Abstract
Ty1 is the most successful of the five endogenous yeast retrotransposons. The life cycle of Ty1 dictates that a number of nucleocapsid (NC)-facilitated events occur although the protein(s) responsible for these events has not been identified. The positioning of the NC peptide is conserved at the carboxy terminus of the Gag protein among most long terminal repeat (LTR)-containing retroelements. An analogous region of Ty1 that simultaneously encodes part of Gag, protease (PR), and the C-terminal p4 peptide was mutagenized. Some of these mutations result in smaller-than-normal virus-like particles (VLPs). The mutants were also found to impair an NC-like functionality contained within the amino terminus of the protease that is distinct and separable from its proteolytic activity. Remarkably, these mutants have distinct defects in reverse transcription.
Ty1, the most abundant retrotransposon in the yeast Saccharomyces cerevisiae, is present at 20 to 35 copies per haploid genome (4, 6, 9). These genomic elements are actively transcribed such that Ty1 mRNA represents approximately 1% of total poly(A)+ RNA within the cell (11). Transcription of genomic Ty1 elements requires transcription factor Spt3p (37). Since the GAL1 promoter is active in spt3 null cells, Ty1 elements driven by the GAL1 promoter can be analyzed in a background free of endogenous element transcription (5).
Ty1 mRNA is translated into two proteins, Gag and the Gag-Pol polyprotein. The Gag protein is the major structural component of Ty1 virus-like particles (VLPs), replication intermediates in which reverse transcription occurs (13, 16, 25, 31). The Gag-Pol polyprotein contains the enzymatic components required for replication, including protease (PR), integrase (IN), and reverse transcriptase (RT). These enzymatic species are liberated from the Gag-Pol polyprotein by proteolytic processing. The element-encoded PR is responsible for proteolytic processing and is absolutely required for retrotransposition (1, 30, 41).
The stoichiometric ratio of Gag to Gag-Pol is determined by the efficiency of a 7-nucleotide (nt) frameshift signal contained within Ty1 mRNA. The frameshift is typically 5 to 10% efficient, which fixes the ratio of Gag to Gag-Pol at 10 to 20 to 1 (3). “Erasing” the frameshift signal by a single-nucleotide deletion blocks retrotransposition. Similarly, (i) altering expression levels of a single-copy tRNA gene that inhibits frameshifting or (ii) expression, in trans, of a protein known to inhibit +1 ribosomal frameshifting blocks retrotransposition (12, 18, 35, 38). Mutations that erase the endogenous frameshift signal can be suppressed in cis by introducing a new frameshift signal at an ectopic site (21).
All of the functions typically associated with a nucleocapsid (NC) functionality such as RNA packaging, positioning of primer tRNA, and efficient strand transfer and reverse transcription occur during the Ty1 retrotransposition process. However, these functions have yet to be ascribed to a specific protein(s). Reverse transcription of Ty1 is primed by the initiator methionine tRNAMeti (7). A basic region of Ty1 CA has recently been shown to facilitate this process in vitro, suggesting that the C-terminal basic region provides an NC-like function (8). Whether or not deletion of this region is compatible with Ty1 transposition in vivo remains to be determined. Unlike its retrovirus counterparts, Ty1 does not encode an NC peptide with a canonical zinc finger motif (15, 27). Indeed, it remains possible that some of the NC duties normally subserved by a single-element-encoded NC peptide in retroviruses are shared among host-encoded and Ty1 element-encoded protein(s).
NC protein coding regions can be readily identified in the gag genes of most retroviruses and retrotransposons, which contain one or two CX2CX4HX4C “Zn knuckle” motifs. The position of the NC coding sequence in the genome of long terminal repeat (LTR)-containing retroelements is evolutionarily conserved. The NC peptide is located at or near the carboxy terminus of Gag downstream of CA and upstream of the Pol coding region. The p4 coding region occupies the analogous position in the Ty1 genome, and we hypothesized that it might specify an NC functionality. However, p4 is downstream of the basic region near the C terminus of CA identified by Cristofari et al. (8).
Certain mutations in the p4 region of Gag and the amino terminus of PR block transposition (27). However, interpretation of the mutant phenotype is complicated by the fact that these mutations simultaneously alter Gag, PR, and the p4 peptide sequences. The transposition defect observed in these mutants could be secondary to loss or gain of function in any or all of these proteins. We demonstrated, through the application of a novel technique called frameshift transplantation, that these mutations specifically affect the Ty1 PR (21). While frameshift transplant mutants implicated the PR protein as being specifically affected, they did not offer any insight into the nature of the defect.
In this report we demonstrate that the amino terminus of the Ty1 PR serves an essential role in Ty1 replication that is separable and distinct from its proteolytic role. Mutations in this region give rise to a unique phenotype: a general failure in reverse transcription. Additionally, elements bearing these mutations produce smaller-than-normal VLPs (27). These findings support the assertion that the amino terminus of the Ty1 PR is required for at least one NC function: facilitating reverse transcription.
MATERIALS AND METHODS
Yeast strains and plasmids.
Yeast strain YH51 (MATa ura3-52 his4-539 lys2-801 spt3-202) was used for all experiments unless otherwise specified. Strain YH10 (MATa ura3-52 his4-539 lys2-801) was used in complementation studies. It is congenic to YH51, differing only at the SPT3 locus. Cells were grown on synthetic complete medium lacking Ura (SC−Ura). Transposition was induced by growth on medium containing 2% galactose.
Mutants s9 through s12 with linker insertion mutations in the p4 region were constructed via a three-piece ligation strategy with two PCR products and pJef1105 digested with HpaI and BstEII. The first PCR product was amplified using primer JB1993 (ATATGTCAGACCACCACCAAT) and primer 1 (Table 1). It was digested with HpaI and BamHI. The second product was amplified with primer 2 (Table 1) and primer JB1994 (TGTCCTGGAAGTGAAATTGTA). It was digested with BstEII and BamHI. d1, s7, and s8 mutations have already been described (27).
TABLE 1.
Primers used to construct PR mutants
| Mutant | Sequence of primera:
|
|
|---|---|---|
| 1 | 2 | |
| s9- | GCGCGGATCCTTCAGCAAGTTTCTGGCCTAAGATGAA | GCGCGGATCCGCCGCCCATACTAATCATTCTGATGAT |
| s10 | GCGCGGATCCGGCGGCATGATTAGTATGATTTACTGTAGA | GCGCGGATCCGGCGGCGGACACCTCCTTCTCGATTCA |
| s11 | GCGCGGATCCGGCAGGGAGTTCATCATCAGAATG | GCGCGGATCCGCCGCCGATTCAGGAGCATCACGAACC |
| s12 | GCGCGGATCCGGAATCGTTGTCCGTGCTGGG | GCGCGGATCCCATCTTAGGCCATGAACCGATTCAATTGAACAAT |
Primer 1 is used in conjunction with JB1993. Primer 2 is used with primer JB1994. BamHI sites contained within the primers are underlined.
