Abstract
Group A rotaviruses are major pathogens causing acute gastroenteritis in children and animals. To determine if group A rotavirus replicates and induces disease in rats, antibody-negative Lewis neonatal or adult rats were inoculated orally with tissue culture-adapted human (Wa, WI61, and HAL1166), simian (rhesus rotavirus [RRV] and SA11), bovine (WC3), lapine (ALA), or porcine (OSU) rotavirus strains, wild-type murine (ECwt) rotavirus strain, or phosphate-buffered saline (PBS). Rotavirus infection in rats was evaluated by (i) clinical findings, (ii) virus antigen shedding or infectious virus titers in the feces or intestinal contents measured by enzyme-linked immunosorbent assay or fluorescent-focus assay, (iii) histopathological changes in the small intestine, (iv) distribution of rotavirus antigen in small-intestine sections by immunofluorescence, and (v) growth rate. Rotavirus infection of 5-day-old but not ≥21-day-old rats resulted in diarrhea that lasted from 1 to 10 days postinoculation. The severity of disease and spread of infection to naÏve littermates differed depending on the virus strain used for inoculation. The duration of virus antigen shedding following infection was considerably prolonged (up to 10 days) in neonatal rats compared to that in 21-day-old rats (1 or 2 days). Based on lack of virus antigen shedding and disease induction, the murine ECwt rotavirus was the only strain tested that did not infect rats. Histopathological changes in the small-intestine mucosa of 5-day-old RRV-inoculated rats but not of PBS-inoculated rats was limited to extensive enterocyte vacuolation in the ileum. In RRV-inoculated neonatal rats, rotavirus antigen was detected in the epithelial cells on the upper half of the intestinal villi of the jejunum and ileum. In addition, infection of neonatal rats with RRV but not with PBS resulted in reduced weight gain. Rats infected with group A rotaviruses provide a new animal model with unique features amenable to investigate rotavirus pathogenesis and the molecular mechanisms of intestinal development, including physiological factors that may regulate age-dependent rotavirus-induced diarrhea.
Group A rotaviruses are the most common causative agents of acute gastroenteritis in children under 2 years of age and are also associated with diarrhea in the young of avian (chicken, turkey, and pigeon) and many mammalian (simian, porcine, bovine, ovine, caprine, equine, canine, feline, lapine, and murine) species (17, 22). Rotavirus infection is primarily restricted to the villus epithelium of the small intestine, and the outcome of infection is age restricted. The impact of rotavirus disease in humans includes over 600,000 annual associated deaths in developing countries, and in the United States, annual economic losses due to rotavirus infections have been conservatively estimated at $1 billion (12, 22, 24, 31, 39, 43, 53, 71). Therefore, development of a safe and effective rotavirus vaccine is a global priority.
The use of animal models of rotavirus infection has provided key insights in our understanding of the pathogenesis of both human and animal rotaviruses. Five models in three large (cow, pig, and sheep) and two small laboratory (rabbit and mouse) animal species have been used to define parameters of rotavirus infection, pathology, disease, immune response, and test vaccine efficacy (4, 6–10, 14–17, 23, 74, 75). Much of the early work on rotavirus pathogenesis utilized large animals, generally either colostrum-deprived or gnotobiotic models (reviewed in references 17, 26, and 59). Most studies in calves used rotaviruses of bovine origin, but some heterologous (nonbovine) rotaviruses can also infect and induce mild disease in calves (17, 26, 45, 52, 55, 59). Piglets are monogastric animals with intestinal physiology that resembles that of humans and are susceptible to infection and severe disease by some rotavirus isolates from other species, including humans (17, 19, 26, 35, 51, 59, 70). Compared with infections in piglets and calves, rotavirus infection in lambs results in less severe histopathological changes and mild clinical disease (17, 26, 59, 64). Large animal models have limited use due to high costs, the restricted availability of isolation or germfree facilities for large animals, and the need for specialized equipment and staff—all factors that preclude their use in large-scale studies.
Small animal models have several advantages over large animal models, including cost-effectiveness, the ability to incorporate large numbers of animals in studies, the feasibility of isolation of large numbers of infected animals, short gestations and multiparous births, and the availability of rotavirus-naÏve animals. Presently, rabbits and mice are two small animal models routinely used to study rotavirus infections. The rabbit model was the first small animal model developed to examine active humoral immunity and protection (6–10, 14–17), followed by the development of the adult mouse model (4, 23, 74, 75). Limitations of the rabbit and adult mouse models of rotavirus infection are that (i) human rotavirus strains do not efficiently replicate in either animal, (ii) clinical disease is only observed in animals of ≤2 weeks of age, (iii) only homologous virus strains (isolated from the same species) replicate efficiently and spread horizontally to uninoculated control animals, whereas heterologous virus strains (isolated from a different species) do not, and (iv) the small size of the intestinal tract of neonatal mice does not allow pathophysiological studies, and while the rabbit’s intestinal tract is of an optimal size, studies that use naÏve rabbits are expensive (4, 6–10, 14–17, 23, 26, 54, 74, 75). Early and recent studies demonstrated that rotavirus disease but not infection is age restricted in mice and rabbits following inoculation of murine and lapine rotavirus strains, respectively (4, 8, 38, 50, 54, 56, 57, 62, 66). It was recently reported that, among 27 different heterologous (nonlapine) or reassortant rotavirus strains, only simian rhesus rotavirus (RRV) strain and human rotavirus strains belonging to the P11[14] serotype replicate or are capable of effective horizontal transmission in adult rabbits (6, 9). In addition, only limited or abortive virus replication occurs in mice infected with heterologous (nonmurine) rotaviruses, as evidenced by lack of virus excretion above input titers and no transmission of infection from inoculated to control animals (4, 17, 23, 26, 54). Therefore, the limitations of both the rabbit and mouse models highlight the need for other small animal models to study homologous and heterologous group A rotavirus infection, pathogenesis, and pathophysiology.
Rotavirus infection of rats was initially ascribed to a group B rotavirus called infectious diarrhea of infant rats (IDIR) (72), a virus antigenically related to human, bovine, and porcine group B rotaviruses (20, 34, 58, 72, 73). No group A rat rotavirus has been identified to date, and data on group A rotavirus infection of rats are limited. The group A simian RRV strain, but not simian SA11, bovine NCDV, or human Wa strains, replicates and induces diarrhea in 3- to 5-day-old rats, but the data were never fully documented because details of experiments and results were not published (77). The simian SA11 strain reportedly infects Fisher 344 germfree neonatal rats (27, 28). Only two reports describe seroepidemiological rates of group A rotaviral infections in rats. One study failed to detect antibodies to group A rotavirus in unspecified albino laboratory rats, wild rats (Rattus rattus), and shrews (Suncus murinus) resident in an animal house (1), while a high incidence (76%) of group A rotavirus antibodies was reported in serum specimens collected from wild rats in the Shizuoka prefecture in Japan (69). Thus, group A rotavirus infections of rats can occur, but the lack of a documented animal model may have obscured this fact and discouraged additional studies to search for rat rotaviruses, especially because, nowadays, animals facilities obey strict husbandry regulations and group A rotavirus infections may no longer be a veterinary problem in such facilities. To complicate matters further, Wistar rats were reported to lack the cellular group A rotavirus receptor (3), although others showed that Wistar rat mucins inhibit the replication of the group A RRV and Wa strains (78). Keeping in mind that (i) the size of the rat intestine is ideal for physiological studies, including Ussing chamber analyses, (ii) the physiology of the gastrointestinal tract of rats is well established, (iii) rats are cost effective, and (iv) the sequence of the rat genome is projected to be available by 2005, the present study was designed to examine in detail if heterologous (nonrat) group A rotaviruses replicate, spread, and induce disease in rats. Our work establishes a new animal model of age-dependent, rotavirus-induced diarrheal disease that includes the ability to study human rotavirus infections in a small animal model.
MATERIALS AND METHODS
Animals.
Two-month-old dams with litters or 21- or 270-day-old rotavirus antibody-free Lewis (LEW/SsNHsd) rats were obtained from Charles River Laboratories, Inc. (Wilmington, Mass.) or Harlan Sprague-Dawley (Houston, Tex.). Control and inoculated rats were housed individually in microisolator cages in the same room under negative pressure in a BL2 containment facility at 21 ± 2°C on a 12-h-12-h light-dark cycle with lights on at 0800 h. Food (Rodent Laboratory Chow 5001; Ralston Purina, St. Louis, Mo.) and deionized water were autoclaved and provided ad libitum from the day of the rats’ arrival at Baylor College of Medicine until the completion of the experiments. To avoid biological variation between dams and their respective litters, known as litter effect, pups were shuffled prior to all experiments and each litter was adjusted to 10 pups per dam. Some litters were weaned by removing each rat from the dam at 21 days of age. Control animals were always handled prior to virus-inoculated animals. Prior to inoculation, all rats were rotavirus antibody negative at a dilution of 1:50 as measured by enzyme-linked immunosorbent assay (ELISA) (M. Ciarlet, M. E. Conner, and M. K. Estes, unpublished data).
Cells, viruses, and antibodies.
The simian RRV 2 strain [RRV-2 (MMU 18006)] (P5B[3], G3) was originally isolated from the feces of a 3-month-old rhesus monkey with diarrhea (67), and a stock from the original isolate was obtained from H. B. Greenberg (Stanford University Medical School, Palo Alto, Calif.). The simian rotavirus strain SA11 (clone 3) (P3B[2], G3) (32) was derived from a stock of the original isolate (SA11) (6, 21, 40) that was plaque purified three times in our laboratory. The porcine rotavirus OSU (P9[7],G5) was obtained from L. J. Saif (Ohio State University, Wooster) and was shown previously to be attenuated in neonatal mice (79). The human rotavirus strains Wa (P1A[8], G1) and WI61 (P1A[8], G9) and the bovine rotavirus strain WC3 (P5[7], G6) were kindly provided by H. F. Clark (Wistar Institute, Philadelphia, Pa.). The human rotavirus strain HAL1166 (P11[14], G8) was provided by Y. Hoshino (National Institutes of Health, Bethesda, Md.). The lapine rotavirus strain ALA (P11[14], G3) was provided by M. Thouless (University of Washington, Seattle) (6). The viruses were propagated in fetal African green monkey kidney MA104 cells in the presence of 1 μg of trypsin as described previously (6, 14). Virus titers were determined by fluorescent-focus assay (FFA) and were expressed as focus-forming units (FFU) (7). The wild-type murine rotavirus ECwt (P[16], G3) was kindly provided by H. B. Greenberg (22) and was propagated in suckling mice (49). The lot of ECwt used for our experiments was the same lot used routinely in our laboratory to productively infect mice.
Purified immunoglobulin G (IgG) from a rabbit hyperimmune-phase serum raised to virus-like particles containing the structural rotavirus proteins VP2 and VP6 (2/6-VLPs) (7) or to a purified peptide corresponding to amino acids 114 to 135 of simian SA11 rotavirus strain nonstructural protein 4 (NSP4) (2) was used to detect rotavirus antigen in frozen small intestine sections. Rabbit IgG was purified by a two-step procedure: (i) albumin and other non-IgG proteins were precipitated with caprylic (octanoic) acid, and (ii) the IgG fraction was precipitated with ammonium sulfate and the IgG in the precipitate was suspended in phosphate-buffered saline (PBS) (pH 7.4) (44).
Animal inoculations and procedures.
Rats of ≥21 days of age were inoculated orally with a syringe and a blunt-ended feeding needle (Popper and Sons, New Hyde Park, N.Y.) with 1 ml of either human (Wa, 4.4 × 106 FFU; WI61, 6 × 106 FFU; and HAL1166, 6 × 105 FFU), simian (RRV, 4 × 107 FFU; and SA11, 2 × 107 FFU), bovine (WC3, 8 × 106 FFU), or lapine (ALA, 2 × 106 FFU) tissue culture-adapted rotavirus strains or 100 μl of the wild-type murine (ECwt, 104 50% adult mouse shedding dose [SD50]) rotavirus strain. Five-day-old rat pups were gavaged with 0.5 ml of either human (Wa, 2.2 × 106 FFU; WI61, 3 × 106 FFU; and HAL1166, 3 × 105 FFU), simian (RRV, 2 × 107 FFU; and SA11, 1 × 107 FFU), bovine (WC3, 4 × 106 FFU), lapine (ALA, 1 × 106 FFU), or porcine (OSU, 5 × 107 FFU) tissue culture-adapted rotavirus strains or 100 μl of the wild-type murine (ECwt, 104 adult mouse SD50) rotavirus strain. Inoculation of 5-day-old pups with simian RRV was performed 10 independent times. Since the aim of this study was to test the replication capability of heterologous (non-rat) rotavirus strains in rats, we inoculated rats with the highest-titered virus available because it has been reported to be important for the replication efficacy of rotavirus strains in heterologous hosts (6, 23). Negative-control 5-day-old and ≥21-day-old rats were mock inoculated with 0.5 and 1 ml of PBS, respectively. A subset of neonatal rats inoculated with RRV or PBS were weighed daily from 0 to 16 days postinoculation (DPI) to monitor gain of weight.
