Abstract
Objective:
Prospective trials have shown that 1-cm and 2-cm margins are safe for melanomas <1 mm thick and ≥1 mm thick, respectively. It is unknown whether narrower margins increase the risk of LR or mortality.
Summary Background Data:
To determine the relationship between histopathologic excision margin, local recurrence (LR) and survival for patients with melanomas ≤2 mm thick.
Methods:
Data were extracted from the Sydney Melanoma Unit database for all patients with cutaneous melanoma ≤2 mm thick, diagnosed up to 1996. Patients with positive excision margins or follow-up <12 months were excluded, leaving 2681 for analysis. Outcome measures were LR (recurrence <5 cm from the excision scar), in-transit recurrence, and disease-specific survival. Factors predicting LR and overall survival were tested with Cox proportional hazards analysis.
Results:
Median follow-up was 83.8 months. LR was identified in 55 patients (median time to recurrence, 37 months). At 120 months, the actuarial LR rate was 2.9%. Five-year survival after LR was 52.8%. In multivariate analysis, only margin of excision and tumor thickness were predictive of LR (both P = 0.003). When all patients with a margin <0.8 cm in fixed tissue (corresponding to a margin of <1 cm in vivo) were excluded from analysis, margin was no longer significant in predicting LR. Thickness, ulceration, and site were predictive of survival, but margin was not (P = 0.49).
Conclusions:
Histopathologic margin affects the risk of LR. However, if the in vivo margin is ≥1 cm, it no longer predicts risk of LR. Patient survival is not affected by margin.
The effects of histopathologic margin width on local recurrence and survival were analyzed in 2681 patients. The risk of local recurrence was inversely related to excision margin. Survival was not affected.
The appropriate margin of excision for cutaneous melanoma has been the subject of argument for many years. Modern recommendations are governed by data from prospective randomized clinical trials. The first, published by Veronesi et al1 and sponsored by the World Health Organization (WHO) Melanoma Group, was a randomized trial for patients with melanomas ≤2 mm thick. Six hundred twelve patients were randomized to a surgical excision margin of either 1 cm or 3 cm. There were no significant differences in either local recurrence (LR) or survival rates after 8 years of follow-up. The Intergroup Melanoma Study, by Balch et al,2 randomized patients with melanomas from 1 to 4 mm in thickness between a 2-cm margin of excision and a 4-cm margin. Again, there were no significant differences in either LR or survival rates. A Swedish trial, published by Ringborg et al,3 also confirmed the safety of a 2-cm margin. Recently, Khayat et al4 reported a randomized trial of patients with melanomas ≤2 mm thick that compared a 2-cm margin to a 5-cm margin. No differences in outcome were found. Finally, a recent trial reported by Thomas et al5 compared a 1-cm surgical margin to a 3-cm surgical margin in patients with melanomas that were >2 mm thick. An increase in locoregional recurrence was found, but there was no difference in overall survival. Most authorities therefore recommend that melanomas <1 mm thick should be removed with a 1-cm margin, melanomas between 1 and 2 mm thick should be excised with an margin of either 1 or 2 cm, and melanomas that are >2 mm thick should be excised with surgical margins of 2 cm.6,7
Despite these prospective data, there are many unanswered questions in relation to excision margins for melanoma. For example, it has been noted that in the WHO trial, there were 4 patients who developed LR, all from the group who had a 1-cm margin of excision and who had primaries between 1 and 2 mm thick.1 This observation has led to concern among some surgeons who consider that a 1-cm margin may be inadequate for melanomas from 1 to 2 mm thick, particularly since there is a high mortality among patients who suffer a LR.6 Others accept the finding that a 1-cm margin is adequate treatment of all patients with 1- to 2-mm-thick melanomas because of the lack of a significant difference in LR or survival between the 2 groups in the WHO trial.8 Although the 1-cm difference between 1- and 2-cm excision margins might be relatively trivial on the back or thigh, it can have significant implications for wound management, cosmetic outcome, and functional result on areas like the face or hands.9 Furthermore, melanomas arising in the head and neck region or on distal extremities were not randomized in these trials.
The adequacy of margins of less than 1 cm has not been tested in a randomized trial, but for cosmetic reasons they are commonly accepted on the face and distal extremities.9 It is not known whether this practice increases the risk of LR. Most fundamentally, it is not known whether there is a relationship between the size of the excision margin and the risk of LR. It has been argued, for example, that a histologically clear margin may be adequate treatment of most melanomas and that LR is actually a form of systemic metastasis.10 The question of whether LR is due to surgical failure or to the biology of the tumor remains unanswered. It is unlikely that any further prospective trials will take place for patients with melanomas <2 mm thick.
The aim of this study was to determine the LR rate for patients with melanomas ≤2 mm thick and to test whether a relationship exists between histopathologic margin of excision and LR. Further, we sought to determine if such a relationship was clinically significant. Because LR is uncommon and these patients have a low mortality risk, information on a very large number of patients was required to perform such an analysis. This information was obtainable from the Sydney Melanoma Unit (SMU) database.