VLP preparation.
VLPs were prepared via equilibrium density centrifugation on 20 to 70% linear sucrose gradients as described previously (13).
Immunoblotting.
Cells were grown overnight in 10 ml of SC−Ura-2% raffinose overnight. Transposition was induced by diluting the culture to an A600 of 0.6, adding galactose to a final concentration of 2%, and incubating the culture at 26°C for 24 h or as indicated. Yeast lysates were prepared from approximately 2 A600 units of cells in a mini-bead beater (Biospec Products) in 40 μl of 100% trichloroacetic acid (TCA) and 100 μl of acid-washed glass beads. The beads were pelleted and washed once with 1 ml of ice-cold 5% TCA and once with 1 ml of ice-cold water. The lysates were removed from the beads by incubation in 100 μl of 6% sodium dodecyl sulfate (SDS)-0.5 M Tris base at 55°C for 15 min. Five microliters of supernatant was used for immunoblotting.
After electrophoresis, proteins were transferred to Immobilon polyvinylidene difluoride membranes at 300 mA for 2 h. Membranes were blocked in 3% milk-0.1% Tween 20 in phosphate-buffered saline (PBS). Primary antibody R2-F (anti-VLP) was applied in blocking solution for 1 h at 1:10,000 dilution. Primary antibody 8B11 ascites fluid (anti-IN) was applied at 1:1,000 dilution. The blots were washed three times for 10 min in PBS-0.1% Tween 20 (PBS-T). The secondary antibody (horseradish peroxidase [HRP]-conjugated anti-rabbit antibody [used with R2-F] or HRP-conjugated anti-mouse antibody [used with 8B11]) (Amersham) was applied at a 1:7,500 dilution in 3% milk in PBS-T for 40 min. The membranes were washed as described above and developed with ECL (Amersham) in accordance with the manufacturer’s instructions.
cDNA synthesis assays.
Yeast cells harboring plasmids were grown for 24 h at 24°C in 10 ml of SC−Ura-2% raffinose. The cells were then diluted to an A600 of 0.6 and induced as described above. The cDNA synthesis is dependent on galactose induction (data not shown). Cells (0.5 ml) at an A600 of 2.0 were then pelleted and washed once in water. Nucleic acids were extracted from the cell pellet by agitation with glass beads in a solution consisting of 400 μl of DNA extraction buffer (0.5 M NaCl, 20 mM Tris-HCl [pH 7.5], 1 mM EDTA, 1% SDS), 200 μl of buffer-saturated phenol, and 200 μl of chloroform using a mini-bead beater (Biospec Products). The lysate was centrifuged for 10 min in an Eppendorf model 5415 microcentrifuge at 14,000 rpm. The supernatant was extracted with an equal volume of chloroform before the nucleic acids were precipitated with ethanol. Ten micrograms of total nucleic acid was digested with 20 U of EcoRI and 2 μg of RNase A in a 15-μl reaction mixture. Southern blotting was performed as described previously (2).
RT assays.
Endogenous and exogenous RT assays were performed essentially as described previously (16, 41).
RNA analysis.
VLPs were prepared as described previously (27). The three sucrose fractions with peak RT activity were pooled and centrifuged at 250,000 × g at 4°C for 1 h to prepare a concentrated VLP pellet. This pellet was resuspended in 300 μl of diethyl pyrocarbonate-treated water. The protein concentrations were determined, and 250 μg of VLPs was used as an RNA source. Anti-Gag immunoblots confirmed that the amounts of Gag protein in the particles were comparable. RNA was extracted by incubating 100 μl of VLPs with 50 μl of buffer-saturated phenol-50 μl of chloroform for 5 min at 65°C. The nucleic acids were ethanol precipitated with 2.5 M ammonium acetate, resuspended in load buffer, and resolved on a 1% agarose-formaldehyde gel.
For dot blots, the nucleic acids extracted as described above were treated with either DNase or RNase A and applied to a GeneScreen (NEN) membrane through a Schleicher and Schuell 96-well dot blot apparatus in 100 μl of water. Northern and dot hybridizations were performed as described previously (2).
Analysis of reverse transcription intermediates.
DNA was prepared from equivalent amounts of VLPs as described above. The resuspended nucleic acids were treated with 2 μg of RNase A for 1 h at 37°C. The reaction mixture was reextracted with phenol and chloroform and precipitated. The DNA was resuspended in 20 μl of water.
tRNA Northern blots.
Total nucleic acids from equivalent amounts of wild-type and mutant VLPs were prepared as described above. The nucleic acids were separated in a 10% polyacrylamide-urea gel at 650 V. The nucleic acids were then transferred to a GeneScreen (NEN) membrane in 1× MOPS (morpholinepropanesulfonic acid) buffer at 20 V for 4 h at 4°C. The nucleic acids were cross-linked to the membrane for 12 min at 125 W. The membrane was then baked in vacuo at 80 oC for 2 h. The membrane was prehybridized at 37oC in 10 ml of 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-1% SDS containing 100 μg of sheared herring sperm DNA/ml. 32P-end-labeled oligonucleotide JB384 (108 cpm; TTCCACTGCGCCACGGCGCT) was added and allowed to hybridize for 5 h. The blot was washed three times for 10 min in 6× SSC-1% SDS at 37°C, wrapped, and exposed to film.
Transposition assays.
Yeasts, grown as patches, were replica plated from SC−Ura-2% glucose to SC−Ura-2% galactose and grown at 26°C for 4 days. The patches were then replica plated to SC−Ura-2% glucose and grown overnight at 30°C. The patches were replica plated to yeast extract-peptone-dextrose (YPD) and, after overnight growth at 30°C, were replica plated to SC−0.1% 5-fluoroorotic acid. A portion of the patch was then diluted and plated on both YPD and YPD-75 μg of G418/ml. The transposition frequency of the wild-type element is defined as 100%.
RESULTS
Mutations in the p4 region inhibit transposition.
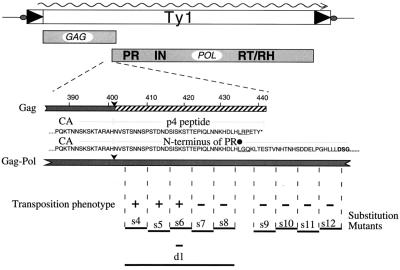
To define the essential role of the amino terminus of Ty1 PR, a series of block substitution mutants were constructed in this region (Fig. 1). The mutated sequences span the amino terminus of the PR up to, but not including, the active site. Some of the mutants (d1, s7, and s8) have been shown to cause a morphological change in Ty1 VLPs. Specifically, the VLPs produced by these mutant elements are smaller and migrate at a reduced rate through sucrose density gradients (27).
