Abstract
Objective:
To assess whether the risk for nonsentinel node metastases may be predicted, thus sparing a subgroup of patients with breast carcinoma and a positive sentinel lymph node (SLN) biopsy completion axillary lymph node dissection (ALND).
Summary Background Data:
The SLN is the only involved axillary lymph node in the majority of the patients undergoing ALND for a positive SLN biopsy. A model to predict the status of nonsentinel axillary lymph nodes could help tailor surgical therapy to those patients most likely to benefit from completion ALND.
Methods:
All the axillary sentinel and nonsentinel lymph nodes of 1228 patients were reviewed histologically and reclassified according to the current TNM classification of malignant tumors as bearing isolated tumor cells only, micrometastases, or (macro)metastases. The prevalence of metastases in nonsentinel lymph nodes was correlated to the type of SLN involvement and the size of the metastasis, the number of affected SLNs, and the prospectively collected clinicopathologic variables of the primary tumors.
Results:
In multivariate analysis, further axillary involvement was significantly associated with the type and size of SLN metastases, the number of affected SLNs, and the occurrence of peritumoral vascular invasion in the primary tumor. A predictive model based on the characteristics most strongly associated with nonsentinel node metastases was able to identify subgroups of patients at significantly different risk for further axillary involvement.
Conclusions:
Patients with the most favorable combination of predictive factors still have no less than 13% risk for nonsentinel lymph node metastases and should be offered completion ALND outside of clinical trials of SLN biopsy without back-up axillary clearing.
In patients with breast carcinoma and a positive sentinel lymph node biopsy, further axillary involvement is correlated with the size of the metastasis in the sentinel lymph node, the number of involved sentinel nodes, and the occurrence of peritumoral vascular invasion in the breast primary.
The very high negative predictive value of axillary sentinel lymph node (SLN) biopsy in staging patients with clinically node-negative breast carcinoma allows almost 65% to 70% of patients to be spared axillary lymph node dissection (ALND) and its associated morbidity because of a metastasis-free SLN.1 Conversely, in case of a positive SLN biopsy, the standard of care remains completion ALND for a more exhaustive staging.2–8 Further axillary involvement, however, will not be identified in the majority of these patients, who will not derive any benefit from axillary dissection. Thus, a predicted small chance of additional axillary metastasis after a positive SLN biopsy might justify avoiding ALND also in a selected cohort of patients with positive SLN biopsy.9,11
Several features of the primary tumors and of the involved SLNs have been investigated for their possible value in predicting the risk for further axillary involvement.2–16 The conclusions of these studies, however, are weakened by the relatively small series of patients investigated, and a recent metaanalysis of the published results failed to identify a validated predictive model, as a result of the lack of standardization of the investigational procedures among the different studies and to their discordant conclusions.17
Despite these caveats, the size of SLN metastasis has emerged as a most powerful independent predictor.10,12,13,15,16 In particular, patients with micrometastatic SLN (ie, SLN-harboring metastases up to 2 mm in maximum diameter) reportedly are at a significantly lower risk for further axillary involvement than patients with SLN metastases larger than 2 mm (13–24% vs. 45–79%). We18 and others3 have further stratified the cohort of patients with micrometastatic SLN in 2 groups with significantly different chances of nonsentinel lymph node metastases according to the size of the micrometastasis (up to 1 mm or larger). The reported prevalence of further axillary involvement in patients with micrometastastic SLNs, however, is still too high to recommend these patients be spared completion ALND outside of clinical trials comparing ALND with clinical follow up.3,17,18
The new edition of the TNM classification of malignant tumors19 has now separately classified patients with isolated tumor cells (ITC) only in the regional lymph nodes within the pN0 (i+) category. ITC have been defined as single tumor cells or small clusters of cells, not more than 0.2 mm in greatest dimension, that do not typically show evidence of metastatic activity or penetration of vascular or lymphatic sinus walls.20 The new category is intended to prevent overstaging and hence overtreatment of the patients.
