Abstract
Objective:
The aim of this prospective multicenter study was to define a scoring system for the prediction of tumor recurrence after potentially curative surgery for gastric cancer.
Summary Background Data:
The estimation of the risk of recurrence in individual patient may be relevant in clinical practice, to apply adjuvant therapies after surgery, and plan an adequate follow-up program. Only a few studies, most of which were retrospective or performed on a limited number of patients, have developed a prognostic score in patients with gastric cancer.
Methods:
A total of 536 patients who underwent UICC R0 resection between 1988 and 1998 at 3 surgical departments in Italy were considered. All patients were followed up using a standard protocol after discharge from the hospital. The mean follow-up period was 56 ± 44 months, and 94 ± 29 months for surviving patients. The scoring system was calculated on the basis of a logistic regression model, where the presence of the recurrence was the dependent variable, and clinicopathologic variables were the covariates.
Results:
Recurrence occurred in 272 of 536 patients (50.7%). The scoring system for the prediction of the risk in individual cases gave values ranging from 1.4 to 99.9; the model distributed most cases in the extremes of the range. The risk of recurrence increased remarkably with score values; it was only 5% in patients with a score below 10, up to 95.4% in patients with a score of 91 to 100. No recurrence was observed in 43 patients with a score below 4, whereas all of the 56 patients with a score over 97 presented a recurrence. The model correctly predicted recurrence in 227 of 272 patients (sensitivity, 83.5%), whereas the absence of recurrence was correctly predicted in 214 of 264 patients (specificity, 81.1%); the overall accuracy was 82.2%. Prognostic score was clearly superior to UICC tumor stage in predicting recurrence. The high effectiveness of the score was confirmed in preliminary data of a validation study.
Conclusions:
The scoring system obtained with a regression model on the basis of our follow-up data is useful for defining subgroups of patients at a very low or very high risk of tumor recurrence after radical surgery for gastric cancer. Final results of the validation study are essential for a clinical application of the model.
In this prospective multicenter study including 536 patients, a scoring system for prediction of tumor recurrence after radical surgery for gastric cancer was built. A high correspondence between score values and risk of recurrence was found, and most patients were classified in subgroups with a very low or a very high risk of recurrence.
Gastric cancer, despite its declining incidence, is still one of the most common causes of death by cancer in Western countries. Surgery is the main method of treatment, and in about two thirds of the patients who have undergone surgery it can be defined as “curative,” ie, without microscopic or macroscopic residual tumor.1,2 However, in many cases recurrence can occur, through several patterns of dissemination (locoregional, hematogenous, peritoneal). At present, no effective therapy exists for recurrent gastric carcinoma; for this reason, the estimation of the risk of recurrence in individual patients may be relevant in clinical practice, to apply adjuvant therapies after surgery, and to plan an adequate follow-up program. A more correct definition of prognosis may be also useful in clinical research, to stratify subgroups with the same risk when analyzing the effectiveness of different treatments.
Numerous studies have investigated the role of patient-related, tumor-related, or treatment-related factors in the prognosis of patients curatively resected for gastric cancer. Depth of tumor invasion and nodal involvement are considered the most important prognostic factors; patients operated on for early gastric cancer have an excellent probability of long-term survival, whereas in patients with serosal involvement and lymph node metastasis prognosis is very poor.3–7 Other potential prognostic factors play a role in affecting prognosis above all in the intermediate stages of the disease.8,9 However, it is difficult to assess the overall impact of the different prognostic factors on individual patients; this is only possible by using a statistical model that takes into account all variables considered, by assigning a risk score to each patient.
The aim of the present prospective study was to build a statistical model for the definition of the risk of recurrence after potentially curative surgery for primary gastric cancer, using a set of variables commonly applied in clinical practice; a population of patients submitted to follow-up examinations according to a standard protocol was taken into consideration.
MATERIALS AND METHODS
Selection of Patients
For the calculation of the score, we selected 536 patients (study group) who underwent resection for primary gastric cancer between January 1988 and June 1998 at 3 surgical departments in Italy (Department of General Surgery and Surgical Oncology, University of Siena; First Division of General Surgery, University of Verona; and First Department of Surgery, “Morgagni” Hospital in Forlì), all part of the Italian Research Group for Gastric Cancer (IRGGC). Patient population consisted of 339 males and 197 females (mean age, 65 ± 11 years; range, 23–86 years).
The following criteria were adopted for including patients in the study group: 1) potentially curative surgery, defined as the absence of tumor residuals at intraoperative macroscopic examination and microscopic examination of resection margins (Union Internationale Contre le Cancer [UICC] R0 resection)1; cases presenting systemic metastases were excluded, even if removable by radical surgery; 2) patients who gave their informed consent and underwent complete periodical follow-up examinations, all according to the same protocol (minimum 5 years in surviving patients); and 3) absence of second primitive malignancies prior to surgery or during follow-up. Patients who died of postoperative complications or other causes during follow-up were also excluded from the study.
Patients operated on between July 1998 and December 1999 were considered for a preliminary validation of the score (validation group). In this period, at the 3 centers, 169 patients were operated for primary gastric cancer. We excluded from the study 30 patients who underwent noncurative surgery, 13 patients who died of causes other than tumor recurrence or developed second primitive malignancies, and 4 patients who were lost at follow-up; as such, 122 patients were selected.
Surgical Treatment
In all patients, a careful preoperative staging of the neoplasm was performed. This included upper digestive endoscopy and biopsy, chest x-ray, liver ultrasound, and abdominopelvic computed tomography scan. After laparotomy, a complete examination of the peritoneal cavity and liver was performed.
