Abstract
Objective:
To review the physiologic effects of carbon dioxide (CO2) pneumoperitoneum in the morbidly obese.
Summary Background Data:
The number of laparoscopic bariatric operations performed in the United States has increased dramatically over the past several years. Laparoscopic bariatric surgery requires abdominal insufflation with CO2 and an increase in the intraabdominal pressure up to 15 mm Hg. Many studies have demonstrated the adverse consequences of pneumoperitoneum; however, few studies have examined the physiologic effects of pneumoperitoneum in the morbidly obese.
Methods:
A MEDLINE search from 1994 to 2003 was performed using the key words morbid obesity, laparoscopy, bariatric surgery, pneumoperitoneum, and gastric bypass. The authors reviewed papers evaluating the physiologic effects of pneumoperitoneum in morbidly obese subjects undergoing laparoscopy. The topics examined included alteration in acid-base balance, hemodynamics, femoral venous flow, and hepatic, renal, and cardiorespiratory function.
Results:
Physiologically, morbidly obese patients have a higher intraabdominal pressure at 2 to 3 times that of nonobese patients. The adverse consequences of pneumoperitoneum in morbidly obese patients are similar to those observed in nonobese patients. Laparoscopy in the obese can lead to systemic absorption of CO2 and increased requirements for CO2 elimination. The increased intraabdominal pressure enhances venous stasis, reduces intraoperative portal venous blood flow, decreases intraoperative urinary output, lowers respiratory compliance, increases airway pressure, and impairs cardiac function. Intraoperative management to minimize the adverse changes include appropriate ventilatory adjustments to avoid hypercapnia and acidosis, the use of sequential compression devices to minimizes venous stasis, and optimize intravascular volume to minimize the effects of increased intraabdominal pressure on renal and cardiac function.
Conclusions:
Morbidly obese patients undergoing laparoscopic bariatric surgery are at risk for intraoperative complications relating to the use of CO2 pneumoperitoneum. Surgeons performing laparoscopic bariatric surgery should understand the physiologic effects of CO2 pneumoperitoneum in the morbidly obese and make appropriate intraoperative adjustments to minimize the adverse changes.
The number of laparoscopic bariatric operations is increasing in the United States. It is important for surgeons performing laparoscopic bariatric surgery to understand potential adverse consequences of carbon dioxide pneumoperitoneum in morbidly obese patients. The authors reviewed the literature and summarize the current understanding of the physiologic effects of pneumoperitoneum on carbon dioxide absorption and excretion, femoral venous flow, and hepatic, renal, and cardiorespiratory function in the morbidly obese.
Obesity is now considered an epidemic in the United States. According to a study from the Center for Disease Control and Prevention, 30.5% of Americans are considered obese with a body mass index (BMI) greater than 30 kg/m2, and 4.7% of Americans are considered morbidly obese (BMI > 40).1 Obesity is also affecting children in the United States. The prevalence of overweight among children and adolescents has increased from 10% in 1971 to 1974 to 31% in 1999 to 2000.2 Obesity contributes to the development of many medical conditions and can also lead to premature death.3 Surgical therapy is an effective sustained means of weight loss for the treatment of morbid obesity according to the 1991 National Institutes of Health Consensus Conference.4 Roux-en-Y gastric bypass (GBP) is currently the most commonly performed bariatric operation in the United States. With the introduction of laparoscopic bariatric surgery in 1994, there has been a tremendous interest by surgeons to incorporate bariatric surgery into their practice.5 Therefore, it is important for surgeons performing laparoscopic bariatric surgery to understand the fundamental differences between the laparoscopic versus open operation and the possible adverse consequences of pneumoperitoneum in the morbidly obese.
The fundamental differences between laparoscopic and open approaches to bariatric surgery are the methods of access and exposure. Surgical access is generally obtained through an upper midline incision in open bariatric surgery and through five abdominal trocars in laparoscopic bariatric surgery. Surgical exposure of the operative field is commonly performed using abdominal wall retractors in open surgery compared with carbon dioxide (CO2) pneumoperitoneum in laparoscopic surgery. Pneumoperitoneum with CO2 has been used in clinical practice since the introduction of laparoscopic cholecystectomy in the late 1980s. The physiologic effects of pneumoperitoneum include 1) systemic absorption of CO2 and 2) hemodynamic and physiologic alteration in a variety of organs due to the increased intraabdominal pressure. CO2 absorption across the peritoneal surface and into the systemic circulation can result in hypercarbia and eventual systemic acidosis. The increased intraabdominal pressure during pneumoperitoneum has been shown in nonobese subjects to result in hemodynamic alteration and changes in femoral venous flow and renal, hepatic, and cardiorespiratory function.6–9 Few studies have examined the effects of CO2 pneumoperitoneum in the morbidly obese. In addition, laparoscopic bariatric surgery, particularly laparoscopic GBP, is a complex operation often associated with a longer operative time than other commonly performed laparoscopic procedures. A longer operative time during laparoscopic GBP translates to longer exposure of the host to the adverse physiologic effects of pneumoperitoneum (Fig. 1). Therefore, surgeons performing laparoscopy in morbidly obese patients should understand the basic physiologic changes occurring during pneumoperitoneum, recognize the clinical changes, and make appropriate intraoperative adjustments to minimize the adverse changes.
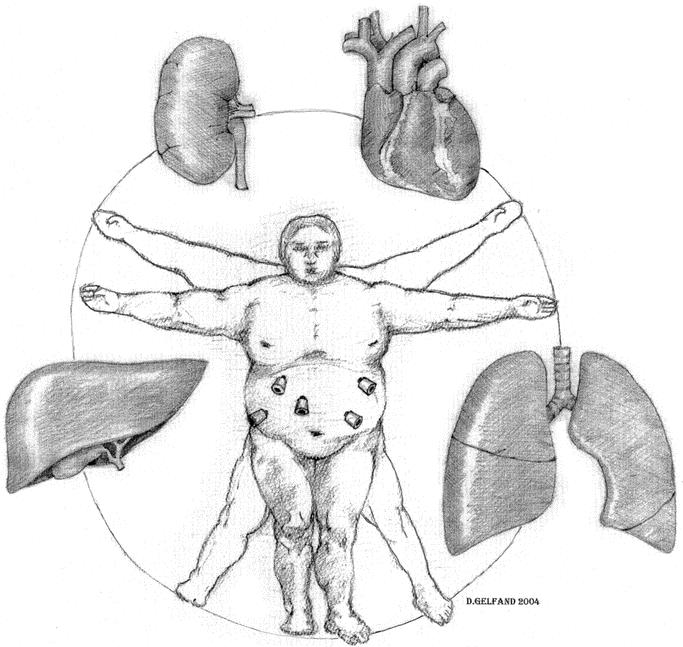
FIGURE 1. Pneumoperitoneum in the morbidly obese can lead to alteration of cardiac, respiratory, hepatic, and renal function.