At 0, 6, 12, 24, 48, 72, 96, 120, 168, and 216 h postinoculation (hpi), 5-day-old rats inoculated with simian RRV or PBS were anesthetized and euthanatized by subcutaneous administration of 200 to 300 μl of a mixture of xylazine, ketamine hydrochloride, and acepromazine maleate (0.19, 3.75, and 0.037 mg/pup, respectively). At each time point, tissues were collected from two control (PBS) pups and four RRV-inoculated rat pups.
Sample collection and processing.
Individual fecal samples of rats that were ≥21 days of age were collected 0 to 10 DPI. Cages and bedding were changed on a daily basis during collection of fecal samples to avoid cross-contamination. In neonatal rats, collection of individual fecal samples and determination of diarrhea were performed once a day by gently pressing the abdomen. Fecal samples were processed as a 10% solution in cold (4°C) PBS (140 mM NaCl, 2.7 mM KCl, 1 mM CaCl2, 0.5 mM MgCl2, 8 mM Na2HPO4, and 1.5 mM KH2PO4) containing penicillin (200 U/ml), streptomycin (200 μg/ml), and gentamicin (2 mg/ml) antibiotics (Gibco BRL-Life Technologies, Grand Island, N.Y.) as described earlier (6, 10). Diarrhea was noted and scored from 1 to 4 based on color, consistency, and amount of stool (2). A score that was ≥ 2 was considered proof of diarrhea, whereas a score that was < 2 was not. Daily percent diarrhea for each group was calculated by dividing the number of diarrheic samples by the number of total samples collected each day, since fecal samples from individual 5-day-old rats could not be obtained every day.
Removal and ligation of distal and proximal ends of the intestinal tract from the subset of euthanatized RRV-inoculated and PBS control rats were performed as previously described (8, 14). Gut homogenates of the cecum, small intestine, and large intestine and three sections (1 cm in length) of small intestine corresponding to the duodenum (proximal), jejunum (middle), and ileum (distal) were collected from each rat pup.
Detection of rotavirus antigen by ELISA.
The presence of rotavirus antigen in rat fecal samples or intestinal contents was determined by ELISA as described previously (6, 14). A positive reaction was defined if the optical density (OD) of the virus well minus the OD of wells lacking antigen was ≥ 0.1 and if this OD was at least 2 standard deviations greater than the ODs of the negative-control samples.
Detection of infectious rotavirus by FFA.
The titers of infectious virus from rat intestinal contents or fecal samples were measured by FFA and expressed in FFU (7). The 10% intestinal contents or fecal suspensions were serially diluted (10-fold), and each dilution was assayed in duplicate on MA104 cell monolayers. Infectivity titers were expressed in FFU. Due to toxicity, the lowest dilution of fecal samples tested for infectivity titers was 1:10. When fluorescent foci in 1/10 dilutions could not be visualized by fluorescence microscopy, the samples were considered negative, and a value of 50 FFU was arbitrarily assigned.
Analysis of RNA by polyacrylamide gel electrophoresis.
To confirm that rats shed only the virus with which they were inoculated, fecal suspensions from virus-inoculated rats that were positive for rotavirus by ELISA were tested by polyacrylamide gel electrophoresis. Nucleic acids of representative input and recovered virus from fecal material were extracted and subjected to electrophoresis in a 7% polyacrylamide gel, and genome segments were visualized by silver staining (5).
Preparation of small intestine tissue sections.
To evaluate histopathology or the distribution of acid and neutral mucopolysaccharides, small intestine sections were fixed in 10% zinc-formalin for 24 h and then transferred to 70% graded ethanol for dehydration. Samples were embedded in paraffin wax and sectioned at 4 μm as described (8).
To detect rotavirus antigen or neutral fats, small intestine sections were placed in plastic molds, covered with the frozen-specimen-embedding medium Cryomatrix (Shandon Lipshaw, Inc., Pittsburgh, Pa.), and frozen in liquid nitrogen-chilled isopentane (2-methylbutane). A pellet of RRV-infected MA104 cells was used as positive antigen control. Small intestine samples and control MA104 cells were sectioned at 4 μm and allowed to air dry overnight at room temperature (RT) on slides.
Evaluation of histopathology.
Histopathological findings were evaluated in small intestine sections of neonatal rats sacrificed at 0, 6, 12, 24, 48, 72, 96, 120, 168, and 216 hpi. Sections were stained with hematoxylin and eosin (8, 18, 52). Stained sections were visualized under the light microscope, and villi were examined for presence of enterocyte injury, inflammation, and vacuolization.
Additional samples from mid-duodenum, -jejunum, and -ileum of neonatal rats sacrificed at 0, 12, 24, 48, and 72 hpi were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) and subsequently with 1% osmium tetraoxide. Fixed samples were embedded in plastic for thin sectioning and electron microscopy (EM) analysis. Sections (400 Å) were stained with uranyl acetate and viewed under a JEOL 1200 transmission electron microscope.
Detection of rotavirus antigen in small intestine sections by immunofluorescence.
Cut frozen sections from rats sacrificed at 0, 6, 12, 24, 48, 72, 96, 120, 168, and 216 hpi were fixed in 95% alcohol for 10 min and allowed to completely air dry. Slides were washed twice with distilled H2O followed by PBS containing 0.05% Tween 20 (Bio-Rad Laboratories, Richmond, Calif.) (PBS-T) prior to blocking with 2% normal goat serum (Dako Diagnostics, Inc., Mississauga, Canada) for 30 min at RT. Excess goat serum was decanted, not rinsed, and slide contents were incubated for 2 h at RT with a 1:100 dilution of either rabbit anti-2/6-VLP or anti-NSP4 (amino acids 114 to 135) hyperimmune-phase serum diluted in ChemMate (Dako Diagnostics, Inc.). Slides were washed twice with PBS-T, and their contents were incubated with goat anti-rabbit Ig conjugated to fluorescein isothiocyanate (Sigma Chemical Co., St. Louis, Mo.) diluted 1:200 in PBS for 2 h at RT. Following incubation, slides were rinsed with PBS-T and immediately covered with Faramount aqueous mounting media (Dako Diagnostics, Inc.) and coverslips. Fluorescence was examined under the UV light with an Olympus IX70 microscope (Olympus America, Inc., Lake Success, N.Y.).
Detection of neutral fats and acid and neutral mucopolysaccharides.
To detect neutral fats, cut frozen sections from neonatal rats sacrificed at 0, 24, 48, and 72 hpi were stained with red oil O (Sigma) (46, 48). Sections were rinsed in 60% isopropyl alcohol and stained in 0.3% oil red O solution for 30 min. Slides were rinsed twice in 60% isopropyl alcohol and were then rinsed in distilled H2O until clear. Subsequently, slides were counterstained in Mayer’s hematoxylin for 1 min followed by rinsing in distilled H2O. Then slides were rinsed in ammonia H2O and distilled H2O before mounting with Aqua-Mount (Dako Diagnostics, Inc.) before being visualized under the light microscope.
The pattern of acid mucopolysaccharides was determined with alcian blue as described (35, 37, 60) with some modifications. Briefly, formalin-fixed, cut small-intestine sections from neonatal rats sacrificed at 0, 24, 48, and 72 hpi were deparaffinized, hydrated in distilled H2O, and placed in 3% acetic acid for 3 min. Without rinsing, slides were placed in 5% alcian blue, pH 2.5 (EM Science, Gibbstown, N.J.) for 30 min at RT. Slides were washed in running tap water for 10 min and then rinsed in distilled H2O before counterstaining in nuclear-fast red solution (Sigma) for 5 min. Slides were washed in running tap water for at least 1 min before dehydration with two changes of 95% alcohol, absolute alcohol, and clear xylene. Slides were mounted in Krystalon (EM Science) and were visualized under the light microscope.
Neutral mucopolysaccharides and basement membranes were studied by the periodic acid-Schiff reaction as described previously (35, 37, 60) with some modifications. Briefly, formalin-fixed, cut small-intestine sections from neonatal rats sacrificed at 0, 24, 48, and 72 hpi were deparaffinized, hydrated in distilled H2O, and placed in 1% periodic acid for 5 min. Slides were washed three times with distilled H2O and placed in Schiff reagent (EM Science) for 15 min and were then placed in warm tap water for 5 min. To develop full color, slides were washed in running tap water for 10 min before counterstaining in Mayer’s hematoxylin for 1 min. Subsequently, the slides were washed in distilled H2O and then in ammonia H2O. Finally, the slides were dehydrated as described above and mounted in Krystalon (EM Science) before visualization under the light microscope.
Statistical analysis.
Statistical analyses were performed using SPSS Version 7.5 for Windows (SPSS, Inc., Chicago, Ill.). Individual data for the mean days of virus shedding and percent diarrhea and disease severity scores for different age groups were compared using the Kruskal-Wallis test followed by the Mann-Whitney U test. Means of the slopes of the cumulative weight gain per day were determined by linear regression and compared using the t test for equality of means. Correlation coefficients were calculated by using Pearson’s correlation coefficient.
RESULTS
Infection of 5-day-old rats inoculated with different group A rotavirus strains.
Although group A rotaviruses have not been identified in rats, we wanted to directly examine whether heterologous (non-rat) group A rotaviruses would replicate, spread, and induce disease in rats. Therefore, we inoculated 5-day-old rats with different tissue culture-adapted group A rotavirus strains isolated from humans (Wa, WI61, and HAL1166), monkeys (RRV and SA11), cows (WC3), rabbits (ALA), or pigs (OSU) or a wild-type rotavirus strain isolated from mice (ECwt) or PBS. Prior to all experiments, pups were shuffled and each litter was adjusted to 10 pups per dam to avoid biological variation between dams and their respective litters. For most of the experiments, eight pups were inoculated with virus and two were inoculated with PBS to determine if rotavirus infection would spread among mock-inoculated littermates. The efficiency of horizontal transmission of RRV was further evaluated by inoculating eight pups with PBS and two pups with RRV. None of the dams were inoculated. One group of 10 pups was PBS inoculated, and another group was inoculated with RRV for each individual experiment as controls. PBS inoculations were performed prior to any virus inoculation.