For tumors located in the middle and lower third of the stomach, a subtotal gastrectomy was generally preferred, provided that a distance of at least 5 cm between the proximal resection margin and the neoplasm was maintained; in the remaining cases, the entire stomach was removed. An intraoperative frozen section of the surgical resection line was examined histologically in cases of doubt. Gastrectomy was always completed by the removal of the greater omentum and perigastric lymph nodes; the type of lymphadenectomy was classified prospectively according to the criteria described by the Japanese Research Society for Gastric Cancer.10 In patients submitted to extended lymphadenectomy, a complete bursectomy was also performed. A median of 12 lymph nodes (range, 3–51 lymph nodes) were removed by limited lymphadenectomy (D1) and 39 lymph nodes (range 7–112) by extended lymphadenectomy (D2-D3). For reconstruction, the Roux-en-Y (after total gastrectomy) and Billroth II (after subtotal gastrectomy) techniques were preferred.
None of the patients included in this study received adjuvant chemo/radiotherapy.
Tumor Classification
The resected specimen was processed by the surgeon and the pathologist according to the criteria described by the Japanese Research Society for Gastric Cancer.10 Diameter of the tumor and location were classified accordingly; tumor location was considered “diffuse” if the entire organ was involved. Each lymph node station was removed and classified either during the operation or from the resected specimen immediately afterward; single lymph nodes were retrieved by the surgeon from the fresh specimen and then submitted to histopathologic examination.
Tumor invasion (T) and lymph node (N) classifications followed UICC criteria.1 Histologic type was classified as intestinal or diffuse-mixed, in accordance with Lauren's classification.
Follow-up
All patients, following discharge from the hospital, were subjected to regular outpatient follow-up examinations.11 These included a clinical checkup, hematological analysis, tumor marker assays (CEA, CA 19–9) (at each examination), abdominal ultrasonography and chest x-ray (every 6 months), digestive endoscopy (every year), computed tomography of the abdomen (in the case of suspected recurrence of disease or increase of tumor markers above pathologic levels), and bone scintigraphy (in the case of suspected bone metastasis). The diagnosis of the recurrence was established on the basis of imaging studies or intraoperative and biopsy findings in cases of reoperation. Computed tomography of the abdomen was always performed after diagnosis of recurrence to complete staging. The type of recurrence was classified as hematogenous, peritoneal, or locoregional, taking into consideration the first site of relapse.
The follow-up of the study group was closed in June 2003. Cases that were classified as disease-free at the last follow-up examination presented completely normal results for all imaging studies as well as normal tumor marker levels. The mean follow-up period was 56 ± 44 months (range, 2–158 months) for the entire patient population (including deceased patients) and 94 ± 29 months (range, 60–158 months) for cases classified as disease-free.
The follow-up of the validation group was closed in April 2004. The mean follow-up period was 39 ± 22 months (range, 3–68 months) for all patients and 58 ± 7 months (range, 42–68 months) for disease-free patients.
Statistical Analysis
Numerical variables were expressed as the mean ± SD of the mean, if not otherwise specified. Pearson's χ2 test and t test were used for the association between clinicopathologic variables and recurrence. Cumulative risk of recurrence was calculated using the Kaplan-Meier method (1 − survival), considering the presence of recurrence as the end point.
For the computation of the score in the study group, a logistic regression model was built; the presence of the recurrence was the dependent variable, whereas clinical and pathologic variables were considered as numerical or categorical covariates. Numerical covariates were age (per year) and tumor size (per cm). Categorical covariates, with the corresponding category and code, were: gender (female = 0, male = 1); tumor location (lower third = 0, middle third = 1, upper third = 2, diffuse = 3); Lauren histotype (intestinal = 0, diffuse-mixed = 1); depth of invasion (pT1 = 0, pT2 = 1, pT3–T4 = 2); nodal status (pN0 = 0, pN1 = 1, pN2 = 2, pN3 = 3); type of gastrectomy (partial = 0, total = 1); extent of lymphadenectomy (limited = 0; extended = 1). The code “0” was assigned to the reference category. In the statistical program, the contrast for the comparison of categories was defined as “simple”; as such, each category of the predictor variable (except the reference category) was compared with the reference category.
The parameters of the model were estimated using the maximum-likelihood method. Significant variables were included in the model by means of the forward stepwise selection: starting with a model containing only the constant, at each step the variable with the smallest significance value entered the model, with a default level of P < 0.05. Significance value of each factor was reassessed at each step; if a variable in a forward stepwise block exceeded a significance level of 0.1, it was removed form the model. Removal testing was based on the probability of the likelihood-ratio statistic.
The final model generated a set of independent prognostic variables, with their β regression coefficients, standard error (SE) of the coefficients and P values. The fit of the model was verified by the Hosmer and Lemeshow goodness-of-fit test.
The probability of the event (recurrence) was estimated by the formula:
where e is the base of natural logarithm and Z is the result deriving from the logistic regression equation:
c is the constant of the logistic regression model, and X1...p are the independent variables identified by the model, with their regression coefficients (B1...p).12,13
With this method, we were able to estimate the probability of recurrence for each patient; the formula was included in a database, and the risk of recurrence calculated automatically.
The Statistical Package for the Social Sciences software (version 8.0) (SPSS, Chicago, IL) was used for statistical analysis.