EFFECTS OF CARBON DIOXIDE ABSORPTION DURING PNEUMOPERITONEUM
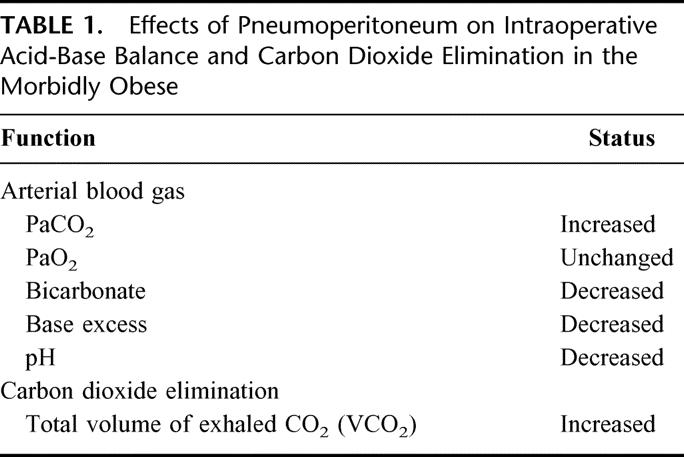
Table 1 summarizes the effects of pneumoperitoneum on acid-base balance and CO2 elimination. Pneumoperitoneum can result in systemic absorption of CO2 and alteration of acid-base balance. Absorption of CO2 across the peritoneum is normally eliminated through the lungs because of its high aqueous solubility and diffusibility. If intraoperative ventilation is impaired, CO2 absorption can result in hypercapnia and acidosis.10 Hypercapnia can cause cardiac arrhythmias, vasoconstriction of the pulmonary vessels, and a mixed response in cardiac function. Acidosis associated with hypercapnia has a depressive effect on myocardial contractility, whereas hypercapnia can stimulate the autonomic nervous system leading to tachycardia and increased myocardial contractility. To prevent hypercapnia, close intraoperative monitoring of end-tidal CO2 (ETCO2) or arterial partial pressure of CO2 (PaCO2) is therefore essential. Although ETCO2 level is an easily accessible monitoring parameter, it often underestimates the true level of PaCO2.11 In patients undergoing laparoscopic adjustable gastric banding, Demiroluk et al12 reported an increase in PaCO2 levels from 34 mm Hg at baseline to 42 mm Hg after abdominal insufflation. In a study of laparoscopic versus open GBP, ETCO2 levels were found to increase by 14% of baseline (from 35 mm Hg to 40 mm Hg), and PaCO2 levels increased by 10% of baseline (from 38 mm Hg to 42 mm Hg) during laparoscopic GBP.13 Although ETCO2 and PaCO2 levels increased during laparoscopy, these levels are still considered as normocapnia because appropriate ventilatory adjustments were performed.13
TABLE 1. Effects of Pneumoperitoneum on Intraoperative Acid-Base Balance and Carbon Dioxide Elimination in the Morbidly Obese
During pneumoperitoneum, appropriate ventilatory changes should be performed to eliminate the increased CO2 load and prevent systemic acidosis. Ventilatory changes consist of increasing the minute ventilation. Dumont et al14 reported that minute ventilation increased by 21% to limit the rise in ETCO2 in patients undergoing laparoscopic gastroplasty. In a study of patients undergoing laparoscopic GBP, ventilatory adjustments were performed by increasing the respiratory rate by 25% and minute ventilation by 21% to counteract the increase in CO2 load and prevent intraoperative acidosis.13
Elimination of the increased CO2 load is performed primarily through the lungs. The total volume of exhaled CO2 (VCO2) during pneumoperitoneum is therefore an indirect method to quantify the amount of CO2 absorbed during laparoscopy. In a study of nonobese patients, Tan et al15 estimated that the volume of CO2 absorbed from the peritoneal cavity ranged from 38 to 42 mL/min during laparoscopy, which represented a 30% increase in the CO2 load. In a study of laparoscopic and open GBP, VCO2 levels were reported to increase from 201 mL/min at baseline to 261 mL/min at 2.5 hours after abdominal insufflation in patients who underwent laparoscopic GBP, which represented a 30% increase in the CO2 load, but VCO2 levels remained unchanged in patients who underwent open GBP.13 If an assumption is made that the measured VCO2 during open GBP is the direct product of metabolism, the amount of absorbed CO2 during laparoscopic GBP can be estimated by taking the difference in VCO2 levels between laparoscopic and open GBP patients. Nguyen et al13 estimated the rate of CO2 absorption during laparoscopic GBP at 19 to 39 mL/min.13 Overall, absorption and excretion of CO2 in morbidly obese subjects appear to be similar to that of nonobese subjects.
EFFECTS OF INCREASED INTRAABDOMINAL PRESSURE DURING PNEUMOPERITONEUM
Pneumoperitoneum results in a state of acutely elevated intraabdominal pressure. Similar to nonobese subjects, the intraabdominal pressure during laparoscopy of the morbidly obese is set at 15 mm Hg to provide adequate visualization and exposure of the operative field. The normal intraabdominal pressure of nonobese individuals is 5 mm Hg or less.16 In contrast, morbidly obese patients have a chronically elevated intraabdominal pressure at 9 to 10 mm Hg.17 This section discusses the physiologic effects of increased intraabdominal pressure during pneumoperitoneum on femoral venous flow and renal, hepatic, and cardiorespiratory function.
