type and chimeric TE virions derived from the human and murine cells banded sharply at 1.14 g/ml (Fig. 5), a value consistent with the density of HIV-1 virions.
FIG. 5.
Chimeric TE virions possess densities similar to those of wild-type (WT) HIV-1 virions. Viral supernatants collected 3 days after transfection of HEK 293-T or murine MGT5.cyT cells were concentrated by pelleting, layered on a 20 to 60% linear sucrose gradient, and centrifuged to equilibrium at 35,000 rpm in an SW40T rotor for 12 h. Ten 0.9-ml fractions were collected, and the p24 CA content in each gradient fraction was assayed by enzyme-linked immunosorbent assay.
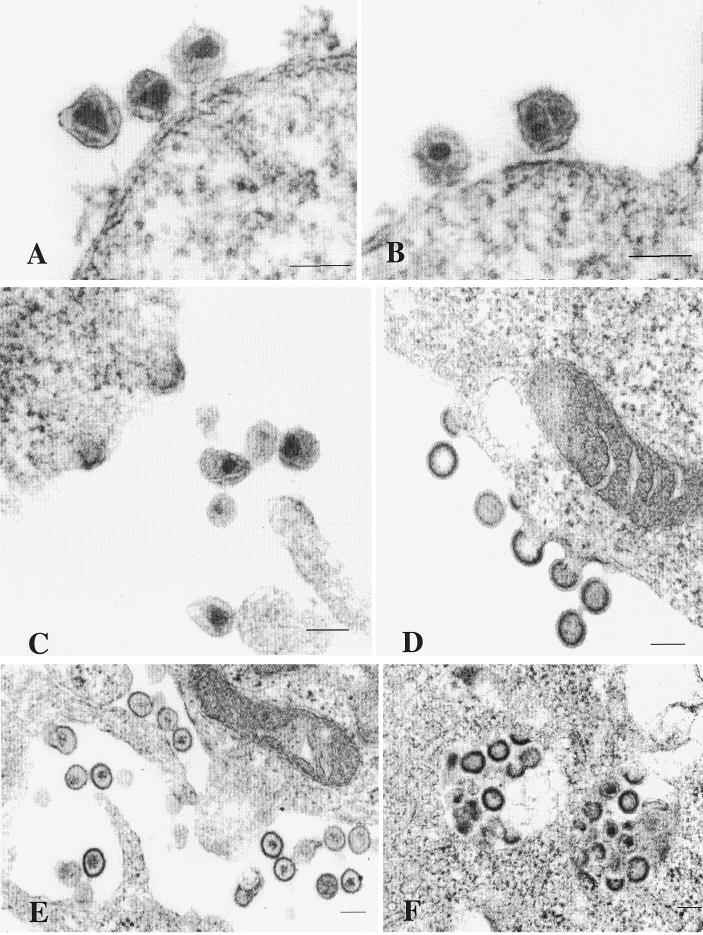
Transmission electron microscopy of the HEK 293 cells transfected with chimeric TE proviruses revealed viral particles in 5 to 10% of the cells (Fig. 6). Particles were not observed in mock-transfected cells. In cells transfected with the chimeric constructs, characteristic budding and extracellular virus particles were frequently observed. The mature extracellular particles exhibited characteristic 100-nm-diameter particles containing a cone-shaped core (Fig. 6A, B, and C). Immature particles containing an electron-dense spherical shell surrounding a translucent interior core region were also present. Budding particles contained an electron-dense crescent-shaped band surrounding the interior of the particles (Fig. 6C and D). Abnormal extracellular mature particles were observed (Fig. 6E) in some cells expressing the TE chimeras, but not in cells expressing wild-type virus. These contained irregular and ill-defined cores surrounded by an electrolucent area. Furthermore, although most of the budding occurred at the plasma membrane, some virus-like particles appeared to bud into small vacuoles and other intracellular regions within some cells (Fig. 6F). Thus, although the chimeric Gag constructs were capable of assembling virions with native morphology, they also resulted in some incorrectly assembled virions and inappropriate budding events.
FIG. 6.