PATIENTS AND METHODS
The SMU database contains prospectively collected data on more than 20,000 patients who have been seen and followed in the unit. Data were extracted for patients who met the following criteria:
Surgery before 31 December 1996
Primary invasive cutaneous melanoma ≤2 mm in thickness
Excision margins that were histologically clear
Narrowest excision margin measured in millimeters by histopathologic examination of tissue sections
At least 12 months of follow-up
Tumor thickness and level recorded
The histopathologic margin was evaluated in a standard way by pathologists at the SMU and recorded in a prospective database. The excised lesion was sliced into sequential 3-mm transverse slices, each embedded in tissue blocks. A single 5-μm-thick section from each 3-mm block was cut and examined microscopically, and the extent of the tumor and clearance from the margin were measured. Where the primary tumor was completely excised, the same calculation was made in the wide excision specimen calculated as distance in millimeters from the scar. The “margin” reported was the minimum or closest margin of normal tissue identified between the tumor or biopsy scar and the edge of the wide excision specimen. Other histopathologic features were recorded, including tumor type, ulceration, and number of mitoses per millimeter squared. Outcome measures were LR, in-transit recurrence, and disease-specific survival. LR was defined as recurrence of melanoma within 5 cm of the excision scar of the primary at any time during the patient's follow-up. In-transit disease was defined as recurrence outside a 5-cm radius but between the primary site and the regional nodal basin.
For the purpose of analysis, patients were divided into those who had a margin ≥0.8 cm. This figure was chosen because it corresponds to a 1-cm margin in vivo, allowing for 20% tissue shrinkage after excision and fixation.11
Patients who had a diagnosis of desmoplastic or acral lentiginous melanoma were excluded from the study because of a putative increased risk of LR.12,13 This left a total of 2681 patients for analysis.
Statistical Analysis
LR and disease-specific survival curves were constructed according to the Kaplan-Meier method. Time of freedom from LR was calculated from the date of the initial wide excision procedure. Melanoma-specific survival curves were calculated from the time of the LR. Differences between curves were assessed using the log-rank method. Factors predicting LR and overall survival were tested with the Cox proportional hazards regression model. Margin, thickness, and mitotic rate were analyzed as continuous variables. Anatomic site was analyzed according to whether the primary melanoma occurred on the trunk or elsewhere. Tumor satellites and positive nodes were reported in a very small number of patients (4 with satellites, 36 node-positive patients) and were not included in the risk analysis for LR.
RESULTS
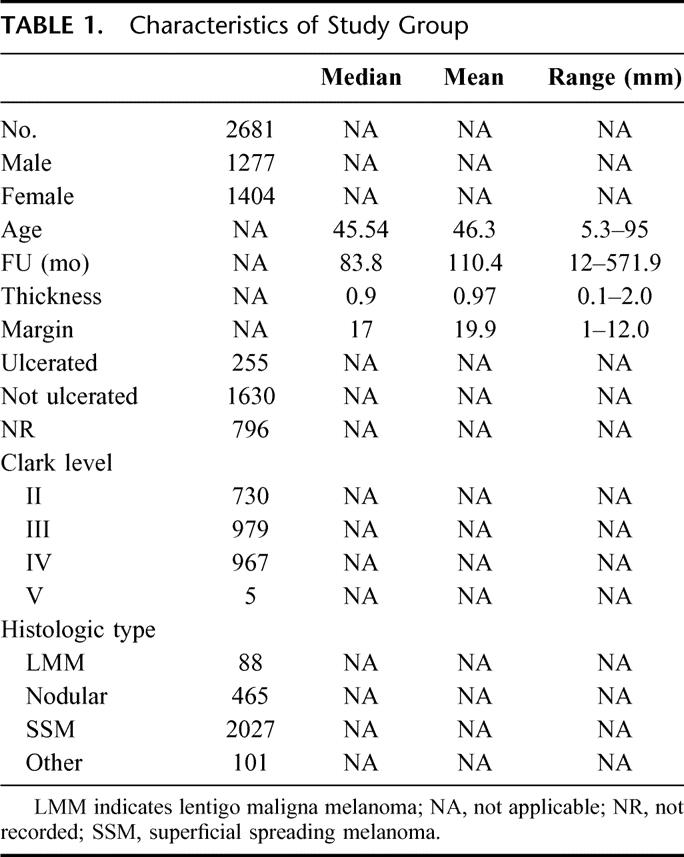
A description of the patient population is shown in Table 1. The date of excision of the primary melanoma ranged from 1951 to December 1996. Median follow-up was 83.8 months.
