Abstract
The effects of genetic adjuvants on humoral and cell-mediated immunity to two human immunodeficiency virus antigens, Env and Nef, have been examined in mice. Despite similar levels of gene expression and the same gene delivery vector, the immune responses to these two gene products differed following DNA immunization. Intramuscular immunization with a Nef expression vector plasmid generated a humoral response and antigen-specific gamma interferon (IFN-γ) production but little cytotoxic-T-lymphocyte (CTL) immunity. In contrast, immunization with an Env vector stimulated CTL activity but did not induce a high-titer antibody response. The ability to modify these antigen-specific immune responses was investigated by coinjection of DNA plasmids encoding cytokine and/or hematopoietic growth factors, interleukin-2 (IL-2), IL-12, IL-15, Flt3 ligand (FL), and granulocyte-macrophage colony-stimulating factor (GM-CSF). Coadministration of these genes largely altered the immune responses quantitatively but not qualitatively. IL-12 induced the greatest increase in IFN-γ and immunoglobulin G responses to Nef, and GM-CSF induced the strongest IFN-γ and CTL responses to Env. A dual approach of expanding innate immunity by administering the FL gene, together with a cytokine that enhances adaptive immune responses, IL-2, IL-12, or IL-15, generated the most potent immune response at the lowest doses of Nef antigen. These findings suggest that intrinsic properties of the antigen determine the character of immune reactivity for this method of immunization and that specific combination of innate and adaptive immune cytokine genes can increase the magnitude of the response to DNA vaccines.
Genetic immunization combines several advantages of diverse traditional vaccine strategies. DNA-based vaccines are noninfectious, are incapable of replication, and do not express unwanted pathogenic proteins, and thus they have enhanced safety features compared to live or attenuated vaccines. Similar to live vector strategies, synthesis of the antigen in vivo may enhance the induction of cell-mediated immunity, and this de novo production of antigen should ensure the presentation of the correctly folded and glycosylated proteins. Construction of recombinant plasmids is technically simpler and more rapid than that of live vectors, and the amount of DNA required for effective immunization is much smaller than the amount of protein necessary to induce similar immune responses (11, 15, 26). Many animal models of infectious diseases have been reported in which DNA vaccines induce antibody, CD4+ T-helper lymphocyte, and CD8+-cytotoxic-T-lymphocyte (CTL) responses that, in some cases, are protective against subsequent pathogenic challenge (9, 32).
The success of a candidate vaccine depends on its ability to generate a long-term protective immune response. Evidence suggests that a successful and efficacious vaccine against human immunodeficiency virus (HIV) should induce a potent memory cell-mediated immune response in addition to neutralizing antibodies against immunodominant antigens from clinically relevant viral subtypes (17). The type of adjuvant used to formulate antigen can modify the immune responses generated by vaccination, and with the development of in vivo gene delivery techniques, it is now possible to use genetic adjuvants to modulate the strength or profile of this response. Multiple groups have demonstrated the potential of coadministering DNAs that express cytokines or costimulatory molecules together with plasmids encoding viral antigens (2, 4, 5, 13, 23).
Several factors are of particular interest. Interleukin-12 (IL-12) is a critical cytokine in the development of a strong T-cell response that is directed towards cell-mediated immunity (29). IL-15 shares many properties of IL-2 with respect to activation of T cells, particularly CD8+ CTLs. Unlike IL-2, it plays a pivotal role in the development, survival, and function of NK cells (31). Flt3 ligand (FL) is a potent hematopoietic factor that induces profound expansion of progenitor cell pools in multiple tissues. In particular, FL is a growth factor for the in vivo and ex vivo generation of dendritic cells and renders NK cell progenitors more responsive to other cytokines that may enhance the development of antitumor immunity (19, 20). Granulocyte-macrophage colony-stimulating factor (GM-CSF) is known to act as a genetic adjuvant in the induction of humoral and cellular immune responses to coinjected viral genes, such as the HIV gp160 gene (12, 14, 22).
In the present report, we investigate the effects of plasmid-encoded immunomodulators on HIV DNA vaccine responses. Modulation by factors that affect T-cell function or innate immunity and hematopoiesis, including IL-2, IL-12, IL-15, GM-CSF, and FL, were studied. Their effects on anti-Env and anti-Nef immune responses were examined. We find that cytokines and hematopoietic factors can increase the immune response to the immunizing antigen, and combinations of immunomodulators that act on T cells and those that affect antigen-presenting cells can enhance the immune responses to low concentrations of antigen. While these responses were quantitatively increased, the balance of cellular and humoral immune responses to different HIV candidate antigens, Nef and Env, remained unchanged.
MATERIALS AND METHODS
Plasmid DNA and antigens.
DNA vaccines expressing full-length HIV type 1 (HIV-1)(HXB2) envelope protein (pNGVL-gp160) and Nef (pNGVL-Nef) and plasmids expressing murine IL-2, IL-12, IL-15, GM-CSF, and FL were cloned and inserted into the pNGVL expression vector by the National Gene Vector Laboratory (University of Michigan). Glutathione S-transferase (GST)-tagged Nef protein was affinity purified from lysates from Escherichia coli BL21 (DE3) cells which were transformed with a pGex-Nef-GST plasmid using Pharmacia (Piscataway, N.J.) reagents. Bacteria were transformed with pGex-GST to produce control GST protein. Two peptides of Nef protein corresponding to amino acids 71 to 90 and 81 to 100, with the sequences PQVPLRPMTYKAAVDLSHFL and KAAVDLSHFLKEGGLEGLI, were provided by the Medical Research Council-directed AIDS Research Collaboration (Centralised Facility for AIDS Reagents, National Institute for Biological Standards and Control, Potters Bar, United Kingdom). Three gp120 peptides, with the sequences RIQRPGRAFVTIGK, PTDPNPQEVVLVNVT, and YGVPVWKEATTTLF, corresponding to residues 110 to 122 of the V3 region and two regions of HIV gp160, were synthesized by 9-fluorenylmethoxycarbonyl chemistry (University of Michigan Protein and Carbohydrate Structure Facility) and were used to restimulate spleen cells from mice immunized with pNGVL-gp160. Recombinant HIV-1 (HXB2) gp120 was obtained from Intracel (Issaquah, Wash.).