FIG. 1.
Schematic of the Ty1 Gag-Pol overlap region. The Gag and Gag-Pol polyproteins as well as the relevant amino acid sequences are shown. Black dot, site of +1 translational frameshifting; ∗, Gag sequence position corresponding to the stop codon. The amino acids encoded fully or in part by the frameshift are underlined. PR active-site residues are in boldface. Arrowheads, PR cleavage sites in Gag and Gag-Pol. The extent of the p4 peptide is indicated. Block substitution mutants are shown under the amino acid residues they replace with the sequence AAGSAA. The transpositional competence of elements bearing these mutations is indicated. The s7, s8, and d1 mutants that block transposition are collectively referred to as the PRdsd mutants.
Ty1 PR activity is essential for transposition, and transposition frequency is reduced over 100-fold in Ty1 elements bearing a mutation in or near the PR active site (Table 2) (41). Transposition frequency is reduced over 20-fold in block substitution and deletion mutants s7 through s12 and d1 (Table 2) (27).
TABLE 2.
Mutant phenotypes
| Ty1 element | Proteolytic processing | Transposition frequencya for:
|
|
|---|---|---|---|
| YH51 | YH10 | ||
| WTb | + | 100 | 100 |
| PR− | − | <1 | <1 |
| d1 | + | <1 | 7.6 |
| s7 | + | <1 | 9.4 |
| s8 | + | <1 | 9.1 |
| s9 | +/−c | <1 | 2.5 |
| s10 | +/− | <1 | 2.3 |
| s11 | +/− | <1 | 2.1 |
| s12 | − | <1 | 2.5 |
Transposition assays were performed and frequencies were measured as described in Materials and Methods. Strain YH10 is SPT3+ and expresses endogenous Ty1 elements. Strain YH51 is congenic to YH10 and is spt3. It does not express endogenous Ty1 elements.
WT, wild type.
+/−, partial processing phenotype.
Analysis of proteolytic processing.
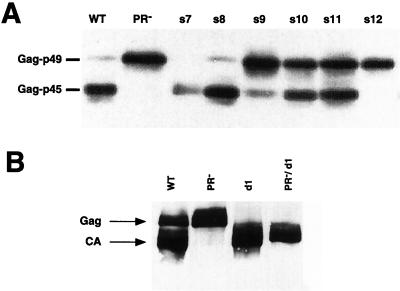
To explore the possibility that transposition is compromised in these mutants as a simple consequence of PR inactivation, we analyzed yeast lysates prepared from cells that were induced for transposition. The in vivo activity of the Ty1 PR can be conveniently assessed by determining the ratio of the Gag-p49 precursor (which has an electrophoretic mobility corresponding to a 58-kDa protein) to the mature form, Gag-p45 (CA), which migrates as a 54-kDa species.
As shown in Fig. 2A, proteolytic processing in the wild-type element results in the conversion of the majority of the Gag-p49 species to the CA species. Processing is completely impaired in the PR active-site mutant and in mutant s12, in which the linker substitution lies closest to the active-site sequence DSG. Processing is impaired to various degrees in mutants s9, s10, and s11. However, mutants s7, s8, and d1 show processing phenotypes that are indistinguishable from that observed in wild-type elements. Importantly, no Gag immunoreactivity is detected in a control strain containing an empty vector, which independently confirms that spt3 mutants do not express endogenous Ty1 Gag proteins (data not shown).
FIG. 2.
Some block substitution mutants process Ty1 Gag. Lysates were prepared from equal numbers of YH51 cells harboring the indicated plasmids after 24 h of galactose induction. An SDS-10% polyacrylamide gel was used to separate the proteins. The immunoblot was probed with polyclonal antibody R2-F, which recognizes Ty1 Gag. (A) Variable processing in a series of block substitutions in the amino terminus of PR. Note that the extent of processing in mutants s7 and s8 is similar to that for the wild type (WT). (B) Processing of Gag is observed in d1 mutants.
The transposition defect observed in mutants s9 through s12 may be explained in part by impaired processing. However, the severe transposition defects of mutants s7 and s8 (mutants s7, s8, and d1 are referred to collectively as the PRdsd mutants, for DNA synthesis defective) cannot be explained by a proteolysis defect because we have shown that they process Ty1 Gag and Gag-Pol normally (see below).
Because the d1 mutant lacks a majority of the p4 region that distinguishes Gag from CA, processing in it cannot be readily assessed by a conventional anti-Gag immunoblot assay. A doublet of bands, one of which comigrates with p45 and one of which migrates slower (by an amount equivalent to an ∼0.5-kDa increase in size), is typically seen in d1 lysates probed with an anti-Gag antibody. The p45.5 species could represent the precursor form of Gag in this d1 mutant; partial processing of the carboxy-terminal five amino acids would yield a mixture of CA and the uncleaved precursor species and account for the observed difference in electrophoretic mobilities. Ty1 Gag is also known to be phosphorylated (39). The doublet observed in d1 lysates could in theory also be the consequence of Gag phosphorylation; however we were unable to resolve the doublet into a singlet via treatment with a variety of phosphatases (J. F. Lawler, Jr., and J. D. Boeke, unpublished results). To demonstrate that this doublet results from partial proteolytic processing, we created a double mutant in which a PR− active-site mutation was created in the context of pJef1105-d1. This construct, pJL527 (pJef1105PR−/d1), produces only the p45.5 species. We therefore conclude that the doublet observed in d1 lysates is the result of partial proteolytic processing (Fig. 2B).
Proteolytic processing of the Gag-Pol polyprotein proceeds through an ordered (or semiordered) pathway with regard to cleavage site utilization (17, 26). The Gag-p4/Gag-PR site is cleaved first. This cleavage is required for subsequent processing of the PR-IN and/or IN-RT sites. The d1 mutant produces an IN species that comigrates with authentic IN produced by the wild-type element (27). The appearance of this mature IN species is evidence of Gag-Pol processing in the d1 mutant.