Although this policy may prove effective for patients treated with complete surgical dissection and histopathologic examination of the regional lymph nodes, it remains to be determined whether the new category is meaningful and can be safely adopted also for staging patients undergoing SLN biopsy. In particular, the question now arises whether patients with breast carcinoma and ITC only in the axillary SLN are at such a low risk for additional nonsentinel lymph node metastases that completion ALND may not be necessary.
In this study, we assessed the actual prevalence of ITC only in the axillary SLN of patients with breast carcinoma and its predictive implications on the status of the remaining axillary lymph nodes. For this purpose, we reclassified according to the new TNM classification the metastatic SLNs and all nonsentinel axillary lymph nodes of 1228 patients treated homogeneously in a single center using standardized investigational protocols. The strength of the size of SLN metastasis and of other clinicopathologic parameters in predicting the risk for further axillary metastases has been also evaluated by multivariate analysis.
METHODS
Patients
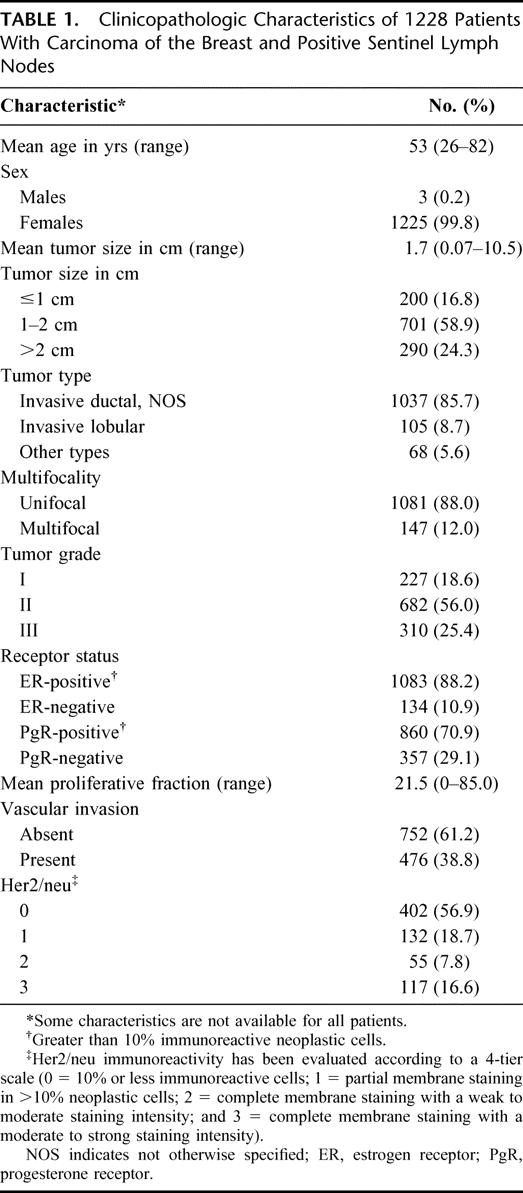
From August 1997 to February 2003, 4207 consecutive patients with clinically node-negative early breast carcinoma (3 cm or less in size) were treated with breast-conserving surgery (quadrantectomy or wide local excision) or mastectomy and SLN biopsy at the European Institute of Oncology in Milan according to the institutional protocols previously described.21–23 Of these patients, 1228 (29.2%) underwent complementary axillary clearance according to Berg's 3 levels24 because of a positive SLN biopsy and have been enclosed in the current study. The relevant clinicopathologic characteristics, including age and sex of these patients, size, type, grade, proliferative fraction (Ki-67 labeling index) estrogen- and progesterone-receptor status, and Her2/neu expression of the primary tumors, multifocality and occurrence of peritumoral vascular invasion were prospectively recorded in a dedicated database and are reported in Table 1.
TABLE 1. Clinicopathologic Characteristics of 1228 Patients With Carcinoma of the Breast and Positive Sentinel Lymph Nodes
Each patient provided an informed consent, and the ethics committee at the European Institute of Oncology approved the study.