TABLE 1. Characteristics of Study Group
LR
Of 2681 patients available for analysis, LR was identified in 55. The median time to recurrence was 37 months (range: 2 to 193). Characteristics of the patients who developed a LR are shown in Table 2. The actuarial rate of LR, calculated according to the Kaplan-Meier method, was 2.9% at 180 months. When analyzed according to thickness, patients with melanomas <1 mm thick suffered a LR rate at 180 months of 1.7%, whereas for patients with melanoma 1 to 2 mm thick, the LR rate at 180 months was 4.3% (P < 0.001) (Fig. 1). There was a total of 88 patients with lentigo maligna melanomas, and 5 of these recurred locally. Five-year survival of the patients who developed a LR, calculated according to the Kaplan-Meier method, was 52.8%. The survival curve is shown in Figure 2.
TABLE 2. Characteristics of Patients With Local Recurrences
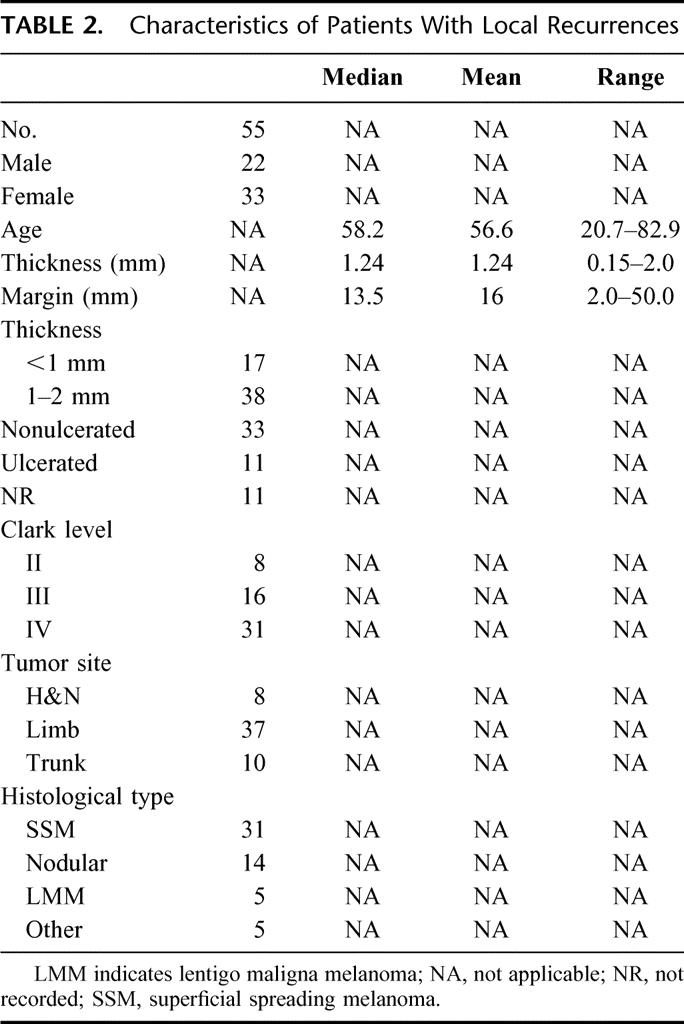
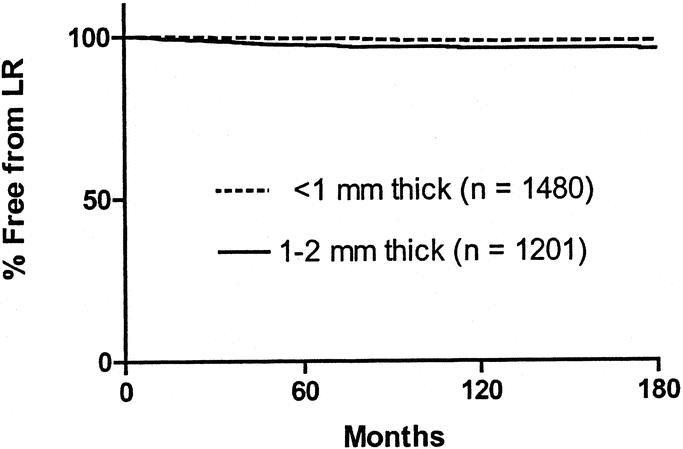
FIGURE 1. Kaplan-Meier curves of LR in the entire patient population according to thickness of the primary melanoma. The curves are significantly different by log-rank analysis (P < 0.001).
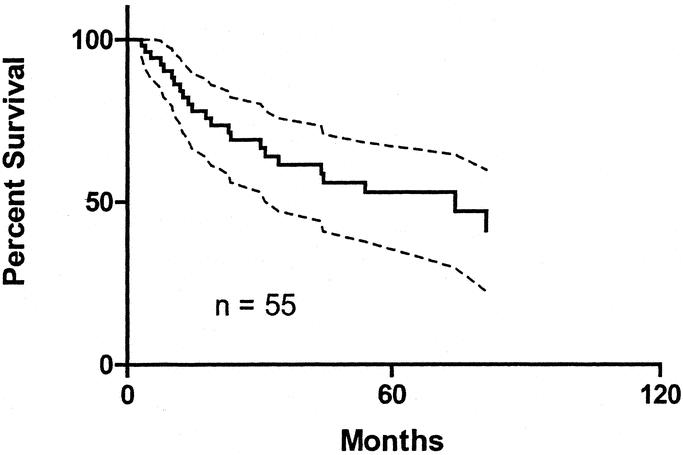
FIGURE 2. Kaplan-Meier survival curve of patients who had a LR. Dotted lines show 95% confidence intervals.
