Abstract
Summary Background Data:
To analyze the effects of a treatment program of intraoperative electron beam radiation therapy (IOERT) and external beam radiation therapy and chemotherapy on the outcome of patients with unresectable or locally advanced pancreatic cancer.
Methods:
From 1978 to 2001, 150 patients with unresectable and nonmetastatic pancreatic cancer received IOERT combined with external beam radiation therapy and 5-fluorouracil–based chemotherapy for definitive treatment.
Results:
The 1-, 2-, and 3-year actuarial survival rates of all 150 patients were 54%, 15%, and 7%, respectively. Median and mean survival rates were 13 and 17 months, respectively. Long-term survival has been observed in 8 patients. Five patients have survived beyond 5 years and 3 more between 3 and 4 years. There was a statistically significant correlation of survival to the diameter of treatment applicator (a surrogate for tumor size) used during IOERT. For 26 patients treated with a small-diameter applicator (5 cm or 6 cm), the 2- and 3-year actuarial survival rates were 27% and 17%, respectively. In contrast, none of the 11 patients treated with a 9-cm-diameter applicator survived beyond 18 months. Intermediate survival rates were seen for patients treated with a 7- or 8-cm-diameter applicator. Operative mortality was 0.6%, and postoperative and late complications were 20% and 15%, respectively.
Conclusions:
A treatment strategy employing IOERT has resulted in long-term survival in 8 of 150 patients with unresectable pancreatic cancer. Survival benefit was limited to patients with small tumors. Enrollment of selected patients with small tumors into innovative protocols employing this treatment approach is appropriate.
One hundred fifty patients with unresectable and nonmetastatic pancreatic cancer received intraoperative electron beam radiation therapy combined with external beam radiation therapy and 5-fluorouracil chemotherapy. The 1-, 2-, and 3-year actuarial survival rates of all 150 patients were 54, 15, and 7, respectively. Five patients have survived beyond 5 years and 3 more between 3 and 4 years.
Pancreatic cancer remains one of the most formidable challenges in oncology. In the year 2004, the American Cancer Society estimates 31,860 new cases of pancreatic cancer in the United States and 31,270 deaths from the disease.1 Thus, pancreatic cancer is expected to be the fourth leading cause of cancer-related death, with less than 5% survival 5 years after diagnosis.2
The disturbing mortality rate of pancreatic cancer is due to the high rate of metastases at the time of diagnosis, its fulminant clinical course, and the lack of effective systemic therapies. At present, surgery offers the only therapeutic means of cure. Unfortunately, only 5% to 25% of patients present with tumors amenable to resection. For patients undergoing resection for localized pancreatic carcinoma, median survivals of 11 to 23 months have been reported. At the other end of the clinical spectrum, a high percentage of patients (40% to 45%) present with metastatic disease, with a survival of only 3 to 6 months.2
Patients with locally advanced carcinoma of the pancreas comprise an intermediate group. Radiotherapeutic approaches have been frequently employed as these patients have unresectable tumors by virtue of local invasion of the portal, mesenteric, or celiac vessels in the absence of clinically detectable metastases. Because of the poor results with conventional radiation therapy and chemotherapy, specialized radiation therapy techniques that increase the radiation dose to the tumor have been used to improve local tumor control without increasing normal tissue morbidity. Intraoperative radiation therapy approaches have included permanent radioactive seed implantations (iodine-125) or electrons as a dose escalation technique in combination with external beam irradiation (EBRT) and chemotherapy.3–5
Since 1978, we have used intraoperative irradiation in patients with unresectable carcinoma of the pancreas. We elected electron beam versus radioactive seed implantation because larger tumors can be treated, the dose delivered is more uniform, the trauma to the peripancreatic tissue is less, the radiation therapy field is broader so that the high dose volume can include a 1- to 2-cm margin outside the gross tumor volume, and the possibility of seeding cancer from an implantation is eliminated.3–5 The intent of this paper is to present our experience with an aggressive combination of external beam and intraoperative irradiation in a group of 150 patients.