Histopathologic Examination of the Sentinel Lymph Node and Estimation of the Size of Metastases
All the SLNs were serially and completely sectioned and examined intraoperatively on frozen sections (for 3932 patients) or on formalin-fixed and paraffin-embedded sections (for the remaining 275 patients) according to procedures developed at the European Institute of Oncology and previously detailed.25,26 Briefly, each lymph node was carefully isolated from the surrounding fatty tissue leaving intact the nodal capsule. The node was then bisected along its major axis and both moieties were processed. Nodes less than 5 mm in thickness were processed uncut. Fifteen pairs of adjacent 5-μm thick sections were cut at 50-μm intervals from both lymph node halves, amounting to 60 sections per node; whenever residual nodal tissue was left, additional pairs of sections were cut at 100-μm intervals until the node was completely sectioned. One section of each pair was routinely stained with hematoxylin and eosin (H&E), whereas the other section was stained for cytokeratins using the MNF116 monoclonal antibody (Dako, Glostrup, Denmark), as previously reported,25 whenever deemed necessary to assess the nature of atypical cells suspicious for malignancy seen in the corresponding H&E preparations.
The original histologic slides of all positive SLNs were reviewed and the actual size of the metastases was assessed as previously described.18 The largest axis of the metastatic nests in the plane of the tissue sections was measured histologically with an ocular micrometer, and the thickness was calculated according to the number of involved contiguous sections and to the sectioning interval between them. To avoid underestimation of the thickness of the metastases, the cutting intervals immediately preceding the first and following the last involved sections were also included. The recorded largest size corresponded to the maximum diameter in the plane of the section or to the thickness of the metastatic foci, whichever was larger. If multiple but distinct (ie, separated by uninvolved tissue sections) metastases were identified in the same SLN, the size of the largest was recorded.
According to the size of the SLN metastases, 4 categories were devised: ITC (according to the current TNM classification),19 small micrometastases (>0.2–1 mm), larger micrometastases (1–2 mm), and (macro)metastases (larger than 2 mm).
Examination of Nonsentinel Axillary Lymph Nodes
Nonsentinel axillary lymph nodes were accurately isolated from the fresh fibrofatty tissue, separated according to the 3 Berg's levels, bisected if greater than 5 mm, fixed in neutral-buffered formalin for 8 to 12 hours, embedded in paraffin, and routinely processed. Three to 6 H&E-stained sections, cut at 100- to 500-μm intervals, were examined histologically per node. The total number of isolated lymph nodes, the number of metastatic lymph nodes, and the TNM classification of the nodal metastases were recorded.
Statistical Analyses
The Fisher exact test was used to assess the association between categorical variables. Univariate and multivariate logistic regression models were used to assess the association between various clinicopathologic parameters, including age of patients, sex, tumor diameter and histology, grade and proliferative fraction, receptor status (dichotomized using 10% immunoreactive neoplastic cells as a cutoff), occurrence of peritumoral vascular invasion, size of SLN metastasis, number of positive SLN, and the presence of additional nonsentinel axillary lymph node metastases. Patients were subsequently stratified according to the clinicopathologic parameters remained most significant in multivariate analysis and the sensitivity (prediction of additional nonsentinel axillary lymph node metastases) was assessed for each group of subjects. Data were analyzed with the SAS software. All P values were based on 2-sided testing.
RESULTS
Prevalence and Size of Additional Metastases to Nonsentinel Nodes According to the Size of Sentinel Lymph Node Metastases
Overall, 1943 SLNs (mean, 1.58 SLN per patient; median, 1.0; range, 1–8) were obtained from the 1228 patients with a positive SLN biopsy and reexamined together with 26,771 nonsentinel axillary lymph nodes (mean, 21.8 lymph nodes per patient; median, 21.0; range, 3–57).
Of the 1228 patients, 116 (9.4%) had ITC only in the SLNs, whereas 318 (26%) and 794 (64.6%) had micrometastases and macrometastases, respectively, as shown in Table 2. Among the patients with micrometastatic SLNs, 212 and 106 patients had tumor deposits up to 1 mm or larger, respectively.
