Abstract
Objective:
To examine the prognostic significance of disseminated tumor cells in blood and bone marrow of patients undergoing surgical resection of colorectal liver metastases.
Summary Background Data:
Despite curative hepatic resection of colorectal liver metastases, a high percentage of patients develop tumor recurrence. These recurrences probably originate from disseminated tumor cells released into the circulation before or during surgery.
Methods:
Thirty-seven patients with potentially curative (R0) resection of colorectal liver metastases were prospectively enrolled into the study. Preoperative bone marrow samples and preoperative, intraoperative, and postoperative blood samples were examined for disseminated tumor cells by CK20 RT-PCR.
Results:
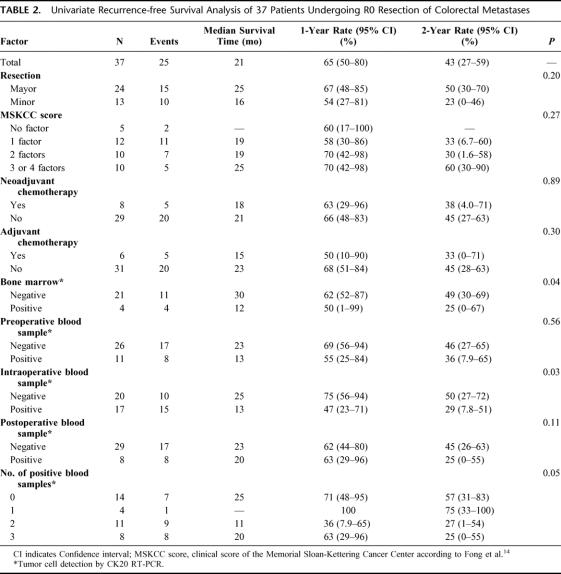
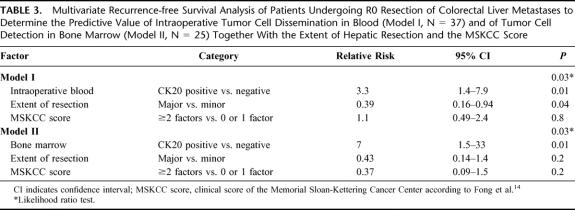
Tumor cells were detected in preoperative blood samples in 11 of 37 (30%) patients, in intraoperative blood samples in 17 of 37 (46%) patients, and in postoperative blood samples in 8 of 37 (22%) patients. Four of 25 (16%) patients tested positive for disseminated tumor cells in bone marrow samples. Median follow-up time for all patients was 38 months (range, 10–63 months). Multivariate analysis confirmed tumor cell detection in intraoperative blood (P = 0.009) and in bone marrow samples (P = 0.013) to be independent prognostic factors of tumor relapse.
Conclusions:
This is the first study demonstrating that detection of hematogenous tumor cell dissemination during hepatic resection of colorectal cancer metastases predicts tumor relapse. Detection of disseminated tumor cells may help to individualize adjuvant therapy for patients with colorectal liver metastases and to develop surgical strategies to prevent intraoperative hematogenous tumor cell shedding.
Blood and bone marrow samples from 37 patients undergoing curative surgical resection of colorectal liver metastases were examined for disseminated tumor cells. Detection of tumor cells in intraoperative blood and in bone marrow samples correlated significantly with disease-free survival of patients and is an independent prognostic factor for tumor relapse.