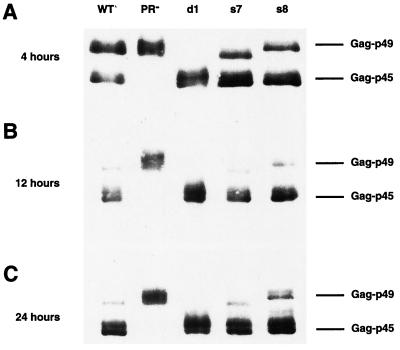
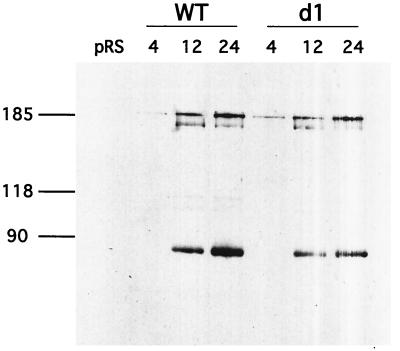
VLP assembly is a dynamic process and, as such, might be sensitive to minor variations in the time course of Gag and Gag-Pol processing (31). To determine whether the transposition defect observed in the PRdsd mutants is the consequence of an altered time course of PR activity, we prepared lysates from cultures at various time points after the onset of galactose induction. Anti-Gag immunoblots of these cultures are shown in Fig. 3A to C. No difference between the wild type and PRdsd mutants can be detected at any of the time points examined. If anything, in this experiment, the PRdsd mutants apparently process Gag faster than the wild type; however, this modest difference is not reproducible (data not shown). Similarly, the time course of processing at the PR-IN and IN-RT junctions did not vary between wild-type and d1 mutant elements (Fig. 4).
FIG. 3.
PRdsd mutants process Gag. Lysates were prepared from equal numbers of YH51 cells harboring the indicated Gal-Ty1 plasmids after 4 (A), 12 (B), or 24 (C) h of galactose induction. SDS-10% polyacrylamide gel electrophoresis was used to separate the proteins. The immunoblot was probed with polyclonal antibody R2-F, which recognizes Ty1 Gag. Fourfold more protein was loaded in the 4-h time point to facilitate detection.
FIG. 4.
d1 mutants competently process Gag-Pol. Lysates were prepared from equal numbers of YH51 cells harboring the indicated Gal-Ty1 plasmids after 4, 12, or 24 h of galactose induction. SDS-8% polyacrylamide gel electrophoresis was used to separate the proteins. The immunoblot was probed with monoclonal antibody 8B11, which recognizes an epitope between amino acids 310 and 435 in Ty1 IN (J. F. Lawler, Jr., and J. D. Boeke, unpublished observations; 13). WT, wild type.
We also determined that introduction of the s7 or s8 mutation does not lead to a change in site selection by the PR at the Gag-PR junction (21). Gag-PR is cleaved between the histidine (amino acid 401) and asparagine residues in the sequence TARAHNVSTS in the s7 and s8 mutants as well as the wild-type element (21). It therefore seems unlikely that a qualitative or quantitative difference in processing is responsible for the transposition defect observed in the PRdsd mutants. We note, however, that introduction of the s7 mutation causes a slight increase in the electrophoretic mobility of Gag-p49. This is also seen when an amino-terminal truncation of the s7 mutant Gag protein is expressed in Escherichia coli (J. F. Lawler, Jr., and J. D. Boeke, unpublished observations).
Galactose-induced overexpression of Ty1 PR active-site mutants is not complemented in trans by endogenous elements (10). To test whether the PRdsd mutants could be complemented in trans, we assayed transposition in congenic yeast strains differing only at the SPT3 locus. SPT3 encodes a transcription factor that is required for the expression of endogenous Ty1 elements but not Gal-Ty1 elements (5, 37).
In complementation assays, mutants s9 through s12 behave more like the PR active-site mutant (PR−) in that their transposition frequencies are markedly reduced (approximately 40- to 47-fold) in both SPT3 and spt3 backgrounds (Table 2). In contrast, mutants s7, s8, and d1 show more-significant residual levels of transposition in the SPT3 background (11- to 13-fold reduced from the wild-type level). This suggests that s7, s8, and d1 are complemented to a greater extent by endogenous elements than the PR− and s9 through s12 mutants and that the functionality they lack can be supplied in trans. In this type of system, it should be noted that the mutant elements are overexpressed and likely constitute the bulk of Ty1 proteins in the cell.
This provides a second piece of evidence that the PRdsd mutants cannot be grouped into the same phenotypic class as mutants with impaired processing and suggests a function separable from catalytic activity that can be complemented in trans. In contrast, other mutations in the Gag-PR cleavage site that modestly reduce processing efficiency have only very minor effects on transposition frequency (27). Therefore, the PRdsd mutants are a functionally distinct class of PR alleles that define a separate nonproteolytic activity of the Ty1 PR.
We have previously demonstrated that the amino terminus of the PR and the carboxy terminus of Gag are generated by a single autoproteolytic event (21, 27). The sequences affected by s7 and s8 mutations lie in a region where GAG and the portion of POL encoding PR overlap. We have also shown that s7 and s8 mutations exert their deleterious effect on transposition through some effect on the PR, as opposed to an effect on Gag. This was done by creating viable frameshift transplant mutants in which the sequences affected by s7 and s8 mutations were only translated as part of POL (21).
Effects on reverse transcription.
Experiments in which the frameshift was transplanted indicated that PR sequences (and not Gag-p49 or the C-terminal Gag-p4 peptide sequences) are the required p4 region sequences defined by mutants s7, s8, and d1. However, those studies did not shed light on the nature of the transposition defect observed in the PRdsd mutants. The Ty1 life cycle serves as a useful framework to begin to address this question. One requirement for successful retrotransposition is the synthesis of Ty1 cDNA from an mRNA template. We analyzed this stage of the Ty1 replication cycle in the PRdsd mutants by determining whether they are capable of cDNA synthesis.
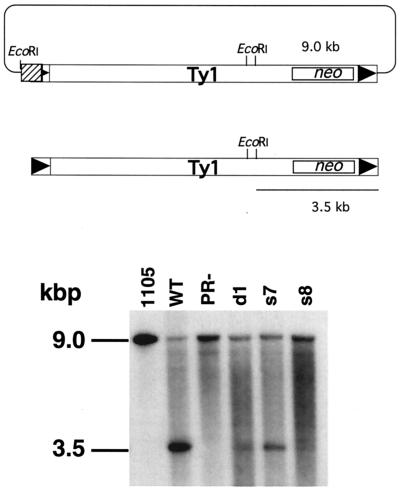
A cDNA synthesis assay developed for a distantly related transposon of fission yeast Schizosaccharomyces pombe was adapted for this purpose (23) (26). In this assay, marked Ty1-neo cDNA can be distinguished from the plasmid-borne copy by digesting total cellular DNA with EcoRI. The Ty1-neo donor plasmid (pJef1105) and Ty1-neo cDNA migrate at 9.0 and 3.5 kbp, respectively, in this assay. A neo probe was used to detect both species. The presence of the donor plasmid signal serves as an internal control for events such as plasmid loss or integration. The transcription factor encoded by the SPT3 gene is required for the synthesis of full-length Ty1 mRNA (5, 37). Therefore an spt3 mutant host strain was used to permit interpretation of the mutant phenotype in the absence of an endogenous wild-type Ty1 transcript.