TABLE 2. Distribution of Metastases to the Sentinel and Nonsentinel Lymph Nodes According to Size
The prevalence of additional metastases to nonsentinel axillary lymph nodes in the whole cohort of patients was 39.4% (484 of 1228 patients), with a mean number of 4.2 (median, 2; range, 1–47) involved lymph nodes. The percentage of additional metastases, according to the size of the SLN metastasis, is reported in Table 2. Of the 116 patients with ITC only in the SLN, 17 (14.7%) had further axillary involvement, as did 68 (21.4%) of the 318 patients with SLN micrometastases (0.2–2 mm in size) (P = 0.15). Patients with macrometastatic disease in the SLNs, however, showed a significantly higher proportion of nonsentinel lymph node metastases (P < 0.0001), which were detected in 50.3% (399 of 794) of the cases (Table 2).
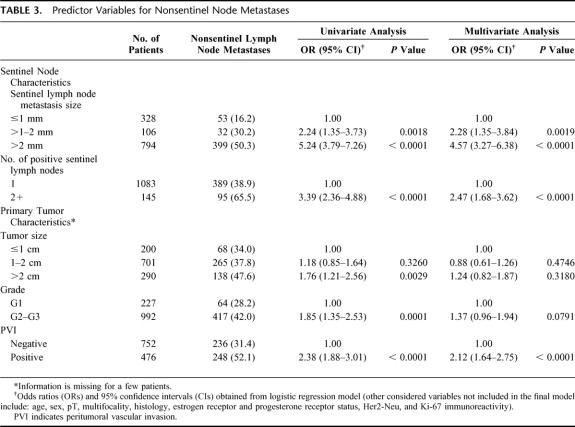
When the patients with micrometastasis to the SLN were further stratified according to the metastasis size (up to 1 mm vs. 1–2 mm), the prevalence of nonsentinel lymph node involvement was 17% (36 of 212) and 30.2% (32 of 106), respectively (P = 0.009). Therefore, patients with a positive SLN biopsy could be stratified in 3 groups at significantly different risk for metastases to nonsentinel axillary lymph nodes (Table 3). Patients with ITC only or SLN micrometastases up to 1 mm had the lowest risk of additional metastases, compared with those with micrometastases 1 to 2 mm in size (odds ratio [OR], 2.24; 95% confidence interval [CI], 1.35–3.73; P = 0.0019) and with those with SLN macrometastases (OR, 5.24; 95% CI, 3.79–7.26; P < 0.0001).
TABLE 3. Predictor Variables for Nonsentinel Node Metastases
In each subgroup of patients with SLN metastases, additional metastases to nonsentinel lymph nodes were mostly of the macrometastatic type, ie, larger than 2 mm. Indeed, as shown in Table 2, the prevalence of macrometastases in nonsentinel lymph nodes ranged from 58.3% (21 of 36 cases) among patients with SLN micrometastases 1 to 2 mm in size, to 90% (360 of 399 cases) among patients with macrometastases in the SLN.
Correlation of Nonsentinel Lymph Node Metastases With Other Clinicopathologic Parameters
In addition to the size of SLN metastases, we also considered all the available clinicopathologic features of the 1228 patients as possible predictors of further axillary involvement.
In univariate analysis, the likelihood of additional metastases was significantly higher for patients with primary tumors larger than 2 cm (P = 0.0029), grade 2 or 3 (P = 0.0001), or exhibiting peritumoral vascular invasion (P <0.0001), and for patients with 2 or more involved SLNs (P <0.0001) (Table 3).
Age of the patients and multifocality, histologic type, and biologic features (estrogen and progesterone receptor status, Her2/neu expression, and proliferative fraction) of the primary tumor did not correlate with the prevalence of nonsentinel lymph node metastases.