LR Compared With In-Transit Recurrence
In-transit disease developed in 50 patients, of whom 14 also developed LR. In-transit disease appeared before LR in 2 patients, after LR in 7, and was simultaneous with LR in 5. The patients who developed in-transit recurrence without LR were compared with those who had LR with no subsequent in-transit disease. Characteristics of these 2 groups are shown in Table 3. Survival for both groups is shown in Figure 3. There was no significant difference between the 2 groups by log-rank analysis, suggesting that there is little difference in the prognosis of these 2 types of recurrence.
TABLE 3. Characteristics of Patients With LR Versus In-Transit Recurrence
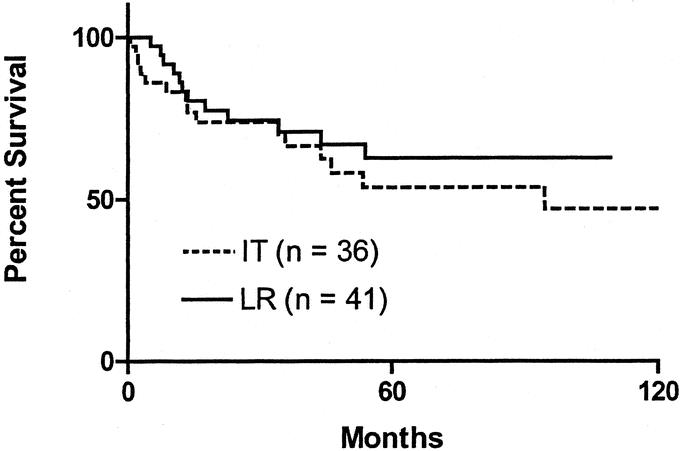
FIGURE 3. Kaplan-Meier survival curves comparing survival of patients with a local recurrence (LR) compared with patients with an in-transit (IT) recurrence. The difference between groups is not significant (log-rank analysis).
Margin of Excision and LR
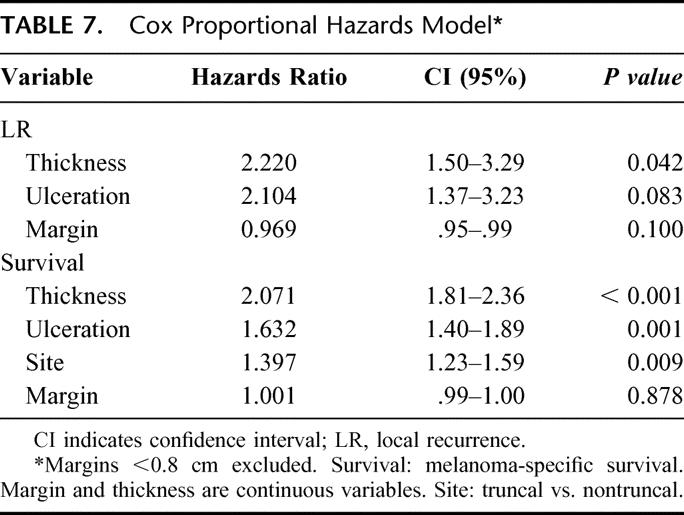
Crude LR rates analyzed according to margin of excision are shown in Table 4. LR rates vary from 1.06% to 3.85%. It is possible that LR may only be an increased risk if a “critical threshold” or minimum margin is not achieved by the treating surgeon. On the basis that a histopathologic margin of 0.8 cm should approximate a clinical margin of 1 cm in vivo, the actuarial LR rate was calculated for the group where the margin of excision was <0.8 cm and compared with the group who had an excision margin of ≥0.8 cm. The results are shown in Figure 4. At 120 months, the LR rates were 6.7% and 2.2%, respectively (P = 0.0003). Factors predicting the risk of LR were analyzed using the Cox proportional hazards model. Variables included in the analysis were gender, site of primary, type of histology, mitotic rate, and Clark level, none of which were significant. Results are shown in Table 5. Only margin of excision was predictive of LR (P = 0.003). When thickness, ulceration, and margin were reanalyzed excluding other factors, both thickness and margin were predictive of LR, whereas ulceration was not (Table 6). All patients with an excision margin less than 0.8 cm were then excluded from the multivariate analysis, leaving 2265 patients. Results are presented in Table 7. In this analysis, excision margin lost its significance in predicting LR, whereas thickness retained significance. Positive nodes and tumor satellites were reported in a very small number of patients (4 with satellites, 36 node-positive patients) and were not included in the risk analysis for LR.
