Abstract
The H5N1 influenza virus, which killed humans and poultry in 1997, was a reassortant that possibly arose in one type of domestic poultry present in the live-poultry markets of Hong Kong. Given that all the precursors of H5N1/97 are still circulating in poultry in southern China, the reassortment event that generated H5N1 could be repeated. Because A/goose/Guangdong/1/96-like (H5N1; Go/Gd) viruses are the proposed donors of the hemagglutinin gene of the H5N1 virus, we investigated the continued circulation, host range, and transmissibility of Go/Gd-like viruses in poultry. The Go/Gd-like viruses caused weight loss and death in some mice inoculated with high virus doses. Transmission of Go/Gd-like H5N1 viruses to geese by contact with infected geese resulted in infection of all birds but limited signs of overt disease. In contrast, oral inoculation with high doses of Go/Gd-like viruses resulted in the deaths of up to 50% of infected geese. Transmission from infected geese to chickens occurred only by fecal contact, whereas transmission to quail occurred by either aerosol or fecal spread. This difference is probably explained by the higher susceptibility of quail to Go/Gd-like virus. The high degree of susceptibility of quail to Go/Gd (H5N1)-like viruses and the continued circulation of H6N1 and H9N2 viruses in quail support the hypothesis that quail were the host of origin of the H5N1/97 virus. The ease of transmission of Go/Gd (H5N1)-like viruses to land-based birds, especially quail, supports the wisdom of separating aquatic and land-based poultry in the markets in Hong Kong and the need for continued surveillance in the field and live-bird markets in which different types of poultry are in contact with one another.
While the present study was in the review process, H5N1 reappeared in the land-based-poultry markets in Hong Kong. The present study provides information on the molecular and biological properties of A/goose/Guangdong/1/96-like (H5N1) viruses (abbreviated Go/Gd), which provided the hemagglutinin (HA) and neuraminidase (NA) genes for the 2001 viruses but does not deal with the reassortant H5N1 viruses that resulted in the slaughter of all live poultry in Hong Kong Special Administrative Region (SAR) in May 2001.
The H5N1 influenza virus that spread directly from poultry to humans in Hong Kong in 1997 caused the deaths of 6 of 18 persons diagnosed with infection with this virus (8, 31). This virus was proposed to be a naturally occurring avian reassortant (8, 24). The HA gene of the human A/Hong Kong/156/97 (H5N1; [abbreviated H5N1/97]) virus is highly homologous with that of the A/goose/Guangdong/1/96 (H5N1) virus (24, 30), whereas the replicative complex of H5N1/97 is highly homologous with that of the A/quail/Hong Kong/G1/97 (H9N2) virus (10) and with that of the A/teal/Hong Kong/W312/97 (H6N1) (11) virus. A/teal/Hong Kong/W312/97 and H5N1/97 contain NAs that are highly homologous with each other (11).
The HA of the putative H5N1/97 reassortant most probably originated from a Go/Gd-like virus, for the HAs differ by only eight amino acids. The HAs of both viruses possessed the same series of basic amino acids at the HA cleavage site (RERRRKKR) (30). The remainder of the Go/Gd genome, including the NA gene, was distinct from those of H5N1/97-like viruses and was most homologous to those of avian influenza viruses from either North America or Asia (30).
Outbreaks of highly pathogenic H5N1 influenza virus infection in geese in the southern Chinese province of Guangdong, which is adjacent to the Hong Kong SAR, were first reported in 1996; during the summer of 1996 as many as 40% of the geese on a goose farm died (26). H5N1 influenza viruses that are genetically indistinguishable from the Go/Gd isolates were found in the stalls that housed geese in Hong Kong in 1999 (7). These viruses were designated A/environment/Hong Kong/437/99 (H5N1) and were highly pathogenic in chickens; these findings demonstrate that precursor viruses that contributed the HA gene to H5N1/97-like viruses continued to circulate in southern China in 1999.
It is unusual for influenza viruses, including highly pathogenic avian influenza viruses, to cause disease in aquatic birds such as ducks (1). Less is known about their pathogenicity in geese. Three different H7N7 influenza viruses that were isolated from geese were nonpathogenic in inoculated geese but were highly pathogenic in chickens (17). Therefore, the isolation of H5N1 influenza viruses from geese raises the questions of whether these viruses are pathogenic in geese under experimental conditions and whether the clinical features of disease seen under field conditions are related to virus pathogenicity or to mixed infection with other agents. Another issue is the potential of these H5N1 viruses to spread to other domestic avian poultry including chickens and quail, which could potentially be infected with the H6N1 and H9N2 viruses that were precursors of the H5N1/97-like viruses.
Because the precursors of the H5N1/97-like viruses are still circulating in southeastern China, a repetition of the reassortment event that generated the H5N1/97-like virus is still possible. The host range of Go/Gd-like viruses in Hong Kong poultry and the possible hosts that could support such a reassortant event remain unknown, although such information about the H9N2 virus is available (9). The purpose of the present study is to identify the molecular and biological properties of Go/Gd-like viruses in poultry in the Hong Kong markets.
MATERIALS AND METHODS
Housing and monitoring of geese in Hong Kong markets.
Poultry in the Hong Kong live markets in December 1997 were slaughtered to eradicate H5N1/97-like viruses. Afterward, the markets were cleaned and the policy regarding holding birds was changed (9, 21). Aquatic birds, including ducks and geese, are now imported to a separate facility in which they are slaughtered, and the chilled carcasses are sold through retail outlets. Live aquatic poultry are not available in the live-poultry markets. In contrast terrestrial poultry continue to be sold live in the retail markets of Hong Kong. All consignments of geese travel by sea from farms in Guangdong Province directly to the Western wholesale market in Hong Kong. The boat trip takes 6 to 7 h. However, some consignments are assembled at the departure point in China for several hours before they are shipped.
Thirteen serum samples are randomly collected from each consignment of geese and ducks imported into Hong Kong and are subjected to HA inhibition (HI) tests to detect antibodies to H5 influenza virus. In addition, cloacal swabs from the birds whose sera are sampled are grouped into four pools and are tested for the presence of hemagglutinating viruses by culturing in 10- or 11-day-old chicken embryos. When a consignment contains bird(s) whose serum contains HI antibodies, swabs of viscera (10 pools of three swabs each) are randomly collected from 30 additional birds or from sick birds. In some instances, additional environmental swabs (e.g., swabs of the cage) are also collected. The cloacal, fecal, and environmental samples are treated with antibiotics as described previously (9) and used to inoculate chicken embryos to isolate influenza viruses (22). Throughout this paper, we refer to the H5N1 virus isolates that were designated A/environment/Hong Kong/437/99 (7) as goose isolates, because they were obtained from goose holding pens that are extensively cleaned between shipments.
Serologic tests.
Antisera specific for the isolated HA and NA antigens of the reference strains of influenza A viruses were prepared in goats (28). Monoclonal antibodies to A/chicken/Pennsylvania/1370/83 (H5N2) were prepared as described previously (13). HA titrations and HI tests were performed in microtiter plates with sera that had been treated with a receptor-destroying enzyme (15). NA titrations and NA inhibition tests were done according to the procedure of Aymard-Henry et al. (4).