Wild-type Ty1 elements synthesize approximately 4- to 65-fold more cDNA than the PR− and PRdsd mutants, which synthesize very little (Fig. 5). Note that the donor plasmid signal does not vary appreciably across the lanes. Mutants s7 and d1 reproducibly synthesize slightly more cDNA than s8 and PR− mutants, although they are all significantly impaired with respect to the wild type. This observation implies that mutants s7 and d1 are “leaky” with respect to cDNA synthesis compared to the other mutants. The marked reduction in the amount of cDNA synthesized by these mutants points to a defect in the Ty1 life cycle before completion of cDNA synthesis.
FIG. 5.
PRdsd and PR− mutants have a cDNA synthesis defect. (Top) Sizes of EcoRI fragments containing the neo gene in the donor plasmid (upper portion) and in Ty1-neo cDNA (lower portion). (Bottom) Total yeast nucleic acids from strain YH51 were digested with EcoRI, treated with RNase A, and separated on a 1% agarose gel. The nucleic acids were transferred, and the membrane was probed with 32P-labeled DNA generated from an XhoI-HindIII fragment of the Tn903 neo gene (boxed) via random hexamer labeling. The upper band (9.0 kbp) represents the Gal-Ty1-neo plasmid product. The lower band represents Ty1-neo cDNA. Equal amounts of DNA were loaded in all lanes. WT, wild type.
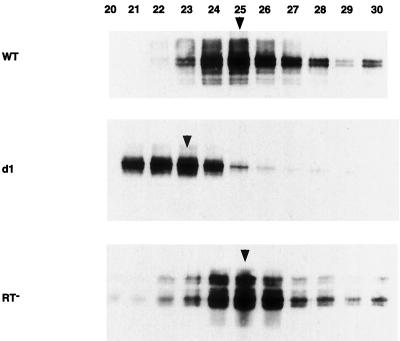
It is tempting to speculate that the smaller size and reduced migration of the PRdsd mutant particles are a direct consequence of their inability to synthesize cDNA. This is not the case however. Elements bearing inactivating point mutations in RT, which are incapable of any cDNA synthesis, form VLPs that comigrate with wild-type VLPs on sucrose density gradients (Fig. 6). Functionally inactivating mutations in the RNase H domain of RT also produce VLPs that migrate normally (J. F. Lawler, Jr., and J. D. Boeke, unpublished results).
FIG. 6.
VLP migration. Samples (2 μl) of each 1.2-ml sucrose gradient fraction were mixed with sample buffer and loaded in each lane. SDS-10% polyacrylamide gel electrophoresis was used to separate the proteins. The immunoblot was probed with polyclonal antibody R2-F, which recognizes Ty1 Gag. Arrowheads, fractions containing peak RT activity. The numbering of the gradient proceeds increasingly from the top (lightest) to the bottom (heaviest). WT, wild type.
RT activity on an exogenously supplied substrate is used to monitor VLP purification from sucrose gradients. We performed an immunoblot with an anti-Gag antibody on fractions with peak RT activity to compare RT activities of wild-type and mutant VLPs. This normalization step is necessary because VLP yield can vary with certain experimental factors such as cell lysis efficiency. As shown in Table 3, the wild-type and PRdsd mutant VLPs all possessed nearly equivalent amounts of RT activity when normalized to the amount of Gag immunoreactivity present in the particles. In contrast, the amount of cDNA produced was reduced 4- to 65-fold (Table 4).
TABLE 3.
RT activities
| Sample | RT activity
|
|
|---|---|---|
| Normalizeda | % of WTb | |
| WT | 2,577 | 100 |
| PR− | 2,518 | 97 |
| d1 | 2,242 | 87 |
| s7 | 2,554 | 99 |
| s8 | 2,155 | 83 |
Anti-Gag immunoblotting was performed on serial twofold dilutions of peak VLP fractions. A dilution factor was calculated based on the amount of Gag immunoreactivity in that fraction. Raw RT activity was normalized by multiplying by the dilution factor.
WT, wild type.
TABLE 4.
Fold decrease of primer RNA and reverse transcription products
The retrovirus NC functions, which include RNA packaging and facilitation of strand transfer, are clearly required for Ty1 replication (15, 19, 22, 42). Retrovirus NC peptides are typically encoded in the region between Gag and PR, which may suggest an evolutionary conservation of NC placement in this region. We next began to look for a defect in functions normally ascribed to NC proteins such as Ty1 mRNA packaging.
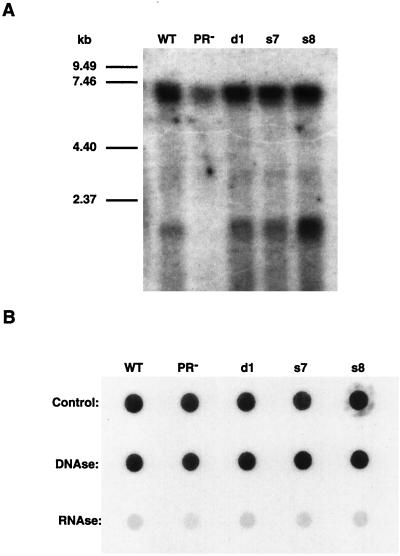
VLPs from wild-type and mutant elements were prepared, and their nucleic acids were extracted. Northern blots of the contents of these VLPs show a discrete, neo-hybridizing band corresponding to the marked Ty1-neo mRNA at approximately 6.5 kb and a second band at approximately 1.7 kb (Fig. 7A). The latter band likely corresponds to transcripts that initiate from within the neo gene that marks the Ty1 element. Consistent with previous reports, less Ty1 mRNA is observed in PR− particles (14, 41). In contrast, the PRdsd mutants encapsidated normal amounts of Ty1-neo mRNA. This observation can be taken as further evidence that the PRdsd mutants, although affecting the PR molecule, do not behave as simple PR active-site mutants.
FIG. 7.
(A) RNA blot of VLP-extracted nucleic acids. The amount of VLP sample used was normalized via anti-Gag immunoblots. The nucleic acids were separated on a 1% agarose-formaldehyde gel, transferred to a GeneScreen membrane, and probed as for Fig. 4. (B) Dot blot of VLP-extracted nucleic acids. Nucleic acids from the same extraction were divided into three pools and treated with DNase or RNase A or were not treated and were probed as described above. WT, wild type.
Various cellular messages have been detected in Ty1 VLPs (J. F. Lawler, Jr., and J. D. Boeke, unpublished data; 40). The unexpected absence of the 1.7-kb neo band in the PR− lane might suggest some reduction in this mutant’s ability to nonspecifically package certain non-Ty1 mRNAs (14, 40, 41). These neo transcripts are sensitive to RNase A treatment. Dot blots of nucleic acids extracted from VLPs also show that most of the neo-hybridizing material is sensitive to RNase A treatment but not DNase treatment (Fig. 7b).
A specific defect in reverse transcription.