Independent Predictors of Nonsentinel Lymph Node Metastases in Multivariate Analysis and Formulation of a Predictive Model
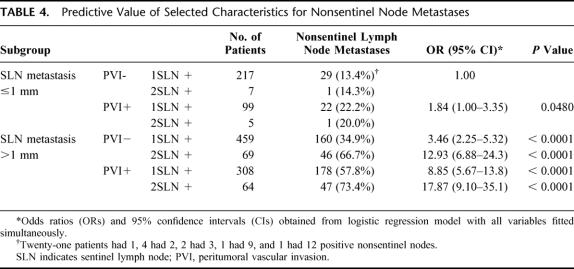
In multivariate analysis, the size of SLN metastases, the involvement of more than 1 SLN, and the occurrence of peritumoral vascular invasion in the primary breast carcinoma emerged as independent predictors of nonsentinel lymph node metastases (all P < 0.0001), whereas primary tumor size and grade did not retain any significant association (Table 3). The 3 significant predictors of nonsentinel node metastases were then included in a predictive model (Table 4), whereby patients with a unique SLN involved by the smallest metastasis (ITC or micrometastases up to 1 mm in size) in the absence of peritumoral vascular invasion in the breast primary have the lowest (13.4%) risk of nonsentinel lymph node metastases, whereas patients with 2 or more SLN harboring metastases larger than 1 mm in the presence of vascular invasion in the breast tumor have a remarkably higher (approximately 20-fold) risk of additional metastases. All other possible combinations of predictive factors correlate with intermediate risks between the 2 extremes.
TABLE 4. Predictive Value of Selected Characteristics for Nonsentinel Node Metastases
DISCUSSION
By reviewing the histologic preparations of both sentinel and nonsentinel axillary lymph nodes of 1228 patients subjected to ALND because of a positive SLN biopsy, we documented that almost 10% (116 of 1228) of these patients had ITC only in the SLN. The prevalence of additional axillary metastases in this cohort of patients was 14.7% (17 of 116 patients). This figure is not significantly different from the 21.4% (68 of 318 patients) obtained in the cohort of patients with SLN micrometastasis (0.2–2 mm), but it is significantly lower than that seen in patients with SLN metastases larger than 2 mm (399 of 794 or 50.3%; P < 0.0001).
This is the first study specifically addressing the risk for further axillary involvement in a large series of patients with ITC only in SLNs. Assuming, however, that in previous studies, most of the patients with SLN micrometastases that were detectable only with immunohistochemistry actually had ITC only, the reported prevalence of nonsentinel lymph node metastases in this cohort of patients ranges from 7.6%27 to 20%,6 perfectly in line with our findings. It should also be added that the risk for further axillary involvement in patients with ITC only in the SLN is likely underestimated, because in the current and previous investigations, nonsentinel lymph nodes were not examined with extensive serial sectioning and immunohistochemical assays as were the corresponding SLNs. More accurate scrutiny of the nonsentinel lymph nodes would have resulted in a 10% to 20% increase in the detection of metastasis.28–30
We did not find any significant difference in the risk for additional metastases in patients with either ITC or true micrometastases in the SLN. However, when the patients with SLN micrometastases were further stratified according to the size of the micrometastases (up to 1 mm vs. 1–2 mm), those with larger micrometastases showed a significantly higher prevalence of additional metastases (30.2 vs. 17.0; P = 0.01), thus confirming previous data.3,18 To summarize, patients with a positive SLN biopsy can be stratified in 3 groups at significantly different risk for involvement of nonsentinel lymph nodes. Patients with ITC or small (up to 1 mm) micrometastases in the SLN have the lowest risk of additional metastases (16.2%), which increases to 30.2% and 50.3% for patients with 1 to 2 mm micrometastases or larger metastases, respectively, as previously indicated in studies of smaller series of patients.10,12,13,15,16
Completion ALND in all the patients with positive axillary SLN biopsy adds clinically meaningful information because in most of these patients, including those with ITC only or micrometastases in the SLNs, nonsentinel node metastases are larger than 2 mm. In the majority of the patients in the current series, the identification of additional positive nonsentinel axillary lymph nodes affected further systemic therapy. Indeed, according to the recommendations of the St. Gallen Consensus,31 adjuvant chemotherapy in addition to endocrine interventions was considered for minimal or average-risk patients with endocrine-responsive tumors and ITC or micrometastases only in the SLN, who had additional (macro)metastases in nonsentinel lymph nodes. For patients with (macro)metastatic disease in the SLN, the extent of further axillary involvement together with the menopausal status and the evaluation of endocrine responsiveness of the primary tumor was taken into account in tailoring the most appropriate adjuvant intervention. Further local treatments, however, were not recommended based on the detection of positive nonsentinel axillary lymph nodes. Again, it should be reemphasized that the true percentage of macrometastases might be lower after a more accurate examination of all the nonsentinel nodes that would likely detect missed micrometastases. Macrometastatic disease in nonsentinel lymph nodes of patients with micrometastatic SLN was also reported in previous studies.3,4,13,18 These additional metastases, which have undisputed prognostic value and dictate upstaging of the patients with smaller SLN metastases, would go undetected if patients would be spared ALDN.