Infection studies of poultry.
The viruses used were grown in embryonated chicken eggs, and aliquots of the virus were stored at −70°C (see tables and Fig. 2 for details). The 50% egg infectious doses (EID50) were determined from samples of groups of three infected eggs at days 3 and 5 after infection.
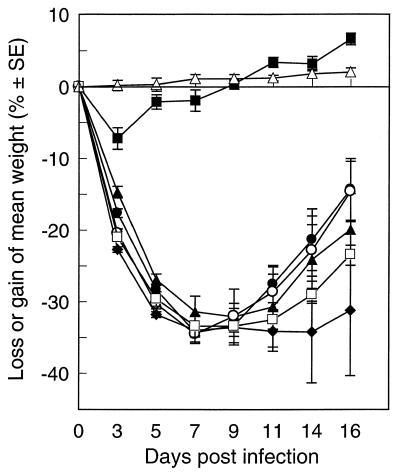
FIG. 2.
Changes in the weights of mice infected with goose H5N1 influenza viruses. BALB/c mice were infected with Gs/HK/437-4/99 (⧫), Gs/HK/437-6/99 (•), Gs/HK/437-8/99 (▴), Gs/HK/437-10/99 (•), Gs/HK/485-3/00 (○), and Gs/HK/485-5/00 (□). Untreated animals (▵) received PBS. The weights of the mice were measured on days 0, 3, 5, 7, 9, 11, 14, and 16 after infection. Each value is the percent change in the initial mean starting weight on day 0. The standard errors (SE) are given.
Chinese white geese, 3 to 4 weeks old, were inoculated with virus in a volume of 1.0 ml via tracheal and oral routes and via the nares and eyes (see Table 3 for doses). Virus infectivity titers were determined from samples of brain, heart, lung, liver, spleen, kidney, and intestine from animals that died of infection or that were very sick and were euthanized. In addition, tracheal and cloacal samples were collected at different intervals, and influenza viruses isolated from these samples were titrated in embryonated eggs.
TABLE 3.
Transmissibility and pathogenicity of A/Gs/HK/99 (H5N1) viruses in geese
| Virus strain | Method of transmission | No. of birds shedding/total no. of birds on day after infection:
|
Sick/dead/totalb | |||||
|---|---|---|---|---|---|---|---|---|
| 3
|
5
|
7
|
||||||
| Trachea | Cloaca | Trachea | Cloaca | Trachea | Cloaca | |||
| Gs/HK/437-4/99 | Inoculationa | 3/3 | 3/3 | 3/3 | 3/3 | 1/1 | 1/1 | 3/3/3 (7–8) |
| Contact | 1/2 | 2/2 | 2/2 | 2/2 | 0/1 | 0/1 | 2/1/2 (7) | |
| Gs/HK/437-6/99 | Inoculation | 3/3 | 3/3 | 3/3 | 3/3 | 0/1 | 0/1 | 3/3/3 (6–9) |
| Contact | 2/2 | 1/2 | 2/2 | 2/2 | 0/2 | 0/2 | 2/0/2 | |
| Gs/HK/437-8/99 | Inoculation | 3/3 | 3/3 | 1/2 | 2/2 | 1/2 | 2/2 | 3/2/3 (3–8) |
| Contact | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 0/2 | 2/0/2 | |
| Gs/HK/437-10/99 | Inoculation | 1/2 | 2/2 | 1/2 | 0/2 | 0/2 | 0/2 | 2/0/2 |
| Contact | 2/2 | 2/2 | 2/2 | 2/2 | 0/2 | 0/2 | 2/0/2 | |
| Gs/HK/485-3/00 | Inoculation | 3/3 | 3/3 | 3/3 | 3/3 | 2/3 | 2/3 | 3/0/3 |
| Contact | 0/2 | 0/2 | 1/2 | 1/2 | 1/2 | 1/2 | 2/1/2 (3) | |
| Gs/HK/485-5/00 | Inoculation | 3/3 | 3/3 | 2/2 | 2/2 | 2/2 | 2/2 | 3/1/3 (5) |
| Contact | 1/2 | 1/2 | 2/2 | 2/2 | 2/2 | 2/2 | 2/0/2 | |
Geese were infected nasally, tracheally, and orally with 106.5 EID50 of virus in a volume of 1.0 ml.
The geese were observed for 10 days after infection. Sick/dead/total, number of geese that were sick/number of geese that died/total number of geese. Numbers in parentheses are days on which birds died.
The pathogenicities of the viruses in chickens were determined as described previously (18). Three-week-old specific-pathogen-free chickens received inoculations with virus intravenously (injection volume, 0.2 ml) or tracheally or orally and into the nares and eyes in a total volume of 1.0 ml. Blood samples were collected from chickens 14 and 21 days after infection for serologic analysis. Tracheal and cloacal swabs were collected and analyzed for virus in chicken embryos. The 50% chicken infectious dose (dose of virus infecting 50% of inoculated chickens; CID50) was determined by infecting groups of four chickens with 10-fold dilutions of virus grown in chicken embryos. Chickens were swabbed tracheally and cloacally on days 3 and 5, and virus was detected by replication in 10-day-old chicken embryos. The 50% chicken lethal dose (CLD50) was determined from the number of deaths in the groups of infected chickens 10 days after infection.
Groups of three young adult quail (Coturnix coturnix) were infected orally, intranasally, and orbitally with 106.5 EID50 of virus in a volume of 0.5 ml. Tracheal and cloacal swabs were obtained, and the amounts of virus present in samples from infected quail were measured as described previously (9). The methods of determining the 50% quail infectious dose (QID50) and the 50% quail lethal dose (QLD50) were the same as those used in determining the CID50 and CLD50.
Young Pekin white ducks (2 months old) were inoculated with approximately 106.5 EID50 of virus in a total volume of 1.0 ml. The sites of inoculation were the trachea, the nares, eyes, and oral cavity (22). Tracheal and cloacal swabs were collected at different intervals, and influenza viruses within these samples were titrated in embryonated eggs.
All studies were carried out in U.S. Department of Agriculture-approved BL3+ facilities with animals that were free of influenza virus that could be detected by serologic or virologic assays. Because all experiments were done in a BL3+ containment facility, the total number of birds used in the experiments was limited by the amount of space in the facility (see Tables 3 to 7 for details). All poultry were observed for signs of disease, and food and water intake was monitored; the research complied with all relevant federal guidelines and institutional policies related to animal care.
TABLE 7.