MATERIALS AND METHODS
From May 1978 to May 2001, 150 patients (70 females and 80 males) with a mean age of 62 years (range, 36 to 80 years) were treated with intraoperative electron beam irradiation (IOERT) at the Massachusetts General Hospital for unresectable carcinoma of the pancreas. This study was approved by IRB committee of the Massachusetts General Hospital. The primary tumor was located in the head of the pancreas in 104 patients and in the body and tail in the other 46 patients. The selection criteria for this form of therapy were (1) biopsy-proven pancreatic ductal adenocarcinoma; (2) localized unresectable tumor capable of inclusion in the high-dose intraoperative “boost” volume; and (3) no distant metastatic disease. Clinical evaluation of these patients included history and physical examination, chest radiograph, and conventional abdominal and pelvic CT scan. Forty-nine patients (33%) had undergone laparotomy with or without gastric/biliary diversion prior to referral. Over the 23 years of this study, an evolution in the staging of these patients has occurred with the abandonment of angiography and laparotomy to the adoption of helical CT scanning with contrast enhancement and thin-section imaging, laparoscopy, and, more recently, endoscopic ultrasound. All patients are currently undergoing helical CT scanning with contrast enhancement and thin section imaging, laparoscopy, and endoscopic ultrasound prior to initiation of radiation therapy.
The techniques of IOERT at the Massachusetts General Hospital have been previously described.6 In brief, the surgeon and radiation oncologist assess the extent of disease at operation and a cylindric applicator of appropriate size (5 to 9 cm in diameter) is selected to cover the tumor comfortably within the field, usually with a 1-cm margin around the pancreatic mass. Therefore, the applicator size is a surrogate for tumor diameter, which is approximately 2 cm less than the applicator size. The operating team then defines the dimensions and extent of tumor. This requires dividing the gastrocolic omentum, reflecting the stomach superiorly, and performing a Kocher maneuver, if indicated. The applicator limits the amount of normal tissue that is irradiated by retracting nearby radiation-sensitive normal tissues (stomach, jejunum, transverse colon, liver, and usually a large portion of the duodenum) outside the cylinder applicator and, thus, outside the region of high radiation dose that is confined to the inside of the applicator. The energy of the electron beam is selected on the basis of the thickness of the tumor. The energy has ranged from 9 to 29 MeV, corresponding to a depth for the 90% isodose line of 2.5 cm to 6 cm, respectively.
From 1978 to 1996, anesthetized patients were transferred with the incision temporarily closed from the third-floor operating room to the linear accelerator suite. Once in the suite, the abdomen was reopened under sterile conditions, and the applicator was repositioned in the abdomen and then attached to the head of the linear accelerator. Since June 1996, all patients have undergone surgery and IOERT in our dedicated operating room suite with a wall-mounted linear accelerator, thus eliminating the transport process. From 1978 to 1983, we were seeking to determine dose tolerance to IOERT. Accordingly, we initially used a dose of 15 Gy, which was increased to 17.5 Gy and then to 20 Gy when evidence of local recurrences developed at the lower dose levels and when the normal tissue tolerance was found to be acceptable. Since 1983, all patients have received 20 Gy. Following completion of IOERT, a gastrojejunostomy was usually performed (if not done previously) to alleviate or prevent gastric outlet obstruction. Biliary bypass was performed if indicated. Actual surgical procedures performed were IOERT only (27 patients), IOERT and gastrojejunostomy (80 patients), and IOERT and gastrojejunostomy and choledochoduodenostomy or choledochojejunostomy (43 patients).
In addition to IOERT, patients usually received EBRT to the primary tumor and regional lymph nodes using 2-field or 4-field techniques prior to exploration for IOERT. Patients received either “low-dose EBRT” of 10 to 20 Gy in 1 to 2 weeks or “high-dose EBRT” of 37 to 50 Gy in 4 to 6 weeks. Exploration for IOERT was performed 1 to 10 days after completion of radiation therapy for low-dose EBRT patients and 4 to 6 weeks for high-dose EBRT patients. After recovery from surgery and IOERT, low-dose EBRT patients received additional postoperative irradiation (30–40 Gy) with a 4-field technique in 3 to 5 weeks, for a total external beam dose of 50 Gy. Following IOERT, no further EBRT was given to high-dose EBRT patients. For patients receiving high-dose EBRT prior to IOERT or 30 to 40 Gy of postoperative irradiation following IOERT, 5-fluorouracil (5-FU) chemotherapy was usually administered as a bolus (500 mg/m2 per day) for 3 consecutive days during the first and last weeks of irradiation or as a protracted venous infusion (225 mg/m2 per 24 hours) throughout the course of radiation therapy. More recently, 7 patients have received concurrent gemcitabine and protracted venous infusion 5-FU during the course of high-dose EBRT.