TABLE 4. Crude LR Rate Grouped by Margin of Excision
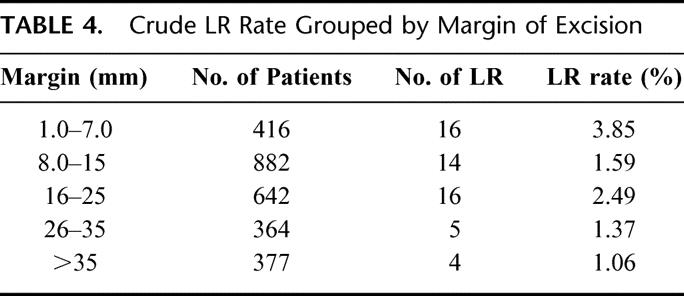
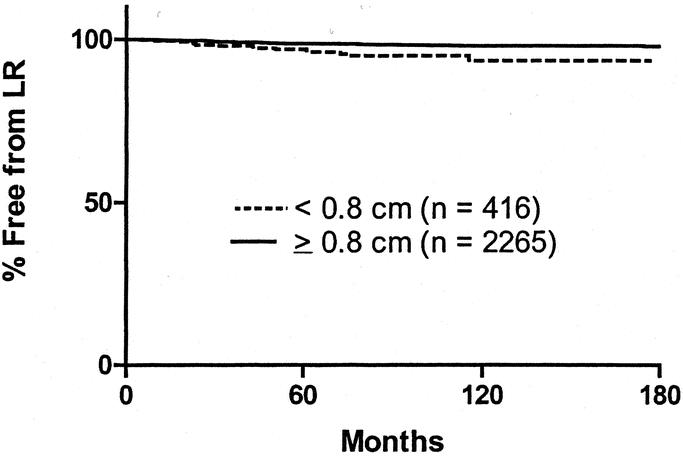
FIGURE 4. Kaplan-Meier curves of LR according to histopathologic margin of excision (<0.8 cm versus ≥0.8 cm). The curves are significantly different by log-rank analysis (P < 0.001).
TABLE 5. Cox Proportional Hazards Model of LR Factors
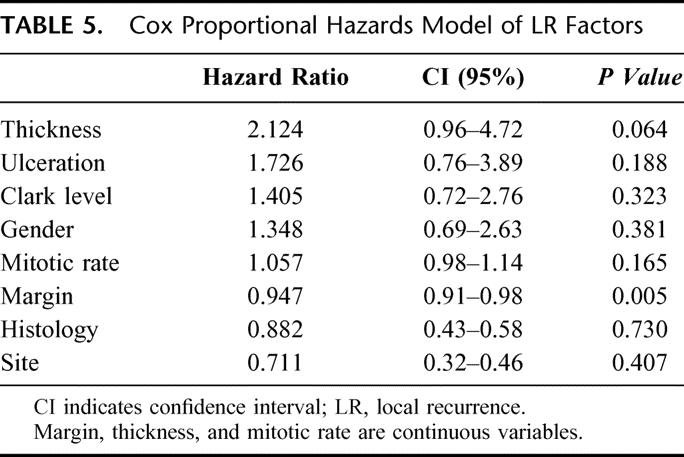
TABLE 6. Results of the Cox Proportional Hazards Model
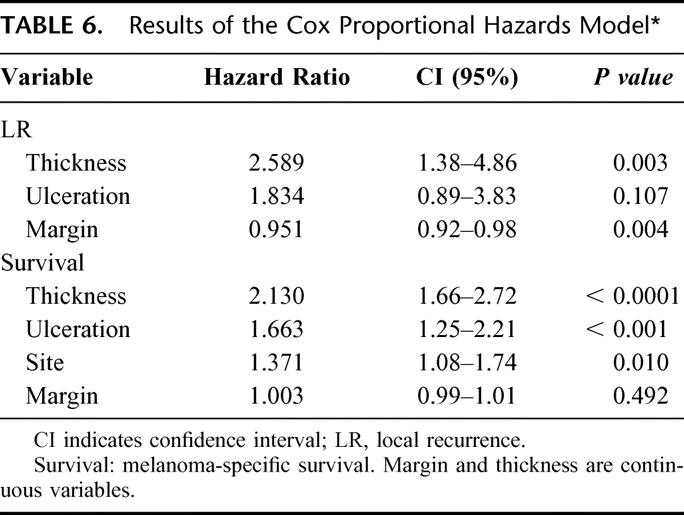
TABLE 7. Cox Proportional Hazards Model
Margin of Excision and Survival
Factors predicting melanoma-specific survival were analyzed using the Cox proportional hazards model. Results are shown in Table 6. Thickness, ulceration and site were highly significant predictors of survival, as expected, but excision margin was not (P = 0.49). When patients who had an excision margin of <0.8 cm, corresponding to a margin of <1 cm in vivo, were excluded from analysis, results were similar (Table 7).