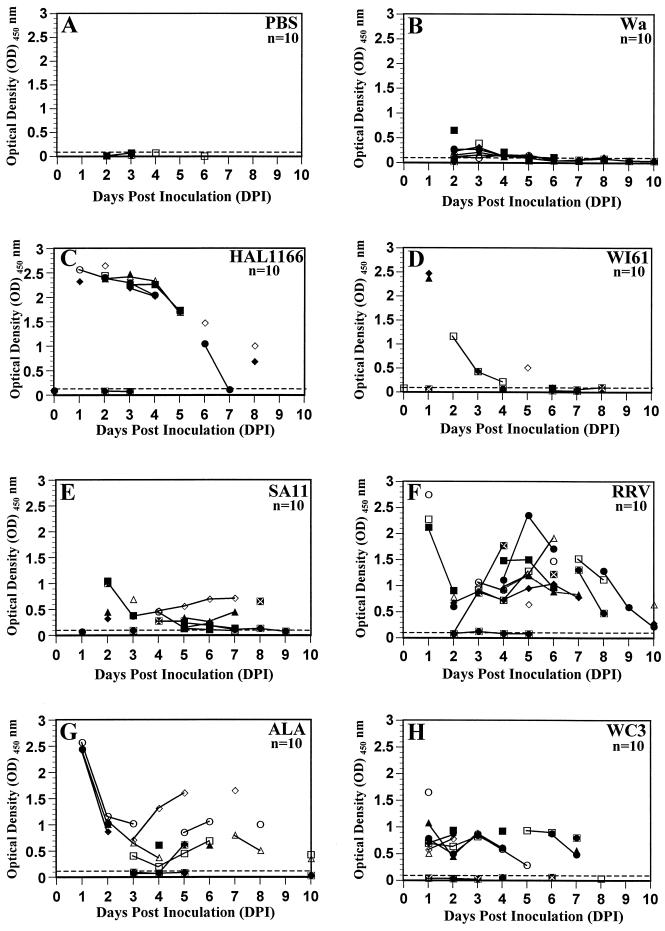
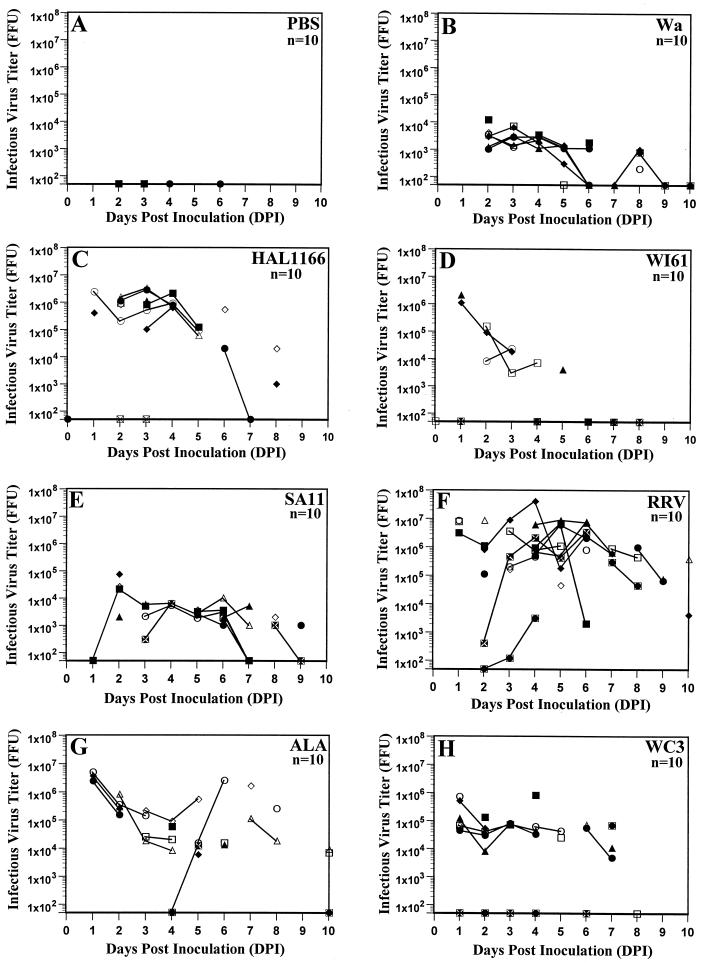
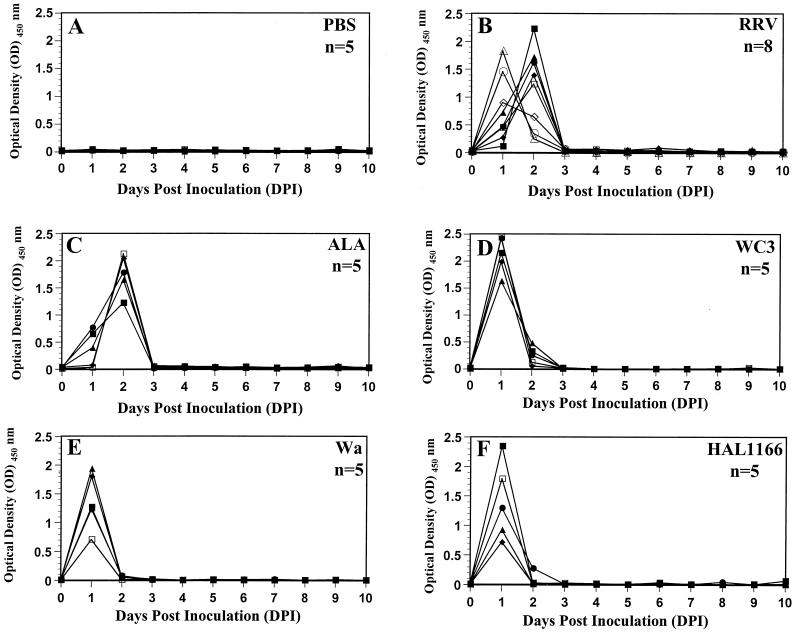
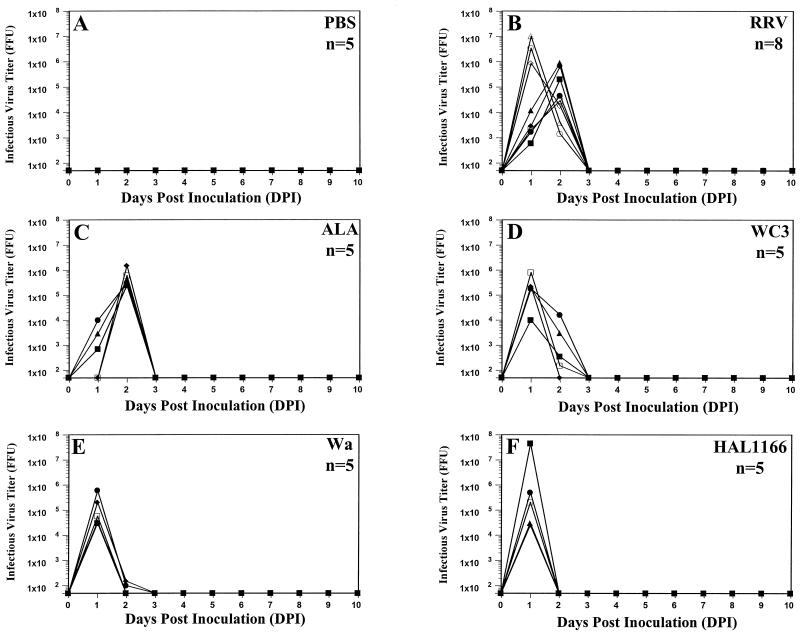
Five-day-old rats inoculated with PBS or their respective dam did not shed virus antigen (Fig. 1A) or infectious virus (Fig. 2A) as measured by ELISA or FFA, respectively, over a 10-day period. Five-day-old rats inoculated with group A tissue culture-adapted human or animal rotavirus strains shed virus antigen (Fig. 1B to H) as well as infectious virus (Fig. 2B to H) over a period of 5 to 10 days, starting as early as 1 DPI. With all the viruses, the amount of virus antigen shedding correlated (r = 0.863, P ≤ 0.002; Pearson’s correlation coefficient) with infectious virus shedding. Neonatal rats inoculated with human rotavirus strains Wa and HAL1166 and with WI61 shed virus antigen and infectious virus for 8 and 5 days, respectively (Fig. 1B to D and Fig. 2B to H). The amount of virus antigen or infectious virus shed by Wa-, SA11-, and WC3-inoculated rat pups (Fig. 1B, E and H and 2B, E, and H) was approximately half of that shed by HAL1166-, RRV-, and ALA-inoculated rat pups (Fig. 1C, F, and G and 2C, F, and G). The amount of virus antigen or infectious virus shed by WI61-inoculated rat pups (Fig. 1D or 2D, respectively) was similar to that shed by OSU-inoculated rat pups (data not shown). Neonatal rats inoculated with simian SA11 and RRV, lapine ALA, and bovine WC3 also shed virus antigen or infectious virus for a period of 7 to 10 days (Fig. 1E to H or 2E to H, respectively). Neonatal rats inoculated with porcine OSU shed infectious virus for 5 days, whereas those inoculated with wild-type murine ECwt could not be measured by FFA due to the inability of ECwt to grow in cell culture (data not shown). Only one neonatal rat inoculated with wild-type murine ECwt shed low amounts (OD ≤ 0.184) of virus antigen from 2 to 4 DPI (data not shown), and this OD was not statistically different from the OD for the PBS group (P ≥ 0.455; Mann-Whitney U test). The large experimental variation observed in 5-day-old rats cannot be directly interpreted or compared because it is likely the reflection of the range of the inoculation doses and the replication efficiencies of each of the rotavirus strains tested. Experiments with RRV were performed 10 independent times, and the results were always reproducible.
FIG. 1.
Viral antigen-shedding curves of individual fecal samples of 5-day-old rats inoculated with 0.5 ml of PBS (n = 10) (A), 2.2 × 106 FFU of human rotavirus Wa (B), 3 × 105 FFU of human rotavirus HAL1166 (C), 3 × 106 FFU of human rotavirus WI61 (D), 107 FFU of simian rotavirus SA11 (E), 2 × 107 FFU of simian rotavirus RRV (F), 106 FFU of lapine rotavirus ALA (G), or 4 × 106 FFU of bovine rotavirus WC3 (H). Viral antigen shedding was assessed by ELISA from 0 to 10 DPI and was expressed as net readings for OD at 450 nm. Readings of ≥0.1 are considered positive. Fecal samples from individual 5-day-old rats could not be obtained every day; therefore, missing data points reflect a lack of sample and not a lack of detection of virus antigen shedding. Each virus-inoculated group consisted of eight virus-inoculated (▪, •, ▴, ⧫, □, •, ▵, and ◊) and two PBS-inoculated (white X in black box and black circle in white box) rat pups to monitor spread among mock-inoculated littermates, bringing the number of animals per group to 10. All PBS inoculations were performed prior to any virus inoculation.
FIG. 2.
Infectious virus-shedding curves of individual fecal samples of 5-day-old rats inoculated with 0.5 ml of PBS (n = 10) (A), 2.2 × 106 FFU of human rotavirus Wa (B), 3 × 105 FFU of human rotavirus HAL1166 (C), 3 × 106 FFU of human rotavirus strain WI61 (D), 107 FFU of simian rotavirus SA11 (E), 2 × 107 FFU of simian rotavirus RRV (F), 106 FFU of lapine rotavirus strain ALA (G), or 4 × 106 FFU of bovine rotavirus strain WC3 (H). Infectious rotavirus shedding was assessed by FFA from 0 to 10 DPI and was expressed in FFU. Fecal samples from individual 5-day-old rats could not be obtained every day; therefore, missing data points reflect a lack of sample and not a lack of detection of virus antigen shedding. Each virus-inoculated group consisted of eight virus-inoculated (▪, •, ▴, ⧫, □, •, ▵, and ◊) and two PBS-inoculated (white X in black box and black circle in white box) rat pups to monitor spread among mock-inoculated littermates, bringing the number of animals per group to 10. All PBS inoculations were performed prior to any virus inoculation.
We confirmed that the excreted virus was the same virus as the inoculum by examining the RNA electropherotype of the virus recovered from selected-pooled fecal samples. In the neonatal rats inoculated with HAL1166, RRV, and ALA, the virus recovered from the stools was identical to the virus inoculum (data not shown). No virus could be detected in pooled stools from neonatal rats inoculated with Wa, WI61, SA11, WC3, OSU, or ECwt (data not shown), probably due to the low amount of virus excreted by these rat pups.
The transmission efficiency of group A rotavirus strains in rats was monitored by its ability to spread horizontally to PBS-inoculated littermate (control) rat pups and the dam housed in the same cage. Horizontal transmission to pups occurred with some but not all of the rotavirus strains tested. PBS-inoculated littermates of SA11-, RRV-, and ALA- but not of Wa-, WI61-, HAL1166-, WC3-, OSU-, or ECwt-inoculated rat pups became infected based on detectable virus antigen or infectious virus shedding starting at 2 or 3 DPI (Fig. 1 and 2; data not shown). The kinetics of shedding of virus antigen and infectious SA11, RRV, and ALA by PBS-inoculated littermates appeared similar to that shed by the respective virus-inoculated rat pups (Fig. 1E to G and 2E to G, respectively), although it cannot be directly compared due to the variation observed within the groups. All human and animal viruses, except porcine OSU and murine ECwt, strains, were readily transmitted to the uninoculated dams. Dams most likely became infected due to the altricial nature of rats: young are born blind and deaf, unable to maintain body temperature, and unable to eliminate wastes without maternal stimulation (29, 30). Virus antigen or infectious virus shedding in the dams commenced 1 or 2 DPI and lasted 2 to 6 days (data not shown). Titers of infectious rotavirus shed by each dam over a period of 10 days ranged from 1.9 × 102 to 8.5 × 102 FFU (Wa), 5 × 101 to 2 × 103 FFU (WI61), 2.5 × 103 to 1.3 × 104 FFU (HAL1166), 5 × 101 to 7.5 × 102 FFU (SA11), 9 × 104 to 8.4 × 105 FFU (RRV), 1.4 × 102 to 2.4 × 104 FFU (ALA), and 3 × 102 to 7.8 × 104 FFU (WC3). Thus, although human Wa, WI61, and HAL1166 and bovine WC3 did not spread efficiently to PBS-inoculated littermates, these viruses spread horizontally to their respective dams. Dams of OSU- or ECwt-inoculated neonatal rats did not shed rotavirus antigen or infectious rotavirus as detected by ELISA or FFA, respectively (data not shown).