The reasons why nonsentinel axillary lymph nodes may harbor larger metastases than those encountered in the SLN of the same patients have not been elucidated thus far. The possibility of an incorrect identification of the SLN, as a result of the fact that the lymphatic flow could be deviated toward a nonsentinel axillary lymph node in case the true SLN is largely replaced by metastatic deposits, cannot be definitely ruled out, although it is very unlikely to hold true in the current series of cases. Indeed, none of the nonsentinel lymph node metastases detected in patients with ITC or micrometastasis in the SLN were large enough to replace the lymph node parenchyma extensively and affect the lymphatic inflow.
Because the actual size of SLN metastasis by itself could not be considered a predictive parameter powerful enough to identify a subgroup of patients for whom completion ALND might be safely avoided despite a positive SLN biopsy, we further analyzed additional clinicopathologic variables for their ability to predict the status of the nonsentinel lymph nodes in these patients. Multivariate analysis of our data demonstrated that, in addition to the size of SLN metastases, which remained the most powerful predictor, the involvement of more than 1 SLN (OR, 2.47; 95% CI, 1.68–3.62; P <0.0001) and the occurrence of peritumoral vascular invasion in the primary breast tumor (OR, 2.12; 95% CI, 1.64–2.75; P <0.0001) were independent predictors of further axillary involvement, as already put forward.2,13,16
In the current study, a primary tumor size larger than 2 cm correlated with an increased risk of nonsentinel node metastases in univariate analysis only and did not emerge as an independent predictive factor in multivariate analysis. This is in line with the experience of some authors,3,14,18 but at variance with other investigations showing a significant correlation of primary tumor size with nonsentinel lymph node metastases in multivariate analysis.2,10,12,13,15,16 The discordant conclusions may well be the result of the fact that we have evaluated a series of patients far larger than those (ranging from 60–389 patients) of the previous studies.
Finally, we included the 3 characteristics most strongly associated with nonsentinel lymph node metastases in the formulation of a predictive model that would be valuable to clinicians until results are available from the ongoing trials. Although the model is effective in discriminating subgroups of patients at significantly different risk for nonsentinel node metastases, even the most favorable combination of factors does not eliminate or even reduce to less than 10% the chance of further axillary involvement, contrary to the findings of Weiser et al,10 who reported absence of additional metastases in 24 patients with small breast cancers, no peritumoral vascular invasion, and SLN micrometastases.
CONCLUSIONS
Patients with primary breast carcinoma and positive SLN biopsy can be stratified in subgroups at significantly different risk for further axillary involvement according to the size of the SLN metastases, the number of positive SLNs, and the occurrence of peritumoral vascular invasion in the breast primary tumor. With regard to the predicted risk for nonsentinel node metastases, the cohort of patients with ITC only in the SLN is not different from that of patients with micrometastatic disease. Accordingly, the pN0 (i+) and pNmi (for micrometastases measuring 0.2–2 mm) categories recently introduced by the latest edition of the TNM classification cannot be safely adopted to tailor the surgical treatment of the axilla for patients undergoing SLN biopsy. A more useful discrimination of the patients at lower and higher risk for nonsentinel node metastases is attainable by stratifying them according to the size (up to 1 mm, including ITC, or larger) of SLN metastases.