Surgical hepatic resection remains the most effective therapy for patients with liver metastases from colorectal cancer.1 In a large number of prospective and retrospectives studies, hepatic resection has been shown to be a safe and effective therapy that prolongs survival.2 The 5-year survival rate after surgical resection ranges from 20% to 51%, depending on preoperative selection criteria.2 However, even after curative liver resection, recurrent disease develops in approximately 75% of patients.3 Tumor recurrence after curative liver resection is probably caused by the spread of tumor cells into the circulation either before or during surgery; however, these cells could not be detected reliably until recently. PCR-based protocols are sensitive and specific assays for the detection of disseminated cancer cells, allowing the identification of approximately one neoplastic cell in 107 normal peripheral mononuclear blood cells.4,5 The detection of disseminated cancer cells by reverse transcription–polymerase chain reaction (RT-PCR) is based on the detection of mRNA. Since blood and bone marrow contain sufficient RNAase to rapidly destroy extracellular RNA, the detection of mRNA in blood and bone marrow samples is generally accepted as an indicator for the presence of viable cells.6,7
Recently, we demonstrated the sensitivity and specificity of a CK20 RT-PCR system in detecting disseminated colorectal cancer cells in blood and bone marrow.8–10 Using this technique, our group has demonstrated that tumor cell release into blood is a frequent event in patients with liver metastases from colorectal cancer undergoing major liver resections and is enhanced by intraoperative tumor manipulation.11 However, the prognostic impact of this observation is currently not defined. It is essential to prove the prognostic relevance as a basis for altering surgical resection techniques to reduce intraoperative shedding of tumor cells into the bloodstream. Furthermore, patients identified with hematogenous tumor cell dissemination might be potential candidates for adjuvant chemotherapy.
The aim of this prospective study was to evaluate whether disseminated tumor cells detected by CK20 RT-PCR in blood and bone marrow of patients undergoing hepatic resection for colorectal liver metastases can reliably predict tumor recurrence.
MATERIALS AND METHODS
Patients
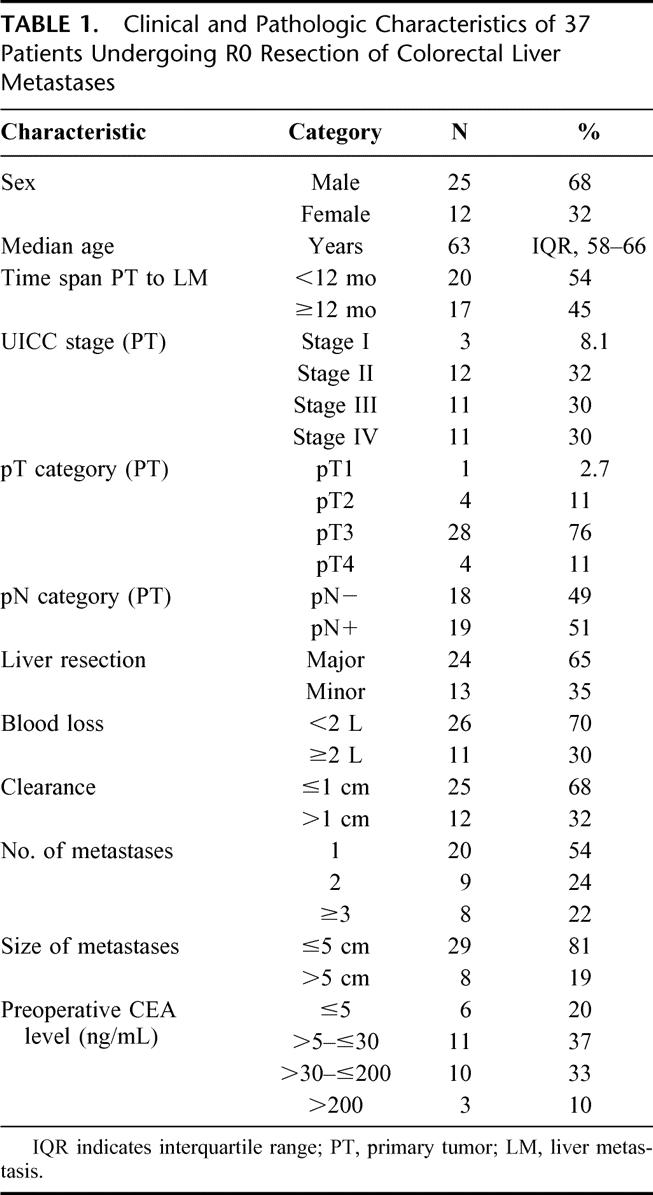
Between May 1996 and March 1999, 37 consecutive patients (25 male, 12 female, median age 63 years) undergoing liver resection for metastatic colorectal carcinoma at the Department of Surgery at the University of Heidelberg with negative microscopic margins (R0) were included. Patients underwent standard liver resection without special attention to manipulation of the liver or the metastases. Six other patients treated in the same time period for colorectal liver metastases were excluded from the study because they didn't undergo R0 resection (peritoneal tumor spread or liver resection with positive microscopic resection margin [R1] on postoperative histology). The likelihood of a curative resection was assessed preoperatively by a standard workup, including spiral computed tomography (CT) of the abdomen and chest. Colonoscopy was routinely performed to rule out tumor recurrence or metachronous colorectal cancer. Intraoperative ultrasound of the liver was performed in all patients. R0 resection was confirmed by postoperative histopathologic examination of the specimen with serial sectioning of the resection margins. As previously described, major liver resection was defined as resection of 2 or more liver segments while minor liver resection comprised resection of only one liver segment.11,12 Follow-up after hepatic resection was performed every 3 months for 2 years and thereafter twice per year. In addition to clinical evaluation, serum CEA levels, CT of the abdomen, chest x-ray, and colonoscopy were routinely performed. When indicated, additional diagnostic workup, including CT of the chest, was undertaken.