Pathogenicity of goose H5N1 influenza virus isolates in micea
| Virus strain | No. of mice dead/totalb | Disease signs | Mean virus titer (log10 EID50 ± SE)c in:
|
||
|---|---|---|---|---|---|
| Lung on day:
|
Brain on day 3 | ||||
| 3 | 7 | ||||
| Gs/HK/437-4/99 | 4/8 | + | 3.2 ± 0.2 | 1.5 ± 0.1 | <0.1 |
| Gs/HK/437-6/99 | 0/10 | − | 4.2 ± 0.2 | 2.5 ± 0.6 | <0.1 |
| Gs/HK/437-8/99 | 4/8 | + | 2.8 ± 0.2 | 2.8 ± 0.4 | <0.1 |
| Gs/HK/437-10/99 | 4/8 | + | 2.3 ± 0.2 | 0.8 ± 0.5 | <0.1 |
| Gs/HK/485-3/00 | 4/10 | + | 2.5 ± 0 | 1.5 ± 0.5 | <0.1 |
| Gs/HK/485-5/00 | 8/10 | + | 3.2 ± 0.4 | 1.7 ± 0.7 | 0.3 ± 0.1 |
Each anesthetized mouse was intranasally inoculated with 106.5 or 106.75 EID50 of virus in a volume of 0.1 ml.
Determined during a period that ended 16 days after infection.
Whole brains and lungs were homogenized and resuspended in 1.0 ml of PBS before the virus titers were determined. The data were recorded for the tissues of three mice per group.
Transmission of H5N1 virus from geese to chickens or to quail.
Three- to 4-week-old Chinese white geese were housed in 0.4-m2 wire mesh cages and fed ad libitum. The birds were inoculated with 105.75 EID50 of A/goose/Hong Kong/437-4/99 (A/Gs/HK/437-4/99; H5N1) virus in a total volume of 1.0 ml by the tracheal and oral routes and via the eyes and nares. The infected birds were put into contact with three geese of the same age. Uninfected quail or specific-pathogen-free white leghorn chickens were placed in cages below and beside the cage containing the infected geese; the distance between the cages was approximately 30 cm. The dropping tray was removed from the goose cage to allow direct contact between the birds below and the feces of infected birds. Air in the containment cabinets flowed from the bottom through the top.
Infection studies of mice.
Female BALB/c mice (weight, 18 to 20 g; The Jackson Laboratory, Bar Harbor, Maine) were used. After the mice were anesthetized by inhaling isoflurane, each was intranasally inoculated with 106.5 or 106.75 EID50 of virus in a volume of 0.1 ml. Eight to 10 mice per group were observed daily for signs of infection and survival until 16 days after infection. The mice were weighed on days 0, 3, 5, 7, 9, 11, 14, and 16 after infection. Three mice from each experimental group were exsanguinated on days 3 and 7, and their lungs and brains were removed. The organs were ground and homogenized in 1 ml of cold phosphate-buffered saline (PBS), solid debris was pelleted by centrifugation at 2,000 × g for 10 min, and the tissues were used to determine the EID50 in 10-day-old embryonated chicken eggs. Virus titers in mouse lungs and brain are given in units of log10 EID50 per 0.1 ml ± standard error (SE). The limit of virus detection was 0.1 log10 EID50 per 0.1 ml. For dose-response experiments, BALB/c mice were infected with 107.2, 106.5, 105.5, 104.5, or 103.5 EID50 of A/Gs/HK/437-10/99 or A/Gs/HK/485-5/00 isolates in a volume of 0.1 ml.
Gene sequencing and analysis.
Viral gene sequencing and analysis were performed as previously described (10). In brief, we used the RNeasy Mini Kit (Qiagen, Inc., Valencia, Calif.) to extract RNA from viruses in infected allantoic fluid. Reverse transcription-PCR (RT-PCR) was performed with specific primers for each gene segment (primer sequences are available upon request). PCR products were purified with the QIAquick PCR purification kit (Qiagen, Inc.) and sequenced with synthetic oligonucleotides. Reactions were performed with rhodamine DyeTerminator cycle sequencing ready reaction kits and AmpliTaq DNA polymerase FS (Perkin-Elmer/Applied Biosystems, Inc.). Samples were subjected to electrophoresis and analyzed on model 377 DNA sequencers (Perkin-Elmer/Applied Biosystems, Inc.).
The sequence data were edited by using the Wisconsin sequence analysis package, version 10.0 (Genetics Computer Group, Madison, Wis.). Phylogenetic analyses were performed with PAUP (phylogenetic analysis using parsimony), version 4.0 (David Swofford, Illinois Natural History Survey, Champaign, Ill.).
Nucleotide sequence accession numbers.
The nucleotide sequences obtained from this study are available from GenBank under accession no. AF398417 through AF398432.
RESULTS
Isolation of H5N1 influenza virus from geese in Hong Kong.
Influenza viruses were isolated during routine virologic surveillance of geese and ducks imported into Hong Kong from Guangdong Province in February 1999 and February 2000. In February 1999, the HI titers of a consignment infected with Go/Gd-like virus ranged from 32 to 128 when the sera were tested against H5N1 virus. After treatment with receptor-destroying enzyme, five high-titer sera remained positive in the HI test. The associated geese showed no signs of disease. Four H5N1 virus isolates were obtained from feces collected from the floor and the walls of the cage of the infected geese.
In February 2000, the range of HI titers of the affected consignment was from 16 to 64. However, these sera lost HI activity after receptor-destroying enzyme treatment. Although these geese showed no signs of disease, two of the cloacal swab pools from geese in this consignment contained H5N1 virus.
Antigenic characterization of H5N1 goose influenza virus isolates.
To determine the antigenic subtype of the influenza viruses from geese and their relationship to other influenza viruses, the six goose influenza virus isolates were examined with a panel of reference sera to the 15 HA and 9 NA subtypes and with a panel of monoclonal antibodies to A/chicken/Pennsylvania/1370/83 (H5N2). Antigenic analysis with hyperimmune goat antisera to A/tern/South Africa/61 (H5N3) and monoclonal antibodies to A/chicken/Pennsylvania/1370/83 (H5) showed that the viruses are of the H5 subtype and are closely related, if not identical, to H5N1/97 (H5N1) (Table 1). One goose isolate (Gs/HK/437-6/99) was antigenically distinguishable from the rest when analyzed with hyperimmune sera and with monoclonal antibody CP-46. This result indicates some antigenic heterogeneity between Gs/HK/437-6/99 and the other isolates. NA inhibition tests showed that the NA of each isolate belongs to the N1 subtype.
TABLE 1.
Antigenic analysis of goose H5N1 influenza virus isolates
| Virus strain | HI titer for:
|
|||
|---|---|---|---|---|
| Polyclonal antiseraa | Monoclonal antibodies to CK/PA/1370/83b (H5)
|
|||
| CP-24 | CP-46 | CP-58 | ||
| Gs/HK/437-4/99 | 640 | 400 | 6,400 | 6,400 |
| Gs/HK/437-6/99 | 80 | 100 | 100 | 3,200 |
| Gs/HK/437-8/99 | 640 | 400 | 3,200 | 6,400 |
| Gs/HK/437-10/99 | 320 | 200 | 800 | 3,200 |
| Gs/HK/485-3/00 | 320 | 3,200 | 6,400 | 3,200 |
| Gs/HK/485-5/00 | 320 | 3,200 | 6,400 | 3,200 |
| HK/156/97 | 320 | 200 | 800 | 3,200 |
| Tern/South Africa/61 | 160 | <c | 1,600 | 400 |
| CK/PA/1370/83 | 160 | 3,200 | 6,400 | 3,200 |
Serum from a goat immunized with the isolated HA from A/tern/South Africa/61 HA (H5).