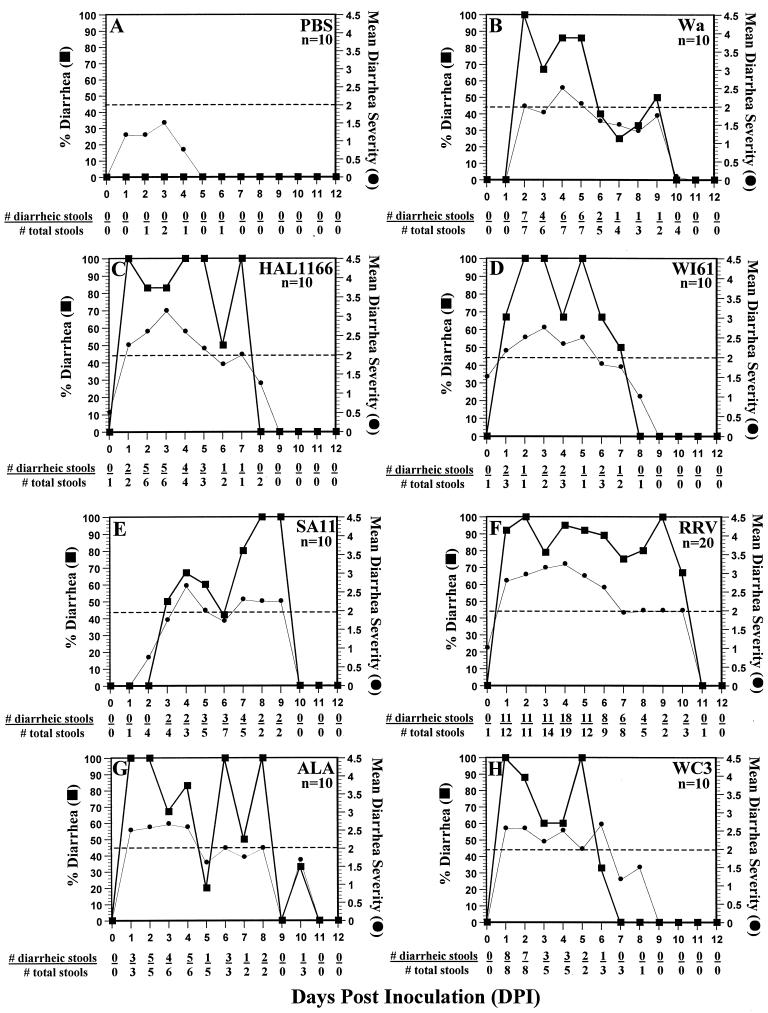
All virus-inoculated but not PBS-inoculated 5-day-old rats developed diarrhea (Fig. 3). None of the dams developed diarrhea (data not shown). However, PBS-inoculated littermates of ALA-, SA11-, and RRV-inoculated groups developed diarrhea of similar severity to their respective virus-inoculated littermates, as a result of efficient horizontal transmission, whereas the PBS-inoculated littermates of Wa-, WI61-, HAL1166-, and WC3-inoculated groups developed either a mild diarrhea (score of 2) or no diarrhea (score < 2). Since fecal samples from each neonatal rat could not be collected every day, the percent diarrhea for each group per day was calculated by dividing the number of diarrheic samples by the number of total samples collected each day. The mean disease severity was determined by dividing the sum of all diarrhea scores (1 to 4) by the number of total samples scored each day. No significant difference (P ≥ 0.187; Mann-Whitney U test) in percent diarrhea or disease severity was observed among groups of rats inoculated with the Wa, WI61, HAL1166, SA11, ALA, and WC3 strains (Fig. 3B to E and G to H). However, percent diarrhea and disease severity induced by RRV were significantly more severe (P ≤ 0.042; Mann-Whitney U test) than either the percent diarrhea or disease severity induced by the Wa, WI61, SA11, or WC3 strain but was equivalent (P ≥ 0.084; Mann-Whitney U test) to those induced by the HAL1166 or ALA strain (Fig. 3). The percent diarrhea and disease severity induced by porcine OSU in neonatal rats (data not shown) was equivalent (P = 0.389; Mann-Whitney U test) to that of human WI61 (Fig. 3D). Neonatal rats inoculated with wild-type ECwt murine rotavirus developed mild disease (score of 2) at 2 and 3 DPI, accounting for 66 and 50% of the inoculated animals (data not shown). The peak of percent diarrhea correlated with peak days of virus antigen or infectious virus shedding in each of the virus groups (Fig. 1 to 3), except for the SA11-inoculated rats (Fig. 1E, 2E, and 3E). The onset of disease induced by all group A rotavirus strains except simian SA11 or wild-type murine ECwt generally occurred at 1 DPI, achieving 100% by 1 or 2 DPI. The onset of disease of the animals inoculated with SA11 occurred at 3 DPI (Fig. 3E). Therefore, the results demonstrate that infection, disease, and transmission of a variety of group A rotaviruses occur in rats but that the severity of disease and transmissibility differ from strain to strain.
FIG. 3.
Percent diarrhea (▪) and mean diarrhea severity (•) in 5-day-old neonatal rat litters inoculated with PBS (A), human rotavirus strain Wa (B), human rotavirus strain HAL1166 (C), human rotavirus strain WI61 (D), simian rotavirus strain SA11 (E), simian rotavirus strain RRV (F), lapine rotavirus strain ALA (G), or bovine rotavirus strain WC3 (H) from 0 to 12 DPI. No diarrhea was observed in any rat beyond 12 DPI (data not shown). Percent diarrhea for each group per day was calculated by dividing the number of diarrheic samples by the number of total samples collected each day, since fecal samples from each 5-day-old neonatal rat could not be collected every day. A score of ≥2 was considered diarrhea, whereas a score of <2 was considered normal. The mean disease severity was determined by dividing the sum of all diarrhea or not-diarrhea scores (1 to 4) by the number of total samples scored each day.
In all 10 of our experiments with RRV, the two sham-inoculated pups always got infected. In an additional experiment, the efficiency of the horizontal transmission of RRV was confirmed further when RRV was able to spread from two RRV-inoculated pups to all eight PBS-inoculated littermates and to the dam as determined by virus antigen or infectious virus shedding measured by ELISA or FFA, respectively (data not shown). The percent diarrhea and disease severity of all the pups in this experiment were similar (P = 0.3 and 0.410; Mann-Whitney U test, respectively) to those obtained when eight pups were inoculated with virus and two were inoculated with PBS (Fig. 1F, 2F, and 3F).
Since the simian RRV strain induced the most severe disease in neonatal rats, we next determined the 50% diarrhea dose (DD50) and the SD50 of RRV in rat pups. Five-day-old rat pups (n = 10) were gavaged with 0.5 ml of 10-fold dilutions of RRV from 2 × 103 to 2 × 106 FFU. Based on induction of disease and virus antigen shedding (data not shown), the DD50 and SD50 of RRV in rat pups calculated by using the Karber equation were 6.6 × 104 and 5 × 103 FFU, respectively.
Distribution of RRV in intestinal contents of 5-day-old rats.
To determine the kinetics of RRV replication in the intestine of 5-day-old rats, the presence of rotavirus antigen or infectious virus was assayed in gut homogenates of PBS- and RRV-inoculated rats collected at 0 to 216 hpi by ELISA and FFA, respectively. Gastrointestinal transit time in adult rats is <8 h and is approximately 16 h in suckling rats (30, 36, 63, 65). As determined previously (7, 9), detection of virus shedding by ELISA and detection of infectious virus by FFA correlated (P < 0.001 and r = 0.792; Pearson’s correlation coefficient). Whenever fecal samples were available, infectious virus was detected and diarrhea was observed in RRV-inoculated rats beginning at 12 or 24 hpi (data not shown).
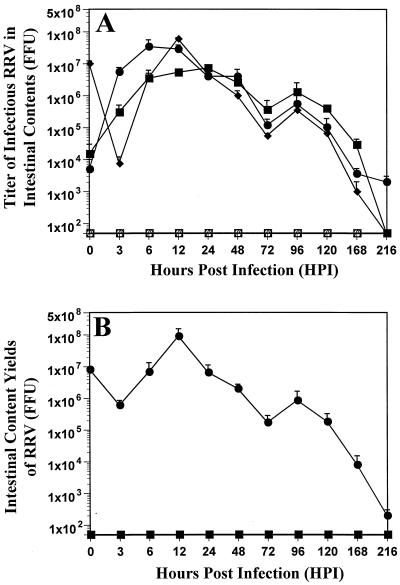
No infectious rotavirus was detected in any of 5-day-old PBS-inoculated rats at any time points in any part of the intestine (Fig. 4). Virus growth curves of RRV replication in the intestine of the 5-day-old neonatal rats showed that virus titers (about 107 FFU) in the small intestine, corresponding to the inoculum at 0 hpi, decreased >1,000-fold by 3 hpi (Fig. 4A), possibly reflecting the eclipse phase. Following the decrease of virus titers in the small intestine at 3 hpi, virus titers in the small intestine increased and reached maximal values by 12 hpi (Fig. 4A). Thereafter, virus titers in the small intestine declined gradually. In the intestine, infectious virus was detected from 3 to 216 hpi with individual titers ranging from 1.3 × 103 to 6.2 × 107 FFU (small intestine), 2.9 × 104 to 7.25 × 106 FFU (large intestine), and 2 × 103 to 3.4 × 107 FFU (cecum) (Fig. 4A). The peaks of infectious titers in the cecum and the large intestine of RRV-inoculated neonatal rats occurred at 6 and 24 hpi, respectively. The virus titers in the cecum exceeded the input dose from 6 to 12 hpi by approximately fivefold, reflecting accumulation of infectious virus from the inoculum and newly made virus. The peak of infectious virus titers in the intestine was followed by a progressive decline until 48 hpi. By 72 hpi, virus titers in the intestine decreased slightly (about 100-fold in the small intestine and 10-fold in the large intestine and cecum) and a modest second peak of infectious virus was detected at 96 hpi. Following the appearance of the second peak, a sharp decline of infectious titers occurred in all intestinal sections. By 216 hpi, no infectious virus was detected in the small or large intestine. Small amounts (2 × 103 FFU) of infectious virus were still detected in the cecum (possibly reflecting accumulation of infectious virus), although no virus antigen could be detected by ELISA at this time point (data not shown). The total intestinal yields of RRV, expressed in FFU, demonstrated that output virus was almost 10-fold more than that of input virus (Fig. 4B). Liquid or partially formed stools in the large intestine were present in all RRV-inoculated, but not PBS-inoculated, neonatal rats from 12 to 216 hpi (data not shown).
FIG. 4.
Group A simian RRV replication in neonatal rats. (A) Growth curves of simian RRV inoculated into 5-day-old neonatal rats. Titers of infectious virus (FFU) from the contents of the small intestine (⧫), large intestine (▪), and cecum (•) after inoculation with 2 × 107 FFU of RRV were determined by FFA from 0 to 216 hpi. For infectious titers of <100, 50 was used to calculate the mean infectious titer. An infectious titer of 50 was considered negative. No infectious virus from the intestinal contents of the small intestine (□), large intestine (•), and cecum (▵) was detected after inoculation with PBS. (B) Total intestinal content yield of RRV-inoculated (•) rat pups measured in FFU. The total amount of infectious RRV recovered (9 × 107 FFU) from the intestinal contents surpassed the amount of the virus inoculum (2 × 107 FFU). No infectious virus was detected in the intestinal contents of PBS-inoculated (▪) rat pups. Each data point represents the average infectious rotavirus titers of a total of three independent experiments ± standard error of the mean (total PBS n = 6 and total RRV n = 12 per time point).