Patients were seen 6 to 8 weeks after all treatment and then at 3- to 6-month intervals. Follow-up included physical examination and routine blood studies. Abdominal and pelvic CT scans were performed 3 to 6 months or as needed. The mean and median follow-up times were 12 and 17 months, respectively. Survival times were measured from the initiation of radiation therapy. No patient was lost to follow-up. Life table probabilities of survival were calculated using the actuarial method of Kaplan-Meier. Survival distributions were compared using the 1-sided long rank test. Prognostic variables were evaluated using the Cox proportional hazards model as well as the log-rank test, with survival time as the outcome variable.
RESULTS
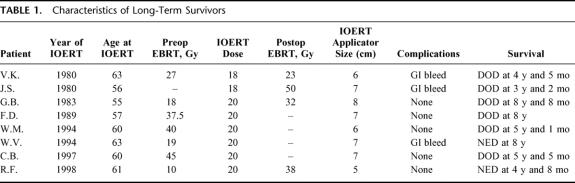
The 1-, 2-, and 3-year actuarial survival rates of all 150 patients were 54%, 15%, and 7%, respectively (Fig. 1). Median and mean survival rates were 13 and 17 months, respectively. Long-term survival has been observed in 8 patients. Following treatment, 3 patients have survived 3 to 4 years and 5 patients beyond 5 years (Table 1). The original biopsies of these 8 patients were rereviewed and the diagnosis of pancreatic ductal adenocarcinoma confirmed in all.
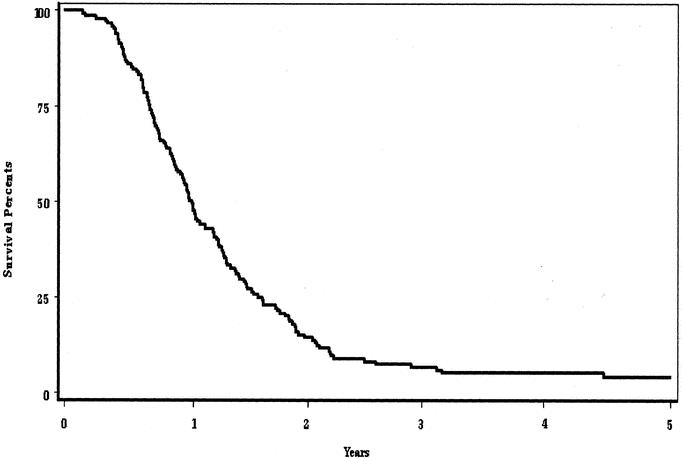
FIGURE 1. Actuarial survival rates of 150 patients with unresectable pancreatic cancer receiving intraoperative electron beam radiation therapy.
TABLE 1. Characteristics of Long-Term Survivors
There was a statistically significant correlation of survival to the diameter of treatment applicator used during IOERT (Fig. 2). For 25 patients treated with a small-diameter applicator (5 cm or 6 cm), the 2- and 3-year actuarial survival rates were 27% and 17%, respectively. In contrast, none of the 11 patients treated with a 9-cm diameter applicator survived beyond 18 months. Intermediate survival rates were seen for patients treated with a 7- or 8-cm-diameter applicator. No statistically significant differences in survival were seen by location of tumor within the pancreas (head versus body and tail) or low-dose EBRT versus high-dose EBRT prior to IOERT (data not shown).