RESULTS
Tumor Recurrence
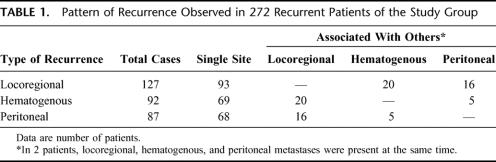
Recurrence of gastric cancer during the follow-up was detected in 272 (50.7%) of 536 patients included in the study group; 127 patients (23.7%) showed locoregional recurrence, 92 patients (17.2%) hematogenous metastases, and 87 patients (16.2%) peritoneal dissemination; in 42 of 536 patients (7.8%), recurrence occurred at multiple sites (Table 1). The cumulative risk of recurrence in the study group is represented in Figure 1. Mean time to recurrence in 272 recurrent patients was 19 ± 17 months (range, 2–96 months). In most cases (201 of 272, 73.9%), recurrence occurred within 2 years after surgery; in only 9 cases (3.3%), it was found after 5 years following surgery.
TABLE 1. Pattern of Recurrence Observed in 272 Recurrent Patients of the Study Group
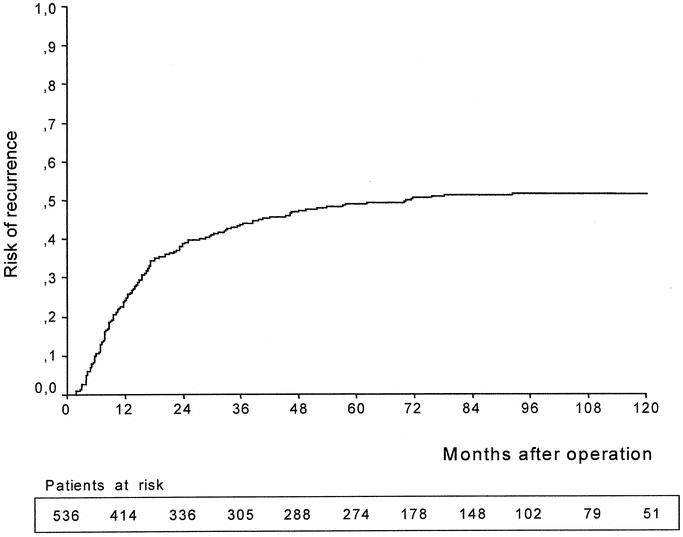
FIGURE 1. Cumulative risk of recurrence (Kaplan-Meier method) in 536 patients submitted to curative surgery for gastric cancer (study group). The estimated risk of recurrence (± standard error) was 49% ± 2% at 5 years and 52% ± 2% at 10 years after surgery. The number of patients at risk in each subperiod is indicated in the table.
Risk Factors for Recurrence
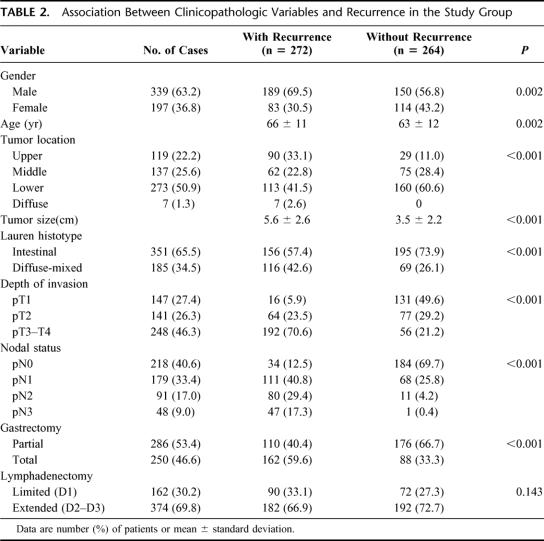
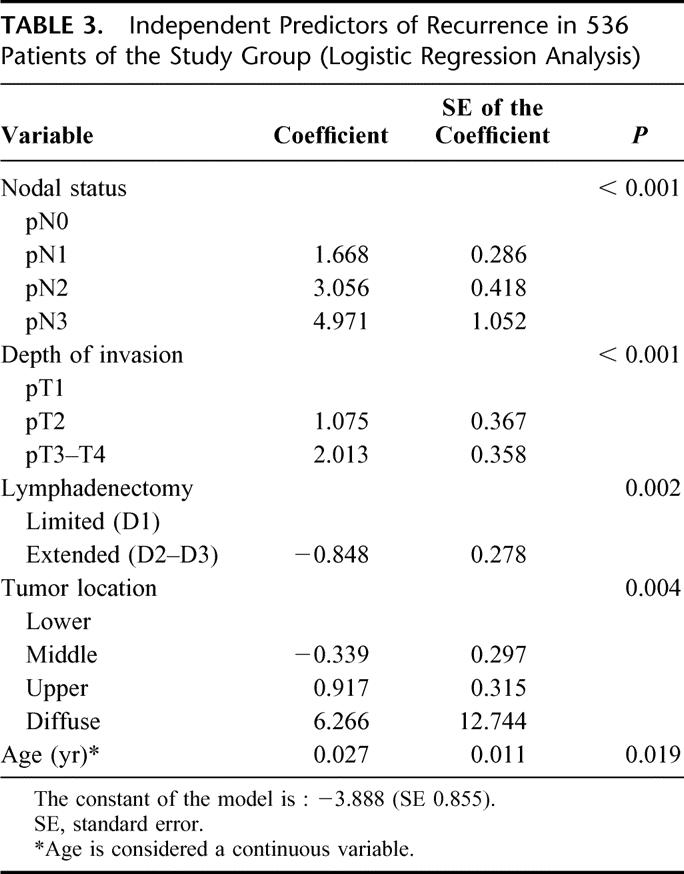
The association between clinicopathologic variables and recurrence in the study group is reported in Table 2. All of the variables analyzed, with the exception of lymph node dissection, were related to the risk of relapse, with various levels of statistical significance. Recurrences were particularly frequent in the upper third or a diffuse tumor location, neoplasms involving the serosa, and when there was a pN2 or pN3 nodal status; on the contrary, they were rare in early forms and in node-negative patients. Multivariate analysis of prognostic variables was performed by means of a logistic regression model, as previously described. The final model identified nodal status, depth of invasion, extent of lymphadenectomy, tumor location, and advanced age as independent predictors of recurrence; the corresponding β regression coefficients, SE of the coefficients, and P values are reported in Table 3. The Hosmer and Lemeshow goodness-of-fit test of the final model was P = 0.948, thus indicating that the model fit the data very adequately.