Mice and immunizations.
Female 6- to 8-week-old BALB/c mice (Charles River Laboratories, Wilmington, Del.) were injected in the right and left quadriceps muscles with 80 μg of a plasmid, pNGVL, with no insert (control vector) or the same plasmid expressing HIV-1 gp160 (pNGVL-gp160) or Nef (pNGVL-Nef) in 200 μl of phosphate-buffered saline three times at 2-week intervals. Plasmids that contained no insert (pNGVL, control vector) or those that expressed immunomodulators were mixed with the antigen-expressing plasmid so that each mouse received a total of 20 μg of immunomodulator-expressing DNA per immunization.
Assessment of T-cell responses.
Spleen cells from immunized mice were suspended at 2 × 106/ml in RPMI 1640 medium supplemented with 8% fetal calf serum (complete medium). The cells were cultured in triplicate wells of 96-well microtiter plates at 37°C and 5% CO2 in the presence of antigen, concanavalin A (2.0 μg/ml), or medium alone. Cells from mice immunized with pNGVL-gp160 were restimulated with the V3 peptide (40, 20, 10, or 5 μM), with an irrelevant Nef peptide, or with recombinant HIV-1 gp120 protein (5 and 1 μg/ml). Nef-specific responses were tested by restimulating spleen cells from immunized mice with the Nef-GST fusion protein (5 and 1 μg/ml) or GST (5 μg/ml). Supernatants were removed after 72 h to test for IL-4, IL-5, and gamma interferon (IFN-γ) production. The concentrations of these cytokines were determined by specific enzyme-linked immunosorbent assay (ELISA), using commercially available antibodies (PharMingen, San Diego, Calif.). The mean cytokine concentrations in supernatants from triplicate cultures were derived from a standard curve prepared with recombinant cytokines at known concentrations. The limits of sensitivity of the assays were 0.1 ng/ml for IFN-γ and 20 pg/ml for IL-4 and IL-5. Negative control animals were injected with the same plasmid backbone, pNGVL, but lacking an insert.
CTL responses.
CTL effector cells were prepared by culturing spleen cells from immunized mice in complete medium at an initial concentration of 5 × 106/ml and were sensitized with peptides or stable cells. For peptide stimulation, spleen cells from immunized mice were pulsed with the V3 loop peptide of gp120 or with the two Nef peptides, giving a final concentration of 40 μM. For stable cells, irradiated BC14H cells (stably transfected BC10ME cells expressing gp160) (7) or irradiated P815-Nef (stably transfected P815 cells expressing Nef by being transduced with recombinant retrovirus expressing Nef using the method described before [34]) were added to the spleen cells in a 1-to-10 ratio. The cells were cultured for 7 days, with the addition of recombinant IL-2 (5 U/ml) on day 4. For peptide-sensitized cells, 107 target P815 (H-2d) cells (American Type Culture Collection, Manassas, Va.) in log-phase growth were pulsed with peptide for 4 h, washed twice, and then incubated with 100 μCi of sodium [51Cr]chromate for 90 min at 37°C. For spleen cells sensitized with stable cells, target stable BC14H, BC10ME (control cell line), P815-Nef, and P815 (control cell line) cells (107) were also incubated with 100 μCi of sodium [51Cr]chromate for 90 min at 37°C. 51Cr-pulsed target cells were extensively washed in complete medium, resuspended at 105/ml, and assayed as previously described (33).
Anti-HIV Env and anti-HIV Nef antibody titers.
Serum samples were obtained by tail vein bleed 2 weeks after the second and third immunizations and were tested by ELISA for anti-HIV Env or anti-HIV Nef immunoglobulin and immunoglobulin G (IgG) subclasses. Serial threefold dilutions of serum were added to microtiter plates coated with recombinant gp120 or purified Nef-GST (1.0 μg/ml) or GST (1.0 μg/ml), and bound antibodies were detected using alkaline-phosphatase-conjugated antibodies specific for whole IgG or for IgG subclasses (PharMingen). Results are expressed as endpoint titers calculated by regression of the straight part of a curve of optical density versus serum dilution to a cutoff of 2 standard deviations above background control values.
Immunoprecipitation and Western blot antibody analysis.
Nef-GST or gp160 from transfected 293 cell supernatants were immunoprecipitated with serum from immunized mice. Monoclonal antibodies against Nef or polyclonal antibodies against gp160 were used as positive controls. Immunoprecipitates were detected by Western blot analysis with horseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG (Santa Cruz Biotechnology Inc., Santa Cruz, Calif.).
Statistical analysis.
Statistical significance between groups was analyzed by t test using the SigmaStat software program version 2.0.
RESULTS
Inherent differences in immune responses to Env and Nef after DNA vaccination.