Histopathological lesions in the small intestine of 5-day-old rats.
To determine if RRV infection causes histopathological lesions in the small intestine of neonatal rats, 5-day-old rats were inoculated with 0.5 ml of PBS or 2 × 10 7 FFU of RRV and euthanatized at 0 to 216 hpi. At necropsy, no gross changes of the intestinal tract of control rats sacrificed at any of the time points were observed. In RRV-infected rats, accumulation of fluid in the small intestine was first observed at 12 hpi and persisted through 120 to 168 hpi along with distention throughout the small and large intestines (data not shown).
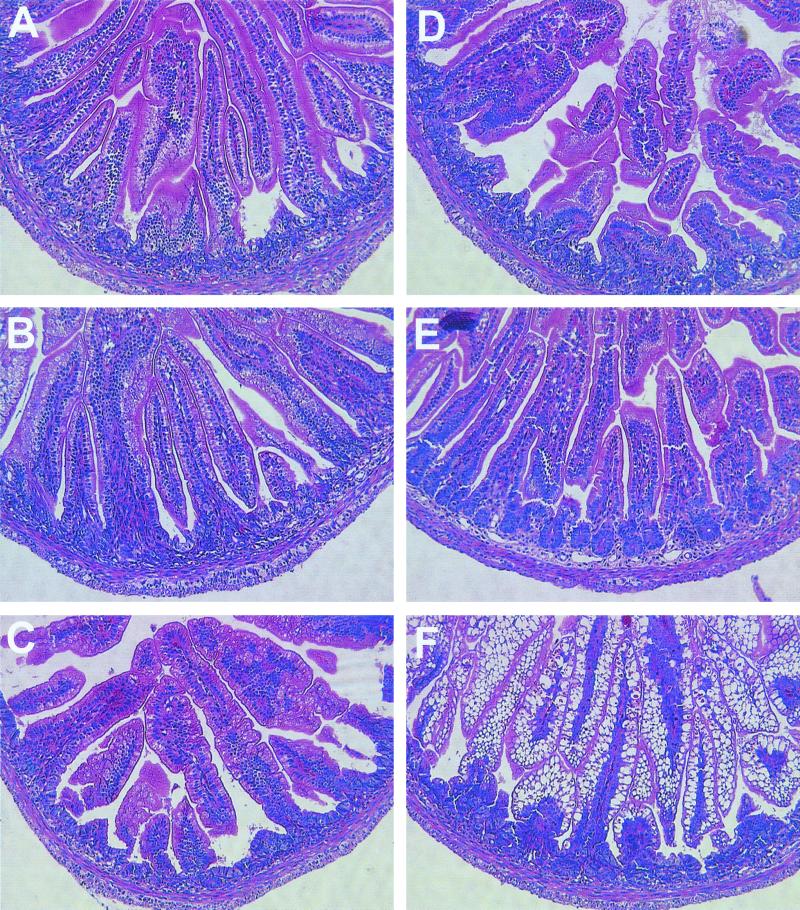
No lesions were seen in any of the 5-day-old control (PBS) rats at any of the time points (Fig. 5A to C). In the RRV-inoculated 5-day-old rats, there was little to no inflammation, villus height and width were maintained, and histopathological lesions were virtually absent except for large vacuoles in the enterocytes lining most of the surface of the villi in the ileum (Fig. 5D to F). Rare or minimal vacuolization was also noticeable at the tips of the villi in some PBS control rat pups but was always less than that observed in RRV-inoculated rat pups and was typically detected in the duodenum. Vacuolization was maximal in the ileum by 48 to 72 hpi and lessened by 96 hpi. There was a trend towards resolution of histopathological changes as measured by a decrease in size and number of vacuoles by 168 hpi (data not shown). The vacuoles did not stain for neutral or acid mucopolysaccharides with periodic acid-Schiff or alcin blue stains, respectively (data not shown). The vacuoles did not stain for lipid in frozen sections, and only duodenal enterocytes contained lipid droplets; there were no differences between PBS control and experimental animals (data not shown). All other features of the intestines of RRV- and PBS-inoculated animals, including enterocyte injury, sloughing, villus height/crypt depth ratio, mitotic activity, and leukocyte content and blood vessels of the lamina propia were identical (data not shown).
FIG. 5.
Photomicrograph of small intestine of duodenal (A), jejunal (B), and ileal (C) mucosa of 5-day-old PBS mock-infected control neonatal rat or duodenal (D), jejunal (E), and ileal (F) mucosa of 5-day-old group A simian RRV-infected neonatal rat at 72 hpi. In RRV-infected rat pups, histopathological lesions are limited to large vacuoles in the enterocytes lining most of the surface of the villi in the ileum. Hematoxylin and eosin staining; original magnification, ×240.
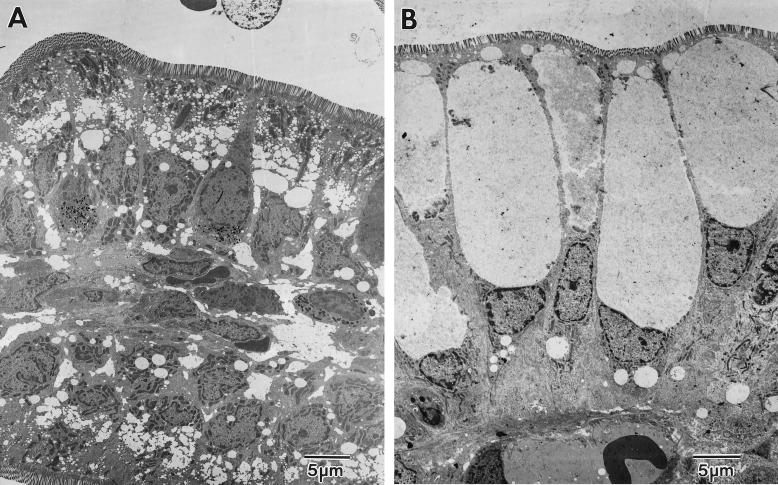
EM analysis of small intestine samples of 5-day-old rats demonstrated supranuclear cytoplasmic vacuoles present in the ileum of both PBS- and RRV-inoculated rat pups (Fig. 6). However, the vacuoles were obviously expanded in the virus-exposed rats (Fig. 6B). Cellular organelles were pushed aside, and nuclei were compressed in markedly enlarged enterocytes. The vacuoles contained small, ill-defined fragments of cytoplasmic debris in a general amorphous or finely granular background and appear therefore to be expansions of normal transport vesicles. No viral particles could be detected in the vacuoles or in any region of the intestine of the RRV-inoculated rats at any time point by EM (Fig. 6 and data not shown).
FIG. 6.
Electron micrographs of ultrastructural appearance of the ileum of PBS-inoculated (A) or RRV-inoculated (B) 5-day-old rats at 48 hpi. Extensive cytoplasmic vacuolization occurred after RRV infection (B), including relocation of the nucleus. Vacuoles are devoid of virus particles, and the brush border system appears intact. Original magnification, ×2,400.
Detection of rotavirus antigen in sections of the small intestine of 5-day-old rats.
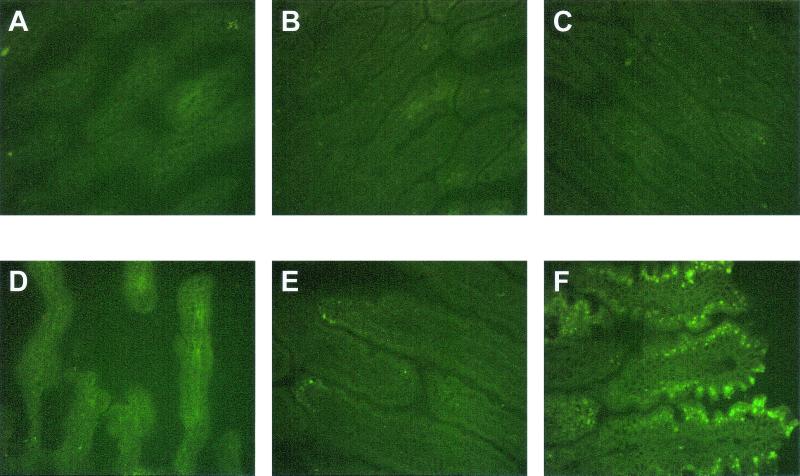
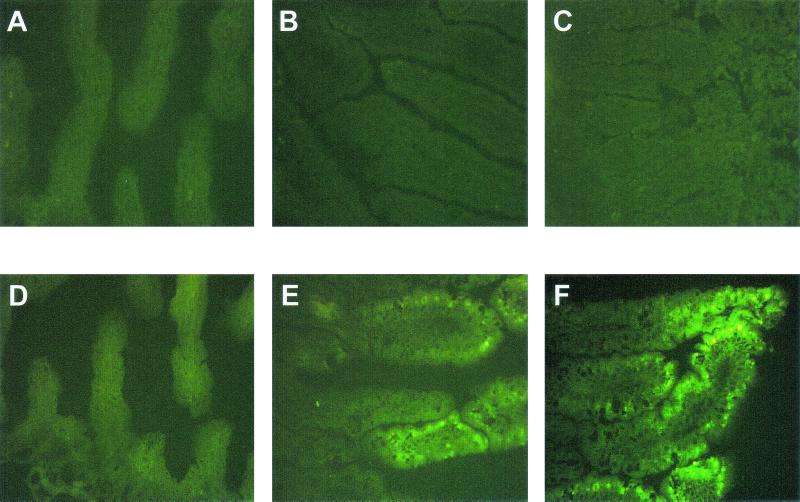
Frozen sections of the duodenum, jejunum, and ileum of the 5-day-old rats inoculated with RRV were stained by immunofluorescence to detect the distribution of rotavirus structural protein VP6 (Fig. 7) and nonstructural protein NSP4 (Fig. 8). Rotavirus antigen was not detected in any section of the small intestine of 5-day-old control (PBS) rats (Fig. 7A to C and 8A to C). At 24 hpi, rotavirus VP6 or NSP4 protein was detected in the epithelial cells located on the upper half of the intestinal villi in the jejunum and the ileum (Fig. 7E and F and 8E and F) but not in the duodenum (Fig. 7D and 8D) of RRV-infected 5-day-old rats. There was no evidence of rotavirus antigen present in the lower half of the intestinal villi or in the crypts in any region of the small intestine of RRV-infected 5-day-old rats at any time point. At 48 and 72 hpi, the distribution of rotavirus antigen was the same as at 24 hpi, although the detection of NSP4 was stronger than at 24 hpi (data not shown). No or little rotavirus antigen was detected beyond 96 hpi in any part of the small intestine. Detection of NSP4 antigen, a nonstructural protein not found in the mature infectious virus particle, in small intestine sections of RRV-inoculated rat pups provides additional evidence that RRV was replicating in the neonatal rats, because to detect NSP4, the NSP4 mRNA had to be synthesized and expressed.
FIG. 7.
Photomicrograph of the distribution of specific immunofluorescence staining against rotavirus VP6 antigen of duodenum (A), jejunum (B), or ileum (C) of 5-day-old PBS mock-infected control neonatal rat and duodenum (D), jejunum (E), or ileum (F) of 5-day-old RRV-infected neonatal rat at 24 hpi. Rotavirus VP6 antigen is distributed in the epithelial cells of the upper half of the intestinal villi of the jejunum and ileum. Original magnification, ×240.
FIG. 8.
Photomicrograph of the distribution of specific immunofluorescence staining against rotavirus NSP4 antigen of duodenum (A), jejunum (B), or ileum (C) of 5-day-old PBS mock-infected control neonatal rat and duodenum (D), jejunum (E), or ileum (F) of 5-day-old RRV-infected neonatal rat at 24 hpi. Rotavirus NSP4 antigen is distributed in the epithelial cells of the upper half of the intestinal villi of the jejunum and ileum. Original magnification, ×240.
Effect of group A RRV infection on weight gain of neonatal rats.
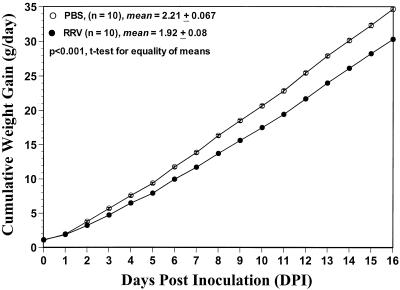
We also monitored the daily gain of weight of 5-day-old neonatal rats from one litter each inoculated with either 0.5 ml of PBS (n = 10; six males and four females) or 2 × 107 FFU of RRV (n = 10; four males and six females) from 0 to 16 DPI, the time at which the rats were weaned and sexed.