TABLE 2. Association Between Clinicopathologic Variables and Recurrence in the Study Group
TABLE 3. Independent Predictors of Recurrence in 536 Patients of the Study Group (Logistic Regression Analysis)
Computation of the Score
From the results of the logistic regression model, the coefficient Z was calculated as:
Z = −3.888 − 0.339(middle third) + 0.917(upper third) + 6.266(diffuse location) + 0.027(age) + 1.075(pT2) + 2.013(pT3-T4) + 1.668(pN1) + 3.056(pN2) + 4.971(pN3) − 0.848(D2–D3 dissection)
The value of parametric variables was 0 (negative) or 1 (positive), whereas age was considered as a continuous variable.
For each patient, the value of the coefficient Z obtained was included in the formula:
which gives risk values ranging from 0 to 100.
Score values were obtained for all patients included in the study group. The mean value of the score was 50.7 ± 35.3 (range, 1.4–99.9). The distribution of the score is reported in Figure 2; values were stratified into 10 subgroups. The model distributed most cases in the extremes of the range; 170 cases (31.7%) were classified below 20, and 171 (31.9%) over 81. Only a few patients were located in the middle of the range, ie, in the groups with an intermediate risk of recurrence.
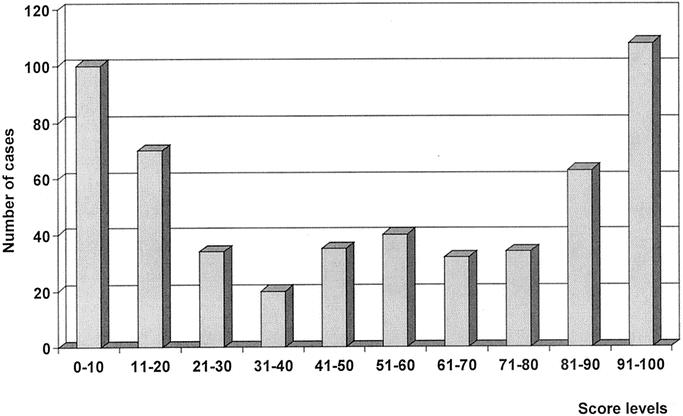
FIGURE 2. Distribution of score values in patients included in the study group. Most patients were located in the extremes of the range, whereas few patients were classified in intermediate risk groups.
Scoring System and Recurrence
An internal validation of the score was performed in the study group. The model correctly predicted recurrence in 227 of 272 patients (sensitivity, 83.5%), whereas the absence of recurrence was correctly predicted in 214 out of 264 patients (specificity, 81.1%); the overall accuracy was 82.2%.
Mean score values in patients with recurrence and disease-free patients were 75.4 ± 25.1 (range, 4.1–99.9) versus 25.4 ± 24.9 (range, 1.4–96.9), respectively (P < 0.001). The relationship between score values and follow-up time is reported in Figure 3. Most patients with high score values presented a recurrence with a short time interval. No recurrence was observed in 43 patients with a score below 4 (with a mean follow-up time of 95 ± 31 months; range, 61–158 months). On the contrary, all of the 56 patients with a score higher than 97 recurred, with a mean time interval of 12 ± 9 months (range, 2–37 months). The incidence of recurrent cases according to score strata is reported in Figure 4. The risk of recurrence increased remarkably with score values; it was only 5% in 100 patients with a score of 0 to 10 and 95.4% in 108 patients with a score of 91 to 100. Only 2 patients out of 59 (3.4%) with a score below 5 presented a recurrence. Figure 5 shows the predicted risk of recurrence according to score values in the study group.
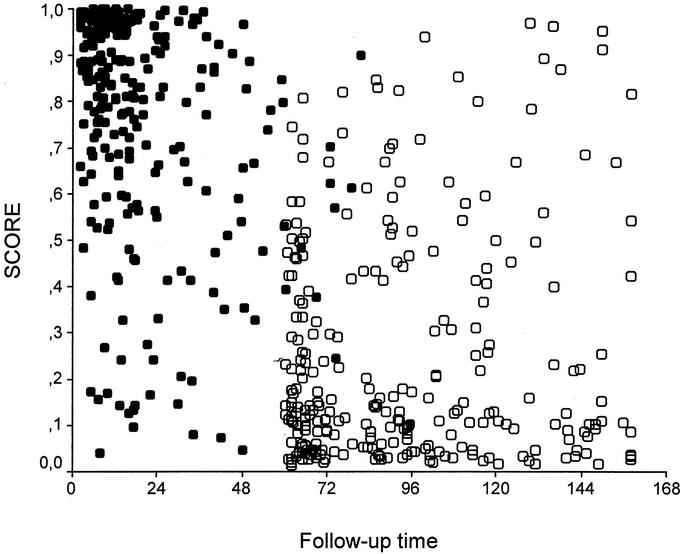
FIGURE 3. Relationship between score values and follow-up time (months) in 536 patients of the study group: ○, disease-free patients; •, patients with recurrence. Most patients with a high score level presented a recurrence within 2 years after surgery; on the contrary, patients with low score values were disease-free with a long follow-up time.