Hemodynamic Changes and Cardiac Function
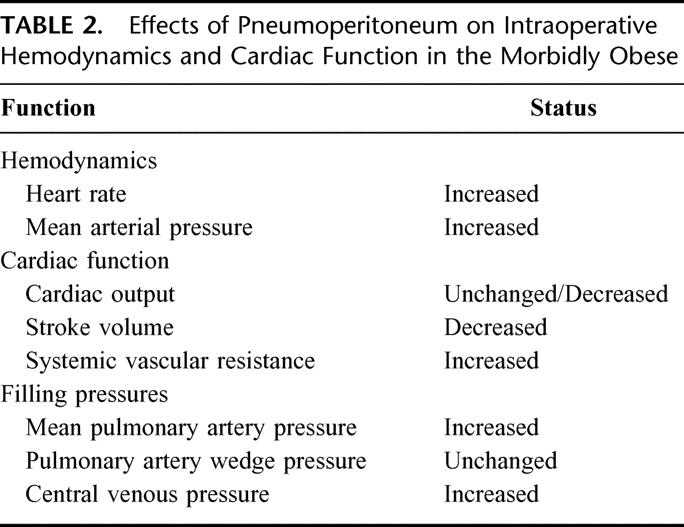
Abdominal insufflation has been shown to alter mean arterial pressure and heart rate (Table 2). Heart rate and mean arterial blood pressure commonly increase during laparoscopy.18,19 In a study of morbidly obese patients, heart rate and mean arterial pressure increased during both laparoscopic and open GBP and remained elevated throughout the operation.20 Comparing between obese and nonobese individuals, Fried et al21 reported that heart rate increased after pneumoperitoneum in both nonobese and obese individuals; however, obese individuals had a more pronounced increase in the heart rate level.
TABLE 2. Effects of Pneumoperitoneum on Intraoperative Hemodynamics and Cardiac Function in the Morbidly Obese
The hemodynamic effects of pneumoperitoneum on cardiac function have been extensively examined in nonobese individuals. Several investigators have demonstrated a reduction in cardiac output during pneumoperitoneum,22–23 whereas others24–25 have reported minimal change in cardiac function. Westerband et al22 reported a 30% reduction in cardiac index in patients who underwent laparoscopic cholecystectomy. McLaughlin et al23 also demonstrated that cardiac index decreased by 30% within 30 minutes of initiation of pneumoperitoneum. In a comparative study of cholecystectomy performed by the abdominal wall lifting method versus laparoscopy, Ninomiya et al26 reported that cardiac output was depressed during pneumoperitoneum but not during the abdominal wall lifting technique and suggested that the increased intraabdominal pressure during pneumoperitoneum is the main factor responsible for alteration of cardiac function.
Few studies have examined the effects of pneumoperitoneum on cardiac function in the obese. Fried et al21 compared cardiac function of 6 morbidly obese individuals with 6 normal body weight subjects; morbidly obese subjects had a 12% increase in cardiac output after abdominal insufflation. Using Swan-Ganz catheterization, Nguyen et al20 reported a decrease in cardiac output and stroke volume upon abdominal insufflation by 6% and 8% of baseline, respectively, during laparoscopic GBP. In contrast to the results observed in the nonobese, studies in morbidly obese subjects demonstrated minimal cardiac depression with initiation of pneumoperitoneum. We hypothesized that the cardiac dysfunction during pneumoperitoneum is minimized or better tolerated in morbidly obese patients with an intrinsically elevated intraabdominal pressure at 9 to 10 mm Hg compared with nonobese patients with an intrinsic intraabdominal pressure of less than 5 mm Hg.
Many factors influence cardiac function including preload, afterload, cardiac contractility, heart rate, and myocardial compliance. Hypovolemia reduces the preload and hence reduces cardiac output. Therefore, a euvolemic preoperative volume status is very important to minimize any cardiac depression associated with pneumoperitoneum. Measurement of filling pressures is the most accurate method for estimation of intravascular volume; however, cardiac filling pressure can be falsely elevated during pneumoperitoneum. The increased intraabdominal pressure during pneumoperitoneum causes cephalad shift of the diaphragm and increases the pleural pressure that is transmitted to the cardiac chambers. Nguyen et al20 reported a significantly higher central venous and mean pulmonary artery pressure during laparoscopic GBP compared with open GBP even though the two groups received a similar amount of intraoperative fluid.
Factors specific to laparoscopy that can affect intraoperative cardiac function include increased intraabdominal pressure, reverse Trendelenburg positioning, and hypercarbia. Hypercarbia is normally avoided during laparoscopy because of appropriate ventilatory adjustments. In most clinical studies, a moderate rise in PaCO2 levels (<45 mm Hg) should not contribute to cardiac impairment. In addition, reverse Trendelenburg positioning is also not a major factor in alteration of hemodynamic status.27 The increased intraabdominal pressure is the main factor that may account for cardiac depression. The mechanisms for reduction in cardiac output after abdominal insufflation include an increase in afterload and a decrease in preload by impeding venous return. Declan Fleming et al28 reported that the afterload, as measured by systemic vascular resistance, increased by 25% of baseline after abdominal insufflation and decreased with desufflation. Nguyen et al20 noted that open GBP was not associated with any alteration in the systemic vascular resistance while laparoscopic GBP resulted in an immediate increase in the systemic vascular resistance by 34% of baseline upon insufflation and returned to baseline value within 1.5 hours after initiation of pneumoperitoneum. The timing for the increase in systemic vascular resistance correlated with the reduction in cardiac output and stroke volume.20 The results from these studies suggest that an increase in the systemic vascular resistance is the primary event leading to a reduction in cardiac output. In addition, cardiac depression during pneumoperitoneum is often transient as the body compensates for the altered physiology. Zuckerman and Heneghan29 reported that reduction in cardiac index occurred immediately after abdominal insufflation during laparoscopic cholecystectomy but returned to baseline levels within 10 to 15 minutes after abdominal insufflation. Similar results were observed in morbidly obese subjects; cardiac output levels recovered after a transient depression at 2.5 hours after abdominal insufflation during laparoscopic GBP.20
Hepatic Function
Table 3 summarizes the effects of pneumoperitoneum on intraoperative portal venous flow and postoperative changes in liver enzymes. In animal and human studies, the increased intraabdominal pressure at 15 mm Hg has been shown to reduce portal venous flow.30 In a clinical study of laparoscopic cholecystectomy, Jakimowics et al6 reported a 53% reduction in portal blood flow with abdominal insufflation to 14 mm Hg. A reduction in portal venous blood flow during pneumoperitoneum may lead to hepatic hypoperfusion and acute hepatocyte injury. Portal hypoperfusion can lead to transient elevation of liver enzymes. Halevy et al31 reported transient increases in the level of hepatic transaminases (ALT and AST) after laparoscopic cholecystectomy, which returned to baseline by 72 hours postoperatively. Understanding the effect of pneumoperitoneum on portal venous flow is particularly important in the morbidly obese as these patients often have preexisting liver disease. Gholam et al32 noted that 84% of subjects who underwent Roux-en-Y GBP had steatosis, and Spaulding et al33 reported that there is a 56% prevalence of nonalcoholic steatohepatitis in morbidly obese subjects.