CK/PA/1370/83, chicken/Pennsylvania/1370/83.
<, below the detection limit of the test.
Genetic analysis of goose H5N1 viruses.
To determine the extent of genetic relatedness between the Go/Gd-like isolates collected in 2000 and those collected in 1996 and 1999 (7, 30), one virus (Gs/HK/485-3/00) was completely sequenced and compared with three representative viruses that were partially sequenced (Table 2). The analyzed gene segments from the two H5N1 Go/Gd-like isolates collected in 2000 were 99.6 to 100% homologous with each other. In addition, the gene segments of these H5N1 isolates were highly related to those of the isolates collected in 1996 and 1999. The PB2 and NA gene segments of the February 2000 isolates were 97.6 and 97.9% homologous with those of goose/Guangdong/1/96 and 99.2% homologous with those of the 1999 isolates (Table 2).
TABLE 2.
Homology between the gene segments of Gs/HK/485-3/00 (H5N1) and those of earlier isolates
| Gene product | Region of comparison (nucleotides) | % Homology with influenza virus:
|
||
|---|---|---|---|---|
| Gs/GD/1/96b | Gs/HK/437-10/99 | HK/156/97 | ||
| PB2 | 32–2274 | 97.6 | 99.2 | 86.3 |
| PB1 | 779–2304 | 98.3 | 99.7 | 93.1 |
| PA | 23–1441 | 98.5 | 99.6 | 89.3 |
| HA | 70–1733 | 98.4 | 98.9 | 98.1 |
| NP | 22–1533 | 98.6 | 99.1 | 93.3 |
| NA | 25–1433 | 97.9 | 99.2 | 86.3 (89.9)a |
| M | 66–974 | 98.7 | 98.9 | 91.9 |
| NS | 41–823 | 98.7 | 99.4 | 70.1 |
The homology shown in parentheses is that found when the deletions are not included in the comparison.
Gs/GD/1/96, goose/Guangdong/1/96.
Sequence analysis showed a limited number of amino acid changes scattered throughout the HA molecules. One change in the 2000 Gs/Gd isolates with potential functional relevance was the arginine-to-glycine substitution at residue −6 of HA1, resulting in shortening the series of basic amino acids associated with the HA of highly pathogenic avian influenza viruses. This substitution reduced the number of basic amino acids at the cleavage site from six to five. Sequence analysis showed no changes in the length of the stalk of the NA molecules.
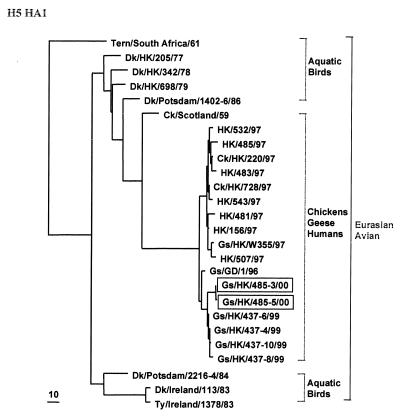
Phylogenetic analysis of each gene segment of the 2000 H5N1 goose isolates revealed genetic relationships similar to those previously reported for the 1996 and 1999 goose H5N1 isolates (7, 30). Phylogenetic analysis of the HA1 molecules showed that each goose H5N1 isolate from 1996, 1999, and 2000 is part of a separate sublineage (Fig. 1), whereas the HAs of H5N1 viruses isolated from poultry and humans in 1997 form a separate sublineage. Phylogenetic analysis of the seven other gene segments of the goose H5N1 isolates collected in 2000 showed a high degree of relatedness to goose H5N1 isolates from 1996 and 1999 but showed a marked difference from H5N1 isolates from humans and chickens in 1997 (results not shown).
FIG. 1.
Phylogenetic analysis of the HA1 genes of H5 influenza A viruses. The tree was generated with PAUP by using a maximum-parsimony algorithm. Nucleotides 77 through 1037 (961 bp) of the HA gene were analyzed. The tree is rooted to A/Japan/305/57 (H2N2). Only Eurasian lineages are shown. The lengths of the horizontal lines are proportional to the minimum numbers of nucleotide differences required to join nodes. Vertical lines are for spacing branches and labels. Abbreviations: Ck, chicken; Dk, duck; Env, environment; Gs, goose; Ty, turkey; GD, Guangdong; HK, Hong Kong.
Transmission and pathogenicity of goose H5N1 influenza viruses in geese.
In the field, A/goose/Guangdong/1/96 (H5N1) influenza virus reportedly killed 40% of infected geese (26). In contrast, no deaths occurred and no signs of disease were present among geese in the poultry market that contained H5N1 Go/Gd-like viruses in the present studies. These H5N1 viruses were isolated from goose holding pens or from cloacal samples at the port of entry into Hong Kong after shipment by boat from southern China.
To determine the pathogenicity and transmissibility of these H5N1 viruses, geese were inoculated with four isolates collected in 1999 and two from 2000. Four groups of geese were infected with one each of the four isolates and put into contact with uninfected geese. Three of the four H5N1 isolates collected in 1999 killed the inoculated animals, and one of the 2000 isolates also killed one of three inoculated geese (Table 3). The birds that died initially showed only mild signs of disease; some of these birds experienced weight loss, lethargy, and diarrhea. Virus was detected in tracheal and cloacal samples from all infected geese, and some surviving animals continued to shed virus for 12 days (data not shown). Titration of the organs from a dead goose (Gs/HK/485-5/00) revealed virus in the organs examined, the highest titers being in the liver (5.5 log10 EID50), kidney (4.50 log10 EID50), and lung (4.25 log10 EID50); lower titers were found in the heart (3.5 log10 EID50), brain (2.75 log10 EID50), and spleen (2.50 log10 EID50).
Each H5N1 isolate collected in 1999 was transmitted to geese that were in contact with the experimentally infected birds, and one goose died after being infected with the Gs/HK/437-4/99 isolate through contact. The other three isolates were detected in the trachea and cloaca for up to 5 days. These animals showed no overt signs of disease. In later transmission experiments, we used the single isolate that caused the death of one goose after it was infected through contact (i.e., Gs/HK/437-4/99) (data not shown). In these experiments, none of the four birds in contact with the infected birds, though infected, showed signs of disease or died of influenza infection. Despite the small number of geese that we were able to test, the overall findings indicate that high doses of virus can kill geese but that, when viruses are transmitted to geese by contact, the mortality rate is relatively low.
Replication and transmission of A/Gs/HK/99 (H5N1) influenza viruses in ducks.