Six patients received neoadjuvant chemotherapy prior to hepatic resection, 4 patients received chemotherapy after hepatic resection, and 2 patients received neoadjuvant and adjuvant chemotherapy. As no standard regimen for chemotherapy in the setting of liver resection of colorectal metastases exists, indication for chemotherapy was left to the discretion of the treating oncologist. The PCR results of the majority of the patients included in this study were reported earlier by our group.11 Clinical records and survival data were obtained for all patients.
Blood and Bone Marrow Samples
Three blood samples (10 mL) were obtained from each patient through a central venous catheter in the superior vena cava: the first after induction of anesthesia, the second immediately after completed liver resection and the third 24 hours after the operation. Bone marrow samples (10 mL) were obtained after induction of general anesthesia by aspiration from both iliac crests. Twelve patients refused bone marrow aspiration; therefore, bone marrow samples were only taken from 25 of the 37 patients.
The blood and bone marrow samples were diluted with 10 mL phosphate-buffered saline. After density centrifugation through Ficoll-Paque (Pharmacia) (30 minutes, 400 g) mononuclear cells were harvested from the interphase and washed twice in phosphate-buffered saline. The cell pellet was then shock frozen in liquid nitrogen and stored at −70°C.
RNA Extraction
RNA extraction from peripheral mononuclear blood cells, from bone marrow samples, and from frozen tissue sections of tumors was previously described.8
RT-PCR
CK20-RT-PCR was performed as previously described.8 PCR products were analyzed by electrophoresis on 2% agarose gels. PCR products were blotted onto nylon membranes (Hybond N+, Amersham Life Science, Buckinghamshire, UK) and hybridized with a chemoluminescence-labeled oligonucleotide probe.9
RNA quality and performance of reverse transcription of all analyzed samples were confirmed by RT-PCR amplification of glyceraldehyde phosphate dehydrogenase transcripts as previously described.8
Sensitivity of CK20 RT-PCR
The sensitivity of the CK20 RT-PCR assay was determined in previous cell spiking experiments, allowing the detection of 10 colon cancer cells (HT 29) in 10 mL blood.8
Ethics
Informed consent was obtained from all patients, and the study protocol was approved by the ethics committee of the University of Heidelberg.
Statistics
SAS software (Release 8.02, SAS Institute, Inc., Cary, NC) was used for statistical analysis. The primary end point of this study was disease-free survival measured from the date of hepatic resection. Patients alive without local recurrence or distant metastases at the last follow-up date were censored. During the follow-up period, all observed deaths were tumor related. Disease-free survival was calculated by the method of Kaplan-Meier.13 Correlations between tumor cell detection and disease-free survival were analyzed with the log-rank test. To consolidate the number of clinicopathologic factors potentially associated with recurrence, a recently established clinical risk score for predicting recurrence after hepatic resection14 was used for the analysis. Because tumor cell release is significantly enhanced intraoperatively in patients with liver metastases from colorectal cancer undergoing major liver resection,11 the extent of hepatic resection was also included in the analysis of disease-free survival. Hazard ratios and corresponding 95% confidence intervals were computed using the Cox proportional hazards regression analysis.15 The likelihood ratio was taken to analyze the overall significance of the two final models. P values of less than 0.05 were considered statistically significant. All tests were performed two-sided.