TABLE 3. Effects of Pneumoperitoneum on Hepatic Function in the Morbidly Obese
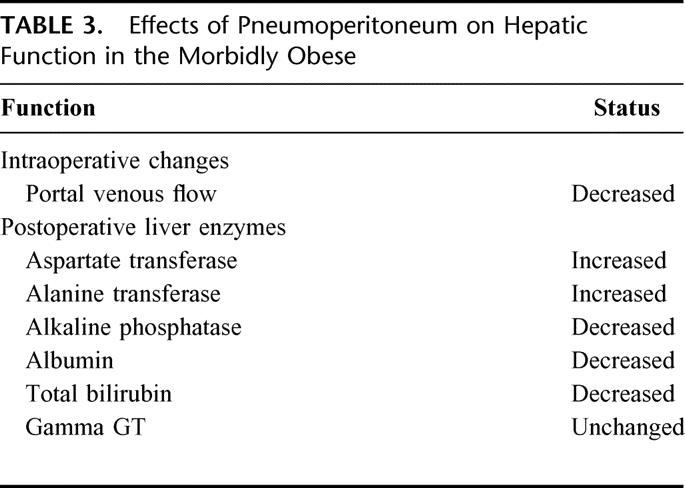
Few studies have examined the effects of pneumoperitoneum on hepatic function in the morbidly obese. Transient elevation of hepatic transaminase levels (ALT and AST) after laparoscopic and open GBP have been reported.34,35 The transaminase levels after laparoscopic GBP have been shown to increase by 6-fold, peaking at 24 hours postoperatively, and returned to baseline by the third postoperative day.34 Although a mild increase in hepatic enzymes is not a specific indicator for liver damage, an increase in hepatic transaminase levels exceeding 3 times that of baseline may suggest acute hepatic damage. The mechanisms for alteration of postoperative hepatic function include direct operative trauma to the liver, the use of general anesthetics, and the reduction of portal venous flow during pneumoperitoneum. The operative trauma associated with mechanical retraction of the left lobe of the liver is the primary mechanism for elevation of liver enzymes after open GBP.35 Certain anesthetic agents, metabolized through the liver, can be hepatotoxic and result in elevation of postoperative hepatic enzymes. Lastly, an acute increase in the intraabdominal pressure during laparoscopy has been shown to decrease portal venous flow, as the normal portal pressure is often less than 10 mm Hg.6,30 Although acute elevation of hepatic transaminases were observed after laparoscopic GBP, it was only transient and subsided by the third postoperative day without long-term sequelae. Therefore, pneumoperitoneum in the morbidly obese is considered safe in patients with normal baseline liver function. Further studies are needed to evaluate the safety of pneumoperitoneum in patients with preexisting severe liver dysfunction.
Intraoperative Pulmonary Mechanics
The increased intraabdominal pressure at 15 mm Hg during laparoscopy can adversely affect intraoperative pulmonary mechanics. Pneumoperitoneum in nonobese subjects has been shown to decrease the respiratory compliance and increase airway pressure.36,37 The mechanism for these physiologic changes is the increased intraabdominal pressure with cephalad shift of the diaphragm. Table 4 summarizes the effects of pneumoperitoneum on intraoperative respiratory mechanics.
TABLE 4. Effects of Pneumoperitoneum on Intraoperative Respiratory Mechanics in the Morbidly Obese
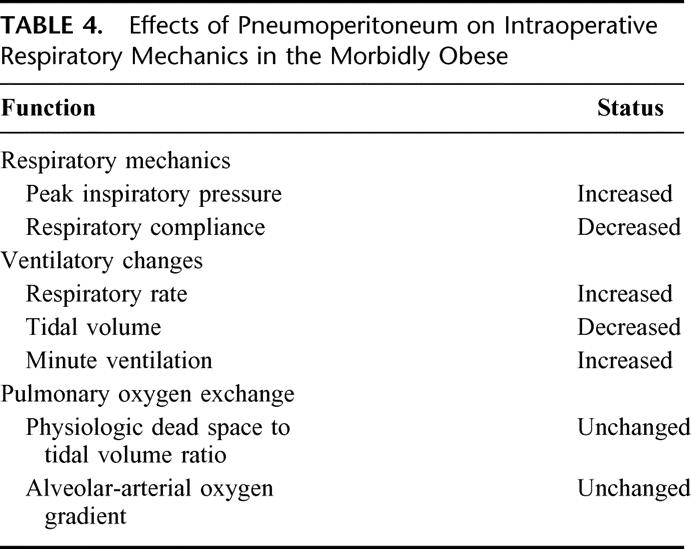
Respiratory compliance consists of both lung and chest wall compliance. In a randomized trial comparing pulmonary mechanics during cholecystectomy performed by an abdominal wall lift method or pneumoperitoneum, Lindgren et al36 reported higher respiratory compliance during the abdominal lift method than during pneumoperitoneum. Similar findings occurred in morbidly obese subjects. Dumont et al14 reported a reduction in pulmonary compliance by 31% in patients who underwent laparoscopic gastroplasty. In a study of laparoscopic and open GBP, respiratory compliance decreased by 42% during laparoscopic GBP compared with 29% during open GBP.13 Reduction in respiratory compliance during open GBP is related to the use of rigid mechanical retractors on the abdominal wall, whereas the reduction in respiratory compliance during laparoscopic GBP is related to the increased intraabdominal pressure.