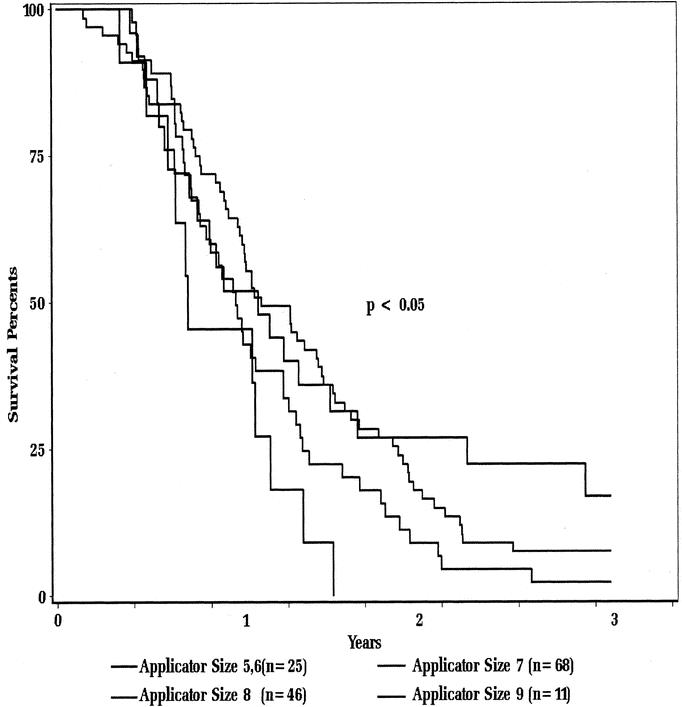
FIGURE 2. Actuarial survival as function of applicator size.
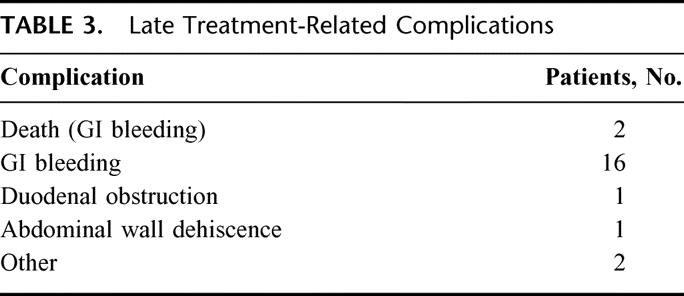
Postoperative complications are summarized in Table 2. Mortality was 0.6%, accounted for by 1 patient with cirrhosis that died postoperatively of liver failure. Early postoperative complications occurred in 20% of patients (Table 2) and late treatment-related complications in 15% of patients (Table 3). Upper gastrointestinal bleeding secondary to duodenal ulcer or erosion requiring medical intervention (medication, transfusion) occurred in 16 patients, and 2 patients died of treatment-related complications of upper GI bleeding at 37 months and 53 months, respectively.
TABLE 2. Postoperative Complications

TABLE 3. Late Treatment-Related Complications
DISCUSSION
Over the past 25 years, a number of studies have evaluated IOERT in the treatment of patients with locally advanced and unresectable pancreatic cancer. European and Japanese investigators have frequently used a large single dose of electron beam IOERT (20–40 Gy) without EBRT.7 Because of the potential toxicity of such high-dose irradiation, other investigators have employed IOERT at lower doses (10–20 Gy) combined with a treatment program of EBRT and chemotherapy.
Initial investigations during the 1970s and 1980s examined the feasibility, toxicity, and value of this treatment approach.8–10 Investigators from the Mayo Clinic used 20 Gy of IOERT first followed by high-dose postoperative EBRT. Data from their initial studies revealed a highly significant advantage in local control with IOERT and EBRT, in comparison with external irradiation alone (40–60 Gy). The actuarial local control at 1 year for those who received IORT was 82% compared with 48% for those who did not and at 2 years it was 66% and 20%, respectively. The significant advantage in local control did not translate into a survival advantage in the IOERT group, because of the high incidence of abdominal failure (>50%) in both groups. Median survival from the day of exploration was 12.6 months in the external irradiation alone group and 13.4 months in the IOERT group, and the 2-year overall survival was 16.5% and 12%, respectively.