Because domestic ducks are considered a possible intermediate host between wild aquatic ducks and other domestic poultry in the natural transmission of influenza viruses, we studied the ability of domestic ducks to support the replication of the goose H5N1 viruses. Two H5N1 isolates collected in 1999 were used to inoculate Pekin white ducks, which were subsequently put into contact with uninfected ducks (Table 4). Each H5N1 isolate replicated in the four inoculated animals in each group and were detected in the cloaca on days 3 or 5; the virus titers ranged from <2.0 to >5.0 log10 EID50 per 0.1 ml. Viruses were also detected in the tracheas of most birds; virus titers were from <2.0 to >5.5 log10 EID50 per 0.1 ml. Detection of H5N1 viruses in ducks that were in contact with the experimentally infected ducks varied; because the number of birds used in the experiment was small, we can only conclude that the viruses can be transmitted to ducks through contact and can be detected in the tracheas and the cloacae of ducks infected through contact. None of the ducks showed signs of disease, and they consumed normal amounts of food.
TABLE 4.
Replication and transmission of A/Gs/HK/99 (H5N1) virus in ducksa
| Virus strain | Method of transmission | Virus shedding on postinfection day: signs
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 3
|
5
|
||||||||
| Trachea
|
Cloaca
|
Trachea
|
Cloaca
|
||||||
| No. of birds shedding/total | Infectivity titer (log10/0.1 ml) range | No. of birds shedding/total | Infectivity titer (log10/0.1 ml) range | No. of birds shedding/total | Infectivity titer (log10/0.1 ml) range | No. of birds shedding/total | Infectivity titer (log10/0.1 ml) range | ||
| Gs/HK/437-4/99 | Inoculation | 2/4 | ≤2.0–≥5.2 | 2/4 | ≤2.0–2.2 | 2/4 | ≤2.0–2.5 | 4/4 | ≤2.2–3.3 |
| Contact | 2/2 | 2.2–3.5 | 1/2 | 2.0–2.5 | 0/2 | <b | 1/2 | ≤2.0–≤2.2 | |
| Gs/HK/437-6/99 | Inoculation | 4/4 | 2.8–≥5.5 | 4/4 | 3.5–4.2 | 0/4 | < | 2/4 | ≤2.0–2.5 |
| Contact | 1/2 | ≤2.0–2.5 | 1/2 | ≤2.0–≥5.2 | 0/2 | < | 1/2 | ≤2.0–≤2.2 | |
Ducks were inoculated with approximately 106.5 EID50 as given in Materials and Methods. No inoculated ducks exhibited signs of disease.
<, no detectable virus.
Pathogenicity of goose H5N1 influenza viruses in chickens.
The intravenous injection of virus-containing allantoic fluid into chickens is the U.S. Department of Agriculture’s approved method of determining the pathogenicity of influenza viruses in chickens. If the viruses are highly pathogenic, they will kill six of eight inoculated birds in 10 days (18). In addition, the amino acid sequence at the connecting peptide region of HA0 is expected to possess a series of basic amino acids.
After intravenous injection into chickens, each of the six goose isolates collected in 1999 or 2000 caused disease signs typical of those seen in chickens infected with highly pathogenic influenza viruses, e.g., swollen heads and blue combs, and killed seven or all eight of the infected birds (Table 5). Oral or nasal inoculation of chickens with the 1999 goose isolates produced disease signs typical of those seen in chickens infected with highly pathogenic avian influenza viruses, but these routes of inoculation resulted in the deaths of only one or two of the five birds (Table 5). In contrast the 2000 isolates killed three-fourths of chickens when inoculated by the oral or nasal route. Thus, our findings indicate that both the 1999 and 2000 goose H5N1 isolates are highly pathogenic in chickens and that each virus has a series of basic amino acids adjacent to the HA cleavage site (Table 5).
TABLE 5.
Pathogenicity of goose H5N1 influenza viruses in chickens and quail
| Virus strain | No. of chickens sick/dead/total after:
|
No. of quail sick/dead/total after oral and nasal inoculation | Sequence through connecting pep-tide of HA | |
|---|---|---|---|---|
| Intravenous inoculation | Oral and nasal inoc-ulation | |||
| Gs/HK/437-4/99 | 8/7/8 | 5/1/5 | NTa | TPQRERRRKKR |
| Gs/HK/437-6/99 | 8/8/8 | 5/2/5 | 8/7/8 | TPQRERRRKKR |
| Gs/HK/437-8/99 | 8/7/8 | 5/1/5 | 8/6/8 | TPQRERRRKKR |
| Gs/HK/437-10/99 | 8/7/8 | 5/1/5 | 8/6/8 | TPQRERRRKKR |
| Gs/HK/485-3/00 | 8/8/8 | 4/3/4 | 8/8/8 | TPQREGRRKKR |
| Gs/HK/485-5/00 | 8/8/8 | 3/3/4 | 8/8/8 | TPQREGRRKKR |
| HK/156/97 | 8/8/8 | 8/8/8 | NT | TPQRERRRKKR |
NT, not tested.
Pathogenicity of goose H5N1 viruses in quail.
Because quail were the source of the A/quail/Hong Kong/G1/97 (H9N2) virus, which may have been the donor of genes encoding internal proteins for the H5N1/97-like viruses, we examined the ability of quail to support the replication of the goose H5N1 viruses, the reported donors of the HA gene for the H5N1/97-like viruses (30). Each goose H5N1 virus was highly pathogenic in quail that had been inoculated orally and intranasally (Table 5). The birds became lethargic by the third day and died between days 3 and 7 after inoculation. Titration of the organs of quail that died on days 3 to 5 revealed virus in all organs examined, the highest titers being detected in the brains (4.3 to 7.5 log10 EID50) and pancreases (0 to >5.0 log10 EID50), with lower titers in the lungs (3.55 to 4.5 log10 EID50) and liver (<1 to 2.8 log10 EID50). Thus each of the goose H5N1 viruses was highly pathogenic in quail.
Transmission of A/Gs/HK/437-4/1999 (H5N1) from geese to chickens and to quail.
Given the role of the goose in the genesis of the H5N1 virus in 1997, we determined whether H5N1 influenza viruses isolated from geese could spread from geese to other domestic poultry, i.e., chickens and quail. Chinese white geese were infected with the representative Gs/HK/437-4/99 isolate and put into the same cages as the uninfected birds. Transmission by contact with feces was also studied by housing quail or chickens in cages below the geese. Aerosol transmission of virus was studied by placing quail or chickens in cages that were beside the cages of infected geese at a distance of approximately 0.3 m.
Infection of geese with Gs/HK/437-4/99 resulted in the deaths of more than 50% of the birds (Table 6), but none of the geese that were in contact, with the infected ones died despite acquiring infection through heavy fecal contamination of the cages and water troughs. In contrast, all four chickens that had contact with goose feces became infected and two died after they showed signs typical of highly pathogenic influenza infection. Tracheal and cloacal samples collected on days 3, 5, 7, 9, and 14 contained virus (details not shown); virus was isolated more consistently from cloacal samples than from tracheal samples. In contrast to the results for fecal transmission to chickens, there was no evidence of aerosol transmission of virus to chickens. Studies of transmission of Gs/HK/437-4/99 (H5N1) to quail revealed that all birds (three of three) that were in contact with goose feces and two of the four birds with exposure to aerosol spread died after they had displayed signs typical of highly pathogenic influenza infection. In the quail studies, viruses were isolated more consistently from tracheal samples than from cloacal samples (results not shown). Thus, our findings indicate that quail were infected by aerosol exposure to geese infected with Gs/HK/437-4/99, whereas chickens were not.