RESULTS
Characteristics of the Patients
A total of 37 patients undergoing R0 resection of colorectal liver metastases were prospectively included. Extrahepatic disease had been excluded in all patients with the standard diagnostic tests prior to surgery to ensure that a potentially curative resection was indeed possible.
Follow-up was successfully completed for all patients. Thirteen of 37 (35%) patients died of tumor relapse. Median follow-up time of patients alive was 38 months (range, 10–63 months). One-, 2-, and 3-year estimated overall survival rates were 92%, 83%, and 68%, respectively. During follow-up, 25 patients developed tumor recurrence: only hepatic in 11 patients, only extrahepatic (lung, bone, colon, and/or lymph nodes) in 7 patients, and both intrahepatic and extrahepatic in 1 patient. Tumor localization was not exactly defined before death in the remaining 6 patients. Clinical and pathologic characteristics of the patients are presented in Table 1.
TABLE 1. Clinical and Pathologic Characteristics of 37 Patients Undergoing R0 Resection of Colorectal Liver Metastases
Specificity of CK20 RT-PCR
The specificity of the CK20-RT-PCR assay was determined in former studies, in which 174 blood samples from 98 healthy individuals and bone marrow samples from 30 patients without malignant disease consistently tested negative for CK20 expression.8–10,16,17 In a previous work, we excluded exfoliation of CK20 expressing normal liver cells during surgery by examining blood samples from 10 patients undergoing liver resection for benign disease. All these samples tested negative.11
Tumor Cell Detection in Blood Samples and Bone Marrow
Tumor cells were detected in preoperative blood samples of 11 of 37 (30%) patients. Seventeen of 37 (46%) patients revealed disseminated tumor cells in blood samples taken intraoperatively. Eight of 37 (22%) patients showed tumor cells in postoperative blood samples (taken 24 hours after the operation). Four of 25 (16%) patients tested positive for disseminated tumor cells in their bone marrow samples. Tumor cell detection rates in blood and bone marrow samples were not statistically different in patients receiving neoadjuvant chemotherapy as compared with patients without this treatment.
Correlation of CK20 RT-PCR With Survival and Prognosis
The results of the univariate analysis of disease-free survival are shown in Table 2. Intraoperative detection of tumor cell dissemination was correlated with a significantly shorter disease-free survival (Fig. 1). Median disease-free survival of CK20-positive patients was 13 months versus 25 months for CK20-negative patients (P = 0.03). Fifteen of 17 patients with tumor cells in intraoperative blood samples developed tumor recurrences, whereas only 10 of 20 patients without tumor cells in intraoperative blood samples developed tumor relapse.
TABLE 2. Univariate Recurrence-free Survival Analysis of 37 Patients Undergoing R0 Resection of Colorectal Metastases
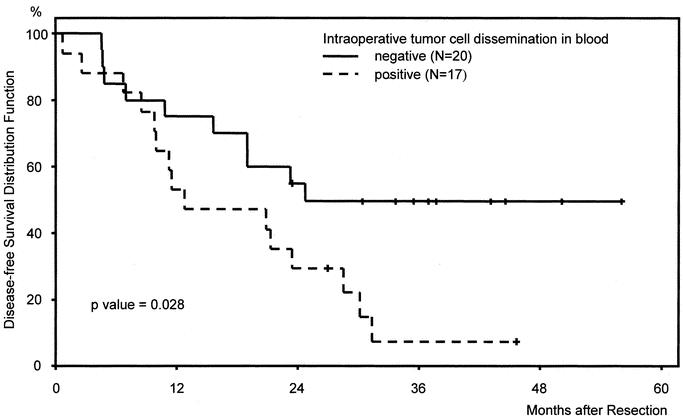
FIGURE 1. Kaplan-Meier disease-free survival curves of patients (n = 37) after curative hepatic resection of colorectal liver metastases stratified according to CK20 RT-PCR results in intraoperative blood samples.