DISCUSSION
It is often said that although a cancer surgeon cannot always cure the patient, the least that must be accomplished is locoregional control. Despite the fact that many studies have examined the influence of surgical margin on LR and survival in melanoma patients, there is still much debate on the subject.10,14,15 With one exception, prospective trials that have compared 2 different clinical margins have shown no difference between outcomes.1–5 The recently published trial from the United Kingdom by Thomas et al5 randomized 900 patients with melanomas ≥2 mm thick to undergo either a 1-cm margin or a 3-cm margin of excision. This study showed a higher rate of combined locoregional recurrence in the group of patients who had a narrower excision. However, there was no significant difference in LR alone or in overall survival. Furthermore, the sentinel node biopsy procedure was not used in the Thomas et al5 trial. It seems likely that selective lymphadenectomy might have affected regional recurrence rates and, therefore, overall locoregional recurrence. Available results from randomized trials do not allow conclusions to be drawn about margins of even narrower width. Furthermore, LR is rare after excision of melanomas that are <2 mm thick, so by necessity, inferences about causes are drawn from very few patients, even in large trials.2,16 Finally, definitions of margin and LR vary between reports making comparisons difficult. One study, for example, reported the margin of excision based on retrospective evaluation of dictated surgical reports.17 Although prospective trials have convincingly demonstrated that a measured 2-cm surgical margin is safe for most cutaneous melanoma patients with lesions 1 to 2 mm thick, the appropriateness of a 1-cm margin remains controversial. There are also ample data confirming the safety of 1-cm margins to treat lesions less than 1 mm thick, but even removal of this minimum radial margin of skin can sometimes leave a significant cosmetic defect. Is it safe to reduce surgical margins even further? This study reports on a large number of patients with complete data on measured histopathologic margin and other important prognostic variables, including tumor thickness and ulceration. Confounding variables such as desmoplastic melanomas, known to have a particularly high risk of LR, have been excluded from analysis. As far as we are aware, this is the largest and most complete retrospective analysis yet performed on the relationship between excision margin and outcome for patients with cutaneous melanomas ≤2 mm thick.
Definition of Margin
The excision margin measured by an operating surgeon for a clinical trial and the margin measured by a pathologist on a fixed specimen can vary considerably. The latter is a more accurate measurement of the closest radial margin of normal tissue surrounding the excised tumor. The histopathologic margin is thus a more appropriate measurement to determine a biologic relationship between excision width and LR. The use of a 20% correction factor to account for tissue shrinkage in the fixed specimen is only an approximation but it is based on sound studies of the relationship between in vivo and fixed melanoma specimens11.
Definition of LR
LR has been defined in various ways. In the WHO excision margins trial, LR was defined as recurrent melanoma appearing within 1 cm of the excision scar.1 In the Intergroup Trial, it was defined as recurrence appearing within 2 cm of the excision site.2 In this study, LR was defined as recurrence within 5 cm of the scar because that is the way recurrence was recorded in the SMU database since the beginning of data collection. There is no way to determine whether these are “true” LRs or the result of local lymphatic spread or even hematogenous metastasis. Until there is a mechanism to trace the source of a tumor deposit that grows adjacent to an excision scar, any definition of LR must remain arbitrary.
LR Rate
For patients with 1- to 2-mm-thick melanomas in the Intergroup trial, at a median follow-up of 120 months, the LR rate was 1% for lesions of the trunk and proximal extremities and 3.8% for lesions of the head and neck and distal extremities.2 In the Swedish trial that studied patients with melanomas <2 mm thick, randomization was between 2-cm and 5-cm margins. The rates of LR in the 2 groups at a median follow-up of 67 months were 0.8% and 1%, respectively.3 Khayat et al4 reported a LR in 5 of 326 (1.5%) study patients randomized to either a 2-cm or 5-cm margin. In our study, the actuarial rate of LR was 2.9% at 15 years, with a median follow-up 83.8 months. Although it is possible that longer follow-up would increase the rate of LR, most recurrences should be evident within 5 years.18
Patient Outcome After LR
There is no doubt that LR is a significant event for the patient and portends a serious prognosis. In various reports, long-term survival rates have ranged widely from 69% to 5%.2,16,19–21 Dong et al18 found that survival after LR was influenced by thickness of the primary. In that study, 5-year survival after LR for patients with lesions 0.75 mm thick was 70.1% and for patients with melanomas 0.75 to 1.5 mm thick, 63.1%. These figures are consistent with our data where survival at 5 years after LR for patients with melanomas ≤2 mm thick was 52.8%.
Mechanisms of LR
Possible mechanisms of LR have been discussed in detail elsewhere.15 Briefly, there may be residual disease, local infiltration of either tissue or lymphatics or, least likely, local metastases seeded from other systemic sites of disease. The latter theory has been supported by prospective data from the Intergroup Melanoma Surgical Trial where both 10-year survival data and the subsequent pattern of metastatic disease in patients with LR more closely resembled systemic disease than a purely regional problem.2 However, there were only 28 patients in the Intergroup trial who developed a LR. In several reports, Heenan10,24 and Heenan et al22,23 have argued that a LR resulting from retained local disease can be differentiated from hematogenous metastasis to the excision scar from a systemic source on the basis of its histopathologic appearance. This hypothesis has been disputed and has yet to be confirmed by others.