Ultra-thin-section electron microscopic analysis of HEK 293 EBNA cells transfected with chimeric TE proviruses. A total of 2 × 106 HEK 293 EBNA cells were plated in 10-cm-diameter dishes and transfected 1 day later with 10 μg of proviral DNA (TE versions) by using FuGene reagent. Three days posttransfection, the cells were fixed in 2.5% gluteraldehyde. The cell pellets were washed, collected, and postfixed in 1% osmium tetraoxide. They were then stained with 2% aqueous uranyl acetate, dehydrated in a graded ethanol series, infiltrated, and embedded in Spurr’s plastic resin. The embedded cells were polymerized overnight at 70°C. Sections 60 to 80 nm thick were cut with an ultramicrotome, mounted on 200 mesh copper grids, poststained with lead citrate, and examined in a JEOL 1200 EX transmission electron microscope at 60 kV. (A and B) Mature MA-ACG virions. (C) Budding structures and mature virions of MAfuseATG. (D) Budding and immature virions of MA-ACG (E) Abnormal mature particles of MA-ACG. (F) MAfuseATG particles budding intracellularly. Each bar represents a scale of 0.1 μm.
Because of the lower transfection efficiency of MGT5.CyT cells, budding events were rarely observed for the chimeric viruses. However by examining samples that had been concentrated and pelleted from transfected MGT5.CyT cells, mature chimeric virions possessing wild-type morphology were found in all of the samples (data not shown).
Infectivity of chimeric TE viruses.
To measure the ability of the chimeric TE viruses to infect and replicate in human and murine cells, pseudotypes of wild-type and chimeric TE viruses were prepared by transfection of HEK 293 EBNA with equal amounts of proviral DNA and Mo-MuLV Env expression vector. The viruses were used to infect MGT5.cyT and GHOST.R5, a reporter cell line derived from transformed human osteosarcoma cells that expresses hu-CD4 and hu-CCR5 and contains an integrated LTR-EGFP reporter. Pseuodtyping the viruses with MuLV Env allowed us to achieve a higher rate of infection of the murine cells (9), enabling us to discern differences in the efficiencies of postentry events more sensitively. We quantitated the proportion of GFP-fluorescent GHOST.R5 cells (plated at 2.7 × 104 cells per well the day before infection) 3 days after infection with 40, 120, and 350 ng of p24 CA per ml of input virus. Wild-type viruses produced 28, 73, and 85% fluorescent cells, respectively; MAfuseATG TE viruses gave 0, 0.5, and 5% fluorescent cells; and MA-ACG TE viruses gave 0.8, 2.2, and 11.3% fluorescent cells, respectively. Infection with negative control NL4eNeo particles did not result in any observable fluorescent cells. Thus, although the chimeric viruses were competent for the early steps of the virus replication cycle, they were clearly less infectious than wild-type HIV-1. At similar doses of input p24 CA, MA-ACG infected more cells than MAfuseATG chimera. Small but significant numbers of fluorescent cells were observed in MGT5.cyT cells infected with 1 μg of p24 CA of chimeric TE viruses (data not shown).
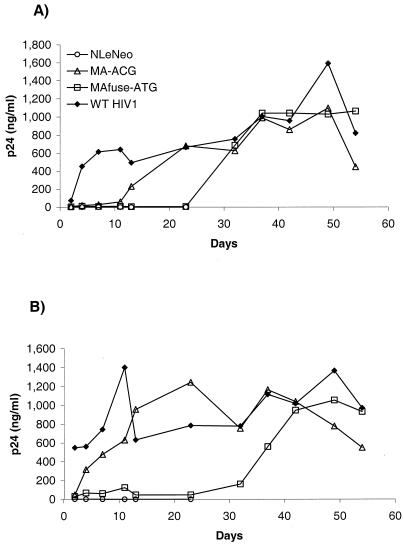
To compare the growth kinetics of the chimeric TE viruses with those of wild-type HIV-1, we infected the human T-cell line MT-4 with increasing amounts of viruses derived from HEK 293 EBNA transfections and monitored p24 CA levels in the culture supernatant. Infection of MT-4 with 5 ng of p24 CA input virus did not result in productive infection (data not shown); however, at higher doses of input virus, both chimeras replicated in MT-4 after a prolonged delay and achieved p24 levels comparable to those of wild-type HIV-1 (Fig. 7). At both 30 ng (Fig. 7A) and 125 ng (Fig. 7B) of input p24 CA, the rise in MAfuseATG infection lagged behind that of MA-ACG, consistent with its lower infectivity observed earlier. The reduced infectivity of the MAfuseATG chimera may reflect the disruptive effects of the additional MA residues in the chimera. Addition of zidovudine to the cultures abolished or significantly retarded the growth of the chimeric viruses (data not shown). The delay in the growth of both chimeras may be due to the necessity for adaptive changes to be acquired during the lag period when the viruses were replicating at low levels. We performed similar infections of stimulated human peripheral blood mononuclear cells (PBMCs) with the chimeric viruses. Although all of the viruses could initially infect PBMCs, the infection did not spread, and peak levels of p24 CA were 2 logs lower than for MT-4 cells (data not shown).