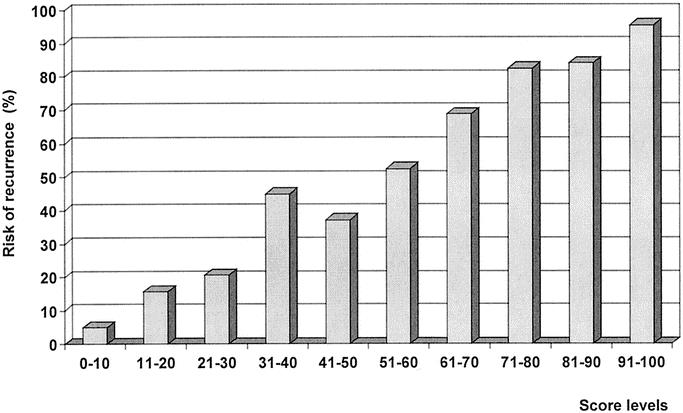
FIGURE 4. Incidence of recurrence in score subgroups. A high correlation between score levels and incidence of recurrence was found.
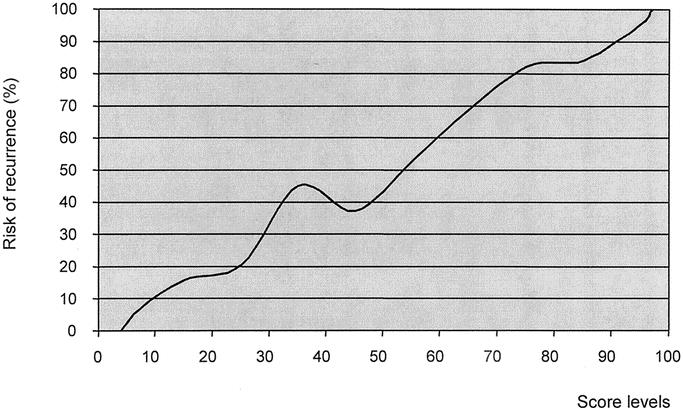
FIGURE 5. Linear definition of the risk of recurrence according to score level.
To evaluate the effectiveness of the scoring system in predicting recurrence compared with UICC staging system, a multivariate analysis was performed, including recurrence as a dependent variable and score and stage as covariates. Only score level proved to be statistically significant (coefficient: 5.70, SE: 0.43, P < 0.001), whereas tumor stage did not prove to be a significant predictive variable (P = 0.828).
Preliminary Validation Data
A preliminary evaluation of effectiveness of the score was conducted in 122 patients who underwent potentially curative surgery between July 1998 and December 1999 (validation group), a different patient population with respect to patients considered for the computation of the score. Of the 122 patients considered, 59 (48.4%) developed a tumor recurrence, with a mean time interval of 18 ± 11 months (range, 3–48 months). Locoregional recurrence was found in 24 patients (19.7%), peritoneal recurrence in 20 patients (16.4%), and hematogenous metastases in 23 patients (18.9%); recurrences were multiple in 8 patients (6.6%). The distribution of the score in the validation group and the concordance between predicted and observed risk of recurrence are shown in Figure 6. Similar to the study group, most patients were classified in the extremes of the range: 41 cases (33.6%) were classified below 20 and 36 (30%) over 81. Score level was 21.9 ± 22.6 (range, 1.8–85.9) in disease-free patients versus 78.3 ± 23 (range, 13.2–100) in patients with recurrence (P < 0.001). No recurrence was observed in patients with a score below 13, whereas all patients with a score over 86 recurred.
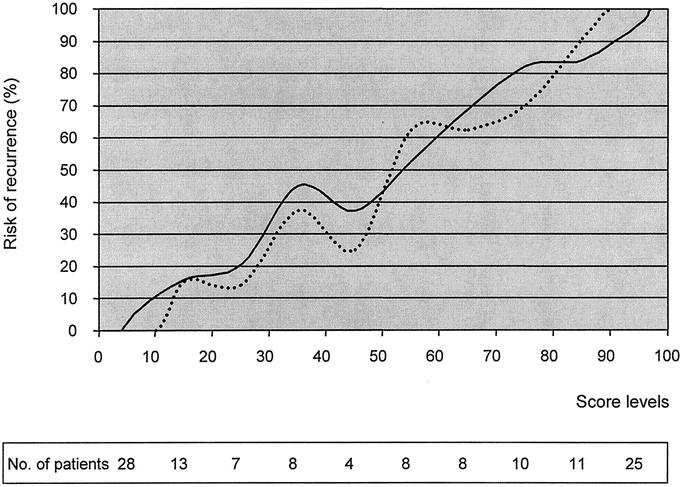
FIGURE 6. Predicted (solid line) and observed (broken line) risk of recurrence according to score level in 122 patients of the validation group. The distribution of patients in score subgroups is indicated in the table.