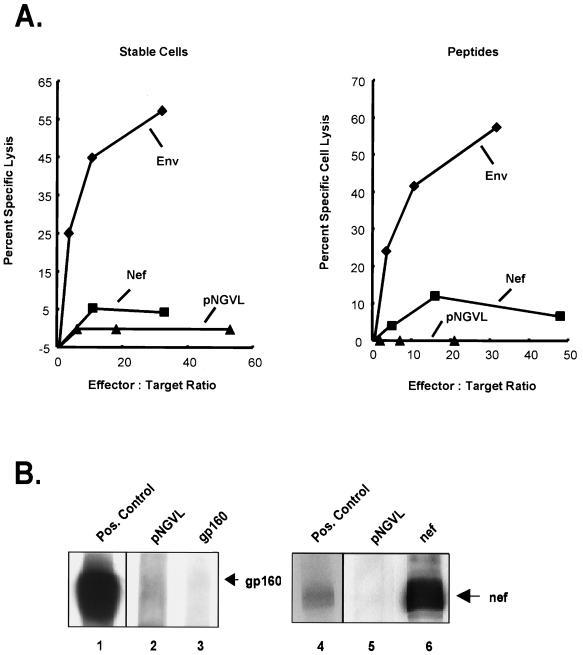
The immune responses to Env and Nef were characterized following DNA immunization. Intramuscular immunization with a Nef expression vector plasmid generated a strong humoral response but little CTL immunity (Fig. 1). In contrast, immunization with an Env vector stimulated CTL activity, against both stably transduced cells and peptide-pulsed targets (Fig. 1A), but did not induce a high-titer antibody response by Western blot analysis (Fig. 1B), though levels were measurable with more sensitive assays, such as immunoprecipitation or ELISA (B. K. Chakrabarti, W.-P. Kong, B.-Y. Wu, Z.-Y. Yang, J. Friborg, X. Ling, S. R. King, D. C. Montefiori, and G. J. Nabel, submitted for publication). Quantitative differences in expression did not account for the disparate immune responses to Env and Nef. Codon modification of the plasmid expression vectors was used to ensure high levels of gene expression, and both gene products were readily detected by Western blotting (Fig. 1) (Chakrabarti et al, submitted). Further quantitation of expression levels revealed that the Env expression vector directs synthesis of 59.6 μg of Env while the pNGVL-Nef expression vector makes 89.7 μg of Nef per 106 293 cells in 48 h, i.e., levels of protein synthesis for the two vectors were comparable. Thus, the predominant cellular and humoral components of the response to these two gene products differed, possibly related to qualitative differences in antigen presentation and/or immune-cell recognition.
FIG. 1.
Antigen plays a role in determining the induction of predominant CTL or antibody responses. Mice were intramuscularly immunized with plasmid DNA expressing Nef or Env. (A) Ten days after the third immunization, the cytolytic activity of spleen cells from mice was tested against the relevant stably transfected target cells (left), BC14H cells which express gp160, and P815-Nef cells which express Nef, or against peptide-pulsed P815 target cells (right) as detailed in Materials and Methods. The standard deviation for each triplicate point was ≤10%. Mice were injected with Env expression vectors encoding gp160 (left) or gp150 (right), respectively. (B) The antibody response was determined by immunoprecipitation followed by Western blot analysis with horseradish peroxidase-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology Inc.). Each lane corresponds to the serum from an animal immunized with either the control vector (lanes 2 and 5) or a plasmid that expresses either Env or Nef (lanes 3 and 6). Mouse polyclonal serum to gp160 (HIV-1 V3 monoclonal [IIIB-V3-13], National Institutes of Health AIDS Reagent Program) and monoclonal antibody to Nef (Intracel) were used as positive controls (lanes 1 and 4, respectively).
DNA immunization with a plasmid expressing HIV-1 Nef induces a Th1 cytokine secretion pattern.
Immunization of mice with pNGVL-Nef resulted in a high level of IFN-γ production in spleen cells stimulated with Nef protein (5 μg) in vitro (Fig. 2A). In the ELISA, two different concentrations of antigen were used to examine the dose response of the antigen. Although IL-4 and IL-5 production were observed in response to mitogen stimulation in these samples, these and other Th2 cytokines were undetectable in the supernatants of spleen cells restimulated with any concentration of Nef (data not shown). This cytokine secretion pattern was antigen specific, as no cytokines were produced in response to restimulation with the fusion tag, GST, at the same concentrations as Nef-GST (data not shown). Furthermore, immunization with a control vector with no insert alone, pNGVL, did not induce cytokine responses (Fig. 2A).
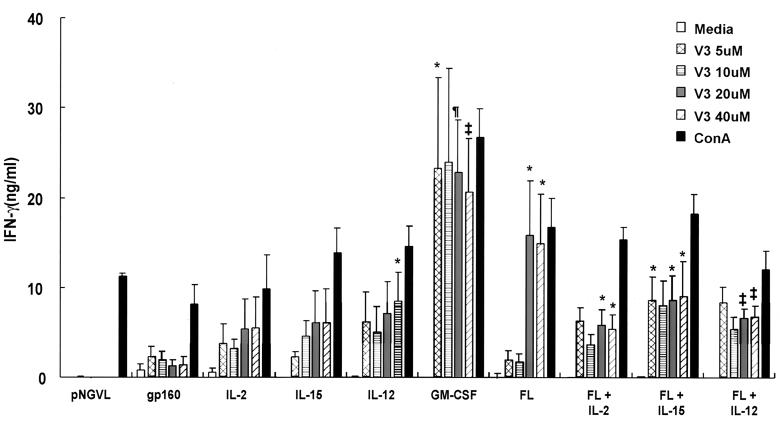
FIG. 2.