FIG. 7.
Chimeric TE viruses replicate in MT-4 cells with delayed kinetics. MT-4 cells (5 × 105) were infected with 30 (A) and 125 (B) ng of p24 CA of input virus derived from HEK 293 EBNA transfections. The cells were washed the following day and split regularly. Culture medium was collected for p24 CA assay at intervals.
To determine whether chimeric MAfuseATG and MA-ACG Gag could rescue wild-type virus assembly in the murine cells, we cotransfected MGT5.cyT with equal amounts of chimeric TE and wild-type HIV-1 constructs and monitored pr55gag polyprotein processing and virus release. The use of an anti-p17 HIV-1 MA antibody allowed us to discern products originating from the wild-type HIV-1 constructs. Although cotransfected murine cells released appreciable quantities of particle-associated p24 CA, no p17 MA was detected (data not shown). This suggests that the p24 CA originated from the chimeric constructs, not from the wild-type HIV-1 provirus. Thus, coexpression of the chimeras and wild-type Gag in MGT5.cyT cells did not lead to the rescue of wild-type Gag processing, consistent with a failure of the chimeric and wild-type Gag to coassemble. To ensure that the lack of complementation was not simply the result of low cotransfection efficiency, we used an MuLV chimera that contained the HIV-1 MA and that can be rescued into viral particles when cotransfected with wild-type MuLV into murine 3T3 fibroblasts (3). We cotransfected MGT5.cyT cells with the MuLV-HIV-1 MA chimera and wild-type MuLV. Under our transfection conditions, we were able to reproduce this finding, arguing that our cotransfection efficiency was sufficiently high to detect rescue had this occurred.
We have shown here that the substitution of MuLV MA sequences into HIV-1 alleviates the block to virion assembly and Gag processing in murine cells. This finding is consistent with a model in which, in mouse cells, Gag fails to target to the plasma membrane and as a result, virions fail to assemble, be processed, and be released. This appears to be the result of a required host cofactor that is nonfunctional in the murine fibroblasts (1, 8). The MuLV MA substitution may have removed the requirement for this cofactor or allowed interaction with the murine form of the cofactor. The inability of chimeric Gag to rescue wild-type HIV-1 Gag processing is consistent with a requirement for Gag to be transported to the plasma membrane for Gag multimerization to occur. The failure of wild-type HIV-1 Gag to traffic to the plasma membrane would preclude its rescue by the chimeric Gag. It is unlikely that the Gag-Gag interaction domains in the chimeras have been disrupted, because the chimeric Gags can by themselves assemble into particles. It is conceivable that the chimeric Gags preferentially assemble with like molecules rather than with wild-type HIV-1 pr55gag. However, the interaction domains primarily map to the NC region and the carboxyl terminus of CA (4), both of which are unaltered in the chimeras.
The chimeric TE viruses were poorly infectious for human and murine cells, although they did replicate with delayed kinetics in MT-4 cells. This finding is reminiscent of HIV-1 mutants in which MA was replaced by a heterologous src myristyl anchor and which contained a truncated Env (13). In MT-4 (12), the gp41 cytoplasmic tail is not required for virus replication, making it unlikely that its absence accounts for the reduced replication of the chimeras. Additional defects relating to the incompatibility of the MuLV MA for HIV-1 functions are also likely to be responsible for their compromised replication efficiency in human cells. The failure of the chimeric viruses to replicate in MGT5.cyT cells may indicate that these defects are more pronounced in mouse cells or that additional blocks are present. The reciprocal chimera (HIV-1 MA in MuLV) is also replication competent, albeit with reduced efficiency (2). Thus, although MA from divergent retroviruses can functionally substitute for one another for portions of the virus replication cycle, they do not for others. Further adaptation or engineering of these chimeras may restore viral replication, and such viruses would be useful for establishing a murine model for HIV-1 replication.