The hypothesis that LR is related to local dermal lymphatic invasion seems to be better supported by available data. The presence of satellites or in-transit disease at the time of diagnosis is now recognized to have a similar prognostic impact to regional nodal disease, and this observation is reflected in the new American Joint Committee on Cancer staging system.25 The presence of either of these types of tumor deposit might reasonably be expected to predispose to LR, although data are lacking because of the rarity of satellites or in-transit disease for relatively thin melanomas. Our data concur with those of several other authors who have demonstrated that LR does not confer the same dismal prognosis as systemic recurrence and that long-term cure is possible in almost half the patients with LR, similar to the cure rates reported for in transit recurrence or regional nodal disease.18,20,21,26 Furthermore, tumor features such as lymphovascular invasion have been reported to be predictive of both LR and in-transit recurrence, consistent with the hypothesis that local dermal lymphatic invasion is the most likely mechanism.27
LR and Margin
Is LR a function of inadequate surgery or a reflection of the biologic behavior of the disease? Given the uncertain mechanism for LR, this question cannot be answered at present. However, the data from the present study show, within the limits of a retrospective analysis, that given a clear histopathologic margin, there is still a statistical relationship between the margin of excision and the likelihood of LR. Nevertheless, this relationship does not seem to persist if the melanoma is excised with an in vivo margin of at least 1 cm. These findings concur with those of Ng et al,26 where the risk of LR was increased in the group of patients whose margin of excision was less than a calculated “ideal” margin of 1 cm for lesions <1 mm thick and 1.5 cm for lesions 1 to 2 mm thick.
Clinical Significance
Analysis of these data cannot answer the question of whether a 1-cm or a 2-cm margin leads to better results in treating melanomas from 1 mm to 2 mm in thickness. However, for the reasons stated above, a prospective trial to answer this question is unlikely to occur. What can be said is that there is no demonstrable relationship between margin and LR, given a minimal histopathologic margin of 0.8 cm (equivalent to a 1-cm margin in vivo). It is possible that once a certain excision margin is achieved, 1 cm for example, further excision of normal tissue to 2 cm or beyond has no effect on LR rate. Our data are consistent with this hypothesis. Furthermore, there is no apparent effect of any width of surgical margin on survival. Therefore, these data support the safety of a 1-cm margin for melanomas that are 1 to 2 mm in thickness.
Although LR is an uncommon event, the fact that we have shown a relationship between margin and LR suggests that margins of less than 1 cm in vivo, common in the treatment of melanomas of the face, for example, may be inadequate. Simple histopathologic clearance of the specimen may not be enough to ensure that local control will be optimal. However, the surgeon must continue to balance the cosmetic outcome of the excision with the low likelihood of recurrence. Even with histopathologic margins less than 0.8 cm, the LR rate was still low, 6.7% at 180 months. Detailed marginal assessment has been shown to be more reliable for acral lentiginous and lentigo maligna melanomas than for superficial spreading or nodular types, where intradermal spread may be noncontiguous.28 In our data, 5 of 88 lentigo maligna melanomas recurred locally. Although these data suggest a higher recurrence rate than for other subtypes of melanoma, the numbers are insufficient to draw a firm conclusion.
Conclusions
The results of this study suggest that for patients with melanomas ≤2 mm thick, the risk of LR, although small, is inversely related to the margin of excision. However, if a histopathologic margin of 0.8 cm is achieved, corresponding to an in vivo margin of 1 cm, this relationship is lost. There is no identifiable relationship between margin width and survival.
ACKNOWLEDGMENTS
The authors thank Marjorie Colman for invaluable assistance in managing and analyzing the SMU database.
Footnotes
Reprints: J.G. McKinnon, MD, FRCSC, Division of Surgical Oncology, Tom Baker Cancer Center, 1331-29 Street N.W., Calgary, Alberta, Canada T2N 4N2. E-mail: mckinnon@ucalgary.ca.