All parameters of rotavirus-induced diarrhea and virus antigen or infectious virus shedding (data not shown) were similar to those of previous experiments (Fig. 1 and 2). Induction of disease in 5-day-old neonatal rats following inoculation with group A RRV strain resulted in a concomitant loss of weight gain per day starting at 2 DPI, which was clearly observed at 3 DPI compared to what was seen for PBS-inoculated 5-day-old rats, which did not develop disease (Fig. 9). No animals died following rotavirus infection. Once diarrhea ceased (by 9 or 10 DPI), the growth rate of RRV-inoculated rats became similar to that of the PBS-inoculated rats, although the actual weights of the infected group still lagged behind. The mean of the slopes by linear regression of each group was compared to examine the difference in cumulative weight gain per day between the RRV- and PBS-inoculated groups. The mean of the slope (2.21 ± 0.067) of the PBS-inoculated rats was significantly greater (P < 0.001; t test for equality of means) than that of RRV-inoculated rats (1.92 ± 0.08). These results indicate that group A rotavirus infection of neonatal rats causes a self-limiting, acute diarrheal disease that affects pup growth.
FIG. 9.
Effect of group A RRV infection in body weight gain of 5-day-old neonatal rats. Following infection with 2 × 107 FFU of RRV, individual neonatal rats were weighed daily to monitor weight gain compared to that of PBS-inoculated, 5-day-old neonatal rats. All RRV-inoculated (n = 10, four males and six females) but not PBS-inoculated (n = 10, six males and four females) neonatal rats shed virus antigen (as measured by ELISA) or infectious virus (as measured by FFA) and developed diarrhea from 1 to 9 DPI (data not shown). Each group of rats was from the same litter and was sexed at 16 DPI.
Infection of 21- or 270-day-old rats inoculated with different group A rotavirus strains.
Group A rotavirus infection causes disease only in rabbits and mice of ≤2 weeks of age (5, 8, 38, 54, 56, 57, 59, 62, 74, 75), and group B rotavirus infection causes disease in rats of ≤2 weeks of age (72). Although there are no data on group B rotavirus infection in adult rats (72), group A rotavirus antigen shedding is also age restricted in rabbits and mice; in neonates, shedding is prolonged in neonates compared to that in animals of ≥2 weeks of age (5, 8, 54, 74, 75). Therefore, we examined if rotavirus infection or disease was age dependent by infecting 21-day-old rats with different group A rotavirus strains. Rats of 21, not 15, days of age were chosen because rats are weaned at 21 days of age, allowing easier and more convenient collection of daily fecal samples than in rats at 15 days of age. Also, 270-day-old rats (n = 2) were inoculated with 4 × 107 FFU of RRV.
Similar to what is found in mice and rabbits (5, 8, 38, 54, 56, 57, 59, 62, 74, 75), rotavirus disease was age restricted in neonatal rats. None of the 21- or 270-day-old rats inoculated with rotavirus developed disease (data not shown). All the rotavirus-inoculated rats, except those inoculated with ECwt, shed virus antigen for 1 or 2 days from 1 to 2 DPI (Fig. 10). Based on lack of virus antigen shedding, rats inoculated with PBS (Fig. 10A) or the wild-type murine rotavirus strain ECwt (data not shown) were not infected. The duration of virus antigen shed by 21-day-old rats was significantly reduced (P < 0.001; Mann-Whitney U test) compared to that of neonatal rats inoculated with the corresponding rotavirus strains, indicating that, similar to the situation in rabbits and mice, rotavirus infection in rats is also age restricted. Rats of 270 days of age had shown patterns of RRV antigen shedding similar to those for rats of 21 days of age (data not shown). To ensure that virus antigen detection was not simply due to residual virus inoculum, we also measured the amount of infectious virus in the fecal samples of the virus-inoculated rats (Fig. 11). The titers of infectious rotavirus shed on peak days of antigen shedding (Fig. 10) ranged from 2.9 × 104 to 1 × 107 FFU (RRV), 2.4 × 105 to 1.5 × 106 FFU (ALA), 1 × 104 to 9 × 105 FFU (WC3), 3 × 103 to 8.5 × 105 FFU (Wa), or 5 × 104 to 5 × 107 FFU (HAL1166) (Fig. 11B to F). The reason why 21-day-old rats only shed virus antigen (Fig. 10) or infectious virus (Fig. 11) from 1 to 2 DPI is not clear, but the amount of virus shed cannot be inert intestinal passage of the input inoculum, due to the size and dilution of each fecal sample collected and because the titers of excreted virus exceeded virus input. Thus, rotavirus replication occurred in 21-day-old rats, albeit for fewer days than in 5-day-old rats.
FIG. 10.
Fecal virus antigen-shedding curves of individual 21-day-old rats inoculated with PBS (A), simian RRV (4 × 107 FFU) (B), lapine rotavirus ALA (2 × 106 FFU) (C), bovine rotavirus WC3 (8 × 106 FFU) (D), human rotavirus Wa (4.4 × 106 FFU) (E), or human rotavirus HAL1166 (6 × 105 FFU) (F). Fecal rotavirus antigen shedding was assessed by ELISA from 0 to 10 DPI and expressed as net readings of OD at 450 nm. Readings of ≥0.1 are considered positive.
FIG. 11.
Fecal infectious virus-shedding curves of individual 21-day-old rats inoculated with PBS (A), simian RRV (4 × 107 FFU) (B), lapine rotavirus ALA (2 × 106 FFU) (C), bovine rotavirus WC3 (8 × 106 FFU) (D), human rotavirus Wa (4.4 × 106 FFU) (E), or human rotavirus HAL1166 (6 × 105 FFU) (F). Fecal infectious virus shedding was assessed by FFA from 0 to 10 DPI and was expressed in FFU.
DISCUSSION
Our results firmly establish clinical and virological parameters of group A rotavirus infection in rats by showing that (i) viral replication, disease, and transmission of several tissue-culture adapted rotavirus strains of simian (RRV and SA11), bovine (WC3), lapine (ALA), porcine (OSU), or human (Wa, WI61, and HAL1166) origin can occur from birth to adulthood as measured by virus antigen or infectious virus shedding; (ii) disease is age dependent; (iii) histopathological changes do not occur in neonatal rats; (iv) rotavirus antigen distribution in the small intestine is limited to the upper half of the intestinal villi of the jejunum and ileum; and (v) rotavirus infection of neonatal rats results in reduced weight gain and growth during the early phase of infection.
In rabbits and mice, only homologous strains (isolated from the same species) replicate efficiently and spread horizontally to uninoculated control animals, whereas heterologous virus strains (isolated from a different species) do not (this includes human rotaviruses) (6, 8, 17, 23, 54). In contrast, infection, disease, and transmission of group A rotaviruses in neonatal rats occur with heterologous (non-rat) rotavirus strains of simian, bovine, lapine and human origin, although disease severity and transmission efficiency differ depending on the virus strain used. Differences in the replication efficacy of these nonrat rotavirus strains tested may be attributed to the viral dose delivered to rats and/or specific combinations of rotavirus genes that influence the replication outcome in a heterologous host. Among all group A rotavirus strains tested, simian RRV is the most efficient at replicating, causing disease, and spreading. In fact, RRV is capable of horizontal transmission even when 20% of the littermates are initially inoculated with the virus, and RRV-infected rats shed the highest amount of infectious virus with a calculated SD50 of 5 × 103 FFU, indicating that RRV replicates effectively in rats at doses lower than 107 FFU. However, the SD50 and DD50 of RRV in rat pups vary by approximately 10-fold, indicating that, at lower doses, not all infections lead to disease, which could be because RRV is not a group A rat rotavirus strain. The level of infectious virus excretion of neonatal rats inoculated with HAL1166 and ALA was slightly less than for RRV, while simian SA11, bovine WC3, porcine OSU, or human Wa or WI61 were decreased compared to those of RRV. Porcine OSU, shown to be attenuated in neonatal mice (79), induced mild disease in neonatal rats, suggesting that this strain may also be attenuated in rats. Unexpectedly, a rotavirus strain from another rodent species, the murine, wild-type rotavirus ECwt strain, which is known to infect mice in our laboratory, was the only heterologous rotavirus that failed to replicate in neonatal or 21-day-old rats.
Analysis of intestinal gut homogenates of neonatal rats demonstrated that a complete virus replication cycle occurred in RRV-infected neonatal rats. The detection of NSP4 in villus epithelial cells further supports the contention that RRV replicated in neonatal rats. In another study (77), simian SA11 and human Wa infection of Wistar rats did not result in virus replication, but the inoculum doses used were not provided. Since we used Lewis rats for our studies and others (3, 27, 77, 78) have used either germfree Fisher 344 or Wistar rats, it is possible that different strains of rats may differ in their susceptibility to group A rotavirus infection. Although heterologous rotavirus replication is less restricted in rats than in mice and rabbits, the capacity of the rat intestine to support replication of a group A rotavirus may be limited compared to the replication capability of the group B rotavirus IDIR (72, 73). Rotavirus VP6 and NSP4 antigens were detected only in the upper half of the intestinal villi of the jejunum and ileum. The fact that NSP4, a nonstructural protein, was detected in the small intestine of neonatal rats not only provides evidence that RRV replicates but also represents the first time that a rotavirus nonstructural protein has been detected in small intestine tissues.
Similar to infection of neonatal rats with the IDIR group B rotavirus (20, 33, 72, 73), infection of neonatal rats with group A rotaviruses results in diarrhea. Moreover, group A rotavirus-induced diarrhea is age dependent in rats. Even though all rotavirus strains tested induced an age-dependent disease, not all of them were equally virulent. RRV was the most virulent strain tested as measured both by percent diarrhea and disease severity (P ≤ 0.042; Mann-Whitney U test). Although the DD50 of RRV in neonatal rats is estimated to be approximately 6.6 × 104 FFU, the DD50 of the other rotavirus strains tested in neonatal rats remains to be determined. Human HAL1166 and lapine ALA closely followed RRV in terms of virulence. However, with the possible exception of human HAL1166 or lapine ALA strains, lower doses than those used for the other viruses may not result in disease induction. As in mice, group A NSP4 induces disease in young rats (J. M. Ball and M. K. Estes, unpublished data).
Horizontal transmission of rotavirus from infected pups to their dams was more efficient than that to PBS-inoculated littermates probably due to the altricial nature of rats (29, 30). Infection of neonatal rats with the different rotavirus strains resulted in different patterns of transmission to dams and control (PBS) mock-inoculated littermates, which could be dose related or because of the different replication capabilities in rats of each of the rotavirus strain tested. Simian RRV and lapine ALA were readily transmitted to PBS-inoculated littermates, while transmission of bovine WC3, porcine OSU, and human Wa, WI61, and HAL1166 rotavirus strains did not occur as determined by lack of infectious virus shedding. However, with the exception of OSU, all viruses were transmitted to the corresponding dams. In contrast with ≥21-day-old inoculated rats, which shed virus for 1 or 2 days, dams that acquired infection from their pups shed infectious virus for 2 to 6 days. The longer duration of virus shedding in dams was likely due to constant exposure to infectious virus being shed by the pups. We found that RRV was the most replication efficient and virulent of all viruses tested in rats. Horizontal transmission of heterologous viruses is rare in mice and rabbits, although we recently showed that RRV can also replicate productively and spread horizontally in rabbits (6, 9). RRV also replicates in humans and was the basis for the RRV-tetravalent vaccine (53). Like the IDIR group B rotavirus (72, 73), group A rotaviruses probably transmit horizontally via direct contact with contaminated feces, fomite transmission, human contact, or possibly airborne spread of contaminated dust or bedding.