IFN-γ responses following coimmunization of DNA expressing Nef with DNA expressing cytokine or hematopoietic factors. Mice were intramuscularly immunized with DNA without an insert (control) or plasmid expressing Nef alone (80 μg and 20 μg of pNGVL per mouse) or with Nef plus IL-2, IL-15, IL-12, GM-CSF, or FL (20 μg/mouse); FL and IL-2, IL-15, or IL-12 (10 μg of each cytokine/mouse), Nef, and FL on the first immunization and Nef and IL-2 or IL-12 on the second and third immunizations (2+3); or empty vector (pNGVL). Two weeks after the third immunization, spleens from immunized mice were restimulated in vitro with Nef protein in the form of a GST fusion-tagged protein at 5 (A) or 1 (B) μg/ml. IFN-γ secretion was assessed by ELISA in supernatants removed at 72 h. Responses are mean (plus standard deviation) concentrations, with the statistically insignificant background subtracted, for 5 to 16 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.005 versus pNGVL-Nef-immunized mice at the same in vitro dose of Nef protein.
Cytokine vectors enhance Nef-specific T-cell responses.
The effects of genetic adjuvants on the pNGVL-Nef vaccine response were analyzed next. Mice were immunized three times with pNGVL-Nef in combination with plasmids expressing IL-2, IL-12, IL-15, FL, GM-CSF, or control vector (5:1 antigen/cytokine plasmid ratio). Humoral and cellular immune responses to Nef were tested 2 weeks after the final immunization. Spleen cells from mice immunized with pNGVL-Nef and pNGVL-IL-12 produced the most significant increase in IFN-γ production after stimulation with 5 μg of Nef/ml (Fig. 2A). In contrast to IL-2, IL-15 induced an increase in IFN-γ production when spleen cells were restimulated at the highest dose of Nef (Fig. 2A). Coinjection with the GM-CSF or FL expression vector with Nef modestly increased antigen-specific IFN-γ production.
No IFN-γ production was observed at a lower concentration of Nef (1 μg/ml), and minimal cytokine production was observed in mice administered pNGVL-Nef and an individual immunomodulator expression vector (Fig. 2B); however, coinjection of FL and a cytokine gene, together with pNGVL-Nef, induced a highly significant increase in IFN-γ production when spleen cells from these immunized mice were stimulated with a lower dose of Nef (1 μg/ml) in vitro (Fig. 2B). This response was reduced when FL was administered during the first immunization with pNGVL-Nef and IL-2 or IL-12 was administered during subsequent immunizations. Thus, timing and cytokine cooperativity determined the efficacy of FL, which was stimulatory under specific conditions.
Induction of anti-Nef antibodies and effects of cytokine vectors on pNGVL-Nef humoral responses.
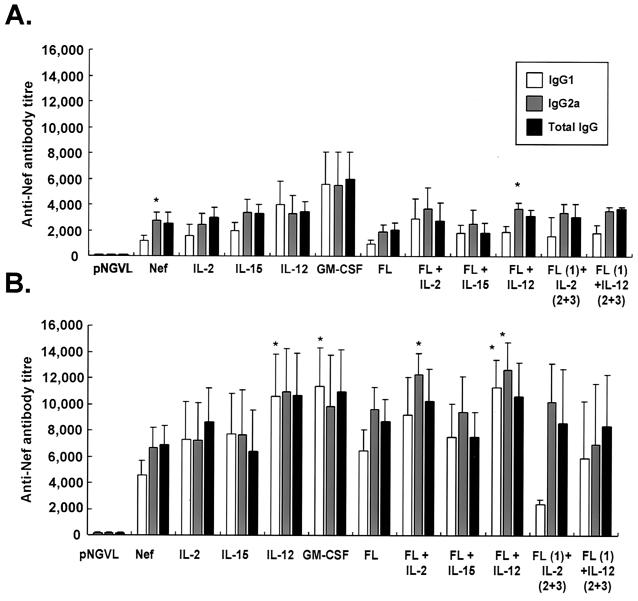
Two DNA immunizations with pNGVL-Nef induced a strong antigen-specific antibody response (Fig. 3A). The total IgG response was significantly increased when mice received another immunization (P < 0.05) (Fig. 3B). The IgG2a subtype dominated the serum antibody response after two immunizations (Fig. 3A), though a third immunization resulted in a significant (P < 0.001) increase in IgG1 anti-Nef titers (Fig. 3B). The Nef-specific IgG response in serum was also confirmed by immunoprecipitation followed by Western blot analysis (Fig. 1B).
FIG. 3.
Coinjection with plasmids expressing immunomodulator molecules enhances the induction of serum anti-Nef specific IgG antibodies. Mice were immunized as described in the legend to Fig. 2. The levels of anti-Nef antibodies, total IgG, and IgG subclasses were determined by ELISA on serum samples recovered 2 weeks after the second (A) and third (B) immunizations. The results are mean (plus standard deviation) endpoint titers for 5 to 16 mice per group. *, P < 0.05 versus IgG1 titer observed in the same group of mice (A) or P < 0.05 versus pNGVL-Nef-immunized mice (B).
Two immunizations with pNGVL-Nef in combination with any of the immunomodulator expression vectors did not significantly alter the serum antibody response observed in Nef-immunized mice (Fig. 3A); however, a third immunization resulted in a further increase in anti-Nef titer in these mice. Coinjection of the IL-12 expression vector resulted in a significant increase in IgG1 titers. FL vector in combination with pNGVL-Nef and IL-12 increased both IgG1 and IgG2a titers. In contrast, coadministration of the FL and IL-2 vectors with pNGVL-Nef led to a significant increase in IgG2a titers, suggesting a more prominent Th1 response.