Increased intraabdominal pressure during laparoscopy also increases the airway pressure. Without ventilatory changes, peak inspiratory pressure (PIP) can increase by 17% to 109% during laparoscopy.11,14,37 Galizia et al37 reported a significant increase in PIP in patients who underwent laparoscopic cholecystectomy but no change in PIP in patients who underwent cholecystectomy by the abdominal wall lifting method. In morbidly obese patients who underwent laparoscopic gastroplasty, Dumont et al14 reported an increase in airway pressure by 17%. Nguyen et al13 reported no significant change in PIP during open GBP, but PIP levels increased by 12% during laparoscopic GBP. To limit the rise in PIP, ventilatory adjustment is commonly performed by decreasing the tidal volume.
Although pneumoperitoneum alters respiratory compliance and airway pressure, the pulmonary gas exchange is unaffected during laparoscopic cholecystectomy.15 Similarly, no significant intraoperative changes in the physiologic dead space to tidal volume ratio or the alveolar-arterial oxygen gradient occurred in morbidly obese patients undergoing laparoscopic GBP.13 Sprung et al38 also reported no significant change in the alveolar-arterial oxygen gradient, and Demiroluk et al12 reported no significant difference in PaO2 levels in morbidly obese patient undergoing laparoscopy.
Renal Function
The increased intraabdominal pressure during laparoscopy has been shown to alter renal function (Table 5). A reduction in intraoperative urine output has been well documented during laparoscopic operations.39,40 In a trial comparing laparoscopic adrenalectomy with gasless laparoscopic adrenalectomy, Nishio et al39 demonstrated that urine output decreased significantly with abdominal insufflation and improved upon desufflation. In a swine model, McDougall et al40 demonstrated that the degree of intraoperative oliguria is dependent on the level of increased intraabdominal pressure; higher intraabdominal pressures resulted in a greater degree of oliguria. The mechanism for oliguria is related to the acute increased intraabdominal pressure. In a study of critically ill patients, Kron et al41 reported that an acute increase in the intraabdominal pressure to greater than 25 mm Hg resulted in acute renal insufficiency and abdominal decompression caused immediate improvement in renal function. Similar to nonobese subjects, intraoperative urine output in morbidly obese subjects decreased immediately after initiation of pneumoperitoneum during laparoscopic GBP and remained 31% to 64% lower than during open GBP.42
TABLE 5. Effects of Pneumoperitoneum on Intraoperative Urine Output and Postoperative Renal Function in the Morbidly Obese
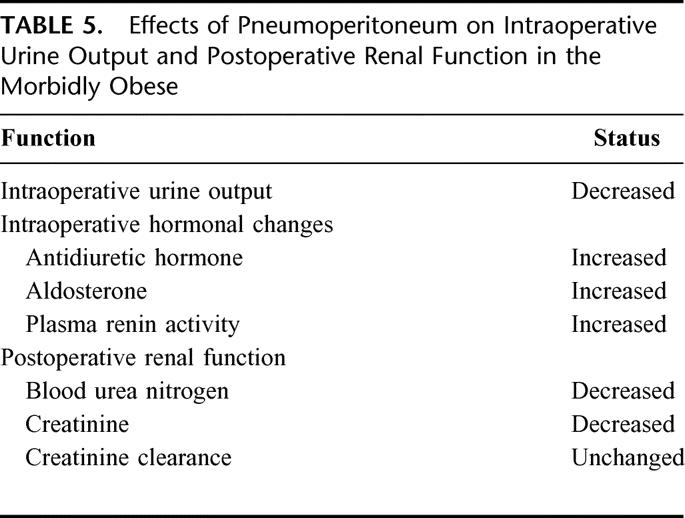
There are several mechanisms for diminished urine output during laparoscopy. Pneumoperitoneum has a direct pressure effect on the renal cortical blood flow. In a swine model, Chiu et al8 reported that superficial renal cortical perfusion decreased by 60% with abdominal insufflation and returned to baseline level after desufflation. Pneumoperitoneum also has a direct pressure effect on the renal vasculature, resulting in reduced renal blood flow. In a swine study, Are et al43 demonstrated that renal blood flow decreased by 36% below baseline as measured by radioactive microspheres. In addition, intraoperative releases of certain hormones such as antidiuretic hormone (ADH), plasma rennin activity, and serum aldosterone may diminish urine output. ADH facilitates water reabsorption in the distal tubules and concentrates the urine. Ortega et al44 reported a precipitous rise in ADH concentrations during laparoscopic cholecystectomy, which was not observed during open cholecystectomy. In a study of morbidly obese subjects, Nguyen et al42 reported that levels of ADH, plasma rennin activity, and aldosterone significantly increased during laparoscopic GBP.
Despite the intraoperative oliguria, pneumoperitoneum at 15 mm Hg is considered clinically safe. Nishio et al39 reported no changes in serum creatinine after laparoscopic adrenalectomy when compared with gasless laparoscopic adrenalectomy. In a study of morbidly obese subjects, no significant changes in blood urea nitrogen or serum creatinine levels were observed in the perioperative period after laparoscopic GBP.42 Additionally, the creatinine clearance measured in patients who underwent laparoscopic GBP was in the normal range both on the first and second postoperative day.17
Venous Stasis
The true incidence of deep venous thrombosis after laparoscopic compared with open operation is unknown; however, some of the factors relating to Virchow's triad (endothelial injury, hypercoagulability, and venous stasis) are altered during laparoscopy. The main factor that is adversely affected during laparoscopy is venous stasis. The increased intraabdominal pressure and reverse Trendelenburg position during laparoscopy have been shown to reduce femoral venous flow.45,46 The increased intraabdominal pressure during laparoscopy has a direct compressive effect on the inferior vena cava and iliac veins and decreases lower extremity venous flow. By the force of gravity during reverse Trendelenburg position, the abdominal viscera can also produce a compressive effect on the iliac veins resulting in a decrease in femoral venous flow. In a study of laparoscopic cholecystectomy, Millard et al45 reported that a combination of pneumoperitoneum and 30° reverse Trendelenburg position decreased peak systolic velocity of the common femoral vein by 42%. Similarly, Ido et al46 reported that abdominal insufflation reduced femoral vein velocity and the addition of reverse Trendelenburg has an additive effect by further reducing femoral vein velocity.