TABLE 6.
Transmission of A/Gs/HK/437-4/99 (H5N1) from geese to chickens and quail
| Animals | Method of transmission | No. of birds dead/total no. (days that birds died) | No. of birds shedding virus/total no. on day 5
|
Days on which virus was shed
|
||
|---|---|---|---|---|---|---|
| Trachea | Cloaca | Trachea | Cloaca | |||
| Geese | Direct inoculation | 4/7 (1–7) | 3/3 | 3/3 | 3–11 | 3–14 |
| Physical contact | 0/4 | 4/4 | 4/4 | 3–14 | 3–14 | |
| Chickens | Fecal contact | 2/4 (6–10) | 1/4 | 4/4 | 3–7 | 3–14 |
| Aerosol contact | 0/4 | 0/4 | 0/4 | 0 | 0 | |
| Quail | Fecal contact | 3/3 (7–9) | 3/3 | 2/3 | 3–9 | 3–9 |
| Aerosol contact | 2/4 (11–14) | 3/4 | 0/4 | 3–9 | 3–9 | |
Dose of A/Gs/HK/437-4/99 (H5N1) required to infect quail and chickens.
The above findings indicating that a representative H5N1 isolate (Gs/HK/437-4/99) can be transmitted by the aerosol route to quail but not to chickens raise the question of whether quail are more susceptible than chickens to infection with this virus. To answer this question, we determined the QID50 and QLD50 in quail and the CID50 and CLD50 in chickens of the Gs/HK/437-4/99 (H5N1) virus. The QID50 and QLD50 required 1.7 and 2.5 log10 EID50 of virus, respectively (average of two independent experiments), while the CID50 and CLD50 required 3.8 and 4.0 log10 EID50 of virus, respectively. Therefore, quail are more susceptible to infection with Gs/HK/437-4/99 (H5N1) than chickens and less virus is needed to kill quail than to kill chickens.
Pathogenicity of goose H5N1 influenza viruses in mice.
Infection of mice with each of the six isolates resulted in virus replication in the lungs; titers ranged from 2.3 to 4.2 log10 EID50 on day 3 after infection. On that day, low levels of virus were detected in the brains of mice infected with Gs/HK/485-5/00, but no virus was detected in the brains of mice infected with the other goose isolates.
The six goose isolates demonstrated differences in pathogenicity in mice: with the exception of Gs/HK/437-6/99, the goose viruses collected in 1999 killed 4 of 8 mice in each group, whereas the isolates collected in 2000 killed 4 to 8 mice in groups of 10 (Table 7). Isolate Gs/HK/437-6/99 failed to cause disease or death in any of the 10 mice infected. Five of the six goose isolates caused signs of infection, including ruffled fur and weight loss. The greatest percentage of weight loss occurred on day 7 after infection: the initial weight decreased by as much as 34.5% (Fig. 2).
We determined whether the degree of viral pathogenicity in mice was related to the dose of inoculated virus. Two of the more pathogenic isolates (Gs/HK/437-10/99 and Gs/HK/485-5/00) served as representative viruses. The degree of pathogenicity decreased as the dose of inoculating virus decreased. Gs/HK/437-10/99 caused the deaths of mice infected with 7.2, 6.5, and 5.5 log10 EID50 (data not shown). The Gs/HK/485-5/00 isolate was even more pathogenic, as virus inoculation at a dose of 4.5 log10EID50 killed two out of eight mice (data not shown). For most viruses the number of mice that died of infection and the number of mice that showed signs of disease were positively correlated with the level of virus in the lungs and the amount of weight that was lost. However Gs/HK/437-6/99 had higher titers in the lungs of mice on day 3 but caused no mortality, illustrating that these are a heterogeneous group of viruses in biological behavior.
DISCUSSION
Go/Gd (H5N1)-like viruses continue to be isolated from geese in southeastern China. These viruses were one of the proposed precursors of H5N1/97, which was transmitted to and, in some instances, caused the deaths of humans in Hong Kong in 1997. In the present investigation of the transmissibility and pathogenicity of the Go/Gd-like viruses, we found that quail are more susceptible to infection by these viruses than chickens or ducks and that Go/Gd-like viruses can be transmitted to quail by aerosols. When geese were inoculated with high doses of Go/Gd-like viruses, half of the geese experienced a generalized systemic infection, whereas natural transmission between geese resulted in few deaths. Our findings suggest that the H5N1 viruses are likely to be transmitted to other poultry in southern China, especially to quail in a poultry market setting.
A/quail/Hong Kong/G1/97 (H9N2)-like influenza viruses, whose six genes encoding internal proteins are similar to those of H5N1/97 (H5N1), continue to circulate in quail (9). The detection of A/quail/Hong Kong/G1/97-like viruses in up to 16% of quail cages in the Hong Kong live-poultry markets and the serologic evidence of infection in approximately 60% of poultry imported into Hong Kong (9) emphasize the widespread distribution of this virus genotype in southeastern China. Studies have indicated that H9N2 influenza viruses are widespread throughout Eurasia and Africa: they have been isolated from birds in Germany, Italy, Ireland, Iran, Pakistan, Saudi Arabia, and South Africa (2, 3). The gene segments of the A/chicken/Pakistan/2/99 (H9N2) isolate are closely related (97 to 99% homology) to those of the H9N2 viruses isolated from humans and quail in Hong Kong in 1999 (6). H6N1 viruses that are closely related to A/teal/Hong Kong/W312/97 (H6N1) also continue to circulate in domestic poultry in Hong Kong, especially in quail (unpublished results). Therefore, the possible ancestors of the H5N1 viruses that were transmitted to humans in 1997 are still present in Asia: the precursor HA genes continue to circulate in geese, and viruses containing the H5N1/97-like genes encoding internal proteins continue to circulate in quail. The high susceptibility of quail to Go/Gd (H5N1)-like viruses, the continued circulation of H9N2 viruses containing the replication gene complex of HK/156 (H5N1)-like viruses, and the presence of H6N1 viruses similar to A/teal/HongKong/W312/97-like viruses in quail raise the possibility that quail were the host for the reassortment event that resulted in the emergence of the H5N1/97 virus. Such findings validate the decision to change the market system so that aquatic birds are no longer housed in live-poultry markets in Hong Kong and are only available as killed, chilled poultry. Because the Go/Gd-like viruses can be transmitted by aerosol to quail, it is important to keep quail well separated from aquatic birds to prevent the re-emergence of H5N1/97-like viruses.
The H5N1 viruses isolated from goose stalls in 1999 and 2000 were pathogenic in geese when high doses were administered but killed only 1 of 12 geese that were infected through contact (combined results in Tables 3 and 6). This finding suggests that apparently healthy geese can shed virus and that, when they are coinfected with other agents or receive very high doses of virus, signs of disease develop. In the field, cocirculating bacteria and other agents may lead to increased mortality. The rather long period of virus shedding (up to 14 days) from the respiratory tract and in feces is noteworthy. The viruses could be maintained in a flock of geese that show no evidence of disease for an extended period.