Enhancement of HIV-1 gp160 cellular immune responses by coadministration of immunomodulatory expression vectors.
Mice were immunized with a plasmid that expressed Env from HIV-1 (HXB2), and spleen cells from these mice were restimulated in vitro with the V3 loop peptide. Coinjection of an IL-2, IL-12, IL-15, or FL plasmid with pNGVL-gp160 significantly enhanced the weak peptide-specific IFN-γ production that was observed when spleen cell cultures from mice immunized with pNGVL-gp160 alone were stimulated with high doses (40 μM) of V3 peptide (Fig. 4). Coadministration of the GM-CSF plasmid increased IFN-γ production at all concentrations of V3 peptide. In contrast, inclusion of the FL plasmid with pNGVL-gp160 resulted in a significant increase in IFN-γ production when spleen cells from these mice were stimulated in vitro with a high (20 and 40 μM) but not a low (5 and 10 μM) antigen concentration. Coinjection of the IL-12 plasmid alone or with the FL vectors with pNGVL-gp160 also markedly enhanced the Th1 response at lower peptide concentrations (10 and 5 μM, respectively).
FIG. 4.
IFN-γ responses following coimmunization of DNA expressing HIV gp160 with DNA expressing cytokine or hematopoietic factors. Mice were intramuscularly immunized with DNA expressing gp160 alone (80 μg and 20 μg of pNGVL per mouse) or with gp160 plus plasmid expressing IL-2, IL-15, IL-12, GM-CSF, or FL (20 μg/mouse); FL and IL-2, IL-15, or IL-12 (10 μg of each cytokine/mouse); or empty vector (pNGVL). Two weeks after the third immunization, spleens from immunized mice were restimulated in vitro with medium alone or with the Env V3 loop peptide at a concentration of 40, 20, 10, or 5 μM or with concanavalin A (ConA). IFN-γ secretion was assessed by ELISA in supernatants removed at 72 h. The responses are mean (plus standard deviation) concentrations for 5 to 16 mice per group. *, P < 0.05; ‡, P < 0.01; ¶, P < 0.005 versus pNGVL-gp160-immunized mice at the same in vitro dose of V3 peptide.
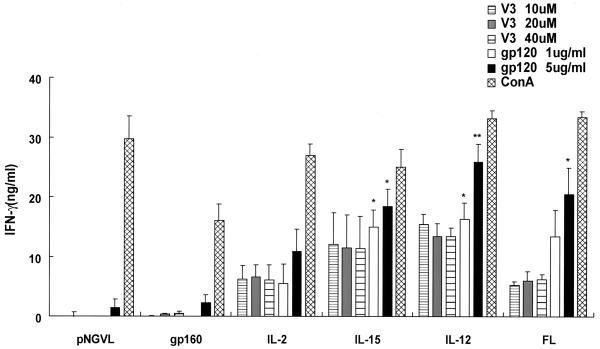
To compare the pattern of IFN-γ production between the V3 loop peptide and the complete protein, spleen cells from immunized mice were also restimulated with recombinant gp120 protein (Fig. 5). Th1 cytokine production was enhanced when spleen cells from all mice were restimulated with gp120 compared to that with the peptide epitope. Inclusion of the IL-15, IL-12, or FL vector resulted in a significant enhancement of gp120-specific IFN-γ production at both antigen concentrations. Thus, the patterns of IFN-γ secretion were comparable between the V3 loop peptide and the complete protein.
FIG. 5.
IFN-γ responses due to restimulation with recombinant gp120 following coimmunization of DNA expressing HIV gp160 with DNA expressing cytokine or hematopoietic factors. Mice were immunized as described in the legend to Fig. 4. In addition to restimulation of spleen cells with V3 peptide, cells were also restimulated with recombinant gp120 at a concentration of 5 or 1 μg/ml. The responses are mean (plus standard deviation) concentrations, with the background subtracted, for five mice per group. *, P < 0.05; **, P < 0.01 versus pNGVL-gp160-immunized mice at the same in vitro dose of gp120 protein.
Induction of CTL responses by immunization with Env.
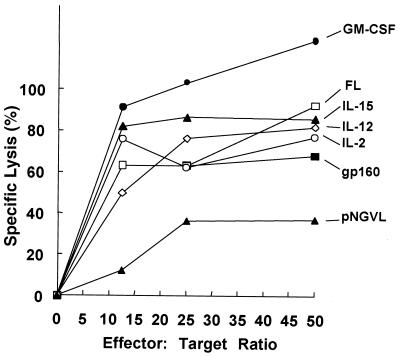
The induction of an anti-gp160 effector CTL response was modulated when the gp160 expression plasmid was coinjected with cytokine, FL, or GM-CSF genes (Fig. 6). Coadministration of the GM-CSF expression plasmid consistently resulted in the largest increase in CTL activity. Injection of the IL-15 gene also resulted in a strong increase in epitope-specific CTL activity. Coinjection of the IL-2 or IL-12 vector had only a modest effect on the CTL response compared to pNGVL-gp160 alone (Fig. 6). Though the CTL assay is limited in its ability to quantify immune responses in vivo because of the requirement for in vitro sensitization, it has been used to directly quantify levels of epitope-specific killing present in ex vivo cell cultures, allowing indirect comparisons of vaccine effects on in vivo immune responses to be made.
FIG. 6.