The observation that the PRdsd mutant VLPs package normal amounts of Ty1 mRNA implies a defect during the reverse transcription process. The first step in the reverse transcription process is minus-strand strong-stop cDNA synthesis. In this step, tRNAMeti is bound to the primer binding site within Ty1 mRNA and serves to prime reverse transcription. It has recently been demonstrated, using T7 transcripts, that the primer tRNA-Ty1 RNA annealing process can be facilitated in vitro by a basic domain in the carboxy terminus of CA (8). The general reverse transcription defect that we observed in our mutants could, in theory, be due to a specific defect in minus-strand strong-stop cDNA synthesis or in its transfer. We designed an experiment to distinguish between these two possibilities.
Ty1 RT synthesizes a cDNA strand complementary to the first 91 nt of the Ty1 mRNA (7). This DNA/RNA hybrid is digested by a Ty1-encoded RNase H activity, which permits the minus-strand strong-stop cDNA to be transferred to the 3′ LTR of a Ty1 message and continue minus-strand synthesis (28, 29). Various reverse transcription intermediates have been detected in Ty1 VLPs (20, 32).
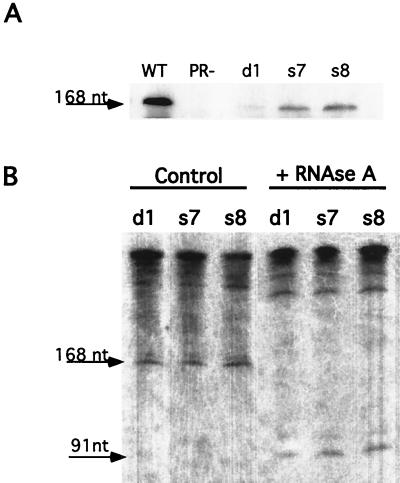
VLPs from wild-type, PR−, and PRdsd mutant cells were purified and assayed for minus-strand strong-stop cDNA. As shown in Fig. 8A, when equal amounts of VLPs were analyzed, there was significantly more minus-strand strong-stop cDNA in wild-type particles than in the PRdsd mutant particles. These results were quantified (Table 4).
FIG. 8.
(A) DNA blot of VLP-extracted nucleic acids. This blot was transferred to a GeneScreen membrane and subsequently probed with a random-primed 32P-labeled probe corresponding to minus-strand strong-stop cDNA. These samples were normalized to the Gag content of the VLPs. WT, wild type. (B) Blot of VLP-extracted nucleic acids that were either treated with RNase A or not treated. The corresponding shift in electrophoretic mobility is consistent with the presence of a covalently bound tRNAMeti. These samples were normalized to A260.
As expected from the transposition frequency, the d1 mutant had the “tightest” phenotype in terms of amount of strong-stop DNA. Interestingly, PR− VLPs contained no detectable minus-strand strong-stop cDNA, consistent with their inability to incorporate label in endogenous reverse transcription reactions (41). This observation extends the recent finding that PR− VLPs do not possess dimeric RNA whereas wild-type VLPs do and suggests that the dimers might be required for efficient primer tRNA packaging and strong-stop DNA synthesis (14). The initial minus-strand strong-stop cDNA product contains a covalently bound tRNAMeti primer that is removed at a later step in the reverse transcription process. Relatively little of this product exists within PRdsd mutant VLPs. The product detected in both wild-type and mutant particles is 168 nt long and can be shifted to 91 nt long following treatment with RNase A. Thus no qualitative difference between the strong-stop DNAs produced in wild-type and mutant VLPs was detected (Fig. 8B).
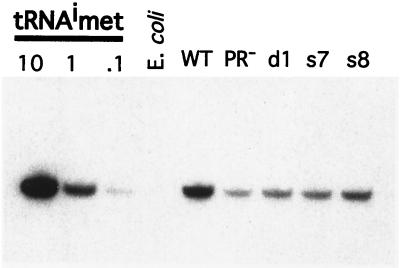
Thus there is a clear defect in reverse transcript abundance in PRdsd mutants. However, a formal possibility is that the PRdsd mutant particles do not package sufficient primer tRNA. To explore this possibility, we performed RNA blotting to quantitate the amount of tRNAMeti present in the wild-type and mutant particles. Wild-type particles contain about twofold more tRNAMeti than PRdsd mutant particles (Fig. 9; Table 4). The PR− mutant has even less tRNAMeti than the PRdsd mutants. However, it also packages less Ty1 mRNA. Indeed, a comparison of the signal intensities shows that there is twofold more tRNAMeti signal in the wild-type particles than in the PRdsd mutants. Unlike what is observed in minus-strand strong-stop synthesis, where the d1 mutant phenotype is the most severe and the s8 mutant is the least severe, there is no apparent difference in tRNAMeti levels between the various PRdsd mutants.
FIG. 9.
Mutant elements package tRNAMeti. Total nucleic acids were prepared from equal amounts of Ty1 VLPs and separated on a 10% polyacrylamide-urea gel. The amount of VLP sample used was normalized via anti-Gag immunoblots. A standard curve of purified yeast tRNAMeti was also resolved on this gel. The gel was exposed overnight with an intensifying screen.
DISCUSSION
Genome organization in retroviruses and retrotransposons is evolutionarily conserved and NC (identified by its Zn knuckle motif) is always found at the carboxy terminus of the Gag protein. We have previously shown that a function essential for successful Ty1 replication is contained within the region common to the C terminus of Gag, PR, and the p4 peptide. This region, which we refer to as the p4 region, was analyzed via a series of block substitution mutations to determine whether an essential Ty1 NC functionality resides there. Mutations in the carboxy terminal portion of this region block transposition, whereas mutations residing in the amino-terminal portion do not. We have previously demonstrated, through the use of a frameshift transplant technique, that these mutations cause a transposition defect via an effect they exert on the Ty1 PR (21).
The most trivial explanation for the transposition defect seen in these mutants is that they inactivate the Ty1 PR, which is required for transposition. However, we are unable to detect any alterations in proteolytic processing in the mutant elements. Samples harvested at various points after the induction of transposition revealed no differences in the kinetics of processing. Samples harvested after 24 h of induction, a time point by which Ty1 protein levels have likely reached equilibrium, indicate no defect in the extent of processing.
Ty1 elements containing mutations in or near the Gag-PR cleavage site have been described (27). One of these mutants, s3.4, contains an isoleucine substitution at position +1 relative to the His-Asn scissile bond and exhibits partially impaired processing of Gag but transposes with only a very modest reduction in efficiency. The existence this class of mutant implies that, even if a kinetic defect in PR activity that is below our threshold of detection exists, it is unlikely to impair transposition.
The PRdsd mutants are partially complemented by endogenous elements whereas mutations in or near the PR active site are not. We therefore conclude that this essential “activity” of PR is distinct and separable from its proteolytic activity.