The model correctly predicted the presence of recurrence in 52 of 59 patients (sensitivity, 88.1%), whereas the absence of recurrence was predicted in 53 of 63 patients (specificity, 84.1%); overall accuracy was 86.1%.
To compare the prognostic value of the score with respect to UICC staging system in the validation group, a multivariate analysis was performed. Only score level proved to be a significant predictive variable (coefficient, 7.07; SE, 1.10; P < 0.001), whereas UICC tumor stage did not (P = 0.508).
DISCUSSION
The definition of the probability of recurrence in individual patients submitted to curative surgery for gastric cancer is a complex issue. Numerous studies focusing on the potential prognostic factors have been published in the literature; in most of them, pT and pN stage are considered variables with the strongest influence on the risk of recurrence.2,4–6 Many other variables, either used in a clinical routine or investigated by complex biologic-molecular analysis, were indicated as predictors of patient prognosis.3,8,9,14–17 The utility of the scoring system consists of the possibility to simultaneously consider a set of variables to assign a risk score to an individual patient. The use of statistical models to predict recurrence has been increasing in clinical oncology.13,18,19
The main characteristics of the present study conducted by the IRGGC were as follows: 1) a prospective evaluation of patients submitted to radical surgery and periodical follow-up examinations according to a standard protocol, with a long follow-up time for survivors; 2) the analysis of patient-, tumor- and treatment-related variables commonly used in clinical practice; 3) the end point of the study (clinically assessed recurrence of the tumor, rather than survival time obtained from patient records, family physicians or demographic services); and 4) the linear definition of the risk of recurrence applicable to individual patients, rather than the inclusion in a risk category.
All of our patients underwent a careful preoperative and intraoperative clinical staging of the disease; the classification of surgical treatment, lymph node dissection, and pathologic variables followed standard criteria. Recurrence of gastric cancer was identified in about half of our patients. Our data confirm that most recurrences occur within 2 years after surgery, and recurrence is a rare event after 5 years.5,20 Nodal status and depth of invasion were the most important predictors of poor prognosis in the study group; a very high risk coefficient was associated with pN3 status, and only 1 of the 48 patients with more than 15 lymph nodes involved remained disease-free. Advanced age and upper third location also increased the risk of recurrence. Location of tumor in the proximal third has been shown by other authors to be an independent negative prognostic factor.21–23 The influence of advanced age on outcome has not been well clarified2,24; several studies have suggested that the poorer prognosis of aged patients may be related to a higher propensity to develop hematogenous metastases.5,11,25
A reduction in the risk of recurrence was associated with extended lymph node dissection. The effectiveness of lymph node dissection in surgical treatment of gastric cancer patients is a debated issue, with conflicting results reported by prospective randomized and observational studies.2,26,27 Previous studies conducted by our group indicated a survival benefit after D2–D3 lymphadenectomy.11,28 In the present study, the Will Rogers phenomenon (stage migration) might have affected the results of multivariate analysis; however, to define patient prognosis, our model provides an estimation of the risk of recurrence according to the extent of lymphadenectomy, whether related to the quality of treatment or to a more correct staging of the disease.
The variables identified as influencing prognosis, and their pertinent coefficient risk, were included in a statistical model, to predict the risk of recurrence in individual patients.
In this model, most patients were distributed in the extremes of the range; as such, a large proportion of patients were classified as having a very low or very high risk of recurrence after potentially curative surgery. In the study group, a high correspondence between the score and prognosis was observed. Only 5 of 100 patients (5%) with a score below 10 and 11 of 70 patients (15.7%) with a score of 11 to 20 presented a recurrence. No recurrence was observed in 43 patients with a score below 4. On the contrary, a very high risk of recurrence was observed in patients with score values over 71. All 56 patients with a score over 97 presented a recurrence with a short time interval after surgery.
These findings were also confirmed in preliminary data of a validation study. A very high correspondence between predicted and observed risk of recurrence, and even in the distribution of the score was found in 122 patients of the validation group. Overall accuracy was even higher with respect to values observed in the internal validation performed on the study group. All patients with a score level over 86 recurred, whereas all patients with a score below 13 remained disease-free. Multivariate analysis confirmed the superiority of the score with respect to UICC staging system. However, we underline that these are only preliminary data performed in a small group of patients; furthermore, the follow-up period was lower with respect to the study group. An external validation of the score in a larger group of patients is essential for the clinical application of our model. For this reason, several other centers of the IRGGC entered the validation study in January 2001. Final results of this study will be probably available in 2007.
Similar validation studies might be performed in other institutions in the world. If the results of these studies will confirm the effectiveness of the score, this could be applied in a clinical setting. Postoperative therapies and follow-up programs may be individualized to single patients; patients with a score below 4 may be considered at no risk and excluded from follow-up programs. On the contrary, in patients with high score levels, surgical treatment should be considered “noncurative,” and patients should be submitted to postoperative therapies to slow the progression of the disease. At present, no adjuvant radio/chemotherapeutic protocols have demonstrated a clear benefit in gastric cancer, and this limits the clinical utility of the prognostic score.29 The utility may be clinically higher if new effective adjuvant therapies are developed.
Even in our model, some cases (95 patients with a score of 31–60, equal to 17.7% of the total population of the study group) were associated with an intermediate risk (37.1%–52.5%). In these patients, the prediction of outcome after surgery remains a problem; the prognostic value of new biologic markers may be higher in these cases. Our study may be useful in identifying subgroups of patients who may clinically benefit from biologic research on gastric cancer.