CTL responses following coimmunization of DNA expressing HIV gp160 with DNA expressing cytokine or hematopoietic factors. Mice were intramuscularly immunized with DNA expressing gp160 alone or with gp160 plus IL-2, IL-15, IL-12, GM-CSF, FL, or empty vector (pNGVL). Two weeks after the third immunization, cytolytic activities of restimulated pools of spleen cells from four mice were tested against P815 target cells incubated with the V3 loop peptide or with medium alone. The results are expressed as net percent specific lysis, obtained by subtracting the percent specific lysis of unpulsed P815 target cells (range, 0 to 10%) from the lysis of peptide-bearing cells.
DISCUSSION
DNA vaccination offers the potential to induce immunity to viral, bacterial, and tumor antigens as well as allergens (9, 11, 15, 26, 32). This study investigated methods that could possibly enhance this mode of immunization by coinjection of cytokine genes that modulate the magnitude or profile of HIV antigen-specific immune responses. Here, immune analyses were performed using an inbred mouse strain, with the same expression vector for vaccination, the same vector for coadministration of immunomodulators, and comparable levels of protein synthesis. Despite this consistency, different immune responses to Nef and Env were detected, attributable only to the immunogen, i.e., the cDNA insert was the only difference between the alternative vectors. Expression of cytokines and hematopoietic factors from coinjected plasmid vectors can significantly increase antigen-specific cellular or humoral immune responses, and a synergism was observed between selected immunomodulators, but the predominance of a cellular or humoral response was not reversed by the genetic adjuvants tested in this study. Despite similar levels of gene expression, it is unsurprising that the immune responses to these antigens differ. Other factors, such as folding, oligomerization, secretion or localization in different subcellular organelles (endoplasmic reticulum, Golgi apparatus, or cytoplasm), or affinity for major histocompatibility complex-encoded molecules may also affect the character and potency of the response. This finding highlights a significant difference between DNA immunization with genetic adjuvants and traditional subunit or live vector vaccine strategies.
The induction of a strong anti-Nef antibody response and CD4+ Th cell responses indicates that the antigen was not fully sequestered in the cytosol and was capable of priming CD4+ Th and B cells to differentiate and produce cytokines and antibodies. This result suggests that protein may have been secreted from the transfected cell or released from dying cells, as with influenza nucleoprotein (30), or from immune-mediated lysed cells (8). Furthermore, an anti-Nef cytotoxic response can be induced when mice are immunized with recombinant vaccinia virus expressing Nef (21), suggesting that induction of immune responses to an antigen expressed from plasmid DNA can be different from that in response to viral vectors. It is known from previous studies using DNA priming and adenovirus boosting in an Ebola virus model (28), for example, that 10- to 100-fold increases in antibody titer can be seen when using an adenoviral vector boost compared to a boosting immunization with plasmid DNA alone encoding the same antigen in primates. In contrast to the anti-Nef responses, immunization with a plasmid encoding the full-length membrane-bound Env induced a strong CTL response, suggesting that the number of effective antigen-presenting cells was sufficient to induce a persistently strong anamnestic CTL response through presentation of Env epitopes in class I molecules. Recently, mutations have been identified in this codon-modified Env plasmid that elicit substantial antibody responses by DNA immunization, with comparable levels of protein synthesis (1; Chakrabarti et al., submitted). This result demonstrates that regions of the Env protein determine its ability to elicit an antibody response by DNA vaccination.
The lower antibody response to Env suggests that the expressed gp160 antigen may not present conformational epitopes that induce the activation and differentiation of B cells. For immunization with DNA, the induction of a predominantly humoral or cellular immune response, therefore, was also an inherent property of the antigen, in contrast to live vector or subunit vaccine strategies. In the last two cases, the effects of the formulation or the inflammatory properties of the virus can dominate adjuvant determinants of the antigen. Although bacterial DNA can induce innate immune responses (10), pNGVL-Nef and pNGVL-gp160 contain similar numbers and types of known immunostimulatory sequences, suggesting that the DNA sequence of the plasmid is unlikely to account for the differences in Nef and Env immune responses.
It has been observed that primate immune responses induced by DNA vaccination may be less potent than those observed in murine models. However, early results suggest that DNA immunization followed by a heterologous boost may be an extremely effective vaccine regimen in primates (28). A better understanding of interspecies discrepancies will be required to extrapolate the results of rodent studies to humans. On the other hand, if differences in immune responses are related to inherent properties of an antigen, they could be maintained, possibly with quantitative differences, across species.
Repeated immunization with pNGVL-Nef enhanced the induction of humoral responses, with a significant increase in the IgG1 titers, and did not result in the induction of a CTL response or a significant increase in cell-mediated immunity, suggesting that some genetic vaccines may optimize humoral immunity without increasing cellular immunity after multiple injections. The requirement for multiple immunizations to boost initial antigen-specific cellular immunity may be reduced by coinjection of cytokine-expressing plasmids. This method of cytokine delivery may also represent a safer and more effective alternative to repeated systemic administration of the recombinant protein, which can be toxic (16) or short-lived due to a low serum half-life (3, 18, 25). For example, IL-12 exerts side effects when delivered as protein, but coinjection of IL-12 plasmid with the Nef expression vector enhanced Th1 and antibody responses but did not lead to obvious splenomegaly or markedly increased numbers of white blood cells.