Detection of tumor cell dissemination in preoperative blood samples did not significantly correlate with the disease-free survival of the patients, although CK20-positive patients had a poorer median disease-free survival (13 months) compared with CK20-negative patients (23 months).
All patients with detectable tumor cells in postoperative blood samples developed tumor recurrences. The difference between disease-free survival of patients with CK20-positive and CK20-negative postoperative blood samples, however, did not reach statistical significance (Fig. 2).
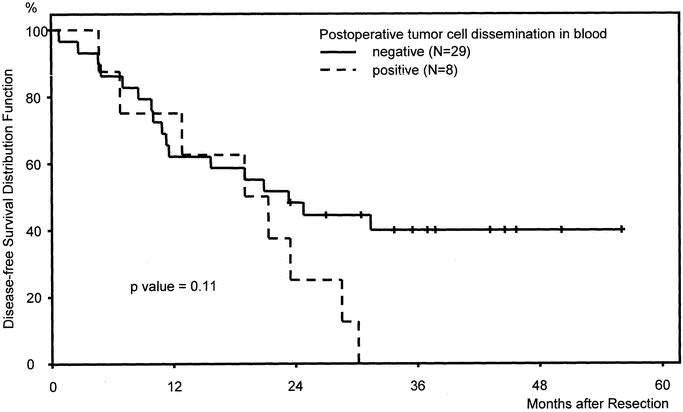
FIGURE 2. Kaplan-Meier disease-free survival curves of patients (n = 37) after curative hepatic resection of colorectal liver metastases stratified according to CK20 RT-PCR results in postoperative blood samples.
The number of positive blood samples seems to have prognostic impact, as patients with 2 or 3 positive samples showed a worse outcome compared with patients with one or none positive sample on univariate analysis.
Patients with disseminated tumor cells in their bone marrow had a significantly worse prognosis compared with CK20-negative patients (Fig. 3). Median disease-free survival of CK20-positive patients was 12 months versus 30 months in CK20-negative patients (P = 0.04). All patients with CK20 positive bone marrow samples developed tumor recurrences.
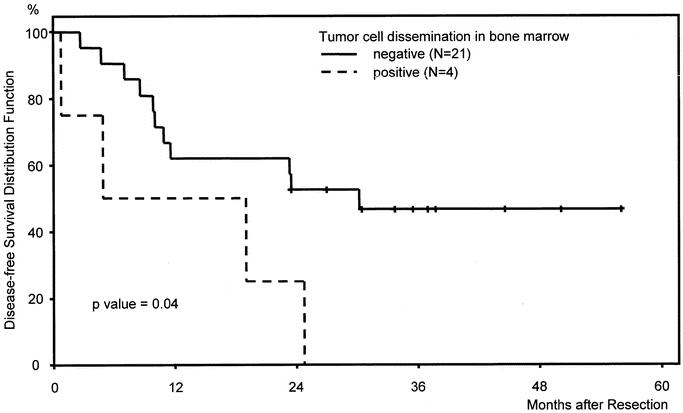
FIGURE 3. Kaplan-Meier disease-free survival curves of patients (n = 25) after curative hepatic resection of colorectal liver metastases stratified according to CK20 RT-PCR results in bone marrow samples.
To determine the prognostic value of tumor cell dissemination in patients undergoing curative resection for colorectal liver metastases a multivariate disease-free survival analysis was performed (Table 3). Factors included in this multivariate model were the extent of hepatic resection, the clinical risk score according to Fong et al,14 and either intraoperative tumor cell dissemination in blood (model I) or tumor cell dissemination in bone marrow (model II). Intraoperative tumor cell dissemination in blood and tumor cell detection in bone marrow were shown to be independent prognostic factors. In our patient cohort, the extent of hepatic resection was also a prognostic factor in the multivariate analysis whereas the Fong score was not.