Another limitation of our study is the difficulty in the prediction of the exact site of tumor recurrence. We tried to develop different models for the prediction of each type of recurrence (peritoneal, locoregional, hematogenous), considering the type of recurrence as the dependent variable. The 3 models had a fairly good sensitivity for the single pattern of relapse but a very low specificity (data not shown). For example, patients with a high risk to develop locoregional recurrence had a similar high risk to develop hematogenous metastases. As such, in our opinion, it is difficult to predict the exact site of recurrence with the parameters at present available in clinical routine. Probably, the analysis of additional variables (peritoneal cytology, new molecular markers, and above all biologic characteristics of the tumor) might allow in the future a more correct prediction of tumor spread after radical surgery for gastric cancer.17,30
In several studies, mathematical models designed to predict the prognosis of gastric cancer patients have been developed.17,31–34 The first scoring system was described by Marubini et al.31 Interestingly, in their study, the significant variables identified by multivariate analysis were in accordance with our findings (pT stage, nodal status, age, tumor location); however, the extent of lymphadenectomy was not included in their analysis, and nodal status was defined as “N-negative” or “N-positive.” The definition of the relative risk associated with the level of nodal involvement according to the latest UICC TNM classification is essential for a better definition of prognosis after surgery for gastric cancer.35
Kologlu et al reported a prognostic score based upon a lot of different variables.33 However, that study included a limited number of subjects (128 patients), and an R0 resection was performed in only 35% of the cases; the prognosis of patients after noncurative surgery is invariably poor, independently of the influence of clinical and pathologic variables.2,4,6 Furthermore, in their study, patients were classified into 3 risk groups, and most of them (47%) were located in the group with an intermediate prognosis.
The score proposed by Inoue et al is based upon cDNA microarray analysis, and at present it is difficult to apply in a clinical setting.17 Similar difficulties can be found in the score proposed by Victorzon et al, who included in their analysis Sialyl Tn antigen and ploidy of tumor cells.32
A retrospective study conducted on a large number of patients at Memorial Sloan Kettering Cancer Center in New York was recently reported.34 The end point of that study was tumor-related death of patients, and it allowed a good estimation of 5-year and 9-year probability of survival after an R0 resection, even if no treatment-related variable was included. Our study is slightly different, as it provides a prediction of the risk of tumor recurrence after curative surgery and was performed according to a prospective design, with standard preoperative staging, surgical treatment, and follow-up.
The formula obtained from our model, described in the Materials and Methods and Results sections, can be easily included in every database program, and the prognostic value of the score verified, and in case used, in other institutions. The inclusion of the formula in the database program, with the automatic calculation of the score, could make its use simple and available worldwide. If confirmed by validation studies, it will be possible, upon discharge of the patient, to predict the probability of the recurrence, to plan appropriate adjuvant therapies and follow-up examinations. An adequate selection of patients may increase the effectiveness of adjuvant therapies, and an early diagnosis of relapse may offer a chance of a cure in recurrent patients.36,37 Finally, the definition of subgroups of patients with the same prognosis may play an essential role in clinical research on gastric cancer.
ACKNOWLEDGMENTS
The authors thank Giuseppe Verlato (Unit of Epidemiology and Medical Statistics, University of Verona) for statistical revision of the manuscript
Footnotes
Reprints: Franco Roviello MD, Via De Gasperi 5, 53100 Siena, Italy. E-mail: Roviello@unisi.it.
REFERENCES
- 1.Sobin LH, Wittekind C. TNM Classification of Malignant Tumours, 5th ed. New York: John Wiley & Sons, 1997. [Google Scholar]
- 2.Siewert JR, Bottcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison JD, Fielding JW. Prognostic factors for gastric cancer influencing clinical practice. World J Surg. 1995;19:496–500. [DOI] [PubMed] [Google Scholar]
- 4.Kim JP, Lee JH, Kim SJ, et al. Clinicopathologic characteristics and prognostic factors in 10,783 patients with gastric cancer. Gastric Cancer. 1998;1:125–133. [DOI] [PubMed] [Google Scholar]
- 5.Yoo CH, Noh SH, Shin DW, et al. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. [DOI] [PubMed] [Google Scholar]
- 6.Roukos DH, Lorenz M, Karakostas K, et al. Pathological serosa and node-based classification accurately predicts gastric cancer recurrence risk and outcome, and determines potential and limitation of a Japanese-style extensive surgery for Western patients: a prospective with quality control 10-year follow-up study. Br J Cancer. 2001;84:1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folli S, Morgagni P, Roviello F, et al. Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol. 2001;31:495–499. [DOI] [PubMed] [Google Scholar]