In the report by Cauthen et al. (7), the H5N1 influenza viruses isolated from goose holding pens were designated A/environment/Hong Kong/437-4/99, A/environment/Hong Kong/437-6/99, A/environment/Hong Kong/437-8/99, and A/environment/Hong Kong/437-10/99. Although these designations are technically correct, we argue that it would have been useful to include the name of the probable host in the designation because the shipment of geese had antibodies to H5. Many virus-containing samples from domestic and wild birds are from feces under birdcages or in close association with pelagic birds. Although some doubt about the origin of samples not directly collected from animals exists, the absence of information about the probable host omits potentially important ecological information.
Previous studies on the pathogenicity of the H5N1 Go/Gd-like viruses collected in 1999 revealed that these viruses replicate in the lungs of mice but do not cause weight loss or death (7). The present studies showed that Go/Gd-like isolates Gs/HK/437-4/99, Gs/HK/437-8/99, and Gs/HK/437-10/99 caused decreases of up to 30% in initial weight and killed up to 50% of infected mice (Table 7). The Go/Gd-like H5N1 isolates collected in 2000 (Gs/HK/485-3/00 and Gs/HK/485-5/00) also caused weight loss and death; low titers of virus were detected in the brains of mice infected with the Gs/HK/485-5/00 isolate. We speculate that the differences in pathogenicity are due to the presence of minor subpopulations of virus, but the present studies do not attempt to resolve this possibility. The differences between our findings and those that were previously published (7) probably result from differences in the doses of virus administered. In our case, high doses of virus were used (6.5 to 6.75 log10 EID50), but in dose-response studies with two viruses, weight loss and death were not observed when doses of 4.5 log10 EID50 or lower of virus were administered intranasally.
The role of ducks in the natural history of influenza A viruses is well established (29). However, the available evidence suggests that wild ducks do not maintain H5 influenza viruses in nature (20). Nonpathogenic forms of H5 influenza viruses have been more consistently isolated from seabirds, particularly shorebirds (25). The natural reservoirs of H5 and H7 viruses have not really been identified, because no systematic study has been done (20). Shorebirds and gulls (Charadrii formes) are the likely source of these viruses, but the evidence supporting this statement is largely circumstantial. The first known isolate of H5 virus (A/tern/South Africa/61 [H5N3]) was recovered from dead terns in South Africa (5) and was highly pathogenic in chickens. Since that time, highly pathogenic H5 influenza viruses have not been associated with the deaths of wild aquatic birds. Moreover, the sequence of the connecting peptide of avirulent H5 HAs is typically QRETR/G. After transmission to domestic poultry, including turkeys, chickens, and quail, the H5 influenza viruses can become highly pathogenic, but these viruses are nonpathogenic in ducks (1). A high degree of pathogenicity is associated with multiple basic amino acids at the cleavage site of HA (for example, the sequence in A/chicken/Scotland/59 [H5N1] is QRKKR) (reviewed in reference 12). The number of basic amino acids varies and can be influenced by an adjacent carbohydrate (13). These differing lengths of basic amino acids have been postulated to be the result of stuttering by the polymerase during replication (16).
Limited information on the susceptibility and transmissibility of influenza viruses in quail is available. Studies with A/turkey/Ontario/7732/66 (H5N9) in quail indicate tracheal replication and limited pathogenicity on initial inoculation (23, 27), but after adaptation to quail the virus became highly pathogenic (27). It is noteworthy that virus was transmitted between quail by contact, suggesting the possibility of aerosol transmission (27).
The present transmission studies of the A/goose/Guangdong/1/96 (H5N1)-like viruses in geese indicate that the virus can be transmitted to geese and cause high morbidity but limited mortality. Of greatest concern is the aerosol transmission of these H5N1 viruses to quail, which are more susceptible to infection and death than chickens. The cocirculation of Eurasian lineage H9N2 influenza viruses in poultry can influence the pathogenicity of H5N1 viruses, if the birds were recently infected with H9N2 viruses. Studies have demonstrated that infection of chickens with H9N2 influenza viruses that emerged in Asia can result in protection against lethal H5N1 infection (14, 19). This protection is mediated by CD8+ T cells but not by CD4+ T cells, and the chickens shed H5N1 viruses from their trachea and in their feces (19). Thus, the lethal effects of the H5N1 viruses can be masked in apparently healthy birds, but these birds can continue to shed highly pathogenic H5N1 viruses. Preliminary studies indicate that the cocirculation of H9N2 viruses can protect chickens against the appearance of disease signs and death caused by Go/Gd-like viruses.
The continuing circulation of the precursor viruses of the H5N1/97-like virus is of concern because, if the Gs/Gd-like viruses are transmitted to land-based poultry such as quail and chickens that are immune to H9N2 viruses, then they could be reintroduced into the Hong Kong poultry markets.
The concern expressed in the above paragraph was validated in May 2001 when H5N1 virus reassortants containing Go/Gd-like HA and NA were transmitted to land-based poultry in Hong Kong SAR. The reappearance of H5N1 in land-based poultry resulted in the decision to slaughter all live poultry in Hong Kong for a second time in 4 years. The continued circulation of the precursor viruses of H5N1/97-like viruses in southeastern China in 2001 is a concern that emphasizes the need for persistent influenza surveillance.
Acknowledgments
This study was supported by Public Health Research grants AI29680 and AI95357 from the National Institute of Allergy and Infectious Diseases, Wellcome Trust grant 057476/Z/99/Z, Cancer Center Support (CORE) grant CA-21765, and the American Lebanese Syrian Associated Charities. Support for Robert G. Webster to work in Hong Kong on this project was provided by the University of Hong Kong under the distinguished visiting scholars scheme.
We gratefully acknowledge the continued support of the officers of the Food and Environmental Hygiene Department, Hong Kong. We thank L. J. Zhang, P. Ghose, Jennifer Humberd, Patrick Seiler, C. Y. Cheung, and C. H. Lee for excellent technical support of this study, Alice Herren and Laurie Twit for preparation of the manuscript, and Julia Cay Jones for editorial assistance.
REFERENCES
- 1.Alexander, D. J., G. Parsons, and R. J. Manvell. 1986. Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtypes for chickens, turkeys, ducks and quail. Avian Pathol. 15:647–662. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, D. J. 1998. Avian influenza in the eastern hemisphere (excluding the Pacific basin) during 1992–1997, p. 9–13. In D. E. Swayne and R. D. Slemons (ed.), Proceedings of the Fourth International Symposium on Avian Influenza, U.S. Animal Health Association, Tallahassee, Fla.