The reduced size of PRdsd mutant particles that was previously reported and confirmed in this study may suggest a defect in VLP maturation (27). It is clear from comparisons between wild-type, PRdsd mutant, and RT− particles that the synthesis of full-length Ty1 cDNA is not required for normal particle morphology.
PRdsd mutant elements have a significant defect in synthesis of cDNA but package Ty1 mRNA in normal quantities, pointing to defects in reverse transcription. This is another aspect of the PRdsd mutants that distinguishes them from PR− mutants, which package far less Ty1 mRNA than wild-type elements. An analysis of reverse transcription intermediates indicates that these mutants fail to synthesize normal amounts of cDNA products despite the presence of nearly normal amounts of primer tRNAMeti. The d1 mutant appears to be defective in initiating reverse transcription, whereas s7 and s8 appear defective in transfer or extension of the strong-stop DNA (Table 4).
There are a number of potential explanations consistent with these data. First, it has recently been shown that Ty1 mRNA is dimeric in wild-type particles and monomeric in PR− particles (14). It is possible that the Ty1 mRNA in PRdsd mutant particles, although normal in abundance, remains monomeric despite encoding a functional PR. Monomeric Ty1 mRNA may not be a suitable substrate for Ty1 RT or may not anneal properly with its primer tRNAMeti.
This explanation raises the larger question of why this might be so. One explanation is that normal particle morphology may be required for proper mRNA folding and primer tRNAMeti annealing in vivo. Experiments designed to test this hypothesis were attempted by supplying PRdsd mutant VLPs with an exogenous oligonucleotide to prime reverse transcription (28, 29). We were never able to detect synthesis of minus-strand strong-stop cDNA by PRdsd mutant elements in these “in viro” assays (data not shown).
It has recently been shown that a 103-amino-acid carboxy-terminal fragment of Ty1 CA from amino acids 299 to 401 possesses primer tRNA annealing activity and can initiate the Ty1 reverse transcription reaction in vitro (8). It is possible that the PRdsd mutations, by altering either the kinetics or spatial parameters of particle assembly, prevent this part of the protein from accessing its substrates. This hypothesis is consistent with the observation that PRdsd mutants are partially complemented in trans. Alternatively, normal particle morphology may be required for Ty1 RT to be appropriately positioned. In the absence of appropriate positioning, Ty1 RT may be unable to extend an existing primer template. Previous work (27) has shown, and this work confirms, that PRdsd mutant VLPs are smaller than wild-type VLPs.
The observation that an NC function is contained within a viral PR is precedented, although not for retroelements (24, 33). Some plant viruses, including potyviruses, also encode NC functions within their PR gene open reading frame (34). In one case, it has been shown that this NC functionality contained within the PR is essential for systemic movement of viral RNA (36). The inclusion of an NC functionality in the context of an active PR is likely a genome-streamlining strategy that enables the virus and element to maintain a smaller genome. It is not clear whether the NC function assigned to the p4 region in this work is direct or indirect.
In conclusion, we have shown that the N terminus of the Ty1 PR plays an essential role in facilitating reverse transcription. This activity is distinct and separable from its proteolytic activity and can be partially complemented in trans.
Acknowledgments
We thank Gerard Keith for providing a sample of purified tRNAMeti and Abram Gabriel for providing plasmids and for critical reading of the manuscript. We thank Jill Keeney for insightful suggestions regarding tRNA detection methods. We thank members of the Boeke lab for helpful suggestions.
This work was supported by NIH grant GM 36481 to J.D.B. and Medical Scientist Training Grant GM-07309 to J.F.L.
References.
- 1.Adams, S. E., J. Mellor, K. Gull, R. B. Sim, M. F. Tuite, S. M. Kingsman, and A. J. Kingsman. 1987. The functions and relationships of Ty-VLP proteins in yeast reflect those of mammalian retroviral proteins. Cell 49:111–119. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1988. Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, New York, N.Y.
- 3.Belcourt, M. F., and P. J. Farabaugh. 1990. Ribosomal frameshifting in the yeast retrotransposon Ty: tRNAs induce slippage on a 7 nucleotide minimal site. Cell 62:339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeke, J. D., D. Eichinger, D. Castrillon, and G. R. Fink. 1988. The Saccharomyces cerevisiae genome contains functional and nonfunctional copies of transposon Ty1. Mol. Cell. Biol. 8:1432–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeke, J. D., C. A. Styles, and G. R. Fink. 1986. Saccharomyces cerevisiae SPT3 gene is required for transposition and transpositional recombination of chromosomal Ty elements. Mol. Cell. Biol. 6:3575–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron, R. R., E. Y. Loh, and R. W. Davis. 1979. Evidence for transposition of dispersed repetitive DNA families in yeast. Cell 16:739–751. [DOI] [PubMed] [Google Scholar]
- 7.Chapman, K. B., A. S. Bystrom, and J. D. Boeke. 1992. Initiator methionine tRNA is essential for Ty1 transposition in yeast. Proc. Natl. Acad. Sci. USA 89:3236–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cristofari, G., D. Ficheux, and J.-L. Darlix. 2000. The Gag-like protein of the yeast Ty1 retrotransposon contains a nucleic acid chaperone domain analogous to retroviral nucleocapsid proteins. J. Biol. Chem. 275:19210–19217. [DOI] [PubMed] [Google Scholar]
- 9.Curcio, M. J., and D. J. Garfinkel. 1994. Heterogeneous functional Ty1 elements are abundant in the Saccharomyces cerevisiae genome. Genetics 136:1245–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curcio, M. J., and D. J. Garfinkel. 1992. Posttranslational control of Ty1 retrotransposition occurs at the level of protein processing. Mol. Cell. Biol. 12:2813–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curcio, M. J., A. M. Hedge, J. D. Boeke, and D. J. Garfinkel. 1990. Ty RNA levels determine the spectrum of retrotransposition events that activate gene expression in Saccharomyces cerevisiae. Mol. Gen. Genet. 220:213–221. [DOI] [PubMed] [Google Scholar]
- 12.Dinman, J. D., and R. B. Wickner. 1992. Ribosomal frameshifting efficiency and gag/gag-pol ratio are critical for yeast M1 double-stranded RNA virus propagation. J. Virol. 66:3669–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eichinger, D. J., and J. D. Boeke. 1988. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: cell-free Ty1 transposition. Cell 54:955–966. [DOI] [PubMed] [Google Scholar]