Finally, because a reliable predictive model for identifying patients with a very low risk (eg, <10%) of additional nonsentinel lymph node metastases is still lacking, we concur with other researchers2–8 that, outside of clinical trials of SLN biopsy without backup axillary node clearance, completion ALND is still recommended in patients with any evidence of an involved SLN.
ACKNOWLEDGMENTS
The authors thank Dr. Stefania Andrighetto for her invaluable help with data managing.
Footnotes
Reprints: Giusuppe Viale, MD, FRCPath, Department of Pathology, European Institute of Oncology, Via Ripamonti 435, 20141 Milan, Italy. E-mail: giuseppe.viale@ieo.it.
REFERENCES
- 1.Schwartz GF, Giuliano AE, Veronesi U, et al. Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast, April 19–22, 2001, Philadelphia, Pennsylvania. Cancer. 2002;94:2542–2551. [DOI] [PubMed] [Google Scholar]
- 2.Wong SL, Edwards MJ, Chao C, et al. Predicting the status of the nonsentinel axillary nodes: a multicenter study. Arch Surg. 2001;136:563–568. [DOI] [PubMed] [Google Scholar]
- 3.Rahusen FD, Torrenga H, van Diest PJ, et al. Predictive factors for metastatic involvement of nonsentinel nodes in patients with breast cancer. Arch Surg. 2001;136:1059–1063. [DOI] [PubMed] [Google Scholar]
- 4.den Bakker MA, van Weeszenberg A, de Kanter AY, et al. Non-sentinel lymph node involvement in patients with breast cancer and sentinel node micrometastasis: too early to abandon axillary clearance. J Clin Pathol. 2002;55:932–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sachdev U, Murphy K, Derzie A, et al. Predictors of nonsentinel lymph node metastasis in breast cancer patients. Am J Surg. 2002;183:213–217. [DOI] [PubMed] [Google Scholar]
- 6.Mignotte H, Treilleux I, Faure C, et al. Axillary lymph-node dissection for positive sentinel nodes in breast cancer patients. Eur J Surg Oncol. 2002;28:623–626. [DOI] [PubMed] [Google Scholar]
- 7.Nos C, Harding-MacKean C, Freneaux P, et al. Prediction of tumour involvement in remaining axillary lymph nodes when the sentinel node in a woman with breast cancer contains metastases. Br J Surg. 2003;90:1354–1360. [DOI] [PubMed] [Google Scholar]
- 8.de Widt-Levert LM, Tjan-Heijnen VCG, Bult P, et al. Stage migration in breast cancer: surgical decisions concerning isolated tumor cells and micro-metastases in the sentinel lymph node. Eur J Surg Oncol. 2003;29:216–220. [DOI] [PubMed] [Google Scholar]
- 9.Liang WC, Sickle-Santanello BJ, Nims TA. Is a completion axillary dissection indicated for micrometastases in the sentinel lymph node? Am J Surg. 2001;182:365–368. [DOI] [PubMed] [Google Scholar]
- 10.Weiser MR, Montgomery LL, Tan LK, et al. Lymphovascular invasion enhances the prediction of non-sentinel node metastases in breast cancer patients with positive sentinel nodes. Ann Surg Oncol. 2001;8:145–149. [DOI] [PubMed] [Google Scholar]
- 11.Guenther JM, Hansen NM, DiFronzo LA, et al. Axillary dissection is not required for all patients with breast cancer and positive sentinel nodes. Arch Surg. 2003;138:52–56. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds C, Mick R, Donohue JH, et al. Sentinel lymph node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol. 1999;17:1720–1726. [DOI] [PubMed] [Google Scholar]