- 3.Alexander, D. J. 2000. A review of avian influenza in different bird species. Vet. Microbiol. 74:3–13. [DOI] [PubMed] [Google Scholar]
- 4.Aymard-Henry, M., M. T. Colman, W. R. Dowdle, W. G. Laver, G. C. Schild, and R. G. Webster. 1973. Influenza virus neuraminidase-inhibition test procedures. Bull. W. H. O. 48:199–202. [PMC free article] [PubMed] [Google Scholar]
- 5.Becker, W. B. 1966. The isolation and classification of tern influenza virus A/tern/South Africa/1961. J. Hyg. 64:309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron, K. R., V. Gregory, J. Banks, I. H. Brown, D. J. Alexander, A. J. Hay, and Y. P. Lin. H9N2 subtype influenza A viruses in poultry in Pakistan are closely related to the H9N2 viruses responsible for human infection in Hong Kong. Virology 278:36–41. [DOI] [PubMed]
- 7.Cauthen, A. N., D. E. Swayne, S. Schultz-Cherry, M. L. Perdue, and D. L. Suarez. 2000. Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans. J. Virol. 74:6592–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claas, E. C. J., A. D. M. E. Osterhaus, R. Van Beck, J. C. de Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A (H5N1) virus related to a highly pathogenic avian influenza virus. Lancet 251:472–477. [DOI] [PubMed] [Google Scholar]
- 9.Guan, Y., K. F. Shortridge, S. Krauss, P. S. Chin, K. C. Dyrting, T. M. Ellis, R. G. Webster, and M. Peiris. 2000. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J. Virol. 74:9372–9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan, Y., K. F. Shortridge, S. Krauss, and R. G. Webster. 1999. Molecular characterization of H9N2 influenza viruses; were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. USA 96:9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann, E., J. Stech, I. Leneva, S. Krauss, C. Scholtissek, P. O. Chin, M. Peiris, K. F. Shortridge, and R. G. Webster. 2000. Characterization of the influenza A gene pool in avian species in southern China: was H6N1 a derivative or a precursor of H5N1? J. Virol. 74:6309–6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito, T., and Y. Kawaoka. 1998. Avian influenza, p. 126–136. In K. G. Nicholson, R. G. Webster, and A. J. Hay (ed.). Textbook of influenza. Blackwell Sciences Ltd., Oxford, United Kingdom.
- 13.Kawaoka, Y., C. W. Naeve, and R. G. Webster. 1984. Is virulence of H5N2 influenza viruses in chickens associated with loss of carbohydrate from the hemagglutinin? Virology 139:303–316. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill, E., S. Seo, D. Woodland, K. Shortridge, and R. G. Webster. Infection with H9N2 influenza viruses confers immunity against lethal H5N1 infection. Options Control Influenza IV, in press.
- 15.Palmer, D. F., M. T. Colman, W. R. Dowdle, and G. C. Schild. 1975. Advanced laboratory techniques for influenza diagnosis. Immunol. Ser. 6:51–52. [Google Scholar]
- 16.Perdue, M. L., M. Garcia, D. Senne, and M. Fraire. 1997. Virulence-associated sequence duplication at the hemagglutinin cleavage site of avian influenza viruses. Virus Res. 49:173–186. [DOI] [PubMed] [Google Scholar]
- 17.Röhm, C., J. Suss, V. Pohle, and R. G. Webster. 1996. Different hemagglutinin cleavage site variants of H7N7 in an influenza outbreak in chickens in Leipzig, Germany. Virology 218:253–257. [DOI] [PubMed] [Google Scholar]
- 18.Senne, D. A., B. Panigrahy, Y. Kawaoka, J. E. Pearson, J. Suss, M. Lipkind, H. Kida, and R. G. Webster. 1996. Survey of the hemagglutinin (HA) cleavage site sequences of H5 and H7 avian influenza viruses: amino acid sequence at the HA cleavage site as a marker of pathogenicity potential. Avian Dis. 40:425–437. [PubMed] [Google Scholar]
- 19.Seo, S., and R. G. Webster. 2001. Cross-reactive cell-mediated immunity and protection of chickens from lethal H5N1 influenza virus infection in the Hong Kong poultry markets. J. Virol. 75:2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharp, G. B., Y. Kawaoka, S. M. Wright, B. Turner, V. Hinshaw, and R. G. Webster. 1993. Wild ducks are the reservoir for only a limited number of influenza A subtypes. Epidemiol. Infect. 110:161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shortridge, K. F. 1999. Poultry and the influenza H5N1 outbreak in Hong Kong, 1997: abridged chronology and virus isolation. Vaccine 17:S26–S29. [DOI] [PubMed] [Google Scholar]
- 22.Shortridge, K. F., N. N. Zhou, Y. Guan, P. Gao, T. Ito, Y. Kawaoka, S. Kodihalli, S. Krauss, D. Markwell, K. G. Murti, M. Norwood, D. Senne, L. Sims, A. Takada, and R. G. Webster. 1998. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology 252:331–342. [DOI] [PubMed] [Google Scholar]
- 23.Slemons, R. D., and B. C. Easterday. 1972. Host response differences among 5 avian species to an influenza virus—A/turkey/Ontario/7732/66 (Hav5N?). Bull W. H. O. 47:521–525. [PMC free article] [PubMed] [Google Scholar]
- 24.Subbarao, K., A. Klimov, J. Katz, H. Regenery, W. Lim, H. Hall, M. Perdue, D. Swayne, C. Bender, J. Huang, M. Hemphill, T. Rowe, M. Shaw, X. Xu, K. Fukuda, and N. Cox. 1998. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279:393–396. [DOI] [PubMed] [Google Scholar]
- 25.Suss, J., J. Schafer, H. Sinnecker, and R. G. Webster. 1994. Influenza virus subtypes in aquatic birds of eastern Germany. Arch. Virol. 135:101–114. [DOI] [PubMed] [Google Scholar]
- 26.Tang, X., G. Tian, J. Zhao, and K. Y. Zhou. 1998. Isolation and characterization of prevalent strains of avian influenza viruses in China. Chin. J. Anim. Poult. Infect. Dis. 20:1–5. (In Chinese.) [Google Scholar]
- 27.Tashiro, M., M. Reinacher, and R. Rott. 1987. Aggravation of pathogenicity of an avian influenza virus by adaptation to quails. Arch. Virol. 93:81–95. [DOI] [PubMed] [Google Scholar]
- 28.Webster, R. G., V. A. Isachenko, and M. Carter. 1974. A new avian influenza virus from feral birds in the USSR: recombination in nature? Bull. W. H. O. 51:324–332. [PMC free article] [PubMed] [Google Scholar]
- 29.Webster, R. G., W. J. Bean, O. T. Gorman, T. M. Chambers, and Y. Kawaoka. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56:152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu, S. K., K. Subbarao, N. J. Cox, and Y. Guo. 1999. Genetic characterization of the pathogenic influenza A/goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 261:15–19. [DOI] [PubMed] [Google Scholar]
- 31.Yuen, K. Y., P. K. S. Chan, M. Peiris, D. N. C. Tsang, T. L. Que, K. F. Shortridge, P. T. Cheung, W. K. To, E. T. F. Ho, R. Sung, A. F. B. Cheng, and members of the H5N1 Study Group. 1998. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 351:467–471. [DOI] [PubMed] [Google Scholar]