TABLE 3. Multivariate Recurrence-free Survival Analysis of Patients Undergoing R0 Resection of Colorectal Liver Metastases to Determine the Predictive Value of Intraoperative Tumor Cell Dissemination in Blood (Model I, N = 37) and of Tumor Cell Detection in Bone Marrow (Model II, N = 25) Together With the Extent of Hepatic Resection and the MSKCC Score
DISCUSSION
Despite the potential for long-term survival, patients undergoing resection of colorectal liver metastases are at a substantial risk for tumor recurrence.18 Many studies have therefore examined prognostic criteria to improve patient selection for surgical therapy and/or adjuvant or neoadjuvant chemotherapy.3,19–21 Several patient- and tumor-related factors have been identified that correlate with poor survival and prognosis in patients after surgical resection for colorectal liver metastases. Two large studies, by Fong et al14 and Nordlinger et al,22 have developed prognostic scores to predict long-term outcome of patients undergoing surgical resection for colorectal liver metastases. These scores incorporate several clinical factors, such as number of hepatic metastases, nodal status of the primary tumor, preoperative CEA levels, size of largest tumor nodule, and time interval between primary tumor and discovery of liver metastasis. While these statistically defined prognostic scores apply to patient populations, the exact risk for each individual patient after surgical resection for colorectal liver metastases cannot be determined.
Therefore, research has focused on identifying specific molecular or genetic markers that may define patients at high risk for tumor relapse or tumor progression. New molecular techniques have demonstrated the presence of occult tumor cells in different body compartments (eg, blood, bone marrow, or lymph nodes) after resection of solid tumors.23 Lindemann et al showed, for the first time, the prognostic significance of tumor cells detected in the bone marrow of patients with primary colorectal cancer using immunohistochemical methods.24 However, no study has addressed this question in patients with liver metastases for colorectal cancer. Therefore, the aim of the present study was to examine whether the detection of disseminated tumor cells by CK20 RT-PCR identifies patients at high risk for disease recurrence after curative hepatic resection of colorectal liver metastases. This was of special interest in the context of our recent finding, that tumor cell shedding into the bloodstream is enhanced during resection of colorectal liver metastases.11
Although the concept of dissemination of malignant cells during surgical procedures is not new, the possibility that such disseminated cells may indeed be clinically relevant is again attracting attention.25–27 The relationship between circulating tumor cells and the development of metastatic disease is still unclear. Tumor cell shedding into the peripheral circulation appears to be a relatively common event, but most of these cells do not survive.28 However, in the appropriate environment, a large hematogenous bolus of viable tumor cells may give rise to distant metastases. In addition, major hepatic resections are associated with profound immunologic changes and with the activation of clotting factors, which might enhance the metastatic potential of tumor cells disseminated during surgery.29,30 The prognostic impact of tumor cells detected during surgery is confirmed by our study in which intraoperative tumor cell dissemination was identified to be a prognostic factor for disease-free survival of patients undergoing curative resection of colorectal liver metastases (Fig. 1; Table 3). We therefore conclude that intraoperative tumor manipulation, which is known to result in an enhanced release of malignant cells, causes intrahepatic or extrahepatic tumor recurrence, at least in a part of patients undergoing liver resection for colorectal metastases.
Tumor cell detection in blood taken 24 hours postoperatively was more than halved compared with intraoperative tumor cell dissemination and was not significantly correlated to a poorer prognosis of the patients (Fig. 2). Interestingly, all patients with detectable tumor cells in postoperative blood samples developed tumor recurrence (Table 2). This observation is confirmed by a recent study demonstrating that clearance of circulating tumor cells within 24 hours of colorectal cancer resections was greatest in the subgroup of patients with the best prognosis.31 When looking at the Kaplan-Meier curves, survival seems to be almost identical for the first 18 to 24 months postoperatively; however, after this time interval, patients with positive blood samples show a worse survival compared with patients without tumor cell detection. Detecting tumor cells in blood samples might therefore have special prognostic relevance in regard to postoperative survival after the first 2 years have passed; however, this has to be substantiated in further studies.
Tumor cell dissemination in bone marrow detected by CK20 RT-PCR was also significantly correlated with shorter disease-free survival of patients after curative hepatic resection of colorectal liver metastases (Fig. 3) and was shown to be a prognostic factor on multivariate analysis (Table 3). This observation supports the hypothesis that detection of disseminated tumor cells in bone marrow might serve as a valid surrogate marker for systemic hematogenous tumor cell spread in an individual patient.