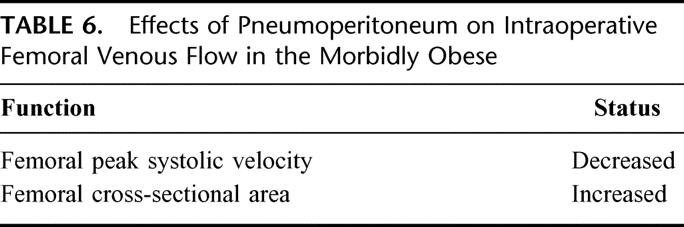
Development of venous stasis during laparoscopy in morbidly obese subjects has been observed (Table 6). Nguyen et al47 reported that increased intraabdominal pressure and reverse Trendelenburg positioning are independent factors that resulted in decreased femoral peak systolic velocity. Increased intraabdominal pressure during laparoscopic GBP reduced peak femoral systolic velocity by 43% and increased the femoral cross-sectional area by 52%.47 By combining pneumoperitoneum with reverse Trendelenburg position, peak femoral systolic velocity was further reduced to 57% of baseline values.47
TABLE 6. Effects of Pneumoperitoneum on Intraoperative Femoral Venous Flow in the Morbidly Obese
The use of sequential compression devices (SCD) during laparoscopy has been shown to reverse the adverse effects of pneumoperitoneum on femoral venous flow in nonobese subjects.45 The SCD provide a sequential pressure gradient on the lower extremities, which accelerates venous flow and facilitates venous emptying. Millard et al45 and Schwenk et al48 reported that the use of SCD during laparoscopic cholecystectomy was effective in reversing the reduced femoral systolic velocity to baseline values. In contrast, the use of SCD in morbidly obese subjects was only partially effective in augmenting the femoral peak systolic velocity. In a study of laparoscopic GBP, a combination of abdominal insufflation and reverse Trendelenburg positioning significantly decreased femoral peak systolic velocity; however, the reduced femoral systolic velocity level was only partially reversed with the use of SCD and was lower than baseline values by 38%.47 The ineffectiveness of SCD in returning femoral peak systolic velocity to baseline value in morbidly obese patients is attributed to the larger calves and thighs of these individuals.47 Patients with large calves and thighs may need higher compression pressure in the SCD to augment venous flow. Since the use of SCD was only partially effective in reversing the reduction of femoral venous flow, the use of antithrombotics in combination with SCD may be necessary for deep venous thrombosis prophylaxis in morbidly obese patients.
PATIENT SELECTION AND INTRAOPERATIVE MANGEMENT
Surgeons performing laparoscopic surgery on the morbidly obese should select appropriate patients based on the above physiologic data. We do not advocate the use of laparoscopic surgery in morbidly obese patients with severe cardiac, pulmonary, hepatic, or renal dysfunction. For example, patients with severe right-sided heart failure, congestive heart failure, moderate/severe coronary artery disease, obesity hypoventilation syndrome, severe liver cirrhosis, or acute renal failure should not undergo laparoscopic bariatric surgery as these patients are at high risk for intraoperative and postoperative complications relating to the use of CO2 pneumoperitoneum.
Appropriate intraoperative management should be performed to minimize the adverse consequences of laparoscopic surgery in the morbidly obese. The use of sequential compression devices is important to counteract the reduction in femoral systolic velocity during pneumoperitoneum. In addition, surgeons should limit the amount of time the patient is positioned in reverse Trendelenburg as it is an independent factor for development of venous stasis. Basic intraoperative monitoring should include ETCO2, urine output, and PaCO2 levels in selected cases. Morbidly obese patients with cardiac and pulmonary dysfunction undergoing laparoscopic surgery should have more extensive monitoring, including Swan-Ganz catheterization and radial arterial catheter with routine blood gas measurement. Cardiac depression is common and occurs immediately upon insufflation; however, cardiac output normally recovers by 1.5 hours after insufflation. If cardiac output or cardiac index continues to be depressed in patients with preexisting cardiac dysfunction, surgeons should consider interrupting pneumoperitoneum and/or consider conversion to laparotomy. Ventilatory adjustments should be performed by increasing minute ventilation to eliminate the increased CO2 load. In general, the respiratory rate is increased to limit the rise in PaCO2 and the tidal volume is reduced to limit the rise in peak inspiratory pressures. In certain instances, laparoscopy should be interrupted and pneumoperitoneum should be released in order for the anesthesiologist to control excessive hypercapnia. Similarly, oliguria is an expected intraoperative finding but not anuria. Assuming a euvolemic status, intraoperative management for anuria includes evaluation of the Foley catheter for patency and laparoscopic examination of the bladder. If the laparoscopic evaluation demonstrated a collapsed bladder and the Foley catheter is patent, then the diagnosis of anuria should be entertained. Desufflation of the abdomen should be performed until urine output resumes. In cases whereby urine output does not resume with desufflation, conversion to laparotomy should be performed. The role of dopamine to enhance intraoperative urine output is unclear. In a randomized trail evaluating intraoperative urine output of patients undergoing laparoscopic colectomy, Perez et al49 reported that patients receiving low doses of dopamine had higher urine output compared with patients who did not receive dopamine. Overall, the surgeon must be aware of the physiologic consequences of prolonged laparoscopy, limit the patient's exposure to CO2 pneumoperitoneum, and make the decision for conversion based on the patients’ preoperative physiologic status and response to CO2 absorption and increased intraabdominal pressure.