We observed an antigen-specific effect on the capacity of individual genetic adjuvants to modulate immune responses. The effects of coinjection differed when pNGVL-gp160 was used instead of pNGVL-Nef; in the former case, GM-CSF, not IL-12, induced the most significant increases in Env-specific CD4+ Th1 and CD8+ CTL responses. These differences in the abilities of the genetic adjuvants to modulate the immune response were dependent on the antigen. The different effects of cytokines on these responses may reflect their underlying mechanisms of action. For example, in the predominantly antibody-driven response to Nef, IL-12 was most effective, likely due to its fundamental role in driving Th1 cell differentiation and in the stimulation of non-B-cell responses (29). In contrast, the strong cell-mediated immune response to gp160 may not require IL-12 to increase this already polarized response. Instead, the actions of GM-CSF, by boosting the antigen-presenting population that activates T cells (6, 27), may exert more complementary effects on this response. It is interesting that the FL plasmid did not induce such a potent effect as the GM-CSF plasmid in pNGVL-gp160-immunized mice. The capacity for these two growth factors to stimulate expansion of distinct subsets of dendritic cells may explain this effect (24). These findings suggest that genetic adjuvants must be chosen with consideration of the antigen-driven profile of the immune response, and an individual genetic adjuvant may not act universally for all DNA vaccines.
A cooperative effect in Th1 cytokine induction between FL and other cytokines was observed at lower stimulatory concentrations of Nef or Env peptide. These results demonstrate that FL alone can increase immune responses after DNA immunization; however, the FL-enhanced cell populations, predominantly dendritic cells, may be more effective in the presentation of antigen to T cells modulated by cytokines, such as IL-12, IL-2, or IL-15. Furthermore, in contrast to the effects of individual cytokines, this cooperative effect between FL and other cytokines was independent of the immunizing antigen; however, the expansion of progenitor populations must be simultaneous with modulation of the effector immune response. Cooperativity was reduced when FL was administered only on the first immunization and cytokines were coinjected on subsequent immunizations. This approach to generate a potent immune response by concurrent stimulation of hematopoiesis and the adaptive immune response may prove more useful at lower doses of antigen or viral challenge. This vaccination strategy is also less dependent on the immunizing antigen and may be more convenient than injection of individual cytokines or growth factors. Such genetic strategies may contribute to vaccine development for HIV and other infectious pathogens.
Acknowledgments
We thank Nancy Barrett for preparation of the figures, Betty Wu for preparation of plasmids, Judith Stein for advice, and Cherilyn Davis and Ati Tislerics for manuscript preparation.
REFERENCES
- 1.Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barouch, D. H., A. Craiu, M. J. Kuroda, J. E. Schmitz, X. X. Zheng, S. Santra, J. D. Frost, G. R. Krivulka, M. A. Lifton, C. L. Crabbs, G. Heidecker, H. C. Perry, M. E. Davies, H. Xie, C. E. Nickerson, T. D. Steenbeke, C. I. Lord, D. C. Montefiori, T. B. Strom, J. W. Shiver, M. G. Lewis, and N. L. Letvin. 2000. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. USA 97:4192–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch, D. H., S. Santra, T. D. Steenbeke, X. X. Zheng, H. C. Perry, M. E. Davies, D. C. Freed, A. Craiu, T. B. Strom, J. W. Shiver, and N. L. Letvin. 1998. Augmentation and suppression of immune responses to an HIV-1 DNA vaccine by plasmid cytokine/Ig administration. J. Immunol. 161:1875–1882. [PubMed] [Google Scholar]
- 4.Boyer, J. D., J. Kim, K. E. Ugen, A. D. Cohen, L. Ahn, K. Schumann, K. Lacy, M. L. Bagarazzi, A. Javadian, R. Ciccarelli, R. S. Ginsberg, R. R. MacGregor, and D. B. Weiner. 1999. HIV-1 DNA vaccines and chemokines. Vaccines 17(Suppl. 2):53–64. [DOI] [PubMed] [Google Scholar]
- 5.Cho, J. H., S. W. Lee, and Y. C. Sung. 1999. Enhanced cellular immunity to hepatitis C virus nonstructural proteins by codelivery of granulocyte macrophage-colony stimulating factor gene in intramuscular DNA immunization. Vaccine 17:1136–1144. [DOI] [PubMed] [Google Scholar]
- 6.Chow, Y. H., B. L. Chiang, Y. L. Lee, W. K. Chi, W. C. Lin, Y. T. Chen, and M. H. Tao. 1998. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J. Immunol. 160:1320–1329. [PubMed] [Google Scholar]
- 7.Collins, J. L., P. Q. Patek, and M. Cohn. 1981. Tumorigenicity and lysis by natural killers. J. Exp. Med. 153:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, H. L., C. L. Millan, and S. C. Watkins. 1997. Immune-mediated destruction of transfected muscle fibers after direct gene transfer with antigen-expressing plasmid DNA. Gene Ther. 4:181–188. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly, J. J., J. B. Ulmer, and M. A. Liu. 1998. DNA vaccines. Dev. Biol. Stand. 95:43–53. [PubMed] [Google Scholar]