Further prospective studies now need to clarify whether patients with tumor cells in blood and bone marrow benefit from adjuvant chemotherapy and whether tumor cell detection in blood or bone marrow is a suitable surrogate marker for monitoring the efficacy of adjuvant chemotherapy after hepatic resection. The rationale for adjuvant chemotherapy after curative liver resection is to treat and eradicate suspected disseminated tumor cells or micrometastases in the liver, thereby decreasing the relapse rate and improving patient survival. Several studies have, however, indicated that antiproliferative agents may not be efficient against disseminated tumor cells as these cells rarely express proliferation-associated markers.32,33 Despite conflicting results for adjuvant or neoadjuvant chemotherapy in patients undergoing liver resection, it has recently been demonstrated that postoperative chemotherapy using a combination of hepatic arterial and intravenous infusion may improve outcome of patients after resection of colorectal liver metastases.34 In summary, there is a strong need for a surrogate marker to measure the efficacy of neoadjuvant and adjuvant therapy in patients with hepatic metastases of colorectal cancer. The results of our study are encouraging in this context.
Intraoperative tumor cell dissemination during hepatic resection of colorectal liver metastases can possibly be reduced by alternative surgical strategies, eg, the anterior approach, a less commonly used technique of hepatic resection that avoids major liver manipulation before venous occlusion. The anterior approach was associated with an improved survival of patients with hepatocellular carcinoma in a retrospective study.35 Further studies are required to investigate whether this new surgical strategy improves survival of patients after resection of colorectal liver metastases.
CONCLUSION
This is the first study demonstrating the prognostic significance of tumor cells detected in blood and bone marrow in patients undergoing liver resection for colorectal liver metastases. This new prognostic factor may change the surgical management of patients with colorectal liver metastases and may help to individualize the treatment of these patients with systemic or regional chemotherapy.
ACKNOWLEDGMENTS
The authors thank Professor M.W. Büchler, MD, for his support of our work.
Footnotes
Supported by a grant from the Medical Faculty of the University of Heidelberg.
Reprints: Jürgen Weitz, MD, PhD, Section of Surgical Oncology, Department of Surgery, University of Heidelberg, Im Neuenheimer Feld 110, 69120 Heidelberg, Germany. E-mail: Jürgen_Weitz@med.uni-heidelberg.de.
REFERENCES
- 1.Primrose JN. Treatment of colorectal metastases: surgery, cryotherapy, or radiofrequency ablation. Gut. 2002;50:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodgers MS, McCall JL. Surgery for colorectal liver metastases with hepatic lymph node involvement: a systematic review. Br J Surg. 2000;87:1142–1155. [DOI] [PubMed] [Google Scholar]
- 3.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. [DOI] [PubMed] [Google Scholar]
- 4.Pantel K, von Knebel Doeberitz M. Detection and clinical relevance of micrometastatic cancer cells. Curr Opin Oncol. 2000;12:95–101. [DOI] [PubMed] [Google Scholar]
- 5.Johnson P, Burchill S, Selby P. The molecular detection of circulating tumor cells. Br J Cancer. 1995;72:268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macfarlane DE, Dahle CE. Isolating RNA from whole blood: the dawn of RNA-based diagnosis. Nature. 1993;362:186–188. [DOI] [PubMed] [Google Scholar]
- 7.Ghossein R, Bhattacharya S, Rosai J. Molecular detection of micrometastases and circulating tumor cells in solid tumors. Clin Cancer Res. 1999;5:1950–1960. [PubMed] [Google Scholar]
- 8.Weitz J, Kienle P, Lacroix J, et al. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin Cancer Res. 1998;4:343–348. [PubMed] [Google Scholar]
- 9.Weitz J, Kienle P, Magener A, et al. Detection of disseminated colorectal cancer cells in lymph nodes, blood and bone marrow. Clin Cancer Res. 1999;5:1830–1836. [PubMed] [Google Scholar]