CONCLUSION
Altered physiology has been demonstrated during laparoscopy in the morbidly obese patients similar to that of nonobese patients. The two factors during laparoscopy that can result in adverse physiologic changes are absorption of CO2 and increased intraabdominal pressure. In the morbidly obese, absorption of CO2 during pneumoperitoneum can lead to an increase in PaCO2 levels; however, hypercarbia is commonly avoided with appropriate ventilatory changes. Absorption of CO2 also alters the acid-base balance and increase in CO2 excretion load. Compared with baseline values, the increased intraabdominal pressure during pneumoperitoneum can reduce femoral venous flow, intraoperative urine output, portal venous flow, respiratory compliance, and cardiac output. Appropriate intraoperative changes should be instituted to minimize these physiologic changes; the use of SCD is important to counteract the reduction in femoral venous flow; increase in intravascular volume is important to minimize intraoperative oliguria, and in certain instances, desufflation is necessary to avoid anuria; appropriate ventilatory changes during pneumoperitoneum include increasing the minute ventilation to minimize the elevated CO2 load and decreasing the tidal volume to minimize high peak inspiratory pressure. Surgeons should be constantly aware that shortening the operative time is an important factor in reducing the patient's exposure to CO2 pneumoperitoneum and its adverse consequences. Despite the adverse physiologic changes during pneumoperitoneum, laparoscopic surgery in the morbidly obese is considered safe in patients with normal renal, hepatic, and cardiorespiratory function. Bariatric surgeons should have a thorough understanding of the adverse physiologic effects of pneumoperitoneum and be able to recognize the adverse clinical manifestation and its management.
Footnotes
Reprints: Ninh T. Nguyen, MD, Department of Surgery, 101 The City Drive, Bldg 55, Rm 106, Orange, CA 92868. E-mail: ninhn@uci.edu.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Flegal KM, Carroll MD, et al. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. [DOI] [PubMed] [Google Scholar]
- 4.NIH Conference: Gastrointestinal surgery for severe obesity: consensus development conference panel. Ann Intern Med. 1991;115:956–961. [PubMed] [Google Scholar]
- 5.Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg. 1994;4:353–357. [DOI] [PubMed] [Google Scholar]
- 6.Jakimowics J, Stultiens G, Smulders F. Laparoscopic insufflation of the abdomen reduces portal venous flow. Surg Endosc. 1998;12:129–132. [DOI] [PubMed] [Google Scholar]
- 7.Beebe DS, McNevin MP, Crain JM, et al. Evidence of venous stasis after abdominal insufflation for laparoscopic cholecystectomy. Surg Gynecol Obstet. 1993;176:443–447. [PubMed] [Google Scholar]
- 8.Chiu AW, Chang LS, Birkett DH, et al. The impact of pneumoperitoneum, pneumoretroperitoneum, and gasless laparoscopy on the systemic and renal hemodynamics. J Am Coll Surg. 1995;181:397–406. [PubMed] [Google Scholar]
- 9.Hirvonen EA, Poikolainen EO, Paakkonen ME, et al. The adverse hemodynamic effects of anesthesia, head-up tilt, and carbon dioxide pneumoperitoneum during laparoscopic cholecystectomy. Surg Endosc. 2000;14:272–277. [DOI] [PubMed] [Google Scholar]
- 10.Lindgren L, Koivusalo AM, Kellokumpu I. Conventional pneumoperitoneum compared with abdominal wall lift for laparoscopic cholecystectomy. Br J Anaesth. 1995;75:567–572. [DOI] [PubMed] [Google Scholar]
- 11.Sharma KC, Brandstetter RD, Brensilver JM, et al. Cardiopulmonary physiology and pathophysiology as a consequence of laparoscopic surgery. Chest. 1996;110:810–815. [DOI] [PubMed] [Google Scholar]
- 12.Demiroluk S, Salihoglu Z, Zengin K, et al. The effects of pneumoperitoneum on respiratory mechanics during bariatric surgery. Obes Surg. 2002;12:376–379. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen NT, Anderson J, Fleming NW, et al. Effects of pneumoperitoneum on intraoperative respiratory mechanics and gas exchange during laparoscopic gastric bypass. Surg Endosc. (in press). [DOI] [PubMed]
- 14.Dumont L, Mattys M, Mardirosoff C, et al. Changes in pulmonary mechanics during laparoscopic gastroplasty in the morbidly obese patient. Acta Anaesthesiol Scand. 1997;41:408–413. [DOI] [PubMed] [Google Scholar]
- 15.Tan PL, Lee TL, Tweed WA. Carbon dioxide absorption and gas exchange during pelvic laparoscopy. Can J Anaesth. 1992;39:677–681. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez NC, Tenofsky PL, Dort JM, et al. What is normal intra-abdominal pressure? Am Surg. 2001;67:243–248. [PubMed] [Google Scholar]
- 17.Nguyen NT, Lee SL, Anderson JT, et al. Evaluation of intraabdominal pressure after open and laparoscopic gastric bypass. Obes Surg. 2001;11:40–45. [DOI] [PubMed] [Google Scholar]
- 18.Dexter SP, Vucevic M, Gibson J, et al. Hemodynamic consequences of high- and low-pressure capnoperitoneum during laparoscopic cholecystectomy. Surg Endosc. 1999;13:376–381. [DOI] [PubMed] [Google Scholar]
- 19.Meininger D, Byhahn C, Bueck M, et al. Effects of prolonged pneumoperitoneum on hemodynamics and acid-base balance during totally endoscopic robot-assisted radical prostatectomies. World J Surg. 2002;26:1423–1427. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen NT, Ho HS, Fleming NW, et al. Cardiac function during laparoscopic vs open gastric bypass: a randomized comparison. Surg Endosc. 2002;16:78–83. [DOI] [PubMed] [Google Scholar]
- 21.Fried M, Krska Z, Danzig V. Does the laparoscopic approach significantly affect cardiac functions in laparoscopic surgery? Pilot study in non-obese and morbidly obese patients. Obes Surg. 2001;11:293–296. [DOI] [PubMed] [Google Scholar]