- 14.Feng, Y. X., S. P. Moore, D. J. Garfinkel, and A. Rein. 2000. The genomic RNA in Ty1 virus-like particles is dimeric. J. Virol. 74:10819–10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabus, C., D. Ficheux, M. Rau, G. Keith, S. Sandmeyer, and J. L. Darlix. 1998. The yeast Ty3 retrotransposon contains a 5′-3′ bipartite primer-binding site and encodes nucleocapsid protein NCp9 functionally homologous to HIV-1 NCp7. EMBO J. 17:4873–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garfinkel, D. J., J. D. Boeke, and G. R. Fink. 1985. Ty element transposition: reverse transcriptase and virus-like particles. Cell 42:507–517. [DOI] [PubMed] [Google Scholar]
- 17.Garfinkel, D. J., A.-M. Hedge, S. D. Youngren, and T. D. Copeland. 1991. Proteolytic processing of pol-TYB proteins from the yeast retrotransposon Ty1. J. Virol. 65:4573–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakami, K., S. Pande, B. Faiola, D. P. Moore, J. D. Boeke, P. J. Farabaugh, J. N. Strathern, Y. Nakamura, and D. J. Garfinkel. 1993. A rare tRNA-Arg(CCU) that regulates Ty1 element ribosomal frameshifting is essential for Ty1 retrotransposition in Saccharomyces cerevisiae. Genetics 135:309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keeney, J. B., K. B. Chapman, V. Lauermann, D. F. Voytas, S. U. Astrom, U. von Pawel-Rammingen, A. Bystrom, and J. D. Boeke. 1995. Multiple molecular determinants for retrotransposition in a primer tRNA. Mol. Cell. Biol. 15:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauermann, V., K. Nam, J. Trambley, and J. D. Boeke. 1995. Plus-strand strong-stop DNA synthesis in retrotransposon Ty1. J. Virol. 69:7845–7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawler, J. F., G. V. Merkulov, and J. D. Boeke. 2001. Frameshift signal transplantation and the unambiguous analysis of mutations in the yeast retrotransposon Ty1 Gag/Pol overlap region. J. Virol. 75:6769–6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lener, D., V. Tanchou, B. P. Roques, S. F. Le Grice, and J. L. Darlix. 1998. Involvement of HIV-I nucleocapsid protein in the recruitment of reverse transcriptase into nucleoprotein complexes formed in vitro. J. Biol. Chem. 273:33781–33786. [DOI] [PubMed] [Google Scholar]
- 23.Levin, H. L. 1995. A novel mechanism of self-primed reverse transcription defines a new family of retroelements. Mol. Cell. Biol. 15:3310–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maia, I. G., and F. Bernardi. 1996. Nucleic acid-binding properties of a bacterially expressed potato virus Y helper component-proteinase. J. Gen Virol. 77:869–877. [DOI] [PubMed] [Google Scholar]
- 25.Mellor, J., S. M. Fulton, M. J. Dobson, W. Wilson, S. M. Kingsman, and A. J. Kingsman. 1985. A retrovirus-like strategy for expression of a fusion protein encoded by yeast transposon Ty1. Nature 313:243–246. [DOI] [PubMed] [Google Scholar]
- 26.Merkulov, G. V., J. F. Lawler, Y. Eby, and J. D. Boeke. 2001. Ty1 proteolytic cleavage sites are required for retrotransposition: all sites are not created equal. J. Virol. 75:638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merkulov, G. V., K. M. Swiderek, C. B. Brachmann, and J. D. Boeke. 1996. A critical proteolytic cleavage site near the C terminus of the yeast retrotransposon Ty1 Gag protein. J. Virol. 70:5548–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mules, E. H., O. Uzun, and A. Gabriel. 1998. In vivo Ty1 reverse transcription can generate replication intermediates with untidy ends. J. Virol. 72:6490–6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mules, E. H., O. Uzun, and A. Gabriel. 1998. Replication errors during in vivo Ty1 transposition are linked to heterogeneous RNase H cleavage sites. Mol. Cell. Biol. 18:1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller, F., K. H. Bruhl, K. Freidel, K. V. Kowallik, and M. Ciriacy. 1987. Processing of TY1 proteins and formation of Ty1 virus-like particles in Saccharomyces cerevisiae. Mol. Gen. Genet. 207:421–429. [DOI] [PubMed] [Google Scholar]
- 31.Palmer, K. J., W. Tichelaar, N. Myers, N. R. Burns, S. J. Butcher, A. J. Kingsman, S. D. Fuller, and H. R. Saibil. 1997. Cryo-electron microscopy structure of yeast Ty retrotransposon virus-like particles. J. Virol. 71:6863–6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pochart, P., B. Agoutin, S. Rousette, R. Chanet, V. Doroszkiewicz, and T. Heyman. 1993. Biochemical and electron microscope analyses of the DNA reverse transcripts present in the virus-like particles of the yeast transposon Ty1. Identification of a second origin of Ty1 DNA plus strand synthesis. Nucleic Acids Res. 21:3513–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rojas, M. R., F. M. Zerbini, R. F. Allison, R. L. Gilbertson, and W. J. Lucas. 1997. Capsid protein and helper component-proteinase function as potyvirus cell-to-cell movement proteins. Virology 237:283–295. [DOI] [PubMed] [Google Scholar]
- 34.Scheid, O. M. 1999. Plant viruses. New tool for Swiss army knife. Nature 397:25. [DOI] [PubMed] [Google Scholar]
- 35.Tumer, N. E., B. A. Parikh, P. Li, and J. D. Dinman. 1998. The pokeweed antiviral protein specifically inhibits Ty1-directed +1 ribosomal frameshifting and retrotransposition in Saccharomyces cerevisiae. J. Virol. 72:1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verchot, J., and J. C. Carrington. 1995. Evidence that the potyvirus P1 proteinase functions in trans as an accessory factor for genome amplification. J. Virol. 69:3668–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winston, F., K. J. Durbin, and G. R. Fink. 1984. The SPT3 gene is required for normal transcription of Ty elements in S. cerevisiae. Cell 39:675–682. [DOI] [PubMed] [Google Scholar]
- 38.Xu, H., and J. D. Boeke. 1990. Host genes that influence transposition in yeast: the abundance of a rare tRNA regulates Ty1 transposition frequency. Proc. Natl. Acad. Sci. USA 87:8360–8364. (Erratum, 88:2612, 1991.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu, H., and J. D. Boeke. 1991. Inhibition of Ty1 transposition by mating pheromones in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:2736–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu, H., and J. D. Boeke. 1990. Localization of sequences required in cis for yeast Ty1 element transposition near the long terminal repeats: analysis of mini-Ty1 elements. Mol. Cell. Biol. 10:2695–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Youngren, S. D., J. D. Boeke, N. J. Sanders, and D. J. Garfinkel. 1988. Functional organization of the retrotransposon Ty from Saccharomyces cerevisiae: Ty protease is required for transposition. Mol. Cell. Biol. 8:1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zapp, M. L., and M. R. Green. 1989. Sequence-specific RNA binding by the HIV-1 Rev protein. Nature 342:714–716. [DOI] [PubMed] [Google Scholar]