- 10.Koch M, Weitz J, Kienle P, et al. Comparative analysis of tumor cell dissemination in mesenteric, central and peripheral venous blood in patients with colorectal cancer. Arch Surg. 2001;136:85–89. [DOI] [PubMed] [Google Scholar]
- 11.Weitz J, Koch M, Kienle P, et al. Detection of hematogenic tumor cell dissemination in patients undergoing resection of liver metastases of colorectal cancer. Ann Surg. 2000;232:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bismuth H, Houssin DH, Castaing D. Major and minor segmentectomies in liver surgery. World J Surg. 1982;6:10–24. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox DR. Regression models and life tables [with discussion]. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 16.Weber T, Lacroix J, Weitz J, et al. Expression of cytokeratin 20 in thyroid carcinomas and peripheral blood detected by reverse transcription polymerase chain reaction. Br J Cancer. 2000;82:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Güdemann C, Weitz J, Kienle P, et al. Detection of hematogenous micrometastasis in patients with transitional cell carcinoma. J Urol. 2000;164:532–536. [PubMed] [Google Scholar]
- 18.Scheele J, Altendorf-Hofmann A, Grube T, et al. Resection of colorectal liver metastases: which prognostic factors should govern patient selection? Chirurg. 2001;72:547–560. [DOI] [PubMed] [Google Scholar]
- 19.Scheele J, Stangl R, Altendorf-Hofmann A, et al. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery. 1991;110:13–29. [PubMed] [Google Scholar]
- 20.Schlag P, Hohenberger P, Herfarth C. Resection of liver metastases in colorectal cancer: competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol. 1990;16:360–365. [PubMed] [Google Scholar]
- 21.Yamada H, Kondo S, Okushiba S, et al. Analysis of predictive factors for recurrence after hepatectomy for colorectal liver metastases. World J Surg. 2001;25:1129–1133. [DOI] [PubMed] [Google Scholar]
- 22.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 23.Pantel K, von Knebel Doeberitz M. Detection and clinical relevance of micrometastatic cancer cells. Curr Opin Oncol. 2000;12:95–101. [DOI] [PubMed] [Google Scholar]
- 24.Lindemann F, Schlimok G, Dirschedl P, et al. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet. 1992;340:685–689. [DOI] [PubMed] [Google Scholar]
- 25.Romsdahl MM, McGrath RG, Hoppe E, et al. Experimental model for the study of tumor cells in the blood. Acta Cytol. 1965;9:141–145. [PubMed] [Google Scholar]
- 26.Liotta LA, Kleinermann J, Saidel GM, et al. Quantitative relationships of intravascular tumor cells, tumor vessels and pulmonary metastasis following tumor implantation. Cancer Res. 1974;34:997–1004. [PubMed] [Google Scholar]
- 27.Nishizaki T, Matsumata T, Kanematsu T. Surgical manipulation of VX2 carcinoma in the rabbit liver evokes enhancement of metastasis. J Surg Res. 1990;49:92–97. [DOI] [PubMed] [Google Scholar]
- 28.Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51(suppl 18):5054–5059. [PubMed] [Google Scholar]
- 29.Jarnagin WR, Delman K, Kooby D, et al. Neoadjuvant interleukin-12 immunogene therapy protects against cancer recurrence after liver resection in an animal model. Ann Surg. 2000;231:762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colacchio TA, Yeager MP, Hildebrand LW. Perioperative immunomodulation in cancer surgery. Am J Surg. 1994;167:174–179. [DOI] [PubMed] [Google Scholar]
- 31.Patel H, Le Marer N, Wharton RQ, et al. Clearance of circulating tumor cells after excision of primary colorectal cancer. Ann Surg. 2002;235:226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braun S, Kentenich C, Janni W, et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol. 2000;18:80–86. [DOI] [PubMed] [Google Scholar]
- 33.Pantel K, Schlimok G, Braun S, et al. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J Natl Cancer Inst. 1993;85:1419–1424. [DOI] [PubMed] [Google Scholar]
- 34.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. [DOI] [PubMed] [Google Scholar]
- 35.Liu CL, Fan ST, Lo CM, et al. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]