- 8.Roviello F, Marrelli D, Vindigni C, et al. p53 accumulation is a prognostic factor in intestinal-type gastric carcinoma but not in the diffuse type. Ann Surg Oncol. 1999;6:739–745. [DOI] [PubMed] [Google Scholar]
- 9.Kooby DA, Suriawinata A, Klimstra DS, et al. Biologic predictors of survival in node-negative gastric cancer. Ann Surg. 2003;237:828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma [2nd English ed]. Gastric Cancer. 1998;1:10–24. [DOI] [PubMed] [Google Scholar]
- 11.Marrelli D, Roviello F, de Manzoni G, et al. Italian Research Group for Gastric Cancer. Different patterns of recurrence in gastric cancer depending on Lauren's histological type: longitudinal study. World J Surg. 2002;26:1160–1165. [DOI] [PubMed] [Google Scholar]
- 12.Hosmer DW Jr, Wang CY, Lin IC, et al. A computer program for stepwise logistic regression using maximum likelihood estimation. Comput Programs Biomed. 1978;8:121–134. [DOI] [PubMed] [Google Scholar]
- 13.Vander Poorten VL, Balm AJ, Hilgers FJ, et al. The development of a prognostic score for patients with parotid carcinoma. Cancer. 1999;85:2057–2067. [PubMed] [Google Scholar]
- 14.Maehara Y, Oshiro T, Baba H, et al. Lymphatic invasion and potential for tumor growth and metastasis in patients with gastric cancer. Surgery. 1995;117:380–385. [DOI] [PubMed] [Google Scholar]
- 15.Kodera Y, Yamamura Y, Torii A, et al. The prognostic value of preoperative serum levels of CEA and CA 19–9 in patients with gastric cancer. Am J Gastroenterol. 1996;91:49–53. [PubMed] [Google Scholar]
- 16.Blok P, Craanen ME, Offerhaus GJ, et al. Gastric carcinoma: clinical, pathogenic, and molecular aspects. QJM. 1997;90:735–749. [DOI] [PubMed] [Google Scholar]
- 17.Inoue H, Matsuyama A, Mimori K, et al. Prognostic score of gastric cancer determined by cDNA microarray. Clin Cancer Res. 2002;8:3475–3479. [PubMed] [Google Scholar]
- 18.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20:791–796. [DOI] [PubMed] [Google Scholar]
- 19.Smaletz O, Scher HI, Small EJ, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20:3972–3982. [DOI] [PubMed] [Google Scholar]
- 20.Shiraishi N, Inomata M, Osawa N, et al. Early and late recurrence after gastrectomy for gastric carcinoma: univariate and multivariate analyses. Cancer. 2000;89:255–261. [DOI] [PubMed] [Google Scholar]
- 21.Ohno S, Tomisaki S, Oiwa H, et al. Clinicopathologic characteristics and outcome of adenocarcinoma of the human gastric cardia in comparison with carcinoma of other regions of the stomach. J Am Coll Surg. 1995;180:577–582. [PubMed] [Google Scholar]
- 22.Pacelli F, Papa V, Caprino P, et al. Proximal compared with distal gastric cancer: multivariate analysis of prognostic factors. Am Surg. 2001;67:697–703. [PubMed] [Google Scholar]
- 23.Talamonti MS, Kim SP, Yao KA, et al. Surgical outcomes of patients with gastric carcinoma: the importance of primary tumor location and microvessel invasion. Surgery. 2003;134:720–727. [DOI] [PubMed] [Google Scholar]
- 24.Maehara Y, Emi Y, Tomisaki S, et al. Age-related characteristics of gastric carcinoma in young and elderly patients. Cancer. 1996;77:1774–1780. [DOI] [PubMed] [Google Scholar]
- 25.Okuno K, Shigeoka H, Tanaka A, et al. Clinicopathological evaluation of T2-gastric cancer among age groups. Hepatogastroenterology. 2000;47:1180–1182. [PubMed] [Google Scholar]
- 26.De Manzoni G, Verlato G, Guglielmi A, et al. Prognostic significance of lymph node dissection in gastric cancer. Br J Surg. 1996;83:1604–1607. [DOI] [PubMed] [Google Scholar]
- 27.Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–914. [DOI] [PubMed] [Google Scholar]
- 28.Roviello F, Marrelli D, Morgagni P, et al. Survival benefit of extended D2 lymphadenectomy in gastric cancer with involvement of second level lymph nodes: a longitudinal multicenter study. Ann Surg Oncol. 2002;9:894–900. [DOI] [PubMed] [Google Scholar]
- 29.Cuschieri A. Does chemoradiotherapy after intended curative surgery increase survival of gastric cancer patients? Gut. 2002;50:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimori H, Yasoshima T, Denno R, et al. A novel experimental mouse model of peritoneal dissemination of human gastric cancer cells: different mechanisms in peritoneal dissemination and hematogenous metastasis. Jpn J Cancer Res. 2000;91:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marubini E, Bonfanti G, Bozzetti F, et al. A prognostic score for patients resected for gastric cancer. Eur J Cancer. 1993;29A:845–850. [DOI] [PubMed] [Google Scholar]
- 32.Victorzon M, Lundin J, Haglund C, et al. A risk score for predicting outcome in patients with gastric cancer, based on stage, sialyl-Tn immunoreactivity and ploidy: a multivariate analysis. Int J Cancer. 1996;67:190–193. [DOI] [PubMed] [Google Scholar]
- 33.Kologlu M, Kama NA, Reis E, et al. A prognostic score for gastric cancer. Am J Surg. 2000;179:521–526. [DOI] [PubMed] [Google Scholar]
- 34.Kattan MW, Karpeh MS, Mazumdar M, et al. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. 2003;21:3647–3650. [DOI] [PubMed] [Google Scholar]
- 35.Karpeh MS, Leon L, Klimstra D, et al. Lymph node staging in gastric cancer: is location more important than Number? An analysis of 1,038 patients. Ann Surg. 2000;232:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujimoto S, Takahashi M, Mutou T, et al. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer. 1997;79:884–891. [DOI] [PubMed] [Google Scholar]
- 37.Ambiru S, Miyazaki M, Ito H, et al. Benefits and limits of hepatic resection for gastric metastases. Am J Surg. 2001;181:279–283. [DOI] [PubMed] [Google Scholar]