- 13.Turner RR, Chu KU, Qi K, et al. Pathologic features associated with nonsentinel lymph node metastases in patients with metastatic breast carcinoma in a sentinel lymph node. Cancer. 2000;89:574–581. [DOI] [PubMed] [Google Scholar]
- 14.Abdessalam SF, Zervos EE, Prasad M, et al. Predictors of positive axillary lymph nodes after sentinel lymph node biopsy in breast cancer. Am J Surg. 2001;182:316–320. [DOI] [PubMed] [Google Scholar]
- 15.Cserni G. Sentinel lymph-node biopsy-based prediction of further breast cancer metastases in the axilla. Eur J Surg Oncol. 2001;27:532–538. [DOI] [PubMed] [Google Scholar]
- 16.Hwang RF, Krishnamurthy S, Hunt K, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol. 2003;10:248–254. [DOI] [PubMed] [Google Scholar]
- 17.Degnim AC, Griffith KA, Sabel MS, et al. Clinicopathologic features of metastasis in nonsentinel lymph nodes of breast carcinoma patients. A metaanalysis. Cancer. 2003;98:2307–2315. [DOI] [PubMed] [Google Scholar]
- 18.Viale G, Maiorano E, Mazzarol G, et al. Histologic detection and clinical implications of micrometastases in axillary sentinel lymph nodes for patients with breast carcinoma. Cancer. 2001;92:1378–1384. [DOI] [PubMed] [Google Scholar]
- 19.Sobin LH, Wittekind CH. TNM Classification of Malignant Tumours. 6th ed. New York: Wiley-Liss; 2002. [Google Scholar]
- 20.Hermanek P, Hutter RV, Sobin LH, et al. Classification of isolated tumor cells and micrometastasis. Cancer. 1999;86:2668–2673. [PubMed] [Google Scholar]
- 21.Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864–1867. [DOI] [PubMed] [Google Scholar]
- 22.Veronesi U, Paganelli G, Viale G, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;91:368–373. [DOI] [PubMed] [Google Scholar]
- 23.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–553. [DOI] [PubMed] [Google Scholar]
- 24.Berg JW. The significance of axillary node level of breast cancer. Cancer. 1955;8:776–778. [DOI] [PubMed] [Google Scholar]
- 25.Viale G, Bosari S, Mazzarol G, et al. Intraoperative examination of axillary sentinel lymph nodes in breast carcinoma patients. Cancer. 1999;85:2433–2438. [PubMed] [Google Scholar]
- 26.Luini A, Gatti G, Frasson A, et al. Sentinel lymph node biopsy performed with local anesthesia in patients with early-stage breast carcinoma. Arch Surg. 2002;137:1157–1160. [DOI] [PubMed] [Google Scholar]
- 27.Kamath VJ, Giuliano R, Dauway EL, et al. Characteristics of the sentinel lymph node in breast cancer predict further involvement of higher-echelon nodes in the axilla: a study to evaluate the need for complete axillary lymph node dissection. Arch Surg. 2001;688–692. [DOI] [PubMed] [Google Scholar]
- 28.Cote RJ, Peterson HF, Chaiwun B, et al. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer. Lancet. 1999;354:896–900. [DOI] [PubMed] [Google Scholar]
- 29.Chu KU, Turner RR, Hansen NM, et al. Sentinel node metastasis in patients with breast carcinoma accurately predicts immunohistochemically detectable nonsentinel node metastasis. Ann Surg Oncol. 1999;6:756–761. [DOI] [PubMed] [Google Scholar]
- 30.Sabel MS, Zhang P, Barnwell JM, et al. Accuracy of sentinel node biopsy in predicting nodal status in patients with breast carcinoma. J Surg Oncol. 2001;77:243–246. [DOI] [PubMed] [Google Scholar]
- 31.Goldhirsch A, Wood WC, Gelber RD, et al. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21:3357–3365. [DOI] [PubMed] [Google Scholar]