Investigators at the NCI (Bethesda, MD) reported results of IOERT in the treatment of patients with unresectable pancreatic cancer.11 Thirty-two patients with unresectable stage III (locally advanced, positive nodes) or IV (visceral or peritoneal metastases) pancreatic cancer underwent biliary and gastric bypasses and were randomized to receive either IOERT of 25 Gy and postoperative EBRT of 50 Gy to the upper abdomen or postoperative EBRT of 60 Gy without IOERT. Both groups were treated with postoperative 5-FU. Median survival times for patients with stage III and IV disease were not different between the IOERT and EBRT groups (8 months); however, for those with stage III disease, median survival time and time to disease progression were superior in the IOERT group. Complications in patients treated at the NCI included late duodenal hemorrhage in 3 of 16 patients. They had 1 early death from respiratory failure in their IOERT group.
In 1985, the Radiation Therapy Oncology Group initiated a study of IOERT plus EBRT for patients with locally unresectable, nonmetastatic pancreatic cancer.12 Patients were treated with a combination of 20 Gy of IOERT and postoperative EBRT to 50.4 Gy in combination with 5-FU. Eighty-six patients were entered on the study through June 1, 1988, and analyzed through April 1990. Fifty-one patients were fully analyzable. Median survival time of the 51 patients was 9 months, with an 18 month actuarial survival of 9%. Local control could not be adequately evaluated in this multi-institutional study. Major postoperative complications were not excessive and occurred in 12% of patients. Two patients had major late morbidity leading to death, one from duodenal bleeding and the second from biliary obstruction. This study demonstrated the feasibility of IOERT in a multi-institutional setting.
To improve these results, treatment approaches have evolved on different fronts, including changes in treatment schedule of external beam radiation therapy, the use of hypoxic cell sensitizers during IOERT, and contemporary uses of chemotherapy concurrently and after radiation therapy.
In an effort to improve patient selection for IOERT, Mayo Clinic investigators have treated patients with full-dose EBRT before IOERT.10 This sequence allows restaging at 2 to 2.5 months after initiation of treatment. Of the initial 51 patients enrolled in this treatment schedule, 14 (27%) did not receive IOERT because of progression of disease. The actual incidence of local control at 1 and 2 years was 86% and 68%, respectively. The median survival of 14.9 months in their current series compares favorably with survivals in other IOERT and external-beam series. When compared with 56 patients treated during the same period at the Mayo Clinic with a different treatment sequence (IOERT followed by high dose EBRT), the median and overall 2-year survival (calculated from the date of diagnosis) was statistically higher (median, 14.9 months compared with 10.5 months; 2-year survival, 27% compared with 6%).
Survival improvements seen in the high-dose preoperative group of IOERT patients probably reflect altered and improved patient selection, rather than treatment effect. These difference suggest that giving a full component of EBRT with 5-FU before exploration and IOERT may be more appropriate than giving IOERT as an initial component of treatment. The altered sequence did result in 27% of patients not receiving IOERT as additional treatment, because of already documented disease progression.
It is well known that hypoxic cells are likely a major limitation to tumor radiocurability when using high single doses per fraction as cells in the hypoxic fraction dominate the survivors following a large dose. The use of intraoperative radiation therapy for patients with unresectable pancreatic cancer would seem an ideal model to test the efficacy of hypoxic cell sensitizers. Our group evaluated the hypoxic cell sensitizer misonidazole in conjunction with 15 to 20 Gy of IOERT in 41 patients with unresectable pancreatic cancer.13 The local control and survival results did not demonstrate any advantage to the use of misonidazole, and this approach has not been pursued.
A more contemporary approach to the use of concurrent and maintenance chemotherapy was reported by investigators from Thomas Jefferson Medical Center. In this study, 49 patients with locally advanced pancreatic cancer received 10 to 20 Gy of IOERT followed by postoperative irradiation with concurrent and maintenance 5-FU and leucovorin chemotherapy.14 Median survival was 16 months, with a 2-year survival rate of 22%, figures significantly better than historic controls. Local failure was seen in 31%. Early postsurgical morbidity was observed in 7 of 49 patients (14%) and late treatment-related morbidity in 8 of 43 (19%) alive beyond 6 months.