REFERENCES
- 1.Veronesi U, Cascinelli N. Narrow excision (1-cm margin): a safe procedure for thin cutaneous melanoma. Arch Surg. 1991;126:438–441. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Soong SJ, Smith T, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1–4 mm melanomas. Ann Surg Oncol. 2001;8:101–108. [DOI] [PubMed] [Google Scholar]
- 3.Ringborg U, Andersson R, Eldh J, et al. Resection margins of 2 versus 5 cm for cutaneous malignant melanoma with a tumor thickness of 0.8 to 2.0 mm: randomized study by the Swedish Melanoma Study Group. Cancer. 1996;77:1809–1814. [DOI] [PubMed] [Google Scholar]
- 4.Khayat D, Rixe O, Martin G, et al. Surgical margins in cutaneous melanoma (2 cm versus 5 cm for lesions measuring less than 2.1-mm thick). Cancer. 2003;97:1941–1946. [DOI] [PubMed] [Google Scholar]
- 5.Thomas JM, Newton-Bishop J, A'Hern R, et al. Excision margins in high-risk malignant melanoma. N Engl J Med. 2004;350:757–766. [DOI] [PubMed] [Google Scholar]
- 6.Reintgen D. Establishing a standard of care for the patient with melanoma. Ann Surg Oncol. 2001;8:91. [DOI] [PubMed] [Google Scholar]
- 7.Balch CM. Surgical margins for melanoma: is 2 cm too much? Aust N Z J Surg. 2002;72:251–252. [DOI] [PubMed] [Google Scholar]
- 8.Cascinelli N. Resection margins for stage I cutaneous melanoma. Br J Plast Surg. 1994;47:582. [DOI] [PubMed] [Google Scholar]
- 9.Tseng JF, Tanabe KK, Gadd MA, et al. Surgical management of primary cutaneous melanomas of the hands and feet. Ann Surg. 1997;225:544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heenan PJ. Melanoma: margins for error. Aust N Z J Surg. 2002;72:300–303. [DOI] [PubMed] [Google Scholar]
- 11.Silverman MK, Golomb FM, Kopf AW, et al. Verification of a formula for determination of preexcision surgical margins from fixed-tissue melanoma specimens. J Am Acad Dermatol. 1992;27:214–219. [DOI] [PubMed] [Google Scholar]
- 12.Kuchelmeister C, Schaumburg-Lever G, Garbe C. Acral cutaneous melanoma in Caucasians: clinical features, histopathology and prognosis in 112 patients. Br J Dermatol. 2000;143:275–280. [DOI] [PubMed] [Google Scholar]
- 13.Beenken S, Byers R, Smith JL, et al. Desmoplastic melanoma: histologic correlation with behavior and treatment. Arch Otolaryngol Head Neck Surg. 1989;115:374–379. [DOI] [PubMed] [Google Scholar]
- 14.Piepkorn M, Barnhill RL. A factual, not arbitrary, basis for choice of resection margins in melanoma. Arch Dermatol. 1996;132:811–814. [PubMed] [Google Scholar]
- 15.McCarthy WH. Melanoma: margins for error: another view. Aust N Z J Surg. 2002;72:304–306. [DOI] [PubMed] [Google Scholar]
- 16.Urist MM, Balch CM, Soong S, et al. The influence of surgical margins and prognostic factors predicting the risk of local recurrence in 3445 patients with primary cutaneous melanoma. Cancer. 1985;55:1398–1402. [DOI] [PubMed] [Google Scholar]
- 17.Heaton KM, Sussman JJ, Gershenwald JE, et al. Surgical margins and prognostic factors in patients with thick (>4mm) primary melanoma. Ann Surg Oncol. 1998;5:322–328. [DOI] [PubMed] [Google Scholar]
- 18.Dong XD, Tyler D, Johnson JL, et al. Analysis of prognosis and disease progression after local recurrence of melanoma. Cancer. 2000;88:1063–1071. [DOI] [PubMed] [Google Scholar]
- 19.Drzewiecki KT, Andersson AP. Local melanoma recurrences in the scar after limited surgery for primary tumor. World J Surg. 1995;19:346–349. [DOI] [PubMed] [Google Scholar]
- 20.Soong SJ, Harrison RA, McCarthy WH, et al. Factors affecting survival following local, regional, or distant recurrence from localized melanoma. J Surg Oncol. 1998;67:228–233. [DOI] [PubMed] [Google Scholar]
- 21.Wildemore JKT, Schuchter L, Mick R, et al. Locally recurrent malignant melanoma characteristics and outcomes: a single-institution study. Ann Plast Surg. 2001;46:488–494. [DOI] [PubMed] [Google Scholar]
- 22.Heenan PJ, English DR, Holman CD, et al. The effects of surgical treatment on survival and local recurrence of cutaneous malignant melanoma. Cancer. 1992;69:421–426. [DOI] [PubMed] [Google Scholar]
- 23.Heenan PJ, Weeramanthri T, Holman CD, et al. Surgical treatment and survival from cutaneous malignant melanoma. Aust N Z J Surg. 1985;55:229–234. [DOI] [PubMed] [Google Scholar]
- 24.Heenan PJ. Melanoma: margins for error. Aust N Z J Surg. 2003;73:550. [DOI] [PubMed] [Google Scholar]
- 25.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. [DOI] [PubMed] [Google Scholar]
- 26.Ng AK, Jones WO, Shaw JH. Analysis of local recurrence and optimizing excision margins for cutaneous melanoma. Br J Surg. 2001;88:137–142. [DOI] [PubMed] [Google Scholar]
- 27.Borgstein PJ, Meijer S, van Diest PJ. Are locoregional cutaneous metastases in melanoma predictable? Ann Surg Oncol. 1999;6:315–321. [DOI] [PubMed] [Google Scholar]
- 28.Breuninger H, Schlagenhauff B, Stroebel W, et al. Patterns of local horizontal spread of melanomas: consequences for surgery and histopathologic investigation. Am J Surg Pathol. 1999;23:1493–1498. [DOI] [PubMed] [Google Scholar]