- 10.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927–974. [DOI] [PubMed] [Google Scholar]
- 11.Hasan, U. A., A. M. Abai, D. R. Harper, B. W. Wren, and W. J. Morrow. 1999. Nucleic acid immunization: concepts and techniques associated with third generation vaccines. J. Immunol. Methods 229:1–22. [DOI] [PubMed] [Google Scholar]
- 12.Kato, H., H. Bukawa, E. Hagiwara, K. Q. Xin, K. Hamajima, S. Kawamoto, M. Sugiyama, M. Sugiyama, E. Noda, M. Nishizaki, and K. Okuda. 2000. Rectal and vaginal immunization with a macromolecular multicomponent peptide vaccine candidate for HIV-1 infection induces HIV-specific protective immune responses. Vaccine 18:1151–1160. [DOI] [PubMed] [Google Scholar]
- 13.Kim, J. J., K. A. Simbiri, J. I. Sin, K. Dang, J. Oh, T. Dentchev, D. Lee, L. K. Nottingham, A. A. Chalian, D. McCallus, R. Ciccarelli, M. G. Agadjanyan, and D. B. Weiner. 1999. Cytokine molecular adjuvants modulate immune responses induced by DNA vaccine constructs for HIV-1 and SIV. J. Interferon Cytokine Res. 19:77–84. [DOI] [PubMed] [Google Scholar]
- 14.Kusakabe, K., K. Q. Xin, H. Katoh, K. Sumino, E. Hagiwara, S. Kawamoto, K. Okuda, Y. Miyagi, I. Aoki, K. Nishioka, D. Klinman, and K. Okuda. 2000. The timing of GM-CSF expression plasmid administration influences the Th1/Th2 response induced by an HIV-1-specific DNA vaccine. J. Immunol. 164:3102–3111. [DOI] [PubMed] [Google Scholar]
- 15.Leitner, W. W., H. Ying, and N. P. Restifo. 1999. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine 18:765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard, J. P., M. L. Sherman, G. L. Fisher, L. J. Buchanan, G. Larsen, M. B. Atkins, J. A. Sosman, J. P. Dutcher, N. J. Vogelzang, and J. L. Ryan. 1997. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 90:2541–2548. [PubMed] [Google Scholar]
- 17.Letvin, N. L. 1998. Progress in the development of an HIV-1 vaccine. Science 280:1875–1880. [DOI] [PubMed] [Google Scholar]
- 18.Lynch, D. H. 1998. Induction of dendritic cells (DC) by Flt3 Ligand (FL) promotes the generation of tumor-specific immune responses in vivo. Crit. Rev. Immunol. 18:99–107. [DOI] [PubMed] [Google Scholar]
- 19.Lynch, D. H., A. Andreasen, E. Maraskovsky, J. Whitmore, R. E. Miller, and J. C. Schuh. 1997. Flt3 ligand induces tumor regression and antitumor immune responses in vivo. Nat. Med. 3:625–631. [DOI] [PubMed] [Google Scholar]
- 20.Maraskovsky, E., K. Brasel, M. Teepe, E. R. Roux, S. D. Lyman, K. Shortman, and H. J. McKenna. 1996. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 184:1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michel, F., A. Hoffenbach, P. Froussard, P. Langlade-Demoyen, M. Kaczorek, M. P. Kieny, and F. Plata. 1992. HIV-1 env, nef, and gag-specific T-cell immunity in mice: conserved epitopes in nef p27 and gag p25 proteins. AIDS Res. Hum. Retrovir. 8:469–478. [DOI] [PubMed] [Google Scholar]
- 22.Okada, E., S. Sasaki, N. Ishii, I. Aoki, T. Yasuda, K. Nishioka, J. Fukushima, J. Miyazaki, B. Wahren, and K. Okuda. 1997. Intranasal immunization of a DNA vaccine with IL-12- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigens. J. Immunol. 159:3638–3647. [PubMed] [Google Scholar]
- 23.Pasquini, S., Z. Xiang, Y. Wang, Z. He, H. Deng, M. Blaszczyk-Thurin, and H. C. Ertl. 1997. Cytokines and costimulatory molecules as genetic adjuvants. Immunol. Cell Biol. 75:397–401. [DOI] [PubMed] [Google Scholar]
- 24.Pulendran, B., J. L. Smith, G. Caspary, K. Brasel, D. Pettit, E. Maraskovsky, and C. R. Maliszewski. 1999. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA 96:1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulendran, B., J. L. Smith, M. Jenkins, M. Schoenborn, E. Maraskovsky, and C. R. Maliszewski. 1998. Prevention of peripheral tolerance by a dendritic cell growth factor: flt3 ligand as an adjuvant. J. Exp. Med. 188:2075–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Restifo, N. P., H. Ying, L. Hwang, and W. W. Leitner. 2000. The promise of nucleic acid vaccines. Gene Ther. 7:89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z.-Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605–609. [DOI] [PubMed] [Google Scholar]
- 29.Trinchieri, G. 1994. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 84:4008–4027. [PubMed] [Google Scholar]
- 30.Ulmer, J. B., J. J. Donnelly, S. E. Parker, G. H. Rhodes, P. L. Felgner, V. J. Dwarki, S. H. Gromkowski, R. R. Deck, C. M. DeWitt, and A. Friedman. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745–1749. [DOI] [PubMed] [Google Scholar]
- 31.Waldmann, T. A., and Y. Tagaya. 1999. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 17:19–49. [DOI] [PubMed] [Google Scholar]
- 32.Watts, A. M., and R. C. Kennedy. 1999. DNA vaccination strategies against infectious diseases. Int. J. Parasitol. 29:1149–1163. [DOI] [PubMed] [Google Scholar]
- 33.Xu, L., A. Sanchez, Z. Yang, S. R. Zaki, E. G. Nabel, S. T. Nichol, and G. J. Nabel. 1998. Immunization for Ebola virus infection. Nat. Med. 4:37–42. [DOI] [PubMed] [Google Scholar]
- 34.Yang, Z., R. Delgado, L. Xu, R. F. Todd, E. G. Nabel, A. Sanchez, and G. J. Nabel. 1998. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science 279:1034–1037. [DOI] [PubMed] [Google Scholar]