The series of 150 patients reported here is the largest one in the literature. Even though it spans nearly 25 years, it is relevant because it shows for the first time that long-term survival is possible for patients with unresectable pancreatic cancer. Even though the 3- and 5-year survival rates (7%, 4%) are modest, they are not markedly different than the results reported in contemporary trials of resected pancreatic cancer patients (20%, 10%) or patients undergoing palliative pancreaticoduodenectomy (6.3%, 1.6%), especially when taking into account those patients with smaller tumors as measured by the size of the IOERT treatment applicator.15,16 For 25 patients treated with a small-diameter applicator (5 cm or 6 cm), the 2- and 3-year actuarial survival rates were 27% and 17%, respectively. Furthermore, our study shows that postoperative and late treatment-related toxicity rates were acceptable. Because of the retrospective nature of this study, it was not possible to assess the palliative effects of this therapy or local control results. These study results support further study of selected patients with small unresectable tumors into innovative protocols employing IOERT.
Footnotes
Reprints: Christopher G. Willett, MD, Department of Radiation Oncology, Duke University Medical Center, Box 3085, Durham, NC. E-mail: willett@radonc.duke.edu.
REFERENCES
- 1.Jemal A, Tiwari RC, Thomas A, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. [DOI] [PubMed] [Google Scholar]
- 2.Evans DB, Abbruzzese JL, Willett CG. Cancer of the pancreas. In: Devita VT, Hellman S, Rosenberg SH, eds. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott-Reiner; 2000. [Google Scholar]
- 3.Shipley WU, Nardi GL, Cohen AM, et al. Iodine-125 implant and external beam irradiation in patients with colorectal pancreatic cancer. Cancer. 1980;45:709–714. [DOI] [PubMed] [Google Scholar]
- 4.Shipley WU, Wood WC, Tepper JE, et al. Intraoperative electron beam irradiation for patients with unresectable pancreatic carcinoma. Ann Surg. 1984;200:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood WC, Shipley WU, Gunderson LL, et al. Intraoperative irradiation for unresectable pancreatic carcinoma. Cancer. 1982;49:1272–1275. [DOI] [PubMed] [Google Scholar]
- 6.Willett CG, Warshaw AL. Intraoperatic electron beam irradiation in pancreatic cancer. Front Biosci. 1998;3:207–213. [DOI] [PubMed] [Google Scholar]
- 7.Abe M, Shibanoto Y, Ono K, et al. Intraoperative radiation therapy for carcinoma of the stomach and pancreas. Front Radiat Ther Oncol. 1991;29:258. [DOI] [PubMed] [Google Scholar]
- 8.Gunderson LL, Martin JK, Kvols LK, et al. Intraoperative and external beam irradiation ± 5-FU for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1987;13:319–329. [DOI] [PubMed] [Google Scholar]
- 9.Roldan GE, Gunderson LL, Nagorney D, et al. External beam versus intraoperative and external beam irradiation for locally advanced pancreatic cancer. Cancer. 1988;61:1110–1116. [DOI] [PubMed] [Google Scholar]
- 10.Garton GR, Gunderson LL, Nagorney DM, et al. High dose preoperative external beam and intraoperative irradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1993;27:1153–1157. [DOI] [PubMed] [Google Scholar]
- 11.Sindelar WF, Kinsella TJ. Randomized trial of intraoperative radiotherapy in unresectable carcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1986;12:148–149. [Google Scholar]
- 12.Tepper JE, Noyes D, Krall JM, et al. Intraoperative radiation therapy of pancreatic carcinoma: a report of RTOG-8505. Int J Radiat Oncol Biol Phys. 1991;21:1145–1149. [DOI] [PubMed] [Google Scholar]
- 13.Tepper JE, Shipley WU, Warshaw AL, et al. The role of misonidazole combined with intraoperative radiation therapy in the treatment of pancreatic carcinoma. J Clin Oncol. 1987;5:579–584. [DOI] [PubMed] [Google Scholar]
- 14.Mohiuddin M, Regine WF, Stevens J, et al. Combined intraoperative radiation and perioperative chemotherapy for unresectable cancers of the pancreas. J Clin Oncol. 1995;13:2764–2768. [DOI] [PubMed] [Google Scholar]
- 15.Klinkenbijl JH, Jeekel J, Tarek S, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Ann Surg. 1999;230:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lillemoe K, Cameron J, Yeo C, et al. Pancreaticoduodenectomy: does it have a role in the palliation of pancreatic cancer? Ann Surg. 1996;223:718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]