- 22.Westerband A, Van De Water JM, Amzallag M, et al. Cardiovascular changes during laparoscopic cholecystectomy. Surg Gynecol Obstet. 1992;175:535–538. [PubMed] [Google Scholar]
- 23.McLaughlin JG, Scheeres DE, Dean RJ, et al. The adverse hemodynamic effects of laparoscopic cholecystectomy. Surg Endosc. 1995;9:121–124. [DOI] [PubMed] [Google Scholar]
- 24.D'Ugo D, Persiani R, Pennestri F, et al. Transesophageal echocardiographic assessment of hemodynamic function during laparoscopic cholecystectomy in healthy patients. Surg Endosc. 2000;14:120–122. [DOI] [PubMed] [Google Scholar]
- 25.Dorsay DA, Greene FL, Baysinger CL. Hemodynamic changes during laparoscopic cholecystectomy monitored with transesophageal echocardiography. Surg Endosc. 1995;9:128–133. [DOI] [PubMed] [Google Scholar]
- 26.Ninomiya K, Kitano S, Yoshida T, et al. Comparison of pneumoperitoneum and abdominal wall lifting as to hemodynamics and surgical stress response during laparoscopic cholecystectomy. Surg Endosc. 1998;12:124–128. [DOI] [PubMed] [Google Scholar]
- 27.Zuckerman R, Gold M, Jenkins P, et al. The effects of pneumoperitoneum and patient position on hemodynamics during laparoscopic cholecystectomy. Surg Endosc. 2001;15:561–565. [DOI] [PubMed] [Google Scholar]
- 28.Declan Fleming RY, Dougherty TB, Feig BW. The safety of helium for abdominal insufflation. Surg Endosc. 1997;11:230–234. [DOI] [PubMed] [Google Scholar]
- 29.Zuckerman RS, Heneghan S. The duration of hemodynamic depression during laparoscopic cholecystectomy. Surg Endosc. 2002;16:1233–1236. [DOI] [PubMed] [Google Scholar]
- 30.Jakimowics J, Stultiens G, Smulders F. Laparoscopic insufflation of the abdomen reduces portal venous flow. Surg Endosc. 1998;12:129–132. [DOI] [PubMed] [Google Scholar]
- 31.Halevy A, Gold-Deutch R, Negri M, et al. Are elevated liver enzymes and bilirubin levels significant after laparoscopic cholecystectomy in the absence of bile duct injury? Ann Surg. 1994;219:362–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gholam PM, Kotler DP, Flancbaum LJ. Liver pathology in morbidly obese patients undergoing Roux-en-Y gastric bypass surgery. Obes Surg. 2002;12:49–51. [DOI] [PubMed] [Google Scholar]
- 33.Spaulding L, Trainer T, Janiec D. Prevalence of non-alcoholic steatohepatitis in morbidly obese subjects undergoing gastric bypass. Obes Surg. 2003;13:347–349. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen NT, Braley S, Fleming NW, et al. Comparison of postoperative hepatic function after laparoscopic versus open gastric bypass. Am J Surg. 2003;186:40–44. [DOI] [PubMed] [Google Scholar]
- 35.Saranita J, Soto RG, Paoli D. Elevated liver enzymes as an operative complication of gastric bypass surgery. Obes Surg. 2003;13:314–316. [DOI] [PubMed] [Google Scholar]
- 36.Lindgren L. Koivusalo AM, Kellokumpu I. Conventional pneumoperitoneum compared with abdominal wall lift for laparoscopic cholecystectomy. Br J Anaesth. 1995;75:567–572. [DOI] [PubMed] [Google Scholar]
- 37.Galizia G, Prizio G, Lieto E, et al. Hemodynamic and pulmonary changes during open, carbon dioxide pneumoperitoneum and abdominal wall-lifting cholecystectomy. Surg Endosc. 2001;15:477–483. [DOI] [PubMed] [Google Scholar]
- 38.Sprung J, Whalley DG, Falcone T, et al. The effects of tidal volume and respiratory rate on oxygenation and respiratory mechanics during laparoscopy in morbidly obese patients. Anesth Analg. 2003;97:268–274. [DOI] [PubMed] [Google Scholar]
- 39.Nishio S, Takeda H, Yokoyama M. Changes in urinary output during laparoscopic adrenalectomy. Br J Urol Intl. 1999;83:944–947. [DOI] [PubMed] [Google Scholar]
- 40.McDougall EM, Monk TG, Wolf JS, et al. The effect of prolonged pneumoperitoneum on renal function in an animal model. J Am Coll Surg. 1996;182:317–328. [PubMed] [Google Scholar]
- 41.Kron IL, Harman PK, Nolan SP. The measurement of intra-abdominal pressure as a criterion for abdominal re-exploration. Ann Surg. 1984;199:28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen NT, Perez RV, Fleming N, et al. Effect of prolonged pneumoperitoneum on intraoperative urine output during laparoscopic gastric bypass. J Am Coll Surg. 2002;195:476–483. [DOI] [PubMed] [Google Scholar]
- 43.Are C, Kutka M, Talamini M, et al. Effect of laparoscopic antireflux surgery upon renal blood flow. Am J Surg. 2002;183:419–423. [DOI] [PubMed] [Google Scholar]
- 44.Ortega AE, Peters JH, Incarbone R, et al. A prospective randomized comparison of the metabolic and stress hormonal responses of laparoscopic and open cholecystectomy. J Am Coll Surg. 1996;183:249–256. [PubMed] [Google Scholar]
- 45.Millard JA, Hill BB, Cook PS, et al. Intermittent sequential pneumatic compression in prevention of venous stasis associated with pneumoperitoneum during laparoscopic cholecystectomy. Arch Surg. 1993;128:914–919. [DOI] [PubMed] [Google Scholar]
- 46.Ido K, Suzuki T, Kimura K, et al. Lower-extremity venous stasis during laparoscopic cholecystectomy as assessed using color Doppler ultrasound. Surg Endosc. 1995;9:310–313. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen NT, Cronan M, Braley S, et al. Duplex ultrasound assessment of femoral venous flow during laparoscopic and open gastric bypass. Surg Endosc. 2003;17:285–290. [DOI] [PubMed] [Google Scholar]
- 48.Schwenk W, Bohm B, Fugener A, et al. Intermittent pneumatic sequential compression (ISC) of the lower extremities prevents venous stasis during laparoscopic cholecystectomy: a prospective randomized study. Surg Endosc. 1998;12:7–11. [DOI] [PubMed] [Google Scholar]
- 49.Perez J, Taura P, Rueda J, et al. Role of dopamine in renal dysfunction during laparoscopic surgery. Surg Endosc. 2002;16:1297–1301. [DOI] [PubMed] [Google Scholar]