Histopathological changes caused by rotavirus infection in other small (mice and rabbits) and large (cows, pigs, and sheep) animal models are primarily restricted to the villus epithelium of the small intestine (8, 17, 19, 26, 33, 45, 50–52, 54, 61, 64, 66). Neonatal mice (≤2 weeks of age) develop disease with no or minimal histology (4, 50, 54, 64, 66). Infection of neonatal rats with RRV failed to cause histopathology in the small intestine except for extensive vacuolization in the ileum. Our results are consistent with the limited histopathology observed following group A SA11 infection of neonatal Fisher 344 germfree rats (27, 28). Enterocytes were characterized by nuclei localized at their base and by a large supranuclear area occupying almost the whole apical cytoplasm. Enterocytes exhibiting the marked vacuolization in the ileum did not contain rotavirus antigen, as detected by immunofluorescence or EM. Similar results are observed following oral inoculation of mice with heterologous (nonmurine) rotaviruses (47, 54). The reason why no virions are detected in the vacuoles is not known. Although these results suggest that neither viral infection nor viral replication is directly responsible for the extensive vacuolization observed in the ileum of neonatal rats inoculated with RRV, the vacuoles in rat enterocytes do not contain lipid, as revealed by oil red O staining of neutral fats; the vacuoles may represent dilated vesicles resulting from virus infection. Rotavirus infection of polarized epithelial intestinal Caco-2 and HT-29 cells also results in extensive cytoplasmic vacuolization, including relocation of the nucleus along with the disappearance of the tight junctions (11, 68). Rotavirus infection of MA104 cells results in blocking of normal cellular vesicle trafficking from the endoplasmic reticulum to Golgi apparatus by accumulation of NSP4 in a post-endoplasmic reticulum, microtubule-associated membrane compartment (76).
Since vacuoles are considerably more numerous in RRV-inoculated neonatal rats, they may indicate an effect of rotavirus infection on intestinal absorption. In support of this hypothesis, the mean weight gain of RRV-inoculated rats was significantly lower (P < 0.001; t test for equality of means) than the mean weight gain of PBS-inoculated rats at 48 hpi. Both group A and B rotavirus infections cause a self-limiting, acute diarrheal disease and associated, transient growth retardation related to human rotavirus infections (41, 42, 61). Group A RRV infection in neonatal rats was nonfatal and did not cause any long-term effects on growth and development. These findings are similar to those in natural human rotavirus infection and in mice, piglets, and lambs (13, 17, 25, 26, 41, 42, 61, 64, 70). Loss of weight occurred at the onset of disease and virus shedding, and the drop in weight gain of infected rats was overcome rather quickly (5 or 6 DPI) as reported by Salim et al. (61).
Since group A rotaviruses replicate in rats, it is interesting to speculate why a group A rotaviruses has not been identified in or isolated from rats. The most obvious reason is the limited number of research groups that have thoroughly evaluated and properly analyzed the possibility or prevalence of group A infection in rats. We anticipate that the finding that group A rotaviruses are indeed capable of replicating in rats will allow the identification of group A rat rotaviruses if research efforts are directed toward this goal. If true, proper seroepidemiological studies should reveal high rates of group A infection in rats. In time, the epidemiology, the interaction with the host, and the significance in nature of group A rat rotaviruses can be understood.
This newly identified rat model of group A rotavirus infection possesses unique characteristics amenable to the study of rotavirus gastrointestinal pathophysiology and pathogenesis and the molecular regulation of intestinal development and nutritional control of age-dependent disease. This model may be important for physiological studies of rotavirus pathogenesis, such as understanding the mechanisms of rotavirus-induced diarrhea due to the convenience of the size of the intestinal tract of neonatal rats over that of neonatal mice. Also, this model should be useful to identify the cellular receptor for rotavirus, since there are a number of cDNA libraries for rat intestinal cell lines and monoclonal antibodies to rat intestinal cell markers. One major advantage that distinguishes the rat model from the rabbit and mouse models is the increased susceptibility to infection by rotavirus strains isolated from different species, including humans. Our data show that rats up to at least 9 months of age are susceptible to infection with RRV. The long period of susceptibility will allow the use of the rat model to examine the primary and secondary active immune responses to the same or to different group A rotaviruses (Ciarlet et al., unpublished).
Acknowledgments
We thank Sue Crawford, Susan J. Henning (Department of Pediatrics, Baylor College of Medicine), Judith Ball (Department of Pathobiology, Texas A&M University, College Station, Tex.), and A. Duncan Steele (MRC/MEDUNSA, Diarrheal Pathogens Research Unit, Pretoria, South Africa) for valuable suggestions, Robert F. Ramig for critically revising the manuscript, and Sharon Krater and Shiñawe Jiménez for expert tissue culture technical assistance. We also thank Dorene M. Rudman, Angela Major, and James Barrish for sectioning and staining for rotavirus antigen in frozen small-intestine samples, staining for neutral fats and acid and neutral mucopolysaccharides in frozen or formalin-fixed small-intestine samples, and EM analysis in glutaraldehyde-fixed intestinal samples, respectively.
This work was supported by Advanced Technology Program grant 004949-062 and by U.S. Public Health Service grants AI 24998, DK30144, and DK56338, which funds the Texas Gulf Coast Digestive Disease Center.
REFERENCES
- 1.Awang, A., and K. L. Yap. 1990. Group A rotavirus infection in animals from an animal house and in wild-caught monkeys. J. Diarrhoeal Dis. Res. 8:82–86. [PubMed] [Google Scholar]
- 2.Ball, J. M., P. Tian, C. Q.-Y. Zeng, A. P. Morris, and M. K. Estes. 1996. Age-dependent diarrhea is induced by a viral nonstructural glycoprotein. Science 272:101–104. [DOI] [PubMed] [Google Scholar]
- 3.Bass, D., E. Mackow, and H. B. Greenberg. 1991. Identification and partial characterization of a rhesus rotavirus binding glycoprotein on murine enterocytes. Virology 183:602–610. [DOI] [PubMed] [Google Scholar]
- 4.Burns, J. W., A. A. Krishnaney, P. T. Vo, R. V. Rouse, L. J. Anderson, and H. B. Greenberg. 1995. Analyses of homologous rotavirus infection in the mouse model. Virology 207:143–153. [DOI] [PubMed] [Google Scholar]
- 5.Ciarlet, M., and F. Liprandi. 1994. Serological and genomic characterization of two porcine rotaviruses with serotype G1 specificity. J. Clin. Microbiol. 32:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciarlet, M., M. K. Estes, C. Barone, R. F. Ramig, and M. E. Conner. 1998. Analysis of host range restriction determinants in the rabbit model: comparison of homologous and heterologous rotavirus infections. J. Virol. 72:2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciarlet, M., S. E. Crawford, C. Barone, A. Bertolotti-Ciarlet, R. F. Ramig, M. K. Estes, and M. E. Conner. 1998. Subunit rotavirus vaccine administered parenterally to rabbits induces active protective immunity. J. Virol. 72:9233–9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciarlet, M., M. A. Gilger, C. Barone, M. McArthur, M. K. Estes, and M. E. Conner. 1998. Rotavirus disease, but not infection and development of intestinal histophatological lesions, is age-restricted in rabbits. Virology 251:343–360. [DOI] [PubMed] [Google Scholar]
- 9.Ciarlet, M., M. K. Estes, and M. E. Conner. 2000. Simian rhesus rotavirus (RRV) is a unique heterologous (non-lapine) rotavirus strain capable of productive replication and horizontal transmission in rabbits. J. Gen. Virol. 81:1237–1249. [DOI] [PubMed] [Google Scholar]
- 10.Ciarlet, M., and M. E. Conner. 2000. Evaluation of rotavirus vaccines in small animal models. Methods Mol. Med. 34:147–187. [DOI] [PubMed] [Google Scholar]
- 11.Ciarlet, M., S. E. Crawford, and M. K. Estes. 2001. Differential infection of epithelial cell lines of sialic acid-dependent and sialic acid-independent rotavirus strains. J. Virol. 75:11834–11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark, H. F., P. A. Offit, R. W. Ellis, D. Krah, A. R. Shaw, J. J. Eiden, M. Pichichero, and J. J. Treanor. 1996. WC3 reassortant vaccines in children. Arch. Virol. 12(Suppl.):187–198. [DOI] [PubMed] [Google Scholar]
- 13.Coelho, K. I. R., A. Bryan, C. Hall, and T. Flewett. 1981. Pathology of rotavirus infection in suckling mice: a study by conventional histology, immunofluorescence, ultrathin sections, and scanning electron microscopy. Ultrastruct. Pathol. 2:59–69. [DOI] [PubMed] [Google Scholar]
- 14.Conner, M. E., M. K. Estes, and D. Y. Graham. 1988. Rabbit model of rotavirus infection. J. Virol. 62:1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conner, M. E., M. A. Gilger, M. K. Estes, and D. Y. Graham. 1991. Serologic and mucosal immune response to rotavirus infection in the rabbit model. J. Virol. 65:2562–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conner, M. E., S. E. Crawford, C. Barone, and Estes, M. K. 1993. Rotavirus vaccine administered parenterally induces protective immunity. J. Virol. 67:6633–6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conner, M. E., and R. F. Ramig. 1996. Enteric diseases, p. 713–743. In N. Nathanson, R. Ahmed, F. González-Scarano, D. E. Griffin, K. V. Homes, F. A. Murphy, and H. L. Robinson (ed.), Viral pathogenesis. Lippincott-Raven Publishers, Philadelphia, Pa.
- 18.Cross, R. F., and P. D. Moorhead. 1969. An azure and eosin rapid stain technique. Can. J. Comp. Med. 33:317. [PMC free article] [PubMed] [Google Scholar]
- 19.Crouch, C. F., and G. N. Woode. 1978. Serial studies of virus multiplication and intestinal damage in gnotobiotic piglets infected with rotavirus. J. Med. Microbiol. 11:325–334. [DOI] [PubMed] [Google Scholar]
- 20.Eiden, J., S. Vonderfecht, K. Theil, A. Torres-Medina, and R. Yolken. 1986. Genetic and antigenic relatedness of human and animal strains. J. Infect. Dis. 154:972–982. [DOI] [PubMed] [Google Scholar]
- 21.Estes, M. K., D. Y. Graham, R. F. Ramig, and B. L. Ericson. 1982. Heterogeneity in the structural glycoprotein (VP7) of simian rotavirus SA11. Virology 122:8–14. [DOI] [PubMed] [Google Scholar]
- 22.Estes, M. K. 2001. Rotaviruses and their replication, p. 1747–1785. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 23.Feng, N., J. W. Burns, L. Bracey, and H. B. Greenberg. 1994. Comparisons of the mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with homologous or heterologous rotaviruses. J. Virol. 68:7766–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass, R. I., P. Kilgore, R. Holman, S. Jin, J. Smith, P. Woods, M. Clarke, M.-S. Ho, and J. R. Gentsch. 1996. The epidemiology of rotavirus diarrhea in the United States: surveillance and estimates of disease burden. J. Infect. Dis. 174:S5–S11. [DOI] [PubMed] [Google Scholar]
- 25.Gouvea, V., A. Alencar, O. Barth, L. de Castro, A. M. Fialho, H. Araujo, S. Majerowicz, and H. G. Pereira. 1986. Diarrhoea in mice infected with a human rotavirus. J. Gen. Virol. 67:577–581. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg, H. B., H. F. Clark, and P. A. Offit. 1994. Rotavirus pathology and pathophysiology, p. 255–283. In R. F. Ramig (ed.), Rotaviruses. Springer-Verlag, Berlin, Germany. [DOI] [PubMed]
- 27.Guérin-Danan, C., J.-C. Meslin, F. Lambre, A. Charpilienne, M. Serezat, C. Bouley, J. Cohen, and C. Andrieux. 1998. Development of a heterologous model in germfree suckling rats for studies of rotavirus diarrhea. J. Virol. 72:9298–9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guérin-Danan, C., J.-C. Meslin, A. Chambard, A. Charpilienne, P. Relano, C. Bouley, J. Cohen, and C. Andrieux. 2001. Food supplementation with milk fermented by Lactobacillus casei DN-114 001 protects suckling rats from rotavirus-associated diarrhea. J. Nutr. 131:111–117. [DOI] [PubMed] [Google Scholar]
- 29.Henning, S. J. 1981. Postnatal development: coordination of feeding, digestion, and metabolism. Am. J. Physiol. 241:G199–G214. [DOI] [PubMed] [Google Scholar]
- 30.Henning, S. J., D. C. Rubin, and R. J. Shulman. 1994. Ontogeny of the intestinal mucosa, p. 571–610. In L. R. Johnson (ed.), Physiology of the gastrointestinal tract, 3rd ed. Raven Press, New York, N.Y.