Acknowledgments
We thank R. Shibata and M. Martin (NIAID) for the DH-12 clone, P. Patten for project advice, M. Emerman (FHCRC) for the MuLV-HIV-1 MA chimera, and J. Bernbaum (EMBS) for electron microscopy services.
This work was supported by grant 07-01-0240 from the National Institutes of Standards and Technology, Advanced Technology Program. N.R.L. is an Elizabeth Glaser Fellow of the Pediatric AIDS Foundation.
REFERENCES
- 1.Bieniasz, P. D., and B. R. Cullen. 2000. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 74:9868–9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deminie, C. A., and M. Emerman. 1994. Functional exchange of an oncoretrovirus and a lentivirus matrix protein. J. Virol. 68:4442–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deminie, C. A., and M. Emerman. 1993. Incorporation of human immunodeficiency virus type 1 Gag proteins into murine leukemia virus virions. J. Virol. 67:6499–6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freed, E. O. 1998. HIV-1 Gag proteins: diverse functions in the virus life cycle. Virology 251:1–15. [DOI] [PubMed] [Google Scholar]
- 5.Freed, E. O., J. M. Orenstein, A. J. Buckler-White, and M. A. Martin. 1994. Single amino acid changes in the human immunodeficiency virus type 1 matrix protein block virus particle production. J. Virol. 68:5311–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohl, N. E., R. E. Diehl, E. Rands, L. J. Davis, M. G. Hanobik, B. Wolanski, and R. A. F. Dixon. 1991. Expression of active human immunodeficiency virus type 1 protease by noninfectious chimeric virus particles. J. Virol. 65:3007–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mammano, F., F. Salvatori, S. Indraccolo, A. De Rossi, L. Chieco-Bianchi, and H. G. Göttlinger. 1997. Truncation of the human immunodeficiency virus type 1 envelope glycoprotein allows efficient pseudotyping of Moloney murine leukemia virus particles and gene transfer into CD4+ cells. J. Virol. 71:3341–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariani, R., B. A. Rasala, G. Rutter, K. Wiegers, S. M. Brandt, H.-G. Krausslich, and N. R. Landau. 2001. Mouse-human heterokaryons support efficient human immunodeficiency virus type 1 assembly. J. Virol. 75:3141–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariani, R., G. Rutter, M. E. Harris, T. J. Hope, H.-G. Kräusslich, and N. Landau. 2000. A block to human immunodeficiency virus type 1 assembly in murine cells. J. Virol. 74:3859–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McBride, M. S., and A. T. Panganiban. 1997. Position dependence of functional hairpins important for human immunodeficiency virus type 1 RNA encapsidation in vivo. J. Virol. 71:2050–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moosmayer, D., H. Reil, M. Ausmeier, J.-G. Scharf, H. Hauser, K. D. Jentsch, and G. Hunsmann. 1991. Expression and frameshifting but extremely inefficient proteolytic processing of the HIV-1 gag and pol gene products in stably transfected rodent cell lines. Virology 183:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami, T., and E. O. Freed. 2000. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc. Natl. Acad. Sci. USA 97:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reil, H., A. A. Bukovsky, H. R. Gelderblom, and H. G. Gottlinger. 1998. Efficient HIV-1 replication can occur in the absence of the viral matrix domain. EMBO J. 17:2699–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnierle, B. S., J. Stitz, V. Bosch, F. Nocken, H. Merget-Millitzer, M. Engelstadter, R. Kurth, B. Groner, and K. Cichutek. 1997. Pseudotyping of murine leukemia virus with the envelope glycoproteins of HIV generates a retroviral vector with specificity of infection for CD4-expressing cells. Proc. Natl. Acad. Sci. USA 94:8640–8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibata, R., M. D. Hoggan, C. Broscius, G. Englund, T. S. Theodore, A. Buckler-White, L. O. Arthur, Z. Israel, A. Schultz, H. C. Lane, and M. A. Martin. 1995. Isolation and characterization of a syncytium-inducing, macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J. Virol. 69:4453–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]