- 31.Ho, M., R. Glass, P. Pinsky, N. Young-Okoh, W. Sappenfield, J. Buehler, N. Gunter, and L. Anderson. 1988. Diarrheal deaths in American children: are they preventable? JAMA 260:3281–3285. [PubMed] [Google Scholar]
- 32.Hoshino, Y., R. Jones, and A. Z. Kapikian. 1998. Serotypic characterization of outer spike protein VP4 of vervet monkey rotavirus SA11 strain. Arch. Virol. 143:1233–1244. [DOI] [PubMed] [Google Scholar]
- 33.Huber, A., R. Yolken, L. Mader, J. Strandberg, and S. Vonderfecht. 1989. Pathology of infectious diarrhea of infant rats (IDIR) induced by an antigenically distinct rotavirus. Vet. Pathol. 26:376–385. [DOI] [PubMed] [Google Scholar]
- 34.Hung, T., G. Chen, C. Wang, H. Yao, Z. Fang, T. Chao, Z. Chou, W. Ye, X. Chang, S. Den, X. Liong, and W. Chang. 1984. Waterborne outbreak of rotavirus diarrhoea in adults in China caused by a novel rotavirus. Lancet ii:1139–1142. [PubMed] [Google Scholar]
- 35.Iida, F., and A. Sato. 1975. Study of mucous barrier as a defensive factor of gastric mucosa. Jpn. J. Gastroenterol. 72:1569–1578. [PubMed] [Google Scholar]
- 36.Izbéki, F., T. Wittmann, S. Csáti, E. Jeszenszky, and J. Lonovics. 2001. Opposite effects of acute and chronic administration of alcohol on gastric emptying and small bowel transit in rat. Alcohol Alcohol 36:304–308. [DOI] [PubMed] [Google Scholar]
- 37.Kira, K. 1973. Pathogenesis of peptic ulcer and changes of protective mechanism of the gastric and duodenal mucosa. Jpn. J. Gastroenterol. 70:1182–1200. [PubMed] [Google Scholar]
- 38.Kraft, L. M. 1957. Studies on the etiology and transmission of epidemic diarrhea of infant mice. J. Exp. Med. 106:743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeBaron, C. W., J. Lew, R. I. Glass, J. Weber, and G. Ruiz-Palacios. 1990. Annual rotavirus epidemic patterns in North America: results of a five-year retrospective survey of 88 centers in Canada, Mexico, and the United States. JAMA 264:983–988. [DOI] [PubMed] [Google Scholar]
- 40.Malherbe, H. H., and M. Strickland-Cholmley. 1967. Simian virus SA11 and the related O agent. Arch. Gesamte Virusforsch. 22:235–245. [DOI] [PubMed] [Google Scholar]
- 41.Mata, L., A. Simhon, J. Urrutia, R. Kronmal, R. Fernández, and B. García. 1983. Epidemiology of rotaviruses in a cohort of 45 Guatemalan Mayan Indian children observed from birth to the age of thee years. J. Infect. Dis. 148:452–461. [DOI] [PubMed] [Google Scholar]
- 42.Mata, L. 1982. Diarrheal disease as a cause of malnutrition. Am. J. Trop. Med. Hyg. 47:16–27. [DOI] [PubMed] [Google Scholar]
- 43.Matson, D. O., and M. K. Estes. 1990. Impact of rotavirus infection at a large pediatric hospital. J. Infect. Dis. 162:598–604. [DOI] [PubMed] [Google Scholar]
- 44.McKinney, M., and A. Parkinson. 1987. A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J. Immunol. Methods 96:271–278. [DOI] [PubMed] [Google Scholar]
- 45.Mebus, C. A., L. Stair, N. Underdahl, and M. Twiehaus. 1971. Pathology of neonatal calf diarrhea induced by a reo-like virus. Vet. Pathol. 8:490–505. [Google Scholar]
- 46.Moghadasian, M., L. Nguyen, S. Shefer, B. McManus, and J. Frohlich. 1999. Histologic, hematologic, and biochemical characteristics of apo E-deficient mice: effects of dietary cholesterol and phytosterols. Lab. Investig. 79:355–364. [PubMed] [Google Scholar]
- 47.Mori, Y., M. Sugiyama, M. Takayama, Y. Atoji, T. Masegi, and N. Minamoto. 2001. Avian-to-mammal transmission of an avian rotavirus: analysis of its pathogenicity in a heterologous mouse model. Virology 288:63–70. [DOI] [PubMed] [Google Scholar]
- 48.Nunnari, J., T. Zand, I. Joris, and G. Majno. 1989. Quantitation of oil red O staining of the aorta in hypercholesterolemic rats. Exp. Mol. Pathol. 51:1–8. [DOI] [PubMed] [Google Scholar]
- 49.O’Neal, C., S. E. Crawford, M. K. Estes, and M. E. Conner. 1997. Rotavirus VLPs administered mucosally induce protective immunity. J. Virol. 71:8707–8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osborne, M., S. Haddon, A. Spencer, J. Collins, W. Starsky, T. Wallis, G. Clarke, K. Worton, D. Candy, and J. Stephen. 1988. An electron microscopic investigation of time-related changes in the intestine of neonatal mice infected with murine rotavirus. J. Pediatr. Gastroenterol. Nutr. 7:236–248. [DOI] [PubMed] [Google Scholar]
- 51.Pearson, G. R., and M. S. McNulty. 1977. Pathological changes in the small intestine of neonatal pigs infected with a pig reovirus-like agent (rotavirus). J. Comp. Pathol. 87:363–375. [DOI] [PubMed] [Google Scholar]
- 52.Pearson, G. R., M. S. McNulty, and E. F. Logan. 1978. Pathological changes in the small intestine of neonatal calves naturally infected with reo-like virus (rotavirus). Vet. Rec. 102:454–458. [DOI] [PubMed] [Google Scholar]
- 53.Pérez-Schael, I., M. Guntiñas, M. Pérez, V. Pagone, A. Rojas, R. González, W. Cunto, Y. Hoshino, and A. Z. Kapikian. 1997. Efficacy of the rhesus rotavirus-based quadrivalent vaccine in infants and young children in Venezuela. New Engl. J. Med. 337:1181–1187. [DOI] [PubMed] [Google Scholar]
- 54.Ramig, R. F. 1988. The effects of host age, virus dose, and virus strain on heterologous rotavirus infection of suckling mice. Microb. Pathog. 4:189–202. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds, D., G. Hall, T. Debney, and K. Parsons. 1985. Pathology of natural rotavirus infection in clinically normal calves. Res. Vet. Sci. 38:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riepenhoff-Talty, M. P. Lee, P. Carmody, H. Barrett, and P. L. Ogra. 1982. Age-dependent rotavirus-enterocyte interactions. Proc. Soc. Exp. Biol. Med. 170:146–154. [DOI] [PubMed] [Google Scholar]
- 57.Riepenhoff-Talty, M., T. Dharakul, E. Kowalski, D. Sterman, and P. Ogra. 1987. Rotavirus infection in mice: pathogenesis and immunity. Adv. Exp. Med. Biol. 216B:1015–1023. [PubMed] [Google Scholar]
- 58.Saif, L. J., and B. Jiang. 1994. Nongroup A rotaviruses of humans and animals, p. 339–371. In R. F. Ramig (ed.), Rotaviruses. Springer-Verlag, Berlin, Germany. [DOI] [PubMed]
- 59.Saif, L. J., B. Rosen, and A. Parwani. 1994. Animal rotaviruses, p. 279–367. In A. Z. Kapikian (ed.), Viral infections of the gastrointestinal tract, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 60.Sakamoto, K., H. Hirose, A. Onizuka, M. Hayashi, N. Futamura, Y. Kawamura, and T. Ezaki. 2000. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J. Surg. Res. 94:99–106. [DOI] [PubMed] [Google Scholar]
- 61.Salim, A., A. Phillips, J. Walker-Smith, and M. Farthing. 1995. Sequential changes in small intestinal structure and function during rotavirus infection in neonatal rats. Gut 36:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheridan, J. F., R. Eydelloth, S. L. Vonderfecht, and L. Aurelian. 1983. Virus-specific immunity in neonatal and adult mouse rotavirus infection. Infect. Immun. 39:917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shinohara, H., C. S. Williams, D. L. McWilliam, O. Koldovsky, A. F. Philipps, and B. Dvořák. 2001. Transforming growth factor-alpha delays gastric emptying and small intestinal transit in suckling rats. Scand. J. Gastroenterol. 36:356–360. [PubMed] [Google Scholar]
- 64.Snodgrass, D. R., A. Ferguson, F. Allan, K. Angus, and B. Mitchell. 1979. Small intestinal morphology and epithelial cell kinetics in lamb rotavirus infections. Gastroenterology 76:477–481. [PubMed] [Google Scholar]
- 65.Soontornchai, S., D. Krüger, and R. Grossklaus. 1998. Small intestinal transit and digestibility of lactitol in Wistar rats. Z. Ernaehrwiss. 37:358–362. [DOI] [PubMed] [Google Scholar]
- 66.Starkey, W., J. Collins, T. Wallis, G. Clarke, A. Spencer, S. Haddon, M. Osborne, D. Candy, and J. Stephen. 1986. Kinetics, tissue specificity and pathological changes in murine rotavirus infection of mice. J. Gen. Virol. 67:2625–2634. [DOI] [PubMed] [Google Scholar]
- 67.Stucker, G., L. Oshiro, and N. Schmidt. 1980. Antigenic comparisons of two new rotaviruses from rhesus monkeys. J. Clin. Microbiol. 11:202–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Svensson, L., B. B. Finlay, D. Bass, C.-H. von Bonsdorff, and H. B. Greenberg. 1991. Symmetric infection of rotavirus on polarized human intestinal epithelial (Caco-2) cells. J. Virol. 65:4190–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi, E., Y. Inaba, K. Sato, H. Kurogi, H. Akashi, K. Satoda, and T. Omori. 1979. Antibody to rotavirus in various animal species. Nat. Inst. Anim. Health Q. 19:72–73. [PubMed] [Google Scholar]
- 70.Theil, K., E. Bohl, R. Cross, E. Köhler, and A. Agnes. 1978. Pathogenesis of porcine rotaviral infection in experimentally inoculated gnotobiotic pigs. Am. J. Vet. Res. 39:213–220. [PubMed] [Google Scholar]
- 71.Vesikari, T. 1996. Trials of oral bovine and rhesus rotavirus vaccines in Finland: a historical account and present status. Arch. Virol. 12(Suppl.):177–186. [DOI] [PubMed] [Google Scholar]
- 72.Vonderfecht, S., A. Huber, J. Eiden, L. Mader, and R. Yolken. 1984. Infectious diarrhea of infant rats produced by a rotavirus-like agent. J. Virol. 52:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vonderfecht, S., J. Eiden, R. Miskuff, and R. Yolken. 1988. Kinetics of intestinal replication of group B rotavirus and relevance to diagnostics methods. J. Clin. Microbiol. 26:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ward, R. L., M. M. McNeal, and J. F. Sheridan. 1990. Development of an adult mouse model for studies on protection against rotavirus. J. Virol. 64:5070–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ward, R. L., M. M. McNeal, and J. F. Sheridan. 1992. Evidence that active protection following oral immunization of mice with live rotavirus is not dependent on neutralizing antibody. Virology 188:57–66. [DOI] [PubMed] [Google Scholar]
- 76.Xu, A., A. R. Bellamy, and J. A. Taylor. 2000. Immobilization of the early secretory pathway by a virus glycoprotein that binds microtubules. EMBO J. 19:6465–6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yolken, R., S. Arango-Jaramillo, J. Eiden, and S. Vonderfecht. 1988. Lack of genomic reassortment following infection of infant rats with group A and group B rotaviruses. J. Infect. Dis. 158:1120–1123. [DOI] [PubMed] [Google Scholar]
- 78.Yolken, R., C. Ojeh, I. Khatri, U. Sajjan, and J. Forstner. 1993. Intestinal mucins inhibit rotavirus replication in an oligosaccharide-dependent manner. J. Infect. Dis. 169:1002–1006. [DOI] [PubMed] [Google Scholar]
- 79.Zhang, M., C. Q.-Y. Zeng, Y. Dong, J. M. Ball, L. J. Saif, A. P. Morris, and M. K. Estes. 1998. Mutations in rotavirus nonstructural glycoprotein NSP4 are associated with altered virulence. J. Virol. 72:3666–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]